Abstract
Purpose: The aim of this work was to investigate the volatiles released from aerial parts of Scrophularia subaphylla (Scrophulariaceae) which is a perennial herb growing in Azarbaijan province in Iran.
Methods: A combination of GC-MS and GC-FID were applied for analyzing the chemical compositions of the essential oil extracted by hydro-distillation from the aerial parts of Scrophularia subaphylla (S. subaphylla).
Results: Thirty six compounds, representing 97.32% of total oil were identified. High content of terpenoids (60.02%) were identified in the essential oil with Linalool (22.35%), phytol (15.74%) and geraniol (7.27%) as the most dominant compounds, while other main components were representatives of fatty acids (24.31%), indicated mainly by palmitinic acid (17.29%). DPPH assay was used for assessing the antioxidant properties of compounds. However, no remarkable free radical scavenging activity was observed. Furthermore, Disc diffusion method was applied for evaluating the antimicrobial activity of essential oil vs. gram positive and gram negative bacteria strains. The examined oil showed weak antibacterial effect.
Conclusion: Main constituents of S. subaphylla were terpenoids. In comparison with other genesis of Scrophularia, antioxidant and anti bacterial properties of S. subaphylla essential oil were not noticeable.
Keywords: Scrophularia subaphylla, Scrophulariaceae, Essential oil composition, GC-MS, DPPH assay, Antibacterial activity
Introduction
The genus Scrophularia (commonly known as figworts) is one of the largest genera of the Scrophulariaceae family which is distributed widely in Asia, North America and central Europe, especially in the Mediterranean region.1 This genus is represented in Iranian flora by 42 species, of which 19 are endemic.1,2 Scrophularia subaphylla (S. subaphylla) is one of the endemic species growing in East Azarbaijan province of Iran. It is a multi- stemmed perennial herb with square stems, woody rhizomes, opposite leaves and open two-lipped flowers.3 Several investigations on this genus have revealed various pharmacological activities such as wound healing, anti-inflammatory, antibacterial, cardiovascular, diuretic, protozoacidal, fungicidal, cytotoxic and anti-nociceptive.4-10 Most of these mentioned characteristic were proven to attribute non-volatile components, which were known as secondary metabolites. Iridoids, phenylpropanoids, phenolic acids and flavonoids have been identified as main secondary metabolites.4-10 Despite the high number of Scrophularia species, there are a few studies about the chemical composition of the essential oils of these plants.11-13 Moreover, to our knowledge, biological activities of the essential oils from Scrophularia genus, especially Iranian endemic taxa, have not yet been exhaustively investigated except two recent reports by Pasdaran et al which indicated antioxidant and antimicrobial properties of essential oils of S. amplexicaulis and, insecticidal effects of oil obtained from aerial parts of S. oxycepala.11,12 Therefore, in this work we present for the first time the chemical composition of the essential oil from aerial parts of S. subaphylla occurring in East Azarbaijan province as well as its antioxidant and antimicrobial properties.
Material and Methods
Plant material
Flowering aerial parts of S. subaphylla was collected during July 2013 from Mishodagh Mountain near the Marand city (Yam) in East Azarbaijan province, Iran. Voucher specimen was identified and retained in the Herbarium of Faculty of Pharmacy, Tabriz University of Medical Sciences, Iran, under the accession code Tbz-fph 747.
Essential oil Isolation
The air-dried aerial parts of plant (100 g) were cut into small pieces and submitted to hydro-distillation for 3 h in a Clevenger-type apparatus using hexane (2 mL) as collector solvent. The pale yellow-colored essential oil was dried over anhydrous sodium sulphate, then the solvent was evaporated and the oil was stored in sealed vials at - 4° before analyses.
