Abstract
Type 1 diabetes (T1D) is an autoimmune disease that occurs in genetically susceptible individuals. Regulatory T cells (Tregs) have been shown to be defective in the autoimmune disease setting. Thus, efforts to repair or replace Tregs in T1D may reverse autoimmunity and protect the remaining insulin-producing β cells. On the basis of this premise, a robust technique has been developed to isolate and expand Tregs from patients with T1D. The expanded Tregs retained their T cell receptor diversity and demonstrated enhanced functional activity. We report on a phase 1 trial to assess safety of Treg adoptive immunotherapy in T1D. Fourteen adult subjects with T1D, in four dosing cohorts, received ex vivo–expanded autologous CD4+CD127lo/−CD25+ polyclonal Tregs (0.05 × 108 to 26 × 108 cells). A subset of the adoptively transferred Tregs was long-lived, with up to 25% of the peak level remaining in the circulation at 1 year after transfer. Immune studies showed transient increases in Tregs in recipients and retained a broad Treg FOXP3+CD4+CD25hiCD127lo phenotype long-term. There were no infusion reactions or cell therapy–related high-grade adverse events. C-peptide levels persisted out to 2+ years after transfer in several individuals. These results support the development of a phase 2 trial to test efficacy of the Treg therapy.
Introduction
Type 1 diabetes (T1D) is an autoimmune disease that occurs in genetically susceptible individuals, influenced by the environment and stochastic events (1). These conditions result in immune dysregulation, leading to the generation of pathogenic T cells and destruction of β cells in the islets of Langerhans. T1D is one of the most prevalent chronic diseases of childhood. Despite advances in insulin formulations, insulin delivery systems, and glucose monitoring, less than one-third of patients meet clinical care targets needed to prevent secondary end-organ complications such as retinal, renal, and neurological disease (2, 3). Thus, it is not surprising that the past two decades of research have focused on developing new therapeutics to prevent and treat this devastating disease. Several immunomodulatory therapies, including anti-CD3 (teplizumab) (4), LFA3Ig (alefacept) (5), and anti-thymocyte globulin (thymoglobulin) in combination with granulocyte colony- stimulating factor with or without cyclophosphamide (6), and bone marrow transplantation (6, 7), have shown some promise for the treatment of T1D, yet none has induced permanent immune tolerance (that is, nonresponsiveness to self-tissues or foreign tissues without the need for continuous immune suppression) or resulted in long-term insulin independence. However, one common finding that has emerged from these studies is that the major immunomodulatory effect was to induce, or preferentially support, a regulatory T cell (Treg) subset that is likely to be responsible for the drug efficacy (8). Indeed, this key discovery has provided increased emphasis on the development of Treg and Treg-supportive therapies for the treatment of this challenging disease.
Tregs were initially described as a population of CD4+CD25+ T cells that are critical for controlling autoimmunity and tolerance (9, 10). Tregs inhibit effector T cell (Teff) responses both in vitro and in vivo through a variety of activities including cell-cell contact and soluble factors (11). The identification of the transcription factor FOXP3 as a lineage marker for Tregs has been instrumental in advancing the field. Mutations or deficiency in the FOXP3 gene in scurfy mice or immuno-dysregulation polyendocrinopathy enteropathy X-linked syndrome (IPEX) patients results in a reduced and/or nonfunctional Treg compartment, leading to a fatal multiorgan autoimmune disease (12). FOXP3 controls many aspects of Treg biology, including their development, transcriptional program, and suppressive function in vitro and in vivo. Thus, CD4+CD25+FOXP3+ Tregs are an essential immunosuppressive cell population for the extrinsic control of immune homeostasis and control of autoimmunity and have a unique and highly robust therapeutic profile. Although Tregs require specific T cell receptor (TCR)-mediated activation to develop regulatory activity, their effector function regulates local inflammatory responses through a combination of cell-cell contact and suppressive cytokine production (11, 13, 14). Thus, Tregs specific for a limited number of antigens can efficiently suppress a polyclonal autoreactive response due to dominant antigen nonspecific immunoregulation termed “bystander suppression.” Moreover, activated Tregs can recruit additional regulatory cell populations through a process termed infectious tolerance to achieve long-lasting disease protection (14). There is increasing evidence in mouse models that the adoptive transfer (AT) of Tregs in multiple disease settings, including T1D, results in disease prevention and, in many cases, disease remission (15, 16). Recently, we and others have shown that Tregs are defective in a wide variety of autoimmune diseases, including T1D (17). These defects are manifested by loss of Treg number in inflamed tissues, reduced signaling through the interleukin-2 (IL-2) receptor [based on reduced signal transducer and activator of transcription 5 (STAT5) phosphorylation], and instability of the suppressive activities of the cells in vitro and in vivo (18). These observations have opened an important new concept of drug intervention in autoimmunity, namely, Tregs as immunotherapeutics, particularly if the abnormalities observed in Tregs in vivo can be corrected through their expansion.
Unlike mice, isolation of Tregs based on the CD4 and CD25 markers is not sufficient to isolate most of the FOXP3+ Tregs without the risk of contamination with potentially autoreactive Teffs. We have shown that a combination of cell surface markers—CD4, CD25, and CD127—provides a robust cocktail to isolate FOXP3+ Tregs by fluorescence-activated cell sorting (FACS) from peripheral blood of subjects with T1D (19). On the basis of this selection method, we developed a clinical-scale expansion process for obtaining, in many instances, greater than 3 × 109 Tregs after in vitro expansion of cells sorted from peripheral blood lymphocytes from a single donor (20).
Here, we describe the results of a phase 1 trial in recent-onset T1D. The study included four dosing cohorts (a total of 14 adult patients) that received expanded polyclonal Tregs (polyTregs) ranging from ~5 × 106 to ~2.6 × 109 cells in a single infusion. The expansion process resulted in Tregs with enhanced STAT5 phosphorylation in response to IL-2, increased Treg suppressive activity in vitro, and long-term survival in vivo (greater than 1 year). The cell therapy was well tolerated with no evidence of short-term toxicities (including infusion reactions or cytokine release syndrome), precipitous decline in endogenous insulin production, or opportunistic infections.
Results
Study design
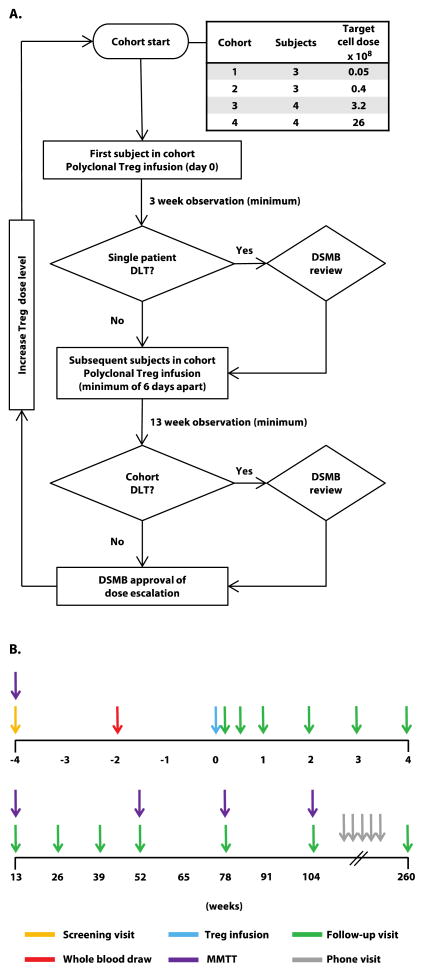
The trial was a phase 1, two-center, open-label, dose-escalation study conducted at the University of California, San Francisco (UCSF) and Yale University in which participants with recent-onset T1D received a single infusion of ex vivo–expanded autologous CD4+CD127lo/−CD25+polyTregs in four dosing cohorts (Fig. 1). The first subject in each cohort was observed for 3 weeks after infusion, after which time the study team met to review cumulative safety data. If no grade 3 or higher adverse event was observed, subsequent subjects in that cohort could be treated. After the 13-week follow-up visit of the last subject in each cohort, an independent data and safety monitoring board (DSMB) reviewed cumulative safety data for approval to escalate the dose and progress to the next cohort. Primary outcome measures were adverse events, laboratory abnormalities, and other signs of toxicity. Secondary diabetes-related outcome measures included C-peptide response during mixed meal tolerance tests (MMTTs), insulin use, and hemoglobin A1c (HbA1c). Figure 1 highlights the schedule of events that includes relevant time points for patient blood sampling for various assessments described below. Table 1 describes the endpoints for the study as defined. This study was approved by institutional review boards at UCSF and Yale University and is registered with ClinicalTrials.gov (NCT01210664).
Fig. 1. Study Design.
