Abstract
Purpose: The bacterial cultivation conditions for obtaining anti-TNF-α single chain variable fragment (scFv) antibody as the soluble product in E. coli was investigated.
Methods: To avoid the production of inclusion bodies, the effects of lactose, IPTG, incubation time, temperature, shaking protocol, medium additives (Mg+2, sucrose), pH, osmotic and heat shocks were examined. Samples from bacterial growth conditions with promising results of soluble expression of GST-hD2 scFv were affinity purified and quantified by SDS-PAGE and image processing for further evaluation.
Results: The results showed that cultivation in LB medium under induction by low concentrations of lactose and incubation at 10 °C led to partial solubilization of the expressed anti-TNF-α scFv (GST-hD2). Other variables which showed promising increase in soluble expression of GST-hD2 were osmotic shock and addition of magnesium chloride. Furthermore, addition of sucrose to medium suppressed the expression of scFv completely. The other finding was that the addition of sorbitol decreased the growth rate of bacteria.
Conclusion: It can be concluded that low cultivation temperature in the presence of low amount of inducer under a long incubation time or addition of magnesium chloride are the most effective environmental factors studied for obtaining the maximum solubilization of GST-hD2 recombinant protein.
Keywords: Single-chain antibodies, Recombinant protein expression, Inclusion bodies, Growth optimization, Escherichia coli, Tumor necrosis factor-alpha
Introduction
Anti-TNF-α antibodies have reported as promising factors in the treatment of some diseases like rheumatoid arthritis and Crohn's disease.1,2 But the possibility of long-term utilization of antibodies, depends on the rate of toxicity in body and the production cost.2 The total cost of antibody injection per year for each patient is markedly high and it can be considered as an obstacle for its use.2 To overcome this dilemma, recombinant DNA technology along with possibility of cloning and the expression of monoclonal antibody fragments in prokaryotes, have revealed a new promising platform for the development of more affordable drugs.3 For example, the variable regions of heavy (VH) and light (VL) fragments of monoclonal antibody can be coupled by a flexible peptide linker to form a polypeptide known as single chain fragment variable (scFv).4,5 Although the scFv antibodies can be produced in both prokaryotic and eukaryotic hosts, however, the prokaryotic systems can be economic and easy to handle, compared to eukaryotic biological systems.6 However, the expression of scFv antibodies in bacterial hosts sometimes leads to the aggregation of recombinant proteins due to partial folding. These aggregates called inclusion bodies are needed to be refolded into their proper structure. Refolding of inclusion body to a soluble protein is costly, laborious and time consuming.6-8 There are several approaches to avoid the production of inclusion body in bacteria such as optimization of the cultivation temperature, type and the concentration of inducers, medium additives, and utilization of heat and osmotic shocks.9-13 Escherichia coli is one of the common host prokaryotic organisms for the production of recombinant proteins because of facile genetic engineering and optimization of recombinant protein expression.14,15 Total amount of recombinant protein has an important role in its degradation rate. It has revealed that when the protein content exceeds 50 percent of the total protein, it reduces the opportunity for proper folding of the product.6 Expression of scFv in E. coli cytoplasm in numerous cases have shown leads to insoluble inclusion bodies, and therefore some strategies were adopted to achieve soluble expression of the target protein16,17 Using low concentration of the inducers like IPTG and lactose reduces the rate of recombinant protein production in the cells.6 The soluble expression rate of scFv in bacteria can vary based on medium and cultivation conditions.18-20 The presence of some additives like sorbitol and sodium chloride in the medium triggers osmotic shock, and the high incubation temperature for a short period of time produces a heat shock response.12 Either osmotic or heat shocks cause increased manufacturing of chaperone molecules which play essential roles in proper folding of de novo synthesized proteins in cells at harsh conditions and eventually catalyze proper folding of proteins.12,18 Metal ions have described as solubilizing factors for some recombinant proteins.20 Especially magnesium solubilization activity is proposed to be higher than any other metal ions.20 Sucrose has been shown to increase the solubility of scFv molecules ranging from 15 to 150 folds.9 Some studies have proved that the low temperatures (10 °C or lower) increase the solubility of recombinant proteins which are otherwise expressed in the form of inclusion body when the incubation temperature is set to 15 and 20 °C.11,21 As mentioned above, the expression of scFv antibodies in E. coli cytoplasm generally leads to the production of inclusion bodies,17,22-24 so optimized growth conditions are required to achieve soluble expression of the target scFv. Therefore, in this study, we have examined the effects of temperature, shaking condition, concentration of inducers (lactose or IPTG), incubation time, pH, sucrose and Mg2+ concentrations, and osmotic or heat shocks on the soluble expression of a 52 kD GST-fusion recombinant protein called GST-hD2 scFv in Escherichia coli. GST-hD2 is a humanized anti-TNF-α single chain variable fragment (scFv) antibody fused to GST protein, which is designed25 using CDRs replacement strategy based on a murine scFv anti-TNF-α antibody.26
Materials and Methods
Bacterial strain, plasmid, and target protein
E. coli Origami (DE3) harboring recombinant plasmid pGEX-6P-1/hD2 was obtained generously from Biotechnology Research Center Tabriz (BRC, Iran). hD2 is a GST fusion protein cloned in pGEX-6P-1 and encodes a single chain fragment variable antibody against TNF-α.
