Abstract
About 10–15 % of adult gastrointestinal stromal tumors (GISTs) and 85 % of pediatric GISTs do not have mutations in the KIT or PDGFRA genes and are generally classified as KIT/PDGFRA wild type (WT). Recent studies have shown that this group of KIT/PDGFRA WT GISTs is quite heterogeneous in terms of clinical phenotype, genetic etiology, and molecular pathways. Succinate dehydrogenase subunit (SDH)-deficient GISTs, which include tumors that are part of multiple endocrine neoplasia syndromes, are the newest group of KIT/PDG-FRA WT GIST to be molecularly elucidated. This review aims to describe the different genetic subgroups of KIT/ PDGFRA WT GIST, with a special focus on the SDH-deficient GIST.
Keywords: SDH-deficient GIST, BRAF, NF1, Paraganglioma, Chondroma
Introduction
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal neoplasms of the gastrointestinal tract [1]. GISTs occur with an incidence between 6.8 and 20 per million [2–4]. Activating mutations in the KIT or PDGFRA genes have been described in GISTs [5, 6] and several kindreds with familial GISTs due to germline KIT or PDGFRA mutations have been reported [7–12]. Conventional chemotherapy is ineffective [13], while imatinib and other tyrosine kinase inhibitors (TKI) including suni-tinib and regorafenib have shown variable activity in KIT/ PDGFRA-mutated GIST, based on the specific genotype [14–16]. However, imatinib has poor efficacy in KIT/ PDGFRA-“wild type” (WT) GIST. Additional studies are needed to evaluate the efficacy of newer TKIs, such as sunitinib or regorafenib in these tumors [17, 18].
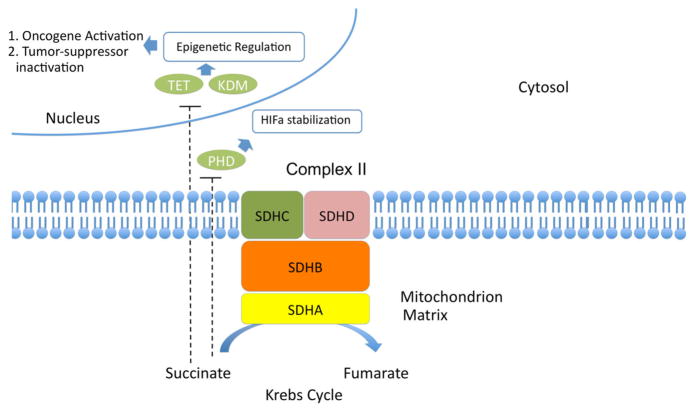
SDH-deficient GIST includes both the syndromic GIST as part of the Carney Triad (CTr) [19, 20] and the Carney–Stratakis Syndrome (CSS) [21], as well as sporadic KIT/ PDGFRA WT GIST [22]. SDH has a central role in energy production, participating in both the Krebs cycle and the respiratory chain. SDH-deficiency leads to accumulation of succinate which subsequently inhibits ten–eleven translocation (TET) and histone lysine (K) demethylases (KDM) enzymes, part of a family of enzymes that are called dioxygenases and participate in the degradation of hypoxia inducible factor 1 alpha (HIF-1a) [23] and the demethylation of DNA and histones [24]. Hydroxylation of 5-methylcyt-osine (5mC) groups and generation of 5-hydroxymethyl-cytosine (5hmc) were only recently described [25]. It has been shown that SDH-deficient GIST is characterized by a significant deficiency of 5hmc, consistent with a failure in TET2 maintenance demethylation [24]. The inhibition of this process by succinate accumulation is another example of how Krebs cycle dysfunction may lead to oncometabolite accumulation and epigenetic effects [26–28] (Fig. 1).
Fig. 1.
Succinate dehydrogenase (complex II) and epigenetic regulation. TET ten–eleven translocation, KDM lysine (K) demethylase, PHD prolyl hydroxylase
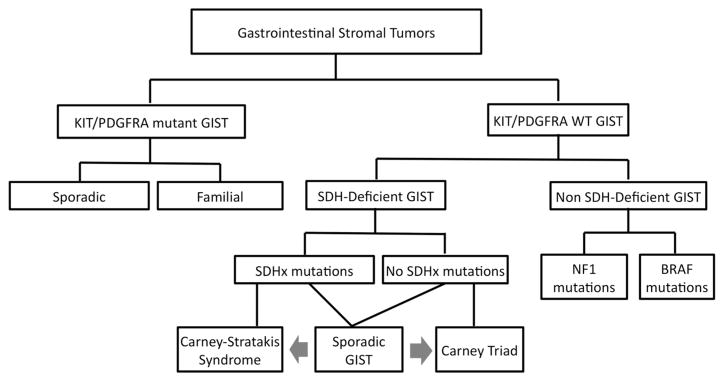
BRAF mutations have also been described in KIT/ PDGFRA WT GIST [29]. It is also known that patients with neurofibromatosis have frequently multifocal GIST [30]. This review aims to provide the current data for the clinical and genetic heterogeneity of KIT/PDGFRA WT GISTs. We focus in the SDH-deficient GISTs, which include syndromic (CTr, CSS) and not syndromic GISTs (Fig. 2).
Fig. 2.
A classification of gastrointestinal stromal tumors
SDH-deficient GISTs
Carney triad
In 1977, Carney described the association of gastric leio-myosarcomas (now known to represent GISTs) with functional paragangliomas and pulmonary chondromas (PCH) [19]. This disorder affected primarily young women. The multifocality in multiple organs and the young age of the patients suggested a genetic disorder. This association was later referred as CTr [31]. Later, patients with CTr were found to have additional tumors such as esophageal leiomyoma and adrenal cortical adenoma [32].
