Abstract
Purpose: A simple and available reversed-phase high performance liquid chromatography (HPLC) method with UV detection has been developed and validated for mycophenolic acid (MPA) assay in human plasma.
Methods: MPA was extracted from plasma with protein precipitation method by acetonitrile: percholeric acid: methanol (75:5:20 v/v/v). The drug separation was achieved using a C8 analytical column and a mobile phase of 0.1M triethylammonium phosphate (pH=5.4)-acetonitril (65:35, v/v), with a flow rate of 1.5 ml/min. The detection wavelength was 304 nm. Limit of detection (LOD) of the method was determined as the lowest MPA concentration producing a signal-to-noise (S/N) ratio of about 3. Limit of quantitation (LOQ) was determined as the lowest MPA concentration capable of being quantitated with enough accuracy and precision.
Results: The method showed significant linear response-concentration relationship throughout the MPA concentration range of 0.2-10 µg/ml. A typical linear regression equation of the method was: y = 8.5523 x + 0.094, with x and y representing MPA concentration (in µg/ml) and peak height respectively, and the regression coefficient (r) of 0.9816. The average within-run and between-run variations of 7.81 and 4.78 percent. The average drug recovery from plasma was 95.24 percent throughout the linear concentration range. The limits of detection (LOD) and quantitation (LOQ) of the method were 0.05 and 0.2 µg/ml, respectively. The practical applicability of the method was proven throughout a bioequivalence study.
Conclusion: The results showed the acceptable degree of linearity, sensitivity, precision, accuracy and recovery for the method. The method was used successfully for quantitation of MPA in plasma samples of healthy volunteers throughout a bioequivalence study.
Keywords: Mycophenolic acid (MPA), Mycophenolic acid (MPA) assay, Reversed-phase HPLC
Introduction
Mycophenolic acid (MPA) is an immunosuppressant drug that has been widely and successfully used in transplant recipients as well as in patients with immune disorders.1-2 MPA is administered as either an ester prodrug or a sodium salt and is extensively metabolized by UDP-glucuronosyltransferases (UGTs) to two glucuronidated metabolites. Mycophenolic acid (MPA, Figure 1) derivatives (Cellcept® or Myfortic®) choice in renal transplantation in combination with a calcineur in inhibitor and steroids.3 Their introduction in clinical medicine represented an advance because they improve graft survival rates. Optimal use of these new immunosuppressive drugs requires knowledge of their pharmacobiology. For example, presystemic hydrolysis of Cellcept® and by systemic esterases release MPA, the active compound.4 MPA is bound to albumin. The ratio between the free and bound components is affected by a variety of conditions including renal insufficiency and concomitant cyclosporin, tacrolimus or steroid therapy.4-6 These variables are commonly present in transplant patients, a situation that argues for monitoring of MPA level. There are more evidences to suggest that the measurement of MPA plasma concentrations. Several HPLC methods have been developed for the determination of MPA in human plasma.7-16 Some of these methods permit quantitation of MPA and its glucuronide metabolite.11-14 Those methods require the use of gradient elution system,8,10,16 use of more than one stationary phase13 or application of tedious sample extraction procedures.8,11 More sensitive methods for MPA assay in plasma have been developed, but they used liquid chromatography with tandem mass spectrometry.17-18 In this paper, we describe the development, optimization and validation of simple, and sensitive HPLC-UV detection method for the assay of MPA in human plasma. Furthermore, we demonstrate the application of this method to quantify MPA levels in human plasma following the oral administration of one single dose of 500 mg Cellcept. The distinct advantages of this method over other reported methods include its simplicity, inexpensive and the method’s reproducibility.
Figure 1.
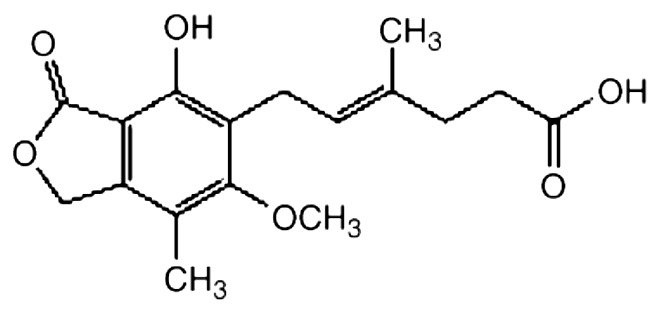
Chemical structure of MPA
Materials and Methods
Materials
Cellcept test capsules (Zahravi), cellcept reference capsules (Roche) and MPA reference standard (99.9% purity) were supplied and identified by Roche. Other chemicals and solvents were from chemical lab or HPLC purity grades, whenever needed, and were purchased locally. Drug-free human plasma was provided by Iranian Blood Transfusion Organization after routine safety evaluations.
