Supplemental digital content is available in the text.
KEY WORDS: Acute kidney injury, perioperative medicine, postoperative complications
Abstract
BACKGROUND
Acute kidney injury (AKI) is an important complication in surgical patients. Existing biomarkers and clinical prediction models underestimate the risk for developing AKI. We recently reported data from two trials of 728 and 408 critically ill adult patients in whom urinary TIMP2•IGFBP7 (NephroCheck, Astute Medical) was used to identify patients at risk of developing AKI. Here we report a preplanned analysis of surgical patients from both trials to assess whether urinary tissue inhibitor of metalloproteinase 2 (TIMP-2) and insulin-like growth factor–binding protein 7 (IGFBP7) accurately identify surgical patients at risk of developing AKI.
STUDY DESIGN
We enrolled adult surgical patients at risk for AKI who were admitted to one of 39 intensive care units across Europe and North America. The primary end point was moderate-severe AKI (equivalent to KDIGO [Kidney Disease Improving Global Outcomes] stages 2–3) within 12 hours of enrollment. Biomarker performance was assessed using the area under the receiver operating characteristic curve, integrated discrimination improvement, and category-free net reclassification improvement.
RESULTS
A total of 375 patients were included in the final analysis of whom 35 (9%) developed moderate-severe AKI within 12 hours. The area under the receiver operating characteristic curve for [TIMP-2]•[IGFBP7] alone was 0.84 (95% confidence interval, 0.76–0.90; p < 0.0001). Biomarker performance was robust in sensitivity analysis across predefined subgroups (urgency and type of surgery).
CONCLUSION
For postoperative surgical intensive care unit patients, a single urinary TIMP2•IGFBP7 test accurately identified patients at risk for developing AKI within the ensuing 12 hours and its inclusion in clinical risk prediction models significantly enhances their performance.
LEVEL OF EVIDENCE
Prognostic study, level I.
Acute kidney injury (AKI) is a significant public health problem1,2 and is common in surgical patients of all types.3,4 AKI occurring after surgery is associated with short-term morbidity, an increased risk of de novo or progressive chronic kidney disease, and adverse cardiovascular events and it also decreases hospital survival rates and increases hospital costs.5–10 Surgical inpatients, particularly those with comorbid disease and those undergoing complex procedures, are at increased risk for developing AKI.5,7,11 Patients who develop AKI in the postoperative period are at high risk for fluid overload, infection (surgical site, pulmonary and urinary tract), and other complications that may directly affect outcome after surgery.3,4,12 In comparison with other risk factors for AKI (e.g., sepsis and nephrotoxic antibiotics), complex surgical procedures expose patients to a combination of pathophysiologic processes, including inflammation, oxidative stress, iron and heme proteins, tissue injury, blood product transfusion, and hemodynamic instability.13–18
Although recent international Kidney Disease Improving Global Outcomes (KDIGO) guidelines have further advanced consensus regarding uniform diagnostic criteria and preventive strategies for AKI,19 the inability to identify AKI early at a potentially reversible stage is still the rate-limiting step.1,20 Traditional biomarkers and physiologic indicators such as serum creatinine and urine output are understood to be late and nonspecific markers of renal dysfunction—yet in the absence of better alternatives, they are in widespread clinical use.21 Even with the new KDIGO guidelines, the impact of AKI on surgical outcomes is still not fully recognized, as demonstrated by a recent study showing the underestimation of risk associated with AKI in postoperative patients.3
Surgical patients represent a unique population for early AKI risk stratification because of the presence of readily modifiable risk factors (e.g., fluid type and amount, blood product administration, hemodynamic management). However, the etiologic differences in surgical AKI patients when compared with their nonsurgical peers mean that it is important to demonstrate that novel biomarkers of AKI risk perform in a useful and robust fashion in patients whose main risk for AKI is their surgical procedure and its attendant management. Accordingly, we conducted a preplanned analysis of surgical patients using data from two multicenter clinical studies that successfully identified intensive care unit (ICU) patients at risk for developing AKI within the ensuing 12 hours of biomarker assessment.22,23 Our goal for this new analysis was to test the hypothesis that the TIMP2•IGFBP7 test would correctly identify a broad range of critically ill surgical patients at high risk for developing AKI.
PATIENTS AND METHODS
Study Design
This was a preplanned subgroup analysis of critically ill surgical patients enrolled in either of our two previously reported studies in which the discovery (Sapphire)23 and subsequent validation (Topaz)22 of TIMP2•IGFBP7 were performed. Surgical patients were further categorized as either cardiothoracic or noncardiothoracic because of known differential risk factors, for example, use of cardiopulmonary bypass.24 All patients were deemed at high risk for AKI, characterized as those with respiratory or cardiovascular dysfunction, as previously reported (Fig. 1).22,23 The design, execution, and reporting of this study meet the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE)25 and the Standards for Reporting of Diagnostic Accuracy (STARD)26 criteria. Data were collected by the investigators and analyzed by external statisticians. Both study protocols were approved by the Western Institutional Review Board (Olympia, Washington) and also by the institutional review board or ethics committee of each study site if required. All patients provided written informed consent. In this article, we present data from the Sapphire study, which defined AKI as KDIGO stages 2 to 3, and from the Topaz study, which used clinical adjudication for AKI, to examine the performance of the TIMP2•IGFBP7 test for risk assessment of AKI in surgical patients. Although these biomarkers have previously been shown to have excellent ability to identify the risk of AKI in an unselected group of acutely ill adult ICU patients,23 their performance in a broad range of surgical patients has not been previously described. Given the likely etiologic differences in surgical and medical AKI and the biologic nature of these markers (indicators of cellular stress), this is an important issue that needs to be addressed before the test can be included in clinical decision making for these patients.
