Abstract
Improvements in the control of haemorrhage after trauma have resulted in survival of many people who would otherwise have died from the initial loss of blood. However, the danger is not over once bleeding has been arrested and blood pressure restored. Two-thirds of patients who die following major trauma now do so as a result of causes other than exsanguination. Trauma evokes a systemic reaction that include an acute, non-specific, immune response associated, paradoxically, with reduced resistance to infection. The result is damage to multiple organs caused by the initial cascade of inflammation aggravated by subsequent sepsis to which the body has become susceptible. This Series examines the biological mechanisms and clinical implications of the cascade of events caused by large-scale trauma that leads to multiorgan failure and death, despite the stemming of blood loss. Furthermore, the stark and robust epidemiological finding – namely, that age has a profound influence on the chances of surviving trauma irrespective of the nature and severity of the injury – will be explored. Advances in our understanding of the inflammatory response to trauma, the impact of ageing on this response, and how this information has led to new and emerging treatments aimed at combating immune dysregulation and reduced immunity after injury will also be discussed.
Introduction
According to the World Health Organisation, trauma accounts for 10% of deaths and 16% of disabilities worldwide - considerably more than malaria, tuberculosis and HIV/AIDS combined.1 The proportion of deaths that are due to injury is rising worldwide, so that road traffic accidents alone are projected to be the fifth largest cause of death and disability by 2020.2 The peak age group of patients with traumatic injuries is in the second decade of life; however, older trauma victims have become more frequent as populations age. For reasons that will be discussed subsequently, they have higher mortality even after adjustment for comorbidity and extent of injury.
Without medical care most people with a severe bodily injury will bleed to death. This began to change in the 16th Century when French military surgeon, Ambroise Paré, first ligated arteries during amputation. Care improved gradually over the ensuing centuries and then more rapidly after the outbreak of the Second World War. Most patients who would previously have died now survive as a result of advances in the management of haemorrhage. The control of haemorrhage and coagulopathy after major trauma has been reviewed recently in the Lancet.3 Topics such as methods to stop bleeding, selection of blood products, finding the balance between underperfusion and overperfusion, and antifibrinolytic and procoagulation therapy were covered. However, many survivors of massive haemorrhage now go on to develop multiorgan failure often accompanied by sepsis.4 Multiorgan failure and sepsis are a result of the systemic response to severe injury, which compounds the original injury (Figure 1). Patients who experience severe trauma are now able to survive because of advances in the control and correction of massive blood loss. Thus, responses that were once protective after mild or moderate trauma are now exaggerated and destructive following massive trauma that is now survivable. Increased understanding of the biology of organ damage has spawned a new generation of proposed treatments for major trauma.5,6 In our review, we are concerned with the phase of injury in which the immediate threat of exsanguination has been averted, but the patient remains at risk of multiorgan failure and sepsis. Furthermore, we will review possible causes for the substantially worse prognosis experienced by older patients with traumatic injuries, independent of injury severity.
Figure 1. Pathways leading to tissue and organ damage after trauma.
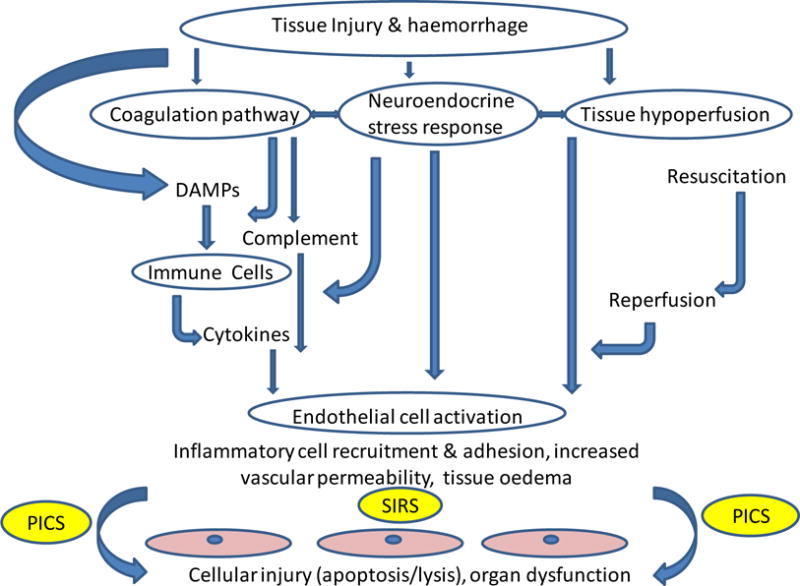
Tissue injury and haemorrhage can lead to systemic inflammatory response syndrome (SIRS), which, if persistent, may cause multiorgan failure. A loss of endothelial integrity can result from tissue hypoperfusion, and activation of coagulation and neuroendocrine pathways, thereby enabling humoral and cellular factors to damage tissues far from the site of the original injury.
DAMPs: damage-associated molecular patterns; PICS: Persistent Inflammation/Immunosuppression and Catabolism Syndrome.
Immune Response to Traumatic injury
The systemic response to severe injury involves interactions across the haemostatic, inflammatory, endocrine and neurological systems, aggravating initial damage caused by hypoperfusion (shock) and reperfusion (Figure 1). Endothelium activated by exposure to inflammatory cytokines becomes more porous, allowing mediators of tissue damage to gain access to the intercellular space. The systemic responses to major trauma are also associated with lowered ability to fight infection, leading to sepsis and further activation of the destructive inflammatory response.
Inflammatory response to injury (Figure 2)
Figure 2. Activation of the immune system after trauma and tissue damage, accompanied by increased susceptibility to infection.
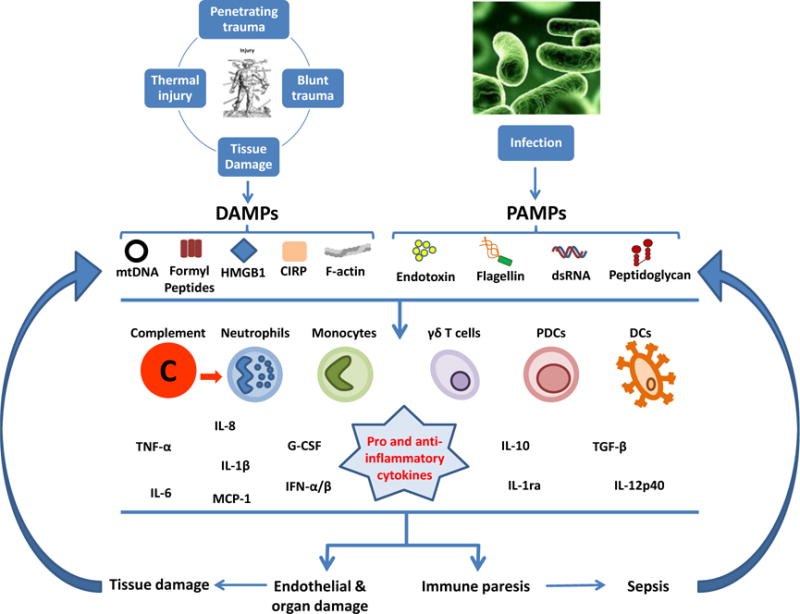
Tissue damage leads to the extracellular release of Damage Associated Molecular Patterns (DAMPs) that will trigger an inflammatory response in the absence of infection. Infection is associated with exposure of the immune system to a range of non-self molecules, collectively referred to as Pathogen Associated Molecular Patterns (PAMPs). PAMPs and DAMPs stimulate cells of the innate immune system and complement, which can lead to endothelial and organ damage, and immunoparesis. Organ damage and sepsis lead to the release of further DAMPs and exposure to additional PAMPs, respectively, resulting in a vicious cycle, with continued inflammation and immune activation.
mtDNA, Mitochondrial DNA; HMGB1, High mobility group box 1 protein; CIRP, Cold-inducible RNA-binding protein; dsRNA, double-stranded RNA; PDCs, Plasmacytoid dendritic cells; DCs, Dendritic cells, TNF-α, Tumour necrosis factor-alpha; G-CSF, Granulocyte-colony stimulating factor; MCP-1, Monocyte chemoattractant protein 1; TGF-β, Transforming growth factor-beta; IFN, interferon; IL, interleukin.
Severe injury is associated with the Systemic Inflammatory Response Syndrome (SIRS).7 This response starts within thirty minutes of a major injury, and is an inflammatory response to blood loss and tissue damage rather than infection.
SIRS results from the release of endogenous factors termed damage-associated molecular patterns (DAMPs) or ‘alarmins’7,8 after tissue injury.9 They are secreted by activated immune cells such as neutrophils8 or released from necrotic cells.10 Peptides and mitochondrial DNA released during necrosis provoke a particularly vigorous foreign body reaction, probably because they are ultimately derived from intracellular bacteria. DAMPs directly activate several immune cells, including neutrophils and monocytes, via cell-surface DAMP receptors.8 DAMPs are also potent activators of complement, leading to rapid generation of C3a and C5a.11,13 Activation of complement and of inflammatory cells triggers the production and release of inflammatory mediators such as interleukins, thereby generating the systemic response seen in SIRS.14 Many therapies to prevent multiorgan failure are directed at targets on the SIRS pathway.