(A) Dose escalation plan. Subjects were enrolled in 4 cohorts with target doses ranging from 0.05 to 26 × 108, where the dose was escalated by 8-fold for each subsequent cohort. The first subject in each cohort received a single infusion of polyclonal Tregs and was observed for a minimum of 3 weeks for dose limiting toxicities (DLT) after which time clinical data was extracted from the database and study team safety review was conducted. If no grade 3 or higher adverse event was observed, treatment of subsequent subjects in that cohort could proceed. Otherwise, treatment would be suspended for DSMB review. Following treatment of the last subject in each cohort, subjects were observed for a minimum of 13 weeks. The study team reviewed cumulative data to assess for any grade 3 or higher related adverse event, any related serious adverse event, undetectable C-peptide in a MMTT at week 13 in 2 subjects, or any other significant safety concerns based on other considerations. The study team’s review decision was reported to the DSMB for approval prior to proceeding to the next dosing cohort. (B) Subject schedule of events. Blood (target of 400 ml) for Treg manufacturing was drawn at week −2, and Treg infusion was given on day 0. Subjects were seen for follow-up assessments on day 4, then weekly for the first 4 weeks, then every 13 weeks for the first year, then every 6 months for 2 years, and then contacted by phone every 6 months for years 3–5 to assess for adverse events.
Table 1.
Primary and secondary study objectives and endpoints.
| Objectives | Endpoints |
|---|---|
Primary Objective
|
Primary Endpoints
|
Secondary Objectives
|
Secondary Endpoints
|
Patient characteristics
Twenty-six patients were screened, 16 met eligibility criteria and were enrolled, and 14 subjects received a single infusion of polyTregs Table 2 shows the demographic and baseline characteristics of the treated subjects. Of the 14 treated subjects, 6 were female and 8 were male. The mean age was 30.3 ± 8.7 years, and the mean disease duration was 39 ± 26.4 weeks at the time of screening. The mean follow-up at the time of data cutoff for this article was 124 weeks (cohort 1: 182 weeks; cohort 2: 156 weeks; cohort 3: 104 weeks; and cohort 4: 78 weeks). The two subjects who did not receive expanded Tregs were not included in the data analysis.
Table 2. Subject characteristics.
Subjects are listed in order of enrollment.
| Cohort | Subject ID | Age at enrollment (years) | Sex | Time from diagnosis at screening (Weeks) | HbA1c at screening (%) |
|---|---|---|---|---|---|
| 1 | 002-002 | 35 | F | 17 | 6.4 |
| 002-003 | 26 | M | 19 | 4.9 | |
| 002-004 | 32 | M | 20 | 5.3 | |
|
| |||||
| 2 | 002-005 | 35 | F | 86 | 5.7 |
| 002-007 | 40 | M | 30 | 5.2 | |
| 002-008 | 32 | M | 14 | 6.0 | |
|
| |||||
| 3 | 007-101 | 19 | M | 30 | 6.0 |
| 002-015 | 43 | M | 104 | 5.3 | |
| 007-102 | 21 | F | 42 | 9.9 | |
| 002-017 | 24 | M | 29 | 7.1 | |
|
| |||||
| 4 | 002-018 | 43 | M | 27 | 7.0 |
| 002-019 | 34 | F | 52 | 6.5 | |
| 007-103 | 18 | F | 28 | 6.2 | |
| 002-022 | 22 | F | 42 | 4.7 | |
Treg isolation and expansion
We have established a robust selection and expansion method for polyTregs from individuals with T1D, where three cell surface markers— CD4, CD25, and CD127—were used to FACS purify the FOXP3+ Tregs present in the peripheral blood as described previously (20). Purified Tregs were cultured with clinical-grade Dynabeads coated with anti-CD3 and anti-CD28 plus recombinant IL-2. As seen in Table 3, a unit of blood yielded between 4.2 × 106 and 11.8 × 106 purified CD4+CD127lo/−CD25+ Tregs, which on average expanded 554.7 ± 370.2–fold (SD) (ranging from 29.8-fold to 1366.8-fold), with each incremental cohort averaging a greater fold expansion as our experience progressed (Table 3). The expression of FOXP3 has been the most reliable marker of Tregs. On average, the expanded Treg preparations that were infused were 92.2% FOXP3+ (range, 76 to 96.9%), with only two preparations<90% positive. The Treg preparations met the additional release criteria of high viability (cutoff ≥85%, actual >98%), high CD4+ percentage (≥95%), and low CD8+ cell contamination (cutoff ≤5%, actual <2.5%) (Table 4). Of 16 attempted expansions, two preparations of expanded Tregs did not meet release criteria. The cell preparation for patient 002–011 had a population (7.36%) of CD4+CD8+ “double-positive” Tregs in the expanded population, which exceeded the release criterion of ≤5% CD8+ cells. However, follow-up analyses showed that the double-positive cells were FOXP3+, did not change during the culture period, and suppressed efficiently in vitro. Moreover, the percentage of double-positive cells did not change during the culture period, suggesting that these cells were indeed present in the circulation of this donor, perhaps representing a small population of tissue-derived Tregs as has been suggested for Teffs previously (21). Preparation for patient 007–014 contained a population of CD4+ Teffs in the expanded cultures (about 80%). Subsequent studies showed that the Teff “contamination” was due to a low level of expression of CD127 on Teffs, making it difficult, with current clinical-grade anti-CD127 monoclonal antibodies, to completely separate the Teff from Treg even after CD4 and CD25 gating.
Table 3. Initial Treg purity and expansion.
The number of Tregs isolated form ~400 ml of whole blood, the Treg purity (%CD4+CD127lo/−CD25+) after FACS, total number of Tregs after 14-day expansion, fold increase from number of Tregs seeded and, and the total number of Tregs infused.
| Cohort | Subject ID | Number Tregs pre-expansion (× 106) | Treg purity (%) | Number Tregs post-expansion (× 109) | Fold expansion | Number Tregs infused (× 108) |
|---|---|---|---|---|---|---|
| 1 | 002-002 | 6.5 | 99.6 | 0.96 | 148.9 | 0.056 |
| 002-003 | 6.3 | 98.7 | 0.19 | 29.8 | 0.050 | |
| 002-004 | 6.8 | 98.7 | 1.34 | 197.7 | 0.056 | |
|
| ||||||
| 2 | 002-005 | 4.2 | 98.8 | 1.35 | 322.2 | 0.436 |
| 002-007 | 4.5 | 97.9 | 2.57 | 576.8 | 0.425 | |
| 002-008 | 6.0 | 99.1 | 3.05 | 505.0 | 0.398 | |
|
| ||||||
| 3 | 007-101 | 5.4 | 97.3 | 2.96 | 544.8 | 3.81 |
| 002-015 | 5.8 | 98.1 | 2.70 | 495.9 | 3.46 | |
| 007-102 | 8.0 | 99.2 | 11.0 | 1366.8 | 3.68 | |
| 002-017 | 6.3 | 99.4 | 3.11 | 518.1 | 3.31 | |
|
| ||||||
| 4 | 002-018 | 10.4 | 96.3 | 13.0 | 1242.4 | 26.8 |
| 002-019 | 11.8 | 98.5 | 7.20 | 613.2 | 26.9 | |
| 007-103 | 6.3 | 97.9 | 3.30 | 527.1 | 29.4 | |
| 002-022 | 4.7 | 98.1 | 3.20 | 677.2 | 23.5 | |
Table 4. Final Treg release specifications and results.
Final cell product was assessed for identity (≥60% FOXP3+ and ≥95% CD4+ cells), purity (≤5% CD8+ cells, 100 beads per 3 × 106 cells, and endotoxin≤3.5 EU/ml), sterility (negative for mycoplasma, anaerobic and aerobic bacteria, gram stain, fungal culture, KO exam), and viability (>85%).
