Abstract
Over the past two decades, baculoviruses have become workhorse research tools for transient transgene expression. Although they have not yet been used directly as a gene therapy vector in the clinical setting, numerous preclinical studies have suggested the highly promising potential of baculovirus as a delivery vector for a variety of therapeutic applications including vaccination, tissue engineering, and cancer treatment. As such, there is growing interest in using baculoviruses as human gene therapy vectors, which has led to advances in baculovirus bioprocessing methods. This review provides an overview of the current approaches for scaled-up amplification, concentration, purification, and formulation of AcMNPV baculoviruses, and highlights the key regulatory requirements that must be met before gene therapy clinical trials can be initiated.
Introduction: baculovirus as gene therapy vectors
Gene therapy provides potential treatment options for many diseases difficult to treat by conventional means. Viral vectors have been utilized in gene therapy interventions to introduce therapeutic genes into patient’s cells through ex vivo or in vivo methods. Recombinant baculoviral vectors derived from the insect baculovirus (BV) Autographa californica multiple nucleopolyhedrovirus (AcMNPV), when equipped with a mammalian expression cassette, are highly effective in mediating transient transgene expression in human cells.1,2 The high transduction efficiency provided by BV vectors in noninsect cells was initially reported in hepatocytes and has since been demonstrated in a broad range of cell types that include most mammalian cell lines, primary cells, adult and embryonic stem cells, and those that are difficult to transduce by other viral vectors.3–6
BVs are enveloped viruses with a circular double-stranded DNA genome commonly found in nature. As a biopesticide, the occluded Heliothis zea BV does not cause any side effects including inflammation and allergy as demonstrated in feeding tests with volunteer human subjects.7 Animal studies with recombinant Bombyx mori BVs also noted no obvious toxicity after subcutaneous injection.8 Even at relatively high titers, BVs do not have obvious cytopathic effects on the transduced cell and remain nonintegrating, hence limiting the risk of genotoxicity caused by insertional mutagenesis.3 Unlike conventionally-used human viral vectors such as adenovirus, retrovirus and adeno-associated virus, BVs are inherently incapable of replicating in human cells and there is no detectable pre-existing immunity to BV in humans.9–11 From a bioprocessing perspective, BV vectors are easy to manipulate genetically, have a large cloning capacity (allowing at least 38 kbp of inserts), and can be readily produced in serum-free culture medium and purified at high titers in Biosafety Level 1 laboratories. In comparison, adenoviruses are highly immunogenic leading to strong immune responses that result in significant toxicity and limit readministration of the vector;12 adeno-associated viruses have a small packaging capacity (only up to 4.5 kbp);13 and retroviral vectors have a significant risk of insertional mutagenesis leading to aberrant transformations.14 For these reasons, recombinant BVs are attractive as novel gene therapy vectors, especially suited for short-term and high-level transgene expression.1–3,15
Although BV vectors have yet to reach clinical stage, preclinical animal and ex vivo studies using human cells have demonstrated the feasibility of BV-mediated gene transfer in several gene therapy applications including vaccination, tissue engineering/regenerative medicine, and cancer therapy. BV vectors have been shown to be effective as vaccine carriers in mice and nonhuman primates, through expression of antigens and/or surface display of exogenous peptides fused with BV envelop proteins, to induce both humoral and cellular immune responses for prevention or treatment of human/animal infectious diseases including malaria, influenza, and rabies.16–19 Recent findings of bone healing with adipose-derived stem cells genetically modified with BV vectors to express various growth factors in animal studies demonstrate the great potential of BV gene therapy in tissue engineering.20–24 BV transduction-based approaches have also been explored for cancer treatment, involving expression of suicide genes, tumor suppressors, or other antitumor genes in target tumor cells.25–30 BVs loaded with immunopotentiating genes are able to elicit strong host immunity against malignancies of the lungs, prostate, bladder, and brain. In a recent bladder cancer mouse study, we observed strong immunopotentiation and increased survival following intravesical instillation of BV vectors expressing cytokines, CD40 ligand and IL-15, into mice bearing established orthotopic bladder tumors.31,32 These encouraging results in animal studies accentuate the promise of BV vectors for gene therapy clinical applications.
To use BV vectors for human gene therapy, the development of robust, reliable, and scalable production processes for the vectors is crucial. This review discusses the current knowledge on the manufacturability of AcMNPV BV vectors for clinical use.
Upstream processes to produce baculoviral vectors for clinical use
Production of master cell bank
An overview of the manufacturing process of clinical-grade recombinant AcMNPV BVs for gene therapy is depicted in Figure 1. The first step in the manufacturing process is the setting up of insect cell banks—master/working cell banks (MCB/WCB)—to be used for amplifying the virus. Ideally, the chosen cells for banking should be easily expanded, capable of consistent and continuous propagation, competent in producing high titers of transduction-capable BV vectors, and free of endogenous viruses and adventitious contaminants.
Figure 1.
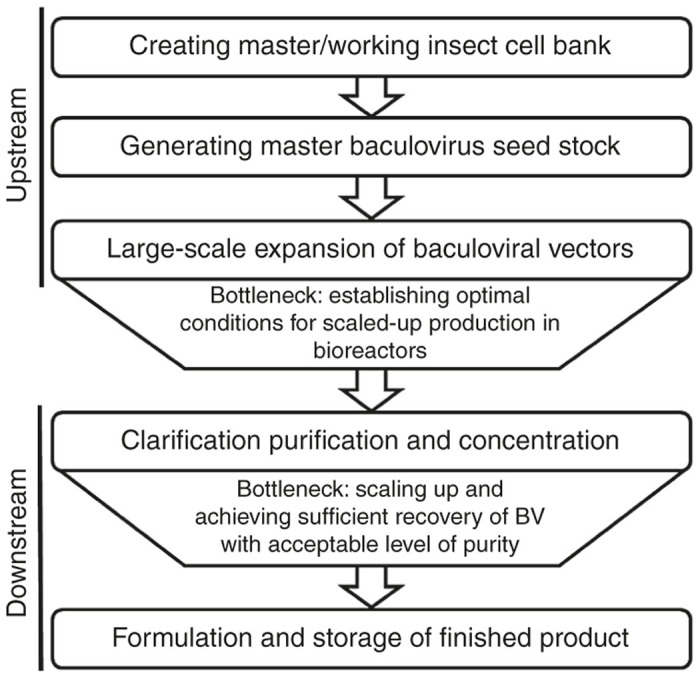
Flow chart of the manufacturing process of baculovirus for clinical gene therapy including existing bottlenecks.