Instrument and HPLC method
The HPLC system consisted of pump (KNAUER, model 1000, Germany), a variable wavelength UV detector (KNAUER, model 2800(DAD), Germany) used at a wavelength of 304 nm with the outputs recorded and analysed using a compatible software (ChromGate, KNAUER, Germany). The drug separation was achieved using a C8 analytical column (250mm×4.6mm, particle size 5µm; Perfectsill, MZ-Analysen technik, Germany) equipped by a guard column of the same packing. The mobile phase was consisted of 0.1M triethylammonium phosphate (pH=5.4)-acetonitril (65:35, v/v) with a flow rate of 1.5 ml/min. Sample injection to system (100µl) was made by a loop.
Standard preparation
A stock solution of 100 µg/ml MPA in methanol was prepared, from which the concentrations of 10, 5, 7, 2.5,1,0.5 and 0.2 µg/ml were prepared by serially diluting this solution with the proper amount of methanol. A series of spiked plasma samples with MPA concentrations of 10, 5, 7, 2.5,1,0.5 and 0.2 µg/ml were prepared by 1:10 dilution of the described solutions with drug-free human plasma.
Assay procedure
To 500µL calibration standards, QC samples, or plasma samples, 12µL perchloric acid (HClO4 75% aqueous solution) and 400 µL methanol and 100 µL acetonitrile were added. The mixtures were vortex mixed for 20 s. After centrifugation at 15 000×g in an eppendorf microcentrifuge tubes for 20 min, 100µL of the supernatant was injected directly onto the analytical column for immediate HPLC analysis.
Analysis Validation Tests
Standard curve (Linear range)
The plasma samples with a series of known concentrations, prepared as described, were analyzed in three separate runs and, in each case, the linear regression analysis was carried out on known concentrations of MPA against the corresponding peak heights and, then, the regression coefficient (r), slope, and y-intercept of the resulting calibration curves were determined.
Within-run variations
In one run, three samples with concentrations of 10, 5, and 0.2 µg/ml (from high, middle, and low regions of the standard curve) were prepared in triplicate and analyzed by developed HPLC method. Then, the coefficient of variations (CV %) of the corresponding determined concentrations were calculated in each case.
Between-run variations
On three different runs, samples from upper, intermediate, and lower concentration regions used for construction of standard curve (the same as within-run variations test) were prepared and analyzed by HPLC method. Then, the corresponding CV% values were calculated.
Absolute recovery (accuracy)
For each sample tested for within- and between-run variations, the absolute recovery of the method was determined as the percent ratio of the measured concentration (determined using standard curve) to the corresponding nominal added concentration.
Relative recovery (matrix effect)
Three samples with concentrations of 10, 5, and 0.2 µg/ml (from high, middle, and low regions of the standard curve) were prepared in triplicate and analyzed by developed HPLC method. Then, the ratio of the recorded peak heights to the peak heights resulted from the direct injection of the aqueous solutions of MPA with the same concentrations were determined as percentage in each case.
Limits of detection and quantitation
Limit of detection (LOD) of the method was determined as the lowest MPA concentration producing a signal-to-noise (S/N) ratio of about 3. Limit of quantitation (LOQ) was determined as the lowest MPA concentration capable of being quantitated with enough accuracy and precision.
Clinical study design
Twelve male subjects were enrolled in a randomized, two-treatment, two-period, single- dose crossover study with a week washout between the first dosing in period I and the first dosing of period II. Single dose study Subjects fasted from the night before dosing until 2 h after dosing for each session. For cellcept refrence group, the 500 mg cellcept formulation was administered and blood samples were obtained prior to dose administration (time 0) and at 0.25, 0.5, 1.0, 1.5, 2.0, 3.0, 4.0, 6.0, 8.0, 10.0 and 24.0 h after the dose. For cellcept -test group, the 500 mg cellcept test formulation was administered and blood samples were obtained prior to dose administration (time 0) and at 0.25, 0.5, 1.0, 1.5, 2.0, 3.0, 4.0, 6.0, 8.0, 10.0 and 24.0 h after the dose. The blood samples were immediately centrifuged at 1600×g for 10 min. The plasma was removed and stored at −20 °C until analysis was done.
