Abstract
Purpose
To examine the relationship between smoking and health-related quality of life (HRQOL) and the impact of quitting smoking on changes in HRQOL among women in the two Nurses’ Health Study (NHS) cohorts (n = 158,736) who were 29 to 71 years of age in 1992/1993 when they reported data on smoking status and completed the Short Form-36® version 1 (SF-36®).
Methods
At baseline, the SF-36® physical component scores (SF-PCS) and mental component scores (SF-MCS) were examined by smoking status (never, 56%, former, 32%, and current, 13%) within 10-year age groups. Smoking characteristics were analyzed as correlates of SF-36®. Changes in smoking status and SF-PCS and SF-MCS, adjusted for comorbid disease and other covariates, were reassessed at 4-year intervals among current smokers in 1992/1993 and those who either continued smoking after 4 and 8 years or reported not smoking at both intervals.
Results
Smokers had lower HRQOL (SF-PCS and SF-MCS) as compared to never and former smokers. Current smoking, cigarettes per day and time since quitting were associated with significantly lower SF-PCS and SF-MCS. Continuing smokers and those who quit had significant declines in SF-PCS over time and significant improvements in SF-MCS at 8 years. There was minimal difference between groups, with some greater improvements in SF-MCS among those reporting non-smoking at 8 years. These findings support the lower ratings of HRQOL by smokers, but quitting alone, after an average of 21 years of smoking, did not improve HRQOL. Further study focused on the HRQOL impact of quitting smoking is needed.
Keywords: Smoking, Smoking cessation, Health-related quality of life, Quality of life, Women, Tobacco
Introduction
Smoking cessation reduces mortality and morbidity [1] and is postulated to improve health-related quality of life (HRQOL). A number of population-based cross-sectional studies report poorer HRQOL among current smokers than either former or never smokers, especially in the physical domain [2–9]. However, data to support the long-term benefits from smoking cessation on HRQOL are limited [6, 10]. The negative health impact of smoking on women is well established [11, 12], but few studies have described the impact of smoking cessation on changes in HRQOL and none have specially addressed changes among women.
Longer duration of smoking [4] and greater number of cigarettes per day (cpd) [9] are linked to lower HRQOL. Fewer cpd also are significantly related to improved perceptions of health status [13]. As the number of years since quitting increases, improvement in mental health may influence perceptions of HRQOL [14].
The health consequences of smoking resulting in increased comorbidity, as well as the co-occurrence of mental illness and depression, may confound investigations of the impact of smoking cessation on HRQOL [1, 15–17], especially among aging smokers. For example, smoking and obesity are often co-occurring conditions, increasing the risk for poor health and negatively impacting appraisals of HRQOL [18].
The purposes of this study were to describe HRQOL by smoking status and other characteristics among women and to evaluate the impact of quitting smoking on changes in HRQOL. We hypothesized that: (1) HRQOL would be lowest among current smokers as compared with former and current smokers; (2) higher cpd (current smokers), number of years smoked (current and former smokers), and shorter time since quitting (former smokers) would be associated with lower HRQOL; and (3) quitting smoking would be associated with more positive (or less negative) changes in HRQOL over time as compared to changes in HRQOL among continuing smokers.
Methods
This study included data from participants in the two Nurses’ Health Study (NHS) cohorts. Details of the NHS procedures are described elsewhere [19, 20]. Briefly, the original cohort (NHS) includes 121,700 married women who were registered nurses, 30–55 years of age, and residing in the 11 most populous states when they gave their informed consent and responded to an initial questionnaire in 1976. The second cohort (NHS II) recruited 116,686 younger female nurses, 25–42 years of age, from 14 populous states, with an initial mailing in 1989. Questionnaires have been mailed every 2 years in order to identify incident diseases and to collect health-related data. Questions on HRQOL were included in the surveys in 1992 (NHS) and 1993 (NHS II) and every 4 years thereafter. Response rates to biennial questionnaires have remained high, at 90% or more.
Sample
For this analysis, we merged the two NHS cohorts into one study population. The sample for the 1992/1993 baseline cross-sectional analysis included participants aged 29–71 years who reported their smoking characteristics and responded to the HRQOL survey (n = 158,734). Changes in HRQOL were examined among those who were current smokers at baseline who either continued to smoke (n = 12,194) or had quit smoking (n = 3,619) by the next HRQOL assessment in 1996/1997, and those who continued to smoke (n = 8,763) or continued to abstain from smoking (n = 3,046) in 2000/2001.
This study was approved by the institutional review boards at the University of California, Los Angeles (#05-388), and the Brigham and Women's Hospital, Boston (#2005-P-002146/1), and is in accordance with the ethical standards laid down by the 1964 Declaration of Helsinki.
Smoking characteristics
On the initial questionnaire in each NHS cohort, participants were asked if they were current smokers (“Do you currently smoke cigarettes?”) or had ever smoked cigarettes regularly. Smoking status was updated on subsequent biennial questionnaires. At the 1992/1993 baseline for this study, current smokers were self-reported, never smokers were defined as women who never reported smoking on any questionnaire, and former smokers were defined as having a reported history of smoking.
The initial cohort questionnaires also asked for age at which regular smoking began, age at quitting, and the usual number of cpd. The cpd were reported every 2 years by category (1–4, 5–14, 15–24, 25–34, 35–44, ≥45), and median values within these categories were used in the analyses. Ages at smoking initiation and cessation were continuous values in the NHS cohort but were collected categorically in NHS II (<15, 15–19, 20–24, 25–29, 30–35 years), therefore, median values were assigned for the calculations. Smoking duration and years since quitting were calculated with each new biennial report of smoking status, and, therefore, their accuracy is within 2 years.
Health-related quality of life
The Medical Outcomes Short Form-36 (SF-36®) version 1 [21] was used to assess physical and emotional HRQOL. This instrument has been used in several studies to examine the relationship of smoking to HRQOL [4, 7, 8, 22–24]. The measure consists of 36 questions, from which eight subscales (physical functioning, role limitations due to physical problems, bodily pain, general health perceptions, mental health, role limitations due to emotional problems, social functioning, and vitality) are created. The subscale scores are scored from 0 to 100, with higher scores indicating better health/HRQOL. Two-component summary scores capture the overall physical and mental health (physical component score or PCS and mental component score or MCS) [21]. These scores are calculated from the eight subscale scores and are transformed so that a mean score of 50 (standard deviation [SD] = 10) reflects the mean in the general US population. Higher scores reflect better HRQOL than the general population.
