Abstract
Ischemia-reperfusion injury (IRI), an innate immunity-driven local inflammation, remains the major problem in clinical organ transplantation. T cell immunoglobulin and mucin domain (TIM-3) – Galectin-9 (Gal-9) signaling regulates CD4+ Th1 immune responses. Here, we explored TIM-3 – Gal-9 function in a clinically relevant murine model of hepatic cold storage and orthotopic liver transplantation (OLT). C57BL/6 livers, preserved for 20h at 4°C in UW solution, were transplanted to syngeneic mouse recipients. Up-regulation of TIM-3 on OLT-infiltrating activated CD4+ T cells was observed in the early IRI phase (1h). By 6h of reperfusion, OLTs in recipients treated with a blocking anti-TIM-3 Ab were characterized by: 1/ enhanced hepatocellular damage (sALT levels, liver Suzuki's histological score); 2/ polarized cell infiltrate towards Th1/Th17-type phenotype; 3/ depressed T cell exhaustion markers (PD-1, LAG3); and 4/ elevated neutrophil and macrophage infiltration/activation. In parallel studies, adoptive transfer of CD4+ T cells from naïve WT, but not from TIM-3 Tg donors, readily recreated OLT damage in otherwise IR-resistant RAG−/− test recipients. Furthermore, pre-treatment of mice with rGal-9 promoted hepatoprotection against preservation-association liver damage, accompanied by enhanced TIM-3 expression in OLTs. Thus, CD4+ T cell-dependent “negative” TIM-3 costimulation is essential for hepatic homeostasis and resistance against IR stress in OLTs.
Introduction
Liver transplantation is the standard of care in patients with end-stage liver disease and those with tumors of hepatic origin (1). However, the cellular damage surrounding organ removal and storage impacts transplantation outcomes because it represents a major risk factor for primary graft non-function, acute and chronic rejection, as well as it contributes to acute donor organ shortage. Despite obvious significance, however, our appreciation of complex immune mechanisms that account for liver ischemia-reperfusion injury (IRI) is still limited (2, 3). Controlling IRI remains an unmet clinical need in organ transplantation.
It has been generally accepted that hepatic IRI results from glycogen consumption and adenosine triphosphatase (ATP) depletion, which triggers a complex innate immune-dominated inflammatory cascade, leading to reactive oxygen species (ROS) production and progressive tissue damage (3). Although hepatic IRI may develop in syngeneic grafts, ex vivo, or under sterile conditions, we have shown that T cells particularly of CD4 phenotype are indispensable for the activation of TLR4-mediated pro-inflammatory immune sequel (4). The question arises as to how adaptive T cells may function in the predominantly TLR4-rich innate immune milieu and in the absence of exogenous antigen stimulation in IR-stressed livers?
Interactions between T cell Immunoglobulin Mucin (TIM) family of co-stimulatory proteins constitute a novel T cell – macrophage molecular signaling network at the innate – adaptive interface in organ transplantation (5). We have shown that treatment of WT mice with anti-TIM-1 mAb ameliorated the hepatocellular damage in livers subjected to either “warm” ischemia in situ (6) or prolonged “cold” storage and transplantation (7), data supported by results from a renal IRI mouse model (8). The TIM-3–Galectin-9 (Gal-9) pathway, on the other hand, constitutes a “negative” T cell costimulation signal between Th1 and macrophages, consistent with our recent report in which TIM-3 blockade worsened tissue damage in IR-stressed livers (9). The importance of “negative” T cell signaling in preventing innate immune activation is supported by our experiments in which disruption of PD-1 (B7) - PD-L1 (H1) pathway promoted IR-hepatocellular damage (10). Thus, multiple T cell costimulatory pathways, both positive and negative, may function in a “two-way” traffic fashion to promote or inhibit TLR4-dependent innate immune responses against IR-insult.
This study was designed to extend our previous findings on the role of TIM-3 costimulation from a mouse model of liver partial “warm” ischemia into a clinically-relevant model of prolonged hepatic cold storage and syngeneic orthotopic liver transplantation (OLT). Our results show that CD4+ T cell-dependent “negative” TIM-3 signaling is critical to maintain hepatic homeostasis in preservation-associated liver damage, whereas enhancing TIM-3 expression by exogenous rGal-9 protects liver grafts against IR stress.
Materials and Methods
Animals
C57BL/6 (WT), B6.129S7-Rag1tm1Mom/J (C57BL/6, RAG−/−) mice at 8-12 weeks of age were used (Jackson Laboratory, Bar Harbor, ME). TIM-3Tg mice (at C57BL/6 background), kindly provided by Dr. Vijai Kuchroo (Harvard University, Boston), were generated by expressing the full-length TIM-3 cDNA under the control of the human CD2 promoter. Animals were housed in the UCLA animal facility under specific pathogen-free conditions and received humane care according to the criteria outlined in Guide for the Care and Use of Laboratory Animals (prepared by the National Academy of Sciences; NIH publication 86-23, revised 1985).
Model of liver “cold” IRI in WT mice
Isogeneic OLTs were performed, as described (11). C57BL/6 livers, stored for 20h at 4°C in UW solution, were transplanted orthotopically by using the cuff technique. The bile duct was connected via ligation over the stent. Anhepatic time was 15–18min. At 1h prior to reperfusion, animals were given a single dose (0.5 mg/mouse i.v.) of antagonistic anti-TIM-3 mAb (RMT3-23) or control Ig (Rat IgG, Bio X Cell, West Lebanon, NH); or human stable-form Gal-9 (rGal-9; 100ug/mouse i.v.; a gift from Prof. Mitsuomi Hirashima, Kagawa University, Japan). Liver and sera samples were collected at time intervals post-transplant for analyses.
Model of liver “cold” IRI in RAG−/− mice
Syngeneic spleen CD4+ T cells (5×106 i.v.), separated from WT or TIM-3 Tg donors by using magnetic cell sorting kit (StemCell Technologies, Vancouver, Canada), were adoptively transferred into severely T-, B-, and NK T-cell deficient RAG−/− test recipients. These animals received WT liver grafts, which were subjected to 20h of cold storage. Liver/sera samples were collected at 6h of reperfusion. Separate groups of RAG−/− mice were treated with control Ig or anti-TIM-3 mAb prior to OLT (at -1h).