Results and Discussion
Method Development
Considering the complex biological matrix of the samples to be analyzed and the nature of the method to be used for drug assay, the method development efforts were made in two different areas of sample preparation and analyte separation which are discussed in detail in the following sections:
Sample preparation
Protein precipitation was necessary and important because this technique can not only purify but also concentrate the sample. Methanol, percholeric acid and acetonitrile were all attempted and acetonitrile: percholeric acid: methanol (75:5:20 v/v/v) was finally adopted because of its high extraction efficiency and less interference. Precipitation with and without adding 0.1M NaOH (100 µl) were both tried, and obvious differences were not observed, so the precipitation using acetonitrile without adding 0.1M NaOH was used at last.
Analyte separation
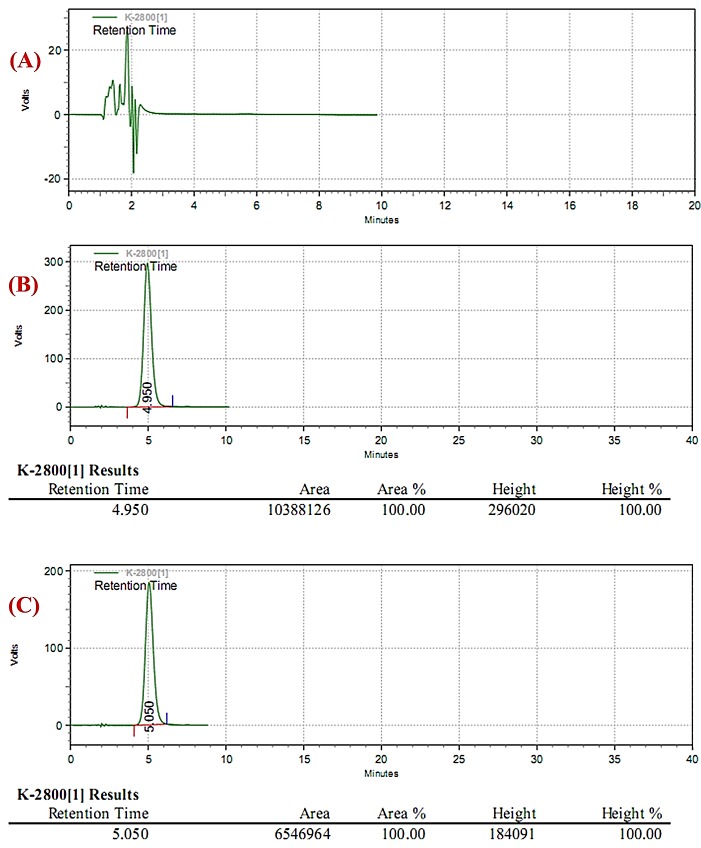
In response to lack of an available, reliable, and easy-to-use analysis method for MPA assay in plasma as an essential part of pharmacokinetic and bioequivalence evaluation projects on the drug we developed a simple and available HPLC method with UV detection based on the available equipments found in most pharmaceutical laboratories. To this end, initially a series of isocratic as well as gradient conditions using different usual mobile phase compositions, polarities, ionic strengths, and pH values were tested in order to determine the best condition for the analyte separation. Typical chromatograms produced from the developed method are shown in Figure 2.
Figure 2.
Typical chromatograms of the HPLC method developed for MPA assay in human plasma: a) human blank (drug-free) plasma; b) human plasma spiked to a 5 µg/ml drug concentration; c) a typical plasma sample from one of volunteers at 8 hrs post-dose following a 500 mg MPA capsule. In chromatograms the peak evident in about 5 min corresponds to MPA.
Method validation tests
Linearity
The method produced linear responses throughout the MPA concentration range of 0.2-10 µg/ml, which is suitable for intended purposes. A typical linear regression equation of the method was: y = 8.5523 x + 0.094, with x and y representing MPA concentration (in µg/ml) and peak height (in arabitiary units), respectively, and the regression coefficient (r) of 0.9816.