Optimization parameters
A single colony of the recombinant bacteria was cultivated overnight in LB medium containing 100 µg mL-1 ampicillin at 37 °C, while shaking at 175 rpm. Then, it was diluted in 1:100 ratio by 50 mL fresh medium in a 500 mL flask. To explore the effects of inducers, the induction of GST-hD2 protein expression was performed at final concentrations 0.1 and 1 mM for IPTG, or 0.03, 0.15, 0.3 and 0.6 mM for lactose in subculture grown at 37 °C while shaking at 180 rpm until an OD600= 0.6-0.8.
To study the effect of temperature and incubation time on scFv solubilization, the above mentioned cultures were incubated at 10, 20 and 37 °C, while samples were harvested at 0, 3, 6 and 18 h after induction. Also, at 10°C, the incubation time was extended further for 48 h after the induction.10 To examine the shaking effect, two different approaches were carried out. Subculture was grown in absence of inducer at 37 °C, while shaking at 200 rpm for 4 h. Then, the shaking speed was decreased to 50 rpm for 18 h and the samples were harvested at 0, 4 and 22 h after subculturing. In another procedure, starter culture and subculture were grown at 30 °C, 120 rpm. The subculture was induced by 0.1 mM IPTG and the cultivation condition was changed to 30 rpm at 25 °C for 15 h.27 To assess the effects of MgCl2 and sucrose on the soluble expression of GST-hD2, 0.1 and 10 mM of MgCl2 and 0.4 M sucrose in final concentration were added to each subculture, respectively.9,20 In this procedure, induction was achieved using 0.6 mM lactose and the cells were harvested 6 and 18 h after induction. The medium pH was adjusted at 5, 6, 7 or 8 and its effects on soluble expression of GST-hD2 were investigated.28 To examine heat and osmotic shock responses, the study were performed according to the protocol by Oganesyan et al12 with some minor modification. Harvesting of cells were performed 3 and 15 h after induction.
GST-hD2 expression and SDS-PAGE analyses
To harvest equal number of cells from different cultures, first the optical densities of the cultures were measured at 600 nm and then the adjusted volume withdrawn from each culture was equated to be 1/OD600 mL (equal to 0.3 mg cell pellet). Then the cells were harvested by centrifugation at 13000 g for 3 min at room temperature. The bacterial pellet was resuspended in 60 µL of lysis buffer (50 mM Tris (pH=8.0), 100 mM NaCl, 1.4 mM PMSF, 0.1% B-Mercaptoethanol, 1% Triton X-100) and kept for 30 min on ice. After three freeze-thaw cycles, the soluble fraction was isolated from insoluble fraction by centrifuging at 12000 g for 10 min at 4 °C. Then, to the soluble and insoluble fractions were added 20 and 80 µL of phosphate saline buffer (PBS), respectively. Subsequently, to the samples was added 10 µL of 2X sample solvent buffer (SDS 4%, Glycerol 20%, 2-mercaptoethanol 10%, Bromophenol blue 0.2%, Tris-Base 50 mM) and heated at 95 °C for 10 min. The expression of GST-hD2 fusion protein was investigated using SDS-PAGE analysis on 12% (V/V) polyacrylamide gel using an electrophoresis apparatus (Bio-Rad, USA) according to Sambrook and Russell instruction after staining by Coomassie Brilliant Blue G250.29 The purified GST-hD2 from the previous study25 called Ab in the current study and was used as a protein size marker in electrophoresis analyses.
Purification and quantification of soluble GST-hD2
For purification of fusion GST-hD2, cells were collected from a 50 mL culture grown as outlined above by centrifugation at 7000 g for 15 min at 4 °C and then were resuspended in lysis buffer (7 mL lysis buffer for each gram of cell pellet) while incubating for 30 min on ice. For maximum cell disruption, 3 cycles of freeze-thaw steps were applied following by intermittently ultra-sonication on ice for 30 s at 60 W with 30 s allowed for cooling for total of 5 min. Afterward lysate were centrifugation at 10,000 g for 20 min at 4 °C and supernatants was used for concentrating and affinity purification. Supernatant was loaded on 20 µL of Glutathione Sepharose 4B bead for 2 h at 4 °C. The bead was isolated by centrifugation at 2300 g for 3 min and washed 5 times with wash buffer (50 mM Tris, 100 mM NaCl, 0.1% 2-β mercaptoethanol). Presence of recombinant antibody in soluble fraction was analyzed using SDS-PAGE. The gels from SDS-PAGE analyses were scanned and their images were saved as TIF files. The relative concentrations of the soluble and purified recombinant antibody samples were quantified based on the band densities to that of known concentration of the standard Ab protein using image processing program called ImageJ (National Institute of Health, Bethesda, MD).