Lymphocyte isolation and flow cytometry analysis
Lymphocytes were isolated from OLTs at 6h post-transplant by using Percoll density gradient (4). Briefly, livers were perfused in situ with collagenase-PBS, and hepatocytes were removed by low-speed centrifugation. The lymphocyte fraction was separated by 25%/50% discontinuous Percoll density gradient (Pharmacia, New York, NY). After RBC lysis, hepatic CD4+ T cells were separated by a magnetic cell sorting kit (StemCell Technologies) for quantitative PCR. For flow cytometry, liver-infiltrating lymphocytes were harvested at 1h post-transplantation, and stained with rat anti-mouse PE-Cy5-CD3 (145-2C11), FITC-CD4 (RM4-5), APC-CD4 (RM4-5), FITC-PD-1 (RMP1-30), PE-TIM-3 (RMT3-23), PE-CD69 (H1.2F3) or certain isotype control rat IgG (eBioscience, San Diego, CA).
Hepatocellular function
Serum alanine aminotransferase (sALT) levels, indicator of hepatocellular injury, were measured by IDEXX Laboratory (Westbrook, ME).
Histology and immunofluorescence
Liver paraffin sections (4μm) were stained with hematoxylin-eosin (H&E). The severity of liver IRI was graded blindly by modified Suzuki's criteria on a scale of 0–4 (12). Immunofluorescence staining was performed on frozen sections with primary mAb against mouse neutrophils Ly-6G (1A8, BD Biosciences, San Jose, CA), macrophages CD68 (FA-11; AbD Serotec, Raleigh, NC) and Alexa Fluor® 488 actin conjugate (Life Technologies). The secondary Ab for Ly-6G/CD68 was goat anti-rat Alexa Fluor® 555 (Invitrogen). Slides, mounted with VECTASHIELD medium/DAPI (Vector Labs, Burlingame, CA)/F-actin were evaluated blindly by counting labeled cells in 10 high-power fields (HPF). Results were expressed as average number of positive cells/HPF (x400)
Myeloperoxidase activity assay
The presence of myeloperoxidase (MPO) was used as an index of neutrophil accumulation in the liver (7). One absorbance unit (AU) of MPO activity was defined as the quantity of enzyme degrading 1mol peroxide/min at 25°C/gram of tissue.
Quantitative RT-PCR
PCRs were performed with platinum SYBR green quantitative PCR kit (Invitrogen, Carlsbad, CA) by the Chromo 4 detector (MJ Research, Waltham, MA). Primers to amplify specific gene fragments were published (7, 10). Target gene expressions were calculated by their ratios to the housekeeping HPRT gene.
Statistical analysis. All values are expressed as the mean ± standard deviation (SD). Data were analyzed with unpaired two-tailed Student's-t test. P<0.05 was considered statistically significant.
Results
TIM-3+ CD4+ T cells infiltrate IR-stressed OLTs
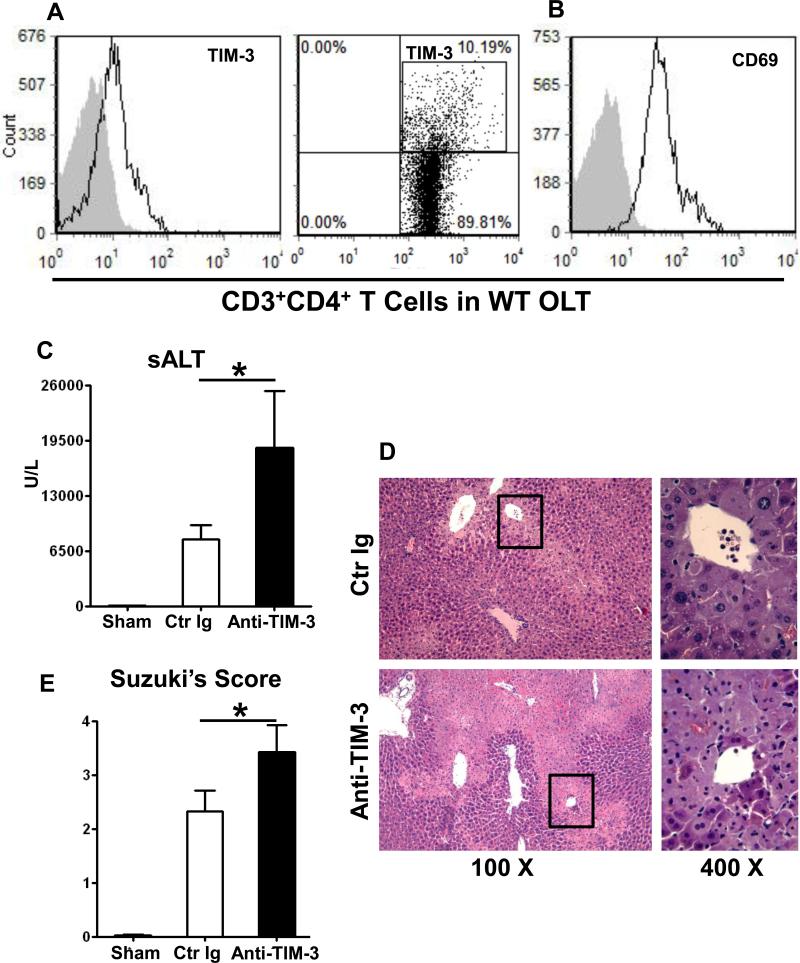
In our principal model, livers from C57B6 donor mice were stored at 4°C in UW solution for 20h, and then transplanted orthotopically to syngeneic recipients. At 1h post-transplant, the time of maximal T cell infiltration in this model (7, 11), OLT-infiltrating CD3+CD4+ T cells were screened for TIM-3 and CD69 expression by flow cytometry. Unlike negative staining for TIM-3 and CD69 in sham controls (Supplementary Fig. 1G and H), CD4+ T cells in IR-stressed OLTs were activated, as shown by CD69 staining (Fig. 1B: 38.4± 0.2 vs. 3.4±0.3 isotype control) and expressed TIM-3 (Fig. 1A: 8.8±1.6 vs. 3.4±0.3 isotype control).
Figure 1.