GC-MS analysis
GC-MS analysis was performed on a Shimadzu GCMS QP 5050A gas chromatograph-mass spectrometer fitted with a fused capillary column DB-1 (60 m, 0.25 mm id, film thickness 0.25 µm), with the following temperature program: 3 min at 50°C, then at 3 °C/min to 260°C, held for 5 min, for a total run of 80 min. Injector and transfer line temperatures were 240°C. Helium was utilized as the carrier gas, at a flow rate of 1.3 ml/min. Essential oil were diluted 1:100 in hexane, and the volume injected was 1µL; split ratio, 1:29; acquisition mass range, 30-600 m/z, ion source temperature 270 °C; quadrupole 100 °C; Solvent delay 2 min; scan speed 2000 amu/s and EV voltage 3000 volts. All mass spectra were acquired in electron-impact (EI) mode with an ionization voltage of 70 eV. Qualitative identification of essential oil constituents was based on direct comparison of the Kovats Indices (KI) and MS data with those for the standard compounds as well as using the spectrophotometer database like NIST NBS54K and Wily 229 library along with comparison of their retention indices (RT) and MS fragmentation pattern with the authentic reference compounds that were reported in the Adam'S literature.14 Also flame ionization detector (FID) which was operated in ionization potential mode at 70 ev, was used for quantification purpose for calculating the relative area percentage (area %) without the use of correction factors.
Antimicrobial assay
Lyophilized form of two gram positive and gram negative bacteria, Staphylococcus aureus ATCC (6538) and Pseudomonas aeroginosa ATCC (9027) respectively, were purchased from institute of Pasture, Iran. They were used to evaluate the antibacterial activity of S. subaphylla essential oil. Activated bacteria were maintained in suitable agar media at 4°C as stock cultures for further uses. A single colony after transferring in to Muller Hinton Broth media incubated at 37°C for 24h then centrifuged. For providing an optical density equal to 108 CFU/ml of bacterial concentration, turbidity was corrected with adding a saline solution. After that, sterilized discs (6 mm diameter, Whatman paper) were impregnated with different concentration of test sample (which were dissolved in 10% aqueous DMSO previously) as well as positive reference (Amikacine). Negative control were prepared from the same solvent (DMSO) employed to dissolve the plant oil. Afterward, petridishes transferred in to the refrigerator to facilitate the diffusion of oil approximately for 30 min, thereafter, plates were incubated at 37 °C for starting the growth of bacteria. Finally, the diameter of the inhibition zones was considered as the antibacterial activity of the test sample.15
Antioxidant activity
The in vitro antioxidant property of the essential oil was evaluated through the modified DPPH assay. 2 ml of 0.08 gr/ml DPPH which was prepared in chloroform was added in to different dilutions of essential oil and negative control (chloroform). Afterwards, when the mixture was homogenous, was kept in room temperature for 30 min to any reaction to occur. The same procedure was repeated for positive control (quercetin). Finally, the absorbance of test specimens was recorded against negative control at 517 nm using UV/Visible Spectrophotometer 160A (USA). Percentage of DPPH reduction was calculated according to the following equation:
Also RC50 was extrapolated from dose-response curve. The experiment was performed in duplicate.16
Results and Discussion
The composition of the essential oil obtained from the aerial parts of S. subaphylla is compiled in Table 1, where the components are listed in order to their elution on the DB-1 column.
Table 1. Chemical constituent of the essential oil from aerial parts of S. subaphylla .