Figure 1.
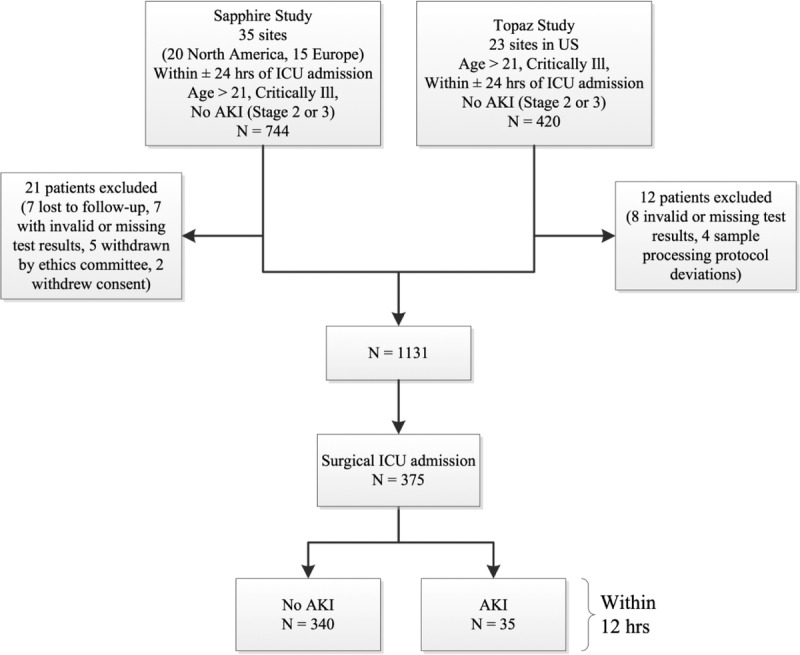
Study design and number of patients in cohorts. AKI was defined as KDIGO AKI stage 2 or 3 for Sapphire and was determined by clinical adjudication for Topaz (based on KDIGO stage 2–3 AKI).
Biomarker Assays
Urine samples were analyzed for TIMP-2 and IGFBP7 using a clinical immunoassay (NephroCheck Test and Astute140 Meter; Astute Medical Inc., San Diego, CA) by technicians who were unaware of the clinical data. The Astute140 Meter automatically multiplies the concentrations of the two biomarkers together and divides this product by 1,000 to report a single numeric test result with units of (ng/mL)2/1,000 (the units for all TIMP2•IGFBP7 values in this report). Each sample was tested once for TIMP2•IGFBP7 in the Sapphire study and three times in the Topaz study. The single value from Sapphire and the median of the three replicates in Topaz were used for analysis.
Statistical Analysis
The primary objective was to evaluate the ability of urinary TIMP2•IGFBP7 to identify critically ill surgical patients who went on to develop moderate or severe AKI in the immediate 12 hours after measurement. Ability to predict this event (i.e., development of moderate or severe AKI) was assessed using the area under the receiver operating characteristic curve (AUC-ROC) for urinary TIMP2•IGFBP7. As a secondary analysis, we used integrated discrimination improvement (IDI), category-free (continuous) net reclassification improvement (cfNRI), and change in AUC-ROC to investigate the improvement in the prediction of the primary end point resulting from the addition of the TIMP2•IGFBP7 data to a clinical model.27 Variables from Table 1 that were associated with the end point (p < 0.1) were selected for inclusion in the clinical model, which was then analyzed using multivariate logistic regression. Logistic regression analyses used the logarithmic transformed and standardized serum creatinine and TIMP2•IGFBP7 test results from time-paired blood and urine samples, respectively, collected at the time of enrollment. All continuous variables in the clinical models were standardized by subtracting the mean and then dividing by twice the standard deviation for their coefficients to be comparable to the coefficients of binary predictors,28 which were coded as 0 or 1. Model performance was assessed with the Hosmer-Lemeshow test for the goodness of fit. Six subjects in the Sapphire study did not have a TIMP2•IGFBP7 test result at the time of enrollment, and therefore, test results from the second collection scheduled 12 hours after enrollment were used for these subjects.
Table 1.