Within-run variations and accuracy
The within-run variations of the developed HPLC method as well as the corresponding absolute recoveries are shown in Table 1.
Table 1. Within–run variations and accuracy of the HPLC method for quantitation of MPA (n=3).
| Nominal Added Concentration (µg/ml) | Sample Number | Measured Concentration (µg /ml) | Mean (SD) | CV% |
| 10 | 1 | 11.34 | 10.29 (1.12) | 10.89 |
| 2 | 10.43 | |||
| 3 | 9.11 | |||
| 5 | 1 | 4.81 | 4.96 (0.15) | 3.02 |
| 2 | 5.12 | |||
| 3 | 4.96 | |||
| 0.2 | 1 | 0.20 | 0.21 (0.02) | 9.52 |
| 2 | 0.21 | |||
| 3 | 0.24 |
Between-run variations and accuracy
The between-run variations of the developed HPLC method as well as the corresponding absolute recoveries are shown in Table 2.
Table 2. Between–run variations and accuracy of the HPLC method for quantitation of MPA (n=3) .
| Nominal Added Concentration (µg/ml) | Run Number | Measured Concentration (µg /ml) | Mean (SD) | CV% |
| 10 | 1 | 10.28 | 10.75 (0.44) | 4.12 |
| 2 | 11.16 | |||
| 3 | 10.81 | |||
| 5 | 1 | 5.21 | 5.09 (0.12) | 2.35 |
| 2 | 4.96 | |||
| 3 | 5.12 | |||
| 0.2 | 1 | 0.19 | 0.19 (0.015) | 7.89 |
| 2 | 0.18 | |||
| 3 | 0.21 |
Relative recovery
The relative recovery of MPA using the developed assay method is shown in Table 3.
Table 3. Relative recovery of MPA by the HPLC method (N=3) .
| Nominal Added Concentration (µg/ml) | Sample Number | Recovery (%) | Mean (SD) |
| 10 | 1 | 90.11 | 95.17 (4.40) |
| 2 | 97.32 | ||
| 3 | 98.09 | ||
| 5 | 1 | 97.20 | 94.08 (4.33) |
| 2 | 89.14 | ||
| 3 | 95.89 | ||
| 0.2 | 1 | 101.00 | 96.47 (6.46) |
| 2 | 93.34 | ||
| 3 | 89.08 |
Limit tests
The limits of detection (LOD) and quantitation (LOQ) of the method were 0.05 and 0.2 µg/ml, respectively.
In general, the results of the validation tests indicated that the developed method has a remarkable degree of accuracy, repeatability, reproducibility, and recovery with application limits being in the desired range for routine applications.
Bioequivalence Study
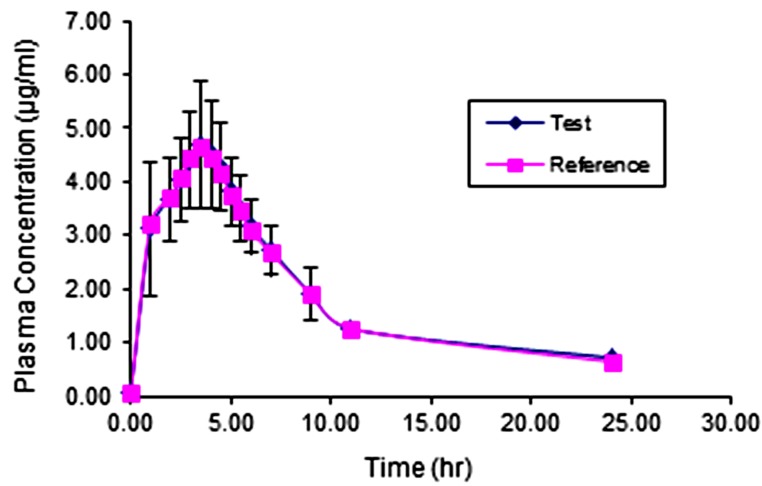
The comparative pharmacokinetic profiles of MPA following the oral administration of 500 mg capsules of test and reference products are shown in Figure 3. Also, the primary and secondary pharmacokinetic parameters of the individual volunteers are shown in Table 4 and Table 5 respectively. These collections of data, while indicating the bioequivalence of these two products, which is out of the scope of the present article, clearly show the practical applicability of the developed method in a real clinical study and these data showed that the method can be applied throughout the in vivo pharmacokinetic studies on MPA products.
