Abstract
Purpose: It is necessary for local anesthetics to pass through the stratum corneum to provide rapid pain relief. Many techniques have been reported to enhance intradermal penetration of local anesthetics such as vesicular lipid carriers. Ethosomes are lipid vesicles containing phospholipids, ethanol at relatively high concentration. We hypothesized that synergistic effects of phospholipids and high concentration of ethanol in formulation could accelerate penetration of nanoethosomes in deep layers of skin.
Methods: Lidocaine-loaded nanoethosomes were prepared and characterized by size and zeta analyzer, scanning electron microscopy (SEM) and X-ray diffractometer (XRD). Furthermore, encapsulation efficiency (EE), loading capacity (LC), and skin penetration capability were evaluated by in vitro and in vivo experiments.
Results: results showed that the particle size, zeta potential, EE and LC of optimum formulation were 105.4 ± 7.9 nm, -33.6 ± 2.4 mV, 40.14 ± 2.5 %, and 8.02 ± 0.71 respectively. SEM results confirmed the non-aggregated nano-scale size of prepared nanoethosomes. Particle size of ethosomes and EE of Lidocaine were depended on the phospholipid and ethanol concentrations. XRD results demonstrated the drug encapsulation in amorphous status interpreting the achieved high drug EE and LC values. In vitro and in vivo assays confirmed the appropriate skin penetration of Lidocaine with the aid of nanoethosomes and existence of deposition of nanoethosomes in deep skin layers, respectively.
Conclusion: The developed nanoethosomes are proposed as a suitable carrier for topical delivery of anesthetics such as Lidocaine.
Keywords: Local anesthetics, Dermal Drug delivery, Ethosome, Lidocaine, Nanoparticle
Introduction
Local anesthetics cause a reversible loss of sensation in a portion of the body, and they reversibly block impulse conduction along nerve axons and other excitable membranes. Anesthesia in skin is useful for relieving pain in procedures involving the skin, such as venipuncture, lumbar puncture, skin biopsy, and hair removal in hirsutism.1,2 Deep analgesia is conventionally achieved through direct intradermal injection of local anesthesia. “Needle anxiety,” however, may cause discontent, especially in pediatric subjects, and can distort the injection site. Therefore, topical formulations may offer significant benefits for preventing procedural pain.3,4 Several problems can arise with conventional topical preparations, e.g., negligible uptake because of the barrier function of the stratum corneum and late onset of action. To overcome these limitations, nanoparticulate drug carriers, particularly lipid-based systems, have been introduced to disrupt and weaken the highly organized intercellular lipids, thereby enhancing intradermal drug penetration, increasing the duration of local action, and preventing systemic absorption of drugs, and thus reducing the side effects associated with the systemic absorption of anesthetics.5-9 Different vesicular systems, especially liposomes, have been studied by numerous investigators. Vesicles are made of one or more bilayers surrounding aqueous spaces and have attracted great interest because of their numerous benefits, such as chemical stability, lower cost, and accessibility of materials. Several studies have focused on the use of liposomes to improve percutaneous delivery; however, because of the absence of deep skin penetration and because liposomes remain limited to the upper layer of the stratum corneum, it is commonly agreed that conventional liposomes are not suitable as carriers for transdermal drug delivery.10-14 Ethosomes, introduced by Touitou et al. in 2000, are modified forms of liposomes which are unilamellar or multilamellar vesicles made of high concentrations (20-45%) of phospholipids and ethanol and have been used for the administration of several drugs. The presence of ethanol, a well-known permeation enhancer, aids ethosomes in permeating deeper into skin layers and entrapping drugs of different physiochemical properties. Ethanol gives them a flexible structure; thus, they can squeeze through the pores in the skin and release drugs into deeper layers.15-17 Lidocaine is an effective and reliable local anesthetic with rapid onset, intermediate action, and moderate systemic toxicity.18,19 It is hypothesized that an ethosomal formulation of Lidocaine will be a suitable carrier for drug encapsulation and rapid and efficient percutaneous penetration. The incorporation of Lidocaine into vesicular systems, which provides efficient drug delivery through skin, may open up a new market for pharmaceutical and cosmetic industries.
Materials and Methods
Materials
Lidocaine was obtained from Darou Pakhsh Pharm. Chem. Co. (Iran). Cholesterol and Rhodamine B were prepared from Merck Company (Germany). Chloroform (Dae-Junge, Korea), methanol (Caledon, Canada), ethanol (Scharlau Chemicals, Spain) and diethyl ether (KianKaveh Pharmaceutical and Chemical Company, Iran) were used as received.
Preparation of Lidocaine-loaded nanoethosomes
Lidocaine-loaded nanoethosomal formulations were prepared according to the modified ethanol injection method.20 Briefly, phosphatidylcholine, cholesterol, and Lidocaine were dissolved in ethanol and injected slowly into the 100 mL aqueous medium under mixing by homogenizer (DIAX 900, Heidolph, Germany) at 20000 rpm.21,22 The composition of different formulations was summarized in Table 1. We also prepared hydroethanolic solution of Lidocaine as a control in the same drug and ethanol amounts of optimized formulation used in skin penetration experiments.