In 1999, Carney reported 79 cases of the triad. Only one-fifth of the patients had all three tumors, and because of the rarity of the disease, the presence of any two of the components was sufficient for the diagnosis. Two of the 79 patients, each with two elements of the triad (gastric sarcoma and paraganglioma) had a sibling with a paraganglioma, raising the possibility that the dyad of gastric sarcoma (GISTs) and paraganglioma constitute a familial syndrome, distinct from CTr [20].
CTr remains until today without clear etiology. Comparative genomic hybridization in 37 patients with Carney Triad has shown genetic alterations in the 1q, 1p, and less frequently in the 14q and 22q chromosomal loci [33]. While SDHB is located on chromosome 1p36.1–p35 and SDHC in 1q21–q23.3, sequencing for SDHB, -C and -D genes did not identify any mutations in the majority of patients with CTr (8).
CTr GISTs have negative staining for SDHB by immunohistochemistry (ICH) regardless of the absence of SDHx mutations [34]. GISTs in CTr patients compared with KIT- or PDGFRA-mutated GIST has unique features: predominance in young women, epithelioid histology, gastric location, multifocality, and frequent lymph node metastases [35].
Carney–Stratakis syndrome
CSS was firstly described in 2002 as a separate condition, characterized by the development of gastric GISTs and paragangliomas, and transmitted by autosomal dominant inheritance with incomplete penetrance [20]. Later, inactivating mutations in -C or -D genes were found in CSS patients [21, 36]. Loss of heterozygosity around the SDH gene chromosomal loci suggests a tumor-suppressor function of SDH subunits in this disease [36].
GISTs in CSS patients has also negative staining for SDHB by ICH [34]. CSS patients present with an almost 1:1 female: male ratio, older age than CTr patients, but do share with CTr patients the epithelioid histology, primarily gastric location, multifocal character, and frequent lymph node metastases [35].
Sporadic SDH-deficient GIST
Frequently, patients with KIT/PDGFRA WT GIST and no personal or family history of other tumors like paragangliomas or chondromas have SDHB deficiency by IHC, with or without SDHx mutations. In a recent report, four of 34 patients (12 %) with sporadic KIT/PDGFRA WT GIST were found to have mutations in SDHB or SDHC and subsequent SDHB deficiency by IHC [22], while patients can have also isolated SDHB-deficient GISTs without SDHx mutations. It is unclear if these isolated SDH-deficient GISTs are sporadic GISTs or the first presentation of CTr or CSS. These patients have to be monitored frequently for symptoms of paragangliomas. Sporadic SDH-deficient GIST shares some of the characteristics of GIST in CTr and/or CSS patients: female predominance (in CTr), gastric location, multifocality, epithelioid histology, and metastasis to regional lymph nodes.
Recent data demonstrate that SDHA is the most commonly mutated SDH subunit in sporadic WT GIST, with approximately one-fourth of SDHB-deficient GISTs by IHC to harbor SDHA mutations. Only one family, including two patients with SDHA-mutated GIST has been reported so far [37]. In one study, nine out of 33 (27 %) of SDH-deficient GISTs lacked expression of SDHA; nonsense or missense SDHA mutations were found in all these patients [38]. In another study, 36 out of 127 (28 %) SDH-deficient GISTs had loss of SDHA expression. Compared with patients with GISTs with positive staining by IHC, the patients with SDHA-deficient GISTs had a lower female-to-male ratio and an older median age [39].
Non-SDH-deficient GIST
NF1-associated GIST
Von Recklinghausen disease, is an inherited, autosomal dominant disease due to mutations to NF1 gene, phenotypically characterized by multiple café au lait spots, freckling, Lisch nodules, development of neurofibromas, and in some case includes also other malignancies like brain tumors, leukemia, and GIST. GISTs in NF1 patients are located in the small intestine (including the duodenum), have spindle histology, are multifocal, lack somatic KIT and PDGFRA mutations, and ICH for SDHB is positive [40–42]. NF1-mutated GISTs do not overexpress IGF1R [43]. There is no clear female predominance in this subgroup of KIT/PDGFRA WT GIST. There is little data regarding response of NF1-mutated GIST to imatinib and sunitinib. One patient has been reported with initial response to imatinib, who eventually became resistant and progressed [44]. In another study, there was no response of NF1-mutated GISTs to Imatinib [45].
GIST with BRAF mutations
BRAF belongs to the RAF family of serine/threonine protein kinases participating in the RAS-RAF-ERK pathway that regulates cell cycle through activation of MAPK pathway. Mutations in BRAF have been identified in other cancers like melanoma and thyroid cancer. In a study that was screening imatinib-naïve and imatinib-resistant GISTs, a BRAF exon 15 V600E mutation was identified in three of 61 imatinib-naïve GIST patients. One identical V600E BRAF mutation was identified in one of 28 imatinib-resistant GISTs. The tumors were located primarily at the small bowel, were strongly KIT immunoreactive with high mitotic rate, and had a high risk for metastases [46]. In another, more recent study, BRAF exon 15 V600E mutation was detected in 9 (13 %) of 70 patients with KIT/ PDGFRA WT GIST [29].
Mutations in BRAF have been described also as a mechanism of resistance. In naive GISTs carrying activating mutations in KIT or PDGFRA a concomitant activating mutation in BRAF (about 2 %) genes was consistent with resistance. In this study, in vitro experiments showed that imatinib was able to switch off the mutated receptor KIT but not the downstream signaling triggered by the BRAF mutation [47]. There are no sufficient data of efficacy of imatinib in BRAF-mutated GISTs. There is one case of response of BRAF-mutated GIST to dabrafenib, a potent ATP-competitive inhibitor of BRAF. In this study, the patient treated with dabrafenib had prolonged antitumor activity [48].