| Result (%) (subject ID)
| ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Assay (specification) | Cohort 1
|
Cohort 2
|
Cohort 3
|
Cohort 4
|
||||||||||
| (002–002) | (002–003) | (002–004) | (002–005) | (002–007) | (002–008) | (007–101) | (002–015) | (007–102) | (002–017) | (002–018) | (002–019) | (007–103) | (002–022) | |
| Percentage of FOXP3+ cells (≥60%) | 76.0 | 92.0 | 95.6 | 82.6 | 96.8 | 94.2 | 94.3 | 96.9 | 92.4 | 93.8 | 94.0 | 95.3 | 94.9 | 92.0 |
| Percentage of CD4+ cells (≥95%) | 96.0 | 95.0 | 97.3 | 95.2 | 98.5 | 98.7 | 98.5 | 98.3 | 97.9 | 97.3 | 98.4 | 97.5 | 98.2 | 96.5 |
| Percentage of CD8+ cells (≤5%) | 2.4 | 1.9 | 0.8 | 0.1 | 0.2 | 0.1 | 0.1 | 0.6 | 0.1 | 0.9 | 0.4 | 0.2 | 0.6 | 0.4 |
| Viability (≥85%) | 100.0 | 99.5 | 98.5 | 98.5 | 99.0 | 99.4 | 98.7 | 99.8 | 99.4 | 99.3 | 99.3 | 98.6 | 99.0 | 99.3 |
Phenotypic and TCR analysis of expanded polyTregs
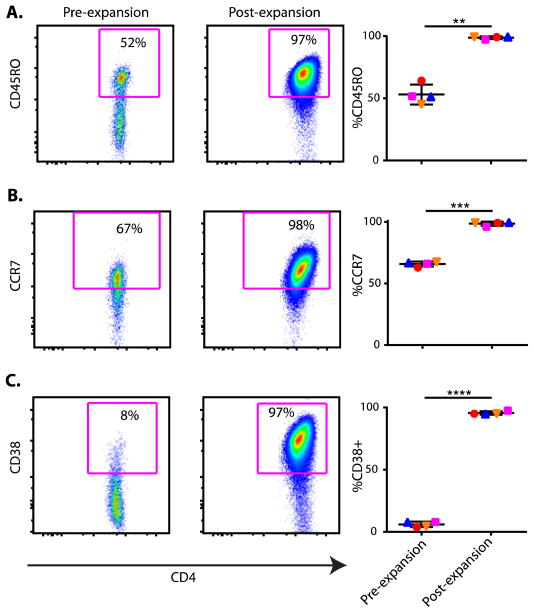
Key cell surface markers, CD4 and CD127, used to isolate the Tregs were largely unchanged after expansion, although CD25 expression increased, which reflected the feedback induction caused by culturing the cells with IL-2. Our previous data demonstrated that the naïve CD45RA+ Tregs preferentially expand in the cultures; however, the CD45RA+RO− cells down-regulate CD45RA and up-regulate CD45RO+ over the expansion period (20). An example is shown in Fig. 2A, where the percent CD45RO+ Tregs went from 52% before culture to 97% after culture. In addition, the cell expansion led to up-regulation of the cell surface markers CCR7 and CD38 increasing from 67% and 8% fresh, to 98% and 97% in expanded populations, respectively (Fig. 2, B and C). CCR7 has been shown to enhance Treg trafficking to lymph nodes (22). CD38, a multifunctional ectoenzyme that catalyzes the synthesis and hydrolysis of cyclic adenosine diphosphate (ADP)–ribose from nicotinamide adenine dinucleotide (NAD+) to ADP-ribose, is reported to be essential for the regulation of intracellular Ca2+ and associated with enhanced Treg function (23). A summary of all the tested patients for each marker is included on the right panels of Fig. 2. These data (right panels) suggest that the potent activation induced in vitro by anti-CD3, anti- CD28, and IL-2 led to a highly activated phenotype with the potential for enhanced Treg function in vivo. The TCRβ repertoire of the expanded Tregs was analyzed and compared to the freshly isolated populations to determine the polyclonality of the expanded Tregs. The expanded cells exhibited polyclonality indistinguishable from the preexpansion cultures based on high-throughput TCRβ sequencing (Adaptive Biotechnologies) (fig. S1), suggesting that the Tregs remained a highly diverse population after expansion.
Fig. 2. Increased expression of CD45RO, CCR7, and CD38 on Tregs after in vitro expansion.
CD4+CD25+CD127loTregs, as shown in Supplemental Figure S6, were stained for (A) CD45RO, (B) CCR7, and (C) CD38 before and after expansion. Representative FACS plots are shown for each of these stains on the left and graphs plotting data for all subjects (n=4) on the right. Data points for each subject are represented in a unique and consistent symbol (blue upright triangle, red circle, orange inverted triangle, and magenta square). Significance was determined by paired t test and indicated with an asterisk. **P = 0.0014, ***P = 0.0001, and ****P < 0.0001
Functional analysis of expanded polyTregs
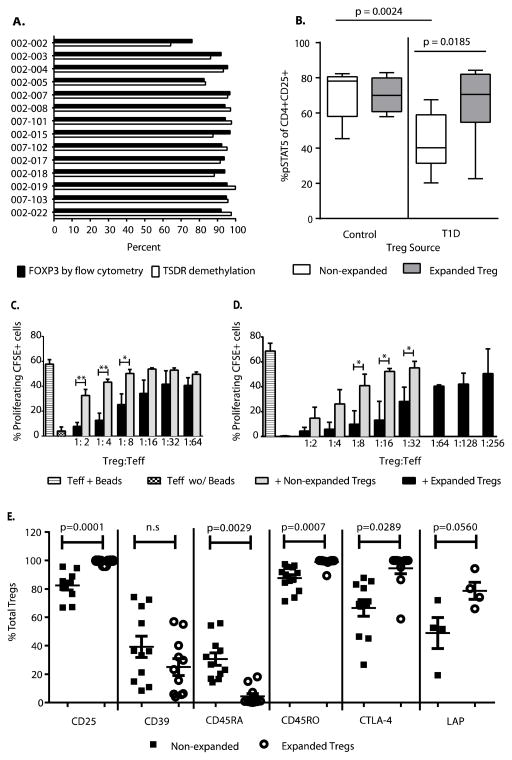
FOXP3 protein can increase transiently in Teffs in response to activation signals. However, the DNA methylation state of enhancer region of the FOXP3 locus [Treg-specific demethylated region (TSDR)] remains methylated in all but bona fide Tregs (24). We analyzed methylation of each preparation. The FOXP3 TSDR remained demethylated in the expanded Tregs, and FOXP3 protein levels correlated with overall demethylation status at the FOXP3 TSDR, indicating overall purity and stability of the expanded Tregs (Fig. 3A). Impaired function of Tregs in patients with T1D has been ascribed to a reduced phosphorylation of STAT5 (pSTAT5) in response to IL-2 (18). pSTAT5 is a key transcription factor essential for IL-2–driven Treg expansion and survival. Exposure to IL-2 in vivo results in an improvement of IL-2–driven pSTAT5 (25), suggesting that ex vivo expansion of Treg with high-dose IL-2 could enhance the IL-2 response in T1D Treg. Indeed, we found that after 14 days of expansion with IL-2, T1D Tregs demonstrate an increase in pSTAT5 in response to IL-2 (P = 0.0185), a finding that is most profound in the Treg that had an IL-2 (pSTAT5) level below the mean of Treg from controls (paired t test = 0.0024) (Fig. 3B). Notably, after 14 days of expansion, the response to IL-2 was not different from Tregs from between T1D patients and healthy control subjects. This was unrelated to the relative expression of CD25 but reflected changes in the signaling through the TCR, CD28, and CD25 cell surface receptors.
Fig. 3. Treg identity and function.
(A) Percent of expanded cells expressing FOXP3 protein as determined by flow cytometry and percent of DNA demethylated at the FOXP3 Treg Specific Demethylation Region (TSDR) was determined by Epiontis as described in Methods section. (B) Percent of STAT5 phosphorylation in response to IL-2 stimulation in CD4+CD25+ Tregs pre- and post-expansion in healthy controls (n=5) and subjects with T1D (n=10). Staining and gating was done as previously described (25). Significance was determined by Mann Whitney test. (C) In an in vitro culture, CFSE labeled Teff cells were cultured for 4 days in the presence of anti-CD3/CD28 antibody coated beads in the presence or absence of expanded/natural Tregs of same donor. T-cell proliferation in these cultures was analyzed by flow cytometry for CFSE dilution as previously described (41). Each condition was set-up in duplicate wells and compared to cultures with Teff alone. Data are represented as mean + SEM for n=4 healthy individuals. Statistical difference between non-expanded and expanded Tregs was determined by t test, where the difference seen at 1:2 is P=0.0050, 1:4 is P=0.0025, and 1:8 is P=0.0354 (*P < 0.05 and **P < 0.01). (D) Suppression assays using CFSE-labeled CD4+CD127+CD25− cells sorted from standard PBMC as Teff cells cultured alone or activated with anti-CD3/anti-CD28 coated beads, and/or co-incubated with Treg cells (from patients enrolled in this trial) show a consistent level of suppression. Comparison of mean of % proliferating CFSE+ cells with increasing ratio of Treg:Teff is shown for non- expanded Treg as compared to expanded Treg (n=3). Statistical difference between non-expanded and expanded Tregs from T1D patients was determined by t test, where the difference seen at 1:8 is P=0.0195, 1:16 is P=0.0112, and 1:32 is P=0.0333 (*P < 0.05). (E) Treg functional markers CD25, CD39, CD45RA, CD45RO, CTLA-4, and LAP were analyzed on expanded Tregs (solid squares) and non-expanded Tregs (open circles) from same healthy control donor by flow cytometry (n>4 individuals as depicted). Representative plots from the FACS analysis can be seen in Supplemental Figure S7. Significance was determined by t test.
We have previously described Treg “instability” in patients with T1D based on our identification of their production of interferon-γ (IFN-γ), a T helper 1 (TH1)-type effector molecule that has been ascribed to participate in the pathogenesis of disease (26), and other studies have shown that expanded Tregs can begin to produce type 2 cytokines such as IL-4 and IL-5 (27, 28). Therefore, we examined supernatants harvested from in vitro expansion cultures and ex vivo– expanded Tregs stimulated overnight with phorbol 12-myristate 13-acetate (PMA) and ionomycin and analyzed for type 1 and 2 cytokines by cytometric bead array (Becton Dickinson) to determine how the expansion had affected their function. Similar to our previous studies (20), there were only a limited amount of Teff cytokines (that is, IFN-γ and IL-17) produced in the culture, and the levels of these cytokines did not change after ex vivo expansion based on intracellular flow cytometric or culture supernatants analyses.