The immune system contains a series of feedback loops to restore homeostasis. SIRS is associated with a compensatory anti-inflammatory response characterised by raised levels of anti-inflammatory cytokines (e.g. IL10 and TGFβ) and cytokine antagonists (e.g. IL1-Ra).15 Depending on the balance of pro- and anti-inflammatory factors, the response might return to baseline or progress to Persistent Inflammation, Immunosuppression and Catabolism Syndrome (PICS) with an increased risk of multiorgan dysfunction and sepsis.16 The risk of such a sustained inflammatory response increases with age for a given severity of trauma.17
Paradoxically, heightened non-specific immunity in SIRS is accompanied by suppression of the body’s ability to mount a defence against invading pathogens. The result is increased susceptibility to infection and sepsis, with the invading microbes further stimulating immune cells via their Pathogen-Associated Molecular Patterns (PAMPs) such as lipopolysaccharide. A vicious circle ensues, with SIRS resulting in inflammation and immunoparesis, which, in turn, leads to sepsis with further inflammation and risk of multiorgan failure (Figure 2). The inflammatory response also includes rapid activation of the complement system – initial activation is followed by consumption and a subsequent imbalance in the components of the complement cascade,11 which is one of many factors that reduces the ability of the body to defend against microorganisms.
Haemostasis and inflammation
According to the cell-based theory of coagulation, activated platelets provide a surface that promotes interaction of clotting factors in the development of a clot.18 Platelets activated by trauma and coagulation release proinflammatory mediators that excite the immune system,19 thereby promoting SIRS. Activation of the immune system increases platelet activity, generating a self-perpetuating cycle.19 Platelets form leukocyte-platelet aggregates, which are potent activators of immune cells and cause endothelial cell damage.20 Platelets21 and neutrophils22 are also major sources of microvesicles and exosomes, which express surface markers and can contain various molecules (including cytokines, miRNA, metabolites and lipids)23 that propagate SIRS. These molecules might prove to be useful targets for treatment. The humoral elements of the coagulation and complement pathways act together to initiate the inflammatory response, with C3a, C5a, and fibrin all known to be neutrophil chemoattractants.13 Plasmin, an important antifibrinolytic molecule, also stimulates the complement cascade.
Neutrophils
High concentrations of proinflammatory cytokines increase the activity of neutrophils causing them to migrate across damaged endothelium and become sequestrated in so-called ‘bystander’ organs.24 Here, the destructive armamentarium of the neutrophil, including proteases (such as neutrophil elastase) and reactive oxygen species, are deployed against healthy tissue. This process exacerbates inflammation and leads to the development of localised organ damage, such as that seen in Acute Respiratory Distress Syndrome (ARDS). Neutrophils have been shown to release their DNA to trap and kill pathogens extracellularly.25 However, this benefit is to some extent negated by histones in the DNA net acting as DAMPs to initiate further inflammation. Importantly, although neutrophils are initially activated as a result of SIRS, their bactericidal function is markedly impaired in the days that follow.26 This decrease might be compounded by release of immature banded neutrophils expressing low levels of the Fcγ receptor CD16 into the circulation.27 Several treatment strategies have been targeted at reduction of the cascade of events triggered by activated neutrophils.
The endocrine system
Cortisol released in response to injury is profoundly anti-inflammatory, inhibiting the activation of innate and adaptive immune cells, and inducing apoptosis in lymphocytes. Furthermore, cortisol strongly induces the release of neutrophils from the bone marrow and from the ‘marginated pool’ within tissues.28 Activation of the hypothalamus-pituitary-adrenal axis usually leads to concomitant release of dehydroepiandrosterone (DHEA) and its sulphated ester DHEAS. However, following trauma, DHEAS levels fall and cortisol steroidogenesis predominates.29 The resulting increased cortisol:DHEA ratio is associated with increased infection rates after trauma.30 Supplementation with DHEA reduced sepsis-related mortality in animals,31 and provides a promising hypothesis for clinical studies.
Non-protein factors in response to injury
Over the last decade, kinetics and amplitude of acute inflammatory responses have been shown to also be regulated by non-protein effectors, including lipid mediators, such as protectins, maresins, resolvins,32 and microRNAs. The discovery that some lipids (e.g. resolvins and maresins) reduce the acute inflammatory response has led to intervention studies using fish oil supplementation to modulate immune response after trauma (discussed later). Several trials are underway to assess the role of statins and dietary modifications as anti-inflammatory agents in trauma (Appendix page 4 and 6).
The brain and inflammation
The interaction between the brain and nervous system is bidirectional; the traumatised brain exacerbates both SIRS and immunoparesis through parasympathetic and sympathetic pathways, respectively.33,34 Furthermore, the complement system has been shown in human and experimental traumatic brain injury models to be an early mediator of post-traumatic neuroinflammation and secondary neuronal damage, ultimately leading to behavioural, emotional, and cognitive problems.35.The brain is also affected by a purely somatic injury. Endothelial damage as a result of raised proinflammatory cytokines and shock reduces the integrity of the blood-brain barrier.36 Not only does this reduced integrity allow humoral mediators of inflammation to enter the brain parenchyma, but it also permits migration of monocytes which then take on the morphological appearance of microglial cells.37 Receptors for pro-inflammatory cytokines are highly expressed in the hippocampus,36 a structure involved in memory consolidation and neuroplasticity, and chronic inflammatory cell activation in this organ may lead to irreversible cognitive decline, especially in the elderly people.
Age and sex differences
Elderly people have a significantly worse prognosis after trauma independent of the nature or severity of their injury, and despite adjustment for comorbidities.38,39 The association between age and outcome of injury is potentially confounded, even after risk adjustment, by factors that contribute to the risk of sustaining an injury in the first place. However, such residual confounding is unlikely to explain the extent of the association between age and mortality which varies 3–4 times between the second and ninth decade of life.40,41 Similar age effects are seen in animals.42 A biological definition of ageing is the “increase in frailty of an organism with time that reduces its ability to deal with stress”. It can be conceptualised as a reduction in the range of stress over which the body can maintain its milieu intérieur. Although the precise mechanisms that relate age to survival of the stress of trauma are not fully elucidated, several plausible hypotheses have been proposed.42 Elderly people are known to have a low-grade inflammatory state at baseline, termed “inflammageing”.43 When injury occurs, the SIRS response can be exaggerated in old people, perhaps because the body has been ‘primed’ by elevated baseline levels of cytokines, or as a result of their reduced ability to produce anti-inflammatory cytokines such as IL10.17 Moreover, the capacity to respond to pathogens is more severely suppressed owing to the age-related decline in both the non-specific (innate) and specific (adaptive) immune pathways; a deterioration known as immunosenescence.43,44 A raised cortisol:DHEAS ratio is seen in all patients after trauma, but the age-related loss of DHEA and DHEAS (adrenopause) could exacerbate this increase contributing to immunoparesis in older trauma patients.30
Although no consensus has been reached,45 large retrospective studies have reported male sex, in addition to old age, as a major risk factor for pneumonia and sepsis after major trauma.46 This finding might be the result of estrogen modulation of pro- and anti-inflammatory pathways.45
Clinical mitigation strategies
Classification of interventions in the post-resuscitation phase
To identify interventions used in the post-resuscitation phase, we systematically searched for relevant randomised controlled trials (RCTs) and reviews of RCTs, and selected articles with clinical endpoints (see Box and Appendix page 2).47 Treatment strategies to prevent multiorgan failure following acute resuscitation can be categorised as those that aim to modulate SIRS, stimulate natural immunity, and prevent microbial proliferation.
Box. Search strategy and selection criteria.
We aimed to produce a usable and accessible document through compilation of evidence from multiple systematic reviews of interventions according to the Cochrane guidelines.47 We searched Medline and Embase for reports published in English between Jan 1, 2008, and Dec 5, 2013, related to trauma and injuries (full search terms are listed on Appendix page 2). We scrutinised abstracts and identified systematic reviews of trials with clinical endpoints. We also searched for studies on trauma care in the Cochrane Database of Systematic Reviews (which includes reviews produced by the Cochrane Groups for Injuries, Anaesthesia and Bone, Joint and Muscle Trauma). Because not all reviews were up to date and some key trials might not have been included in our initial search, we supplemented our overview as follows. First, we searched the Cochrane Central Register of controlled trials for trials of trauma care. Second, we added seminal papers known to authors and suggested by referees. Finally, we searched the reference lists of retrieved papers for reviews of studies that had not already been retrieved, including those in related fields of intensive care and surgery. We examined the reviews and individual papers for methodological issues (such as failure to adhere to intention-to-treat principles) and other salient features (such as heterogeneous results). In addition we searched WHO International Clinical Trials Registry Platform for ongoing trials by combining the term ‘trauma’ with ‘immune’ or ‘inflammation’. We selected ongoing trials that assessed immunomodulatory interventions in trauma patients, including those investigating only surrogate outcomes, and present details in the Appendix (page 4).