Demographic and other health characteristics
Demographic and other characteristics identified as potential confounders which could influence the ratings of HRQOL (SF-PCS and SF-MCS) included age, living alone, body mass index (BMI), physical activity, and the presence of comorbid conditions, including tobacco-related comorbid conditions. Date of birth was determined at study entry and age was calculated at each questionnaire cycle. Living alone or with others was reported in 1992/1993. Weight was assessed on every biennial questionnaire, and BMI was calculated as weight in kilograms divided by height in meters squared, using the height reported at cohort entry. Physical activity was assessed by the self-reported time spent per week performing various recreational activities (e.g., walking) and was transformed into metabolic equivalents based upon the intensity of performed activities [25]. Comorbid diseases, collected as part of the biennial questionnaires, were defined by the self-reported presence of major diseases (see Appendix). Self-reported tobacco-related comorbid conditions were identified from a listing from the 2004 Surgeon General Report [1].
Data analysis
Descriptive statistics were used to profile the sample demographics, smoking status, smoking characteristics, and HRQOL (SF-PCS and SF-MCS) outcomes at the 1992/1993 baseline. The mean and standard errors for the SF-PCS and SF-MCS were calculated and examined by smoking status (never, former, current) within approximate 10-year age groups (29–34, 35–44, 45–54, 55–64, and 65–71 years). Also, the women were classified into four low-to-high categories of SF-PCS and SF-MCS, using age-based percentile cut-points for US females (<25th, 25–49th, 50–74th, ≥75th) [21] (see Appendix), to examine the distribution of smoking and other characteristics by HRQOL scores.
In order to examine the relationship of smoking to HRQOL, a series of multivariate linear regression models were run using data from the sample in 1992/1993 (smoking status and characteristics) as correlates of HRQOL (SF-PCS, SF-MCS, and SF-36® subscale scores). The first model included all participants and examined smoking status as a correlate of HRQOL (never smokers served as the reference group). In the second model, years of smoking (per 10-year intervals) and cpd (per 10 cpd) were examined among current smokers only. The third and fourth models included only former smokers, with one examining smoking duration (per 10 years) and the other examining time since quitting (per 5 years) as predictors of HRQOL. These variables were run in separate models due to high colinearity. All models were adjusted for age, BMI, physical activity, living alone, and the presence of comorbidity.
The sample available for examining changes in HRQOL (SF-MCS and SF-PCS) consisted of current smokers in 1992/1993 who had HRQOL data in 1996/1997 and were classified as a current (n = 12,194) or former (n = 3,619) smoker. This sample included 80% of the current smokers at baseline. In 2000/2001, HRQOL (SF-36®) data were available for 8,763 women who continued to smoke and 3,046 women who continued to abstain. To determine changes in HRQOL at 4 years, we subtracted the SF-PCS and SF-MCS baseline scores in 1992/1993 from the 1996/1997 scores. For changes at 8 years, baseline scores were subtracted from the 2000/2001 scores. Negative changes indicate a decline in SF-PCS or SF-MCS and positive changes indicate improvement. The statistical software package SAS version 9 was utilized for the analysis.
For this study, we used 0.25 of the SD as the minimal threshold to determine clinically meaningful changes in SF-MCS and SF-PCS at 4 and 8 years. Only considering the statistically significant differences was not adequate due to the large sample size. The 0.25 SD value is slightly higher than the 0.20 SD difference used by Hays et al. [24] in evaluating differences in SF-36® PCS and MCS by smoking status in a large sample of Medicare beneficiaries. We also compared the SF-MCS and SF-PCS scores with population-dependent scores (e.g., normative scores of SF-36®) [21].
Results
The baseline characteristics of the sample are displayed in Table 1, including descriptives within four categories based upon age-specific percentile cut-points for US women [21]. In 1992/1993, 12.5% were current smokers. For the SF-PCS, the largest number of women were in the >75th percentile group (35%), indicating that the NHS women had higher PCS than the US sample of women of the same age. For SF-MCS, the scores were more similar for NHS and US women. For both PCS and MCS, the lowest category had the highest percentage of current smokers. Years of smoking among current and former smokers were similar across categories of PCS and MCS, whereas the mean number years since quitting among the former smokers increased with increasing scores and the mean cpd among current smokers decreased with increasing scores. Women in the lowest PCS and MCS categories were more likely to live alone, had higher BMI, and were less active. Comorbid disease was most common in the low PCS category, but was unrelated to MCS. Among continuing smokers only, tobacco-related diseases were also the most common in the lowest PCS category. In contrast, tobacco-related diseases were somewhat more prevalent in the highest category of MCS.
Table 1.