Pathogenesis, clinical implications, and treatment choices in KIT/PDGFRA WT-GISTs
GISTs are hypothesized to originate from the interstitial cells of Cajal (or a partially committed precursor cell) [49], the pacemaker cells which regulate peristalsis at the gastrointestinal tract. Since GISTs originate from Cajal cells, they share also immunohistochemical staining for KIT. Other common markers in GISTs are CD34 (hematopoietic progenitor cell antigen) and DOG-1 (ANO1, anoctamin 1) [50]. As we have already mentioned, GISTs in patients with CTr, CSS, and occasionally sporadic GISTs have SDHB deficiency by IHC [34], and NF1-mutated GISTs have normal SDHB staining [42], while there are no data yet for SDHB staining in BRAF-mutated tumors. GISTs with SDHA mutations also have negative SDHB IHC but also do not stain positive for SDHA [22, 51]. SDHB-deficient GIST tends to have epithelioid cytology, lympho-vascular invasion, and a characteristic multinodular or plexiform growth pattern [52, 53]. Cells of GISTs with BRAF and NF1 mutations have primarily spindle-like histology.
The exact mechanism of SDH deficiency without SDHx mutations is still unclear. One possible mechanism for the observed loss of SDHB expression and complex II function in the KIT/PDGFRA WT GISTs samples without any SDHx defects is epigenetic modification resulting in decreased mRNA expression of one of the subunits of the SDH complex. Although mRNA expression of SDHB, SDHC, and SDHD has not been found significantly different between KIT/PDGFRA WT and mutant GISTs [22], larger studies are needed to confirm this. Another potential mechanism would be mutations in SDHAF2, which has been described previously in paragangliomas [54]; SDHAF2 mutation analysis, which has been conducted in 42 of the KIT/PDGFRA WT GIST cases previously, has not revealed any mutations [22]. It is possible that SDHB deficiency in patients with CTr is due to alterations of genes that regulate SDH stability and function.
As it was discussed previously, SDH deficiency is leading to succinate accumulation, which inhibits the demethylation process of DNA and histones. SDH-deficient GIST has been shown to have a hypermethylator phenotype versus KIT mutant GIST [24] (Fig. 1). In the same study, comparison of SDH-mutant GIST with isocitrate dehydrogenase-mutant glioma revealed comparable measures of global hypo-and hypermethylation. In another study in paragangliomas, SDHx-mutated tumors demonstrated a similar hypermethylator phenotype, associated with downregulation of key genes involved in neuroendocrine differentiation [55]. SDHx mutations have also been found to predispose to pituitary and renal cell carcinomas [56–58].
While it is known that SDH deficiency is leading to a hypermethylator phenotype, it is unknown how epigenetic dysfunctions can drive tumorigenesis in these tumors. Although KIT is overexpressed in KIT/PDGFRA WT GIST, it is unclear if it plays an oncogenic role or is overexpressed because of origin from the interstitial cells of Cajal. In a recent analysis of miRNAs in GIST, a cluster of miRNAs, located in 14q32, was found to be downregulated [59]. Among those, miR-494 have been found in a previous study to downregulate KIT in GIST882 cells [60]. Other confirmed targets by miRNAs located in 14q32 are BCL-6 (miR-127), CCND2 (miR-154), and HDAC6 (miR-433) [59].
Although there is sufficient evidence that the response of SDH-deficient GIST to imatinib is poor, more studies are needed to evaluate the response to other TKIs-like sunitinib that targets angiogenesis, or regorafenib. Patients with SDH-deficient GIST had a median progression-free survival of 12 months [61], while another patient with SDH-deficient GIST had a prolonged response to pazopanib [62]. Another treatment approach would be to target the IGFR pathway. IGF1R overexpression in KIT/PDGFRA WT GIST has been reported in several studies. Indeed, IGF1R is expressed and activated in all GISTs but is markedly overexpressed in KIT/PDGFRA WT GISTs compared with mutant KIT/PDGFRA GISTs [43, 63, 64]. In a recent study, in a set of 11 patients with KIT/PDGFRA WT GIST, eight tumors were SDHB-negative by IHC and showed IGF1R overexpression, while three NF1-related tumors were SDHB-positive and IGF1R-negative, suggesting that IGF1R overexpression could be a feature of SDHB-deficient GIST [65]. Results in an ongoing study using linsitinib, an IGF1R inhibitor, in patients with WT GIST (NCT01560260) are currently pending.
There is currently another ongoing trial using vandetanib in KIT/PDGFRA WT GIST patients (NCT02015065). Vandetanib targets RET oncogene and is approved for medullary thyroid carcinoma [66, 67]. It has been shown that MTC patients have increased amino-acid coding polymorphisms in SDHB as well as G12S and H50R changes in SDHD, especially patients with familial tumors, suggesting a functional connection of coding SDHx polymorphisms to activating Ret mutations [68]. Finally, since tumors with SDH deficiency seem always to be related with a hypermethylator phenotype, epigenetic modulators like decitabine, either alone or in combination with TKIs could be potentially a reasonable therapeutic approach in KIT/PDGFRA WT GIST patients. This is supported by recent preclinical studies, where the migratory phenotype established by hypermethylation in SDHB-deficient chromaffin cells was reversed by decitabine treatment [55].