The cell lines Sf21 and its sub-clone Sf9, derived from Spodoptera frugiperda, and High Five, derived from Trichoplusia ni, have been widely used for the production of AcMNPV BV vectors and recombinant proteins for both research and manufacturing.33–35 They exhibit rapid growth rates, tolerance to shear stress, and robust production of budded AcMNPV BVs and recombinant proteins over several passages in suspension culture with serum-free medium.35–37 Sf9 cells are capable of producing greater numbers of infectious BVs in total than High Five cells (100-fold greater),38 in part due to the higher cell densities achieved by Sf9 cultures.36,38,39 Both High Five and Sf9 cell lines have gained regulatory acceptance for manufacturing of BV-based clinical biologics, e.g., Cervarix and Flublok respectively. Furthermore, since most studies on BV-based gene transfer into mammalian cells utilized AcMNPV vectors that were produced in Sf9 cells, Sf9 remains the choice cell line for AcMNPV BV production.3,28,29
A major safety issue concerning insect cells is the presence of insect viruses.40 High Five cells were found to be latently infected with an alphanodavirus,41 whereas Sf9 (and Sf21) cells were recently discovered to be infected with a novel rhabdovirus, Sf-rhabdovirus.42 While these insect-specific viruses do not appear to replicate in mammalian cells, much is still unknown about their safety profile and their presence may hinder regulatory approval of Sf9 or High Five cells for manufacturing of BV vectors for gene therapy. Therefore, there is need to establish virus-free insect cell lines or remove these contaminating viruses from BV products in downstream processes. An alphanodavirus-free cell line QB-CL-B has been derived from High Five cells and displays similar BV and protein productivities with High Five, and may potentially be used for scaled-up BV production.43 Thus far no Sf-rhabdovirus-free Sf9 cell line exists.
Cell banking should be carried out according to the standards of current good manufacturing practices (cGMP) delineated by the European Medicines Agency (EMA) or the US Food and Drug Administration (FDA).44,45 Several of these guidelines are listed in a review by Lesch et al.46 The purpose of cell banking is to provide assurance that the integrity and quality of the virus packaging cells remain consistent for batch-to-batch virus production. Establishing an MCB involves full characterization of the cells. Information about the identity, origin, karyotype, isoenzyme profile, morphology, growth profile, and BV productivity of the chosen insect cells should be documented. Sterility tests must be performed to demonstrate that the cells are free from adventitious contamination such as bacteria, fungi, mycoplasma, spiroplasma, and relevant human and animal viruses. Additionally, the level of endogenous insect-specific viruses, such as Sf-rhabdovirus in Sf9 cells and alphanodavirus in High Five cells, should be assessed.
Production of master virus seed stock
Setting up a master virus seed stock (MVSS) is essential for quality assurance of the starting material for manufacturing a clinical virus product. A BV MVSS is manufactured from a starting recombinant BV stock that has been well characterized. There are several methods to construct recombinant AcMNPV genomes, namely transposition-based methods (e.g., Bac-to-Bac system) and homologous recombination methods (e.g., BacPAK and flashBAC systems).47,48 Clinical-grade AcMNPV vectors are preferably synthesized with homologous recombination methods as the resultant BV genome will not contain residual antibiotic resistance marker and bacterial sequences.47–50
An MVSS is prepared by amplifying the starting BV stock in cells from the MCB/WCB using a low multiplicity of infection (MOI) under cGMP conditions.46 MVSS preparation involves full characterization of the recombinant AcMNPV vector, providing information on its origin and construction, verifying the integrity of the virus genome and transgene(s) via sequencing or polymerase chain reaction (PCR) analysis, and performing assays to demonstrate purity, sterility, safety, potency, and long-term stability.44,51 Similar to MCB preparation, MVSSs must be free from adventitious contaminants. Insect-specific viruses (e.g., Sf-rhabdovirus or alphanodavirus) relevant to the BV producer cells should also be quantified in the MVSS by quantitative real-time PCR (qPCR). Some methods to determine the infectivity (infectious titer) and potency of MVSSs are highlighted below under the section “Quality control and characterization of AcMNPV baculovirus vectors”. BVs produced in serum-free medium remain stable at or below −80 °C for at least 300 days and is a feasible format for storage of MVSSs as crude virus stocks.52
Large-scale cGMP production strategies
When contemplating the production of AcMNPV vectors for clinical use, it is crucial to have robust, yet cost-effective, cGMP-compliant manufacturing processes at industrial scale. Suspension cultures are preferred for large-scale production and several factors that affect BV yield in suspension culture include cell density at infection, MOI, dissolved oxygen, hydrodynamic forces, pH, temperature, and the time of harvest. Generally for effective BV vector expansion in Sf9 cell cultures, cells are infected at starting densities of 1–3 × 106 cells/ml with a low MOI (0.01–1 pfu/cell).53 Using a low MOI minimizes the formation of defective interfering particles, which compromise the quality of the virus product.54,55 Infected Sf9 cells are cultivated at 27–28 °C with pH kept between 6.0–6.5; oscillating the culture temperature between 24 to 28 °C at a 12-hour period is able to increase BV yield.56 Harvesting of the budded BVs is typically carried out about 3 days postinfection with cell viability above 50%. The above-mentioned parameters may require monitoring and optimization according to the insect cell line and culturing system used. Methods for on-line monitoring of the growth status of BV-infected cultures are available.57,58
Large-scale expansion of AcMNPV vectors is best performed in batch mode as host insect cells lyse during BV infection, and because defective interfering BVs accumulate and productivity decreases with extended passaging of BVs.59 Serum-free, animal component-free culture medium is preferred for cGMP manufacturing of BV vectors to minimize the risk of adventitious contamination and to eliminate the need to remove serum impurities during downstream processing; the review article by Aucoin et al.34 lists several commercially available media. For medium-scale production, a simple setup of Fernbach flask cultures in shaker incubators (at 100–120 rpm) can easily produce up to 10 l of BV supernatant per batch, and with titers above 2 × 108 pfu/ml routinely being achieved (unpublished data), which translates to over a hundred 1010-pfu doses—the average human equivalent dose estimated from various gene therapy studies in mice.29 Nevertheless, at larger volumes, this approach becomes labor-intensive and inefficient. Bioreactor systems, such as the stirred tank, airlift, packed-bed and wave bioreactors, are more suited for large-scale production of up to several hundred liters of AcMNPV BV (reviewed in Contreras-Gómez et al. 2014).60–62
Downstream processing: clarification, concentration, purification, and formulation
For the production of clinical-grade BV vectors, downstream processing (DSP) is essential to remove impurities and insect cell materials from a crude viral product. As BV-mediated gene therapy is still in its nascent stage, there is a lack of established DSP protocols to achieve large-scale processing of BV vectors and satisfy regulatory demands. Notwithstanding, there is growing focus on DSP of BV vectors for clinical use. The main factors to consider in DSP include efficiency, robustness, scalability, and cost-effectiveness. Since DSP typically constitutes up to 70% of the overall production cost, there is need for cost-effective strategies capable of generating sufficient quantity of high-quality/purity BV products within a reasonably short time.63
Clarification
Clarification, the first step in DSP, involves removing cell debris and aggregates from crude BV supernatant while retaining the quality/quantity of the BV product.64 As the budded form of BV is harvested, lysing of cells prior to clarification is not necessary. Low-speed centrifugation (batch or continuous) and membrane microfiltration are two approaches commonly used to clarify BV supernatant. Centrifugation is the gold standard for cell sedimentation in the clarification process, with continuous flow centrifugation as the main method for industrial-scale processing (capable of processing up to 100 l/hour).65 However, the centrifugation setup requires high upfront costs. Alternatively, membrane microfiltration, specifically depth and tangential flow filtration, provides a cheaper option that can be easily scaled up for cGMP production.64 Depth-filters are available in a range of pore sizes (0.1–10 µm) and in disposable format, which eliminates cleaning/sterilization procedures.63 They have been shown to be effective for clarifying BV vectors.66 Microfiltration may also be performed in tangential flow mode using a 0.22-µm retention pore membrane unit, albeit membrane fouling and shear-induced cell lysis may pose a problem.64,67
Concentration
Concentration is an essential step to obtain high-titer virus stocks necessary for human gene therapy and to reduce bulk volume to ease handling, storage, and transport. Sucrose gradient ultracentrifugation is commonly used to concentrate and purify small volumes of BV vectors, but it is time and labor intensive, limited in scalability and suffers from low recovery of BV (usually no greater than 50%), hence is not adopted for large-scale processing.68 We instead tested a high-speed centrifugation method (adapted from ref. 69) to concentrate larger amounts of BV supernatant. By centrifugation of 1-l samples of BV vectors at 15,900 g (JLA8.1 rotor; Beckman) for 1 hour 20 minutes, we could easily achieve 50-fold concentration with an overall recovery of 56.6% (Table 1). About 24% of infectious BV was not pelleted down and an estimated 20% was lost probably due to viral particle aggregation. Essentially, up to 6 l can be processed per cycle—sufficient for medium-scale production.