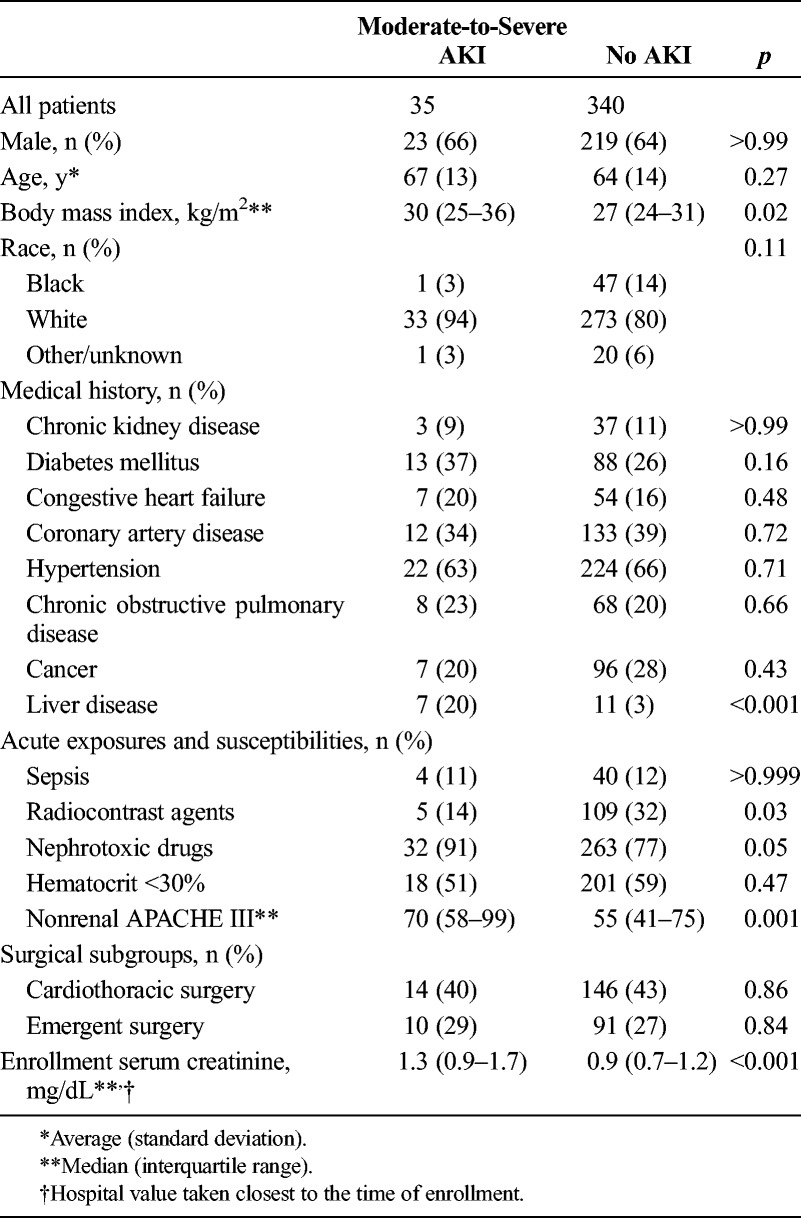
Baseline Patient Characteristics Shown by Primary End Point
Statistical analyses were performed using R 3.1.0.29 For all analyses, two-sided values of p < 0.05 and one-sided values of p < 0.025 were considered statistically significant. Categorical variables were analyzed using Fisher's exact test, χ2 test, or logistic regression. All biomarker performance statistics (AUC, sensitivity, specificity, and relative risk) were calculated as empirical estimates, and their confidence intervals (CIs) were obtained by bootstrap analysis.
RESULTS
Baseline Characteristics
In the Sapphire and Topaz trials, there were 247 and 128 surgical patients, respectively, and these were combined to form the analysis cohort of 375 patients (Fig. 1). Baseline characteristics for all patients are provided in Table 1 and are shown separately by study in the supplemental appendix (see Supplemental Digital Content 1, http://links.lww.com/TA/A693). Patients developing AKI had higher baseline creatinine and tended to have more comorbidities but were otherwise similar to patients without AKI. Patients in both trials had similar baseline characteristics with regard to both acute severity of illness (Acute Physiology and Chronic Health Evaluation [APACHE] III score without the renal component) and chronic disease burden. However, there were more diabetic patients in Sapphire (31% vs. 20%) and more emergency surgery patients (37% vs. 22%) and nephrotoxic medication use (88% vs. 74%) in Topaz. The overall rate of moderate to severe AKI within 12 hours of sampling was slightly higher in Topaz (12.5%) compared with that in Sapphire (7.7%), but this difference was not statistically significant. The 12-hour incidence of moderate-to-severe AKI was 9% (35 of 375 patients) for the pooled surgical cohorts (Fig. 1).
Biomarker Performance
Urinary TIMP2•IGFBP7 performed well in detecting those subjects who developed moderate to severe AKI within 12 hours of sample collection (Figs. 2–3). As reported previously for heterogeneous (medical and surgical) cohorts of critically ill patients,22,30 when the prespecified cutoff TIMP2•IGFBP7 value of 0.3 was used, the sensitivity of the test was 89% (95% CI, 77–97), with an accompanying specificity of 49% (95% CI, 43–54), a positive likelihood ratio of 1.72 (95% CI, 1.44–2.01), and a negative likelihood ratio of 0.24 (95% CI, 0.06–0.49) (see Supplemental Digital Content 1, http://links.lww.com/TA/A693, for additional details). Patients with urinary TIMP2•IGFBP7 test values greater than 0.3 had more than six times the risk for AKI compared with those with a test value at or below the 0.3 cutoff. Put another way, when the TIMP2•IGFBP7 result is less than or equal to 0.3, only one in 42 surgical patients (2.4% absolute risk) will develop moderate or severe AKI within the next 12 hours. At the prespecified test cutoff of 2.0 (again as was the case in heterogeneous cohorts of critically ill patients),22,30 the test specificity improves to a very robust 94%, with a positive likelihood ratio of 6.8 (95% CI, 3.5–12.7) and a substantial increase in risk (full operating characteristics at various cut points are shown in Supplemental Digital Content 1, http://links.lww.com/TA/A693).
Figure 2.