Interventions to modulate SIRS
Numerous interventions have been postulated to attenuate or truncate excessive systemic immune activation (Table 1). A large number of therapies (e.g. prostaglandin E1, monoclonal antibodies against β2 intergrin, anti-L-selectin [CD62L], and leukocyte reduced blood) have targeted activated neutrophils but yielded null results. Trials of antioxidants and continuous venous haemofiltration have also produced null results. Likewise, the only trial56 of statins that assessed the systemic effects of trauma yielded a null result (Table 1), although many more trials of statin therapy are underway (Appendix page 4). By contrast with these null results, systemic hydrocortisone was reported in one RCT to halve hospital-acquired pneumonia and increase mechanical ventilation-free days.49 As noted by the authors, this finding conflicts with the results of CRASH 1 – a study59 of the use of steroids in 10,008 patients with head injury that showed an increased death rate with this intervention. The reported increased mortality is often attributed to the systemic (non-brain) effects of steroids in aggravating immunosuppression.
Table 1.
Proposed interventions to modulate systemic immune response in acute trauma* (fluid resuscitation strategies are discussed in the text)
| Postulated immune-modulating mechanism | Evidence on effectiveness | Study | |
|---|---|---|---|
| Leucoreduced blood | Minimisation of the immunomodulatory effects of leukocytes in transfused blood | No significant difference versus non-reduced blood in terms of effect on infection (RR 0·84, 95% CI 0·55–1·30, 30% reduced blood vs. 36% non-reduced blood) or mortality (RR 1·20, 95% CI 0·74–1·90, 19% vs. 15%) | One RCT (n=268), Nathans et al. (2006)48 |
| Hydrocortisone | Attenuation of trauma-related corticosteroid insufficiency and inflammatory response | Reduction vs. placebo in hospital-acquired pneumonia (HR 0·51, 95% CI 0·30 to 0·83) and increase in mechanical ventilation-free days (by 4 days, 95% CI 2 to 7) | One RCT (n=149), Roquilly et al. (2011)49 |
| Prostaglandin E1 | Suppressing neutrophil activities | No significant difference vs. placebo in terms of effect on mortality (RR 0·93, 0·37 to 2·37)a, sepsis (RR 1·09, 0·45 to 2·.62)a, units of blood transfused (mean difference −5·00, 95% CI −13·32 to 3·32) or severe respiratory failure (RR 0·41, 0·12 to 1·35)a | One RCT (n=48), Vassar et al. (1991)50 |
| Monoclonal antibody against CD18 (rhuMab CD18) | Prevention of resuscitation injury caused by neutrophils by blocking neutrophil-endothelial interaction | No significant difference vs. placebo in terms of effect on mortality (RR 0·87, 95% CI 0·18 to 4·26, 5·8% vs. 6·7%)a or major or minor infection (RR 0·96, 0·57 to 1·60, 38% vs. 40%)a | One RCT (n=116), Rhee et al. (2000)51 |
| Anti-L-selectin (aselizumab) | Prevention of resuscitation injury caused by neutrophils by blocking L-selectin, which is an adhesion molecule involving in the migration of neutrophils across the vascular endothelium | No significant difference vs. placebo in terms of effect on mortality (RR 0·44, 95% CI 0·16 to 1·23, 11% vs. 25%)a and infection (RR 1·22, 95% CI 0·79 to 1·88, 67% vs. 55%)a | One RCT (n=84), Seekamp et al. (2004)52 |
| Antioxidants (Superoxide dismutase) | Minimisation of damage caused by the increased production of free radicals due to trauma | No significant difference vs. placebo in terms of effect on mortality (RR 2·00, 95% CI 0·21 to 19·23, 17% vs. 8·3%)a | One RCT (n=24), Marzi et al. (1993)53 |
| Continuous venovenous haemofiltration | Elimination of a broad spectrum of inflammatory mediators | No significant difference vs. standard care in terms of effect on occurrence of SIRS (RR 0·91, 95% CI 0·67 to 1·23)a | One RCT (n=24), Bauer et al. (2001)54 |
| Statins | Wide ranging anti-inflammatory effects through HMG-coenzyme A reductase inhibition on cellular processes such as cell migration, reactive oxygen species generation, and secretion of proinflammatory cytokines that contribute to the modulation of the inflammatory response | Quantitative results from one of the three RCTs are shown below (one RCT did not report clinical outcomes and another focus on TBI). Results are for simvastatin only; no significant difference versus placebo in terms of effect on mean time to fracture union (mean difference 0·30 days, 95% CI −10·53 to 11·13) | Three RCTs (total n=103) reviewed by Jansen et al. (2013),55 one RCT (n=62), Patil et al. (2009)56 |
| Tranexamic acid | Clot stabilisation; reduction of the inflammatory effects of plasmin by blockade of its conversion from plasminogen | Reduction in mortality vs. placebo (RR 0·90, 95%CI 0·85 to 0·97), in particular due to bleeding (RR 0·85, 95%CI 0·76 to 0·96) and myocardial infarction (RR 0·32, 95%CI 0·14 to 0·75); no significant effect on the risk of receiving surgical interventions (RR 1·00, 95%CI 0·63 to 1·51) or blood product transfusion (RR 0·.98, 95% CI 0·96 to 1·01). | Two RCTs (including CRASH 2, total n=20367), reviewed by Roberts et al. (2011)57 |
| Aprotinin | Clot stabilisation | No significant difference vs. placebo in terms of effect on mortality (RR 0·14, 95% CI 0·01 to 2·67), need for surgical intervention (RR 1·07, 95% CI 0·87 to 1·33) and volume of blood transfused (mean difference −0·40 units, 95% CI −0·91 to 0·11) | Two RCTs (total n=97), reviewed by Roberts et al. (2011)57 |
| Antithrombin III | Potent anticoagulant with independent anti-inflammatory properties. Inactivates thrombin, decreases interleukin 8 production and sequesters neutrophils | No significant difference vs. control in terms of effect on mortality (two RCTs; RR 2·15, 95% CI 0·81 to 5·72), bleeding event (one RCT, RR 3·43, 95% CI 0·15 to 77·58) and multiorgan failure (one RCT, 20% vs. 30%, RR 0·67, 95% CI 0·22 to 2·01)a | Two RCTs (total n=68), reviewed by Afshari et al. (2008)58 |
CI – confidence interval; HR – hazard ratio’ RCT – randomised controlled trial; RR – relative risk; SIRS – systemic inflammatory response syndrome; TBI – traumatic brain injury.
Relevant articles, for example on granulocyte-macrophage colony-stimulating factor, that were not done (fully or in part) with populations of trauma patients are not included in the table, but are mentioned in the text where relevant. Only interventions where evidence on clinical outcomes is available from randomised controlled trials are included.
The effect size and its confidence interval was calculated by authors of this paper using data reported in the trial.
As discussed previously, the haemostatic and immune systems interact in response to trauma, and plasmin can stimulate complement activation. Tranexamic acid competitively inhibits the conversion of plasminogen to plasmin, which, in turn, inhibits fibrin degeneration. This inhibition of fibrin degeneration through the use of tranexamic acid might have contributed to the improved survival reported in the CRASH 2 trial60 and in the joint British and American observational study of battlefield injuries61 not only by improving clot stability, but also by restricting the inflammatory response that can be provoked by fibrin degradation products. Other factors affecting coagulation (aprotinin57 and antithrombin III58) yielded null results, albeit within wide confidence limits. Post-injury coagulopathy is associated with hyper-inflammation and point-of-care thromboelastography has been suggested with view to early recognition of this condition.62 Use of this method was associated with a significant but small (85 ml) reduction in blood loss in a meta-analysis of nine cardiac surgery and liver transplantation trials; however, other end-points including mortality were not significantly affected.63
Notably, all these trials involved small sample sizes, with the exception of CRASH 2, which was an order of magnitude greater than the largest of the remaining studies.
The type of fluid used in resuscitation is noteworthy because intravenous infusion continues beyond the resuscitation phase and some substances (e.g. dextran and hypertonic saline)64 have immunomodulatory effects. Here, many lessons can be learned from critically ill patients (Appendix page 7). Perhaps the most important finding is that starch is harmful, possibly because it leaks across endothelia damaged in the SIRS process. This finding has been established within narrow confidence limits for critical care as a whole.65 In trauma specifically, the confidence limits are wide but the observed direction of effect is consistent with the aforementioned findings (Appendix page 7). A network meta-analysis in sepsis patients confirms the deleterious effect of starch while also showing that mortality is higher with normal saline than with either albumin or ‘balanced’ crystaloids such as Ringers lactate that contain an organic anion.66 Volume replacement strategy is relevant to the immune response: guidelines67,68 recommend a ‘hypotensive’ strategy69 (maintaining systolic blood pressure at 80 to 90 mm Hg) to stabilise clots prior to surgery, but maintaining this level for prolonged periods aggravates the systemic inflammatory response.70 Restoration of blood pressure once haemostasis is secured therefore seems advisable.71 As a general rule, this restoration should be accomplished with restrictive rather than liberal use of red blood cells. Although trial evidence in trauma is limited,68 a restrictive transfusion strategy has been shown to decrease the risk of infection (relative risk [RR] 0·81, 95% CI 0·66 to 1·00) and hospital mortality (RR 0·77, 95% CI 0·62 to 0·95) in a systematic review of RCTs of predominantly surgical and critically ill patients.72
Interventions that stimulate natural immunity
Treatments that aim to augment the body’s immunity against infection are of two broad types (Table 2). The first group consists of biological agents targeting a specific part of the immune pathway, such as interferon-γ, immunoglobulin, and granulocyte-macrophage colony stimulating factor (GM-CSF). The second group is composed of nutritional therapies usually given enterally, and includes immunonutrition (nutritional supplementation with aminoacids, nucleotides and fish oils), glucans, and probiotics and synbiotics. Interest in assessment of enteral therapies has increased after widespread introduction of early enteral feeding in critically ill patients based on RCT evidence that included patients with traumatic injuries.73
Table 2.