Sample characteristics of women in the Nurses’ Health Study (1992/1993), 29 to 71 years of age, by US age-specific percentilesa of the physical health (PCS) and mental health (MCS) component scores of the Short Form-36®
| Totals | Percentiles |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| PCS |
MCS |
||||||||
| <25th | 25–49th | 50–74th | >75th | <25th | 25–49th | 50–74th | >75th | ||
| Number of participants | 158,734 | 29,541 | 34,866 | 38,649 | 55,678 | 35,351 | 48,886 | 38,332 | 36,165 |
| Smoking status | |||||||||
| Never (%) | 55.6 | 56.3 | 55.0 | 57.0 | 54.5 | 54.2 | 55.4 | 56.8 | 55.9 |
| Former (%) | 32.0 | 30.3 | 31.7 | 31.3 | 33.5 | 30.0 | 31.9 | 32.2 | 33.8 |
| Current (%) | 12.5 | 13.4 | 13.3 | 11.8 | 12.0 | 15.8 | 12.8 | 11.0 | 10.4 |
| Years of smoking | |||||||||
| Mean (SD)b | 21.0 (12.2) | 20.3 (11.4) | 21.3 (12.3) | 20.5 (12.0) | 21.5 (12.7) | 20.4 (11.4) | 21.2 (12.5) | 21.0 (12.4) | 21.3 (12.6) |
| Years since quitting | |||||||||
| Mean (SD)c | 15.4 (10.8) | 13.4 (10.2) | 15.1 (10.9) | 15.2 (10.6) | 16.0 (11.0) | 13.9 (10.0) | 14.9 (10.9) | 15.5 (10.9) | 16.1 (11.0) |
| <3 (%) | 11.1 | 8.9 | 8.2 | 7.4 | 10.0 | 9.1 | 8.1 | 7.2 | |
| 3–7 (%) | 23.3 | 21.6 | 21.3 | 20.2 | 21.8 | 22.0 | 20.7 | 20.6 | |
| ≥8 (%) | 65.6 | 69.5 | 70.6 | 72.4 | 68.2 | 68.9 | 71.3 | 72.2 | |
| Cigarettes per dayd | |||||||||
| Mean (SD) | 16.8 (10.3) | 18.5 (10.8) | 17.2 (10.3) | 16.3 (10.1) | 15.9 (9.9) | 18.0 (10.8) | 16.5 (9.9) | 15.9 (10.0) | 16.5 (10.2) |
| 1–14 (%) | 43.7 | 37.7 | 42.0 | 45.2 | 47.4 | 39.5 | 44.1 | 47.6 | 45.1 |
| 15–24 (%) | 39.1 | 39.4 | 39.7 | 39.4 | 38.5 | 39.3 | 40.4 | 37.8 | 38.4 |
| ≥25 (%) | 17.2 | 22.9 | 18.4 | 15.4 | 14.1 | 21.3 | 15.6 | 14.7 | 16.5 |
| Live alone (%) | 4.9 | 6.2 | 4.5 | 4.6 | 4.7 | 7.4 | 4.6 | 4.1 | 3.8 |
| BMI (kg/m2) | |||||||||
| Mean (SD) | 25.6 (5.4) | 27.9 (7.0) | 26.5 (5.7) | 25.2 (4.8) | 24.2 (4.1) | 26.0 (5.8) | 25.6 (5.4) | 25.2 (5.1) | 25.8 (5.5) |
| <19 (%) | 2.9 | 2.7 | 2.4 | 2.8 | 3.4 | 3.2 | 2.9 | 2.8 | 2.6 |
| 19–30 (%) | 78.9 | 65.5 | 74.0 | 81.7 | 87.0 | 76.0 | 79.4 | 81.3 | 78.4 |
| >30 (%) | 18.3 | 31.8 | 23.6 | 15.5 | 9.7 | 20.9 | 17.7 | 15.9 | 19.1 |
| (met-hrs/wk) | |||||||||
| Mean (SD) | 20.2 (25.8) | 16.6 (23.6) | 17.8 (23.0) | 20.5 (25.2) | 23.5 (28.6) | 18.0 (24.1) | 19.3 (24.9) | 21.5 (26.6) | 22.4 (27.7) |
| Comorbid disease (%) | 34.6 | 44.3 | 39.2 | 31.3 | 28.8 | 35.1 | 34.9 | 32.1 | 36.3 |
| Tobacco-related comorbid disease (%)b | 13.6 | 18.8 | 16.1 | 11.9 | 10.4 | 13.5 | 13.8 | 12.3 | 14.7 |
Percentile cut-points using SF-36® normative scores for US females, according to age
Current and former smokers, n = 70,548
Former smokers
Current smokers
PCS = physical component summary score
MCS = mental component summary score
Met = metabolic
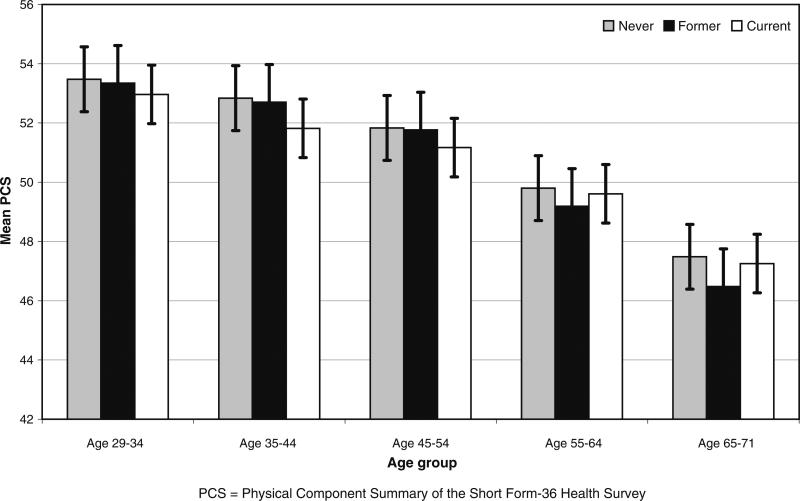
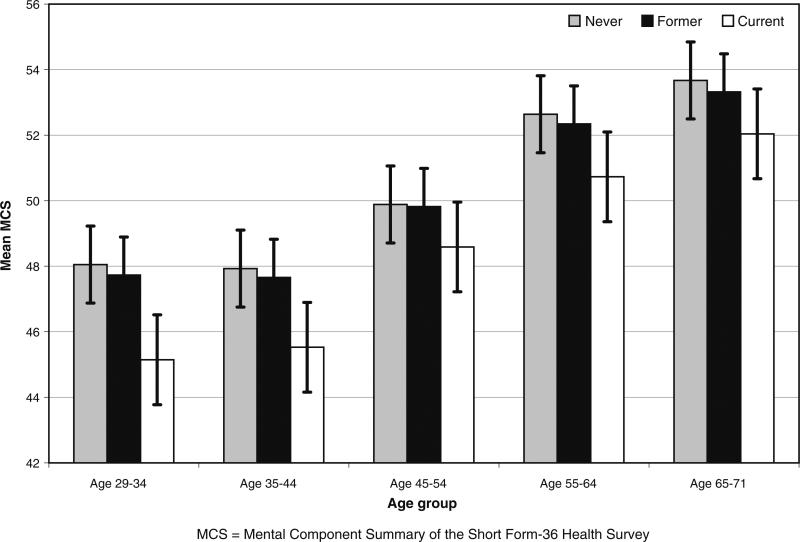
As displayed in Figs. 1 and 2, using data from 1992/1993, the average SF-PCS scores declined across age groups, whereas the SF-MCS scores improved with advancing age. In each age group, current smokers had the lowest mean PCS until the 55–64 and 65–71 age groups, when former smokers had lower scores. Current smokers had lower MCS across all age groups, with the largest difference in mean scores between never (44.5) and current (48.0) smokers in the youngest 29–34 age group.
Fig. 1.
Physical component summary (PCS) bar graphs by smoking status at 1992/1993 within 10-year age groups
Fig. 2.
