Abstract
We report the generation of transgenic mice deficient in the metallothionein MT-I and MT-II genes. The mutations were introduced into embryonic stem cells by homologous recombination. Chimeric mice resulting from the targeted embryonic stem cells transmitted the disrupted alleles through their germ line. Homozygous animals were born alive and appeared phenotypically normal and fertile. Absence of MT proteins was confirmed by direct measurement in liver extracts. Challenging the mutant animals with moderate levels of CdSO4 indicated their greater susceptibility to cadmium toxicity than wild-type animals. These mice should provide a useful model to allow detailed study of the physiological roles of MT-I and MT-II.
Full text
PDF

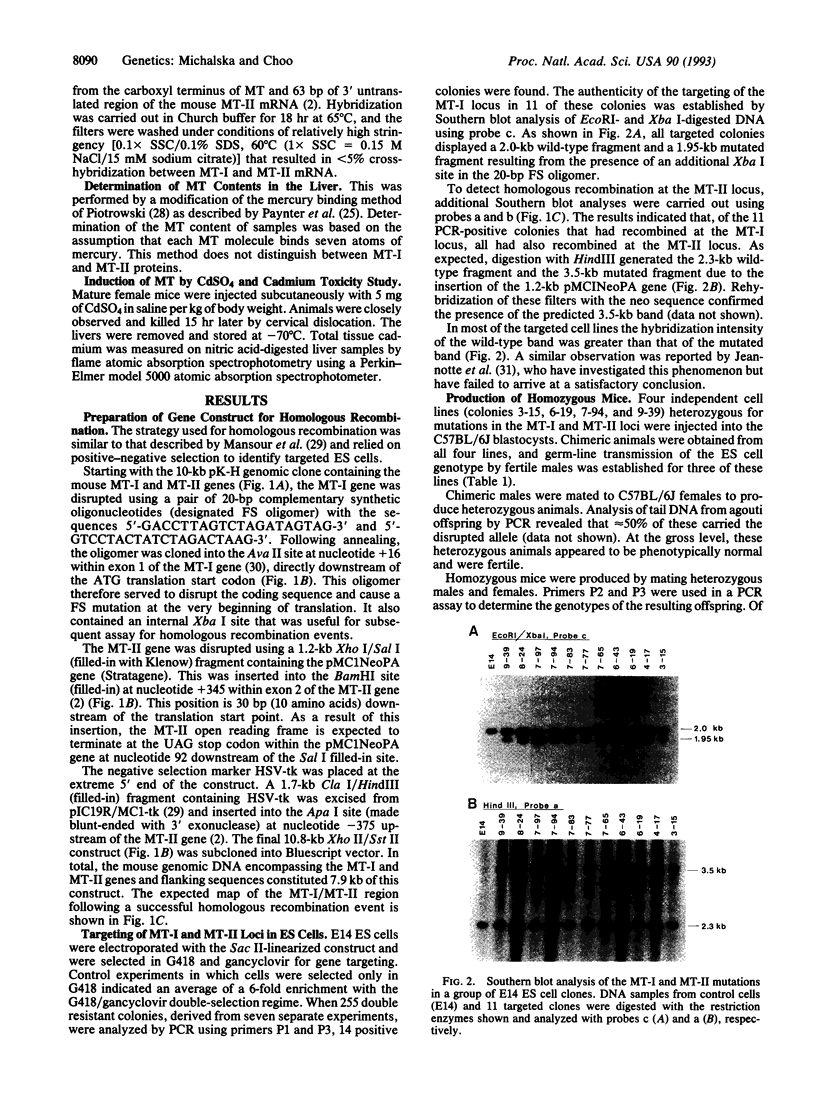

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews G. K., Adamson E. D., Gedamu L. The ontogeny of expression of murine metallothionein: comparison with the alpha-fetoprotein gene. Dev Biol. 1984 Jun;103(2):294–303. doi: 10.1016/0012-1606(84)90317-8. [DOI] [PubMed] [Google Scholar]
- Andrews G. K., Huet-Hudson Y. M., Paria B. C., McMaster M. T., De S. K., Dey S. K. Metallothionein gene expression and metal regulation during preimplantation mouse embryo development (MT mRNA during early development). Dev Biol. 1991 May;145(1):13–27. doi: 10.1016/0012-1606(91)90209-l. [DOI] [PubMed] [Google Scholar]
- Andrews G. K., Huet Y. M., Lehman L. D., Dey S. K. Metallothionein gene regulation in the preimplantation rabbit blastocyst. Development. 1987 Jul;100(3):463–469. doi: 10.1242/dev.100.3.463. [DOI] [PubMed] [Google Scholar]
- Braun T., Rudnicki M. A., Arnold H. H., Jaenisch R. Targeted inactivation of the muscle regulatory gene Myf-5 results in abnormal rib development and perinatal death. Cell. 1992 Oct 30;71(3):369–382. doi: 10.1016/0092-8674(92)90507-9. [DOI] [PubMed] [Google Scholar]
- Bremner I. Nutritional and physiological significance of metallothionein. Experientia Suppl. 1987;52:81–107. doi: 10.1007/978-3-0348-6784-9_5. [DOI] [PubMed] [Google Scholar]
- De S. K., Enders G. C., Andrews G. K. High levels of metallothionein messenger RNAs in male germ cells of the adult mouse. Mol Endocrinol. 1991 May;5(5):628–636. doi: 10.1210/mend-5-5-628. [DOI] [PubMed] [Google Scholar]
- Dobner P. R., Kawasaki E. S., Yu L. Y., Bancroft F. C. Thyroid or glucocorticoid hormone induces pre-growth-hormone mRNA and its probable nuclear precursor in rat pituitary cells. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2230–2234. doi: 10.1073/pnas.78.4.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durnam D. M., Palmiter R. D. Transcriptional regulation of the mouse metallothionein-I gene by heavy metals. J Biol Chem. 1981 Jun 10;256(11):5712–5716. [PubMed] [Google Scholar]
- Durnam D. M., Perrin F., Gannon F., Palmiter R. D. Isolation and characterization of the mouse metallothionein-I gene. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6511–6515. doi: 10.1073/pnas.77.11.6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evering W. E., Haywood S., Bremner I., Trafford J. The protective role of metallothionein in copper overload: I. Differential distribution of immunoreactive metallothionein in copper-loaded rat liver and kidney. Chem Biol Interact. 1991;78(3):283–295. doi: 10.1016/0009-2797(91)90059-g. [DOI] [PubMed] [Google Scholar]
- Glanville N., Durnam D. M., Palmiter R. D. Structure of mouse metallothionein-I gene and its mRNA. Nature. 1981 Jul 16;292(5820):267–269. doi: 10.1038/292267a0. [DOI] [PubMed] [Google Scholar]