Finally, we examined the suppressive activity of the expanded Tregs in vitro. Initial studies were performed using fresh versus expanded Tregs from a series of healthy individuals. The expanded Tregs routinely suppressed the PBMC proliferation at significantly lower conventional T cell (Tconv)/Treg ratios than the nonexpanded cells (Fig. 3C). Similarly, analysis of three expanded Treg preparations from patients in the phase 1 trial demonstrated four- to eightfold greater suppressive activity than nonexpanded Tregs from the same individual (Fig. 3D). Overall, the expanded Tregs from the 14 patients in the phase 1 trial demonstrated suppressive activity greater than 50% at ratios of 1:32 Tconv/Treg or lower, which suggested overall greater activity by the expanded versus nonexpanded Tregs (fig. S2). Finally, we examined a series of cell surface and functional markers on the nonexpanded versus expanded Tregs (Fig. 3E). In addition to the increased suppressive activity, the expanded Tregs showed significant increases in the expression of CD25, CTLA-4, and LAP, all of which have been shown to be involved in Treg function (11, 14, 29). Together, the phenotypic and functional data suggest that the expansion of the Tregs increased not only the overall number of cells for AT but also the functional potential of these cells on a per-cell basis.
Safety
All patients received the target dose according to dose escalation cohort (Table 3). A tabulated cumulative summary of adverse events for all cohorts categorized by system organ class and severity is listed in table S1. There were no infusion reactions. After a mean follow-up of 31 months, there were 11 grade 3 or 4 adverse events, which largely reflected metabolic abnormalities of underlying diabetes. Four serious adverse events were reported. One patient had three episodes of serious hypoglycemia 14, 248, and 463 days after Treg infusion in one subject and one episode of diabetic ketoacidosis 67 days after Treg infusion in a second subject. No opportunistic infections or malignancies were observed. One subject developed grade 2 pharyngitis and had low-copy number cytomegalovirus (CMV) detected on day 7, but not detected at day 28, due to a presumed new infection with CMV occurring before receiving cells. There was no apparent relationship between adverse events and Treg dose (table S2). The full listing of adverse events is in tables S3 to S6.
Metabolic results
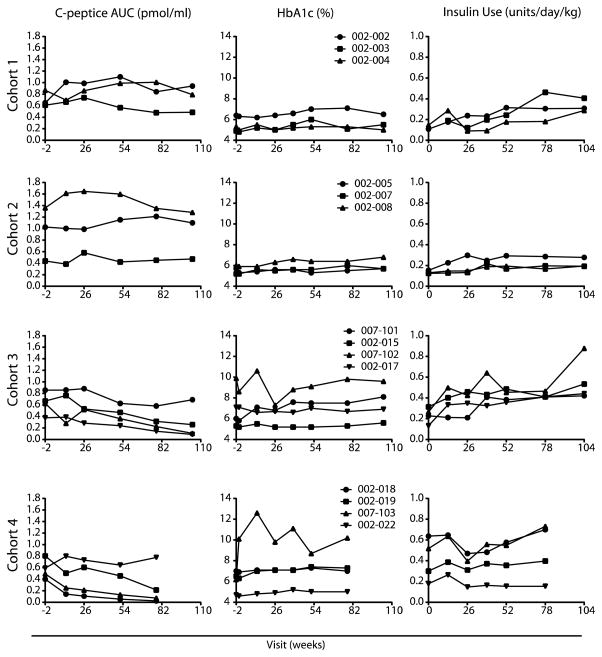
Metabolic function was evaluated by measuring the C-peptide area under the curve (AUC) during and MMTT (Fig. 4). The C-peptide responses were generally unchanged at 1 year and even after 2 years in dose cohorts 1 and 2. Three of four subjects in cohort 3 and three of the four subjects in cohort 4 showed a decline in C-peptide of more than 50% over 78 week of follow-up. Two of four subjects in cohort 4 also had a decline in C-peptide of more than 50% over 52 weeks of follow-up; however, the remaining two patients remained stable from week 13 to 52 weeks. The heterogeneity of diabetes progression and the dependence of progression on age and on duration of diabetes do not allow us to draw a conclusion from these findings in a small number of subjects. The HbA1c levels remained stable in all but one subject in cohort 4, 007–103, whose levels went from 6.2% at screening to 12.6% at week 13, with the individual’s C-peptide diminishing from 0.49 at screening to 0.249 pmol/ml at week 13. Insulin use was generally stable. In summary, there were too few subjects to make a clear statement about stabilization of decay in C-peptide in treated subjects. However, we note that 7 of 14 patients had a C-peptide reduction of <10% of baseline C-peptide, predominantly in the lower-does group, whereas 7 of 14 showed an increase in C-peptide decline at 1 year (predominantly in the higher-dose cohorts). Given the small number of subjects in each cohort, the overall changes in C-peptide in this study fall within the expected decline observed in the natural history of the disease (30).
Fig. 4. Metabolic assessments. (Left column).
C-peptide area under the curve (AUC). C-peptide AUC is reported for fasting 4-hour mixed meal tolerance test without carbohydrate restriction for 3 days preceding test. The target glucose level at the start of the test was between 70 and 200 mg/dL. Regular insulin or short acting insulin analogues was allowed up to 6 and 2 hours before the test, respectively, to achieve the desired glucose level. The baseline blood samples (−10 minutes and 0 minutes) were drawn, and then subjects drank Boost High Protein Nutritional Energy Drink® (Nestle Nutrition) at 6 kcal/kg (1 kcal/mL) to a maximum of 360 mL Blood was drawn at 15, 30, 60, 90, 120, 150, 180, 210, and 240 minutes following Boost dose. C-peptide AUC was calculated using the trapezoid rule. (Middle Column) Hemoglobin A1c (HbA1c). (Right Column) Insulin Use. Subjects self-reported insulin use for the 3 days immediately preceding the scheduled visit. The average total insulin (long acting + short acting) use per day normalized to weight is reported.
T lymphocyte subsets
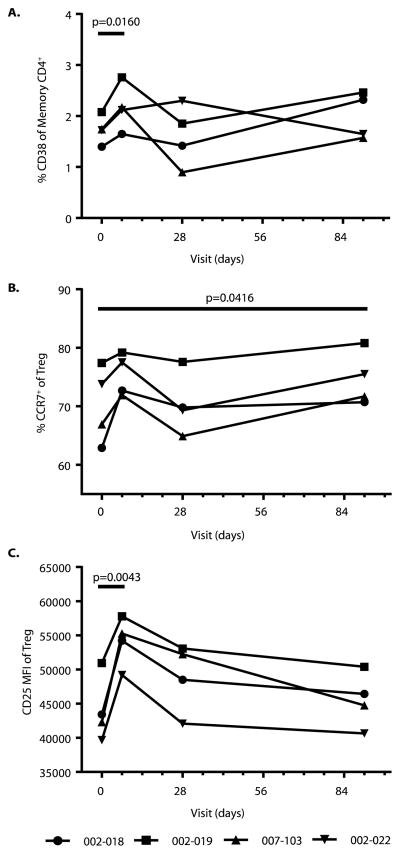
We compared T lymphocyte subsets among participants who had been treated with polyTregs before and after AT. Overall, consistent changes in T cell markers were not observed between Treg-treated patients after AT when compared to pretreatment. A small, transient increase in CD38+ memory T cells was seen in the first week (Fig. 5A). A longer, more pronounced increase was seen in the percentage of CCR7+ Tregs out to day 91 (Fig. 5B). In addition to these changes in T cell marker, an increase in CD25 median fluorescence intensity (MFI) of the Treg population was seen on day 7 after infusion (Fig. %C). All of these changes most likely represent the detection of the transferred polyTreg population. There was also as a significant decrease in CD56hiCD16lo natural killer (NK) cells early after polyTreg injection among all patients enrolled, consistent with the reduction of a more pathogenic type 1 IFN-γ/tumor necrosis factor (TNF)-producing NK population (fig. S3) (31). We did not find a statistically significant change in the titers of anti-GAD65 (glutamic acid decarboxylase 65) or anti-ICA512 (islet cell autoantibody 512) antibodies or differences between the treatment groups.
Fig. 5. Changes in T lymphocyte subsets after Treg infusion.
PBMC samples collected from subjects on days 0, 7, 28, and 91 were frozen from all patients then thawed simultaneously before being stained with CD4, CD45RO, CD25, CD127, CCR7, and CD38 for FACS analysis. Representative FACS plots for all analyses are shown in Supplemental Figure S6. All subjects shown are from cohort 4 (n=4), as detailed in Table 1. (A) An increase in %CD38+ cells was seen within CD4+CD45RO+ T cells subset post Treg infusion. (B) Within the CD4+CD127lo/−CD25+ Tregs the percentage of CCR7+ Tregs increased after infusion of expanded Tregs. (C) MFI of the patients’ CD4+CD127lo/−CD25+Treg population increased upon addition of expanded Tregs. For all plots comparisons were made to day 0 and significance was determined by paired t test.