Figure 3.
Comparative pharmacokinetic profile of MPA following oral administration of 500 mg test and reference products to healthy volunteers.
Table 4. The mean primary pharmacokinetic parameters of MPA in 12 healthy volunteers following oral administration of 500 mg test and reference products.
| PK Parameter | Cmax (µg /ml) | tmax (hr) | AUC0-24 (µg .hr/ml) | AUC0-∞ (µg .hr/ml) | ||||
| Volunteer Code | test | reference | test | reference | test | reference | test | reference |
| A-Gh | 4.89 | 4.88 | 3.00 | 3.50 | 52.14 | 52.20 | 59.00 | 59.36 |
| S-R | 4.85 | 4.87 | 5.00 | 3.50 | 44.60 | 44.10 | 77.56 | 72.15 |
| H-M | 7.21 | 6.23 | 5.00 | 4.00 | 56.67 | 54.85 | 77.02 | 75.54 |
| B-Kh | 3.76 | 3.78 | 5.00 | 3.00 | 34.86 | 35.62 | 40.92 | 41.86 |
| S-N | 3.48 | 3.61 | 5.50 | 3.50 | 33.52 | 37.58 | 34.88 | 39.26 |
| A-Ch | 4.85 | 4.89 | 4.00 | 4.50 | 45.56 | 42.47 | 79.32 | 55.77 |
| K-D | 4.85 | 4.76 | 4.50 | 4.50 | 46.83 | 39.69 | 60.32 | 47.38 |
| Ah-M | 4.87 | 5.12 | 6.00 | 3.00 | 40.71 | 41.69 | 62.53 | 57.20 |
| Ar-L | 3.47 | 3.68 | 3.00 | 4.50 | 32.45 | 34.60 | 33.12 | 35.44 |
| E-S | 4.65 | 4.64 | 4.00 | 4.50 | 49.65 | 48.64 | 93.38 | 51.87 |
| E-O | 6.74 | 5.76 | 4.00 | 5.50 | 51.95 | 50.34 | 71.56 | 70.36 |
| M-E | 4.65 | 5.03 | 3.50 | 4.00 | 49.47 | 49.20 | 56.43 | 98.32 |
| Mean | 4.86 | 4.77 | 4.38 | 4.00 | 44.87 | 44.25 | 62.17 | 58.71 |
| SD | 1.14 | 0.79 | 0.96 | 0.74 | 7.93 | 6.74 | 18.83 | 17.99 |
| CV% | 23.37 | 16.58 | 21.86 | 18.46 | 17.67 | 15.22 | 30.29 | 30.64 |
| Max | 7.21 | 6.23 | 6.00 | 5.50 | 56.67 | 54.85 | 93.38 | 98.32 |
| Min | 3.47 | 3.61 | 3.00 | 3.00 | 32.45 | 34.60 | 33.12 | 35.44 |
| P value1 | 0.829 | 0.294 | 0.839 | 0.649 | ||||
Table 5. The mean secondary pharmacokinetic parameters of MPA in 12 healthy volunteers following oral administration of 500 mg test and reference products.