Mental component summary (MCS) bar graphs by smoking status at 1992/1993 within 10-year age groups
Smoking status and characteristics were examined as correlates of HRQOL (SF-PCS and SF-MCS) (Table 2) using the 1992/1993 data. Current smoking was negatively associated with both SF-PCS and SF-MCS and with all subscale scores. Former smoking was associated with lower SF-MCS and all subscale scores except vitality, though the associations were weaker than those for current smokers. Former smoking was not associated with SF-PCS. Among former smokers, smoking duration was a modest predictor of lower SF-PCS, SF-MCS, and all subscales except role-emotional. Among current smokers, the results were inconsistent, with negative associations with SF-PCS, physical functioning, and general health subscales, but positive associations with SF-MCS and the social functioning, role-emotional, and mental heath subscales. Time since quitting for former smokers was positively associated with SF-PCS and SF-MCS and all subscale scores, indicating improved HRQOL with longer time since quitting. The number of cpd among current smokers was negatively associated with all HRQOL scores, with the greatest impact on the role-emotional, vitality, and role-physical subscales.
Table 2.
Smoking characteristics as correlates of health-related quality of life (Short Form-36®) scores in 1992/1993a
| PCS | MCS | PF | Role-physical | Pain | General health | Vitality | Social functioning | Role-emotional | Mental health | |
|---|---|---|---|---|---|---|---|---|---|---|
| Variables in 1992/1993 | B (SE) | B (SE) | B (SE) | B (SE) | B (SE) | B (SE) | B (SE) | B (SE) | B (SE) | B (SE) |
| Smoking statusb | ||||||||||
| Former | –0.08 (0.05) | –0.32 (0.05)*** | –0.39 (0.09)*** | –0.64 (0.19)*** | –0.79 (0.11)*** | –0.21 (0.10)* | 0.49 (0.10)*** | –0.54 (0.11)*** | –0.71 (0.17)*** | –1.2 (0.08)*** |
| Current | –0.55 (0.06)*** | –2.0 (0.07)*** | –2.1 (0.12)*** | –2.3 (0.26)*** | –1.9 (0.15)*** | –3.4 (0.13)*** | –2.4 (0.14)*** | –3.3 (0.15)*** | –5.3 (0.24)*** | –3.7 (0.12)*** |
| Years of smokingc | ||||||||||
| Former | –0.41 (0.04)*** | –0.14 (0.04)*** | –1.4 (0.08)*** | –1.1 (0.16)*** | –0.36 (0.09)*** | –0.69 (0.08)*** | –0.56 (0.08)*** | –0.54 (0.09)*** | –0.24 (0.14) | –0.70 (0.07)*** |
| Current | –0.37 (0.12).** | 0.57 (0.14)*** | –0.79 (0.24)*** | –0.20 (0.48) | –0.05 (0.29) | –0.53 (0.26)* | 0.47 (0.26) | 1.1 (0.30)*** | 1.3 (0.47)** | 0.47 (0.23)* |
| Time since quittingd | 0.15 (0.02)*** | 0.10 (0.02)*** | 0.59 (0.04)*** | 0.45 (0.07)*** | 0.15 (0.04)*** | 0.25 (0.04)*** | 0.22 (0.04)*** | 0.31 (0.04)*** | 0.21 (0.07)** | 0.33 (0.03)*** |
| cpde | –0.63 (0.06)*** | –0.82 (0.07)*** | –1.5 (0.12)*** | –2.1 (0.24)*** | –1.3 (0.15)*** | –1.8 (0.13)*** | –2.2 (0.13)*** | –1.8 (0.15)*** | –2.3 (0.24)*** | –1.3 (0.11)*** |
P < 0.05
P < 0.01
P < 0.001
Adjusted for age, BMI, physical activity, living alone, and comorbidity
Compared to never smokers
Per 10 years of smoking duration for current and former smokers
Per 5 years since quitting for former smokers
Per 10 cigarettes per day for current smokers
PCS = physical component summary score, SF-36®
MCS = mental component summary score, SF-36®
PF = physical functioning
Table 3 presents the HRQOL data (SF-PCS and SF-MCS, adjusted for covariates) for current smokers at 1992/1993 who had repeat HRQOL data in 1996/1997 and either continued smoking (77%) or changed to non-smoking (23%) and for those who continued to smoke (74%) or remained non-smokers (26%) at the third assessment of HRQOL in 2000/2001. The baseline scores of the two groups were similar and all changes in HRQOL were statistically significant. Continuing smokers experienced “small” (>0.25 SD) clinically important declines in PCS at 8 years; those who quit had “small” declines in PCS at both 4 and 8 years. Similar “small” declines were seen in the physical function subscale for both groups (smokers and those who quit) at 8 years. Those who quit also had clinically important declines at 4 years in PCS. Both groups had a “small” clinically important improvement in SF-MCS at 8 years. Those who quit smoking had a “small” important increase in the mental health subscale at 8 years. There were no other “clinically meaningful” changes over time.
Table 3.