Table 1. Formulation composition of Lidocaine-loaded nanoethosomes.
| Formulation code | Phospholipid (mg) | Ethanol a (mL) | Cholesterol (mg) |
Lidocaine
(mg) |
| F1 | 350 | 10 | - | 35 |
| F2 | 175 | 10 | - | 35 |
| F3 | 175 | 20 | - | 35 |
| F4 | 175 | 40 | - | 35 |
| F5 | 175 | 40 | 35 | 35 |
aThe final volume of formulation was 100 mL.
Characterization of Lidocaine-loaded nanoethosomes
Particle size distribution of formulations was analyzed using dynamic light scattering (DLS) system and reported as intensity-weighted average (z average) and the polydispersity index (PDI), which quantifies size and distribution width, respectively. Zeta potential of prepared nanoethosomes were also analyzed by the same system (Nano ZS, Malvern, UK).20 The morphology of prepared nanoethosomes was obtained using scanning electron microscope (SEM) (MIRA3 TESCAN, UK) operating at 15 kV. The specimens were mounted on a metal stub with double-sided adhesive tape and coated under vacuum with gold (100-150 A°) in an argon atmosphere prior to observation using a direct current sputter technique (EMITECHK450X, England). In order to assess the effect of nanoethosome preparation process on crystallographic patterns of Lidocaine and lipids, XRD analysis was performed using an X-ray diffractometer (D-5000, Siemen, Germany, 2° to 40°) to assess the crystalline structures of Lidocaine, phosphatidylcholine, and vacuum-dried (Binder, Germany) powder of optimized nanoethosomal formulations. The diffraction pattern was measured using a Cu-Kα radiation source (30 mA and 40 kV).
Determination of Entrapment efficiency (EE) and loading capacity (LC)
The EE (%) and LC (%) were expressed as the percentage of entrapped drug to the added drug or to the used lipid, respectively. EE was determined by first separation of the un-entrapped drug by centrifugation method using of Amicon® Ultra-15 (molecular weight cutoff of 100 kDa, Millipore, Germany) tube. The formulation was added to the upper chamber of the Amicon® tube and then the tube was centrifuged (Sigma 3K30, Germany) at 5000 rpm for 15 minutes. The clear solution in the bottom of Amicon® tube was used for Lidocaine determination using a validated HPLC and mathematically calculated according to the following equations:
where, W(Initial drug) is the amount of initial drug used and W(Free drug) is the amount of free drug detected in the lower chamber of Amicon® tube after centrifugation of the nanoethosomal formulations. Accordingly, W(Entrapped drug) is the amount of loaded drug and W(Total lipid) is the amount of used phospholipids and cholesterol in the preparation process.23,24 The formulations in the upper chamber of Amicon® tube were rinsed five times by hydro alcoholic (50%) solution to eliminate unloaded Lidocaine. These rinsed formulations were used for the rest experiments.
Lidocaine was analyzed using a validated reversed-phase HPLC method by Knauer HPLC apparatus (Germany), consisting of a sensitive variable wavelength ultraviolet detector (set at λmax=254 nm) and an ODS column (C18, 10 µm, 4.6 × 250 mm). The samples were eluted using a mobile phase consisting of acetate buffer: acetonitrile (65:35 v/v), adjusted to pH 3.4 with NaOH 1N, at a flow rate of 1 mL/min. At this condition the retention time for Lidocaine was 5.5 min. The calibration curve was linear in the concentration range of 1–50 µg/mL (r2= 0.9998). No interference from the formulation components or skin tissue was observed. The samples were also found to be stable during the study period. The mobile phase was filtered through a 0.45 µm cellulose acetate filter under vacuum and degassed by sonication (Starsonic 35, Italy) in order to protect the column.
In vitro skin permeation and drug deposition studies
Male Wistar rats (weighing 200–250 g) were obtained from Pasteur Institute (Tehran, Iran), housed in animal facilities of the Drug Applied Research Center (Tabriz University of Medical Science) and used for skin permeation and drug deposition studies. All animal experiments were conducted according to the Guide for Care and Use of Laboratory Animals of Tabriz University of Medical Sciences, Tabriz-Iran (National Institutes of Health Publication No 85-23, revised 1985). The abdominal full thickness skin was shaved using an electric razor after sacrificing with excess ether inhalation. The skin was washed and subcutaneous fat was carefully removed. Subsequently, to remove extraneous debris and leachable enzymes, the dermal side of the skin was in contact with a saline solution for 12 h before starting the in vitro skin penetration study. The skins were mounted on the Franz-type diffusion cells with an available diffusion area of 3.8 cm2 and the receptor compartment volume of 25 cm3. The stratum corneum was faced to the donor compartment of the Franz-type diffusion cells (HDT6, Erweka, Germany). Twenty five milliliter of phosphate buffer solution (pH 7.4 and 37 ºC) was used as the receptor medium (stirred with Teflon-coated magnetic stirring bars at 700 rpm) and 200 µL of the formulation was applied on the skin surface in the donor compartment. The donor chamber and the sampling port were covered by parafilm to prevent evaporation during the study. Samples of 250 μL were withdrawn from the receptor compartment through the sampling port of the diffusion cell at determined time intervals (0.5, 1, 2, 4, 6, 12 and 24 h).25 At the end of the experiment, the amounts of drug remaining were quantified. The amount of drug in the withdrawn samples from receptor compartment was also assayed with HPLC apparatus. Each set of experiments was performed in three diffusion cells and was repeated three times in different days.