Results
SDS-PAGE analyses showed that the production of GST-hD2 as inclusion body was proportionally increased with the increase of IPTG or lactose concentrations as well as the incubation time as shown in Figure 1. Different concentrations of IPTG (0. 1 and 1 mM) did not led to soluble expression of GST-hD2. The cultivation of bacteria at different concentrations of lactose ranging from 0.03 to 0.3 mM caused soluble expression of GST-hD2 after 48 h incubation at 10 °C. To optimize culture temperature, the experiments were performed at 20 and 37 °C, however, no sign of soluble expression of recombinant protein was detected (Figure 2). The best result for soluble expression of GST-hD2 was achieved when cells induced by 0.03 to 0.3 mM lactose and incubated at 10 °C for 48 h. Analyzing the soluble fraction obtained from growing the cells in the presence of 0.3 mM lactose as the inducer for 48 h at 10 °C showed that the amount of GST-hD2 expressed as soluble protein was 26.15 µg per one g of bacteria (Figures 3 and 4). Soluble expression of the recombinant protein was not observed after reaching to the OD600 of 0.6-0.8 in bacterial culture with no added inducers (Figure 5). Different shaking speed protocols had no effect on solubilizing the recombinant protein (Figure 6). The various concentrations of magnesium chloride caused the production of slight amount of the recombinant protein. This finding was verified by affinity purification experiment using GSH-Sepharose bead. As shown in Figures 4 and 7, GST-hD2 was observed in the samples prepared form the soluble fraction of cultures grown in the presence of 10 mM magnesium chloride at the levels of 25.21 and 13.92 µg per gbacteria after 6 and 18 h induction times, respectively. By addition of 0.4 M sucrose into the culture media, the expression of recombinant scFv antibody (GST-hD2) was suppressed totally and even the aggregate form of the antibody was not observed as shown in Figure 8 compared to the control (i.e., in the absence of sucrose). The solubility of the recombinant protein did not show any remarkable changes when the pH was varied from 5 to 8 (Figure 9). Sorbitol at final conertatin of 0.5 M in the LB meduim caused diminutioned growth rate of cells in subculturing step. A faint band parallel to our standard size marker protein of GST-hD2 was detected 3 h after induction in all shock treatments. Accorcing to the results shown in Figures 10 and 11, applying the affinity purifacation step on all types of shock experiments (osmotic shock, osmotic shock plus sorbitol, heat shock, heat shock plus sorbitol) revealed that only slight soluble experssion of the recombinant anibody was achieved (5.78 µg per gbacteria) under osmotic shock treatment after 3 h incubation (Figures 4 and 11).
Figure 1.
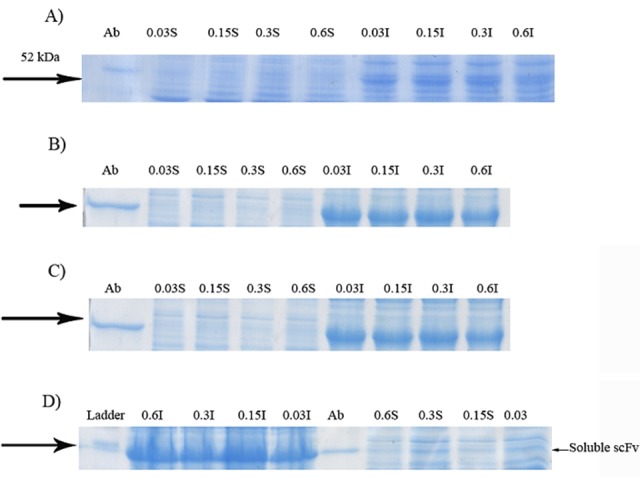
Effect of cultivations at the presence of different concentrations of lactose on soluble expression of GST-hD2. Proteins were resolved on SDS-PAGE prepared using 12% acrylamide and visualized by Coomassie Brilliant Blue G250 29. Cells grown at 10 °C. The digital part of the alphanumerical codes above the lanes show the concentrations of lactose. (S) and (I) symbols of the code denote soluble and insoluble fractions, respectively. Gels in panels A, B, C and D show the results for 3, 6, 24 and 48 h inductions, respectively.
Figure 2.
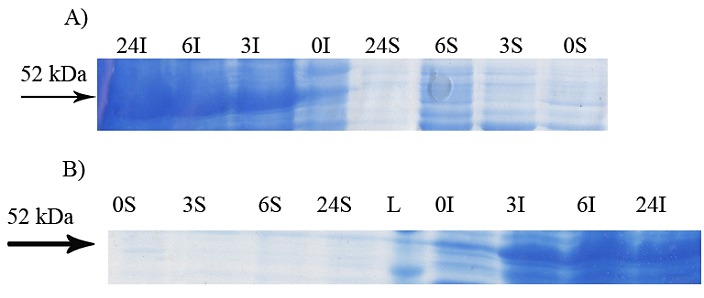
Effect of cultivations at different incubation time on soluble expression of GST-hD2. The cells were induced by 0.1 mMIPTG at 20 °C (panel A) and 37 °C (Panel B). The digital part of the alphanumerical codes above the lanes show incubation times after induction. (L), (S) and (I) symbols of the code denote 50 kDa ladder, soluble and insoluble fractions, respectively.