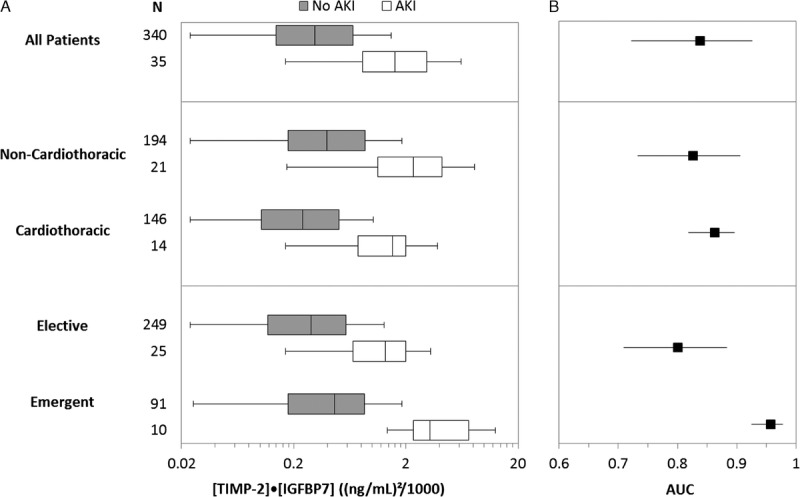
TIMP2•IGFBP7 biomarker performance in surgical subsets. (A) TIMP2•IGFBP7 levels by surgical subgroup and AKI status within 12 hours and (B) AUC by surgical subgroup. Two mutually exclusive subgroup pairs are shown: Noncardiothoracic versus Cardiothoracic and Elective versus Emergent. Boxes and whiskers show interquartile ranges and total observed ranges, censored at 1.5 times the interquartile ranges. Patients with AKI had significantly higher levels of TIMP2•IGFBP7 than patients without AKI for all surgical patients and within each subgroup shown (Wilcoxon rank-sum test adjusted p < 0.001 in all cases). All AUC values were approximately 0.8 or greater and significantly greater than 0.5 (p < 0.001).
Figure 3.
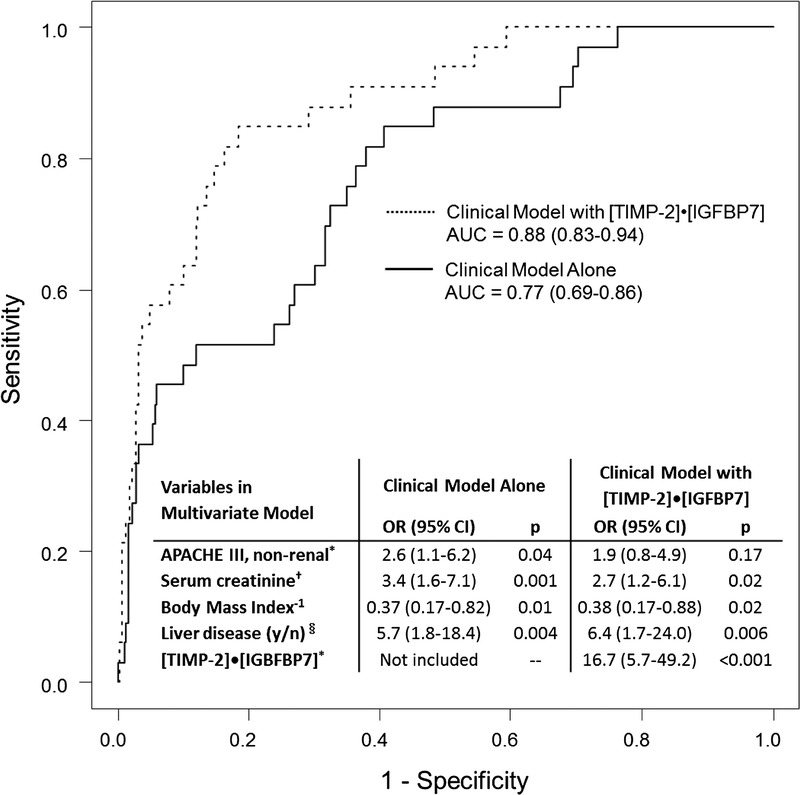
ROC curves and odds ratios from a multivariate clinical model alone and the model with TIMP2•IGFBP7. Stepwise model selection was used to derive the clinical model starting from all variables from Table 1 with p < 0.1 for the end point. All patients with a TIMP2•IGFBP7 value and data for all clinical variables were included (n = 353). The AUC-ROC increases (one-sided p = 0.008) from 0.77 (0.69–0.86) to 0.88 (0.83–0.94) when TIMP2•IGFBP7 is added to the model. *Log10 transform of APACHE III and TIMP2•IGFBP7 were used in the models. †Log2 transform of serum creatinine was used because log10 is not a clinically relevant scale for serum creatinine. Blood for serum creatinine testing was collected at the time of urine collection for TIMP2•IGFBP7 testing. §History of cirrhosis or hepatic failure. The inverse of body mass index was used in the model. All continuous variables were standardized by subtracting the mean and then dividing by 2 standard deviations.
The strong performance of this urinary TIMP2•IGFBP7 test was observed in the surgical cohorts of each individual study as well as when the patients from both cohorts are combined (Figs. 2–3). This is demonstrated by the AUC (95% CI) for the Sapphire (0.80 [95% CI, 0.69–0.92], p < 0.0001) and Topaz (0.88 [95% CI, 0.81–0.96], p < 0.0001) studies and for when cohorts are combined (0.84 [95% CI, 0.76–0.90], p < 0.0001). In comparison, neither urine output nor serum creatinine predicted the development of AKI during the same period as well. Of note, the cohorts were not heterogeneous, as evidenced by the heterogeneity tests for the AUCs between Sapphire and Topaz: Higgin's I2 = 29%; Cochran's Q statistic for measuring heterogeneity is not significant (p = 0.23).
As shown in Figure 2, test characteristics vary somewhat, but remain robust, across different subgroups (cardiac/noncardiac and emergent/elective). A multivariate clinical model was created using a stepwise selection from all the variables in Table 1 that were associated (p < 0.1) with the primary end point. This model was created with and without the inclusion of the biomarker test results. With the addition of the biomarker, the model's ability to predict moderate-to-severe AKI increased, as shown by an increase in the AUC-ROC from 0.77 to 0.88 (p = 0.01) (Fig. 3).