Livers from B6 mice stored for 20h at 4°C were transplanted to syngeneic recipients. (A, B) At 1h post-transplant, graft-infiltrating CD4+ T cells were screened by FACS for CD69 and TIM-3 expression (filled histograms represent isotype control). Representative of 4 experiments is shown). Out of 2.99±0.77×106 of CD3+CD4+ T cells that were isolated from IR-stressed OLTs, 11.22±1.18% were TIM-3+. At 6h post-transplant, the hepatocellular function was analyzed by: (C) sALT levels; (D) liver transplant histology (representative H&E staining; magnification x100 and x400); and (E) Suzuki's histological score of OLT damage (*p<0.05, n=8-10/group).
Disruption of TIM-3 signaling exacerbates IRI in OLTs
To test the function of TIM-3 signaling, we pretreated B6 mice with a single dose (0.5 mg/mouse i.v.) of antagonistic TIM-3 mAb (RMT3-23) or control Ig at 1h prior to reperfusion of IR-stressed OLTs, a protocol known to deplete TIM+ target cells (7). Recipients were then sacrificed at 6h, the peak of hepatocellular damage (11), and liver/peripheral blood samples were collected. Mice given anti-TIM-3 mAb were more susceptible to IRI as compared with Ig-treated controls, evidenced by increased sALT levels (Fig. 1C: 18672±6646 vs. 7819±1719 U/L; p<0.05) and accelerated histological liver injury features. Indeed, disruption of TIM-3 signaling further enhanced the severity of lobular edema, vacuolization, as well as hepatic necrosis (Fig. 1D), accompanied by increased Suzuki's score of OLT damage (Fig. 1E; 3.41±0.50 vs. 2.52±0.38 in Ig-treated controls; p<0.05).
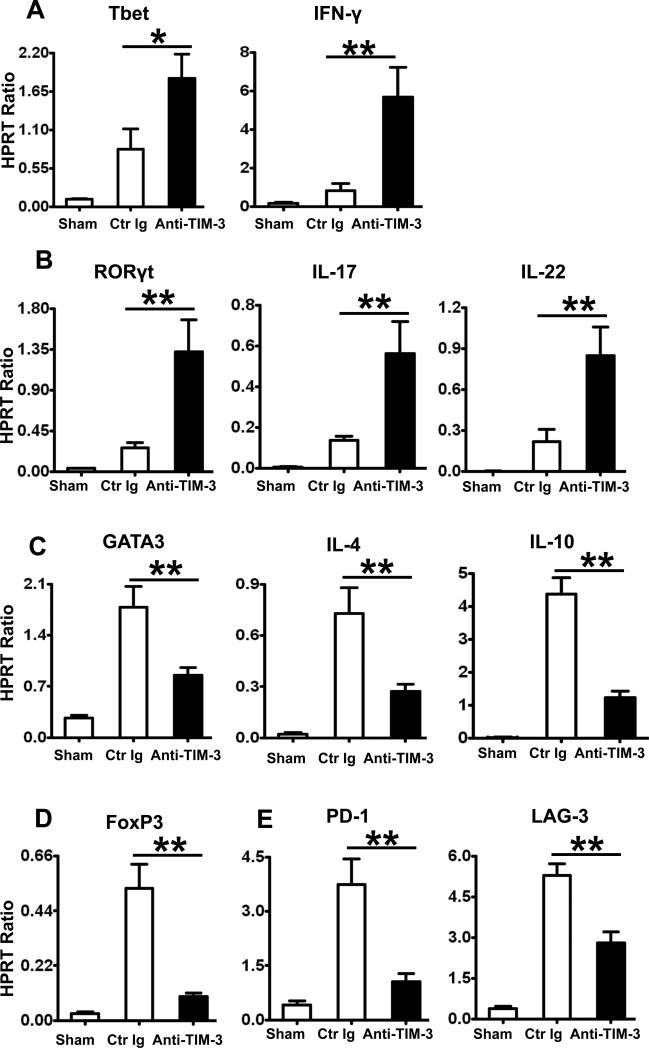
Targeting TIM-3 polarizes CD3+CD4+ T cell transcription and function in IR-stressed OLTs
We analyzed CD4+ T cell cytokine profile in IR-stressed OLTs with or without adjunctive anti-TIM-3 mAb. By 6h of reperfusion, cDNA from liver-infiltrating CD3+CD4+ T cells were isolated and analyzed by quantitative PCR. TIM-3 signaling blockade enhanced expression of Th1-related transcription factor Tbet, IFN-γ, as well as Th17-related transcription factor RORγt, IL-17, and IL-22 (Fig. 2A,B), while simultaneously depressing Th2-related GATA3, IL-4, IL-10 (Fig. 2C) and FoxP3 (Fig. 2D). Consistent with high co-expression of PD-1 (49.5±1.4) on OLT-infiltrating TIM-3+CD4+ T cells (Supplementary Fig. 1D), disruption of TIM-3 signaling down-regulated the expression of genes encoding PD-1 and lymphocyte activation gene 3 (LAG-3) in IR-stressed OLTs (Fig. 2E).
Figure 2.
Quantitative RT-PCR-assisted detection of T cell transcripts and cytokines in CD4+ T cells isolated from IR-stressed OLTs (at 6h of reperfusion): (A) Tbet, IFN-γ; (B) RORγt, IL-17, IL-22; (C) GATA3, IL-4, IL-10; (D) FoxP3, and (E) PD-1, LAG-3. Data normalized to HPRT gene expression (*p<0.05, **p<0.01, n=4-6/group).