Figure 3.
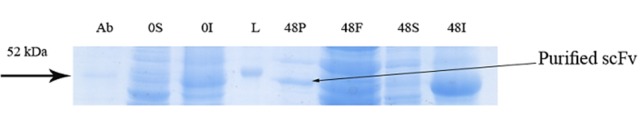
Further assessment of soluble expression of GST-hD2 by applying affinity purification step to the soluble fraction prepared from the culture grown at 10 °C and induced by 0.3 mM lactose (extension to experiment shown in panel D of Figure 1). The digital part of the alphanumerical codes above the lanes show incubation times after induction. (S) and (I) symbols of the code denote soluble and insoluble fractions, respectively. L, P and F represent respectively a 50 kDa ladder protein, purified GST-hd2 and flowthrough.
Figure 4.
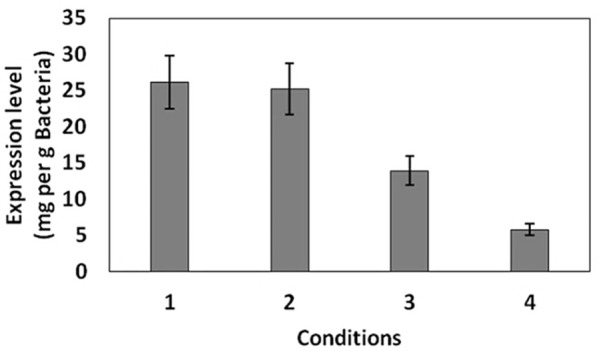
Levels of the soluble GST-hD2 under different conditions explained in Table 1.
Figure 5.
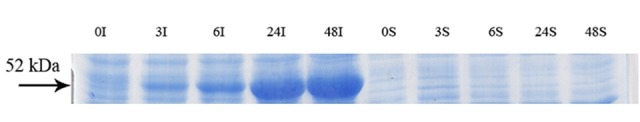
Samples from culture grown in the absence of inducer at 10 °C at 0, 3, 6, 24 and 48 h after reaching to OD600=0.6. The digital part of the alphanumerical codes above the lanes show incubation times. (S) and (I) symbols of the code denote soluble and insoluble fractions, respectively.
Figure 6.
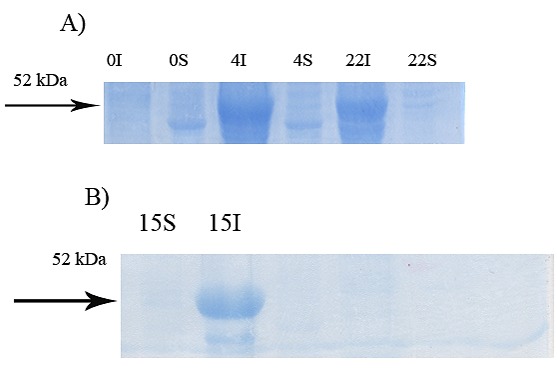
Effect of shakingon soluble expression of GST-hD2. The digital part of the alphanumerical codes above the lanes show incubation times after induction. (S) and (I) symbols of the code denote soluble and insoluble fractions, respectively. A Subculture in absence of inducer and cultivated at 37 °C, 200 rpm for 4 h then, the shaking speed was decreased to 50 rpm. B starter culture and subculture were grown at 30 °C, 120 rpm. The subculture was induced by 0.1 mM IPTG and the cultivation condition was changed to 30 rpm at 25 °C.
Figure 7.
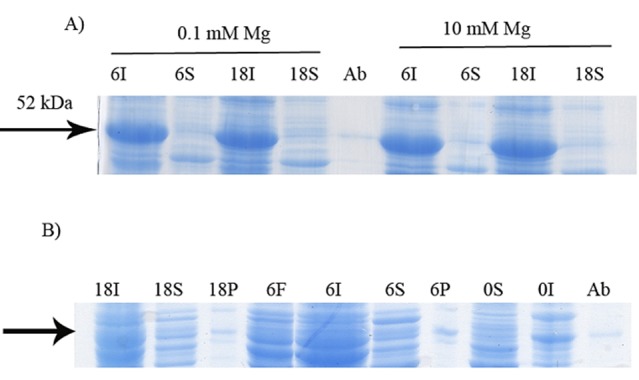
Effect of Mg2+on soluble expression of GST-hD2. The cells were induced by 0.6 mMlactose at 20 °C. The digital part of the alphanumerical codes above the lanes show incubation times after induction. (S) and (I) symbols of the code denote soluble and insoluble fractions, respectively. A Magnesium chloride was used at 0.1 and 10 M concentrations. B Further assessment of soluble expression of GST-hD2 by applying affinity purification step to the soluble fraction prepared from the culture grown at 20 °C and induced by 0.6 mMlactose in the presence of magnesium chloride in 10 mM final concentration. P and F represent purified GST-hD2 and Flowthrough respectively.