In vivo studies
Fluorescence measurement allows to follow the distribution of dyes in skin in the investigated time intervals and therefore, lets to study the effect of carrier on skin distribution of Lidocaine. Rhodamine B labeled (dye content=0.001%) Lidocaine-loaded nanoethosomal formulation was prepared in the same concentration of Lidocaine used in in vitro experiment to study the penetration behavior of nanoethosomal formulation by imaging technique.26-28 The unloaded Rhodamine B was removed from the formulation by dialysis through the cellulose acetate membrane (cut off molecular weight 12 kDa, Sigma, USA). 200 µL of the formulation was applied on the skin surface and after 5 h exposure, rats were sacrified with excess ether inhalation and then the skin was cut into 1.5×1.5 cm2 pieces and rinsed with distilled water. Hydroethanolic solution of Rhodamine B was also used as control. Skins were cut into vertical slices with 15 µm thickness by a freeze microtome (Kryomat 1700, Leitz, Germany) and slices were stored at 4 °C and analyzed within 24 h. The skin slices were investigated under both normal light and fluorescence microscopes (BX50, Olympus, Japan). The images were taken from normal light and fluorescence microscope of the same area and the dye distribution in the skin was evaluated qualitatively.29
Results and Discussion
Characterization of Lidocaine-loaded nanoethosomes
The sizes of prepared Lidocaine-loaded nanoethosomes were analyzed and are presented in Figure 1a and Table 2. Particle size and PDI were in the ranges of 105.4–404.2 nm and 0.173–0.274, respectively. The low PDI value (≤0.2) indicated a narrow size distribution. Optimum formulation had a z-average of 105.4 ± 7.9 nm and PDI of 0.245 ± 0.07. Hopefully, the zeta potential value of the optimized formulation (Figure 1b) will be less than -30 (-33.6 mV), providing a suitable situation for colloidal stability of suspended nanovesicules.30 SEM images of the nanoparticles were well-matched with data regarding size, confirming the narrow size distribution of nanoethosomes (Figure 1c). Particle size analysis results showed that nanoethosome size depended on phospholipid and ethanol concentrations. In the same ethanol concentration, nanoethosome size decreased (from 165.2 nm to 105.4 nm) with decreasing concentrations of phospholipid. Controversy exists regarding the role of lipid concentration in vesicle size. Some articles have reported that vesicle size was increased by increased lipid concentrations, and a few have claimed that increases in lipid concentration decreased vesicle size.15,21,31,32 Increasing the ethanol concentration from 10% to 40% resulted in the production of nanoethosomes with a higher particle size (vesicle size was increased from 105.4 ± 7.9 nm to 404.2 ± 15.6 nm). It was assumed that increasing the ethanol concentration disrupted the vesicle membrane and subsequently increased vesicle size. Cholesterol was added to increase the rigidity of the membrane, which decreased particle size from 404.2±15.6 nm to 239.9 ± 13.5 nm. Contrary results have been reported regarding the role of cholesterol in the size of vesicular systems. Some investigations have indicated that the incorporation of cholesterol increased size, and a few articles have reported that cholesterol had a reducing impact.31,33-36
Figure 1.
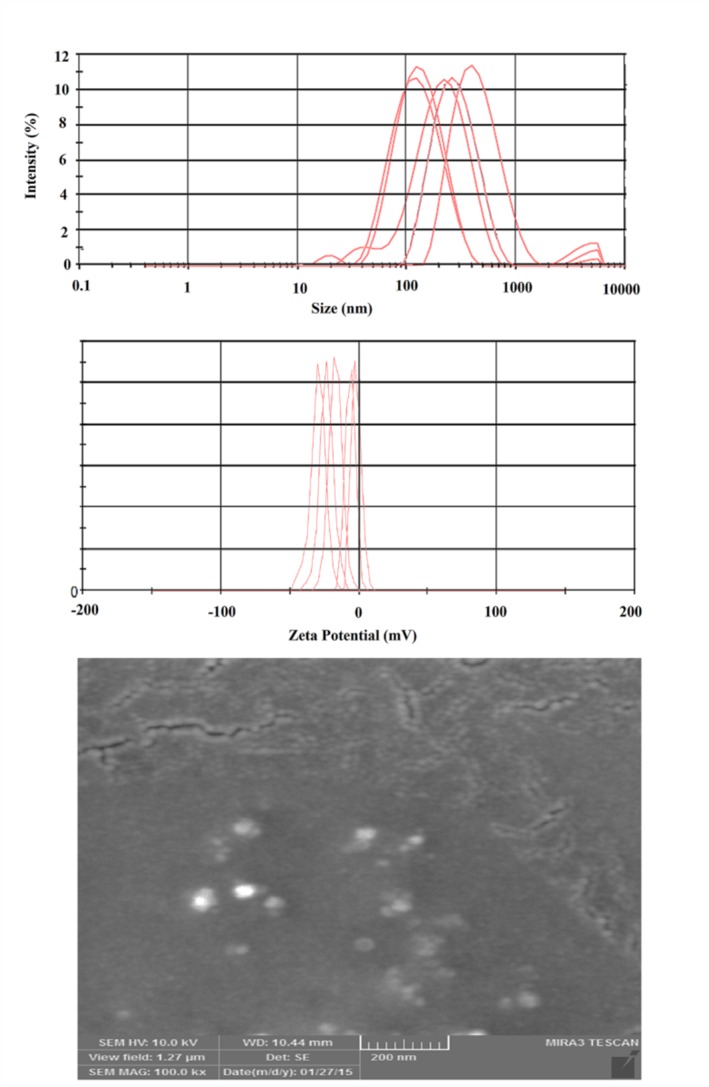
Particle size distributions (top), zeta potential values (middle), and scanning electron microscopy images (down) of Lidocaine-loaded nanoethosomal formulations.