Interventions that aim to augment or stimulate the body’s immunity after acute trauma*
| Postulated mechanism | Evidence on effectiveness | Study | |
|---|---|---|---|
| Interferon-γ | Upregulation of HLA-DR expression | No significant effect vs. placebo on pneumonia or major infection (four RCTs; RR 1·05, 95% CI 0·80 to 1·38, I2=54%)a and mortality (four RCTs, RR 0·67, 0·31 to 1·41, I2=43%)a | Four RCTs (total n=385), reviewed by Spruijt et al. (2010)5 |
| Immunoglobulin | Neutralisation of pathogenic antigens | Reduction in pneumonia vs. placebo (37% vs. 58% in one RCT, 10% vs. 61% in another)b; no significant effect vs. placebo on mortality from infection (one RCT, RR 1·10, 95% CI 0·45 to 2·69)c | Two RCTs (total n=189), reviewed by Spruijt et al. (2010)5 |
| Nutritional support and immunonutrition (including formulas supplemented with arginine and/or glutamine and/or fish oil) | Prevention of immune dysfunction associated with malnutrition; fish oil has anti-inflammatory properties | No significant effect vs. control on mortality (OR 1·03, 95% CI 0·40 to 2·65), infection (OR 0·72, 95% CI 0·27 to 1·91) and length of hospital stay (−0·77 days, 95% CI −15·99 to 14·45) | Seven RCTs (total n=372), reviewed by Marik and Zaloga (2008),73 and one RCT (n=43) by Pérez-Bárcena et al. (2010)74 |
| Glucan | Stimulation of macrophages | Reduction vs. placebo in septic morbidity or sepsis (two RCTs; RR 0·23, 95% CI 0·09 to 0·64)a and mortality related to sepsis or infection (two RCTs; RR 0·14, 95% CI 0·03 to 0·76)a | Two RCTs (total n=79), reviewed by Spruijt et al. (2010)5 |
| Probiotics | Strengthening of host immunity through microorganism-host crosstalk (such as MAMPs of probiotics and PRRs of the gastrointestinal mucosa) | Reduction vs. control in nosocomial infections (five RCTs; RR 0·65, 95% CI 0·45 to 0·94, I2=73%) and ventilator-associated pneumonia (three RCTs, RR 0·59, 95% CI 0·42 to 0·81); no significant effect on mortality (four RCTs, RR 0·63, 95% CI 0·32 to 1·26) | Five RCTs (total n=281), reviewed by Gu et al. (2013)75 |
MAMPs - microorganism-associated molecular patterns. PRRs - pattern recognition receptors.
Only interventions where evidence on clinical outcomes is available from randomised controlled trials are included.
Meta-analysis was not carried out in the cited systematic review. The pooled result and its confidence interval were calculated by authors of this paper. I2 value is shown only when at least moderate heterogeneity was observed (≥30%).
Meta-analysis was not carried out in the cited systematic review. Data was not pooled due to a high level of statistical heterogeneity (>60%).
The effect size and its confidence interval was calculated by authors of this paper using data reported in the trial.
Parenteral biological agents
Immunoglobulin had no effect on mortality, but reduced pneumonia in two trials with very heterogeneous effect sizes: a reduction from 61% to 10% in one study76 and from 58% to 37% in the other.77 The effectiveness of interferon-γ was assessed in a systematic review of four RCTs.5 Infection rates were reduced in one of the four studies and mortality in another, but results were not pooled and some studies used a per-protocol analysis. We therefore did a meta-analysis using all available data (i.e. including early deaths that occurred after randomisation on an intention-to-treat basis), which yielded non-significant results (Table 2). Lastly, GM-CSF and granulocyte colony stimulating factor (G-CSF) were examined in a meta-analysis, but this was in patients with sepsis and not specifically in those with traumatic injuries.78 The results in this broad setting remain equivocal and Bo and colleagues78 concluded that findings based on present trial evidence do not justify routine use of G(M)-CSF.
Nutritional therapies
Numerous RCTs have investigated the effects of immunonutrition in diverse populations of critically ill patients.73,79 Twenty-four RCTs were included in a recent meta-analysis,73 and seven of these studies examined immunonutrition in trauma. No significant effect on mortality was noted when results were pooled across all studies (odds ratio [OR] 0·85, 95% CI 0·64 to 1·13) or trauma studies (OR 1·03, 95% CI 0·40 to 2·65), but a reduction in infection was noted (OR 0·69, 95% CI 0·51 to 0·92). The direction of effect was similar specifically for patients with traumatic injuries, but was not significant (OR 0·72, 95% CI 0·27 to 1·91). However, a randomised trial has emerged, showing that adding immune-modulating treatments (omega-3 fatty acids, glutamine, and antioxidants) had no measurable short-term benefits over standard high-protein enteral nutrition in critically ill patients (including trauma victims) and might increase mortality at 6 months.80 The effect of glucan on mortality related to sepsis and infection was investigated in two trials (RR 0·14, 95% CI 0·03 to 0·76);81,82 however, the 86% reduction in mortality reported was higher than would be expected. Probiotics were associated with reduced nosocomial infections and ventilator-associated pneumonia in trauma patients according to a recent meta-analysis of five small RCTs (281 patients overall).75 However, these findings require confirmation as results from meta-analyses of RCTs in larger samples of patients receiving critical care for all reasons (not specifically trauma) remain inconclusive.83,84
Prevention of microbial proliferation
From the early days of microbiology, two broad approaches to combating infection have been recognised: the patient’s immune response can be augmented or the microorganism can be targeted directly with anti-microbial drugs. Use of prophylactic antibiotics in trauma to overcome the vicious cycle of inflammation and infection has been proposed, and this strategy is supported by good evidence that prophylactic antibiotics are effective in reducing the risk of infection after surgery85 and that the relative effect size is not affected by whether or not the wound is obviously contaminated.86 Specifically in patients with trauma, antibiotics reduced infection after an open limb fracture in a review of RCTs including 1106 patients (RR 0·43, 95% CI 0.29 to 0.65).87 The systematic review comparing prophylactic antibiotics with no prophylaxis in critically ill trauma patients showed that antibiotic treatment combined with selective decontamination of the digestive tract significantly reduced respiratory tract infection (OR 0·38, 95% CI 0·29 to 0·52). The trend in overall mortality was consistent with this finding, but not significant statistically (OR 0·78, 95% CI 0·56 to 1·09).88
Future Research
Owing to our increased understanding, the pathway between injury and organ damage can be mapped in increasing detail. This mapping has resulted in a growing list of therapeutic targets – recent additions include non-protein molecules and cellular fragments such as exosomes. However, clinical trials with adequate statistical power to show worthwhile reductions in mortality following acute trauma are not trivial undertakings; the CRASH 2 trial, which showed a reduction in mortality of nearly 10%, included 20,211 trauma patients from 275 hospitals across 40 countries.60 Thus, trauma exemplifies a pervasive problem for translational medicine – we are now at a point where identification of targets for therapeutic intervention often outstrips capacity to do adequately powered trials.89 The inconclusive results of the many different intervention studies in Table 1 are testament to this problem in the field of trauma.
Two (non-exclusive) solutions to the problem have been proposed; increasing the number and size of clinical trials, and improving the amount of information gathered from a given level of trial activity. A large literature concerning methods to increase the number and size of trials has been reviewed elsewhere.90,91 In the particular case of trauma, capacity to conduct large-scale trials has improved over recent years. First, there has been a clarification of requirements for randomisation of people from whom informed consent cannot be obtained.92 Second, the organisation of trauma care has been systematised through the creation of networks of collaborating major trauma centres worldwide.93 However, it is doubtful that activity can be increased to a sufficient degree to keep pace with discovery – it is a feature of science that one answer generates more than one contingent question.94 We are therefore going to have to “get smarter” at selecting potential therapies and learning from trials. Several methods could contribute towards these aims.
First, the effects of the treatment can be assessed in the group of patients with the greatest capacity to benefit. This can be accomplished by conducting an efficacy trial including only patients thought to have the greatest capacity to benefit in the intervention group. Alternatively, it may be possible to define an adequately powered subgroup of such patients in a pragmatic trial of effectiveness.89 Groups with a high capacity to benefit include those with the most tissue damage (e.g. burn and blast injuries), elderly people, and those with the most severely deranged biomarkers for inflammation and coagulopathy.