SDH-deficient GIST has unique characteristics with important implications in terms of screening, natural history, follow-up, and treatment. Although SDH-deficient GIST has the tendency to metastasize early to liver and lymph nodes, patients frequently have an indolent course, surviving for decades. Risk stratification, by tumor size and mitotic index, using the Miettinen system is not applicable to these tumors [50]. Once a germline SDHx mutation is found in a patient with SDH-deficient GIST, it is very important to screen other members of the family for the same mutation. There are no established guidelines yet for follow-up for asymptomatic carriers of SDHx mutations. In a recent study, 37 asymptomatic SDHx carriers were screened with rapid sequence whole-body MRI. Among those, five patients were diagnosed with six tumors (paragangliomas and one renal cell carcinomas), demonstrating the significance for screening in SDHx carriers [69]. Biochemical screening with yearly measurements of plasma catecholamines should be performed also in these patients. Finally, since patients with SDH-deficient GIST may develop GIST or paraganglioma after many years, a long-term clinical follow-up for the identification of GIST or paragangliomas is essential.
Summary
Both CSS and CTr are linked to SDH deficiency either due to SDHx mutations or due to unknown factors that lead to SDH deficiency. Patients with either “sporadic” GISTs or paragangliomas should always be checked for both tumors, since they may develop these tumors, years later as part of CSS or CTr. The common SDH deficiency leading to a hypermethylator phenotype in CTr, CSS, and the SDH-deficient sporadic GIST raise the need for the use of the term “SDH-deficient GIST, ” which is more specific than KIT/PDGFRA WT GIST.
Contributor Information
Sosipatros A. Boikos, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, MD, USA
Constantine A. Stratakis, Email: sboikos1@jhmi.edu, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD, USA
References
- 1.Corless CL, Fletcher JA, Heinrich MC. Biology of gastrointestinal stromal tumors. J Clin Oncol. 2004;22:3813–3825. doi: 10.1200/JCO.2004.05.140. [DOI] [PubMed] [Google Scholar]
- 2.Perez EA, Livingstone AS, Franceschi D, Rocha-Lima C, Lee DJ, Hodgson N, Jorda M, Koniaris LG. Current incidence and outcomes of gastrointestinal mesenchymal tumors including gastrointestinal stromal tumors. J Am Coll Surg. 2006;202:623–629. doi: 10.1016/j.jamcollsurg.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Nilsson B, Bümming P, Meis-Kindblom JM, Odén A, Dortok A, Gustavsson B, Sablinska K, Kindblom L-G. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era—a population-based study in western Sweden. Cancer. 2005;103:821–829. doi: 10.1002/cncr.20862. [DOI] [PubMed] [Google Scholar]
- 4.Tryggvason G, Gíslason HG, Magnússon MK, Jónasson JG. Gastrointestinal stromal tumors in Iceland, 1990–2003: the ice-landic GIST study, a population-based incidence and pathologic risk stratification study. Int J Cancer. 2005;117:289–293. doi: 10.1002/ijc.21167. [DOI] [PubMed] [Google Scholar]
- 5.Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, Muhammad Tunio G, Matsuzawa Y, Kanakura Y, Shinomura Y, Kitamura Y. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 6.Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen C-J, Joseph N, Singer S, Griffith DJ, Haley A, Town A, Demetri GD, Fletcher CDM, Fletcher JA. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708–710. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 7.Forde PM, Cochran RL, Boikos SA, Zabransky DJ, Beaver JA, Meyer CF, Thornton KA, Montgomery EA, Lidor AO, Donehower RC, Park BH. Familial GI stromal tumor with loss of heterozygosity and amplification of mutant KIT. J Clin Oncol. 2014 doi: 10.1200/JCO.2013.51.6633. [DOI] [PubMed] [Google Scholar]
- 8.Pasini B, Matyakhina L, Bei T, Muchow M, Boikos S, Ferrando B, Carney JA, Stratakis CA. Multiple gastrointestinal stromal and other tumors caused by platelet-derived growth factor receptor alpha gene mutations: a case associated with a germline V561D defect. J Clin Endocrinol Metab. 2007;92:3728–3732. doi: 10.1210/jc.2007-0894. [DOI] [PubMed] [Google Scholar]
- 9.Chompret A, Kannengiesser C, Barrois M, Terrier P, Dahan P, Tursz T, Lenoir GM, Bressac-De Paillerets B. PDGFRA germline mutation in a family with multiple cases of gastrointestinal stromal tumor. Gastroenterology. 2004;126:318–321. doi: 10.1053/j.gastro.2003.10.079. [DOI] [PubMed] [Google Scholar]
- 10.Nishida T, Hirota S, Taniguchi M, Hashimoto K, Isozaki K, Nakamura H, Kanakura Y, Tanaka T, Takabayashi A, Matsuda H, Kitamura Y. Familial gastrointestinal stromal tumours with germline mutation of the KIT gene. Nat Genet. 1998;19:323–324. doi: 10.1038/1209. [DOI] [PubMed] [Google Scholar]
