Abstract
Purpose: Capsaicin (8-methyl-N-vanillyl-6-nonenamide) is a major pungent compound found in hot peppers of the plant genus Capsicum. In vitro effects of dietary capsaicin on redox status in red blood cells during human aging have been explored.
Methods: Total antioxidant potential of capsaicin was evaluated using Ferric reducing ability of plasma (FRAP) assay. GSH was measured as per standard protocol. The in vitro effect of capsaicin was evaluated by incubation of the cells in the assay medium with 10-5M capsaicin (final concentration) for 60 min at 37°C.
Results: Treatment with capsaicin (10-5M) caused a significant (p < 0.01) increase in GSH level in all age groups. Reduced glutathione (GSH) / Oxidized glutathione (GSSG) ratio measures the redox status of the red blood cell. Significant increase in GSH level due to capsaicin, shift the GSH/GSSG ratio, thus alters the redox status of the cell.
Conclusion: The results conclusively prove the efficacy of the antioxidant property of capsaicin and its role in modulating the redox status of red blood cells. This evidence suggests that dietary factors that act as antioxidants to increase GSH level may contribute to a protective effect against age related diseases. This antioxidant effect may, in part, explain the high consumption of capsicum in certain regions of the world.
Keywords: Capsaicin, Aging, Erythrocyte, Redox status, Glutathione, Human
Introduction
Capsaicin is a naturally occurring alkaloid derived from plants of the genus Capsicum, better known as chili pepper fruit. It belongs to vanilloid family of compounds.1 Capsaicin is absorbed by a non active process from the stomach and whole intestine. Maximum blood concentration is seen after 1 hour of administration.2 Capsaicin has been reported in the activation of erythrocyte membrane Na/K and calcium adenosine triphosphatases;3 treatment of post herpetic neuralgia;4 protection of lipid peroxidation, carbonyl formation5 and induced apoptosis in transformed cells6 but effect of capsaicin on redox regulation in red blood cells are not extensively investigated.
Oxidative stress plays a crucial role in the development of the aging process and age dependent diseases.7 Aging is the accumulation process of diverse detrimental changes in the cells and tissues with advancement of age which results, an increase in the risks of disease and death.8 Glutathione is a tripeptide and play a very important role during aging and age related diseases.9 The rate of GSH synthesis depends on the availability of L-cysteine, its influx and efflux plays a crucial role during aging.10,11 GSH is readily oxidized by free radicals and reactive oxygen and nitrogen species to glutathione disulfide (GSSG). GSSG efflux from cells contributes to a net loss of intracellular GSH.12 Due to its high intracellular concentrations; GSH variations in oxidation states can significantly modify the redox environment of red blood cells. Within the erythrocyte, GSH may act as an antioxidant in scavenging reactive oxygen or nitrogen species, as a reductant to maintain protein thiols in a reduced state, or, conversely, as an adjunct in forming mixed disulfides with proteins.9 In recent study, we have shown the protective effect of dietary flavonoids during aging.13 This study was undertaken to evaluate the antioxidant potential of capsaicin and in vitro effect on erythrocyte GSH in view of its importance in the regulation of redox status.
Materials and Methods
Materials
Capsaicin (8-methyl-N-vanillyl-6-nonenamide), TPTZ (2,4,6- tri[2-pyridyl]-s-triazine), and DTNB (5, 5’-dithiobis-2-nitrobenzoic acid) was purchased from Sigma chemical Co. (St. Louis, MO, USA). All other chemicals used were of analytical grade.
Blood collection and isolation of erythrocytes
The study was carried out on total 65 healthy subjects of both sexes. They were divided into three groups (a) Young (n =25) - 20 to 35 years of age, (b) Middle (n = 20) - 36 to 60 years of age and (c) Old (n = 20) - more than 60 years of age. The criteria for selection of subjects were the same as described earlier.10 All persons gave their informed consent for the use of their blood samples for the study. The protocol of study was in conformity with the guidelines of the Institutional Ethical Committee. Human venous blood samples from different healthy volunteers were obtained by venipuncture in heparin. The blood was centrifuged at 1800 x g for 10 min at 4°C. After removal of plasma, buffy coat, the RBCs were washed twice with cold PBS. Washed packed red blood cells were used for GSH estimation and in vitro experiments with capsaicin.