Table 1. Recovery of BV after concentration by centrifugation at 15,900 g for 1 hour 20 minutesa.
| Volume (ml) | Infectious titer (pfu/ml) | Total infectious particles (pfu) | Recovery (%) | |
|---|---|---|---|---|
| BV supernatant | 950 | 3.80E+08 | 3.61E+11 | 100 |
| Concentrated BV (resuspended in phosphate-buffered saline) | 9.5 | 2.15E+10 | 2.04E+11 | 56.6 |
| Remaining supernatant postcentrifugation | 950 | 8.95E+07 | 8.50E+10 | 23.6 |
BV, baculovirus.
BV titers were determined by plaque assays. Results shown are means of triplicate determinations.
Ultrafiltration via tangential flow filtration (TFF) has also been used to concentrate BV vectors, reportedly achieving at least sixfold concentration with 70% recovery yield.66 The TFF method employs membranes with molecular weight cut-off between 100–1,000 kDa for concentrating BV and involves size-based separation where the larger BV particles are retained by the membrane in the retentate while smaller impurities flow through as permeate, thus purifying and concentrating the virus solution concurrently.34,70,71 By incorporating diafiltration into the TFF approach, buffer exchange can also be performed to replace the existing solvent/solution in the feed suspension with a desired buffer to aid further processing and final formulation.72 TFF is an attractive method for cGMP processing of viral products as it is highly scalable, allows processing of greater volumes in shorter times than centrifugation methods, and uses disposable membranes and materials (Table 2).71
Table 2. Methods for purification of BV vectors.
| Method | Details | Key advantage(s) | Drawback(s) | Reported recovery of infectious particles | References |
|---|---|---|---|---|---|
| Tangential flow ultrafiltration | Size-based separation using membranes with molecular weight cut-off between 100–1,000 kDa • Average flow rate of 25 l/m2/hour | Scalable • Available in multiuse, disposable format • At least sixfold virus concentration is achievable | • Complicated procedures • Susceptible to membrane fouling and clogging • Time-consuming clean-in-place and equilibration procedures • Difficulty separating BV from contaminants of similar molecular size | 70% | 66 |
| Sucrose gradient ultracentrifugation | Centrifugation at speeds ≥ 80,000 g • Process up to 1.5 l/cycle | • High resolution of separation, hence able to achieve high purity • Combines concentration and purification in single unit operation | • High cost of equipment • Time-consuming and laborious • Scalability limited by rotor capacity • Causes irreversible viral particle aggregation • Tedious pellet resuspension • Difficulty separating BV from contaminants of similar molecular size | 50% | 34,68 |
| High-speed batch centrifugation | Centrifugation at 15,900 g • Process up to 6 l/cycle | • Simple procedure • Greater processing capacity than ultracentrifugation • 50-fold virus concentration is achievable | • High cost of equipment • Scalability limited by rotor capacity • Causes irreversible viral particle aggregation • Tedious pellet resuspension • Difficulty separating BV from contaminants of similar molecular size | 57% | Reported in this review.69 |
| Ion-exchange membrane chromatography | Separation based on net surface charge. Typically employs polyethersulfone membranes. • Using cation (sulphone or carboxymethanol) or anion (DEAE or quaternary ammonium) exchangers • Maximum flow rate of 25 l/minute with a 5-l bed volume unit | • Scalable and eliminates the need for column packing • Available in multi-use, disposable format • High flow rates are achievable; fast processing time • Relatively low operational cost • Adsorption selectivity of BVs over contaminants based on charge • Negligible diffusional mass transfer resistance • Less shear stress on BVs • 30-fold virus concentration is achievable | • Susceptible to membrane fouling and clogging | Anionic: 65%; cationic: 68–78% | 66,74 |
| Monolithic ion-exchange chromatography | Separation based on net surface charge. Interconnected network of channels with huge diameter ≥ 1,000 nm • Maximum flow rate of 10 l/minute with an 8-l bed volume unit | • Offers the advantages of ion-exchange membrane chromatography Additional benefits: • High porosity and large adsorption area enhance viral binding capacity • High resolution of separation • 52-fold virus concentration is achievable | • Susceptible to lipid fouling and clogging | 87% | 76 |
| Size-exclusion Chromatography | Bead-based porous matrix in a column. | • Less shear stress on BV | • Nonscalable due to column packing issues • Product is diluted in the process • Time-consuming clean-in-place procedures • Low processing speed • Difficulty separating BV from contaminants of similar molecular size | 24% | 73 |
BV, baculovirus; DEAE, diethylaminoethanol.
Purification and polishing
Purification of enveloped AcMNPV BV particles is particularly challenging due to their fragile nature. Some approaches used for BV purification include the concentration methods discussed above (centrifugation and TFF) and size-exclusion chromatography to remove impurities according to size or molecular weight.73 However, these methods are ineffective in removing contaminants with similar size and molecular weight to BV, such as the insect-specific virus Sf-rhabdovirus.42 Further purification and polishing of concentrated BV are mainly carried out using other chromatography-based techniques (reviewed by Nestola et al.63), particularly ion-exchange chromatography. Ion-exchange membrane chromatography provides a scalable and improved alternative to conventional column-based ion-exchange chromatography. Both cation and anion ion-exchange membrane chromatography have been used to purify BV yielding recoveries of 65–78%.66,74 A 30-fold concentration of infective BV was also achieved with cation ion-exchange membrane chromatography using a Mustang S membrane (Pall),74 however, in our preliminary tests, this method was ineffective in removing Sf-rhabdovirus from the BV stock (unpublished data). Another scalable method of ion-exchange chromatography uses monoliths, a type of highly porous materials that allow high flow rate while providing a large adsorption area for efficient virus capture.75 At least 87% recovery with 52-fold enrichment of infective BV can be achieved using anion-exchange monolith.76 A desalting step after purification via ion-exchange chromatography may be required to reduce the salt content of the purified BV for clinical administration.66
A comparison of these purification methods is presented in Table 2. Different combinations of the various methods for clarification, concentration, and purification may be used to achieve the required quality of final BV products for a specified application without compromising on yield.