| Compounds a | K.I | Area % | Identification method |
| 1-Octen-3-ol | 963 | 2.25 | GC-MS, Is |
| n-Octan-3-ol | 980 | 0.68 | GC-MS, Is |
| n-Nonanal | 1083 | 0.85 | GC-MS, Is |
| L-Linalool | 1086 | 22.35 | GC-MS, Is |
| α-Terpineol | 1174 | 5.25 | GC-MS, Is |
| n-Decaldehyde | 1185 | 0.52 | GC-MS, Is |
| 4-α-dimethyl-3-Cyclohexene-1-acetaldehyde | 1192 | 0.25 | GC-MS, Is |
| Z- geraniol | 1211 | 1.94 | GC-MS, Is |
| Geraniol | 1236 | 7.27 | GC-MS, Is |
| Z-2-Decenal | 1238 | 0.49 | GC-MS, Is |
| Undecanal | 1287 | 0.36 | GC-MS, Is |
| E,E-2,4-Decadienal | 1290 | 0.27 | GC-MS, Is |
| Heptylidene acetone | 1319 | 1.08 | GC-MS, Is |
| E-2-dodecenal | 1341 | 0.2 | GC-MS, Is |
| β-Damascenone | 1364 | 1.19 | GC-MS, Is |
| Palmitaldehyde | 1389 | 0.5 | GC-MS, Is |
| Geranyl acetone | 1430 | 0.56 | GC-MS, Is |
| β-Ionone | 1466 | 1.22 | GC-MS, Is |
| Nerolidol B (E OR TZ) | 1549 | 4.21 | GC-MS, Is |
| α-Bisabolol | 1670 | 0.17 | GC-MS, Is |
| Tetradecanal | 1696 | 0.24 | GC-MS, Is |
| Nerolidol | 1702 | 0.48 | GC-MS, Is |
| Myristic acid | 1743 | 1.02 | GC-MS, Is |
| n-Octadecane | 1800 | 0.69 | GC-MS, Ib |
| Hexahydrofarnesyl acetone | 1831 | 3.75 | GC-MS, Is |
| Farnesyl acetone | 1895 | 0.26 | GC-MS, Is |
| Methyl eicosanoate | 1909 | 0.37 | GC-MS, Is |
| Palmitinic acid | 1949 | 17.29 | GC-MS, Is |
| Palmitic acid ethyl ester | 1978 | 0.49 | GC-MS, Is |
| Heneicosane | 2100 | 0.62 | GC-MS, Ib |
| Linoleic acid | 2109 | 1.31 | GC-MS, Ib |
| Oleic Acid | 2116 | 0.91 | GC-MS, Ib |
| Phytol | 2124 | 15.74 | GC-MS, Ib |
| Linolenic acid methyl ester | 2125 | 1.68 | GC-MS, Ib |
| Ethyl linoleate | 2139 | 0.47 | GC-MS, Ib |
| Hexadecanal diallyl acetal | - | 0.39 | GC-MS, Ib |
| Total identified | - | 97.32 | - |
| Non-terpenoid | - | 37.30 | - |
| Terpenoids | - | 60.02 | - |
a Compounds listed in order of elution from a DB-1 column, b Identification Method (Is = Kovats retention index according to authentic standard, Ib = Kovats retention index according to bibliography).
Hydro-distillation gave an odorous light yellow oil with a yield of 0.05 % W/W, based on the dry mass. The very low yield (0.05%) was consistent with those reported in the researches for other species of the genus Schrophularia.11-13 so it seems that this genus contains oil-poor plants. A total of 36 volatiles were identified, accounting for 97.32% of the total volatiles. The components of essential oil were separated into two classes, which were terpenoids (60.02%) and non-terpenoid (37.30%) compounds. Terpenoids were represented mainly by oxygenated monoterpenes (39.42%), with Linalool (22.35%), geraniol (7.27%) and α-terpineol (5.25%) as the most abundant compounds, while the content of oxygenated sesquiterpenes was rather low (4.86%), represented mainly by nerolidol B (4.21%). Among oxygen containing components, alcohols were the most abundant. Moreover, as shown in Figure 1. The oil was characterized by high amount of diterpenes, notably phytol, which accounted for 15.74% of the oil. Among non-terpenoid compounds, fatty acids constituted the main fraction (20.53%) of this part, with palmitic acid (17.29%) as the major components. This finding previously was confirmed by Mitsuo Miyazawa et al in other species of Scrophularia.13 Apart from the main compounds reported above, 1-Octen-3-ol (2.25%) and hexahydrofarnesyl acetone (3.75%) exceeded a content of 2% of the total oil composition, whilst the remaining compounds (n=27) were present in scant amount, most of them existing at contents lower than 1%.