Second, a good argument exists for integration of laboratory and clinical science, both in early phase studies and as nested studies within larger trials. For example, the theoretical idea that the potent vasodilator, dopexamine, would improve intestinal oxygenation (and hence reduce absorption of toxic substances from the gut lumen) was not borne out in a ‘translational’ RCT assessing biological and surrogate outcomes in 30 patients.95
Third, a focus on trauma should not preclude lessons that can be learned from related fields. The pathways leading to organ failure after extensive trauma are largely shared by other critical illnesses, such as acute pancreatitis, and it is possible to ‘borrow strength’ by making cautious inductive inferences from other critical illnesses of this type.96 For example, starch infusions have been discontinued in trauma care largely on the basis of their harmful effects across critical diseases as a whole.65 Research in general surgery might also hold lessons for trauma clinicians. Such ‘controlled trauma’ is associated with less tissue destruction and prolonged shock than is typical of severe trauma scenarios, but evidence might nevertheless be transferable – the importance of prophylactic antibiotics and maintaining body temperature, for example. The rationale for the CRASH 2 trial was based in part on evidence that tranexamic acid reduces blood loss in general surgery.
Lastly, we should search for the most propitious targets for intervention by identifying critical nodes on the pathophysiological pathway. That is to say, simple identification of an inflammatory cascade does not translate into knowledge of where on the pathway to intervene. A system-wide view thus provides clues for research priorities. We have seen that the over-exuberant inflammatory response to major injury is associated with immunoparesis. This finding would suggest that treatments, such as corticosteroids, that reduce both SIRS and immune competence are likely to be less effective than treatments that reduce SIRS but leave immune defences intact (or enhanced). This theoretical argument gains empirical support from the CRASH 1 trial, as discussed above. Treatments that may be selective for the SIRS pathway include those that impact on the initial stages of activation. Such treatments include those (e.g. tranexamic acid) whose effect on the coagulation cascade spills over to reduce complement activation and, hence, inflammation. The statistically significant (36%) reduction in arterial thrombosis (with no increase in venous thrombosis) in the CRASH 2 trial is consistent with such an anti-inflammatory effect.
Although other treatments targeted at the SIRS pathway (Table 1) have not yet proven effective, many studies are ongoing, including one investigating a C1-esterase inhibitor (C1INH).97 Likewise, no less than seven trials of statins are underway. The pleiotropic effects of statins on the SIRS cascade, including reduced leukocyte and platelet adhesion, and inhibition of endothelial nitric oxide synthase production, have led to the implementation of studies investigating their potential therapeutic effect. The effect of statins may be twofold: they not only have anti-inflammatory properties but also promote the bactericidal function of neutrophils.98 Because SIRS is associated with immunoparesis, it seems logical to complement efforts to reduce inflammation with treatments to promote the body’s natural immunity. Again, promising ideas are in the pipeline, including androgens to reverse the change in the cortisol:DHEAS ratio that occurs after trauma, and which is associated with an increased risk of infection. Upregulation of haeme oxygenase predisposes patients with malaria to bacterial infections (such as Salmonella)99 and that inhibition of these enzymes can improve outcomes in animals with sepsis, suggesting a new treatment for assessment in people.100
Although our understanding of the pathways leading to inflammatory organ damage and increased susceptibility to infection after trauma have provided plausible hypotheses, this understanding falls well short of a comprehensive, dynamic picture of how events unfold. A more in-depth systems biology approach is needed if we are to reach the point where we can indeed identify promising therapies by making increasingly valid predictions of effectiveness. Understanding complex biological mechanisms in a dynamic sense (i.e. as they unfold over time through myriad feedback loops) is a subject of increasing attention in medical research. The most ambitious projects seek to model these processes through the use of computer-generated simulation models. Such models have been successful at the cellular level,101,102 and attempts have been made to use such models for large systems,103 including trauma.104 The identification of critical control points in causal pathways where interventions can prevent harmful chain reactions but leave necessary responses intact is central to building such models. The extent to which models that attempt to represent the complexity of pathophysiological pathways will help selection of the most promising candidates or identify genuine surrogate outcomes for adaptive designs, remains unknown. For now, careful laboratory experiments are needed, which may lead to pilot studies in humans for proof-of-principle purposes followed by large-scale assessments of the most promising therapies. The results of these trials will not only be pragmatically useful, but will also enable a more complete understanding of the biological mechanisms in play and even, perhaps, challenge some of the premises in this article.
Supplementary Material
Key messages.
Victims of trauma frequently survive the initial insult owing to rapid volume replacement and haemostasis; however they are at risk of multiorgan failure as a result of an over-exuberant, systemic inflammatory response.
This systemic response not only aggravates the initial organ damage caused by shock, but also reduces the body’s ability to fight infection; this process can lead to an increased risk of sepsis, which, in turn, triggers a further vicious cycle of inflammation, immunoparesis, and infection.
Recent laboratory research has improved our understanding of the complex interaction between the haemostatic, inflammatory, endocrine and neurological systems; new therapeutic targets such as microvesicles, extracellular DNA, and non-protein mediators have been discovered.
The aim of treatment is to reduce inflammation without aggravating immunoparesis; most randomised controlled trials assessing treatments targeting systemic immune responses in trauma were inadequately powered and inconclusive; many trials of promising treatment strategies; including statins, immunonutrition and those targeting neutrophil function, are ongoing.
The beneficial effects of tranexamic acid demonstrated in the CRASH-2 trial might be attributed not only to its antifibrinolytic effect, which reduces bleeding, but also to its immunomodulatory effect.
The use of antibiotic prophylaxis, avoidance of starch for fluid resuscitation, and limited use of red-blood-cell transfusions are supported by several studies that include evidence from patients with major trauma.
Priorities for future research includes the use of a systems biology approach for identification of the most propitious therapeutic targets, nesting studies of biological mechanisms within clinical trials, and judicious selection of results from trials in intensive care and general surgery to support studies in patients with traumatic injuries.
Acknowledgments
Medical Research Council provided funding (see above) for a series of workshops related to trauma research, which lead to the development of this paper. The views expressed in this paper are those of the authors. The funder was not involved in the preparation of the manuscript or the decision to submit it.
Yen-Fu Chen is funded by NIHR SRMRC and NICE (as shown above). Tony Belli’s salary is partially funded by the NIHR SRMRC. Elizabeth Kovacs is supported by a National Institute on Aging grant (NIH AG018859).
Professor Julian Bion helped in identification for trials in intensive care of special relevance to prevention of multi-organ failure in trauma. We thank Pamela Nayyar for her administrative support and Zulian Liu for her help in searching ongoing trials.
Footnotes
Conflict of interest statement
Janet Lord
Receives a salary from the University of Birmingham. She has no conflict of interest to declare.
Mark Midwinter
Is a full time salaried military medical officer. The views in this manuscript are personally endorsed but do not represent official Ministry of Defence opinion. He has no other conflict of interest.
Yen-Fu Chen
Receives funding as salary from the UK National Institute for Health Research Surgical Reconstruction and Microbiology Research Centre (NIHR SRMRC) and the National Institute for Health and Care Excellence (NICE). He has no other conflict of interest.
Antonio Belli
Nothing to declare.
Karim Brohi
Nothing to declare.
Leo Koenderman
Receives his salary from the University Medical Center Utrecht. He receives research funding from the Netherlands Organisation for Scientific Research, Dutch cystic fibrosis foundation, German cystic fibrosis foundation, Dutch Lung foundation. He is inventor on the patent (EP 2 115 470 B1/US 8,460,883 B2), which concerns a simple method to determine refractory neutrophils in peripheral blood of trauma patients.
Elizabeth Kovacs
Receives salary from the Loyola University Chicago’s Stritch School of Medicine. Her research is supported by grants from the National Institutes of Health, the Department of Defense and the Ralph and Marian C. Falk Medical Research Trust. She has no other conflict of interest.
Paul Kubes
No conflicts of interest to declare. He receives funding from the Canadian Institutes of Health.
Richard J Lilford
Acknowledges financial support for the submitted work from the National Institute for Health Research (NIHR) Collaborations for Leadership in Applied Health Research and Care (CLAHRC) for the West Midlands; the Medical Research Council (MRC) Midland Hub for Trials Methodology Research (MHTMR) programme (MRC Grant G0800808); and a National Institute for Health Research (NIHR) Senior Investigator Award. He co-ordinated the successful bid for the NIHR Surgical Reconstruction and Microbiology Research Centre in Birmingham (2011–2016) and was Chief Investigator on the MRC Network Grant on Trauma Research (MRC, 2010–2013: Establishment of a network for trauma research).
Authors’ contributions
JML, MJM and RJL conceptualised the paper and drafted the initial manuscript. YFC carried out literature search, screened the retrieved records for further consideration and produced evidence summary tables. All authors commented on several versions of drafts and contributed to the revision of contents and selection of references.