Four-year and eight-year changes in health-related quality of life scores (Short Form-36®) for current smokers in 1992/1993a comparing those who continued to smoke with those who quit in 1996/1997 and remained abstinent in 2000/2001
| Continuous smoking |
Quit smoking |
|||||
|---|---|---|---|---|---|---|
| 1992/1993 Baseline scores (n = 12,194) | 1996/1997 Current smokers, 4-year change (n = 12,194) | 2000/2001 Current smokers, 8-year change (n = 8,763) | 1992/1993 Baseline scores (n = 3,619) | 1996/1997 Former smokers, 4-year change (n = 3,619) | 2000/2001 Former smokers, 8-year change (n = 3,046) | |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| PCS | 51.0 (8.8) | –1.6 (6.6)* | –3.6 (8.9)* | 51.1 (8.4) | –2.1 (6.6)* | –3.8 (8.2)* |
| MCS | 48.2 (10.0) | 1.5 (8.8)* | 2.7 (8.2)* | 48.8 (9.6) | 1.9 (8.4)* | 3.3 (8.2)* |
| Physical functioning | 86.9 (16.6) | –3.7 (14.1)* | –7.4 (17.8)* | 86.6 (16.2) | –4.7 (15.6)* | –7.8 (18.2)* |
| Role-physical | 77.0 (33.2) | –2.6 (32.0)* | –4.9 (33.6)* | 77.9 (33.8) | –5.0 (32.0)* | –5.7 (33.6)* |
| Pain | 74.3 (19.8) | –0.95 (13.2)* | –4.5 (18.8)* | 75.0 (21.6) | –0.88 (13.2)* | –4.8 (18.8)* |
| General health | 76.9 (17.6) | –1.9 (14.4)* | –4.3 (16.0)* | 77.7 (17.4) | –1.9 (15.1)* | –3.1 (16.0)* |
| Vitality | 57.3 (18.6) | 0.96 (15.4)* | 1.1 (16.8)* | 58.8 (18.6) | 0.84 (15.0)* | 2.2 (16.6)* |
| Social functioning | 84.8 (21.0) | 1.2 (19.8)* | 0.77 (19.6)* | 85.8 (21.0) | 1.1 (19.2)* | 1.1 (19.8)* |
| Role-emotional | 78.1 (32.0) | 0.75 (29.8)* | 2.5 (30.0)* | 79.0 (32.6) | 2.5 (32.6)* | 4.4 (29.8)* |
| Mental health | 71.3 (15.4) | 2.1 (13.2)* | 3.7 (14.0)* | 72.0 (15.6) | 2.2 (15.6)* | 4.1 (10.0)* |
Higher scores indicate better quality of life
P < 0.001
Adjusted for age, BMI, activity, living alone, comorbidity, and baseline score
PCS = physical component summary score, SF-36®
MCS = mental component summary score, SF-36®
Discussion
This is the first study to provide data about HRQOL and smoking status for a large cohort of women and to describe long-term changes in HRQOL after quitting smoking. The findings of lower HRQOL (SF-MCS and SF-PCS) among smokers as compared to former and never smokers are similar to the findings of other studies [4, 9, 24]. In the overview of HRQOL ratings by age groups in 1992/1993, the decline in physical HRQOL by advancing age was expected, along with lower scores among current smokers as compared to never smokers. This is further demonstrated by the higher proportion of current smokers in the lower quartile SF-PCS categories, reflecting poorer physical HRQOL. At older ages, the lower scores in SF-PCS among former smokers as compared to current smokers might reflect the accumulation of comorbid diseases, especially tobacco-related comorbidities. The negative health impact of smoking is a powerful argument for quitting.
Unexpectedly, in 1992/1993, the mental health aspects of HRQOL (SF-MCS and SF-36® subscale scores) were higher for each subsequent age group. Although this may appear to be a paradox, this finding is similar to the results of an analysis of 33 years of cross-sectional data, which found happiness to be increased in older adults, even after adjusting for health status, and regardless of cohort group [26]. This phenomenon of positive mental well-being among older adults, despite poorer physical health status, might explain why those with comorbid disease, as well as those with tobacco-related diseases, populated the groups with the highest level of SF-MCS. SF-MCS was lowest for current smokers in all age groups, with the worst emotional HRQOL in the youngest age group (aged 29–34 years). The mood-altering aspects of tobacco use among women might influence the appraisal of emotional HRQOL [27]. This indicates that consideration of the emotional well-being of young female smokers is especially important during quitting attempts.
As anticipated, smoking status and characteristics were correlates of HRQOL. However, these statistically significant results need to be viewed with caution in this large sample, i.e., small betas. Current smoking was significantly and negatively related to both the composite scores (SF-PCS and SF-MCS) and all subscale scores of the SF-36®, indicating the negative impact on HRQOL. However, former smoking status was not significantly related to SF-PCS, although it was related to subscale scores. This may indicate that the former smokers had diminished physical well-being due to the consequences of smoking. Smokers may quit after becoming symptomatic or developing a tobacco-related comorbid condition [10]. In a cross-sectional analysis of Medicare beneficiaries [24], both recent quitters and current smokers had poorer SF-PCS and SF-MCS.
NHS smokers had an average of 21 years of smoking, much of their adult lives. In 1992, 43% of female smokers in the US had quit (according to National Health Interview Survey estimates), which is greater than the proportion in this analysis (32%); the consumption of almost a pack per day (approximately 17 cpd) in the NHS women was similar to other women at that time (41% of female smokers in 1992 smoked 15–24 cpd, defined as moderate smoking) [1]. As hypothesized, cpd, shorter time since quitting, and the number of years smoking had some relationship to HRQOL. Among current smokers, heavier smoking (based on cpd), which might be indicative of a more profound addiction, was linked with poorer physical and emotional health. Though the effects were generally small, the observed differences in HRQOL by dose of smoking were similar to those reported by others [9, 28, 29], with the greatest differences seen in the role-emotional, vitality, and role-physical subscales. A higher number of cpd was also seen in the lower SF-PCS and SF-MCS quartiles. Among former smokers, the number of years since quitting was positively associated with SF-PCS and SF-MCS, indicating that age-standardized HRQOL improves with longer time since smoking cessation, as would be expected [30]. However, those with a longer and heavier smoking history might have smaller gains in HRQOL after quitting.
Only a minority of smokers in 1992/1993 with follow-up data quit smoking in 1996/1997 and were able to stay quit in 2000/2001, reflecting the struggles to maintain long-term abstinence. The differences in HRQOL by smoking status in the longitudinal analysis are minimal as compared to the impressive differences by smoking status at baseline. The changes in 4- and 8-year computations of SF-PCS and SF-MCS for both continuing smokers and those who quit demonstrate a downward trend for physical well-being and an upward trend for emotional well-being, similar to the findings in the baseline analysis. However, most changes only varied by a few points, although some were in the range of “small” clinical importance (i.e., ≥0.25 SD) [31]. Those who were not smoking at 4 and 8 years had continuing decline in SF-PCS and physical functioning, similar to current smokers. For these women, quitting smoking did not forestall the decline seen with aging. The number of years of tobacco use and the anticipated impact of smoking on health status might be expected to result in lower ratings of physical HRQOL. Tobacco use is associated with increased death; approximately 50% of those who smoke will die of a tobacco-related condition [1], and tobacco-related comorbidity is common. That said, this analysis of HRQOL includes only those women who were alive and able to respond to an HRQOL questionnaire. This may have resulted in a “healthier” subset of current smokers.
Both continuing smokers and those who quit had improved emotional well-being over time. The finding of a “small” but clinically meaningful improvement for those who quit in the mental health subscale, beyond that already seen for current smokers, is similar to that reported in studies finding improved mental health one year post-cessation [14] and at six months post-cessation [7]. Both the cross-sectional and longitudinal analyses demonstrated that the mental health aspects of HRQOL improved over time, regardless of smoking status. The issue of “selective survival” of the women in this study may favorably influence the long-term outcomes of HRQOL. The positive relationship of years of smoking with SF-MCS and several subscales in the cross-sectional analysis may relate to those with worse HRQOL (due to death or morbidity) dropping out of the analysis.