Formulation and storage
It is crucial that BV products are kept stable from the time of final formulation to administration in patients. Conditions such as pH, salt concentration, temperature, and light-exposure are important parameters that affect the stability of BVs. AcMNPV BVs are best stored in the dark at −80 °C (or lower), with salt concentration between 0.1–0.75 M and pH above 5.8 to minimize virus aggregation and loss of infectivity.52,77 Moreover, for human gene therapy applications, the final BV product has to be formulated in a solution that is safe for patient use, such as phosphate-buffered saline. Jorio et al.52 reported that BVs were stable in phosphate-buffered saline supplemented with cryoprotectants such as sucrose (0.25 M), glycerol (2.5%) or dimethyl sulfoxide (2.5%) at −80 °C or in liquid nitrogen for at least 20 days. We further tested and observed that the infectivity of BVs stored at titers greater than 1010 pfu/ml in phosphate-buffered saline supplemented with less cryoprotective glycerol (1% instead of 2.5%) was preserved for at least 4 weeks at −80 °C and was not significantly affected by an additional round of freeze-thawing (Figure 2). There is still need to examine longer-term storage under these conditions.
Figure 2.
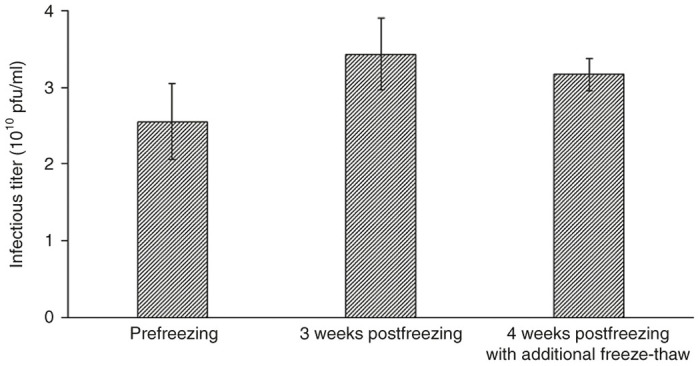
Stability of concentrated baculovirus in phosphate-buffered saline with 1% glycerol after storage in −80 °C. Infectious titers were determined by plaque assay for concentrated baculovirus samples in 1% glycerol-phosphate-buffered saline prior to freezing (prefreezing), after storage for 3 weeks at −80 °C (3 weeks postfreezing), and after subsequent refreezing and storing for an additional week at −80 °C (4 weeks post freezing with additional freeze-thaw). Data are presented as mean ± standard deviation of triplicate determinations.
Quality control and characterization of AcMNPV baculovirus vectors
In the production of clinical grade biologics, several process parameters need to be tightly controlled for reproducibility and quality assurance. Fast and accurate analytical methods are needed to monitor and optimize the production process to maximize output while satisfying the demands of regulatory authorities. The final gene therapy product has to undergo detailed characterization to assess purity, quantity, and potency prior to clinical testing and commercialization. While existing methods to assess the quality of BV-based therapeutics are focused on products derived from the insect cell/BV system rather than BV vector as the final product, as highlighted in a recent review,78 most are still applicable for BV gene therapy vectors, although additional tests are needed to further evaluate safety and potency.
Purity
At present, there is a paucity of specific guidelines pertaining to testing the quality of BV vectors for gene therapy, since none has been approved for clinical trial. However, the general guidelines published by FDA79 or EMA51,80 are still applicable to BV vectors. According to EMA, a final viral vector product must be characterized for identity, purity, potency, and safety for three successive batches to determine production consistency.80 Some analytical methods that have been used to characterize BV vectors include PCR and sequencing of viral genome and expression cassette to verify the identity of BV vectors; Southern blotting to assess the integrity and homogeneity of the BV genome;81 SDS-PAGE and western blot analysis for BV-specific proteins, e.g., gp64, to determine qualitatively the purity of the BV product;78 transmission electron microscopy and dynamic light scattering to examine morphology and size of the viral particles.66
Information about the purity of a final product is invariably linked to safety. As with MVSS preparation, BV products for clinical use need to undergo sterility tests to validate the absence of adventitious agents.44,82 Total DNA/protein and the residual insect-cell-specific DNA/protein content should be assayed using validated methods to establish an upper limit on the acceptable level of impurities. Enzyme-linked immunosorbent assay (ELISA) kits to quantify Sf9 cell proteins are available commercially (e.g., SF9 HCP ELISA kit, Cygnus Technologies). The level of endotoxin in the final product must also be quantified (via Limulus amoebocyte lysate test). Other tests for extraneous agents may be required depending on the raw materials used during production. For instance, using Sf9 cells for BV expansion necessitates detection (via qPCR) of Sf-rhabdovirus in the final product. Attention must be paid to the possible types of contaminants present at each step of the production pipeline and the extent to which they can/must be removed in order to account for the purity of the final product.
Quantification
Virus quantification is a crucial aspect of characterizing the AcMNPV BV product as the virus titer will determine the volume of each treatment dose and has bearing on product potency and purity. Moreover, with MOI being a critical parameter affecting BV yield and quality, accurate and reliable estimation of BV titer is necessary for consistency in production.83,84 There is a wide array of BV titration methods, broadly classified as infectious assays that estimate infectious titer, and viral particle assays that measure the total viral particles.
Conventional infectious assays include the plaque formation and end-point dilution assays.85 However, these assays are time-consuming (require 4–7 days of incubation), laborious and could be variable depending on the incubation conditions and skill of the operator. Other infectious assays such as the alamarBlue and cell size assays offer faster detection but suffer from similar variabilities and involve complex procedures for titer estimation.86–88 Flow cytometric methods have recently been developed to estimate infectious titer from changes to the side scatter of viable cells upon BV infection, and provides fast detection (within 48 hours) with improved accuracy and reliability.89 Importantly, since different insect cell lines display varying susceptibility to BV infection,38 the same cell line should be used for infectious assays performed during manufacturing and when quantifying the final product to provide a consistent standard for comparison.
Viral particle assays quantify total viral particles without discriminating between infectious and noninfectious particles; hence, they typically overestimate the functional virus titer and might confound efficacy studies. Nevertheless, they are mostly fast, highly-specific, and simple to execute. The most common of such assays is qPCR. The method detects the number of BV genomes, regardless from fully-formed viruses or not, using primers corresponding to BV-specific genes, such as gp64 gene.90 BV particles can also be quantified via flow cytometric assays following labeling of viral DNA or envelop proteins with fluorescent-conjugated antibodies or dyes.91,92
Based on the comprehensive evaluation of various BV titration methods by Roldão et al.,88 it is recommended that two or more titration assays be used to quantify a BV product for clinical use. It is also practical to determine the ratio of total to infectious particles (TP/IP) as it functions as an indicator of both product potency and efficiency of the production process.78
Potency
Apart from determining virus titer and infectivity, the transduction efficiency of the therapeutic BV vector has to be assessed in human cells that are as close as possible to the target tissue or cells to fully characterize its potency.78 The functional expression/activity of the therapeutic gene(s) should be quantified by biochemical and immunochemical assays specific to the gene product(s). The limits of acceptable potency should be determined and any loss of potency during storage should be assessed to help specify the validity period of the final product.