Figure 8.
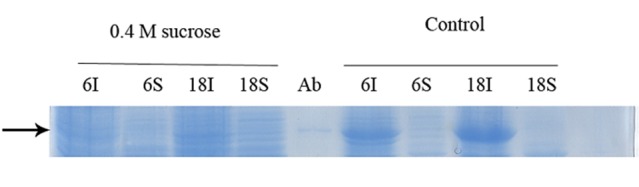
Effect of sucrose on soluble expression of GST-hD2.Sucrose was used at 0.4 M concentration. The culture was induced with 0.6 mM lactose at 20 °C. The digital part of the alphanumerical codes above the lanes show incubation times after induction. (S) and (I) symbols of the code denote soluble and insoluble fractions, respectively. Control experiment was performed without addition of sucrose in subculture process.
Figure 9.
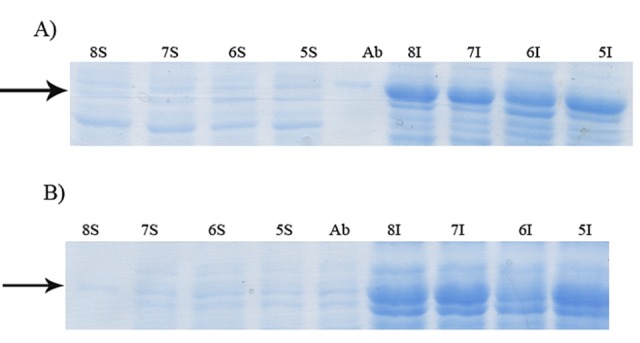
Effect of pH on soluble expression of GST-hD2 at 20°C using 0.6 mM lactose as the inducer. The incubation times were 6 (panel A) and 18 h (panel B) after induction. The digital part of the alphanumerical codes above the lanes show pH of the medium. (S) and (I) symbols of the code denote soluble and insoluble fractions, respectively.
Figure 10.
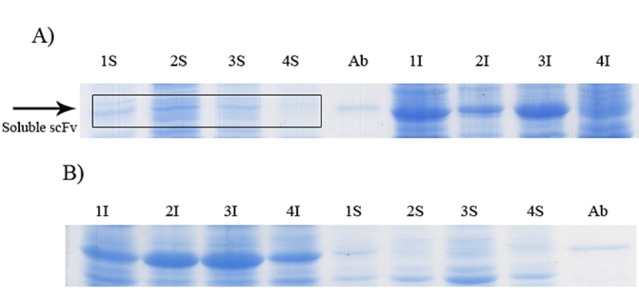
Effect of osmotic and heat shocks on soluble expression of GST-hD2 at 20 °C. The incubation times were 3 (panel A) and 15 h (panel B) after induction. The digital part of the alphanumerical codes above the lanes are as follow, 1: Osmotic shock, 2: Heat shock, 3: Osmotic shock plus sorbitol, 4: Heat shock with sorbitol. (S) and (I) symbols of the code denote soluble and insoluble fractions, respectively.
Figure 11.
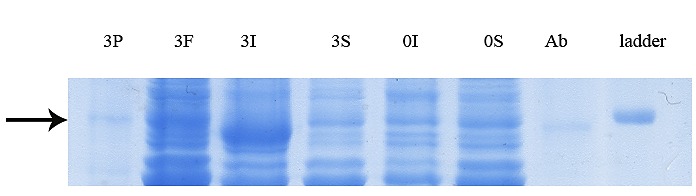
Further assessment of soluble expression of GST-hD2 by applying affinity purification step to the soluble fraction prepared from osmotic shocks (0.5 M NaCl and 1mM glycinebetaine) (extension to experiment shown in panel A of Figure 10). The digital part of the alphanumerical codes above the lanes show incubation times after induction. (S) and (I) symbols of the code denote soluble and insoluble fractions, respectively. P and F represent respectively, purified GST-hD2 and flowthrough.