Unlike the other studies examining HRQOL and smoking status, comorbid diseases were considered as a potential confounder influencing changes in HRQOL and were controlled in the analysis. Although it is not possible to determine from this analysis, illness may have been a motivating factor for quitting. If this were the case, the remaining current smokers may have the “better” HRQOL. Further study is needed to determine how the presence of multiple health conditions or severity of specific illnesses is associated with changes in HRQOL.
The frequency of smoking cessation (23% at 4 years), as calculated from those who responded to the HRQOL survey, may be higher because the calculation only includes women who responded to the HRQOL in 1996/1997. Smokers would be more likely to die before this assessment or be unable to complete the questionnaire because of illness. In addition to the influence of aging and illness, social and political changes were occurring during the 1992–2001 observation period that might have increased personal quitting behaviors. These would include the publication of the first evidence-based recommendations for the effective treatment of tobacco dependence [32] and its second edition in 2000 [33]. Awareness of the health benefits of quitting and the health consequences of smoking for women also gained increased attention [10] and, relevant to this cohort of nurses, so did the coverage of smoking in the nursing literature [34].
Limitations
There are several limitations which should be considered in the interpretations of these findings. Changes in HRQOL by smoking status focused on women who were smoking in 1992/1993 and who either reported quitting or continuing to smoke in 1996/1997 and then were followed through 2000/2001. Women who quit smoking between 1996/1997 and 2000/2001 were not included this analysis.
When we compared age, smoking status, and cpd between women who responded to the 1992/1993 SF-36® (as in Table 1) and those who responded to the general NHS/NHS II questionnaire that year, but did not provide SF-36® data (77% provided SF-36® data), to see if there were differences in drop outs, we found that responders were typically healthier than those who did not respond. Specifically, responders were younger (age 47.9 years for responders and 50.5 years for non-responders), less likely to be smokers (current smokers: 12.5% responders, 16.6% non-responders), and for the current smokers, smoked fewer cpds (16.8 cpd for responders, 18.5 cpd for non-responders). Although the strength of the associations may be somewhat biased by our sample, it is likely that the associations are those that would be generally observed.
By merging data from the younger NHS II women who responded after just one request for participation, with the NHS women, this may have resulted in a healthier cohort than what would be obtained from the first NHS sample alone. The smoking prevalence is different between the two cohorts. In 1988, the NHS I women born in 1946 (age 42) had a smoking prevalence of 18.2%, whereas in 1989, the NHS II women born in 1947 (age 42) had a smoking prevalence of 15.4%. We attribute this difference to the likelihood that nurses who responded to the initial questionnaire in NHS II were more health-conscious than the larger population who received the mailing.
The responsiveness of the SF-36® to changes in smoking cessation may be limited. Efforts have been made to revise this tool by adding items more sensitive to quitting smoking [6]. The data provide support for differences in HRQOL in the short term [29]; however, to our knowledge, there are no reports of utility in long-term outcomes from prospective studies.
Smoking status was based upon self-report at each cycle and was not confirmed through biochemical verification. Women reported their smoking status on biennial questionnaires, but were not specifically asked if they quit smoking, the exact date of quitting, number of quit attempts (including those that were unsuccessful), methods used to quit, or reasons for quitting smoking. Some could have quit just prior to the survey, relapsed, and quit again prior to the next survey. On the other hand, women classified as continuous smokers may have made quit attempts during the interlude prior to the second and third HRQOL assessments, and it is not known for how long these quitting attempts lasted. Others have reported differences in HRQOL in smoking status by sex [3, 27], but these data are only among women.
Additionally, comorbid conditions were self-reported. Even though these women were nurses, they could have misreported a condition. Physical activity and weight were also self-reported.
Although a number of variables were controlled, as postulated by others [8], factors outside of smoking cessation may be more important in contributing to self-perceptions of HRQOL. In this analysis, quitting smoking was not examined in light of other changes which might have diminished self-reported HRQOL, such as weight gain or changes in smoking among others in the household. It is unknown if changes in HRQOL by the smoking status of women in other occupations would be different to these findings for a sample of nurses. This is an area worthy of future research. Finally, this paper focused on the impact of cessation on HRQOL among smokers; changes in HRQOL among never smokers were not examined, thus, conclusions cannot be made as to a difference in the trajectory of change in HRQOL among those who have never smoked.
In conclusion, the cross-sectional analysis supports differences in HRQOL by smoking status, with current smokers generally having worse physical and mental well-being. However, the longitudinal findings show minimal difference in the decline in physical functioning or the improvement in emotional well-being over time for those who quit. These findings challenge the assumption that quitting smoking alone, after years of smoking, will result in substantially improved HRQOL. Although it is expected that smokers will experience health benefits from cessation as compared to continued smokers [11], some of these health improvements may not directly translate into perceptions of improvement in HRQOL, especially if chronic illnesses are present. Those who quit may have quit in response to illness or disability. Realistic expectations for clinicians and smokers about changes in HRQOL after smoking cessation are important. These findings demonstrate that ongoing support is needed for former smokers too so that they stay abstinent and benefit from cessation, even if their perception of HRQOL is not significantly changed. Future prospective studies are needed to examine characteristics associated with improved or diminished HRQOL ratings among smokers and those who quit, and to determine if quitting at certain ages is associated with greater improvements in HRQOL. Additionally, studies might examine the potential moderating effects of long and heavier smoking histories on changes in HRQOL.
Acknowledgments
This research was supported by grants CA87979, CA50385, and K07 CA92696-02 (Cooley) from the National Institutes of Health, and a grant from the Robert Wood Johnson Foundation #55769 (Sarna). We acknowledge Ron Hays for his consultation in issues related to the analysis and interpretation of findings of this study and Dr. Marjorie Wells, School of Nursing, University of California, for her assistance with this project.
Appendix
Comorbid conditions: cardiovascular disease (high blood pressure, myocardial infarction, angina pectoris, peripheral artery disease or claudication, coronary artery surgery, TIA, and stroke), cancer (breast, cervical, uterine, ovarian, colon, rectum, lung, liver, melanoma, pancreas, bladder/kidney, esophageal, leukemia, laryngeal, oral, and stomach), respiratory diseases (asthma, emphysema, chronic bronchitis, and active TB), or other (diabetes mellitus, systemic lupus erythematosis, multiple sclerosis, ALS, cataracts, macular degeneration, and gastric or duodenal ulcer).