Figure 1.
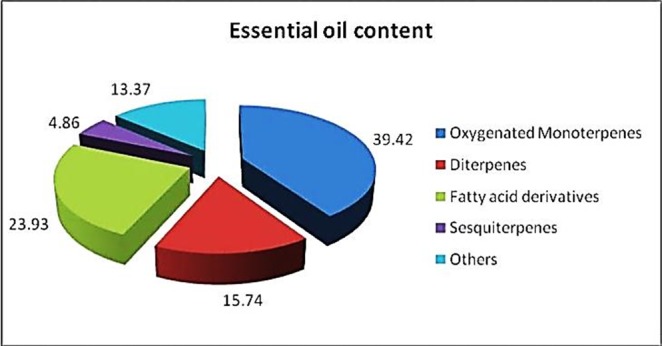
Identified chemical groups from the essential oil of S. subaphylla.
To the best of our knowledge, the current work represents the first study of the essential oil of S. subaphylla but there are some reports about chemical composition of other species of Schrophularia. The comparison of our results with previous researches illustrates considerable differences in terms of chemical composition of the oil. In our examined oil, linalool (22.35%), palmitic acid (17.29%) and phytol (15.74%) were identified as the main components whereas according to a report by Pasdaran et al, eugenol (53.8%), eugenol acetate (24.5%) and caryophyllen oxide (6.4%) were the main components of the essential oil of S. amplexicaulis.11 Moreover, the oil from the aerial parts of S. oxycepala represented phytol (25.3%), methyl benzyl alcohol (9.3%) and dihydroeugenol (6.7%) as principle components.12 It is notable that linalool was found at a relatively high level in our study whereas it was not detected in considerable amount in the oil of the other species.11-13 Conversely, eugenol and its derivatives was absent in our examined oil compared with that of oil composition of other species.11-13 It is noteworthy to mention that phytol as a diterpenoid compound present in all of the investigated Scrophularia species in variable value. 11-13 These findings showed that the genus Schrophularia had a remarkable variation in essential oil composition. Furthermore, in the current study was observed weak antibacterial activity of essential oil (Mean Inhibition Zone Diameter ± SD (MIZD 8 ± 0.3 mm) in comparison to Amikacin (MIZD 15 ± 0.1 mm). Our finding is in contrary to the previous study which were reported the significant antibacterial activity of Scrophularia genesis.11 Also in the case of S. subaphylla was not found remarkable antioxidant activity (RC50 5.2 ×10-2 mg/ml) in comparison to quercetine as a positive control and other species of Scrophularia like S. amplexicaulis oil.11 Due to the fact that the phenolic compounds have been known the most likely constituents responsible for the free radical scavenging activity, lack of these sorts of constituents in our examined oil may be led to weak activity in this assay
Conclusion
Main constituents of S. subaphylla were identified as terpenoides. In comparison with other species of Scrophularia, antioxidant and anti-bacterial properties of S. subaphylla essential oil were not remarkable.
Acknowledgments
This study was financially supported by grant no. 92/1114 from the Drug Applied Research Center of Tabriz University of Medical Sciences. This article was written based on data set of PhD thesis registered in Tabriz University of Medical Sciences (NO. 95).
Ethical Issues
Not applicable.
Conflict of Interest
The authors report no conflicts of interest.