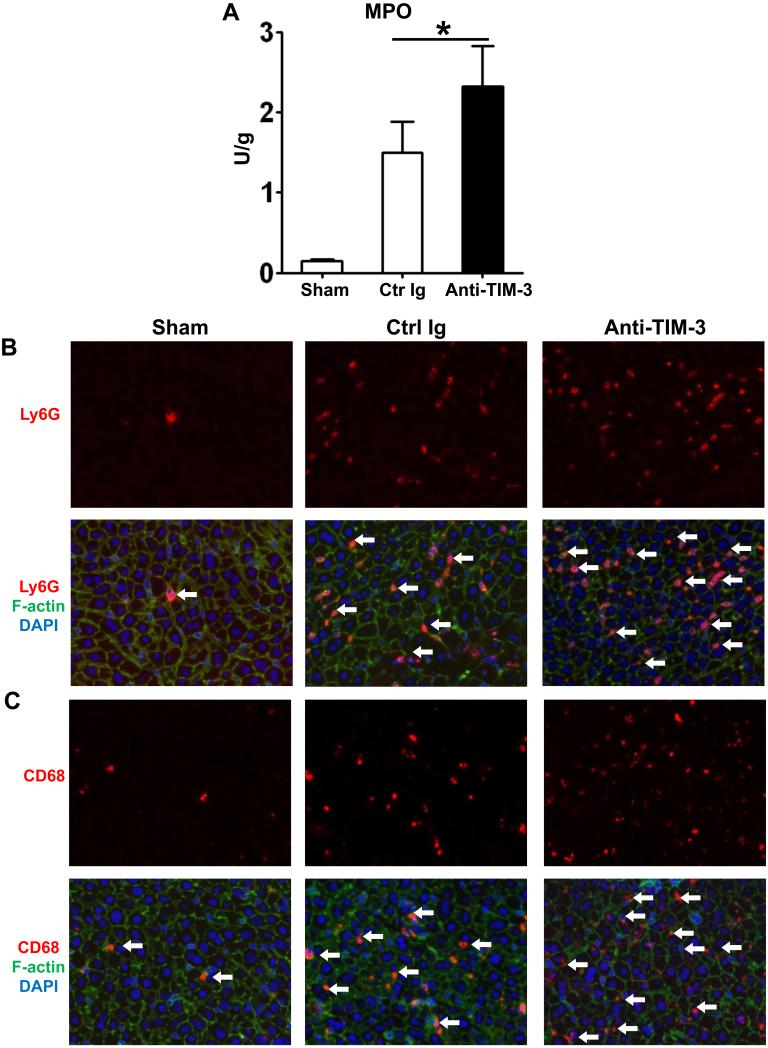
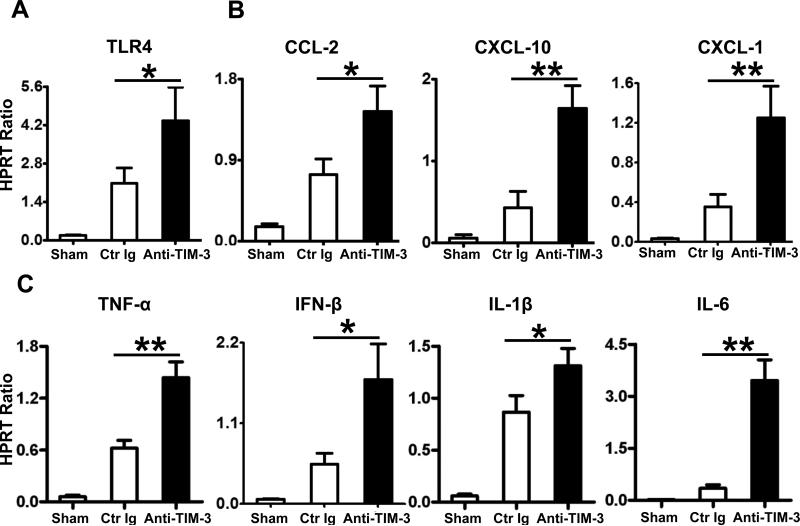
TIM-3 antagonism increases sequestration and function of neutrophils/macrophages in IR-liver grafts
By 6h of transplantation, cold-stored OLTs in mice pretreated with anti-TIM-3 mAb showed increased MPO neutrophil activity (U/g), as compared with controls (Fig. 3A; 2.31±0.50 vs. 1.49±0.37; p<0.05). This was accompanied by increased (p<0.05) frequency of neutrophils (Fig. 3B, 45.25±5.45 vs. 32.50±4.65) and macrophages (Fig. 3C, 50.75±6.85 vs. 25.75±4.27) sequestered in IR-stressed OLTs (Supplementary Fig. 1A, B). Moreover, disruption of TIM-3 pathway enhanced hepatic TLR4 expression (Fig. 4A), along with downstream transcript levels of neutrophil/monocyte-derived proinflammatory chemokine (Fig. 4B; CCL-2, CXCL-10, CXCL-1) and cytokine (Fig. 4C; TNF-α, IFN-β, IL-1β, IL-6) (p<0.01) programs.
Figure 3.
Neutrophil/macrophage sequestration in IR-stressed OLTs after treatment with anti-TIM-3 mAb (at 6h after 20h cold ischemia). (A) MPO levels (*p<0.05, n=4-6/group). Immunofluorescence staining for (B) Ly-6G+ neutrophils; and (C) CD68+ macrophages. Results scored semi-quantitatively by averaging number of positively-stained cells/field (400x magnification). Representative of 4-6 mice/group.
Figure 4.
Quantitative RT-PCR-assisted expression of TLR4 and associated cytokine/chemokine programs in IR-stressed OLTs (at 6h after 20h of cold storage): (A) TLR4; (B) CCL-2, CXCL-10, CXCL-1; and (C) TNF-α, IFN-β, IL-1β, and IL-6. Data normalized to HPRT gene expression (*p<0.05, **p<0.01, n=4-6/group).