Table 2. Particle size, polydispersity index, encapsulation efficiency and loading capacity of nanoethosomal formulations. The results of encapsulation efficiency and loading capacity values are presented in mean ± standard deviation (n=at least 3).
| Formulation code | Size (nm) | Polydispersity index | Zeta potential (mV) | EE a (%) | LC b (%) |
| F1 | 165.2 ± 6.3 | 0.27 | -23.5 ± 2.6 | 66.5 ± 4.3 | 5.4 ± 0.8 |
| F2 | 105.4 ± 7.9 | 0.25 | -33.6 ± 2.9 | 40.1 ±2.5 | 8.0 ± 0.71 |
| F3 | 121.2 ± 06.9 | 0.24 | -16.9 ± 1.9 | 18.7 ± 1.9 | 3.7 ± 0.6 |
| F4 | 404.2 ± 25.3 | 0.26 | -2.15 ± 0.9 | 9.7 ± 1.2 | 1.9 ± 0.3 |
| F5 | 239.9 ± 11.6 | 0.17 | -6.45 ± 0.8 | 12.6 ± 2.0 | 2.5 ± 0.3 |
aEncapsulation Efficiency
bLoading Capacity
As Table 2 indicates, EE (%) and LC (%) values of nanoethosomal formulations were affected by formulation compositions ranging from 10-66% and 2-8%, respectively. The optimum formulation showed EE (%) and LC (%) values of 40.1 and 8.0, respectively. By increasing the ethanol content from 10% to 40%, EE and LC values were reduced from 40.1% to 9.7% and from 8.0% to 1.9%, respectively. These findings indicate that ethanol has a negative effect on Lidocaine loading in nanoethosomal formulations. As expected from the results of previous studies,12,13,37,38 the addition of cholesterol to the formulation increased both EE and LC values. This could be explained by the impact of cholesterol on the rigidity of the bilayer structure of vesicles. Although ethanol has been reported to increase the membrane fluidity of ethosomes, ethanol concentrations >45% (w/w) have been shown to decrease the value of EE, perhaps because the membrane may leak in the presence of high ethanol concentrations. Thus, ethanol concentrations in ethosomes should be controlled within a certain range.39-41
XRD study
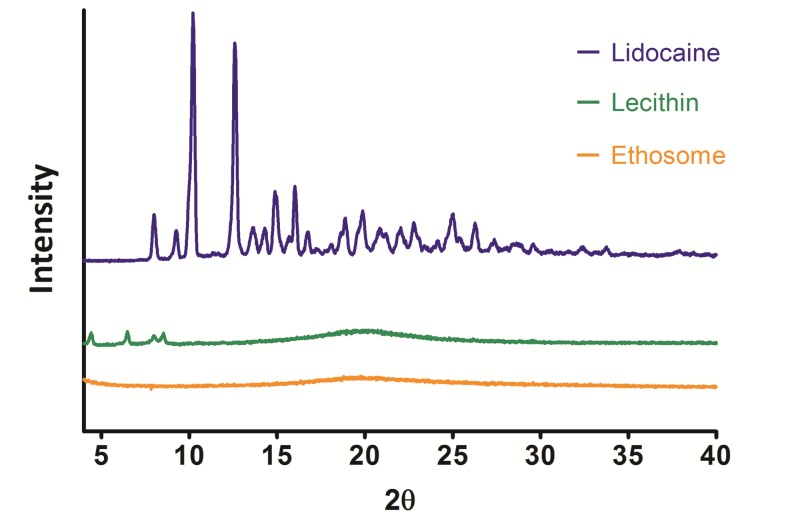
The diffraction pattern of Lidocaine analyzed by the X-ray diffraction method demonstrated several sharp peaks, indicating the crystalline nature of Lidocaine (Figure 2). The characteristic main peaks of Lidocaine were absent in the XRD pattern of the optimized nanoethosomal formulation (F2), suggesting that Lidocaine was uniformly and molecularly dispersed in the nanoethosomes lipid bilayers. This finding supports the explanation of observed high Lidocaine-loading capacity (around 8%) for nanoethosomes. The diffraction pattern of phosphatidylcholine (between 4-10 2θ) also disappeared in the nanoethosomal formulation. This shows that phosphatidylcholine in Lidocaine-loaded nanoethosomes was formed to some extent in the less-ordered crystals. It seems that the drug molecules can be accommodated in between lipid layers, which can increase drug loading and reduce expulsion during storage time.42
Figure 2.