Determination of total antioxidant potential of Capsaicin
The Ferric Reducing Ability of Plasma (FRAP) values were determined, following the method of Benzie & Strain.14 Briefly, 3 mL of FRAP reagent was mixed with 100 µL of capsaicin of different concentration (10-6 to 10-3M); the contents were mixed vigorously for making uniform solution. The absorbance was read at 593 nm at the interval of 30 seconds for 4 minutes.
Determination of erythrocyte GSH
Erythrocyte GSH was measured following the method of Beutler.15 The methods were based on the ability of the sulfhydryl group to reduce 5, 5’-dithiobis-2-nitrobenzoic acid (DTNB) and form a yellow colored anionic product whose optical density was measured at 412 nm. Concentration of GSH was expressed in mg per mL packed red blood cells and was determined from standard plot.
In vitro experiment with capsaicin
The effect of capsaicin on erythrocyte GSH was studied, following the procedure used in earlier studies.5 The in vitro effect of capsaicin was evaluated by incubating the assay medium in the presence of 10-5M (final concentration) of it for 60 min at 37°C. The concentration of capsaicin is in accordance with the results of the previous studies.5
Statistical Analysis
All data analysis was performed on GraphPad Prism version 5.00 for Windows, GraphPad Software (San Diego CA). Results are expressed as mean ± SD. The paired t test and Pearson correlation coefficient were used to identify significant relationship between variables. P value less than or equal to 0.05 was considered statistically significant.
Results and Discussion
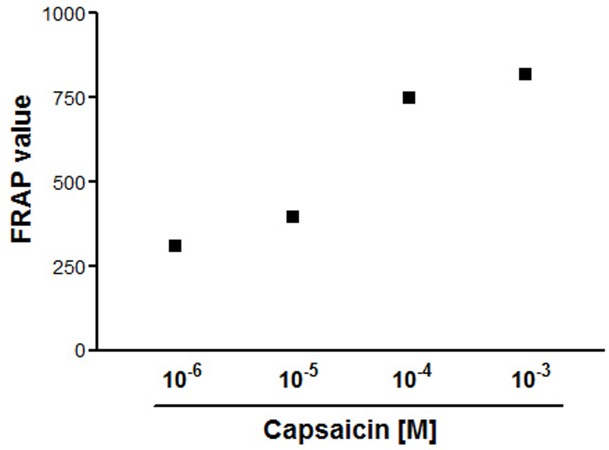
Figure 1 shows total antioxidant potential of capsaicin at different concentration range (10-6 M to 10-3M) in terms of FRAP values. Age-dependent decline in intracellular reduced glutathione (GSH) concentration is shown in Figure 2. Figure 3 represents in vitro effect of capsaicin (final concentration 10-5M) on GSH level in young, middle and old age groups. Results shows a significant (p = 0.0096; 0.0017; 0.0187) increased GSH level in young, middle and old age groups respectively.
Figure 1.
Total antioxidant potential of capsaicin (10-6 M to 10-3M) in terms of Ferric reducing ability of the plasma (FRAP values). FRAP values expressed as µmol Fe (II) per liter of capsaicin.
Figure 2.
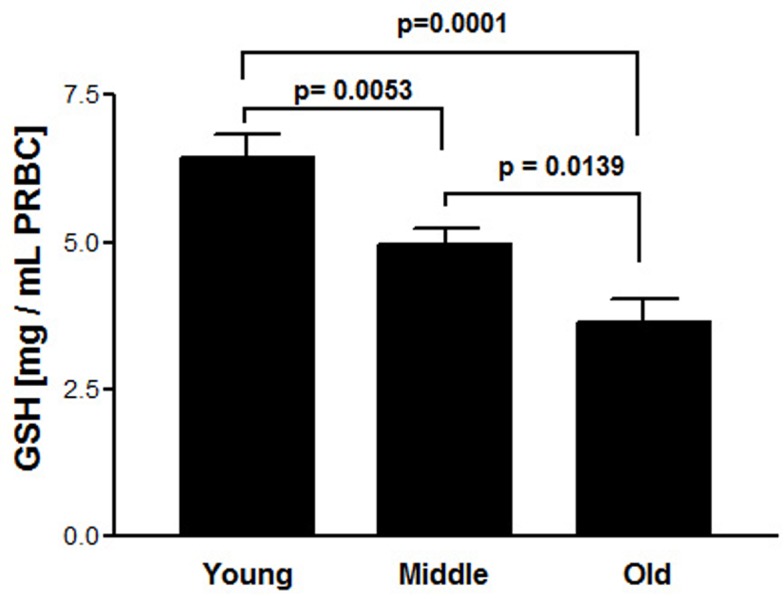
Erythrocytes reduced glutathione in different age groups. GSH level in young were statistically significant (p = 0.0053; r = 0.2924) with middle. A significant correlation (r = 0.5635) was observed between young and old. A significant correlation (r = 0.2924) was observed between middle and old age groups. Concentration of GSH is expressed in mg per mL packed erythrocytes.