The main bugbear in assessing the quality of a BV product is the lack of a well-characterized BV reference material, which makes validating the QC assays and determining the minimum quality standard for clinical-grade product difficult. Currently, there are efforts to define and characterize a reference material for AcMNPV BV vectors.93 This, together with improving QC methods, would provide greater assurance that product safety, efficacy and stability are consistent from batch to batch, and thus facilitate obtaining regulatory approval for AcMNPV gene therapy products.
Towards gene therapy trials and future prospects
Before the first clinical trials can begin, sufficient preclinical studies are required to demonstrate safety of the BV therapy. The FDA and EMA provide guidelines for such studies.51,94,95 Several AcMNPV BV biodistribution studies in animals have been performed that contribute to the growing compendium of safety information regarding in vivo administration of AcMNPV vectors, and are useful towards gaining approval for gene therapy clinical trials.96,97 Although humans have no pre-existing immunity against BVs, readministration of BV vectors may elicit immune responses that inadvertently affect efficacy and safety of the BV gene therapy. This warrants further investigation into the antigenic properties of BV vectors, especially in repeated dosing studies; the information obtained can advise treatment regimens and guide further development of BV therapies.
Although there is greater attention on BV as a product and significant progress has been made in the production and processing of the budded vectors, challenges still remain for large-scale manufacturing. Towards improving BV production, mathematical models have been developed to predict the optimal conditions for scaled-up BV synthesis.34 Moreover, insect cell lines can be modified to enhance their BV production efficiency. As an example, exogenous expression of RNAi suppressor P19 in Sf9 cells significantly increased BV production.98 This highlights the potential utility of establishing a P19-expressing Sf9 cell line for BV manufacturing. An approach to improve virus purification is via surface modification of BV. The surface display of a small biotin acceptor peptide on BV allowed biotinylation and, subsequently, improved affinity purification of the surface-modified BV.99
The rapid inactivation of BV by serum complement, though protects against BV infection, severely restricts its use for in vivo gene delivery to within immune-privileged tissues, such as the brain and testes.100 Generating BVs that display complement-inhibiting proteins, such as decay-accelerating factor, on the envelop surface which resist complement attack may overcome this limitation.101 Other modifications of the BV surface can also improve the transduction efficiency and target specificity of the virus vector.102,103 Nevertheless, the safety of such modifications would have to be evaluated.
In conclusion, with the obvious advantages of BV for gene therapy, the existence of FDA-approved BV-produced vaccines like Flublok, recent solid developments in BV vector production and processing technologies, and integrated efforts towards establishing quality control methods and standards for BV products, the realization of BV-based gene therapy in the clinic within the next 5 to 10 years is a plausible expectation.
Acknowledgments
This work was supported by the Singapore Ministry of Health’s National Medical Research Council (NMRC/CIRG/1367/2013; NMRC/CIRG/1406/2014), Institute of Bioengineering and Nanotechnology (Biomedical Research Council, Agency for Science, Technology and Research, Singapore), and Tessa Therapeutics, Pte Ltd. Singapore.
The authors declare no conflict of interest.
References
- Hu, YC (2008). Baculoviral vectors for gene delivery: a review. Curr Gene Ther 8: 54–65. [DOI] [PubMed] [Google Scholar]
- Kost, TA, Condreay, JP and Jarvis, DL (2005). Baculovirus as versatile vectors for protein expression in insect and mammalian cells. Nat Biotechnol 23: 567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airenne, KJ, Hu, YC, Kost, TA, Smith, RH, Kotin, RM, Ono, C et al. (2013). Baculovirus: an insect-derived vector for diverse gene transfer applications. Mol Ther 21: 739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung, LY, Chen, CL, Lin, SY, Li, KC, Yeh, CL, Chen, GY et al. (2014). Efficient gene delivery into cell lines and stem cells using baculovirus. Nat Protoc 9: 1882–1899. [DOI] [PubMed] [Google Scholar]
- Zeng, J, Du, J, Lin, J, Bak, XY, Wu, C and Wang, S (2009). High-efficiency transient transduction of human embryonic stem cell-derived neurons with baculoviral vectors. Mol Ther 17: 1585–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, J, Du, J, Zhao, Y, Palanisamy, N and Wang, S (2007). Baculoviral vector-mediated transient and stable transgene expression in human embryonic stem cells. Stem Cells 25: 1055–1061. [DOI] [PubMed] [Google Scholar]
- Heimpel, AM and Buchanan, LK (1967). Human feeding tests using a nuclear-polyhedrosis virus of Heliothis zea. J Invertebr Pathol 9: 55–57. [DOI] [PubMed] [Google Scholar]
- Jin, R, Lv, Z, Chen, Q, Quan, Y, Zhang, H, Li, S et al. (2008). Safety and immunogenicity of H5N1 influenza vaccine based on baculovirus surface display system of Bombyx mori. PLoS One 3: e3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahi, YS, Bangari, DS and Mittal, SK (2011). Adenoviral vector immunity: its implications and circumvention strategies. Curr Gene Ther 11: 307–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annoni, A, Goudy, K, Akbarpour, M, Naldini, L and Roncarolo, MG (2013). Immune responses in liver-directed lentiviral gene therapy. Transl Res 161: 230–240. [DOI] [PubMed] [Google Scholar]
- Strauss, R, Hüser, A, Ni, S, Tuve, S, Kiviat, N, Sow, PS et al. (2007). Baculovirus-based vaccination vectors allow for efficient induction of immune responses against plasmodium falciparum circumsporozoite protein. Mol Ther 15: 193–202. [DOI] [PubMed] [Google Scholar]
- Castro, MG, Candolfi, M, Wilson, TJ, Calinescu, A, Paran, C, Kamran, N et al. (2014). Adenoviral vector-mediated gene therapy for gliomas: coming of age. Expert Opin Biol Ther 14: 1241–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merten, O-W, Schweizer, M, Chahal, P and Kamen, AA (2014). Manufacturing of viral vectors for gene therapy: part I. Upstream processing. Pharm Bioprocess 2: 183–203. [Google Scholar]
- Rothe, M, Modlich, U and Schambach, A (2013). Biosafety challenges for use of lentiviral vectors in gene therapy. Curr Gene Ther 13: 453–468. [DOI] [PubMed] [Google Scholar]