Tobacco-related diseases: cardiovascular disease (as described above), cancer (cervical, lung, pancreas, bladder/kidney, esophageal, leukemia, laryngeal, oral, and stomach), respiratory diseases (emphysema, chronic bronchitis), and macular degeneration.
Physical component scores (PCS) and mental component scores (MCS) normative scores for US females according to age [21].
Physical component score (PCS) norms by quartiles
| Age (years) | Mean (SD) | <25th | 25th | 50th | 75th |
|---|---|---|---|---|---|
| 25–34 | 52.46 (7.66) | ≤49.31 | 49.32 | 54.43 | 57.67 |
| 35–14 | 51.36 (8.34) | ≤46.98 | 46.99 | 52.58 | 57.09 |
| 45–54 | 48.95 (9.64) | ≤43.38 | 43.40 | 51.61 | 55.79 |
| 55–64 | 45.03 (11.57) | ≤38.17 | 38.18 | 49.91 | 54.14 |
| ≥65 | 41.02 (11.52) | ≤31.99 | 32.00 | 42.93 | 49.83 |
Mental component score (MCS) norms by quartiles
| Age (years) | Mean (SD) | <25th | 25th | 50th | 75th |
|---|---|---|---|---|---|
| 25–34 | 48.34 (10.12) | ≤41.89 | 41.90 | 51.31 | 55.22 |
| 35–44 | 48.84 (9.49) | ≤43.22 | 43.23 | 51.16 | 55.75 |
| 45–54 | 50.07 (10.18) | ≤45.54 | 45.55 | 53.48 | 56.99 |
| 55–64 | 50.56 (10.16) | ≤44.60 | 44.61 | 53.71 | 57.94 |
| ≥65 | 51.44 (10.54) | ≤43.42 | 43.43 | 55.08 | 58.96 |
Contributor Information
Linda Sarna, School of Nursing, University of California, Los Angeles, CA, USA.
Stella A. Bialous, Tobacco Policy International, San Francisco, CA, USA
Mary E. Cooley, Dana-Farber Cancer Institute, Phyllis F. Cantor Center, Research in Nursing and Patient Care, Boston, MA, USA
Hee-Jin Jun, Channing Laboratory, Department of Medicine, Brigham and Women's Hospital, and Harvard Medical School, Boston, MA, USA.
Diane Feskanich, Channing Laboratory, Department of Medicine, Brigham and Women's Hospital, and Harvard Medical School, Boston, MA, USA.
References
- 1.U.S. Department of Health and Human Services . The health consequences of smoking: A report of the Surgeon General. Centers for Disease Control and Prevention; Atlanta: 2004. [Google Scholar]
- 2.Hirdes JP, Maxwell CJ. Smoking cessation and quality of life outcomes among older adults in the Campbell's Survey on Well-Being. Canadian Journal of Public Health. 1994;85:99–102. [PubMed] [Google Scholar]
- 3.Laaksonen M, Rahkonen O, Martikainen P, Karvonen S, Lahelma E. Smoking and SF-36 health functioning. Preventive Medicine. 2006;42:206–209. doi: 10.1016/j.ypmed.2005.12.003. doi.org/10.1016/j.ypmed.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Lyons RA, Lo SV, Littlepage BNC. Perception of health amongst ever-smokers and never-smokers: A comparison using the SF-36 health survey questionnaire. Tobacco Control. 1994;3:213–215. doi.org/10.1136/tc.3.3.213. [Google Scholar]
- 5.Mody RR, Smith MJ. Smoking status and health-related quality of life: As findings from the 2001 Behavioral Risk Factor Surveillance System data. American Journal of Health Promotion. 2006;20:251–258. doi: 10.4278/0890-1171-20.4.251. [DOI] [PubMed] [Google Scholar]
- 6.Olufade AO, Shaw JW, Foster SA, Leishow SJ, Hays RD, Coons SJ. Development of the Smoking Cessation Quality of Life questionnaire. Clinical Therapeutics. 1999;21:2113–2130. doi: 10.1016/s0149-2918(00)87242-2. doi.org/10.1016/S0149-2918(00)87242. [DOI] [PubMed] [Google Scholar]
- 7.Stewart AL, King AC, Killen JD, Ritter PL. Does smoking cessation improve health-related quality-of-life? Annals of Behavioral Medicine. 1995;17:331–338. doi: 10.1007/BF02888598. doi.org/10.1007/BF02888598. [DOI] [PubMed] [Google Scholar]
- 8.Tillman M, Silcock J. A comparison of smokers' and ex-smokers' health-related quality of life. Journal of Public Health Medicine. 1997;19:268–273. doi: 10.1093/oxfordjournals.pubmed.a024629. [DOI] [PubMed] [Google Scholar]
- 9.Wilson D, Parsons J, Wakefield M. The health-related quality-of-life of never smokers, ex-smokers, and light, moderate, and heavy smokers. Preventive Medicine. 1999;29:139–144. doi: 10.1006/pmed.1999.0523. doi.org/10.1006/pmed.1999.0523. [DOI] [PubMed] [Google Scholar]
- 10.U.S. Department of Health and Human Services . The health benefits of smoking cessation: A report of the Surgeon General. Centers for Disease Control and Prevention; Rockville, MD: 1990. [Google Scholar]
- 11.U.S. Department of Health and Human Services . Women and smoking: A report of the Surgeon General—2001. Centers for Disease Prevention and Control; Atlanta: 2001. [PubMed] [Google Scholar]
- 12.Kenfield SA, Stampfer MJ, Rosner BA, Colditz GA. Smoking and smoking cessation in relation to mortality in women. Journal of the American Medical Association. 2008;299:2037–2047. doi: 10.1001/jama.299.17.2037. doi.org/10.1001/jama.299.17.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolliger CT, Zellweger JP, Danielsson T, van Biljon X, Robidou A, Westin A, Perruchoud AP, Säwe U. Influence of long-term smoking reduction on health risk markers and quality of life. Nicotine & Tobacco Research. 2002;4:433–439. doi: 10.1080/1462220021000018380. doi.org/10.1080/1462220021000018380. [DOI] [PubMed] [Google Scholar]
- 14.Mino Y, Shigemi J, Otsu T, Tsuda T, Babazono A. Does smoking cessation improve mental health? Psychiatry and Clinical Neurosciences. 2000;54:169–172. doi: 10.1046/j.1440-1819.2000.00654.x. doi.org/10.1046/j.1440-1819.2000.00654.x. [DOI] [PubMed] [Google Scholar]