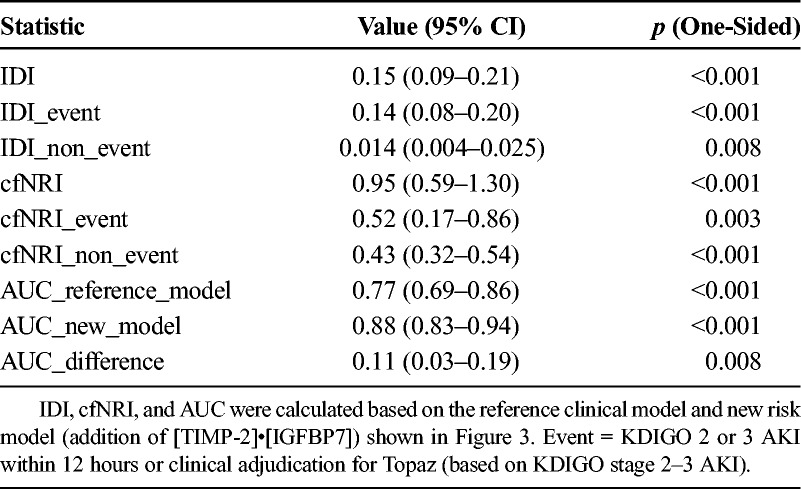
To provide further assessment of the ability of the biomarker to enhance clinical risk prediction, we performed IDI and cfNRI analyses as described in Table 2. The addition of the biomarker to the clinical model resulted in a significant increase in overall ability to predict AKI: IDI = 0.15 (0.09–0.21), p < 0.001; and cfNRI = 0.95 (0.59–1.30), p < 0.001 (Table 2) (see also eFigure 1 in Supplemental Digital Content 1, http://links.lww.com/TA/A693).
Table 2.
IDI and cfNRI for Addition of [TIMP-2]•[IGFBP7] to the Clinical Model
DISCUSSION
To our knowledge, this is the first report using the cell cycle arrest biomarkers TIMP-2 and IGFBP7 to predict the development of moderate-to-severe AKI in a large cohort of heterogeneous, critically ill, postoperative surgical patients. In our study, we used a single measurement of urinary TIMP2•IGFBP7 soon after ICU admission and found an AUC-ROC of 0.84 for development of moderate-to-severe AKI within 12 hours. Using a prespecified cutoff value of 0.3, we found sensitivity and specificity of 89% (77–97%) and 49% (43–54%), respectively; using a prespecified cutoff value of 2.0, we found sensitivity and specificity of 40% (23–57%) and 94% (92–96%). We have demonstrated reassuringly robust performance of a new biomarker test in the prediction of an important clinical outcome and further shown that it is able to significantly enhance clinical risk prediction techniques alone. Importantly, we have also shown that the results of this test remain robust in cardiac and noncardiac surgery and in elective as well as emergent surgery.
Recently, Meersch et al.31 examined the sensitivity and specificity of TIMP2•IGFBP7 for AKI (stage 1 or greater) in a group of patients undergoing cardiac surgery. These investigators found a sensitivity of 0.92 and a specificity of 0.81 for a cutoff value of 0.5 (AUC-ROC of 0.84) using the highest urinary TIMP2•IGFBP7 concentration achieved in the first 24 hours following surgery (composite time point). However, here we report the performance of the test in surgical patients when it is used as a single measure at or near the time of ICU admission to predict the development of moderate-to-severe AKI in the next 12 to 24 hours. This may provide context to clinicians who wish to use the test to help in deciding the immediate care plan for patients in their surgical ICU practice.
The performance of AKI biomarkers can sometimes be diminished in the presence of chronic disease states and also in circumstances where there is activation of the inflammatory cascade.32–39 However, TIMP2•IGFBP7 seems to retain its performance in this surgical cohort and also specifically in the cardiac surgery subgroup (Fig. 2). Current mechanistic thinking in AKI is moving toward the concept of AKI as a secondary injury occurring as danger- and pathogen-associated molecular pattern (DAMP, PAMP) molecules are delivered to the renal tubule via both glomerular filtration and the bloodstream.37 These molecules are detected by pattern recognition receptors on the tubular cell surface where they signal a number of cell responses including alterations in cell cycle progression. When prolonged, cell cycle arrest may lead to senescence and/or apoptosis. However, cell cycle arrest is, itself, a protective mechanism that prevents cells from dividing when they may be injured. The fact that these biomarkers perform so well in surgical patients, and that they are seemingly robust to type and urgency of surgery, suggests that this mechanism of AKI is determined much more by the host response to stress rather than by the type of operation itself.
Both TIMP2 and IGFBP7 have been implicated in the G1 cell cycle arrest phase noted to occur during the very early stages of cellular stress.38–41 Specifically, it has been shown that renal tubular cells go through this G1 cell cycle arrest phase following stress because of a variety of insults.42 In surgical patients, including those undergoing a period of cardiopulmonary bypass, it is reassuring that these markers perform well given the involvement of the innate immune system as a fundamental part of the host response to environmental (surgical) stress.43,44 It is reasonable therefore to suggest that biomarkers whose physiologic origin is in the pathways modulating this response might prove useful as predictors of outcome in this patient population. TIMP2 and IGFBP7 are both implicated in cell cycle arrest and signal transduction during innate immune activation, and as such, it is not surprising that they apparently perform better than previously described biomarkers in surgical patients.