- 11.Postow MA, Robson ME. Inherited gastrointestinal stromal tumor syndromes: mutations, clinical features, and therapeutic implications. Clin Sarcoma Res. 2012;2:16. doi: 10.1186/2045-3329-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kleinbaum EP, Lazar AJF, Tamborini E, Mcauliffe JC, Sylvestre PB, Sunnenberg TD, Strong L, Chen LL, Choi H, Benjamin RS, Zhang W, Trent JC. Clinical, histopathologic, molecular and therapeutic findings in a large kindred with gastrointestinal stromal tumor. Int J Cancer. 2008;122:711–718. doi: 10.1002/ijc.23137. [DOI] [PubMed] [Google Scholar]
- 13.Gold JS, van der Zwan SM, Gõnen M, Maki RG, Singer S, Brennan MF, Antonescu CR, De Matteo RP. Outcome of metastatic GIST in the era before tyrosine kinase inhibitors. Ann Surg Oncol. 2007;14:134–142. doi: 10.1245/s10434-006-9177-7. [DOI] [PubMed] [Google Scholar]
- 14.Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA, Singer S, Janicek M, Fletcher JA, Silverman SG, Silberman SL, Capdeville R, Kiese B, Peng B, Dimitrijevic S, Druker BJ, Corless C, Fletcher CDM, Joensuu H. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–480. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 15.Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, McArthur G, Judson IR, Heinrich MC, Morgan JA, Desai J, Fletcher CD, George S, Bello CL, Huang X, Baum CM, Casali PG. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368:1329–1338. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 16.Demetri GD, Reichardt P, Kang Y-K, Blay J-Y, Rutkowski P, Gelderblom H, Hohenberger P, Leahy M, von Mehren M, Joensuu H, Badalamenti G, Blackstein M, Le Cesne A, Schõffski P, Maki RG, Bauer S, Nguyen BB, Xu J, Nishida T, Chung J, Kappeler C, Kuss I, Laurent D, Casali PG. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:295–302. doi: 10.1016/S0140-6736(12)61857-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janeway KA, Albritton KH, Van Den Abbeele AD, D’Amato GZ, Pedrazzoli P, Siena S, Picus J, Butrynski JE, Schlemmer M, Heinrich MC, Demetri GD. Sunitinib treatment in pediatric patients with advanced GIST following failure of i-matinib. Pediatr Blood Cancer. 2009;52:767–771. doi: 10.1002/pbc.21909. [DOI] [PubMed] [Google Scholar]
- 18.Heinrich MC, Owzar K, Corless CL, Hollis D, Borden EC, Fletcher CDM, Ryan CW, von Mehren M, Blanke CD, Rankin C, Benjamin RS, Bramwell VH, Demetri GD, Bertagnolli MM, Fletcher JA. Correlation of kinase genotype and clinical outcome in the North American Intergroup Phase III Trial of imatinib mesylate for treatment of advanced gastrointestinal stromal tumor: CALGB 150105 Study by Cancer and Leukemia Group B and Southwest Oncology Group. J Clin Oncol. 2008;26:5360–5367. doi: 10.1200/JCO.2008.17.4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carney JA, Sheps SG, Go VL, Gordon H. The triad of gastric leiomyosarcoma, functioning extra-adrenal paraganglioma and pulmonary chondroma. N Engl J Med. 1977;296:1517–1518. doi: 10.1056/NEJM197706302962609. [DOI] [PubMed] [Google Scholar]
- 20.Carney JA, Stratakis CA. Familial paraganglioma and gastric stromal sarcoma: a new syndrome distinct from the Carney triad. Am J Med Genet. 2002;108:132–139. doi: 10.1002/ajmg.10235. [DOI] [PubMed] [Google Scholar]
- 21.McWhinney SR, Pasini B, Stratakis CA. Familial gastrointestinal stromal tumors and germ-line mutations. N Engl J Med. 2007;357:1054–1056. doi: 10.1056/NEJMc071191. [DOI] [PubMed] [Google Scholar]
- 22.Janeway KA, Kim SY, Lodish M, Nosé V, Rustin P, Gaal J, Dahia PLM, Liegl B, Ball ER, Raygada M, Lai AH, Kelly L, Hornick JL, O’Sullivan M, de Krijger RR, Dinjens WNM, Demetri GD, Antonescu CR, Fletcher JA, Helman L, Stratakis CA. Defects in succinate dehydrogenase in gastrointestinal stromal tumors lacking KIT and PDGFRA mutations. Proc Natl Acad Sci USA. 2011;108:314–318. doi: 10.1073/pnas.1009199108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, Pan Y, Simon MC, Thompson CB, Gottlieb E. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 24.Killian JK, Kim SY, Miettinen M, Smith C, Merino M, Tsokos M, Quezado M, Smith WI, Jahromi MS, Xekouki P, Szarek E, Walker RL, Lasota J, Raffeld M, Klotzle B, Wang Z, Jones L, Zhu Y, Wang Y, Waterfall JJ, O’Sullivan MJ, Bibikova M, Pacak K, Stratakis C, Janeway KA, Schiffman JD, Fan J-B, Helman L, Meltzer PS. Succinate dehydrogenase mutation underlies global epigenomic divergence in gastrointestinal stromal tumor. Cancer Discov. 2013;3:648–657. doi: 10.1158/2159-8290.CD-13-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, Agarwal S, Iyer LM, Liu DR, Aravind L, Rao A. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–935. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiao M, Yang H, Xu W, Ma S, Lin H, Zhu H, Liu L, Liu Y, Yang C, Xu Y, Zhao S, Ye D, Xiong Y, Guan K-L. Inhibition of α-KG-dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors. Genes Dev. 2012;26:1326–1338. doi: 10.1101/gad.191056.