- 15.Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: Results from the national epidemiologic survey on alcohol and related conditions. Archives of General Psychiatry. 2004;61:1107–1115. doi: 10.1001/archpsyc.61.11.1107. doi.org/10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- 16.John U, Meyer C, Rumpf HJ, Hapke U. Smoking, nicotine dependence and psychiatric comorbidity—a population-based study including smoking cessation after three years. Drug and Alcohol Dependence. 2004;7:287–295. doi: 10.1016/j.drugalcdep.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Strine TW, Mokdad AH, Dube SR, Balluz LS, Gonzalez O, Berry JT, Manderscheid R, Kroenke K. The association of depression and anxiety with obesity and unhealthy behaviors among community-dwelling US adults. General Hospital Psychiatry. 2008;30:127–137. doi: 10.1016/j.genhosppsych.2007.12.008. doi.org/10.1016/j.genhosppsych.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Healton CG, Vallone D, McCausland KL, Xiao H, Green MP. Smoking, obesity, and their co-occurrence in the United States: Cross sectional analysis. BMJ (Clinical Research Ed.) 2006;333:25–26. doi: 10.1136/bmj.38840.608704.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colditz GA. The Nurses' Health Study: A cohort of US women followed since 1976. Journal of the American Medical Women's Association. 1995;50:40–44. [PubMed] [Google Scholar]
- 20.Myers AH, Rosner B, Abbey H, Willet W, Stampfer MJ, Bain C, Lipnick R, Hennekens C, Speizer F. Smoking behavior among participants in the Nurses' Health Study. American Journal of Public Health. 1987;77:628–630. doi: 10.2105/ajph.77.5.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ware JE, Kosinksi M, Keller SD. SF-36 physical and mental health summary scales: A user's manual. Health Assessment Lab, New England Medical Center; Boston, MA: 1994. [Google Scholar]
- 22.Bellido-Casado J, Martín-Escudero J, Dueñas-Laita A, Mena-Martín F, Arzúa-Mouronte D, Simal-Blanco F. The SF-36 questionnaire as a measurement of health-related quality of life: Assessing short- and medium-term effects of exposure to tobacco versus the known long-term effects. European Journal of Internal Medicine. 2004;15:511–517. doi: 10.1016/j.ejim.2004.06.015. doi.org/10.1016/j.ejim.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 23.Wiggers LC, Oort FJ, Peters RJ, Legemate DA, de Haes HCJ, Smets EM. Smoking cessation may not improve quality of life in atherosclerotic patients. Nicotine & Tobacco Research. 2006;8:581–589. doi: 10.1080/14622200600790005. doi.org/10.1080/14622200600790005. [DOI] [PubMed] [Google Scholar]
- 24.Hays RD, Smith AW, Reeve BB, Spritzer KL, Marcus SE, Clauser SB. Cigarette smoking and health-related quality of life in Medicare beneficiaries. Health Care Financing Review. 2008;29:57–67. [PMC free article] [PubMed] [Google Scholar]
- 25.Wolf AM, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, Corsano KA, Rosner B, Kriska A, Willett WC. Reproducibility and validity of a self-administered physical activity questionnaire. International Journal of Epidemiology. 1994;23:991–999. doi: 10.1093/ije/23.5.991. doi.org/10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- 26.Yang Y. Social inequalities in happiness in the United States, 1972 to 2004: An age-period-cohort analysis. American Sociological Review. 2008;73:204–226. [Google Scholar]
- 27.File SE, Fluck E, Leahy A. Nicotine has calming effects on stress-induced mood changes in females, but enhances aggressive mood in males. The International Journal of Neuropsychopharmacology. 2001;4:371–376. doi: 10.1017/S1461145701002577. doi.org/10.1017/S1461145701002577. [DOI] [PubMed] [Google Scholar]
- 28.Mulder I, Tijhuis M, Smit HA, Kromhout D. Smoking cessation and quality of life: The effect of amount of smoking and time since quitting. Preventive Medicine. 2001;33:653–660. doi: 10.1006/pmed.2001.0941. doi.org/10.1006/pmed.2001.0941. [DOI] [PubMed] [Google Scholar]
- 29.Woolf SH, Rothemich SF, Johnson RE, Marsland DW. Is cigarette smoking associated with impaired physical and mental functional status? An office-based survey of primary care patients. American Journal of Preventive Medicine. 1999;17:134–137. doi: 10.1016/s0749-3797(99)00060-4. doi.org/10.1016/S0749-3797(99)00060-4. [DOI] [PubMed] [Google Scholar]
- 30.Shaw JW, Coons SJ, Foster SA, Leishow SJ, Hays RD. Responsiveness of the Smoking Cessation Quality of Life (SCQOL) questionnaire. Clinical Therapeutics. 2001;23:957–969. doi: 10.1016/s0149-2918(01)80083-7. doi.org/10.1016/S0149-2918(01)80083-7. [DOI] [PubMed] [Google Scholar]
- 31.Sloan JA, Cella D, Hays RD. Clinical significance of patient-reported questionnaire data: Another step toward consensus. Journal of Clinical Epidemiology. 2005;58:1217–1219. doi: 10.1016/j.jclinepi.2005.07.009. doi.org/10.1016/j.jclinepi.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 32.Fiore MC, Bailey WC, Cohen SJ, Dorfman SF, Goldstein MG, Gritz ER, Heyman RB, Holbrook J, Jaen CR, Kottke TE, Landon HA, Mecklenburg RE, Mullen PD, Nett LM, Robinson L, Stitzer ML, Tommasello AC, Villejo L, Wewers ME. Smoking cessation: clinical practice guideline no. 18. U.S. Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research; Rockville, MD: 1996. [Google Scholar]
- 33.Fiore MC, Bailey WC, Cohen SJ, Dorfman SF, Goldstein MG, Gritz ER, Heyman RB, Jaen CR, Kottke TE, Landon HA, Mecklenburg RE, Mullen PD, Nett LM, Robinson L, Stitzer ML, Tommasello AC, Villejo L, Wewers ME. Treating tobacco use and dependence. Clinical practice guideline. U.S. Department of Health and Human Services; Rockville, MD: 2000. [Google Scholar]
- 34.Sarna L, Lillington L. Tobacco: An emerging topic in nursing research. Nursing Research. 2002;51:245–253. doi: 10.1097/00006199-200207000-00005. doi.org/10.1097/00006199-200207000-00005. [DOI] [PubMed] [Google Scholar]