| PK Parameter | λz (hr-1) | MRT (hr) | Cl (L/hr) | Vd,ss (L) | ||||
| Volunteer Code | test | reference | test | Reference | test | reference | test | reference |
| A-Gh | 0.093 | 0.092 | 10.78 | 10.96 | 6.78 | 6.74 | 73.11 | 73.86 |
| S-R | 0.031 | 0.035 | 28.62 | 25.62 | 5.16 | 5.54 | 147.58 | 142.07 |
| H-M | 0.052 | 0.051 | 17.39 | 17.92 | 5.19 | 5.30 | 90.32 | 94.87 |
| B-Kh | 0.082 | 0.081 | 12.20 | 12.19 | 9.77 | 9.56 | 119.26 | 116.52 |
| S-N | 0.146 | 0.143 | 8.17 | 8.55 | 11.47 | 10.19 | 93.66 | 87.17 |
| A-Ch | 0.031 | 0.054 | 28.79 | 15.92 | 5.04 | 7.17 | 145.20 | 114.20 |
| K-D | 0.066 | 0.075 | 15.49 | 12.59 | 6.63 | 8.44 | 102.69 | 106.27 |
| Ah-M | 0.040 | 0.051 | 22.52 | 17.69 | 6.40 | 6.99 | 144.05 | 123.74 |
| Ar-L | 0.177 | 0.169 | 7.43 | 7.34 | 12.08 | 11.29 | 89.69 | 82.89 |
| E-S | 0.091 | 0.121 | 6.62 | 8.75 | 4.28 | 7.71 | 28.36 | 67.51 |
| E-O | 0.050 | 0.049 | 17.99 | 18.58 | 5.59 | 5.69 | 100.56 | 105.62 |
| M-E | 0.090 | 0.081 | 11.09 | 6.96 | 7.09 | 4.07 | 78.64 | 28.33 |
| Mean | 0.079 | 0.083 | 15.59 | 13.59 | 7.12 | 7.39 | 101.09 | 95.25 |
| SD | 0.045 | 0.041 | 7.73 | 5.66 | 2.59 | 2.16 | 34.59 | 30.10 |
| CV% | 57.117 | 49.597 | 49.61 | 41.64 | 36.33 | 29.22 | 34.22 | 31.60 |
| Max | 0.177 | 0.169 | 28.79 | 25.62 | 12.08 | 11.29 | 147.58 | 142.07 |
| Min | 0.031 | 0.035 | 6.62 | 6.96 | 4.28 | 4.07 | 28.36 | 28.33 |
| P value1 | 0.810 | 0.477 | 0.786 | 0.663 | ||||
Conclusion
A simple HPLC method was developed and validated for MPA assay in plasma. The plasma preparation for analysis consisted of a Protein precipitation method. The validation tests on the developed method indicated acceptable degree of linearity, sensitivity, precision, accuracy and recovery for the method. The method was used successfully for quantitation of MPA in plasma samples of healthy volunteers throughout a bioequivalence study. System suitability tests showed that the developed method is of appropriate separation efficiency and peak shape.
Acknowledgments
This work has been supported financially by the Faculty of Pharmacy, Zanjan University of Medical Sciences. The authors wish to thank Zahravi Pharmaceuyticals (Tabriz, Iran) for kindly providing us by cellcept samples. We also would like to thank Iranian Blood Transfusion Organization for providing drug-free plasma.
Ethical Issues
Not applicable.
Conflict of Interest
The authors report no conflicts of interest.
References
- 1.Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of mycophenolate in solid organ transplant recipients. Clin Pharmacokinet. 2007;46(1):13–58. doi: 10.2165/00003088-200746010-00002. [DOI] [PubMed] [Google Scholar]
- 2.Walsh M, James M, Jayne D, Tonelli M, Manns BJ, Hemmelgarn BR. Mycophenolate mofetil for induction therapy of lupus nephritis: A systematic review and meta-analysis. Clin J Am Soc Nephrol. 2007;2(5):968–75. doi: 10.2215/CJN.01200307. [DOI] [PubMed] [Google Scholar]
- 3.Gerbase MW, Fathi M, Spiliopoulos A, Rochat T, Nicod LP. Pharmacokinetics of Mycophenolic acid associated with calcineurin inhibitors: long-term monitoring in stable lung recipients with and without cystic fibrosis. J Heart Lung Transplant. 2003;22(5):587–90. doi: 10.1016/s1053-2498(02)01159-2. [DOI] [PubMed] [Google Scholar]
- 4.Shaw LM, Korecka M, Venkataramanan R, Goldberg L, Bloom R, Brayman KL. Mycophenolic acid pharmacodynamics and pharmacokinetics provide a basis for rational monitoring strategies. Am J Transplant. 2003;3(5):534–42. doi: 10.1034/j.1600-6143.2003.00079.x. [DOI] [PubMed] [Google Scholar]
- 5.Tredger JM, Brown NW, Adams J, Gonde CE, Dhawan A, Rela M. et al. Monitoring mycophenolate in liver transplant recipients: Toward a therapeutic range. Liver Transpl. 2004;10(4):492–502. doi: 10.1002/lt.20124. [DOI] [PubMed] [Google Scholar]