Figure 3.
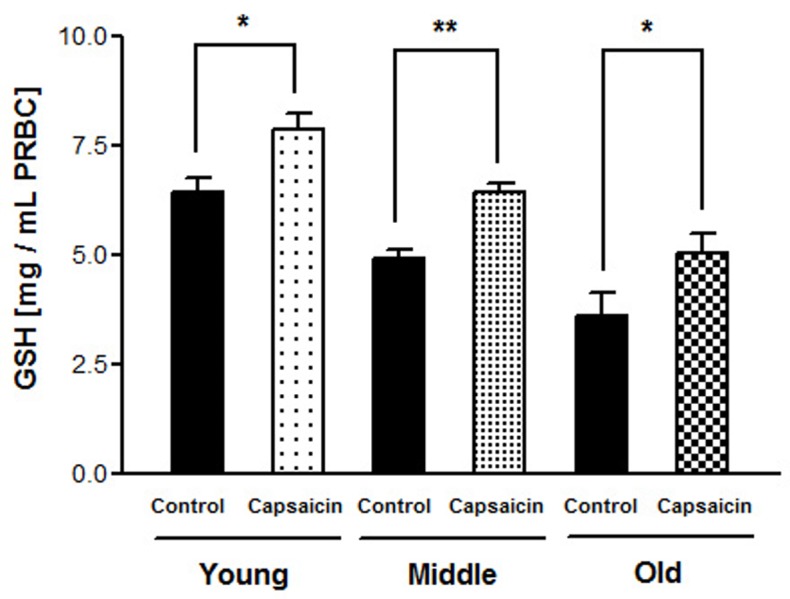
Effect of capsaicin (10-5M) on erythrocyte GSH level in different age groups. Young (p = 0.0187; r = 0.2707); middle (p = 0.0017; r = 0.3010) and old age group (p = 0.0096; r = 0.3181). **p < 0.001; *p < 0.01.
Capsaicin, a highly potent active ingredient of hot chili pepper, has been extensively studied in vivo and in vitro. The neuronal effects of capsaicin are detectable at nanomolar concentrations; while non-neuronal effects require micromolar concentrations.16 Our findings indicate concentration dependent increase in total antioxidant potential. Increased level of GSH due to capsaicin may be explained on its incorporation into the cell membrane which results in altered membrane fluidity. Altered cell membrane permiability induces several other metabolic pathways that result in increased biosynthesis of GSH. Ratio of GSH / GSSG reflects the redox status of the cell, increased GSH level will results, shift in GSH/GSSG ratio, thus modulating the redox status. Our recent findings on shift in GSH/GSSG ratio during human aging also support the above mentioned findings.10 Capsaicin has been shown to incorporate itself into phospholipids membrane and align with phospholipid acyl chain, perturbing the packing of lipid and affecting thermotropic properties.17 The capsaicin induced increase in the activity of the erythrocyte membrane enzymes: Na+/K+-ATPase and Ca2+-ATPase have been reported.3 The exact mechanism underlying the anti-aging effects of capsaicin is unclear.
The erythrocyte redox environment contributes to the increased oxidative stress during aging. GSH is the most abundant low-molecular weight thiol and the principal thiol redox buffer in erythrocytes.12 The red blood cell contributes up to 10% of whole-body GSH synthesis in humans. GSH has many biological functions, including maintenance of membrane protein –SH groups in the reduced form, the oxidation of which can otherwise cause altered cellular structure and function. The GSH/GSSG redox, function in redox signaling and control as well as antioxidant protection, the thiol/disulfide redox states may provide central parameters to link environmental influences and progression of changes associated with aging.
Conclusion
Our findings on the influence of capsaicin on human erythrocyte GSH signify the importance of capsaicin in redox regulation during aging. There is need for evaluating the physiological impact of high capsaicin (capsicum) consumption in some regions of the world. The results presented here could also be useful for the understanding of the non-specific effects of capsaicin during human aging. Further research is needed to understand the mechanism(s) of the action of capsaicin in its nonselective effects on non-neuronal cells in aging and age related diseases.
Ethical Issues
The protocol of study was in conformity with the guidelines of the Amity University Uttar Pradesh Ethical Committee.