In addition to TIMP-2 and IGFBP7, a host of other AKI biomarkers has been previously investigated within the context of surgery.45–50 The majority of these studies have been conducted in post–cardiac surgery patients,45,48 the most comprehensive being the National Institutes of Health–sponsored TRIBE AKI trial wherein multiple AKI biomarkers were tested in more than 1,200 post–cardiac surgery patients.47 These studies have demonstrated that plasma NGAL, urinary NGAL, and urinary IL-18 all performed modestly well in the prediction of AKI, with AUC-ROC values between 0.67 and 0.74. When the biomarkers were added to a clinical model, its performance improved to 0.73 to 0.76. These results are consistent with other cohorts of cardiac and noncardiac surgery patients.
Our study has several limitations. First, our limited sample size only permits exploratory analysis within different surgical subgroups, such as those undergoing cardiac or noncardiac surgery. Second, although consistent with other reports, our overall event rate does not permit large multivariate analyses that may uncover the contribution of other important risk factors such as blood transfusion, fluid administration, or different hemodynamic management strategies. These questions are the focus of our ongoing studies. Despite these limitations, we believe that our data provide busy clinicians caring for surgical patients with reassuring evidence that the TIMP2•IGFBP7 test is able to improve their clinical decision making in the context of AKI risk assessment and stratification. In the future, it will be important to demonstrate which clinical interventions and therapeutic maneuvers are associated with good (and bad) outcomes in patients stratified by biomarker test results. If confirmed in prospective trials, these candidate interventions may then be considered therapeutic goals in patients classified as high risk.
CONCLUSIONS
We have previously shown that the TIMP2•IGFBP7 test accurately identifies a cohort of critically ill adult patients who are at increased risk for developing AKI within the subsequent 12 to 24 hours. Here we provide evidence that this finding is just as accurate, in fact more so, in surgical patients. This is important both because of unique exposures and the presence of several potentially modifiable risk factors in this patient population. Future interventional trials focused on AKI prevention using urinary TIMP2•IGFBP7 as an early marker of renal cellular stress are warranted.
Supplementary Material
AUTHORSHIP
K.J.G., A.D.S., L.S.C., A.B., A.A.-K., K.K., M.L., and J.A.K. conducted the literature search. J.A.K., K.J.G., A.B., A.D.S., L.S.C., A.A.-K., and K.K. designed the study. K.J.G., A.D.S., L.S.C., A.B., A.A.-K., K.K., M.L., and J.A.K. collected data on behalf of the Sapphire and Topaz investigators. J.S. and M.G.W. performed data analysis. J.A.K., K.J.G., A.D.S., L.S.C., A.B., A.A.-K., K.K., M.L., J.S., and M.G.W. contributed to data interpretation. K.J.G., J.A.K., A.D.S., L.S.C., A.B., A.A.-K., K.K., M.L.,J.S., and M.G.W. wrote and critically revised the manuscript.
ACKNOWLEDGMENT
This study was sponsored by Astute Medical.
DISCLOSURE
K.K., A.A.-K., A.B., M.L., J.S., and M.W. declare no conflicts of interest. L.C. has consulting agreements with Abbott Medical, Affymax Medical, Alere Medical, AM Pharma, Astute Medical, Covidien Medical, Gambro Medical, Nxstage, Sanofi, and Bonner Kiernana Law Offices. L.C. has applied for research support from Eli Lilly and owns stock in MAKO Corporation for an orthopedic surgical robot. K.G. has received research grants from Spectral Diagnostics. A.S. has received fees for expert testimony from Abbott Laboratories and is a Medical Advisory Board member for FAST diagnostics for Optical GFR measurement and a Scientific Advisory Board member for NxStage Medical for CRRT in the ICU. J.K. has received consulting fees from Astute Medical, Alere, Opsona, Aetholon, AM Pharma, Cytosorbents, VenBio, Gambro, Baxter, Abbott, Roche, Spectral Diagnostics, Sangart, and Siemens. J.K. has also received research grants from Astute Medical, Alere, Cytosorbents, Gambro, Baxter, Kaneka, Grifols, CR Bard, and Spectral Diagnostics and has a license in unrelated technologies through the University of Pittsburgh to Astute Medical, Cytosorbents, and Spectral Diagnostics.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License, where it is permissible to download and share the work provided it is properly cited. The work cannot be changed in any way or used commercially.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal's website (www.jtrauma.com).
Complete lists of Sapphire and Topaz investigators are available online at http://www.ccm.pitt.edu/sapphire-investigators and http://www.ccm.pitt.edu/topaz-investigators. Clinical Trials Registration: clintrials.gov NCT01209169 and NCT01573962.