- Hamer D. H. Metallothionein. Annu Rev Biochem. 1986;55:913–951. doi: 10.1146/annurev.bi.55.070186.004405. [DOI] [PubMed] [Google Scholar]
- Huang I. Y., Kimura M., Hata A., Tsunoo H., Yoshida A. Complete amino acid sequence of mouse liver metallothionein-II. J Biochem. 1981 Jun;89(6):1839–1845. doi: 10.1093/oxfordjournals.jbchem.a133385. [DOI] [PubMed] [Google Scholar]
- Huang I. Y., Yoshida A. Mouse liver metallothioneins. Complete amino acid sequence of metallothionein-I. J Biol Chem. 1977 Nov 25;252(22):8217–8221. [PubMed] [Google Scholar]
- Jeannotte L., Ruiz J. C., Robertson E. J. Low level of Hox1.3 gene expression does not preclude the use of promoterless vectors to generate a targeted gene disruption. off. Mol Cell Biol. 1991 Nov;11(11):5578–5585. doi: 10.1128/mcb.11.11.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. S., Spiegelman B. M., Papaioannou V. Pleiotropic effects of a null mutation in the c-fos proto-oncogene. Cell. 1992 Nov 13;71(4):577–586. doi: 10.1016/0092-8674(92)90592-z. [DOI] [PubMed] [Google Scholar]
- Karin M. Metallothioneins: proteins in search of function. Cell. 1985 May;41(1):9–10. doi: 10.1016/0092-8674(85)90051-0. [DOI] [PubMed] [Google Scholar]
- Koller B. H., Smithies O. Inactivating the beta 2-microglobulin locus in mouse embryonic stem cells by homologous recombination. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8932–8935. doi: 10.1073/pnas.86.22.8932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li E., Bestor T. H., Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992 Jun 12;69(6):915–926. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- Mansour S. L., Thomas K. R., Capecchi M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988 Nov 24;336(6197):348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Findley S. D., Whitmore T. E., Durnam D. M. MT-III, a brain-specific member of the metallothionein gene family. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6333–6337. doi: 10.1073/pnas.89.14.6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paynter J. A., Camakaris J., Mercer J. F. Analysis of hepatic copper, zinc, metallothionein and metallothionein-Ia mRNA in developing sheep. Eur J Biochem. 1990 May 31;190(1):149–154. doi: 10.1111/j.1432-1033.1990.tb15558.x. [DOI] [PubMed] [Google Scholar]
- Piotrowski J. K., Bolanowska W., Sapota A. Evaluation of metallothionein content in animal tissues. Acta Biochim Pol. 1973;20(3):207–215. [PubMed] [Google Scholar]
- Rudnicki M. A., Braun T., Hinuma S., Jaenisch R. Inactivation of MyoD in mice leads to up-regulation of the myogenic HLH gene Myf-5 and results in apparently normal muscle development. Cell. 1992 Oct 30;71(3):383–390. doi: 10.1016/0092-8674(92)90508-a. [DOI] [PubMed] [Google Scholar]
- Smithies O., Gregg R. G., Boggs S. S., Koralewski M. A., Kucherlapati R. S. Insertion of DNA sequences into the human chromosomal beta-globin locus by homologous recombination. Nature. 1985 Sep 19;317(6034):230–234. doi: 10.1038/317230a0. [DOI] [PubMed] [Google Scholar]
- Snouwaert J. N., Brigman K. K., Latour A. M., Malouf N. N., Boucher R. C., Smithies O., Koller B. H. An animal model for cystic fibrosis made by gene targeting. Science. 1992 Aug 21;257(5073):1083–1088. doi: 10.1126/science.257.5073.1083. [DOI] [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987 Nov 6;51(3):503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Wang Z. Q., Ovitt C., Grigoriadis A. E., Möhle-Steinlein U., Rüther U., Wagner E. F. Bone and haematopoietic defects in mice lacking c-fos. Nature. 1992 Dec 24;360(6406):741–745. doi: 10.1038/360741a0. [DOI] [PubMed] [Google Scholar]