References
- 1.Ardeshiry Lajimi A, Rezaie-Tavirani M, Mortazavi SA, Barzegar M, Moghadamnia SH, Rezaee MB. Study of anti cancer property of scrophularia striata extract on the human astrocytoma cell line (1321) Iran J Pharm Res. 2010;9(4):403–10. [PMC free article] [PubMed] [Google Scholar]
- 2.Mozaffarian V. A dictionary of Iranian plant names, Latin, English, Persian. 4rd ed. Iran: Farhang Mo'aser; 1996. [Google Scholar]
- 3.Rechinger KH. Flora iranica 147. Austria: Graze: Akad. Druck. U. verlagsanstalt; 1981. [Google Scholar]
- 4.Akhmedov SG, Tkachenko DA, Kharchenko NS. pharmacology of flavonoid aglycones of scrophularia grossheimi. Farmakol Toksikol. 1969;32(6):693–4. [PubMed] [Google Scholar]
- 5.Bermejo Benito P, Abad Martinez MJ, Silvan Sen AM, Sanz Gomez A, Fernandez Matellano L, Sanchez Contreras S. et al. In vivo and in vitro antiinflammatory activity of saikosaponins. Life Sci. 1998;63(13):1147–56. doi: 10.1016/s0024-3205(98)00376-2. [DOI] [PubMed] [Google Scholar]
- 6.Emam AM, Diaz-Lanza AM, Matellano-Fernandez L, Faure R, Moussa AM, Balansard G. Biological activities of buddlejasaponin isolated from buddleja madagascariensis and scrophularia scorodonia. Pharmazie. 1997;52(1):76–7. [PubMed] [Google Scholar]
- 7.Ghisalberti EL. Biological and pharmacological activity of naturally occurring iridoids and secoiridoids. Phytomedicine. 1998;5(2):147–63. doi: 10.1016/S0944-7113(98)80012-3. [DOI] [PubMed] [Google Scholar]
- 8.Lacaille-Dubois M-A, Wagner H. Importance pharmacologique des dérivés polyphénoliques. Acta botanica gallica. 1996;143(6):555–62. doi: 10.1080/12538078.1996.10515353. [DOI] [Google Scholar]
- 9.Nishibe S. Bioactive phenolic compounds in traditional medicines. Pure Appl Chem. 1994;66(10-11):2263–6. doi: 10.1351/pac199466102263. [DOI] [Google Scholar]
- 10.Stevenson PC, Simmonds MS, Sampson J, Houghton PJ, Grice P. Wound healing activity of acylated iridoid glycosides from scrophularia nodosa. Phytother Res. 2002;16(1):33–5. doi: 10.1002/ptr.798. [DOI] [PubMed] [Google Scholar]
- 11.Pasdaran A, Delazar A, Nazemiyeh H, Nahar L, Sarker SD. Chemical composition, and antibacterial (against staphylococcus aureus) and free-radical-scavenging activities of the essential oils of scrophularia amplexicaulis benth. Rec Nat Prod. 2012;6:350–5. [Google Scholar]
- 12.Pasdaran A, Nahar L, Asnaashari S, Sarker SD, Delazar A. Gc-ms analysis, free-radical-scavenging and insecticidal activities of essential oil of scrophularia oxysepala boiss. Pharm Sci. 2013;19(1):1–5. [Google Scholar]
- 13.Miyazawa M, Okuno Y. Volatile components from the roots of scrophularia ningpoensis hemsl. Flavour Frag J. 2003;18(5):398–400. doi: 10.1002/ffj.1232. [DOI] [Google Scholar]
- 14.Adams R. Quadrupole mass spectra of compounds listed in order of their retention time on db-5. Identification of essential oils components by gas chromatography/quadrupole mass spectroscopy. USA: Allured Publishing Corporation, Carol. Stream, IL; 2001. [Google Scholar]
- 15.Khodaie L, Delazar A, Lotfipour F, Nazemiyeh H. Antioxidant and antimicrobial activity of pedicularis sibthorpii boiss. And pedicularis wilhelmsiana fisch ex. Adv Pharm Bull. 2012;2(1):89–92. doi: 10.5681/apb.2012.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takao T, Kitatani F, Watanabe N, Yagi A, Sakata K. A simple screening method for antioxidants and isolation of several antioxidants produced by marine bacteria from fish and shellfish. Biosci Biotechnol Biochem. 1994;58(10):1780–3. doi: 10.1271/bbb.58.1780. [DOI] [Google Scholar]