The X-ray diffractions pattern of Lidocaine, Lecithin, and optimized Lidocaine-loaded nanoethosomal formulation.
In vitro drug permeation study
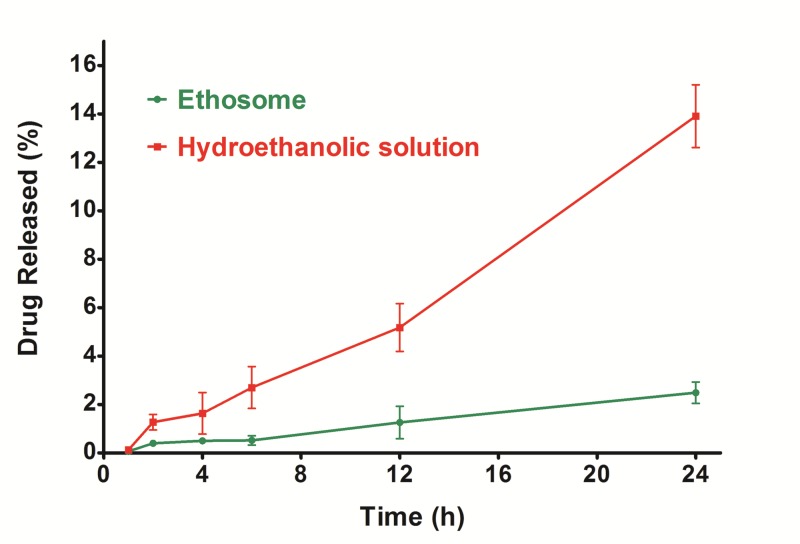
The cumulative percentage of permeated Lidocaine from the optimized nanoethosomal formulation and hydroethanolic solution through exercised rat skin was investigated using Franz diffusion cell for a period of 24 h. As shown in Figure 3, the ratio of drug passed through the skin to the receptor compartment of the Franz diffusion cell was diminished by the encapsulation of Lidocaine in nanoethosomes. Lidocaine itself permeated better than the nanoethosomal formulation. This might be correlated to the characteristics of Lidocaine (molecular weight = 234.34 and Log P = 2.6) which are suitable for skin penetration (MW<500 and Log P 1-4).43,44 Furthermore, nanoethosomes directed only 2.5% of Lidocaine to the receiver compartment of the Franz cell, which is a simulator for systemic drug absorption. Therefore, the ethosomal formulation could be successfully applied for topical drug therapy avoiding systemic drug absorption. This would be ideal for the delivery of drugs such as analgesics, especially for those which may cause adverse systemic side effects, such as Lidocaine. The amounts of unpenetrated Lidocaine remaining on the skin for the hydroethanolic solution and nanoethosomal formulation (F2) were calculated as 18.3 ± 1.9 and 3.5 ± 0.8, respectively, indicating the superiority of nanoethosomes in skin penetration accompanied by skin accumulation of Lidocaine.
Figure 3.
Passed Lidocaine through the stratum corneum from Lidocaine-loaded nanoethosomal formulations and hydro hydroethanolic, determined in vitro on exercised rat skin by Franz diffusion cells.
Although the exact mechanism for superior permeation into deeper skin layers from ethosomes is still not clear, the synergistic effects of a mixture of phospholipids and high concentrations of ethanol in vesicular systems have been suggested to be responsible for deeper distribution and penetration in the skin’s lipid bilayers. The high concentration of ethanol makes the ethosomes unique, which disturbs skin lipid bilayer organization; therefore, when incorporated into a vesicle membrane, it enhances the vesicle’s ability to penetrate the stratum corneum. Furthermore, because of the high ethanol concentration, the lipid membrane is packed less tightly than conventional vesicles, but has equivalent stability, resulting in a more flexible structure and improving the drug distribution ability in stratum corneum lipids.45
In vivo experiment
Rhodamine B has almost the same physicochemical structure (MW = 479.02 and log P = 2.43) as Lidocaine (MW = 234.34 and Log P = 2.6), and both are suitable for skin penetration (MW<500 and Log P 1-4). Therefore, it would be a good model for evaluating the ability of nanoethosomes to penetrate skin and distribute Lidocaine. Figure 4 shows that nanoethosomes could pass Rhodamine B through the stratum corneum to the lower layers of the epidermis, while Rhodamine B in hydroethanolic solution remained mostly on the stratum corneum. These findings verified the in vitro skin penetration study results.
Figure 4.