Contributor Information
Collaborators: on behalf of the Sapphire Topaz investigators
REFERENCES
- 1. Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012; 380 (9843): 756– 766. [DOI] [PubMed] [Google Scholar]
- 2. Kellum JA, Bellomo R, Ronco C. Kidney attack. JAMA. 2012; 307 (21): 2265– 2266. [DOI] [PubMed] [Google Scholar]
- 3. Bihorac A, Brennan M, Ozrazgat-Baslanti T, Bozorgmehri S, Efron PA, Moore FA, Segal MS, Hobson CE. National surgical quality improvement program underestimates the risk associated with mild and moderate postoperative acute kidney injury. Crit Care Med. 2013; 41 (11): 2570– 2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hobson C, Ozrazgat-Baslanti T, Kuxhausen A, Thottakkara P, Efron PA, Moore FA, Moldawer LL, Segal MS, Bihorac A. Cost and mortality associated with postoperative acute kidney injury. Ann Surg. 2015; 261: 1207– 1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bihorac A, Yavas S, Subbiah S, Hobson CE, Schold JD, Gabrielli A, Layon AJ, Segal MS. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann Surg. 2009; 249 (5): 851– 858. [DOI] [PubMed] [Google Scholar]
- 6. Chawla LS, Amdur RL, Shaw AD, Faselis C, Palant CE, Kimmel PL. Association between AKI and long-term renal and cardiovascular outcomes in United States veterans. Clin J Am Soc Nephrol. 2014; 9 (3): 448– 456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hobson CE, Yavas S, Segal MS, Schold JD, Tribble CG, Layon AJ, Bihorac A. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. 2009; 119 (18): 2444– 2453. [DOI] [PubMed] [Google Scholar]
- 8. Hoste EA, Clermont G, Kersten A, Venkataraman R, Angus DC, De Bacquer D, Kellum JA. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006; 10 (3): R73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Murugan R, Karajala-Subramanyam V, Lee M, Yende S, Kong L, Carter M, Angus DC, Kellum JA. Acute kidney injury in non-severe pneumonia is associated with an increased immune response and lower survival. Kidney Int. 2010; 77 (6): 527– 535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wu VC, Wu CH, Huang TM, Wang CY, Lai CF, Shiao CC, Chang CH, Lin SL, Chen YY, Chen YM, et al. Long-term risk of coronary events after AKI. J Am Soc Nephrol. 2014; 25 (3): 595– 605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kheterpal S, Tremper KK, Heung M, Rosenberg AL, Englesbe M, Shanks AM, Campbell DA., Jr Development and validation of an acute kidney injury risk index for patients undergoing general surgery: results from a national data set. Anesthesiology. 2009; 110 (3): 505– 515. [DOI] [PubMed] [Google Scholar]
- 12. White LE, Hassoun HT, Bihorac A, Moore LJ, Sailors RM, McKinley BA, Valdivia A, Moore FA. Acute kidney injury is surprisingly common and a powerful predictor of mortality in surgical sepsis. J Trauma Acute Care Surg. 2013; 75 (3): 432– 438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bellomo R, Wan L, May C. Vasoactive drugs and acute kidney injury. Crit Care Med. 2008; 36 (4 Suppl): S179– S186. [DOI] [PubMed] [Google Scholar]
- 14. Comporti M, Signorini C, Buonocore G, Ciccoli L. Iron release, oxidative stress and erythrocyte ageing. Free Radic Biol Med. 2002; 32 (7): 568– 576. [DOI] [PubMed] [Google Scholar]
- 15. Tinmouth A, Fergusson D, Yee IC, Hébert PC; ABLE Investigators; Canadian Critical Care Trials Group. Clinical consequences of red cell storage in the critically ill. Transfusion. 2006; 46 (11): 2014– 2027. [DOI] [PubMed] [Google Scholar]
- 16. Vermeulen Windsant IC, de Wit NC, Sertorio JT, Beckers EA, Tanus-Santos JE, Jacobs MJ, Buurman WA. Blood transfusions increase circulating plasma free hemoglobin levels and plasma nitric oxide consumption: a prospective observational pilot study. Crit Care. 2012; 16 (3): R95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vermeulen Windsant IC, Hanssen SJ, Buurman WA, Jacobs MJ. Cardiovascular surgery and organ damage: time to reconsider the role of hemolysis. J Thorac Cardiovasc Surg. 2011; 142 (1): 1– 11. [DOI] [PubMed] [Google Scholar]
- 18. Walsh M, Devereaux PJ, Garg AX, Kurz A, Turan A, Rodseth RN, Cywinski J, Thabane L, Sessler DI. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology. 2013; 119 (3): 507– 515. [DOI] [PubMed] [Google Scholar]
- 19. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012; 2 (1): 1– 138. [Google Scholar]
- 20. Murugan R, Kellum JA. Acute kidney injury: what's the prognosis? Nat Rev Nephrol. 2011; 7 (4): 209– 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Star RA. Treatment of acute renal failure. Kidney Int. 1998; 54 (6): 1817– 1831. [DOI] [PubMed] [Google Scholar]
- 22. Bihorac A, Chawla LS, Shaw AD, Al-Khafaji A, Davison DL, Demuth GE, Fitzgerald R, Gong MN, Graham DD, Gunnerson K, et al. Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication. Am J Respir Crit Care Med. 2014; 189 (8): 932– 939. [DOI] [PubMed] [Google Scholar]
- 23. Kashani K, Al-Khafaji A, Ardiles T, Artigas A, Bagshaw SM, Bell M, Bihorac A, Birkhahn R, Cely CM, Chawla LS, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013; 17 (1): R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kumar AB, Suneja M, Bayman EO, Weide GD, Tarasi M. Association between postoperative acute kidney injury and duration of cardiopulmonary bypass: a meta-analysis. J Cardiothorac Vasc Anesth. 2012; 26 (1): 64– 69. [DOI] [PubMed] [Google Scholar]
- 25. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007; 147 (8): 573– 577. [DOI] [PubMed] [Google Scholar]
- 26. Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, Lijmer JG, Moher D, Rennie D, de Vet HC; STARD Group. Toward complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Acad Radiol. 2003; 10 (6): 664– 669. [DOI] [PubMed] [Google Scholar]
- 27. Pencina MJ, D'Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011; 30 (1): 11– 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gelman A. Scaling regression inputs by dividing by two standard deviations. Stat Med. 2008; 27 (15): 2865– 2873. [DOI] [PubMed] [Google Scholar]
- 29. R Development Core Team R: A Language and Environment for Statistical Computing. 2011. Available at: http://www.R-project.org/ Accessed May 18, 2015.