- 6.Sievers TM, Rossi SJ, Ghobrial RM, Arriola E, Nishimura P, Kawano M. et al. Mycophenolate mofetil. Pharmacotherapy. 1997;17(6):1178–97. doi: 10.1002/j.1875-9114.1997.tb03082.x. [DOI] [PubMed] [Google Scholar]
- 7.Ahadi Barzoki M, Rouini MR, Gholami KH, Lesan Pezeshki M, Rezaei S. Determination of mycophenolic acid in human plasma by high-performance liquid chromatography. Daru. 2005;13(3):120–6. [Google Scholar]
- 8.Heller T, van Gelder T, Budde K, de Fijter JW, Kuypers D, Arns W. et al. Plasma Concentrations of Mycophenolic Acid Acyl Glucuronide Are Not Associated with Diarrhea in Renal Transplant Recipients. Am J Transplant. 2007;7(7):1822–31. doi: 10.1111/j.1600-6143.2007.01859.x. [DOI] [PubMed] [Google Scholar]
- 9.Bolon M, Jeanpierre L, El Barkil M, Chelbi K, Sauviat M, Boulieu R. HPLC determination of mycophenolic acid and mycophenolic acid glucuronide in human plasma with hybrid material. J Pharm Biomed Anal. 2004;36(3):649–51. doi: 10.1016/j.jpba.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 10.Jeong H, Kaplan B. Therapeutic Monitoring of Mycophenolate Mofetil. Clin J Am Soc Nephrol. 2007;2(1):184–91. doi: 10.2215/CJN.02860806. [DOI] [PubMed] [Google Scholar]
- 11.Shipkova M, Armstrong VW, Kiehl MG, Niedmann PD, Schutz E, Oellerich M. et al. Quantification of mycophenolic acid in plasma samples collected during and immediately after intravenous administration of mycophenolate mofetil. Clin Chem. 2001;47(8):1485–8. [PubMed] [Google Scholar]
- 12.Millan O, Brunet M, Martorell J, Garcia F, Vidal E, Rojo I. et al. Pharmacokinetics and pharmacodynamics of low dose mycophenolate mofetil in HIV-infected patients treated with abacavir, efavirenz and nelfinavir. Clin Pharmacokinet. 2005;44(5):525–38. doi: 10.2165/00003088-200544050-00006. [DOI] [PubMed] [Google Scholar]
- 13.Teshima D, Kitagawa N, Otsubo K, Makino K, Itoh Y, Oishi R. Simple determination of mycophenolic acid in human serum by column-switching high-performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;780(1):21–6. doi: 10.1016/s1570-0232(02)00410-5. [DOI] [PubMed] [Google Scholar]
- 14.Nolin TD, McMenamin ME, Himmelfarb J. Simultaneous determination of total homocysteine, cysteine, cysteinylglycine, and glutathione in human plasma by high-performance liquid chromatography: Application to studies of oxidative stress. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;852(1-2):554–61. doi: 10.1016/j.jchromb.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qiu K, Tian H, Wang W, Hu XP, Li XB, Gong LL. et al. Pharmacokinetics of enteric-coated mycophenolate sodium in Chinese renal transplantation recipients. Chin Med J (Engl) 2012;125(23):4226–32. doi: 10.3760/cma.j.issn.0366-6999.2012.23.012. [DOI] [PubMed] [Google Scholar]
- 16.Zhong Y, Jiao Z, Yu Y. Simultaneous determination of mycophenolic acid and valproic acid based on derivatization by high-performance liquid chromatography with fluorescence detection. Biomed Chromatogr. 2006;20(4):319–26. doi: 10.1002/bmc.566. [DOI] [PubMed] [Google Scholar]
- 17.Mendonza AE, Gohh RY, Akhlaghi F. Analysis of mycophenolic acid in saliva using liquid chromatography tandem mass spectrometry. Ther Drug Monit. 2006;28(3):402–6. doi: 10.1097/01.ftd.0000211826.65607.05. [DOI] [PubMed] [Google Scholar]
- 18.Figureski MJ, Korecka M, Fields L, Waligorska T, Shaw LM. High-performance liquid chromatography-mass spectroscopy/mass spectroscopy method for simultaneous quantification of total or free fraction of mycophenolic acid and its glucuronide metabolites. Ther Drug Monit. 2009;31(6):717–26. doi: 10.1097/FTD.0b013e3181ba9a0e. [DOI] [PubMed] [Google Scholar]