- Makkonen, KE, Airenne, K and Ylä-Herttulala, S (2015). Baculovirus-mediated gene delivery and RNAi applications. Viruses 7: 2099–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, YC, Yao, K and Wu, TY (2008). Baculovirus as an expression and/or delivery vehicle for vaccine antigens. Expert Rev Vaccines 7: 363–371. [DOI] [PubMed] [Google Scholar]
- Lin, SY, Chung, YC and Hu, YC (2014). Update on baculovirus as an expression and/or delivery vehicle for vaccine antigens. Expert Rev Vaccines 13: 1501–1521. [DOI] [PubMed] [Google Scholar]
- Lu, HY, Chen, YH and Liu, HJ (2012). Baculovirus as a vaccine vector. Bioengineered 3: 271–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhan, S, Prabakaran, M and Kwang, J (2010). Baculovirus as vaccine vectors. Curr Gene Ther 10: 201–213. [DOI] [PubMed] [Google Scholar]
- Li, KC, Chang, YH, Lin, CY, Hwang, SM, Wang, TH and Hu, YC (2015). Preclinical safety evaluation of ASCs engineered by FLPo/Frt-based hybrid baculovirus: in vitro and large animal studies. Tissue Eng Part A 21: 1471–1482. [DOI] [PubMed] [Google Scholar]
- Lin, CY, Lin, KJ, Kao, CY, Chen, MC, Lo, WH, Yen, TC et al. (2011). The role of adipose-derived stem cells engineered with the persistently expressing hybrid baculovirus in the healing of massive bone defects. Biomaterials 32: 6505–6514. [DOI] [PubMed] [Google Scholar]
- Lin, CY, Lin, KJ, Li, KC, Sung, LY, Hsueh, S, Lu, CH et al. (2012). Immune responses during healing of massive segmental femoral bone defects mediated by hybrid baculovirus-engineered ASCs. Biomaterials 33: 7422–7434. [DOI] [PubMed] [Google Scholar]
- Lin, CY, Wang, YH, Li, KC, Sung, LY, Yeh, CL, Lin, KJ et al. (2015). Healing of massive segmental femoral bone defects in minipigs by allogenic ASCs engineered with FLPo/Frt-based baculovirus vectors. Biomaterials 50: 98–106. [DOI] [PubMed] [Google Scholar]
- Lu, CH, Yeh, TS, Yeh, CL, Fang, YH, Sung, LY, Lin, SY et al. (2014). Regenerating cartilages by engineered ASCs: prolonged TGF-β3/BMP-6 expression improved articular cartilage formation and restored zonal structure. Mol Ther 22: 186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, CL, Tseng, YW, Wu, JC, Chen, GY, Lin, KC, Hwang, SM et al. (2015). Suppression of hepatocellular carcinoma by baculovirus-mediated expression of long non-coding RNA PTENP1 and MicroRNA regulation. Biomaterials 44: 71–81. [DOI] [PubMed] [Google Scholar]
- Lykhova, AA, Kudryavets, YI, Strokovska, LI, Bezdenezhnykh, NA, Semesiuk, NI, Adamenko, IN et al. (2015). Suppression of proliferation, tumorigenicity and metastasis of lung cancer cells after their transduction by interferon-beta gene in baculovirus vector. Cytokine 71: 318–326. [DOI] [PubMed] [Google Scholar]
- Swift, SL, Rivera, GC, Dussupt, V, Leadley, RM, Hudson, LC, Ma de Ridder, C et al. (2013). Evaluating baculovirus as a vector for human prostate cancer gene therapy. PLoS One 8: e65557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, CY, Li, F, Yang, Y, Guo, HY, Wu, CX and Wang, S (2006). Recombinant baculovirus containing the diphtheria toxin A gene for malignant glioma therapy. Cancer Res 66: 5798–5806. [DOI] [PubMed] [Google Scholar]
- Wang, S and Balasundaram, G (2010). Potential cancer gene therapy by baculoviral transduction. Curr Gene Ther 10: 214–225. [DOI] [PubMed] [Google Scholar]
- Wu, C, Lin, J, Hong, M, Choudhury, Y, Balani, P, Leung, D et al. (2009). Combinatorial control of suicide gene expression by tissue-specific promoter and microRNA regulation for cancer therapy. Mol Ther 17: 2058–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang, WX, Zhao, Y, Kwang, T, Wu, C, Toh, HC, Mahendran, R, et al. Baculovirus as an immunotherapeutic agent and a gene transfer vector for bladder cancer therapy. Submitted.
- Wang, S, Wu, C, Zhao, Y and LEE, EX (2013). Methods for bladder cancer therapy using baculoviral vectors. Patent WO 2,013,154,503.
- Vaughn, JL, Goodwin, RH, Tompkins, GJ and McCawley, P (1977). The establishment of two cell lines from the insect Spodoptera frugiperda (Lepidoptera; Noctuidae). In Vitro 13: 213–217. [DOI] [PubMed] [Google Scholar]
- Aucoin, MG, Mena, JA and Kamen, AA (2010). Bioprocessing of baculovirus vectors: a review. Curr Gene Ther 10: 174–186. [DOI] [PubMed] [Google Scholar]
- Wickham, TJ, Davis, T, Granados, RR, Shuler, ML and Wood, HA (1992). Screening of insect cell lines for the production of recombinant proteins and infectious virus in the baculovirus expression system. Biotechnol Prog 8: 391–396. [DOI] [PubMed] [Google Scholar]
- Davis, TR, Wickham, TJ, McKenna, KA, Granados, RR, Shuler, ML and Wood, HA (1993). Comparative recombinant protein production of eight insect cell lines. In Vitro Cell Dev Biol Anim 29A: 388–390. [DOI] [PubMed] [Google Scholar]
- Rhiel, M, Mitchell-Logean, CM and Murhammer, DW (1997). Comparison of Trichoplusia ni BTI-Tn-5B1-4 (high five) and Spodoptera frugiperda Sf-9 insect cell line metabolism in suspension cultures. Biotechnol Bioeng 55: 909–920. [DOI] [PubMed] [Google Scholar]
- Wilde, M, Klausberger, M, Palmberger, D, Ernst, W and Grabherr, R (2014). Tnao38, high five and Sf9–evaluation of host-virus interactions in three different insect cell lines: baculovirus production and recombinant protein expression. Biotechnol Lett 36: 743–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto, Y, Zhang, S, Zhang, S, Chen, YR and Blissard, GW (2012). Correction: BTI-Tnao38, a new cell line derived from Trichoplusia ni, is permissive for AcMNPV infection and produces high levels of recombinant proteins. BMC Biotechnol 12: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel, T and Rohrmann, GF (2008). Diversity of errantivirus (retrovirus) sequences in two cell lines used for baculovirus expression, Spodoptera frugiperda and Trichoplusia ni. Virus Genes 36: 583–586. [DOI] [PubMed] [Google Scholar]
- Li, TC, Scotti, PD, Miyamura, T and Takeda, N (2007). Latent infection of a new alphanodavirus in an insect cell line. J Virol 81: 10890–10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, H, Galvin, TA, Glasner, DR, Shaheduzzaman, S and Khan, AS (2014). Identification of a novel rhabdovirus in Spodoptera frugiperda cell lines. J Virol 88: 6576–6585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan, M, Zhang, SY, Jiang, L, Ma, M and Li, GX (2011). Susceptibility to AcMNPV and expression of recombinant proteins by a novel cell clone derived from a Trichoplusia ni QAU-BTI-Tn9-4s cell line. Virol Sin 26: 297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration (2010). Guidance for industry: characterization and qualification of cell substrates and other biological materials used in the production of viral vaccines for infectious disease indications.