- 30. Hoste EA, McCullough PA, Kashani K, Chawla LS, Joanniidis M, Shaw AD, Feldkamp T, Uettwiller-Geiger DL, McCarthy P, Shi J, et al. Derivation and validation of cutoffs for clinical use of cell cycle arrest biomarkers. Nephrol Dial Transplant. 2014; 29 (11): 2054– 2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Meersch M, Schmidt C, Van Aken H, Martens S, Rossaint J, Singbartl K, Görlich D, Kellum JA, Zarbock A. Urinary TIMP-2 and IGFBP7 as early biomarkers of acute kidney injury and renal recovery following cardiac surgery. PLoS One. 2014; 9 (3): e93460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bagshaw SM, Bennett M, Haase M, Haase-Fielitz A, Egi M, Morimatsu H, D'amico G, Goldsmith D, Devarajan P, Bellomo R. Plasma and urine neutrophil gelatinase-associated lipocalin in septic versus non-septic acute kidney injury in critical illness. Intensive Care Med. 2010; 36 (3): 452– 461. [DOI] [PubMed] [Google Scholar]
- 33. Knight EL, Verhave JC, Spiegelman D, Hillege HL, de Zeeuw D, Curhan GC, de Jong PE. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int. 2004; 65 (4): 1416– 1421. [DOI] [PubMed] [Google Scholar]
- 34. Mårtensson J, Bell M, Oldner A, Xu S, Venge P, Martling CR. Neutrophil gelatinase-associated lipocalin in adult septic patients with and without acute kidney injury. Intensive Care Med. 2010; 36 (8): 1333– 1340. [DOI] [PubMed] [Google Scholar]
- 35. Nauta FL, Boertien WE, Bakker SJ, van Goor H, Van Oeveren W, de Jong PE, Bilo H, Gansevoort RT. Glomerular and tubular damage markers are elevated in patients with diabetes. Diabetes Care. 2011; 34 (4): 975– 981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Okura T, Jotoku M, Irita J, Enomoto D, Nagao T, Desilva VR, Yamane S, Pei Z, Kojima S, Hamano Y, et al. Association between cystatin C and inflammation in patients with essential hypertension. Clin Exp Nephrol. 2010; 14 (6): 584– 588. [DOI] [PubMed] [Google Scholar]
- 37. Gomez H, Ince C, De Backer D, Pickkers P, Payen D, Hotchkiss J, Kellum JA. A unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock. 2014; 41 (1): 3– 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Boonstra J, Post JA. Molecular events associated with reactive oxygen species and cell cycle progression in mammalian cells. Gene. 2004; 337: 1– 13. [DOI] [PubMed] [Google Scholar]
- 39. Devarajan P. Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol. 2006; 17 (6): 1503– 1520. [DOI] [PubMed] [Google Scholar]
- 40. Rodier F, Campisi J, Bhaumik D. Two faces of p53: aging and tumor suppression. Nucleic Acids Res. 2007; 35 (22): 7475– 7484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Seo DW, Li H, Qu CK, Oh J, Kim YS, Diaz T, Wei B, Han JW, Stetler-Stevenson WG. Shp-1 mediates the antiproliferative activity of tissue inhibitor of metalloproteinase-2 in human microvascular endothelial cells. J Biol Chem. 2006; 281 (6): 3711– 3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yang QH, Liu DW, Long Y, Liu HZ, Chai WZ, Wang XT. Acute renal failure during sepsis: potential role of cell cycle regulation. J Infect. 2009; 58 (6): 459– 464. [DOI] [PubMed] [Google Scholar]
- 43. Giannoudis PV, Dinopoulos H, Chalidis B, Hall GM. Surgical stress response. Injury. 2006; 37 (Suppl 5): S3– S9. [DOI] [PubMed] [Google Scholar]
- 44. Linde A, Mosier D, Blecha F, Melgarejo T. Innate immunity and inflammation—new frontiers in comparative cardiovascular pathology. Cardiovasc Res. 2007; 73 (1): 26– 36. [DOI] [PubMed] [Google Scholar]
- 45. Arthur JM, Hill EG, Alge JL, Lewis EC, Neely BA, Janech MG, Tumlin JA, Chawla LS, Shaw AD; SAKInet Investigators. Evaluation of 32 urine biomarkers to predict the progression of acute kidney injury after cardiac surgery. Kidney Int. 2014; 85 (2): 431– 438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Herget-Rosenthal S, Marggraf G, Hüsing J, Göring F, Pietruck F, Janssen O, Philipp T, Kribben A. Early detection of acute renal failure by serum cystatin C. Kidney Int. 2004; 66 (3): 1115– 1122. [DOI] [PubMed] [Google Scholar]
- 47. Koyner JL, Garg AX, Coca SG, Sint K, Thiessen-Philbrook H, Patel UD, Shlipak MG, Parikh CR; TRIBE-AKI Consortium. Biomarkers predict progression of acute kidney injury after cardiac surgery. J Am Soc Nephrol. 2012; 23 (5): 905– 914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liang XL, Liu SX, Chen YH, Yan LJ, Li H, Xuan HJ, Liang YZ, Shi W. Combination of urinary kidney injury molecule-1 and interleukin-18 as early biomarker for the diagnosis and progressive assessment of acute kidney injury following cardiopulmonary bypass surgery: a prospective nested case–control study. Biomarkers. 2010; 15 (4): 332– 339. [DOI] [PubMed] [Google Scholar]
- 49. Perry TE, Muehlschlegel JD, Liu KY, Fox AA, Collard CD, Shernan SK, Body SC; CABG Genomics Investigators. Plasma neutrophil gelatinase-associated lipocalin and acute postoperative kidney injury in adult cardiac surgical patients. Anesth Analg. 2010; 110 (6): 1541– 1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wagener G, Gubitosa G, Wang S, Borregaard N, Kim M, Lee HT. Urinary neutrophil gelatinase-associated lipocalin and acute kidney injury after cardiac surgery. Am J Kidney Dis. 2008; 52 (3): 425– 433. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