CD4+ T cell-dependent TIM-3 costimulation confers IR-resistance in OLTs
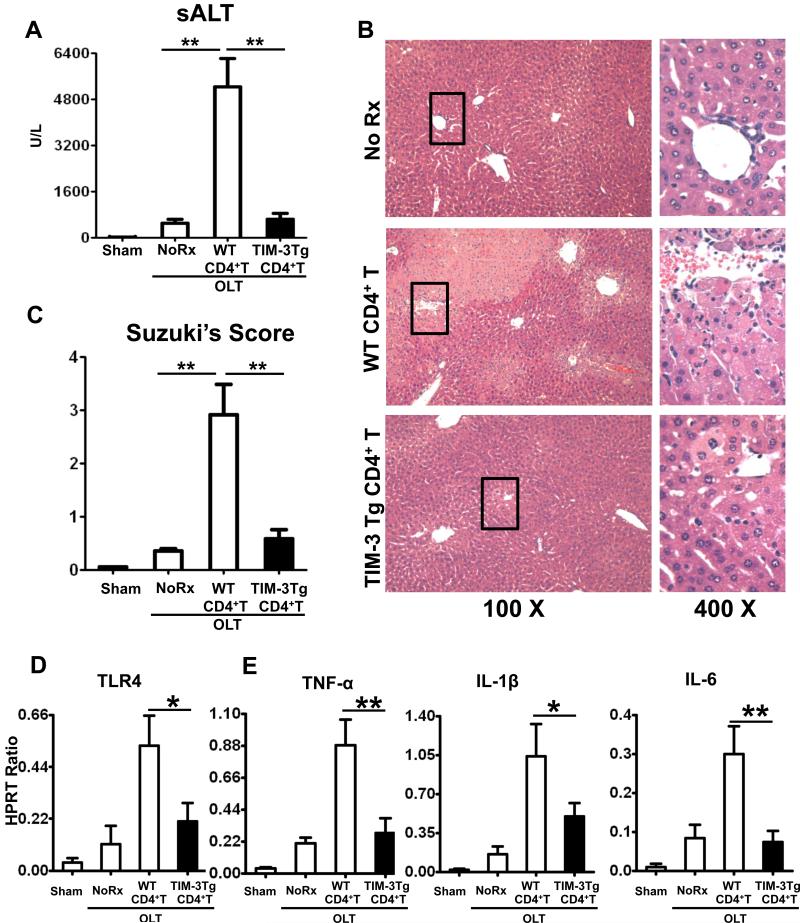
Although originally identified on terminally differentiated Th1 cells, TIM-3 has also been found on macrophages/monocytes, DCs, and NK cells (13). To elucidate the role of CD3+CD4+ T cell-dependent TIM-3 immunomodulation, we utilized a model of preservation-associated liver damage in RAG−/− mice, which are T- B- and NK T cell-deficient (7). RAG−/− test recipients were repopulated with purified (>95%) syngeneic spleen CD4+ T cells (5×106 i.v.) from WT or TIM-3 Tg donors. They were challenged with WT liver grafts that were cold-stored (20h at 4°C in UW). Adoptive transfer of CD4+ T cells from WT naïve donors (<2% of TIM-3+ cells) recreated classic hepatocellular damage in otherwise IR-resistant RAG−/− test mice, evidenced by sALT levels at 6h of reperfusion (Fig. 5A, 5238±981 vs. 495±141 U/L in controls; p<0.01). In contrast, adoptive transfer of CD4+ T cells from TIM-3 Tg donors (50-60% of TIM-3+ cells) failed to trigger liver IRI in RAG−/− recipients (Fig. 5A, sALT: 639±195 vs. 495±141 U/L in controls; p>0.05). OLT resistance against IRI after infusion of TIM-3+CD4+ T cells was confirmed by histology (Fig. 5B, C; Suzuki's score = 2.91±0.56 and 0.58±0.26; p<0.01 after transfer of WT and TIM-3 Tg CD4+ T cells, respectively). The qRT-PCR analysis has shown that transfer of WT but not TIM-3 Tg cells enhanced TLR4 expression (Fig. 5D) and pro-inflammatory IRI signature (TNF-α, IL-1β, IL-6) in OLTs of adoptively transferred RAG−/− mouse recipients (Fig. 5E).
Figure 5.
Groups of RAG−/− mice were repopulated with purified (>95%) spleen CD4+ T cells (5×106 i.v.) from syngeneic WT or TIM-3 Tg donor mice. The WT liver grafts, subjected to 20h cold storage, were transplanted to RAG−/− test recipients. At 6h of reperfusion, the hepatocellular damage in OLTs was assessed by: (A) sALT levels; (B) liver histology (representative H&E staining; magnification x100 and x400); and (C) Suzuki's histological score. The expression of (D) TLR4; and (E) TNF-α, IL-1β, and IL-6 was studied by qRT-PCR (*p<0.05, **p<0.01, n=8-10/group).
Tim-3 - Gal-9 pathway protects OLTs from IRI
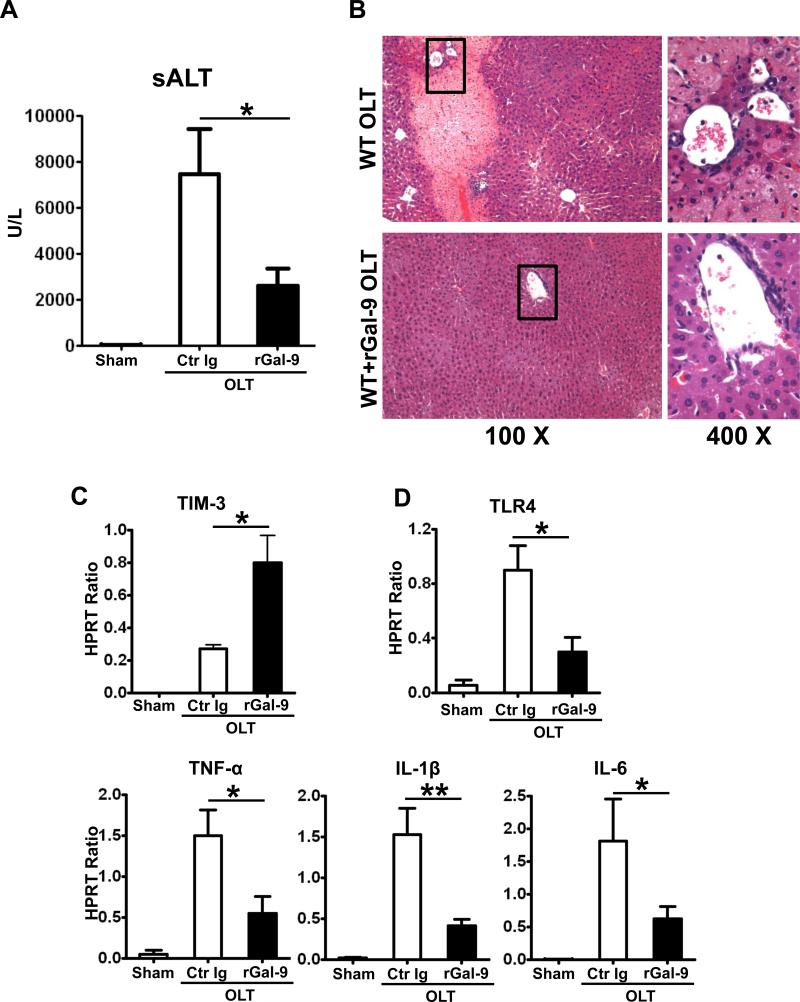
To further assess whether activation of TIM-3 signaling exerts hepatoprotection in OLTs, WT mice were pretreated with a single dose of rGal-9 (100ug/mouse i.v. at -1h). By 6h of reperfusion, WT mice given rGal-9 showed decreased sALT levels, as compared with Ig-treated controls (Fig. 6A: 2578±772 vs. 7455±1971 U/L; p<0.05), along with improved histological features of liver graft injury (Fig. 6B). Moreover, infusion of rGal-9 increased TIM-3 (Fig. 6C) while simultaneously decreasing TLR4 expression, accompanied by diminished TNF-α, IL-1β, IL-6 (Fig. 6D) levels in IR-stressed OLTs.