Table 1. Growth conditions where the soluble expression of GST-hD2 was detected.
| Condition number | Condition | Figure showing the results |
| 1 | Induction by 0.03 to 0.3 mM lactose and Incubation for 48 h at 10 °C | 1.D |
| 2 | Addition 10 mM magnesium and Induction by 0.6 mM lactose and Incubation for 6 at 20 °C | 7 |
| 3 | Addition 10 mM magnesium and Induction by 0.6 mM lactose and Incubation for 15 h at 20 °C | 7 |
| 4 | Osmotic shocks (0.5 M NaCl and 1 mM glycinebetaine) and induction by 0.3 mM IPTG for 3 h at 20 °C | 11 |
Discussion
The correct, active and soluble expression of scFv antibodies and prevention of inclusion body formation in cytoplasm of E. coli have been the focus of many investigations.17,21,24 Inclusion bodies are misfolded proteins due to inefficient interaction between nascent protein and chaperone molecules which causes unsuitable folding of protein.30 Some protocols have been developed based on environmental modifications to produce the soluble form of the expressed proteins in E. coli.6,10,13 In our previous study, no soluble expression of GST-hD2 was observed in cytoplasm when the standard expression methods were applied.29 Very low concentration of inducers has shown to be a good candidate for promoting soluble expression of scFv antibody.31 The results illustrated in Figure 1D show that the extended incubation time of 48 h in the presence of different concentrations of lactose ranging from 0.03 to 0.3 mM at 10 °C was resulted in soluble expression of GST-hD2. This may be the result of slow production rate of scFv antibody at such low temperature and low inducer concentrations providing enough time for the proper folding of the recombinant protein as reported by other investigators as well.9,18,20,32 Soluble expression of GST-hD2 at 20 and 37 °C which were induced by IPTG was not observed (Figure 2). Raising the shaking speed led to increased aeration and growth rate of bacteria and, finally to the increased amount of recombinant protein.33 Therefore, the effect of shaking speed on solubilization of GST-hD2 was examined. However, different shaking speed protocols studied in the current work had no effect on soluble expression of GST-hD2 (Figure 6). Magnesium ionhas been used to solubilize recombinant proteins in the study of Yang et al.20 This is probably due to cellular mechanism and magnesium function as a cofactor in folding of recombinant protein. In a similar manner, we also observed beneficial effect of addition of magnesium chloride (0.1 and 10 mM) on soluble expression of GST-hD2 as shown in Figure 7. In our investigation, sucrose suppressed totally the expression of scFv antibody and the recombinant protein was not detectable in either of soluble and insoluble fractions (Figure 8). This finding is consistent with that of the Heo et al. which described a significant decrease in production of insoluble scFv antibody when sucrose was added to the medium.23 Further study are required to understand the mechanism of suppression of inclusion body by sucrose. Based on our results, pH did not affect the solubility of the recombinant protein at the studied pH range (Figure 9). But some previous studies have shown that pH can affect the solubility rate of the recombinant proteins, this controversy can be due to the differences of the target recombinant proteins.28,35 Osmotic or heat shocks lead to production of heat shock protein (HSP) and also uptake of osmolytes molecules like glycine betaine (as chemical chaperone) which stabilize native protein and probably assist in correct, active and soluble folding of proteins.12 We showed an slight amount of soluble recombinant antibody production 3 h after induction in osmotic shock condition (NaCl 0.5 M, glycine betaine 0.1 mM) which can be attributed to the combined effect of osmotic shock and the role of glycine betaine as a chemical chaperone.12,19 One may deduce that both increased concentration of chaperones due to high concentration of NaCl and chaperone like function of glycine betaine may assist the correct folding of scFv antibody.
Conclusion
In conclusion, our study described the role of temperature, type and concentration of inducer, incubation time, pH, Mg2+, sucrose, heat and osmotic shocks on soluble expression of GST-hD2 recombinant protein. The results showed that the induction by 0.03 to 0.3mMlactose for 48 h at 10 °C or addition of magnesium chloride into medium can demonstrate solubilizing effect on expression of scFv antibody. In general, incubation for 48 h induction by 0.03 to 0.3 mM lactose at low temperature was more effective than incubation time and different concentration of inducers or cultivation without inducer.
Also slight soluble GST-hD2 expression was detected by applying the osmotic shock protocol. Based on experiments mentioned above it is concluded that, the proper folding of GST-hD2 was under the influence the traffic of nascent protein expression and other factor like chaperone molecule which assist correct folding of nascent protein. As the levels of soluble expression of the target protein under the studied conditions were not extremely appropriate for large scale protein production, other strategies such as co-expression of chaperone molecules need to be evaluated for further improvement.11,17,23
Acknowledgments
The authors would like to thank the Research Office, Biotechnology Research Center and Faculty of Advanced Medical Science of Tabriz University of Medical Sciences for providing financial support under Postgraduate Research Grant Scheme toward MSc thesis of Mohammad Sina.