- European Medicines Agency (1998). Note for guidance on quality of biotechnological products: derivation and characterisation of cell substrates Used for production of biotechnological/biological products. (CPMP/ICH/294/95). <http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003280.pdf>. [PubMed]
- Lesch, HP, Makkonen, KE, Laitinen, A, Määttä, AM, Närvänen, O, Airenne, KJ et al. (2011). Requirements for baculoviruses for clinical gene therapy applications. J Invertebr Pathol 107 Suppl: S106–S112. [DOI] [PubMed] [Google Scholar]
- Hitchman, RB, Possee, RD and King, LA (2009). Baculovirus expression systems for recombinant protein production in insect cells. Recent Pat Biotechnol 3: 46–54. [DOI] [PubMed] [Google Scholar]
- Hitchman, RB, Possee, RD and King, LA (2012). High-throughput baculovirus expression in insect cells. Methods Mol Biol 824: 609–627. [DOI] [PubMed] [Google Scholar]
- Kitts, PA and Possee, RD (1993). A method for producing recombinant baculovirus expression vectors at high frequency. Biotechniques 14: 810–817. [PubMed] [Google Scholar]
- Pijlman, GP, van Schijndel, JE and Vlak, JM (2003). Spontaneous excision of BAC vector sequences from bacmid-derived baculovirus expression vectors upon passage in insect cells. J Gen Virol 84(Pt 10): 2669–2678. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency (2010). Guideline on quality, non-clinical and clinical aspects of live recombinant viral vectored vaccines. (EMA/CHMP/VWP/141697/2009). <http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/08/WC500095721.pdf>.
- Jorio, H, Tran, R and Kamen, A (2006). Stability of serum-free and purified baculovirus stocks under various storage conditions. Biotechnol Prog 22: 319–325. [DOI] [PubMed] [Google Scholar]
- Vicente, T, Peixoto, C, Carrondo, MJ and Alves, PM (2009). Virus production for clinical gene therapy. Met Mol Biol 542: 447–470. [DOI] [PubMed] [Google Scholar]
- Wickham, T, Davis, T, Granados, R, Hammer, D, Shuler, M, and Wood, H (1991). Baculovirus defective interfering particles are responsible for variations in recombinant protein production as a function of multiplicity of infection. Biotechnol Lett 13: 483–488. [Google Scholar]
- Zwart, MP, Erro, E, van Oers, MM, de Visser, JA and Vlak, JM (2008). Low multiplicity of infection in vivo results in purifying selection against baculovirus deletion mutants. J Gen Virol 89(Pt 5): 1220–1224. [DOI] [PubMed] [Google Scholar]
- Shao-Hua, C, Hong-Liang, S and Zuo-Hu, L (1998). Effect of temperature oscillation on insect cell growth and baculovirus replication. Appl Environ Microbiol 64: 2237–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiser, A, Elias, CB, Voyer, R, Jardin, B and Kamen, AA (2000). On-line monitoring of physiological parameters of insect cell cultures during the growth and infection process. Biotechnol Prog 16: 803–808. [DOI] [PubMed] [Google Scholar]
- Lecina, M, Soley, A, Gràcia, J, Espunya, E, Lázaro, B, Cairó, JJ et al. (2006). Application of on-line OUR measurements to detect actions points to improve baculovirus-insect cell cultures in bioreactors. J Biotechnol 125: 385–394. [DOI] [PubMed] [Google Scholar]
- Pijlman, GP, van den Born, E, Martens, DE and Vlak, JM (2001). Autographa californica baculoviruses with large genomic deletions are rapidly generated in infected insect cells. Virology 283: 132–138. [DOI] [PubMed] [Google Scholar]
- Deutschmann, SM and Jäger, V (1994). Optimization of the growth conditions of Sf21 insect cells for high-density perfusion culture in stirred-tank bioreactors. Enzyme Microb Technol 16: 506–512. [DOI] [PubMed] [Google Scholar]
- Weber, W, Weber, E, Geisse, S and Memmert, K (2002). Optimisation of protein expression and establishment of the Wave Bioreactor for Baculovirus/insect cell culture. Cytotechnology 38: 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras-Gómez, A, Sánchez-Mirón, A, García-Camacho, F, Molina-Grima, E and Chisti, Y (2014). Protein production using the baculovirus-insect cell expression system. Biotechnol Prog 30: 1–18. [DOI] [PubMed] [Google Scholar]
- Nestola, P, Peixoto, C, Silva, RR, Alves, PM, Mota, JP and Carrondo, MJ (2015). Improved virus purification processes for vaccines and gene therapy. Biotechnol Bioeng 112: 843–857. [DOI] [PubMed] [Google Scholar]
- Vicente, T, Roldão, A, Peixoto, C, Carrondo, MJ and Alves, PM (2011). Large-scale production and purification of VLP-based vaccines. J Invertebr Pathol 107 Suppl: S42–S48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorin, M and Cummings, J. Principles of Continuous Flow Centrifugation. Beckman Coulter, Inc.: Indianapolis, USA, 2015. [Google Scholar]
- Vicente, T, Peixoto, C, Carrondo, MJ and Alves, PM (2009). Purification of recombinant baculoviruses for gene therapy using membrane processes. Gene Ther 16: 766–775. [DOI] [PubMed] [Google Scholar]
- van Reis, R, and Zydney, A (2007). Bioprocess membrane technology. J Membrane Sci 297: 16–50. [Google Scholar]
- Airenne, KJ, Hiltunen, MO, Turunen, MP, Turunen, AM, Laitinen, OH, Kulomaa, MS et al. (2000). Baculovirus-mediated periadventitial gene transfer to rabbit carotid artery. Gene Ther 7: 1499–1504. [DOI] [PubMed] [Google Scholar]
- Wang, CY and Wang, S (2006). Astrocytic expression of transgene in the rat brain mediated by baculovirus vectors containing an astrocyte-specific promoter. Gene Ther 13: 1447–1456. [DOI] [PubMed] [Google Scholar]
- Michalsky, R, Passarelli, AL, Pfromm, PH, and Czermak, P (2010). Concentration of the baculovirus Autographa californica M nucleopolyhedrovirus (AcMNPV) by ultrafiltration. Desalination 250: 1125–1127. [Google Scholar]
- Liu, HF, Ma, J, Winter, C and Bayer, R (2010). Recovery and purification process development for monoclonal antibody production. MAbs 2: 480–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, GY, Chen, CY, Chang, MD, Matsuura, Y and Hu, YC (2009). Concanavalin A affinity chromatography for efficient baculovirus purification. Biotechnol Prog 25: 1669–1677. [DOI] [PubMed] [Google Scholar]
- Transfiguracion, J, Jorio, H, Meghrous, J, Jacob, D and Kamen, A (2007). High yield purification of functional baculovirus vectors by size exclusion chromatography. J Virol Methods 142: 21–28. [DOI] [PubMed] [Google Scholar]
- Wu, C, Soh, KY and Wang, S (2007). Ion-exchange membrane chromatography method for rapid and efficient purification of recombinant baculovirus and baculovirus gp64 protein. Hum Gene Ther 18: 665–672. [DOI] [PubMed] [Google Scholar]
- Podgornik, A and Strancar, A (2005). Convective Interaction Media (CIM)–short layer monolithic chromatographic stationary phases. Biotechnol Annu Rev 11: 281–333. [DOI] [PubMed] [Google Scholar]
- Gerster, P, Kopecky, EM, Hammerschmidt, N, Klausberger, M, Krammer, F, Grabherr, R et al. (2013). Purification of infective baculoviruses by monoliths. J Chromatogr A 1290: 36–45. [DOI] [PubMed] [Google Scholar]
- Jarvis, DL and Garcia, A Jr (1994). Long-term stability of baculoviruses stored under various conditions. Biotechniques 16: 508–513. [PubMed] [Google Scholar]
- Roldão, A, Vicente, T, Peixoto, C, Carrondo, MJ and Alves, PM (2011). Quality control and analytical methods for baculovirus-based products. J Invertebr Pathol 107 Suppl: S94–105. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration (2008). Guidance for FDA reviewers and sponsors: content and review of chemistry, manufacturing, and control (CMC) information for human gene therapy investigational new drug applications (INDs).