References
- 1.WHO. Global health estimates 2014 summary tables: deaths by cause, age and sex. 2000–2012 http://www.who.int/healthinfo/global_burden_disease/estimates/en/index1.html (accessed Aug 4, 2014)
- 2.WHO. Violence and injuries: the facts. Geneva, Switzerland: World Health Organization; 2010. [Google Scholar]
- 3.Gruen RL, Brohi K, Schreiber M, Balogh ZJ, Pitt V, Narayan M, et al. Haemorrhage control in severely injured patients. Lancet. 2012;380(9847):1099–108. doi: 10.1016/S0140-6736(12)61224-0. [DOI] [PubMed] [Google Scholar]
- 4.Wafaisade AP, Lefering RMD, Bouillon BMD, Sakka SGMD, Thamm OCMD, Paffrath TMD, et al. Epidemiology and risk factors of sepsis after multiple trauma: An analysis of 29,829 patients from the Trauma Registry of the German Society for Trauma Surgery. Crit Care Med. 2011;39(4):621–8. doi: 10.1097/CCM.0b013e318206d3df. [DOI] [PubMed] [Google Scholar]
- 5.Spruijt NE, Visser T, Leenen LP. A systematic review of randomized controlled trials exploring the effect of immunomodulative interventions on infection, organ failure, and mortality in trauma patients. Crit Care. 2010;14(4):R150. doi: 10.1186/cc9218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Angus DC, van der Poll T. Severe sepsis and septic shock. New Engl J Med. 2013;369(9):840–51. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464(7285):104–7. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manson J, Thiemermann C, Brohi K. Trauma alarmins as activators of damage-induced inflammation. Brit J Surg. 2012;99(Suppl 1):12–20. doi: 10.1002/bjs.7717. [DOI] [PubMed] [Google Scholar]
- 9.Pugin J. How tissue injury alarms the immune system and causes a systemic inflammatory response syndrome. Ann Intensive Care. 2012;2(1):27. doi: 10.1186/2110-5820-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation.[Erratum appears in Nature 2010 Sep 30;467(7315):622] Nature. 2002;418(6894):191–5. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 11.Burk AM, Martin M, Flierl MA, Rittirsch D, Helm M, Lampl L, et al. Early complementopathy after multiple injuries in humans. Shock. 2012;37(4):348–54. doi: 10.1097/SHK.0b013e3182471795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neher MD, Weckbach S, Flierl MA, Huber-Lang MS, Stahel PF. Molecular mechanisms of inflammation and tissue injury after major trauma-is complement the “bad guy”? J Biomed Sci. 2011;18(1) doi: 10.1186/1423-0127-18-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huber-Lang M, Kovtun A, Ignatius A. The role of complement in trauma and fracture healing. Semin Immunol. 2013;25(1):73–8. doi: 10.1016/j.smim.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Ward PA. The dark side of C5a in sepsis. Nat Rev Immunol. 2004;4(2):133–42. doi: 10.1038/nri1269. [DOI] [PubMed] [Google Scholar]
- 15.Xiao W, Mindrinos MN, Seok J, Cuschieri J, Cuenca AG, Gao H, et al. A genomic storm in critically injured humans. J Exp Med. 2011;208(13):2581–90. doi: 10.1084/jem.20111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gentile LF, Cuenca AG, Efron PA, Ang D, Bihorac A, McKinley BA, et al. Persistent inflammation and immunosuppression: a common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg. 2012;72(6):1491–501. doi: 10.1097/TA.0b013e318256e000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duggal NA, Upton J, Phillips AC, Sapey E, Lord JM. An age-related numerical and functional deficit in CD19(+) CD24(hi) CD38(hi) B cells is associated with an increase in systemic autoimmunity. Aging Cell. 2013;12(5):873–81. doi: 10.1111/acel.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffman M, Monroe DM., 3rd A cell-based model of hemostasis. Thrombosis and haemostasis. 2001;85(6):958–65. [PubMed] [Google Scholar]
- 19.Jenne CN, Urrutia R, Kubes P. Platelets: bridging hemostasis, inflammation, and immunity. Int J Lab Hematol. 2013;35(3):254–61. doi: 10.1111/ijlh.12084. [DOI] [PubMed] [Google Scholar]
- 20.van Gils JM, Zwaginga JJ, Hordijk PL. Molecular and functional interactions among monocytes, platelets, and endothelial cells and their relevance for cardiovascular diseases. J Leukoc Biol. 2009;85(2):195–204. doi: 10.1189/jlb.0708400. [DOI] [PubMed] [Google Scholar]
- 21.Laffont B, Corduan A, Ple H, Duchez AC, Cloutier N, Boilard E, et al. Activated platelets can deliver mRNA regulatory Ago2 microRNA complexes to endothelial cells via microparticles. Blood. 2013;122(2):253–61. doi: 10.1182/blood-2013-03-492801. [DOI] [PubMed] [Google Scholar]
- 22.Timar CI, Lorincz AM, Csepanyi-Komi R, Valyi-Nagy A, Nagy G, Buzas EI, et al. Antibacterial effect of microvesicles released from human neutrophilic granulocytes. Blood. 2013;121(3):510–8. doi: 10.1182/blood-2012-05-431114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19(2):43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Reino DC, Palange D, Feketeova E, Bonitz RP, Xu da Z, Lu Q, et al. Activation of toll-like receptor 4 is necessary for trauma hemorrhagic shock-induced gut injury and polymorphonuclear neutrophil priming. Shock. 2012;38(1):107–14. doi: 10.1097/SHK.0b013e318257123a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–5. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 26.Hietbrink F, Koenderman L, Althuizen M, Leenen LP. Modulation of the innate immune response after trauma visualised by a change in functional PMN phenotype. Injury. 2009;40(8):851–5. doi: 10.1016/j.injury.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Drifte G, Dunn-Siegrist I, Tissieres P, Pugin J. Innate immune functions of immature neutrophils in patients with sepsis and severe systemic inflammatory response syndrome. Crit Care Med. 2013;41(3):820–32. doi: 10.1097/CCM.0b013e318274647d. [DOI] [PubMed] [Google Scholar]
- 28.Davis JM, Albert JD, Tracy KJ, Calvano SE, Lowry SF, Shires GT, et al. Increased neutrophil mobilization and decreased chemotaxis during cortisol and epinephrine infusions. J Trauma. 1991;31(6):725–31. [PubMed] [Google Scholar]
- 29.Wade CE, Lindberg JS, Cockrell JL, Lamiell JM, Hunt MM, Ducey J, et al. Upon-admission adrenal steroidogenesis is adapted to the degree of illness in intensive care unit patients. J Clin Endocrinol Metab. 1988;67(2):223–7. doi: 10.1210/jcem-67-2-223. [DOI] [PubMed] [Google Scholar]
- 30.Butcher SK, Killampalli V, Lascelles D, Wang K, Alpar EK, Lord JM. Raised cortisol:DHEAS ratios in the elderly after injury: potential impact upon neutrophil function and immunity. Aging Cell. 2005;4(6):319–24. doi: 10.1111/j.1474-9726.2005.00178.x. [DOI] [PubMed] [Google Scholar]
- 31.Schmitz D, Kobbe P, Wegner A, Hammes F, Oberbeck R. Dehydroepiandrosterone during sepsis: does the timing of administration influence the effectiveness. J Surg Res. 2010;163(2):e73–7. doi: 10.1016/j.jss.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 32.Serhan CN, Chiang N. Resolution phase lipid mediators of inflammation: agonists of resolution. Curr Opin Pharmacol. 2013;13(4):632–40. doi: 10.1016/j.coph.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu MH, Tian J, Su YP, Wang T, Xiang Q, Wen L. Cervical sympathetic block regulates early systemic inflammatory response in severe trauma patients. Med Sci Monitor. 2013;19:194–201. doi: 10.12659/MSM.883833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hall S, Kumaria A, Belli A. The role of vagus nerve overactivity in the increased incidence of pneumonia following traumatic brain injury. Br J Neurosurg. 2014;28:181–6. doi: 10.3109/02688697.2013.835373. [DOI] [PubMed] [Google Scholar]
- 35.Bellander BM, Singhrao SK, Ohlsson M, Mattsson P, Svensson M. Complement activation in the human brain after traumatic head injury. J Neurotrauma. 2001;18(12):1295–311. doi: 10.1089/08977150152725605. [DOI] [PubMed] [Google Scholar]
- 36.Rothwell NJ, Hopkins SJ. Cytokines and the nervous system II: Actions and mechanisms of action. Trends Neurosci. 1995;18(3):130–6. doi: 10.1016/0166-2236(95)93890-a. [DOI] [PubMed] [Google Scholar]
- 37.Terrando N, Eriksson LI, Kyu Ryu J, Yang T, Monaco C, Feldmann M, et al. Resolving postoperative neuroinflammation and cognitive decline. Ann Neurol. 2011;70(6):986–95. doi: 10.1002/ana.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bruijns SR, Guly HR, Bouamra O, Lecky F, Lee WA. The value of traditional vital signs, shock index, and age-based markers in predicting trauma mortality. J Trauma Acute Care Surg. 2013;74(6):1432–7. doi: 10.1097/TA.0b013e31829246c7. [DOI] [PubMed] [Google Scholar]