Ethical Issues
Not applicable.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Sfikakis PP, Markomichelakis N, Alpsoy E, Assaad-Khalil S, Bodaghi B, Gul A. et al. Anti-tnf therapy in the management of behcet's disease--review and basis for recommendations. Rheumatology (Oxford) 2007;46(5):736–41. doi: 10.1093/rheumatology/kem034. [DOI] [PubMed] [Google Scholar]
- 2.Feldmann M. Development of anti-tnf therapy for rheumatoid arthritis. Nat Rev Immunol. 2002;2(5):364–71. doi: 10.1038/nri802. [DOI] [PubMed] [Google Scholar]
- 3.Pucca MB, Bertolini TB, Barbosa JE, Galina SVR, Porto GS. Therapeutic monoclonal antibodies: Scfv patents as a marker of a new class of potential biopharmaceuticals. Braz J Pharm Sci. 2011;47(1):31–8. doi: 10.1590/S1984-82502011000100005. [DOI] [Google Scholar]
- 4.Skerra A, Pluckthun A. Assembly of a functional immunoglobulin fv fragment in Escherichia coli. Science. 1988;240(4855):1038–41. doi: 10.1126/science.3285470. [DOI] [PubMed] [Google Scholar]
- 5.Griffiths AD, Duncan AR. Strategies for selection of antibodies by phage display. Curr Opin Biotechnol. 1998;9(1):102–8. doi: 10.1016/S0958-1669(98)80092-X. [DOI] [PubMed] [Google Scholar]
- 6.Baneyx F, Mujacic M. Recombinant protein folding and misfolding in Escherichia coli. Nat Biotechnol. 2004;22(11):1399–408. doi: 10.1038/nbt1029. [DOI] [PubMed] [Google Scholar]
- 7.Guo JQ, Li QM, Zhou JY, Zhang GP, Yang YY, Xing GX. et al. Efficient recovery of the functional ip10-scfv fusion protein from inclusion bodies with an on-column refolding system. Protein Expr Purif. 2006;45(1):168–74. doi: 10.1016/j.pep.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 8.Guo JQ, You SY, Li L, Zhang YZ, Huang JN, Zhang CY. Construction and high-level expression of a single-chain fv antibody fragment specific for acidic isoferritin in Escherichia coli. J Biotechnol. 2003;102(2):177–89. doi: 10.1016/S0168-1656(03)00020-8. [DOI] [PubMed] [Google Scholar]
- 9.Kipriyanov SM, Moldenhauer G, Little M. High level production of soluble single chain antibodies in small-scale Escherichia coli cultures. J Immunol Methods. 1997;200(1-2):69–77. doi: 10.1016/S0022-1759(96)00188-3. [DOI] [PubMed] [Google Scholar]
- 10.Donovan RS, Robinson CW, Glick BR. Review: Optimizing inducer and culture conditions for expression of foreign proteins under the control of the lac promoter. J Ind Microbiol. 1996;16(3):145–54. doi: 10.1007/BF01569997. [DOI] [PubMed] [Google Scholar]
- 11.Song JM, An YJ, Kang MH, Lee YH, Cha SS. Cultivation at 6-10°C is an effective strategy to overcome the insolubility of recombinant proteins in Escherichia coli. Protein Expr Purif. 2012;82(2):297–301. doi: 10.1016/j.pep.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 12.Oganesyan N, Ankoudinova I, Kim SH, Kim R. Effect of osmotic stress and heat shock in recombinant protein overexpression and crystallization. Protein Expr Purif. 2007;52(2):280–5. doi: 10.1016/j.pep.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zarei Jaliani H, Farajnia S, Safdari Y, Mohammadi SA, Barzegar A, Talebi S. Optimized condition for enhanced soluble-expression of recombinant mutant anabaena variabilis phenylalanine ammonia lyase. Adv Pharm Bull. 2014;4(3):261–6. doi: 10.5681/apb.2014.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez-Montalban N, Garcia-Fruitos E, Villaverde A. Recombinant protein solubility - does more mean better? Nat Biotechnol. 2007;25(7):718–20. doi: 10.1038/nbt0707-718. [DOI] [PubMed] [Google Scholar]
- 15.Baneyx F. Protein expression technologies: Current status and future trends. Wymondham, Norfolk, UK: Horizon Bioscience; 2004. [Google Scholar]
- 16.Ahmad ZA, Yeap SK, Ali AM, Ho WY, Alitheen NB, Hamid M. Scfv antibody: Principles and clinical application. Clin Dev Immunol. 2012;2012:980250. doi: 10.1155/2012/980250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maeng BH, Nam DH, Kim YH. Coexpression of molecular chaperones to enhance functional expression of anti-bnp scfv in the cytoplasm of Escherichia coli for the detection of b-type natriuretic peptide. World J Microbiol Biotechnol. 2011;27(6):1391–8. doi: 10.1007/s11274-010-0590-5. [DOI] [PubMed] [Google Scholar]
- 18.Donovan RS, Robinson CW, Glick BR. Optimizing the expression of a monoclonal antibody fragment under the transcriptional control of the Escherichia coli lac promoter. Can J Microbiol. 2000;46(6):532–41. doi: 10.1139/w00-026. [DOI] [PubMed] [Google Scholar]