Tracking the adoptively transferred expanded polyTregs
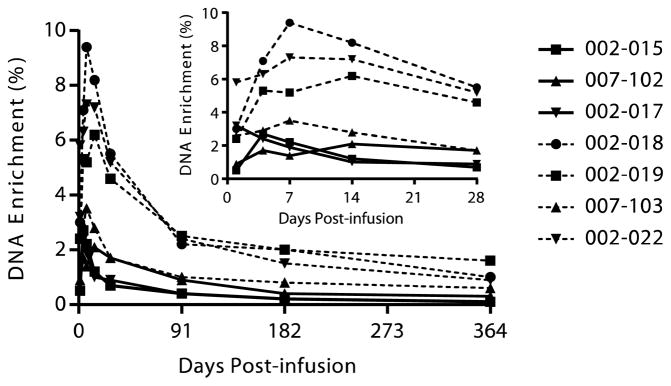
Previous studies of allogeneic adoptive Treg immunotherapy have shown the Tregs to be short-lived (32). Initial studies were performed to examine whether the adoptively transferred cells could be visualized on the basis of the cell surface expression profile of the expanded Tregs. There was a short-term increase in the percentage of CD25 bright cells (fig. S4). In addition, the MFI of CD25 of CD4+CD127loCD25+ cells increased from 385 ± 79 on day 0 to 859 ± 183 on day 1 and stayed up until day 7 (Fig. 5C). We developed a tool to monitor the adoptively transferred cells. Tregs were nonradioactively tagged by labeling the deoxyribose moiety of newly replicated DNA during expansion ex vivo, by metabolic labeling of cells with [6,6-2H2]glucose. This enabled tracking to be performed on the whole Treg population in cohorts 3 and 4. As de- scribed in Materials and Methods, [6,6-2H2]glucose was added to the Treg culture throughout the 14-day expansion. The 2H2 (deuterium) label was incorporated into replicating DNA such that 59.8 ± 1.03% (SD) of the deoxyribose in purine deoxyribonucleosides isolated from cellular DNA was labeled in the expanded Tregs (range, 58.2 to 60.9%). The labeled cells were transferred into the patients, and blood samples were harvested at various time points after administration. Tregs sorted from purified PBMCs, AT, were analyzed for deuterium enrichment in the DNA (Fig. 6 and fig. S5). As seen in Fig. 6 (inset), at day 1, there was a significant percentage of deuterium label in the DNA of circulating Tregs in each individual (Fig. 6, inset). The maximal percentage of the AT polyTregs occurred by 7 to 14 days, after which point there was a decline in the percentage of labeled Tregs in the circulation. Thus, by ~90 days after infusion, about 25% of the peak labeling in cells was still observed in the circulation. This percentage stabilized over the next 9 months, resulting in the prolonged presence of labeled Tregs in the circulation at least 1 year after transfer. Our pharmacokinetic analysis of the survival of the Tregs indicated a two-phase decay curve, with the average half-life of the fast decay phase of about 19.6 days (range, 4.7 to 32.5 days) and a second slow decay phase with a half-life of a year or more in four of seven patients studied (fig. S5 and table S7).
Fig. 6. Survival of infused polyclonal Tregs.
During ex vivo expansion, the 2H label from deuterated glucose (2H2-glucose) contained in the cell culture medium is incorporated into the deoxyribose moiety in replicating DNA through the de novo purine nucleotide synthesis pathway. Three subjects (002–015, 007–102, and 002–017) were treated with a single dose of 2H-labeled Tregs at a target dose of 3.2 ×108cells, and four subjects (002–018, 002–019, 007–103, and 002–022) were treated with a target dose of 26 ×108 cells that were approximately 60% enriched for the 2H-label. Peripheral blood was collected on days 1, 4, 7, 14, 28, 91, 182, and 364 days post infusion, and Tregs were sorted from the peripheral blood. Following isolation and hydrolysis of genomic DNA, the 2H isotopic enrichment of the purine deoxyribonucleosides in Tregs sorted from whole blood was assessed by gas chromatography/mass spectrometry. Background enrichment of unlabeled Tregs was ≤0.1% for each of the seven subjects.
There are several possible reasons for the decrease in the number of circulating Tregs, including cell death, migration to lymphoid tissues and inflammatory sites such as the pancreas, or a high degree of proliferation, thus diluting out the deuterium label. The current study design and data cannot distinguish among these possibilities. It also remained possible that the cells changed their Treg phenotype and the label was not captured in the sorting strategy for Tregs. To address this last possibility, we sorted all the CD4+ cells not in the Treg gate and separated them into naïve, central memory, and effector memory subsets based on the expression of CD45RO and CD62L to see whether deuterium label was found in CD4+ T cell other than Tregs (fig. S6). At no time, up to 365 days, was any deuterium observed in any other subset other than bona fide Tregs within the detection limit of 0.1% (Table 5). Because the labeling level of Tregs ranged from 2 to 8% early and 1 to 2% later, if some fraction of Tregs were converted to Teffs, then the relative contribution from Tregs to any other pool would have to be less than 1:20 to 1:80 early and less than 1:10 to 1:20 later, suggesting that conversion of Tregs into Teffs could have occurred as a rare event, below the level of detection. Overall, the data suggest that the infused Tregs did not transdifferentiate into Teffs.
Table 5. Stability of infused polyTregs.
To address long-term stability of the infused expanded Tregs, samples collected on days 91 (in three of four patients only), 182 and 365 were sorted into Treg and non-Treg subsets as shown in Supplemental Figure S6. Subsets were then analyzed by MS for 2H label, which was incorporated into the infused Tregs during the expansion. Values shown are % enrichment for 2H and have an error of ±0.1.
| Cell Subset |
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DNA enrichment (%)
| |||||||||||
| C012 (002–018)
|
C013 (002–019)
|
C014 (007–103)
|
C016 (002–022)
|
||||||||
| Day 182 | Day 365 | Day 91 | Day 182 | Day 365 | Day 91 | Day 182 | Day 365 | Day 91 | Day 182 | Day 365 | |
| Tregs | |||||||||||
| CD4+CD25+CD127lo | 2.0 | 1.0 | 2.5 | 2.0 | 1.6 | 1.0 | 0.8 | 0.6 | 2.4 | 1.5 | 0.9 |
| Non-Tregs | |||||||||||
| CD45RO+ | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| CD45RO+CD62Lhi | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| CD45RO+CD62Llo | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| CD45ROloCD62Lhi | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
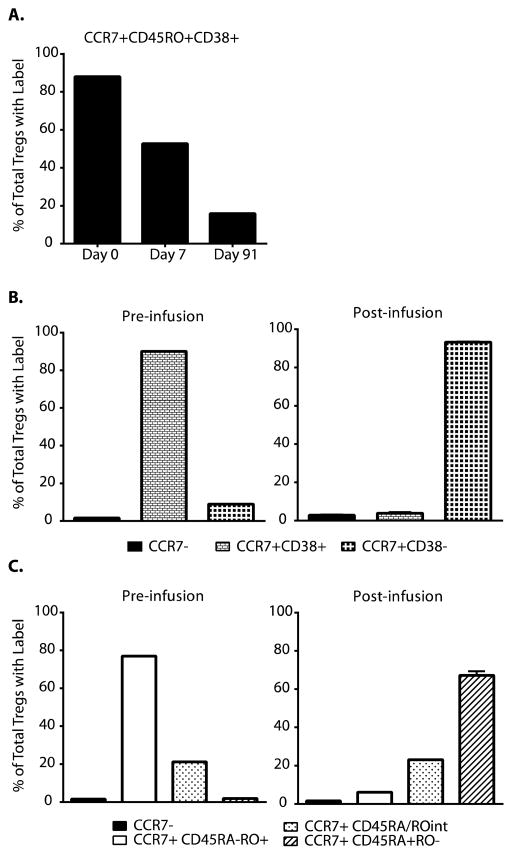
However, there were indeed some changes in the phenotype of the infused Tregs. As mentioned above, the expanded polyTregs converted to a nearly uniform CD45RA−CD45RO+CCR7+CD38high phenotype during ex vivo expansion (Fig. 2). To test the stability of these markers, CD4+CD127lo/−CD25+ Tregs were further divided into separate subsets based on CD45, CCR7, and CD38 expression at various time points before and after infusion and then measured for deuterium enrichment as described above. Although most of the label within the Tregs began in CD45RA−CD45RO+CCR7+CD38high subset, we found that over time most of the Treg-specific label was no longer in this subset (Fig. 7A). Although most of the deuterium-labeled Tregs retained high levels of CCR7 expression (97 to 100%), there was clear evidence that most of the transferred cells lost CD38 expression and there was a switch from CD45RO to CD45RA (Fig. 7, B and C). The fact that the cells regained CD45RA expression could represent either a return to a resting naïve phenotype or a memory phenotype similar to that seen in a subset of CD8+ and CD4+ central memory T cells (33).
Fig. 7. Changes in Tregs phenotypes after transfer.
Frozen samples of expanded Tregs or PBMC collected at time points on days 7–91 post infusion were FACS sorted for CD4+CD25+CD127lo Tregs. These Tregs were further subsetted and sorted based on expression of CCR7, CD38, CD45RA, and CD45RO. Collected Tregs subsets were then analyzed by mass- spectrometry for 2H-label, which was incorporated into the infused Tregs during the expansion. (A) CCR7+CD45RO+CD38+ Tregs (black bars) were analyzed for %enrichment, which represented an increasingly smaller proportion of total Treg 2H enrichment over time. (B) Tregs were subsetted into CCR7− (black bars), CCR7+CD38+ (small grid bars), and CCR7+CD38− (large grid bars) populations for 2H enrichment analysis pre and post infusion. (C) Tregs were subsetted into CCR7− (black bars), CCR7+CD45RA−CD45RO+ (white bars), CCR7+CD45RAintCD45ROint (speckled bars), and CCR7+CD45RA+CD45RO− (striped bars) populations for 2H enrichment analysis pre and post infusion. All plots shown are representative of data collected from subjects in cohort 4. “% of Total Tregs with Label” is defined is Supplemental Figure S8.