- 39.Jones JM, Skaga NO, SØVik S, Lossius HM, Eken T. Norwegian survival prediction model in trauma: modelling effects of anatomic injury, acute physiology, age, and co-morbidity. Acta Anaesthesiol Scand. 2014;58(3):303–15. doi: 10.1111/aas.12256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jackson PC, Hardwicke J, Bamford A, Nightingale P, Wilson Y, Papini R, et al. Revised estimates of mortality from the Birmingham Burn Centre, 2001–2010: a continuing analysis over 65 years. Ann Surg. 2014;259:979–84. doi: 10.1097/SLA.0b013e31829160ca. [DOI] [PubMed] [Google Scholar]
- 41.Perel P, Prieto-Merino D, Shakur H, Clayton T, Lecky F, Bouamra O, et al. Predicting early death in patients with traumatic bleeding: development and validation of prognostic model. BMJ. 2012;345:e5166. doi: 10.1136/bmj.e5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nomellini V, Gomez CR, Gamelli RL, Kovacs EJ. Aging and animal models of systemic insult: trauma, burn, and sepsis. Shock. 2009;31(1):11–20. doi: 10.1097/SHK.0b013e318180f508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128(1):92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 44.Shaw AC, Goldstein DR, Montgomery RR. Age-dependent dysregulation of innate immunity. Nature Reviews Immunology. 2013;13(12):875–87. doi: 10.1038/nri3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choudhry MA, Bland KI, Chaudry IH. Gender and susceptibility to sepsis following trauma. Endocr Metab Immune Disord Drug Targets. 2006;6(2):127–35. doi: 10.2174/187153006777442422. [DOI] [PubMed] [Google Scholar]
- 46.Magnotti LJ, Fischer PE, Zarzaur BL, Fabian TC, Croce MA. Impact of gender on outcomes after blunt injury: a definitive analysis of more than 36,000 trauma patients. J Am Coll Surg. 2008;206(5):984–91. doi: 10.1016/j.jamcollsurg.2007.12.038. discussion 991–2. [DOI] [PubMed] [Google Scholar]
- 47.Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Version 5.1.0 [updated March 2011 ] The Cochrane Collaboration; 2011. http://www.cochrane-handbook.org (accessed April 4,2014) [Google Scholar]
- 48.Nathens AB, Nester TA, Rubenfeld GD, Nirula R, Gernsheimer TB. The effects of leukoreduced blood transfusion on infection risk following injury: a randomized controlled trial. Shock. 2006;26:342–47. doi: 10.1097/01.shk.0000228171.32587.a1. [DOI] [PubMed] [Google Scholar]
- 49.Roquilly A, Mahe PJ, Seguin P, Guitton C, Floch H, Tellier AC, et al. Hydrocortisone therapy for patients with multiple trauma: the randomized controlled HYPOLYTE study. JAMA. 2011;305(12):1201–9. doi: 10.1001/jama.2011.360. [DOI] [PubMed] [Google Scholar]
- 50.Vassar MJ, Fletcher MP, Perry CA, Holcroft JW. Evaluation of prostaglandin E1 for prevention of respiratory failure in high risk trauma patients: a prospective clinical trial and correlation with plasma suppressive factors for neutrophil activation. Prostaglandins Leukot Essent Fatty Acids. 1991;44:223–31. doi: 10.1016/0952-3278(91)90021-v. [DOI] [PubMed] [Google Scholar]
- 51.Rhee P, Morris J, Durham R, Hauser C, Cipolle M, Wilson R, et al. Recombinant humanized monoclonal antibody against CD18 (rhuMAb CD18) in traumatic hemorrhagic shock: results of a phase II clinical trial Traumatic Shock Group. J Trauma. 2000;49(4):611–9. doi: 10.1097/00005373-200010000-00007. discussion 619–20. [DOI] [PubMed] [Google Scholar]
- 52.Seekamp A, van Griensven M, Dhondt E, Diefenbeck M, Demeyer I, Vundelinckx G, et al. The effect of anti-L-selectin (aselizumab) in multiple traumatized patients–results of a phase II clinical trial. Crit Care Med. 2004;32(10):2021–8. doi: 10.1097/01.ccm.0000142396.59236.f3. [DOI] [PubMed] [Google Scholar]
- 53.Marzi I, Buhren V, Schuttler A, Trentz O. Value of superoxide dismutase for prevention of multiple organ failure after multiple trauma. J Trauma. 1993;35(1):110–9. doi: 10.1097/00005373-199307000-00018. [DOI] [PubMed] [Google Scholar]
- 54.Bauer M, Marzi I, Ziegenfuß T, Riegel W. Prophylactic hemofiltration in severely traumatized patients: effects on post-traumatic organ dysfunction syndrome. Intensive Care Med. 2001;27(2):376–83. doi: 10.1007/s001340000824. [DOI] [PubMed] [Google Scholar]
- 55.Jansen JO, Lord JM, Thickett DR, Midwinter MJ, McAuley DF, Gao F. Clinical review: Statins and trauma - a systematic review. Crit Care. 2013;17(3):227. doi: 10.1186/cc12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patil S, Holt G, Raby N, McLellan AR, Smith K, O’Kane S, et al. Prospective, double blind, randomized, controlled trial of simvastatin in human fracture healing. J Orthop Res. 2009;27(3):281–5. doi: 10.1002/jor.20572. [DOI] [PubMed] [Google Scholar]
- 57.Roberts I, Shakur H, Ker K, Coats T, The CRASH-2 Trial collaborators Antifibrinolytic drugs for acute traumatic injury. Cochrane Database Syst Rev. 2011;1:CD004896. doi: 10.1002/14651858.CD004896.pub3. [DOI] [PubMed] [Google Scholar]
- 58.Afshari A, Wetterslev J, Brok J, Moller AM. Antithrombin III for critically ill patients. Cochrane Database Syst Rev. 2008;3:CD005370. doi: 10.1002/14651858.CD005370.pub2. [DOI] [PubMed] [Google Scholar]
- 59.Edwards P, Arango M, Balica L, et al. The CRASH trial collaborators Final results of MRC CRASH, a randomised placebo-controlled trial of intravenous corticosteroid in adults with head injury-outcomes at 6 months. Lancet. 2005;365:1957–59. doi: 10.1016/S0140-6736(05)66552-X. [DOI] [PubMed] [Google Scholar]
- 60.Roberts I, Shakur H, Coats T, Hunt B, Balogun E, Barnetson L, et al. The CRASH-2 trial: a randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients. Health Technol Assess. 2013;17(10):1–79. doi: 10.3310/hta17100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morrison JJ, Dubose JJ, Rasmussen TE, Midwinter MJ. Military Application of Tranexamic Acid in Trauma Emergency Resuscitation (MATTERs) Study. Arch Surg. 2012;147(2):113–9. doi: 10.1001/archsurg.2011.287. [DOI] [PubMed] [Google Scholar]
- 62.Kashuk JL, Moore EE, Sawyer M, Le T, Johnson J, Biffl WL, et al. Postinjury coagulopathy management: Goal directed resuscitation via POC thrombelastography. Ann Surg. 2010;251(4):604–14. doi: 10.1097/SLA.0b013e3181d3599c. [DOI] [PubMed] [Google Scholar]
- 63.Afshari A, Wikkelso A, Brok J, Moller AM, Wetterslev J. Thrombelastography (TEG) or thromboelastometry (ROTEM) to monitor haemotherapy versus usual care in patients with massive transfusion. Cochrane Database Syst Rev. 2011;3:CD007871. doi: 10.1002/14651858.CD007871.pub2. [DOI] [PubMed] [Google Scholar]
- 64.Perera AM, Porter KM. The role of hypertonic saline dextran in trauma resuscitation. Trauma. 2002;4:189–201. [Google Scholar]
- 65.Zarychanski R, Abou-Setta AM, Turgeon AF, Houston BL, McIntyre L, Marshall JC, et al. Association of hydroxyethyl starch administration with mortality and acute kidney injury in critically ill patients requiring volume resuscitation: a systematic review and meta-analysis. JAMA. 2013;309(7):678–88. doi: 10.1001/jama.2013.430. [DOI] [PubMed] [Google Scholar]
- 66.Rochwerg B, Alhazzani W, Sindi A, et al. Fluid resuscitation in sepsis: a systematic review and network meta-analysis. Ann Intern Med. 2014;161:347–355. doi: 10.7326/M14-0178. [DOI] [PubMed] [Google Scholar]
- 67.NICE. Technology Appraisal Guidance 74. National Institute for Clinical Excellence; 2004. Pre-hospital initiation of fluid replacement therapy in trauma. [Google Scholar]
- 68.Spahn DR, Bouillon B, Cerny V, Coats TJ, Duranteau J, Fernandez-Mondejar E, et al. Management of bleeding and coagulopathy following major trauma: an updated European guideline. Crit Care. 2013;17(2):R76. doi: 10.1186/cc12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morrison CA, Carrick MM, Norman MA, Scott BG, Welsh FJ, Tsai P, et al. Hypotensive resuscitation strategy reduces transfusion requirements and severe postoperative coagulopathy in trauma patients with hemorrhagic shock: preliminary results of a randomized controlled trial. J Trauma. 2011;70(3):652–63. doi: 10.1097/TA.0b013e31820e77ea. [DOI] [PubMed] [Google Scholar]
- 70.Doran CM, Doran CA, Woolley T, Carter A, Male K, Midwinter MJ, et al. Targeted resuscitation improves coagulation and outcome. J Trauma Acute Care Surg. 2012;72(4):835–43. doi: 10.1097/TA.0b013e318248347b. [DOI] [PubMed] [Google Scholar]
- 71.Hodgetts T, Mahoney P, Evans G. Defence Medical Education and Training Agency, Joint Service Publication 570. 3. Defence Medical Education and Training Agency; 2006. Battlefield advanced trauma life support. [Google Scholar]