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaelin WG, McKnight SL. Influence of metabolism on epigenetics and disease. Cell. 2013;153:56–69. doi: 10.1016/j.cell.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang M, Pollard PJ. Succinate: a new epigenetic hacker. Cancer Cell. 2013;23:709–711. doi: 10.1016/j.ccr.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 29.Agaimy A, Terracciano LM, Dirnhofer S, Tornillo L, Foerster A, Hartmann A, Bihl MP. V600E BRAF mutations are alternative early molecular events in a subset of KIT/PDGFRA wild-type gastrointestinal stromal tumours. J Clin Pathol. 2009;62:613–616. doi: 10.1136/jcp.2009.064550. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto H, Tobo T, Nakamori M, Imamura M, Kojima A, Oda Y, Nakamura N, Takahira T, Yao T, Tsuneyoshi M. Neurofibromatosis type 1-related gastrointestinal stromal tumors: a special reference to loss of heterozygosity at 14q and 22q. J Cancer Res Clin Oncol. 2009;135:791–798. doi: 10.1007/s00432-008-0514-z. [DOI] [PubMed] [Google Scholar]
- 31.Grace MP, Batist G, Grace WR, Gillooley JF. Aorticopulmonary paraganglioma and gastric leiomyoblastoma in a young woman. Am J Med. 1981;70:1288–1292. doi: 10.1016/0002-9343(81)90840-8. [DOI] [PubMed] [Google Scholar]
- 32.Carney JA. Gastric stromal sarcoma, pulmonary chondroma, and extra-adrenal paraganglioma (Carney Triad): natural history, adrenocortical component, and possible familial occurrence. Mayo Clin Proc. 1999;74:543–552. doi: 10.4065/74.6.543. [DOI] [PubMed] [Google Scholar]
- 33.Matyakhina L, Bei TA, McWhinney SR, Pasini B, Cameron S, Gunawan B, Stergiopoulos SG, Boikos S, Muchow M, Dutra A, Pak E, Campo E, Cid MC, Gomez F, Gaillard RC, Assie G, Füzesi L, Baysal BE, Eng C, Carney JA, Stratakis CA. Genetics of carney triad: recurrent losses at chromosome 1 but lack of germline mutations in genes associated with paragangliomas and gastrointestinal stromal tumors. J Clin Endocrinol Metab. 2007;92:2938–2943. doi: 10.1210/jc.2007-0797. [DOI] [PubMed] [Google Scholar]
- 34.Gaal J, Stratakis CA, Carney JA, Ball ER, Korpershoek E, Lodish MB, Levy I, Xekouki P, van Nederveen FH, den Bakker MA, O’Sullivan M, Dinjens WNM, de Krijger RR. SDHB immunohistochemistry: a useful tool in the diagnosis of Carney–Stratakis and Carney triad gastrointestinal stromal tumors. Mod Pathol. 2011;24:147–151. doi: 10.1038/modpathol.2010.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang L, Smyrk TC, Young WF, Stratakis CA, Carney JA. Gastric stromal tumors in Carney triad are different clinically, pathologically, and behaviorally from sporadic gastric gastrointestinal stromal tumors: findings in 104 cases. Am J Surg Pathol. 2010;34:53–64. doi: 10.1097/PAS.0b013e3181c20f4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pasini B, McWhinney SR, Bei T, Matyakhina L, Stergiopoulos S, Muchow M, Boikos SA, Ferrando B, Pacak K, Assie G, Baudin E, Chompret A, Ellison JW, Briere J-J, Rustin P, Gimenez-Roqueplo A-P, Eng C, Carney JA, Stratakis CA. Clinical and molecular genetics of patients with the Carney–Stratakis syndrome and germline mutations of the genes coding for the succinate dehydrogenase subunits SDHB, SDHC, and SDHD. Eur J Hum Genet. 2008;16:79–88. doi: 10.1038/sj.ejhg.5201904. [DOI] [PubMed] [Google Scholar]
- 37.Oudijk L, Gaal J, Korpershoek E, van Nederveen FH, Kelly L, Schiavon G, Verweij J, Mathijssen RHJ, den Bakker MA, Oldenburg RA, van Loon RLE, O’Sullivan MJ, de Krijger RR, Dinjens WNM. SDHA mutations in adult and pediatric wild-type gastrointestinal stromal tumors. Mod Pathol. 2013;26:456–463. doi: 10.1038/modpathol.2012.186. [DOI] [PubMed] [Google Scholar]
- 38.Wagner AJ, Remillard SP, Zhang Y-X, Doyle LA, George S, Hornick JL. Loss of expression of SDHA predicts SDHA mutations in gastrointestinal stromal tumors. Mod Pathol. 2013;26:289–294. doi: 10.1038/modpathol.2012.153. [DOI] [PubMed] [Google Scholar]
- 39.Miettinen M, Killian JK, Wang Z-F, Lasota J, Lau C, Jones L, Walker R, Pineda M, Zhu YJ, Kim SY, Helman L, Meltzer P. Immunohistochemical loss of succinate dehydrogenase subunit A (SDHA) in gastrointestinal stromal tumors (GISTs) signals SDHA germline mutation. Am J Surg Pathol. 2013;37:234–240. doi: 10.1097/PAS.0b013e3182671178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maertens O, Prenen H, Debiec-Rychter M, Wozniak A, Sciot R, Pauwels P, De Wever I, Vermeesch JR, de Raedt T, De Paepe A, Speleman F, van Oosterom A, Messiaen L, Legius E. Molecular pathogenesis of multiple gastrointestinal stromal tumors in NF1 patients. Hum Mol Genet. 2006;15:1015–1023. doi: 10.1093/hmg/ddl016. [DOI] [PubMed] [Google Scholar]