Figure 6.
Livers from B6 mice, stored for 20h at 4°C, were transplanted to syngeneic recipients. Exogenous rGal-9 (100ug/mouse) was injected i.v. at 1h prior to reperfusion, and OLT function was assessed at 6h after reperfusion by: (A) sALT levels; (B) liver histology (representative H&E staining; magnification x100 and x400); qRT-PCR-assisted hepatic expression of: (C) TIM-3; (D) TLR-4, TNF-α, IL-1β, and IL-6 (*p<0.05, **p<0.01, n=4-6/group).
Discussion
In this study, we established what we believe to be for the first time, that CD4+ T cell-dependent “negative” TIM-3 – Gal-9 costimulation pathway is required to maintain hepatic homeostasis and resistance against innate immunity-driven preservation-inflicted tissue damage in mouse OLTs. By using a model of ex vivo liver cold storage followed by transplantation, we have shown that: 1/ TIM-3 expressing activated CD4+ T cells infiltrated IR-stressed OLTs; 2/ disruption of TIM-3 signaling exacerbated IR-hepatocellular damage, accompanied by polarization towards Th1-type and depression of T cell exhaustion (PD-1 and LAG-3) phenotype; 3/ TIM-3 antagonism enhanced IR-induced TLR4 expression, as well as neutrophil/macrophage trafficking and function in OLTs; 4/ by employing RAG−/− test mice, we have documented the key immunoregulatory role of CD3+CD4+ T cell-dependent TIM-3 pathway in OLT resistance against IR stress; 5/ pretreatment with rGal-9 enhanced hepatic TIM-3 levels, with resultant protection against IRI in OLTs. These findings complement our recent studies in which TIM-3 signaling determined the severity of hepatocellular damage in TLR4-dependent manner (9). However, unlike in “warm” IRI model, we have now: 1/ used a clinically-relevant mouse model of extended (20h) hepatic cold storage followed by transplantation and 2/ identified the protective role of a discrete OLT-infiltrating TIM-3+CD4+ T cell population in IR-hepatic resistance and homeostasis.
Multiple rodent and human immune cell types may express TIM proteins (5, 14). Unlike other negative immunomodulatory molecules, TIM-3 is preferentially expressed on activated CD4+ Th1, CD8+ T cytotoxic 1 (Tc1) and Th17 cells. Indeed, interaction between TIM-3 and its Gal-9 ligand was shown to terminate Th1 responses by inducing TIM-3+ T cell death (5, 15). TIM-3 antagonism, on the other hand, increased the number/activation of macrophages and enhanced the severity of EAE (16). In non-obese diabetic mice, TIM-3 may as a regulatory checkpoint for CD4+CD25+ Treg to dampen Th1-dependent pro-inflammatory and promote tolerogenic responses (17). Increased TIM-3 frequency on T cells was also found during chronic HIV-1 infection, leading to impaired antiviral function, T cell exhaustion, and limited immune activation (18). We have shown that stimulation of PD-1 negative signals ameliorated liver IRI by inhibiting T cell activation and Kupffer cell/macrophage functions (10). Along with other inhibitory receptors, such as PD-1, or LAG3, TIM-3 has been associated with T cell exhaustion in acute myelogenous leukemia (19), endotoxic shock (20) and allograft rejection (5, 21, 22). In the present study, TIM-3 blockade decreased PD-1 and LAG3 levels, suggesting that disruption of TIM-3 signaling may reverse IR-driven T cell exhaustion phenotype by diminishing dysfunctional T cell population in OLTs. This was also the case in a chronic hepatitis C virus (HCV) infection where TIM-3 antagonism rescued dysfunctional CD4+ and CD8+ T cells (23).
Consistent with others (24-26), we have reported on the pathogenic role of T lymphocytes, particularly of CD4 phenotype, in hepatic IRI (4, 27). We have also documented that CD4+ T cells function via CD154 and without de novo Ag-specific activation, as CD40 induction was required to engage CD154-CD40 to facilitate IR-inflammation and tissue injury (4). In the present study, increased expression of TIM-3 and CD69 antigens by OLT-infiltrating T cells is consistent with enhanced CD3+CD4+ T cell-specific TIM-3 signaling in the early post-transplant phase, which precedes the phase of innate immune-driven hepatic activation (3). Furthermore, targeting TIM-3 exacerbated IR-hepatocellular damage, by polarizing OLT molecular signature towards Th1-type (increased Tbet/IFN-γ) and away from Th2-type (depressed IL-4/IL-10 FoxP3) phenotype. Evidence points towards the importance of Th1-type cells and IFN-γ in the activation of Kupffer cells/macrophages and neutrophils in IR innate inflammatory cascade (28). Data from our lab (4) and others (29) demonstrated that CD4+ T cell deficiency leads to hepatic IRI resistance, whereas repopulation with CD4+ T cells restores IRI, implying CD4+ T cell as a key player in the early IRI phase (3, 25). Unlike in sham controls, increased frequency of CD68+ macrophages and enhanced TLR4 activity was observed in IR-stressed OLTs. Notably, mice pretreated with anti-TIM-3 mAb were characterized by elevated CD68+ cell infiltration, enhanced TLR4 responses, and proinflammatory cytokine signature (TNF-α, IFN-β, IL-1β, IL-6) in liver grafts. As disruption of TIM-3 signaling exacerbated TLR4-driven inflammation in OLTs, our results underline the importance of negative TIM-3 signaling in CD4+ T cell - macrophage cross talk in the mechanism of innate immunity-driven IRI.