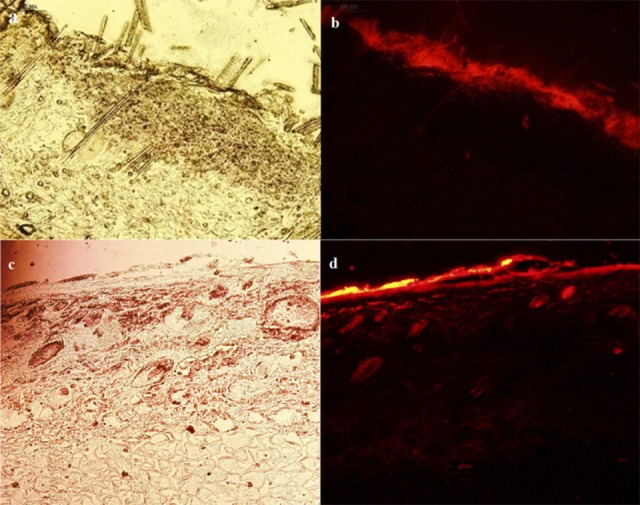
Rhodamine B penetration into rat skin: staining of rat skin following the application of 0.001% Rhodamine B-loaded hydroethanolic solution (a: light microscopic image, b: fluorescent microscopic image) and nanoethosomal (c: light microscopic image, d: fluorescent microscopic image) formulation after 5 h.
Conclusion
Optimization of the nanoethosomal formulation by changes in structural compositions resulted in appropriate characteristics, including small particle size and good encapsulation efficiency, suggesting that nanoethosomes could be used as a carrier of Lidocaine. In vitro studies of Lidocaine skin permeation from nanoethosomes and hydroethanolic solution showed that a higher percentage of drug reached the skin and a lower percentage reached the receiver medium of the Franz cell from nanoethosomes than from hydroethanolic Lidocaine solution, indicating the superiority of nanoethosomes for the dermal delivery of Lidocaine. The in vivo study confirmed the results of the in vitro study by tracking the fluorescent-labeled nanoethosomes in rat skin. The results of this study may pave a way for a novel commercial product for efficient local pain management.
Acknowledgments
This paper was extracted from M.Sc. thesis (No. 92/2-6/3) that was submitted to the Faculty of Advanced Medical Science of Tabriz University of Medical Sciences and financially supported by Drug Applied Research Center of the same university.
Ethical Issues
Not applicable.
Conflict of Interest
The authors report no conflicts of interest.
References
- 1.de Araujo DR, da Silva DC, Barbosa RM, Franz-Montan M, Cereda CM, Padula C. et al. Strategies for delivering local anesthetics to the skin: Focus on liposomes, solid lipid nanoparticles, hydrogels and patches. Expert Opin Drug Deliv. 2013;10(11):1551–63. doi: 10.1517/17425247.2013.828031. [DOI] [PubMed] [Google Scholar]
- 2.Latzke D, Marhofer P, Kettner SC, Koppatz K, Turnheim K, Lackner E. et al. Pharmacokinetics of the local anesthetic ropivacaine after transversus abdominis plane block in healthy volunteers. Eur J Clin Pharmacol. 2012;68(4):419–25. doi: 10.1007/s00228-011-1139-8. [DOI] [PubMed] [Google Scholar]
- 3.Deguzman ZC, O'Mara SK, Sulo S, Haines T, Blackburn L, Corazza J. Bacteriostatic normal saline compared with buffered 1% lidocaine when injected intradermally as a local anesthetic to reduce pain during intravenous catheter insertion. J Perianesth Nurs. 2012;27(6):399–407. doi: 10.1016/j.jopan.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Morris R, McKay W, Mushlin P. Comparison of pain associated with intradermal and subcutaneous infiltration with various local anesthetic solutions. Anesth Analg. 1987;66(11):1180–2. [PubMed] [Google Scholar]
- 5.Prow TW, Grice JE, Lin LL, Faye R, Butler M, Becker W. et al. Nanoparticles and microparticles for skin drug delivery. Adv Drug Deliv Rev. 2011;63(6):470–91. doi: 10.1016/j.addr.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Heuschkel S, Goebel A, Neubert RH. Microemulsions--modern colloidal carrier for dermal and transdermal drug delivery. J Pharm Sci. 2008;97(2):603–31. doi: 10.1002/jps.20995. [DOI] [PubMed] [Google Scholar]
- 7.Mathur V, Satrawala Y, Rajput MS. Physical and chemical penetration enhancers in transdermal drug delivery system. Asian J pharm. 2010;4(3):173. [Google Scholar]
- 8.Gupta M, Vyas SP. Development, characterization and in vivo assessment of effective lipidic nanoparticles for dermal delivery of fluconazole against cutaneous candidiasis. Chem Phys Lipids. 2012;165(4):454–61. doi: 10.1016/j.chemphyslip.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 9. Ghanbarzadeh S, Khorrami A, Arami S. Nonionic surfactant-based vesicular system for transdermal drug delivery. Drug Deliv 2015:1-7. [DOI] [PubMed]
- 10.Samad A, Sultana Y, Aqil M. Liposomal drug delivery systems: An update review. Curr Drug Deliv. 2007;4(4):297–305. doi: 10.2174/156720107782151269. [DOI] [PubMed] [Google Scholar]
- 11.Choi M, Maibach H. Liposomes and niosomes as topical drug delivery systems. Skin Pharmacol Phys. 2005;18(5):209–19. doi: 10.1159/000086666. [DOI] [PubMed] [Google Scholar]
- 12.Ghanbarzadeh S, Arami S. Formulation and evaluation of piroxicam transferosomal gel: An approach for penetration enhancement. J Drug Deliv Sci Technol. 2013;23(6):587–90. doi: 10.1016/S1773-2247(13)50089-X. [DOI] [Google Scholar]