Discussion
Several reports have described abnormalities in the functions of Tregs in patients with T1D, such as impaired responses to IL-2, a critical factor normally needed for Treg growth and survival. Our group also described instability manifest by their expression of pathologic cytokines, suggesting that ex vivo Treg expansion and repair might re-regulate the auto-immunity (34). In nonobese diabetic (NOD) mice, our studies have shown that antigen-specific diabetogenic pathologic T cells may include some cells that have expressed FOXP3 during their development, raising the possibility that the unstable Tregs may acquire pathologic function as a result of acquired factors. Clinical studies with exogenous IL-2 have indicated that expanding Tregs can prevent autoimmunity, but in addition, repair of functional abnormalities is an important part of using these cells therapeutically. Here, we have expanded polyTregs, evaluated their function, and tested their safety after reinfusion in patients with recent-onset T1D. A key achievement to accomplish this was our ability to expand the cells to sufficient numbers for in vivo therapy. Our culture methods and analysis of the expanded cells indicated that the expanded Tregs were not contaminated with significant numbers of Teffs. Furthermore, we have illustrated that FOXP3 expression by FACS directly correlated with percent TSDR demethylation, indicating that the FOXP3+ expression in these cells was stable after the 14-day ex vivo expansion period. The expanded T cells showed robust function in suppression assays in vitro and also corrected the defective pSTAT5 responses to IL-2.
The Treg infusions were well tolerated, and the results of this safety study suggest that the infusions are safe over a more than 500-fold dose range. Cytokine release, infusion reactions, or infectious complications were not seen. We also showed that the infused Tregs did not acquire pathologic phenotypes. The AT Tregs re-expressed CD45RA. It is likely that the changes from CD45RA to CD45RO during expansion were transient in nature. However, it is noteworthy that although central memory T cells are CD45RO+CD45RA−, effector memory T cells express CD45RA (33), suggesting that this change in phenotype for the AT Tregs may reflect further engagement of antigen in vivo and the development of a distinct Treg memory population.
In addition to demonstrating the long life span of a subset of Tregs, the kinetic modeling results may have clinical implications. First, the calculated whole body pool size of Tregs varied widely among T1D subjects. It will be of interest in future studies to determine whether this quantitative metric correlates with immune function or disease progression in individuals. Second, it will also be of interest to determine whether the relative proportion of Tregs that were short- or long-lived (range, 75 to 90% short-lived) has functional importance in Treg AT and whether this kinetic behavior is a feature of the host subject.
Our studies of the Treg pharmacokinetics, based on reinfusion of Tregs labeled ex vivo with stable isotopes, showed a delay in maximal AT Tregs in the circulation and a two-phase decline curve that raises several questions. Other studies have suggested that the delay may be due to regulation of certain adhesion or homing receptors on the ex vivo-expanded Tregs that prevent the cells from immediately entering the circulation but rather promote homing and lodging in the liver or lung for some period of time (35). The apparent precipitous decline in circulating AT polyTregs number between the first and third month after transfer may be due to cell death, perhaps due to a decrease in IL-2 signaling when shifting from the in vitro milieu to in vivo. Consistent with this possibility, on day 1, we observed a significant increase in CD25+ Tregs in the circulation, but this increased CD25 expression diminished rapidly, likely due to the reduced levels of IL-2 over time and perhaps leading to cell death (fig. S4). It should be pointed out that the increase in the percentage of CD25 bright Tregs at day 1 may not fully reflect the presence of AT polyTregs entering the circulation because the relative percentages are higher than might have been expected based on the deuterium labeling. It would appear that the transfer of the ex vivo-expanded Tregs might have carried over some IL-2 bound to the receptor that induced IL-2 receptor (IL-2R) on the endogenous Tregs.
The improvement in Treg function and reversibility of the reduced pSTAT5 suggest that this aspect of Treg function may be amenable to repair at least in the short term and in the context of in vitro expansion, consistent with what was observed in the IL-2/rapamycin study (25). The reduction in pSTAT5 seen in T1D CD4+ T cells reflects several polymorphisms found in the IL-2R and PTPN2, both of which are risk alleles for T1D. However, it may also in part reflect the absence of availability of IL-2 in patients at the time of diagnosis, possibly by consumption by other Teffs. Longer-term studies and analyses of the time of onset of this abnormality may help to resolve these questions. Despite the improvement in pSTAT5, we did not find that the cell culture process increased production of cytokines, such as IL-10, by the expanded Tregs, suggesting that some Treg functions are not enhanced by in vitro expansion. In the future, further studies of the functional phenotype of the Tregs in vivo will address whether these additional functional activities have been enhanced.
Finally, there are several additional conclusions that can be drawn from the studies that have important implications. First, we have demonstrated that the expanded Tregs can be exported to other clinical sites, increasing the feasibility of developing this cell therapy into a true therapeutic. Second, although there were some phenotypic changes that occurred after Treg infusion (such as CD38 and CD45R0 expression), the Tregs were overall quite stable for key parameters such as FOXP3, CD25, CD127 expression, and lack of Teff cytokine production. However, it should be noted that we are only able to sample the cells in the circulation and there may be changes at the site in inflammation in the islets that are not evident when examining the blood. Finally, the enhanced suppressive activity seen in vitro under conditions of Treg activation and expansion with IL-2 might suggest that there will be increased efficacy if the Tregs were combined with IL-2 therapy in vivo. This combination therapy is currently under development. In summary, we have shown that autologous Tregs can be expanded and are well tolerated in patients with recent-onset T1D. The expansion in vitro improves functional defects that have been identified in these cells in patients. Further adequately powered studies will be needed to determine whether the improvement in function and number leads to restoration of immunologic tolerance and prevention of disease progression.
There are several limitations to the study. As a phase 1 study, it was not powered to detect improvement in metabolic function, and therefore, we are unable to assess whether the improvement in the Tregs that we observed in vitro or the greater number of cells after AT will prevent progression of the autoimmune disease. However, the precipitous decline in C-peptide that had been described with high doses of IL-2, which expanded Tregs and NK cells in vivo, was not seen, and several of the patients in this phase 1 study had prolonged C-peptide production, especially in the lower-dose cohorts. Recently, Marek-Trzonkowska et al. reported initial findings of the infusion of Tregs in children with new-onset T1D (36, 37). Their approach to expansion of the cells had many similarities to ours, but they did not assess the effects of the culture on Treg function. Moreover, the dosing that was used in that study was not fixed but given per kilogram, and additional doses were administered on the basis of deterioration in metabolic function - a late occurrence in disease progression. Nonetheless, these investigators suggested the autologous Tregs might be able to maintain C-peptide responses. As with our study, they reported that the infusions were well tolerated without additional safety concerns, most notably no significant risk for infection. Another caveat in these studies is that analyses of the AT polyTregs were limited to sampling of the peripheral blood rather than the sites of autoimmune inflammation. Thus, issues such as local immune suppression, Treg instability, and alteration of Teffs cannot be adequately addressed in this disease setting. Future studies in other autoimmune diseases and organ transplantation should allow for tissue biopsies that can determine the local effects of polyTreg administration.
Thus, in summary, this study reports on the successful isolation, expansion, and reinfusion of polyTregs derived from patients with T1D. This provides a platform for additional clinical trials in this and other autoimmune diseases. The current efforts to use Treg-promoting therapies, such as low-dose IL-2 (38), are likely to be complementary to this current AT effort, potentially resulting in a robust combination therapy, which, when combined with Teff-depleting agents such as teplizumab and alefacept, may lead to durable remission and tolerance in this disease setting (39). Finally, efforts are under way to develop islet antigen–specific Tregs using genetic engineering (chimeric antigen receptors and TCR transduction), which we and others have shown to be even more efficacious to treat autoimmune diabetes in animal models (16).
Materials and Methods
Participants
This study enrolled male and female subjects diagnosed with T1D within 3 to 24 months of screening who were 18 to 45 years of age with peak C-peptide >0.1 pmol/ml during MMTT challenge, were positive for Epstein-Barr antibody, and were positive for at least one of the following antibodies: tyrosine phosphatase–related islet antigen 2 (IA-2), ICA, GAD65, insulin, and zinc transporter 8 (ZnT8). Subjects also had to have adequate venous access to support draw of 400 ml of whole blood and infusion of investigational therapy. Subjects were determined to be ineligible if they had hemoglobin <10.0 g/dl; leukocytes <3000/ml; neutrophils <1500/ml; lymphocytes <800/ml; platelets<100,000/ml; Tregs<10/ml; evidence of active infection [HIV-1/HIV-2, hepatitis B, hepatitis C, Epstein-Barr virus (EBV) or CMV genomes, or positive purified protein derivative (PPD) skin test]; chronic use of systemic glucocorticoids or other immunosuppressive agents or biologic immunomodulators within 6 months before study entry; history of malignancy except adequately treated basal cell carcinoma; or any chronic illness or previous treatment that, in the opinion of the investigator, should preclude participation in the trial. Pregnant or breastfeeding women were excluded from the study, as well as any female who was unwilling to use a reliable and effective form of contraception for 2 years after Treg dosing, and any male who was unwilling to use a reliable and effective form of contraception for 3 months after Treg dosing. All participants provided written informed consent before participating in any study procedures.