Conflict of Interest
The authors report no conflicts of interest.
References
- 1.Hayman M, Kam P. Capsaicin: a review of its pharmacology and clinical applications. Curr Anaesth Crit Care. 2008;19:338–43. doi: 10.1016/j.cacc.2008.07.003. [DOI] [Google Scholar]
- 2.Suresh D, Srinivasan K. Tissue distribution & elimination of capsaicin, piperine & curcumin following oral intake in rats. Indian J Med Res. 2010;131:682–91. [PubMed] [Google Scholar]
- 3.Rizvi SI, Luqman S. Capsaicin-induced activation of erythrocyte membrane sodium/potassium and calcium adenosine triphosphatases. Cell Mol Biol Lett. 2003;8(4):919–25. [PubMed] [Google Scholar]
- 4.Irving GA, Backonja MM, Dunteman E, Blonsky ER, Vanhove GF, Lu SP. et al. A multicenter, randomized, double-blind, controlled study of ngx-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia. Pain Med. 2011;12(1):99–109. doi: 10.1111/j.1526-4637.2010.01004.x. [DOI] [PubMed] [Google Scholar]
- 5.Luqman S, Rizvi SI. Protection of lipid peroxidation and carbonyl formation in proteins by capsaicin in human erythrocytes subjected to oxidative stress. Phytother Res. 2006;20(4):303–6. doi: 10.1002/ptr.1861. [DOI] [PubMed] [Google Scholar]
- 6.Macho A, Calzado MA, Munoz-Blanco J, Gomez-Diaz C, Gajate C, Mollinedo F. et al. Selective induction of apoptosis by capsaicin in transformed cells: The role of reactive oxygen species and calcium. Cell Death Differ. 1999;6(2):155–65. doi: 10.1038/sj.cdd.4400465. [DOI] [PubMed] [Google Scholar]
- 7.Maurya PK, Rizvi SI. Alterations in plasma nitric oxide during aging in humans. Indian J Biochem Biophys. 2009;46(1):130–2. [PubMed] [Google Scholar]
- 8.Rebrin I, Sohal RS. Pro-oxidant shift in glutathione redox state during aging. Adv Drug Deliv Rev. 2008;60(13-14):1545–52. doi: 10.1016/j.addr.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar P, Maurya PK. L-cysteine efflux in erythrocytes as a function of human age: Correlation with reduced glutathione and total anti-oxidant potential. Rejuvenation Res. 2013;16(3):179–84. doi: 10.1089/rej.2012.1394. [DOI] [PubMed] [Google Scholar]
- 10.Rizvi SI, Maurya PK. L-cysteine influx in erythrocytes as a function of human age. Rejuvenation Res. 2008;11(3):661–5. doi: 10.1089/rej.2007.0652. [DOI] [PubMed] [Google Scholar]
- 11.Griffith OW. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic Biol Med. 1999;27(9-10):922–35. doi: 10.1016/s0891-5849(99)00176-8. [DOI] [PubMed] [Google Scholar]
- 12.Kumar P, Maurya PK. Epigallocatechin-3-gallate protects erythrocyte ca(2+)-atpase and na(+)/k(+)-atpase against oxidative induced damage during aging in humans. Adv Pharm Bull. 2014;4(Suppl 1):443–7. doi: 10.5681/apb.2014.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benzie IF, Strain JJ. The ferric reducing ability of plasma (frap) as a measure of "antioxidant power": The frap assay. Anal Biochem. 1996;239(1):70–6. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 14.Beutler E. A Manual of Biochemical Methods. New York: Grunne and Stratton; 1984. [Google Scholar]
- 15.Sharma SK, Vij AS, Sharma M. Mechanisms and clinical uses of capsaicin. Eur J Pharmacol. 2013;720(1-3):55–62. doi: 10.1016/j.ejphar.2013.10.053. [DOI] [PubMed] [Google Scholar]
- 16.Aranda FJ, Villalain J, Gomez-Fernandez JC. Capsaicin affects the structure and phase organization of phospholipid membranes. Biochim Biophys Acta. 1995;1234(2):225–34. doi: 10.1016/0005-2736(94)00293-x. [DOI] [PubMed] [Google Scholar]
- 17.Jones DP. Extracellular redox state: Refining the definition of oxidative stress in aging. Rejuvenation Res. 2006;9(2):169–81. doi: 10.1089/rej.2006.9.169. [DOI] [PubMed] [Google Scholar]