- 41.Miettinen M, Lasota J. Gastrointestinal stromal tumors. Gastroenterol Clin North Am. 2013;42:399–415. doi: 10.1016/j.gtc.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang JH, Lasota J, Miettinen M. Succinate dehydrogenase subunit B (SDHB) is expressed in neurofibromatosis 1-associated gastrointestinal stromal tumors (GISTs): implications for the SDHB expression based classification of GISTs. J Cancer. 2011;2:90–93. doi: 10.7150/jca.2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lasota J, Wang Z, Kim SY, Helman L, Miettinen M. Expression of the receptor for type i insulin-like growth factor (IGF1R) in gastrointestinal stromal tumors: an immunohistochemical study of 1078 cases with diagnostic and therapeutic implications. Am J Surg Pathol. 2013;37:114–119. doi: 10.1097/PAS.0b013e3182613c86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee J-L, Kim JY, Ryu M-H, Kang HJ, Chang HM, Kim T-W, Lee H, Park JH, Kim HC, Kim JS, Kang Y-K. Response to imatinib in KIT- and PDGFRA-wild type gastrointestinal stromal associated with neurofibromatosis type 1. Dig Dis Sci. 2006;51:1043–1046. doi: 10.1007/s10620-006-8003-1. [DOI] [PubMed] [Google Scholar]
- 45.Mussi C, Schildhaus H-U, Gronchi A, Wardelmann E, Hohenberger P. Therapeutic consequences from molecular biology for gastrointestinal stromal tumor patients affected by neurofibromatosis type 1. Clin Cancer Res. 2008;14:4550–4555. doi: 10.1158/1078-0432.CCR-08-0086. [DOI] [PubMed] [Google Scholar]
- 46.Agaram NP, Wong GC, Guo T, Maki RG, Singer S, Dematteo RP, Besmer P, Antonescu CR. Novel V600E BRAF mutations in imatinib-naive and imatinib-resistant gastrointestinal stromal tumors. Genes Chromosom Cancer. 2008;47:853–859. doi: 10.1002/gcc.20589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miranda C, Nucifora M, Molinari F, Conca E, Anania MC, Bordoni A, Saletti P, Mazzucchelli L, Pilotti S, Pierotti MA, Tamborini E, Greco A, Frattini M. KRAS and BRAF mutations predict primary resistance to imatinib in gastrointestinal stromal tumors. Clin Cancer Res. 2012;18:1769–1776. doi: 10.1158/1078-0432.CCR-11-2230. [DOI] [PubMed] [Google Scholar]
- 48.Falchook GS, Trent JC, Heinrich MC, Beadling C, Patterson J, Bastida CC, Blackman SC, Kurzrock R. BRAF mutant gastrointestinal stromal tumor: first report of regression with BRAF inhibitor dabrafenib (GSK2118436) and whole exomic sequencing for analysis of acquired resistance. Oncotarget. 2013;4:310–315. doi: 10.18632/oncotarget.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindblom JM. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol. 1998;152:1259–1269. [PMC free article] [PubMed] [Google Scholar]
- 50.Miettinen M, Wang Z-F, Sarlomo-Rikala M, Osuch C, Rutkowski P, Lasota J. Succinate dehydrogenase-deficient GISTs: a clinicopathologic, immunohistochemical, and molecular genetic study of 66 gastric GISTs with predilection to young age. Am J Surg Pathol. 2011;35:1712–1721. doi: 10.1097/PAS.0b013e3182260752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Nederveen FH, Gaal J, Favier J, Korpershoek E, Oldenburg RA, de Bruyn EMCA, Sleddens HFBM, Derkx P, Rivière J, Dannenberg H, Petri B-J, Komminoth P, Pacak K, Hop WCJ, Pollard PJ, Mannelli M, Bayley J-P, Perren A, Niemann S, Verhofstad AA, de Bruïne AP, Maher ER, Tissier F, Méatchi T, Badoual C, Bertherat J, Amar L, Alataki D, Van Marck E, Ferrau F, François J, de Herder WW, Peeters MPFMV, van Linge A, Lenders JWM, Gimenez-Roqueplo A-P, de Krijger RR, Dinjens WNM. An immunohistochemical procedure to detect patients with paraganglioma and phaeo-chromocytoma with germline SDHB, SDHC, or SDHD gene mutations: a retrospective and prospective analysis. Lancet Oncol. 2009;10:764–771. doi: 10.1016/S1470-2045(09)70164-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rege TA, Wagner AJ, Corless CL, Heinrich MC, Hornick JL. “Pediatric-type” gastrointestinal stromal tumors in adults: distinctive histology predicts genotype and clinical behavior. Am J Surg Pathol. 2011;35:495–504. doi: 10.1097/PAS.0b013e31820e5f7d. [DOI] [PubMed] [Google Scholar]
- 53.Doyle LA, Nelson D, Heinrich MC, Corless CL, Hornick JL. Loss of succinate dehydrogenase subunit B (SDHB) expression is limited to a distinctive subset of gastric wild-type gastrointestinal stromal tumours: a comprehensive genotype-phenotype correlation study. Histopathology. 2012;61:801–809. doi: 10.1111/j.1365-2559.2012.04300.x. [DOI] [PubMed] [Google Scholar]
- 54.Hao H-X, Khalimonchuk O, Schraders M, Dephoure N, Bayley J-P, Kunst H, Devilee P, Cremers CWRJ, Schiffman JD, Bentz BG, Gygi SP, Winge DR, Kremer H, Rutter J. SDH5, a gene required for flavination of succinate dehydrogenase, is mutated in paraganglioma. Science. 2009;325:1139–1142. doi: 10.1126/science.1175689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Letouzé E, Martinelli C, Loriot C, Burnichon N, Abermil N, Ottolenghi C, Janin M, Menara M, Nguyen AT, Benit P, Buffet A, Marcaillou C, Bertherat J, Amar L, Rustin P, De Reyniès A, Gimenez-Roqueplo A-P, Favier J. SDH mutations establish a hypermethylator phenotype in paraganglioma. Cancer Cell. 2013;23:739–752. doi: 10.1016/j.ccr.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 56.Gill AJ, Pachter NS, Chou A, Young B, Clarkson A, Tucker KM, Winship IM, Earls P, Benn DE, Robinson BG, Fleming S, Clifton-Bligh RJ. Renal tumors associated with germline SDHB mutation show distinctive morphology. Am J Surg Pathol. 2011;35:1578–1585. doi: 10.1097/PAS.0b013e318227e7f4. [DOI] [PubMed] [Google Scholar]