The circulating monocytes and PMNs become activated and recruited into IR-livers to amplify local tissue destruction by generating ROS during the second stage of IR-induced liver immune cascade (3, 4). Compared to untreated liver grafts, those pre-treated with anti-TIM-3 mAb showed increased neutrophil sequestration, MPO activity and enhanced expression of IFN-inducible gene products and neutrophil chemoattractant, such as CXCL10, CXCL1 (KC). Pro-inflammatory phenotype after TIM-3 blockade further documents the benefit of negative regulatory function of TIM-3 signaling through cytokine/chemokine networks in cold-stored OLTs.
Since originally discovered on Th1 cells, TIM-3 expression has been also detected on DCs, monocyte/macrophages and mast cells (13). To focus on CD3+CD4+ T cell-dependent TIM-3 signaling, we have used severely T cell-deficient RAG−/− mice as recipients of IR-stressed liver grafts. Consistent with our recent findings (7), adoptive transfer of naïve CD4+ spleen T cells was both necessary and sufficient to facilitate hepatocellular damage in OLTs of otherwise IR-resistant RAG−/− test recipients. In marked contrast, RAG−/− mice remained resistant against IR-induced inflammation/hepatocellular OLT damage following infusion of TIM-3 enriched CD4+ T cells. We found high TIM-3 expression (269.2±20.4) on CD4+ T cells infiltrating TIM-3 Tg OLTs (Supplementary Fig. 1E), along with elevated PD-1 levels (56.9±2.4) on TIM-3+CD4+ T cells (Supplementary Fig. 1F). These results highlight cytoprotectives function of putative T cell exhaustion phenotype in IR-stressed liver isografts. Neither anti-TIM-3 mAb nor control Ig treatment triggered OLT damage in RAG−/− mice without cell transfer, implying that TIM-3+ APCs may not be crucial in facilitating liver IRI in this model (Supplementary Fig. 1C).
Gal-9, a natural TIM-3 ligand, may induce Th1 cell death in TIM-3 dependent manner (30). We now show that treatment with rGal-9 activated TIM-3 expression, diminished IR-triggered TLR4 activation and mitigated proinflammatory cytokine programs, ultimately leading to OLT cytoprotection. These beneficial effects are in agreement with the ability of Gal-9 to ameliorate diet-induced liver steatosis by modulating NKT cell function (31); prolong survival of fully allogeneic cardiac allografts by suppressing Th1/Th17 immune responses (32); or protect corneal allografts from destruction by allo-reactive T cells (33).
In summary, this report supports a novel concept of TIM-3 – Gal-9 “negative” T cell costimulation to promote hepatic homeostasis and cytoprotection against preservation-associated tissue damage in mouse liver transplants. These experimental findings are in agreement with preliminary data of our ongoing clinical study on TIM signaling in human liver transplants suffering from IRI, as: i/ a therapeutic target in IR-mediated liver inflammation; ii/ a biomarker candidate to identify the severity of hepatic IRI; and iii/ a putative predictor of clinical outcomes in liver transplant patients (Kupiec-Weglinski, unpublished).
Supplementary Material
Acknowledgments
This study was supported by NIH Grant RO1 DK062357 (JWKW); W.M. Keck Foundation; The Diann Kim Foundation; The Dumont Research Foundation. HJ is a recipient of the Pilot and Feasibility Grant from CURE Digestive Diseases Research Center and Clinical/Translational Science Institute (CTSI) at UCLA.
Abbreviations
- IRI
ischemia-reperfusion injury
- mAb
monoclonal antibody
- MPO
myeloperoxidase
- ROS
reactive oxygen species
- sALT
serum alanine aminotransferase
- TIM-3
T cell immunoglobulin mucin-3
- Gal-9
Galectin-9
- WT
wild-type
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose, as described by the American Journal of Transplantation.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
References
- 1.Wertheim JA, Petrowsky H, Saab S, Kupiec-Weglinski JW, Busuttil RW. Major challenges limiting liver transplantation in the United States. Am J Transplant. 2011;11:1773–1784. doi: 10.1111/j.1600-6143.2011.03587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhai Y, Petrowsky H, Hong JC, Busuttil RW, Kupiec-Weglinski JW. Ischaemia reperfusion injury in liver transplantation--from bench to bedside. Nat Rev Gastroenterol Hepatol. 2013;10:79–89. doi: 10.1038/nrgastro.2012.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhai Y, Busuttil RW, Kupiec-Weglinski JW. Liver ischemia and reperfusion injury: new insights into mechanisms of innate-adaptive immune-mediated tissue inflammation. Am J Transplant. 2011;11:1563–1569. doi: 10.1111/j.1600-6143.2011.03579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen X, Wang Y, Gao F, Ren F, Busuttil RW, Kupiec-Weglinski JW, et al. CD4 T cells promote tissue inflammation via CD40 signaling without de novo activation in a murine model of liver ischemia/reperfusion injury. Hepatology. 2009;50:1537–1546. doi: 10.1002/hep.23153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yeung MY, McGrath M, Najafian N. The emerging role of the TIM molecules in transplantation. Am J Transplant. 2011;11:2012–2019. doi: 10.1111/j.1600-6143.2011.03727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uchida Y, Ke B, Freitas MC, Ji H, Zhao D, Benjamin ER, et al. The emerging role of T cell immunoglobulin mucin-1 in the mechanism of liver ischemia and reperfusion injury in the mouse. Hepatology. 2010;51:1363–1372. doi: 10.1002/hep.23442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Ji H, Shen X, Cai J, Gao F, Koenig KM, et al. Targeting TIM-1 on CD4 T cells depresses macrophage activation and overcomes ischemia-reperfusion injury in mouse orthotopic liver transplantation. Am J Transplant. 2013;13:56–66. doi: 10.1111/j.1600-6143.2012.04316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rong S, Park JK, Kirsch T, Yagita H, Akiba H, Boenisch O, et al. The TIM-1:TIM-4 pathway enhances renal ischemia-reperfusion injury. J Am Soc Nephrol. 2011;22:484–495. doi: 10.1681/ASN.2010030321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uchida Y, Ke B, Freitas MC, Yagita H, Akiba H, Busuttil RW, et al. T-cell immunoglobulin mucin-3 determines severity of liver ischemia/reperfusion injury in mice in a TLR4-dependent manner. Gastroenterology. 