- 13.Ghanbarzadeh S, Arami S. Enhanced transdermal delivery of diclofenac sodium via conventional liposomes, ethosomes, and transfersomes. Biomed Res Int. 2013;2013:616810. doi: 10.1155/2013/616810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghanbarzadeh S, Khorrami A, Arami S. Preparation of optimized naproxen nano liposomes using response surface methodology. J Pharm Invest. 2014;44(1):33–9. doi: 10.1007/s40005-013-0098-8. [DOI] [Google Scholar]
- 15.Touitou E, Dayan N, Bergelson L, Godin B, Eliaz M. Ethosomes—novel vesicular carriers for enhanced delivery: Characterization and skin penetration properties. J Control Release. 2000;65(3):403–18. doi: 10.1016/S0168-3659(99)00222-9. [DOI] [PubMed] [Google Scholar]
- 16.Touitou E, Godin B, Weiss C. Enhanced delivery of drugs into and across the skin by ethosomal carriers. Drug Dev Res. 2000;50(3‐4):406–15. doi: 10.1002/1098-2299(200007/08)50:3. [DOI] [Google Scholar]
- 17.Elsayed MM, Abdallah OY, Naggar VF, Khalafallah NM. Lipid vesicles for skin delivery of drugs: Reviewing three decades of research. Int J Pharm. 2007;332(1-2):1–16. doi: 10.1016/j.ijpharm.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Wallace MS, Ridgeway B, Jun E, Schulteis G, Rabussay D, Zhang L. Topical delivery of lidocaine in healthy volunteers by electroporation, electroincorporation, or iontophoresis: An evaluation of skin anesthesia. Reg Anesth Pain Med. 2001;26(3):229–38. doi: 10.1053/rapm.2001.22633. [DOI] [PubMed] [Google Scholar]
- 19.Glavas-Dodov M, Goracinova K, Mladenovska K, Fredro-Kumbaradzi E. Release profile of lidocaine hcl from topical liposomal gel formulation. Int J Pharm. 2002;242(1-2):381–4. doi: 10.1016/S0378-5173(02)00221-1. [DOI] [PubMed] [Google Scholar]
- 20.Rasaee S, Ghanbarzadeh S, Mohammadi M, Hamishehkar H. Nano phytosomes of quercetin: A promising formulation for fortification of food products with antioxidants. Pharm Sci. 2015;20(3):96–101. [Google Scholar]
- 21.Ghanbarzadeh S, Valizadeh H, Zakeri-Milani P. The effects of lyophilization on the physico-chemical stability of sirolimus liposomes. Adv Pharm Bull. 2013;3(1):25–9. doi: 10.5681/apb.2013.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghanbarzadeh S, Valizadeh H, Zakeri-Milani P. Application of factorial designs and response surface methodology in formulation development of sirolimus liposome prepared by thin film hydration technique. BioImpacts. 2013;3(2):75–81. doi: 10.5681/bi.2013.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghaderi S, Ghanbarzadeh S, Hamishehkar H. Evaluation of different methods to produce nanoparticle containing gammaoryzanol for potential use in food fortification. Pharm Sci. 2015;20(4):130–4. [Google Scholar]
- 24.Ghanbarzadeh S, Hariri R, Kouhsoltani M, Shokri J, Javadzadeh Y, Hamishehkar H. Enhanced stability and dermal delivery of hydroquinone using solid lipid nanoparticles. Colloids Surf B Biointerfaces. 2015;136:1004–10. doi: 10.1016/j.colsurfb.2015.10.041. [DOI] [PubMed] [Google Scholar]
- 25.Hamishehkar H, Shokri J, Fallahi S, Jahangiri A, Ghanbarzadeh S, Kouhsoltani M. Histopathological evaluation of caffeine-loaded solid lipid nanoparticles in efficient treatment of cellulite. Drug Dev Ind Pharm. 2015;41(10):1640–6. doi: 10.3109/03639045.2014.980426. [DOI] [PubMed] [Google Scholar]
- 26.Trauer S, Richter H, Kuntsche J, Buttemeyer R, Liebsch M, Linscheid M. et al. Influence of massage and occlusion on the ex vivo skin penetration of rigid liposomes and invasomes. Eur J Pharm Biopharm. 2014;86(2):301–6. doi: 10.1016/j.ejpb.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Subongkot T, Wonglertnirant N, Songprakhon P, Rojanarata T, Opanasopit P, Ngawhirunpat T. Visualization of ultradeformable liposomes penetration pathways and their skin interaction by confocal laser scanning microscopy. Int J Pharm. 2013;441(1-2):151–61. doi: 10.1016/j.ijpharm.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 28.Raphael AP, Meliga SC, Chen X, Fernando GJ, Flaim C, Kendall MA. Depth-resolved characterization of diffusion properties within and across minimally-perturbed skin layers. J Control Release. 2013;166(2):87–94. doi: 10.1016/j.jconrel.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 29. Hamishehkar H, Ghanbarzadeh S, Sepehran S, Javadzadeh Y, Adib ZM, Kouhsoltani M. Histological assessment of follicular delivery of flutamide by solid lipid nanoparticles: Potential tool for the treatment of androgenic alopecia. Drug Dev Ind Pharm 2015:1-8. [DOI] [PubMed]
- 30. Tarţău L, Lupusoru R, Bindar D, Melnig V. Experimental research on the effects of nano-vesicles encapsulating ketoprofen in a visceral pain model in mice. Therapeut Pharm Clin Toxicol 2010;14(2).