- 57.Xekouki P, Stratakis CA. Succinate dehydrogenase (SDHx) mutations in pituitary tumors: could this be a new role for mitochondrial complex II and/or Krebs cycle defects? Endocr Relat Cancer. 2012;19:C33–C40. doi: 10.1530/ERC-12-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xekouki P, Pacak K, Almeida M, Wassif CA, Rustin P, Nesterova M, de la Luz Sierra M, Matro J, Ball E, Azevedo M, Horvath A, Lyssikatos C, Quezado M, Patronas N, Ferrando B, Pasini B, Lytras A, Tolis G, Stratakis CA. Succinate dehydrogenase (SDH) D subunit (SDHD) inactivation in a growth-hormone-producing pituitary tumor: a new association for SDH? J Clin Endocrinol Metab. 2012;97:E357–E366. doi: 10.1210/jc.2011-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kelly L, Bryan K, Kim SY, Janeway KA, Killian JK, Schildhaus H-U, Miettinen M, Helman L, Meltzer PS, van de Rijn M, Debiec-Rychter M, O’Sullivan M. Post-transcriptional dysregulation by miRNAs is implicated in the pathogenesis of gastrointestinal stromal tumor [GIST] PLoS ONE. 2013;8:e64102. doi: 10.1371/journal.pone.0064102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim WK, Park M, Kim Y-K, Tae YK, Yang H-K, Lee JM, Kim H. MicroRNA-494 downregulates KIT and inhibits gastrointestinal stromal tumor cell proliferation. Clin Cancer Res. 2011;17:7584–7594. doi: 10.1158/1078-0432.CCR-11-0166. [DOI] [PubMed] [Google Scholar]
- 61.George S, Wang Q, Heinrich MC, Corless CL, Zhu M, Butrynski JE, Morgan JA, Wagner AJ, Choy E, Tap WD, Yap JT, Van den Abbeele AD, Manola JB, Solomon SM, Fletcher JA, von Mehren M, Demetri GD. Efficacy and safety of regorafenib in patients with metastatic and/or unresectable GI stromal tumor after failure of imatinib and sunitinib: a multi-center phase II trial. J Clin Oncol. 2012;30:2401–2407. doi: 10.1200/JCO.2011.39.9394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ganjoo KN, Villalobos VM, Kamaya A, Fisher GA, Butrynski JE, Morgan JA, Wagner AJ, D’Adamo D, McMillan A, Demetri GD, George S. A multicenter phase II study of pazopanib in patients with advanced gastrointestinal stromal tumors (GIST) following failure of at least imatinib and sunitinib. Ann Oncol. 2014;25:236–240. doi: 10.1093/annonc/mdt484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Janeway KA, Zhu M-J, Barretina J, Perez-Atayde A, Demetri GD, Fletcher JA. Strong expression of IGF1R in pediatric gastrointestinal stromal tumors without IGF1R genomic amplification. Int J Cancer. 2010;127:2718–2722. doi: 10.1002/ijc.25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pantaleo MA, Astolfi A, Di Battista M, Heinrich MC, Paterini P, Scotlandi K, Santini D, Catena F, Manara MC, Nannini M, Maleddu A, Saponara M, Lolli C, Formica S, Biasco G. Insulin-like growth factor 1 receptor expression in wild-type GISTs: a potential novel therapeutic target. Int J Cancer. 2009;125:2991–2994. doi: 10.1002/ijc.24595. [DOI] [PubMed] [Google Scholar]
- 65.Chou A, Chen J, Clarkson A, Samra JS, Clifton-Bligh RJ, Hugh TJ, Gill AJ. Succinate dehydrogenase-deficient GISTs are characterized by IGF1R overexpression. Mod Pathol. 2012;25:1307–1313. doi: 10.1038/modpathol.2012.77. [DOI] [PubMed] [Google Scholar]
- 66.Fox E, Widemann BC, Chuk MK, Marcus L, Aikin A, Whitcomb PO, Merino MJ, Lodish M, Dombi E, Steinberg SM, Wells SA, Balis FM. Vandetanib in children and adolescents with multiple endocrine neoplasia type 2B associated medullary thyroid carcinoma. Clin Cancer Res. 2013;19:4239–4248. doi: 10.1158/1078-0432.CCR-13-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wells SA, Gosnell JE, Gagel RF, Moley J, Pfister D, Sosa JA, Skinner M, Krebs A, Vasselli J, Schlumberger M. Vandetanib for the treatment of patients with locally advanced or metastatic hereditary medullary thyroid cancer. J Clin Oncol. 2010;28:767–772. doi: 10.1200/JCO.2009.23.6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Montani M, Schmitt AM, Schmid S, Locher T, Saremaslani P, Heitz PU, Komminoth P, Perren A. No mutations but an increased frequency of SDHx polymorphisms in patients with sporadic and familial medullary thyroid carcinoma. Endocr Relat Cancer. 2005;12:1011–1016. doi: 10.1677/erc.1.00996. [DOI] [PubMed] [Google Scholar]
- 69.Jasperson KW, Kohlmann W, Gammon A, Slack H, Buchmann L, Hunt J, Kirchhoff AC, Baskin H, Shaaban A, Schiffman JD. Role of rapid sequence whole-body MRI screening in SDH-associated hereditary paraganglioma families. Fam Cancer. 2013;13:257–265. doi: 10.1007/s10689-013-9639-6. [DOI] [PubMed] [Google Scholar]