- 19.Barth S, Huhn M, Matthey B, Klimka A, Galinski EA, Engert A. Compatible-solute-supported periplasmic expression of functional recombinant proteins under stress conditions. Appl Environ Microbiol. 2000;66(4):1572–9. doi: 10.1128/AEM.66.4.1572-1579.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Q, Xu J, Li M, Lei X, An L. High-level expression of a soluble snake venom enzyme, gloshedobin, in e. Coli in the presence of metal ions. Biotechnol Lett. 2003;25(8):607–10. doi: 10.1023/A:1023067626846. [DOI] [PubMed] [Google Scholar]
- 21.Sorensen HP, Mortensen KK. Soluble expression of recombinant proteins in the cytoplasm of Escherichia coli. Microb Cell Fact. 2005;4(1):1. doi: 10.1186/1475-2859-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun XW, Wang XH, Yao YB. Co-expression of dsb proteins enables soluble expression of a single-chain variable fragment (scfv) against human type 1 insulin-like growth factor receptor (igf-1r) in e. Coli. World J Microbiol Biotechnol. 2014;30(12):3221–7. doi: 10.1007/s11274-014-1749-2. [DOI] [PubMed] [Google Scholar]
- 23.Heo MA, Kim SH, Kim SY, Kim YJ, Chung J, Oh MK. et al. Functional expression of single-chain variable fragment antibody against c-met in the cytoplasm of Escherichia coli. Protein Expr Purif. 2006;47(1):203–9. doi: 10.1016/j.pep.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Subedi GP, Satoh T, Hanashima S, Ikeda A, Nakada H, Sato R. et al. Overproduction of anti-Tn antibody MLS128 single-chain Fv fragment in Escherichia coli cytoplasm using a novel pCold-PDI vector. Protein Expr Purif. 2012;82(1):197–204. doi: 10.1016/j.pep.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 25. Karimi S. Design and construction of an expression vector encoding for humanized scfv antibody against human tnf-α. Tabriz: Azarbaijan Shahid Madani University; 2013.
- 26.Zhu C, Liu X, Feng J, Zhang W, Shen B, Ou'yang W. et al. Characterization of the neutralizing activity of three anti-human tnf monoclonal antibodies and prediction of their tnf epitopes by molecular modeling and mutant protein approach. Immunol Lett. 2006;102(2):177–83. doi: 10.1016/j.imlet.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Durao P, Chen Z, Fernandes AT, Hildebrandt P, Murgida DH, Todorovic S. et al. Copper incorporation into recombinant cota laccase from bacillus subtilis: Characterization of fully copper loaded enzymes. J Biol Inorg Chem. 2008;13(2):183–93. doi: 10.1007/s00775-007-0312-0. [DOI] [PubMed] [Google Scholar]
- 28.Kopetzki E, Schumacher G, Buckel P. Control of formation of active soluble or inactive insoluble baker's yeast alpha-glucosidase pi in Escherichia coli by induction and growth conditions. Mol Gen Genet. 1989;216(1):149–55. doi: 10.1007/BF00332244. [DOI] [PubMed] [Google Scholar]
- 29. Sambrook J, Russell DW. Molecular cloning: A laboratory manual. 3rd eds. New York: Cold Spring Harbor Laboratory Press; 2001.
- 30.Villaverde A, Carrio MM. Protein aggregation in recombinant bacteria: Biological role of inclusion bodies. Biotechnol Lett. 2003;25(17):1385–95. doi: 10.1023/A:1025024104862. [DOI] [PubMed] [Google Scholar]
- 31.Jun SA, Nam DH, Lo YS, Kim JJ, Chang H, Lee JO. et al. Functional expression of anti-hepatitis b virus (hbv) pres2 antigen scfv by cspa promoter system in Escherichia coli and application as a recognition molecule for single-walled carbon nanotube (swnt) field effect transistor (fet) Biotechnol Bioprocess Eng. 2010;15(5):810–6. doi: 10.1007/s12257-009-3040-1. [DOI] [Google Scholar]
- 32.Shibui T, Nagahari K. Secretion of a functional fab fragment in Escherichia coli and the influence of culture conditions. Appl Microbiol Biotechnol. 1992;37(3):352–7. doi: 10.1007/BF00210991. [DOI] [PubMed] [Google Scholar]
- 33.Berrow NS, Bussow K, Coutard B, Diprose J, Ekberg M, Folkers GE. et al. Recombinant protein expression and solubility screening in Escherichia coli: A comparative study. Acta Crystallogr D Biol Crystallogr. 2006;62(Pt 10):1218–26. doi: 10.1107/S0907444906031337. [DOI] [PubMed] [Google Scholar]
- 34.Weickert MJ, Pagratis M, Glascock CB, Blackmore R. A mutation that improves soluble recombinant hemoglobin accumulation in Escherichia coli in heme excess. Appl Environ Microbiol. 1999;65(2):640–7. doi: 10.1128/aem.65.2.640-647.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simmer JP, Lau EC, Hu CC, Aoba T, Lacey M, Nelson D. et al. Isolation and characterization of a mouse amelogenin expressed in Escherichia coli. Calcif Tissue Int. 1994;54(4):312–9. doi: 10.1007/bf00295956. [DOI] [PubMed] [Google Scholar]