Treg isolation and expansion (40)
About 400 ml of fresh peripheral blood was collected into blood pack units containing citrate phosphate dextrose (Fenwal) and processed within 24 hours for isolation of PBMCs via Ficoll density gradient (GE Healthcare Bio-Sciences). For subjects enrolled at Yale, blood was shipped to UCSF for processing in the good manufacturing practice (GMP) laboratory, and the expanded polyTregs were shipped back to Yale for infusion.
Tregs were isolated on a BD FACSAria II high-speed cell sorter housed in a class 10,000 clean room with the following GMP-grade lyophilized antibodies: CD4-PerCP (peridinin chlorophyll protein) (L200), CD127- PE (phycoerythrin) (40131), and CD25-APC (allophycocyanin) (2A3) (BD Biosciences). The sorted CD4+CD127lo/−CD25+ T cells were collected into 3 ml of X-VIVO 15 medium (Lonza, catalog no. 04-418Q) containing 10% human heat-inactivated pooled AB serum (Valley Biomedical). Treg populations were analyzed for purity after sort and determined to be 98.4% (range, 96.3 to 99.6%) CD4+CD127lo/−CD25+ T cells.
FACS-isolated cells were plated at ~2.5 × 105 Tregs per well in multiple wells of a 24-well plate (Nunc) and activated with Dynabeads CD3/CD28 CTS anti-CD3/anti-CD28–coated microbeads (Life Technologies) at a 1:1 bead/cell ratio. Cells were cultured either in X-VIVO 15 or in X-VIVO 15 customized by Lonza by substituting 100% of the glucose in the base medium with D-glucose ([6,6-2H2], 99%) (Cambridge Isotope Laboratories, catalog no. DLM-349-MPT) supplemented with 10% human heat-inactivated pooled AB serum. At day 2, the culture volume was doubled and IL-2 was added (Proleukin, 300 IU/ml; Prometheus). Cells were resuspended, fresh medium and IL-2 were added at days 5, 7, 9, and 12, and the cells were transferred to cell culture plates and flasks (Nunc), and/or bags (Saint-Gobain) of increasing size to maintain a seeding density of ~2 × 105 to 3 × 105 cells/ml in plates or flasks and a concentration of 500,000/ml in bags. On day 9, cells were restimulated with fresh anti-CD3/anti-CD28–coated beads at a 1:1 ratio. On day 14, cells were consolidated and debeaded using a MaxSep magnet, and bead removal was verified via flow cytometry. Briefly, Dynabeads CD3/CD28 CTS (Invitrogen, catalog no. 402.03D) and Spherobeads (BD, catalog no. 556291) were used as controls for determining instrument settings and defining Dynabeads gate based on forward scatter (FSC) versus side scatter (SSC) followed by FL2 versus FL3 channels on FACSCalibur. Triplicate samples of expanded CD4+CD127lo/−CD25+ Tregs at ~5 × 106 cells/ml were analyzed, and a number of cells and Dynabeads in each sample were collected to determine cell number and bead number contained within each sample. The average bead count and average cell count were used to calculate the bead/cell ratio.
The product was prepared as a cell suspension of fresh, noncryopreserved cells in sterile infusion solution composed of 1:1 PlasmaLyte A/5% dextrose, 0.45% NaCl (Baxter) containing 0.5% human serum albumin (HSA) (Grifols), all supplied as U.S. Food and Drug Administration–approved drugs (PlasmaLyte A and dextrose/NaCl) or licensed products (HSA) for injection and conforming to U.S. Pharmacopeial Convention (USP) standards.
Administration and follow-up
Results of blood chemistries and hematology were reviewed, and a history of any recent illness or fever was obtained before infusion of the cells. Patients received premedication with acetaminophen and diphenhydramine. PolyTregs were infused via a peripheral intravenous line over 10 to 30 min. Vital signs were taken before and after infusion, then every 15 min for at least 1 hour, then every hour for the first 4 hours, and every 4 hours for 20 hours. Chemistries and complete blood count with differential blood count were repeated the next day before discharge from the clinical research unit. Patients were seen for follow-up assessments on day 4 after infusion, then weekly for 4 weeks, then every 13 weeks for 1 year, and then every 26 weeks for 2 years. Telephone monitoring for adverse events continues every 6 months for 5 years after infusion followed by a final clinic visit.
Phenotypic analysis of expanded Treg populations and peripheral blood samples
Freshly expanded cells were evaluated for expression of CD4, CD25, CD127, CD8 (BD Biosciences), and FOXP3. Intracellular staining was performed with Alexa 488 - conjugated anti-FOXP3 (clone 206D) and the FOXP3 staining kit (BioLegend) according to the manufacturer’s instructions and modified as follows: 2 × 106 cells were washed and fixed for 30 min at room temperature using fixation/permeabilization buffer. Cells were washed, resuspended in perm buffer containing deoxyribonuclease I (100 U/ml; Sigma-Aldrich), and incubated for 30 min at room temperature, followed by two washes in perm buffer. Cells were subsequently blocked with human immunoglobulin G (IgG) (5 μg per test) for 5 min and stained for cell surface and intracellular markers along with anti-human FOXP3-Alexa 488 (5 μl per test) or isotype control. Flow cytometric data were collected on a FACSCalibur cytometer (BD Biosciences) and analyzed with FlowJo software (version 9; Tree Star). In experiments to determine phenotype of expanded Tregs and localization of the deuterium labeling, antibodies used for flow cytometric sorting on a BD FACSAria II cytometer included CD45RA-APC (H100) (BioLegend), CD4-Alexa 488 (RPA-T4) (Becton Dickinson), CD38-PerCP-Cy5.5 (HIT2) (BioLegend), CCR7-V450 (150503) (Becton Dickinson), CD45RO-PE-Cy7 (UCHL1) (BioLegend), CD127-PE (hIL-7R-M21) (Becton Dickinson), and CD25-BV786 (M-A251) (Becton Dickinson). FACS files were analyzed with FlowJo software version 9 or greater.
Studies of clinical samples conducted at the Benaroya Research Institute (BRI) were performed as follows. PBMCs collected at baseline and throughout the study were frozen for batched analysis. These PBMC samples were subsequently thawed, labeled, and analyzed as previously described (25) for STAT5 signaling and the immunophenotyping of lymphocyte and T cell subsets. Multicolor flow cytometry was conducted on a BD LSRII flow cytometer and analyzed in FlowJo using standardized panels developed by the Immune Tolerance Network (www.immunetolerance.org).
For studies of nonclinical samples at the BRI, cells (nonexpanded Tregs or D14-expanded Tregs from the same donor) were suspended in FACS buffer [phosphate-buffered saline + 1% fetal bovine serum (FBS) + 0.1% NaN3]. To block Fc receptor-mediated binding of antibodies, cells were suspended in FACS buffer with 1% human serum (MP Biomedicals) for 20 min. These cells were washed, placed on ice for 30 min, and stained with cell surface fluorochrome-conjugated anti-human CD3, CD4, CD45RA, CD45RO, PD-1, and LAP (BioLegend); anti-human CD4, CD25, and CD127 (BD Biosciences); and anti-CD39 (eBioscience). Intracellular staining for FOXP3 (BioLegend) and CTLA-4 (BD Biosciences) was performed using the manufacturer’s protocol. Cells were washed twice in buffer and analyzed by BD LSRII flow cytometer. All data analyses were performed using FlowJo software.
Suppression assays
Treg suppression was assessed by measuring proliferation based on either a [3H]thymidine incorporation or CFSE dilution assay. [3H]-thymidine incorporation Treg suppression assays were performed after expansion for all 14 individuals enrolled in the clinical trial as previously described (20), with the addition of a standard expanded Treg population in each assay to ensure that the data were comparable and could be combined. For all control subjects, suppression was assessed on the basis of CFSE dilution as analyzed by flow cytometry as previously described (41). In brief, CFSE-labeled autologous CD4+ CD25− Teffs were cocultured in round bottom plates with or without expanded Tregs or CD4+CD25hiCD127lo/− Tregs sorted from freshly isolated PBMCs and activated with Dynabeads human T activator CD3/CD28 (Invitrogen) for 4 days. Varying ratios of Teff/Treg were plated in duplicate or triplicate. For comparisons of clinically expanded Tregs to nonexpanded Tregs from the same subject, a small-scale version of the previously described CFSE suppression assay was used, where Teffs were plated at 10,000 cells per well at a 1:10 bead/Teff ratio. For the small-scale CFSE suppression assays, all Tregs were isolated from cryopreserved material and in vitro suppression was assessed on the basis of Treg capacity to suppress the proliferation of a standard allogeneic responder T cells generated from a healthy donor whose PBMCs were cryopreserved in multiple aliquots containing 10 × 106 cells per vial. Additionally, standard nonexpanded Treg and expanded Treg populations were included in each assay to ensure that the data were comparable and could be aggregated.