- 72.Carson JL, Carless PA, Hebert PC. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2012;4:CD002042. doi: 10.1002/14651858.CD002042.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marik PE, Zaloga GP. Immunonutrition in critically ill patients: a systematic review and analysis of the literature. Intensive Care Med. 2008;34(11):1980–90. doi: 10.1007/s00134-008-1213-6. [DOI] [PubMed] [Google Scholar]
- 74.Pérez-Bárcena J, Crespí C, Regueiro V, Marsé P, Raurich JM, Ibáñez J, et al. Lack of effect of glutamine administration to boost the innate immune system response in trauma patients in the intensive care unit. Crit Care. 2010;14(6):R233. doi: 10.1186/cc9388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gu WJ, Deng T, Gong YZ, Jing R, Liu JC. The effects of probiotics in early enteral nutrition on the outcomes of trauma: a meta-analysis of randomized controlled trials. JPEN J Parenter Enteral Nutr. 2013;37(3):310–7. doi: 10.1177/0148607112463245. [DOI] [PubMed] [Google Scholar]
- 76.Douzinas EE, Pitaridis MT, Louris G, Andrianakis I, Katsouyanni K, Karmpaliotis D, et al. Prevention of infection in multiple trauma patients by high-dose intravenous immunoglobulins. Crit Care Med. 2000;28(1):8–15. doi: 10.1097/00003246-200001000-00002. [DOI] [PubMed] [Google Scholar]
- 77.Glinz W, Grob PJ, Nydegger UE, Ricklin T, Stamm F, Stoffel D, et al. Polyvalent immunoglobulins for prophylaxis of bacterial infections in patients following multiple trauma. A randomized, placebo-controlled study. Intensive Care Med. 1985;11(6):288–94. doi: 10.1007/BF00273538. [DOI] [PubMed] [Google Scholar]
- 78.Bo L, Wang F, Zhu J, Li J, Deng X. Granulocyte-colony stimulating factor (G-CSF) and granulocyte-macrophage colony stimulating factor (GM-CSF) for sepsis: a meta-analysis. Crit Care. 2011;15(1):R58. doi: 10.1186/cc10031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mizock BA. Immunonutrition and critical illness: an update. Nutrition. 2010;26(7–8):701–7. doi: 10.1016/j.nut.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 80.Van Zanten ARH, Sztark F, Kaisers UX, et al. High-protein enteral nutrition enriched with immune-modulating nutrients vs standard high-protein enteral nutrition and nosocomial infection in the ICU: a randomized clinical trial. JAMA. 2014;312:514–24. doi: 10.1001/jama.2014.7698. [DOI] [PubMed] [Google Scholar]
- 81.Browder W, Williams D, Pretus H, Olivero G, Enrichens F, Mao P, et al. Beneficial effect of enhanced macrophage function in the trauma patient. Ann Surg. 1990;211(5):605–12. [PMC free article] [PubMed] [Google Scholar]
- 82.de Felippe Junior J, da Rocha e Silva Junior M, Maciel FM, Soares Ade M, Mendes NF. Infection prevention in patients with severe multiple trauma with the immunomodulator beta 1–3 polyglucose (glucan) Surg Gynecol Obstet. 1993;177(4):383–8. [PubMed] [Google Scholar]
- 83.Gu WJ, Wei CY, Yin RX. Lack of efficacy of probiotics in preventing ventilator-associated pneumonia probiotics for ventilator-associated pneumonia: a systematic review and meta-analysis of randomized controlled trials. Chest. 2012;142(4):859–68. doi: 10.1378/chest.12-0679. [DOI] [PubMed] [Google Scholar]
- 84.Barraud D, Bollaert PE, Gibot S. Impact of the administration of probiotics on mortality in critically ill adult patients: a meta-analysis of randomized controlled trials. Chest. 2013;143(3):646–55. doi: 10.1378/chest.12-1745. [DOI] [PubMed] [Google Scholar]
- 85.Nelson RL, Glenny AM, Song F. Antimicrobial prophylaxis for colorectal surgery. Cochrane Database Syst Rev. 2009;1:CD001181. doi: 10.1002/14651858.CD001181.pub3. [DOI] [PubMed] [Google Scholar]
- 86.Bowater RJ, Stirling SA, Lilford RJ. Is antibiotic prophylaxis in surgery a generally effective intervention? Testing a generic hypothesis over a set of meta-analyses. Ann Surg. 2009;249(4):551–6. doi: 10.1097/SLA.0b013e318199f202. [DOI] [PubMed] [Google Scholar]
- 87.Gosselin RA, Roberts I, Gillespie WJ. Antibiotics for preventing infection in open limb fractures. Cochrane Database Syst Rev. 2004;1:CD003764. doi: 10.1002/14651858.CD003764.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.D’Amico R, Pifferi S, Leonetti C, Torri V, Tinazzi A, Liberati A. Effectiveness of antibiotic prophylaxis in critically ill adult patients: systematic review of randomised controlled trials. BMJ. 1998;316(7140):1275–85. doi: 10.1136/bmj.316.7140.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lilford R, Stevens AJ. Underpowered studies. Br J Surg. 2002;89(2):129–31. doi: 10.1046/j.0007-1323.2001.01989.x. [DOI] [PubMed] [Google Scholar]
- 90.Stead M, Cameron D, Lester N, Parmar M, Haward R, Kaplan R, et al. Strengthening clinical cancer research in the United Kingdom. Br J Cancer. 2011;104(10):1529–34. doi: 10.1038/bjc.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Treweek S, Pitkethly M, Cook J, Kjeldstrom M, Taskila T, Johansen M, et al. Strategies to improve recruitment to randomised controlled trials. Cochrane Database Syst Rev. 2010;4:MR000013. doi: 10.1002/14651858.MR000013.pub5. [DOI] [PubMed] [Google Scholar]
- 92.Roberts I, Prieto-Merino D, Shakur H, Chalmers I, Nicholl J. Effect of consent rituals on mortality in emergency care research. Lancet. 2011;377(9771):1071–2. doi: 10.1016/S0140-6736(11)60317-6. [DOI] [PubMed] [Google Scholar]
- 93.The NHS Confederation. Implementing trauma systems: key issues for the NHS. 2010 http://www.nhsconfed.org/~/media/Confederation/Files/Publications/Documents/Implementing_trauma_systems_report.pdf (accessed Aug 4, 2014)
- 94.David AS. Wanted - more answers than questions: literature review. BMJ. 2001;323(7327):1462–3. doi: 10.1136/bmj.323.7327.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Probst C, Hildebrand F, Griensven M, Baur H, Mahlke L, Krettek C, et al. Is dopexamine superior to dopamine in the treatment of multiple trauma patients*#x02013;a prospective, double-blind, randomised study. Injury. 2010;41(5):499–505. doi: 10.1016/j.injury.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 96.Hemming K, Bowater RJ, Lilford RJ. Pooling systematic reviews of systematic reviews: a Bayesian panoramic meta-analysis. Stat Med. 2012;31(3):201–16. doi: 10.1002/sim.4372. [DOI] [PubMed] [Google Scholar]
- 97.Heeres M, Visser T, Wessem KJ, Koenderman AH, Strengers PF, Koenderman L, et al. The effect of C1-esterase inhibitor on systemic inflammation in trauma patients with a femur fracture - The CAESAR study: study protocol for a randomized controlled trial. Trials. 2011;12:223. doi: 10.1186/1745-6215-12-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chow OA, von Köckritz-Blickwede M, Bright AT, Hensler ME, Zinkernagel AS, Cogen AL, et al. Statins enhance formation of phagocyte extracellular traps. Cell Host Microbe. 2010;8(5):445–54. doi: 10.1016/j.chom.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cunnington AJ, de Souza JB, Walther M, Riley EM. Malaria impairs resistance to Salmonella through heme- and heme oxygenasedependent dysfunctional granulocyte mobilization. Nat Med. 2011;18:120–27. doi: 10.1038/nm.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Czaikoski PG, Nascimento DC, Sônego F, et al. Heme oxygenase inhibition enhances neutrophil migration into the bronchoalveolar spaces and improves the outcome of murine pneumonia-induced sepsis. Shock. 2013;39:389–96. doi: 10.1097/SHK.0b013e31828bbcf9. [DOI] [PubMed] [Google Scholar]
- 101.Karr JR, Sanghvi JC, Macklin DN, Gutschow MV, Jacobs JM, Bolival B, Jr, et al. A whole-cell computational model predicts phenotype from genotype. Cell. 2012;150(2):389–401. doi: 10.1016/j.cell.2012.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mogilner A, Allard J, Wollman R. Cell polarity: quantitative modeling as a tool in cell biology. Science. 2012;336(6078):175–9. doi: 10.1126/science.1216380. [DOI] [PubMed] [Google Scholar]
- 103.Langley RJ, Tsalik EL, van Velkinburgh JC, Glickman SW, Rice BJ, Wang C, et al. An integrated clinico-metabolomic model improves prediction of death in sepsis. Sci Transl Med. 2013;5(195):195ra95. doi: 10.1126/scitranslmed.3005893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Folcik VA, An GC, Orosz CG. The Basic Immune Simulator: an agent-based model to study the interactions between innate and adaptive immunity. Theor Biol Med Model. 2007;4:39. doi: 10.1186/1742-4682-4-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