2010;139:2195–2206. doi: 10.1053/j.gastro.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ji H, Shen X, Gao F, Ke B, Freitas MC, Uchida Y, et al. Programmed death-1/B7-H1 negative costimulation protects mouse liver against ischemia and reperfusion injury. Hepatology. 2010;52:1380–1389. doi: 10.1002/hep.23843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen XD, Gao F, Ke B, Zhai Y, Lassman CR, Tsuchihashi S, et al. Inflammatory responses in a new mouse model of prolonged hepatic cold ischemia followed by arterialized orthotopic liver transplantation. Liver Transpl. 2005;11:1273–1281. doi: 10.1002/lt.20489. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki S, Toledo-Pereyra LH, Rodriguez FJ, Cejalvo D. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation. 1993;55:1265–1272. doi: 10.1097/00007890-199306000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Han G, Chen G, Shen B, Li Y. Tim-3: An Activation Marker and Activation Limiter of Innate Immune Cells. Front Immunol. 2013;4:449. doi: 10.3389/fimmu.2013.00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freeman GJ, Casasnovas JM, Umetsu DT, DeKruyff RH. TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol Rev. 2010;235:172–189. doi: 10.1111/j.0105-2896.2010.00903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boenisch O, D'Addio F, Watanabe T, Elyaman W, Magee CN, Yeung MY, et al. TIM-3: a novel regulatory molecule of alloimmune activation. J Immunol. 2010;185:5806–5819. doi: 10.4049/jimmunol.0903435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rangachari M, Kuchroo VK. Using EAE to better understand principles of immune function and autoimmune pathology. J Autoimmun. 2013;45:31–39. doi: 10.1016/j.jaut.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanchez-Fueyo A, Tian J, Picarella D, Domenig C, Zheng XX, Sabatos CA, et al. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat Immunol. 2003;4:1093–1101. doi: 10.1038/ni987. [DOI] [PubMed] [Google Scholar]
- 18.Elahi S, Niki T, Hirashima M, Horton H. Galectin-9 binding to Tim-3 renders activated human CD4+ T cells less susceptible to HIV-1 infection. Blood. 2012;119:4192–4204. doi: 10.1182/blood-2011-11-389585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou Q, Munger ME, Veenstra RG, Weigel BJ, Hirashima M, Munn DH, et al. Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood. 2011;117:4501–4510. doi: 10.1182/blood-2010-10-310425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang F, Hou H, Xu L, Jane M, Peng J, Lu Y, et al. Tim-3 signaling pathway as a novel negative mediator in lipopolysaccharide-induced endotoxic shock. Hum Immunol. 2014 doi: 10.1016/j.humimm.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Gupta S, Thornley TB, Gao W, Larocca R, Turka LA, Kuchroo VK, et al. Allograft rejection is restrained by short-lived TIM-3+PD-1+Foxp3+ Tregs. J Clin Invest. 2012;122:2395–2404. doi: 10.1172/JCI45138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGrath MM, Najafian N. The role of coinhibitory signaling pathways in transplantation and tolerance. Front Immunol. 2012;3:47. doi: 10.3389/fimmu.2012.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Golden-Mason L, Palmer BE, Kassam N, Townshend-Bulson L, Livingston S, McMahon BJ, et al. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. J Virol. 2009;83:9122–9130. doi: 10.1128/JVI.00639-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rabb H, Daniels F, O'Donnell M, Haq M, Saba SR, Keane W, et al. Pathophysiological role of T lymphocytes in renal ischemia-reperfusion injury in mice. Am J Physiol Renal Physiol. 2000;279:F525–531. doi: 10.1152/ajprenal.2000.279.3.F525. [DOI] [PubMed] [Google Scholar]
- 25.Zwacka RM, Zhang Y, Halldorson J, Schlossberg H, Dudus L, Engelhardt JF. CD4(+) T-lymphocytes mediate ischemia/reperfusion-induced inflammatory responses in mouse liver. J Clin Invest. 1997;100:279–289. doi: 10.1172/JCI119533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuboki S, Sakai N, Tschop J, Edwards MJ, Lentsch AB, Caldwell CC. Distinct contributions of CD4+ T cell subsets in hepatic ischemia/reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1054–1059. doi: 10.1152/ajpgi.90464.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen XD, Ke B, Zhai Y, Gao F, Anselmo D, Lassman CR, et al. Stat4 and Stat6 signaling in hepatic ischemia/reperfusion injury in mice: HO-1 dependence of Stat4 disruption-mediated cytoprotection. Hepatology. 2003;37:296–303. doi: 10.1053/jhep.2003.50066. [DOI] [PubMed] [Google Scholar]
- 28.Caldwell CC, Tschoep J, Lentsch AB. Lymphocyte function during hepatic ischemia/reperfusion injury. J Leukoc Biol. 2007;82:457–464. doi: 10.1189/jlb.0107062. [DOI] [PubMed] [Google Scholar]
- 29.Pommey S, Lu B, McRae J, Stagg J, Hill P, Salvaris E, et al. Liver grafts from CD39-overexpressing rodents are protected from ischemia reperfusion injury due to reduced numbers of resident CD4+ T cells. Hepatology. 2013;57:1597–1606. doi: 10.1002/hep.25985. [DOI] [PubMed] [Google Scholar]
- 30.Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6:1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 31.Tang ZH, Liang S, Potter J, Jiang X, Mao HQ, Li Z. Tim-3/galectin-9 regulate the homeostasis of hepatic NKT cells in a murine model of nonalcoholic fatty liver disease. J Immunol. 2013;190:1788–1796. doi: 10.4049/jimmunol.1202814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He W, Fang Z, Wang F, Wu K, Xu Y, Zhou H, et al. Galectin-9 significantly prolongs the survival of fully mismatched cardiac allografts in mice. Transplantation. 2009;88:782–790. doi: 10.1097/TP.0b013e3181b47f25. [DOI] [PubMed] [Google Scholar]
- 33.Shimmura-Tomita M1, Wang M, Taniguchi H, Akiba H, Yagita H, Hori J. Galectin-9-mediated protection from allo-specific T cells as a mechanism of immune privilege of corneal allografts. PLoS One. 2013;8:e63620. doi: 10.1371/journal.pone.0063620. doi: 10.1371/journal.pone.0063620. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.