- European Medicines Agency (2001). Note for guidance on the quality, preclinical and clinical aspects of gene transfer medicinal products. (CPMP/BWP/3088/99). <http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/10/WC500003987.pdf>.
- Possee, RD and Rohrmann, GF (1997). Baculovirus genome organization and evolution. In: Miller, L (ed). The Baculoviruses. Springer US: New York, NY. pp. 109–140. [Google Scholar]
- Food and Drug Administration (1998). Guidance for industry: guidance for human somatic cell therapy and gene therapy.
- Wong, KT, Peter, CH, Greenfield, PF, Reid, S and Nielsen, LK (1996). Low multiplicity infection of insect cells with a recombinant baculovirus: The cell yield concept. Biotechnol Bioeng 49: 659–666. [DOI] [PubMed] [Google Scholar]
- Zhang, YH and Merchuk, JC (2004). A mathematical model of baculovirus infection on insect cells at low multiplicity of infection. Acta Biochim Biophys Sin (Shanghai) 36: 729–740. [DOI] [PubMed] [Google Scholar]
- King, L and Possee, R (1992). Propagation, titration and purification of AcMNPV in cell culture. In: Miller, L (ed). The Baculovirus Expression System: A Laboratory Guide. Springer: Dordrecht, The Netherlands. pp. 106–126. [Google Scholar]
- Janakiraman, V, Forrest, WF, Chow, B and Seshagiri, S (2006). A rapid method for estimation of baculovirus titer based on viable cell size. J Virol Methods 132: 48–58. [DOI] [PubMed] [Google Scholar]
- Pouliquen, Y, Kolbinger, F, Geisse, S and Mahnke, M (2006). Automated baculovirus titration assay based on viable cell growth monitoring using a colorimetric indicator. Biotechniques 40: 282, 284, 286 passim. [DOI] [PubMed] [Google Scholar]
- Roldão, A, Oliveira, R, Carrondo, MJ and Alves, PM (2009). Error assessment in recombinant baculovirus titration: evaluation of different methods. J Virol Methods 159: 69–80. [DOI] [PubMed] [Google Scholar]
- Qi, J, Liu, T, Pan, J, Miao, P and Zhang, C (2015). Rapid baculovirus titration assay based on viable cell side scatter (SSC). Anal Chim Acta 879: 58–62. [DOI] [PubMed] [Google Scholar]
- Hitchman, RB, Siaterli, EA, Nixon, CP and King, LA (2007). Quantitative real-time PCR for rapid and accurate titration of recombinant baculovirus particles. Biotechnol Bioeng 96: 810–814. [DOI] [PubMed] [Google Scholar]
- Shen, CF, Meghrous, J and Kamen, A (2002). Quantitation of baculovirus particles by flow cytometry. J Virol Methods 105: 321–330. [DOI] [PubMed] [Google Scholar]
- Ferris, MM, Stepp, PC, Ranno, KA, Mahmoud, W, Ibbitson, E, Jarvis, J et al. (2011). Evaluation of the Virus Counter® for rapid baculovirus quantitation. J Virol Methods 171: 111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamen, AA, Aucoin, MG, Merten, OW, Alves, P, Hashimoto, Y, Airenne, K et al. (2011). An initiative to manufacture and characterize baculovirus reference material. J Invertebr Pathol 107 Suppl: S113–S117. [DOI] [PubMed] [Google Scholar]
- European Medicinal Agency (2008). Guidelines on the non-clinical studies required before first clinical use of gene therapy medicinal products. (EMEA/CHMP/GTWP/125459/2006). <http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003942.pdf>.
- Food and Drug Administration (2013). Guidance for industry: preclinical assessment of investigational cellular and gene therapy products. <http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/ucm376136.htm>.
- Airenne, KJ, Makkonen, KE, Mähönen, AJ and Ylä-Herttuala, S (2010). In vivo application and tracking of baculovirus. Curr Gene Ther 10: 187–194. [DOI] [PubMed] [Google Scholar]
- Räty, JK, Liimatainen, T, Huhtala, T, Kaikkonen, MU, Airenne, KJ, Hakumäki, JM et al. (2007). SPECT/CT imaging of baculovirus biodistribution in rat. Gene Ther 14: 930–938. [DOI] [PubMed] [Google Scholar]
- Liu, Y, Zhang, L, Zhang, Y, Liu, D, Du, E and Yang, Z (2015). Functional analysis of RNAi suppressor P19 on improving baculovirus yield and transgene expression in Sf9 cells. Biotechnol Lett 37: 2159–2166. [DOI] [PubMed] [Google Scholar]
- Kaikkonen, MU, Viholainen, JI, Närvänen, A, Ylä-Herttuala, S and Airenne, KJ (2008). Targeting and purification of metabolically biotinylated baculovirus. Hum Gene Ther 19: 589–600. [DOI] [PubMed] [Google Scholar]
- Rychlowska, M, Gromadzka, B, Bieńkowska-Szewczyk, K and Szewczyk, B (2011). Application of baculovirus-insect cell expression system for human therapy. Curr Pharm Biotechnol 12: 1840–1849. [DOI] [PubMed] [Google Scholar]
- Kaikkonen, MU, Maatta, AI, Ylä-Herttuala, S and Airenne, KJ (2010). Screening of complement inhibitors: shielded baculoviruses increase the safety and efficacy of gene delivery. Mol Ther 18: 987–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkelä, AR, Enbäck, J, Laakkonen, JP, Vihinen-Ranta, M, Laakkonen, P and Oker-Blom, C (2008). Tumor targeting of baculovirus displaying a lymphatic homing peptide. J Gene Med 10: 1019–1031. [DOI] [PubMed] [Google Scholar]
- Räty, JK, Airenne, KJ, Marttila, AT, Marjomäki, V, Hytönen, VP, Lehtolainen, P et al. (2004). Enhanced gene delivery by avidin-displaying baculovirus. Mol Ther 9: 282–291. [DOI] [PubMed] [Google Scholar]