- 31.Mayer LD, Tai LC, Ko DS, Masin D, Ginsberg RS, Cullis PR. et al. Influence of vesicle size, lipid composition, and drug-to-lipid ratio on the biological activity of liposomal doxorubicin in mice. Cancer Res. 1989;49(21):5922–30. [PubMed] [Google Scholar]
- 32.Johnson SM. The effect of charge and cholesterol on the size and thickness of sonicated phospholipid vesicles. Biochim Biophys Acta. 1973;307(1):27–41. doi: 10.1016/0005-2736(73)90022-9. [DOI] [PubMed] [Google Scholar]
- 33.Szoka F, Jr., Papahadjopoulos D. Comparative properties and methods of preparation of lipid vesicles (liposomes) Annu Rev Biophys Bioeng. 1980;9:467–508. doi: 10.1146/annurev.bb.09.060180.002343. [DOI] [PubMed] [Google Scholar]
- 34.Newman GC, Huang C-H. Structural studies on phosphatidylcholine-cholesterol mixed vesicles. Biochemistry (Mosc) 1975;14(15):3363–70. doi: 10.1021/bi00686a012. [DOI] [PubMed] [Google Scholar]
- 35.Manosroi A, Wongtrakul P, Manosroi J, Sakai H, Sugawara F, Yuasa M. et al. Characterization of vesicles prepared with various non-ionic surfactants mixed with cholesterol. Colloids Surf B Biointerfaces. 2003;30(1):129–38. doi: 10.1016/S0927-7765(03)00080-8. [DOI] [Google Scholar]
- 36.Kuang Y, Liu J, Liu Z, Zhuo R. Cholesterol-based anionic long-circulating cisplatin liposomes with reduced renal toxicity. Biomaterials. 2012;33(5):1596–606. doi: 10.1016/j.biomaterials.2011.10.081. [DOI] [PubMed] [Google Scholar]
- 37.Glavas-Dodov M, Fredro-Kumbaradzi E, Goracinova K, Simonoska M, Calis S, Trajkovic-Jolevska S. et al. The effects of lyophilization on the stability of liposomes containing 5-fu. Int J Pharm. 2005;291(1-2):79–86. doi: 10.1016/j.ijpharm.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 38.Hao Y, Zhao F, Li N, Yang Y, Li K. Studies on a high encapsulation of colchicine by a niosome system. Int J Pharm. 2002;244(1-2):73–80. doi: 10.1016/s0378-5173(02)00301-0. [DOI] [PubMed] [Google Scholar]
- 39.Mbah CC, Builders PF, Attama AA. Nanovesicular carriers as alternative drug delivery systems: Ethosomes in focus. Expert Opin Drug Deliv. 2014;11(1):45–59. doi: 10.1517/17425247.2013.860130. [DOI] [PubMed] [Google Scholar]
- 40.David SRN, Hui MS, Pin CF, Ci FY, Rajabalaya R. Formulation and in vitro evaluation of ethosomes as vesicular carrier for enhanced topical delivery of isotretinoin. Int J Drug Deliv. 2013;5(1):28. [Google Scholar]
- 41.Chandel A, Patil V, Goyal R, Dhamija H, Parashar B. Ethosomes: A novel approach towards transdermal drug delivery. J Pharm Chem Sci. 2012;1(2):563–9. [Google Scholar]
- 42.Li G, Fan C, Li X, Fan Y, Wang X, Li M. et al. Preparation and in vitro evaluation of tacrolimus-loaded ethosomes. Scientific World Journal. 2012;2012:874053. doi: 10.1100/2012/874053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kang L, Yap CW, Lim PF, Chen YZ, Ho PC, Chan YW. et al. Formulation development of transdermal dosage forms: Quantitative structure-activity relationship model for predicting activities of terpenes that enhance drug penetration through human skin. J Control Release. 2007;120(3):211–9. doi: 10.1016/j.jconrel.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 44.Cronin MT, Dearden JC, Moss GP, Murray-Dickson G. Investigation of the mechanism of flux across human skin in vitro by quantitative structure-permeability relationships. Eur J Pharm Sci. 1999;7(4):325–30. doi: 10.1016/S0928-0987(98)00041-4. [DOI] [PubMed] [Google Scholar]
- 45.Singh SY, Aher SS, Saudagar RB. Ethosomes–novel drug delivery system. Res J Topica Cosmet Sci. 2015;6(1):7–14. doi: 10.5958/2321-5844.2015.00002.3. [DOI] [Google Scholar]