In vitro suppression assays were performed in RPMI 1640 (Mediatech) supplemented with 5 mM Hepes, 2 mM L-glutamine, penicillin/streptomycin (50 μg/ml each) (Invitrogen), 50 μM 2-mercaptoethanol (Sigma), 5 mM nonessential amino acids, 5 mM sodium pyruvate (Mediatech), and 10% FBS (Omega Scientific). Cultures were main- tained in 200-μl volumes in U-bottom 96-well plates (Costar) incubated at 37°C and 5% CO2.
TCRβ repertoire analysis
Genomic DNA was extracted from 2.5 × 105 freshly isolated Tregs and 1 × 106 ex vivo-expanded polyTregs. The DNA was submitted to Adaptive Biotechnologies for deep-level TCRβ sequencing. TCR gene frequency analysis was performed using algorithms developed by Adaptive Biotechnologies.
TSDR methylation assay
Genomic DNA from 1 × 106 expanded Tregs was analyzed by Epiontis GmbH according to established protocol. Percentages of demethylated TSDR were calculated as follows: [mean copy numbers of unmethylated DNA/(mean copy numbers of unmethylated DNA + copy numbers of methylated DNA)] × 100. For female Tregs, the percentages calculated above were multiplied by 2 to correct for X-chromosome inactivation.
Treg deuterium tracking
During the 14-day clinical expansion period, the 2H2 label from [6,6-2H2]glucose in the X-VIVO 15 culture medium was incorporated into the DNA of replicating polyclonal CD4+CD127lo/−CD25+ Tregs as previously described (42). Initial qualifying experiments showed no differences in fold expansions, phenotype, or percent TSDR de- methylation between Tregs grown in X-VIVO 15 and Tregs grown in [2H2]glucose-containing X-VIVO 15. Functional suppression assay results showed similar inhibition between Tregs expanded in either type of medium, and cultures were free from bacteria, fungi, mycoplasma, or endotoxin contaminants. MS analyses showed that Tregs expanded in X-VIVO 15 with [6,6-2H2]glucose in the medium at 100% enrichment were ~60% enriched for 2H2 in the deoxyribose moiety of purine deoxyribonucleotides isolated from DNA, which is the theoretical maximum deuterium enrichment observed in deoxyribose in fully replaced cells that divided in the presence of [6,6-2H2]glucose (42, 43). This 60% enrichment level was consistently observed in all seven preparations in this clinical study.
After infusion of the labeled Tregs, peripheral blood was collected from the study participants, purified for Tregs, and analyzed for stable isotope enrichment. In some experiments, the cells were subdivided into Treg versus Teffs, as well as subsets of Tregs, to determine the stability of the Treg surface markers.
Analysis of DNA enrichment by MS
Measurement of deuterium in newly synthesized DNA was performed by GC-MS as described in detail previously (42). Briefly, DNA from proteinase K cell digests was isolated using DNeasy microcolumns (Qiagen) and hydrolyzed using S-1 nuclease and acid phosphatase. Deoxyribose moieties from purine dR-nucleosides were converted to pentafluorobenzylhydroxylamine triacetate derivatives. Enrichment analysis was performed on an Agilent 6890/5973 GC/MS equipped with a 30-m DB-17MS column (inside diameter, 250 μm; film thickness, 25 μm; Agilent) using methane negative chemical ionization and collecting ions in SIM mode at mass/charge ratios (m/z) 435, 436, and 437 (M0, M+1, and M+2, respectively). Enrichment of the [5,5-2H2]deoxy- ribose derivative was determined from measured ratios of the peak abundances of the M+2 ion to the sum of the M+0 to M+2 ions, after subtracting the theoretical (unenriched) natural abundance ratios, which are validated with standard curves of % enrichment.
Laboratory tests
Biochemical autoantibody titers were assayed at the Barbara Davis Center using radioimmunobinding assays, and ICA was measured at the University of Florida. C-peptide and HbA1c were measured at the Northwest Lipid Research Laboratory. Viral loads for EBV and CMV were performed by ViraCor Laboratories. Chemistries and hematology were performed at local clinical laboratories at UCSF and Yale.
Statistics and methods of analysis
Data analyses were performed using GraphPad Prism 6.0 software, and values at P < 0.05 were deemed significant. Cytokine concentrations were determined using SoftMax Pro software (Molecular Devices) with four-parameter data analysis.
Supplementary Material
Acknowledgments
We thank the whole team involved in developing and implementing this clinical trial, including F. Dekovic, T. Ghazi, G. Glunti, Pl Preston-Hurlburt, H. Javier, L. Rink, C. Torok, M. Tatum, and the BRI Diabetes Clinical Research Unit for samples; J. Krischer, E. Whalen, and M. Mason for statistical consultations; N. Warner and BD Biosciences for access to the cGMP (Current Good Manufacturing Processes) antibodies; and the Immune Tolerance Network (UM1AI109565) for the mechanistic study support.
Funding: This work was supported by the Juvenile Diabetes Research Foundation International (Collaborative Center for Cell Therapy; 2-SRA-2014-150 and the clinical trial; 17-2011-661), the Brehm Coalition, The Immune Tolerance Network, BD Biosciences and Caladrius Biosciences
Footnotes
Fig. S1. TCR repertoires of Tregs before and after expansion.
Fig. S2. Treg function of expanded Tregs from patients in phase 1 trial.
Fig. S3. Decreased CD56hi NK cells in circulation after Treg infusion.
Fig. S4. Direct evidence for expanded Tregs in the circulation after injection.
Fig. S5. Clearance of infused Tregs exhibits biphasic exponential decay kinetics.
Fig. S6. Gating strategies for cell sorting and analysis of unfixed CD4+ T cells and subsequent subsets.
Fig. S7. FACS-based phenotyping of nonexpanded and expanded FOXP3+ Treg.
Fig. S8. Equation for calculating “% of Total Tregs with Label”.
Table S1. Tabulation of cumulative number of adverse events for all cohorts categorized by system organ class and severity.
Table S2. Tabulation of cumulative number of adverse events categorized by cohort and severity.
Table S3. Listing of cumulative adverse events reported for cohort 1 by CTCAE term and severity.
Table S4. Listing of cumulative adverse events reported for cohort 2 by CTCAE term and severity.
Table S5. Listing of cumulative adverse events reported for cohort 3 by CTCAE term and severity.
Table S6. Listing of cumulative adverse events reported for cohort 4 by CTCAE term and severity.
Table S7. Two-phase decay model parameters. Raw data for Fig. 2. Raw data for Fig. 3B. Raw data for Fig. 6.
Author contributions: J.A.B. designed and analyzed experiments and wrote the manuscript; K.C.H, P.H.S., and S.E.G. were the clinicians conducting the study. Other authors played various roles in performing, analyzing, and overall contributions in the interpretation and editing of the manuscript.
Competing interests: J.A.B, A.L.P., W.L., and Z.T. are co-inventors on patents (US 20080131445 A1 and US 7722862 B2) filed in connection with the manufacturing of the Treg product. J.A.B. and Q.T. have received funding from Caladrius Biosciences and other in-kind contributions form BD Biosciences. The remaining authors declare that they have no competing interests.
References and Notes
- 1.Bluestone JA, Herold KG. Eisenbarth Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464:1293–1300. doi: 10.1038/nature08933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKnight JA, Wild SH, Lamb MJE, Cooper MN, Jones TW, Davis EA, Hofer S, Fritsch M, Schober E, Svensson J, Almdal T, Young R, Warner JT, Delemer B, Souchon PF, Holl RW, Karges W, Kieninger DM, Tigas S, Bargiota A, Sampanis C, Cherubini V, Gesuita R, Strele I, Pildava S, Coppell KJ, Magee G, Cooper JG, Dinneen SF, Eeg-Olofsson K, Svensson AM, Gudbjornsdottir S, Veeze H, Aanstoot HJ, Khalangot M, Tamborlane WV, Miller KM. Glycaemic control of Type 1 diabetes in clinical practice early in the 21st century: An inter- national comparison. Diabet Med. 2015;32:1036–1050. doi: 10.1111/dme.12676. [DOI] [PubMed] [Google Scholar]
- 3.Miller KM, Foster NC, Beck RW, Bergenstal RM, DuBose SN, DiMeglio LA, Maahs DM, Tamborlane WV. T1D Exchange Clinic Network, Current state of type 1 diabetes treatment in the U.S.: Updated data from the T1D exchange clinic registry. Diabetes Care. 2015;38:971–978. doi: 10.2337/dc15-0078. [DOI] [PubMed] [Google Scholar]
- 4.Herold KC, Gitelman SE, Ehlers MR, Gottlieb PA, Greenbaum CJ, Hagopian W, Boyle KD, Keyes-Elstein L, Aggarwal S, Phippard D, Sayre PH, McNamara J, Bluestone JA AbATE Study Team. Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: Metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes. 2013;62:3766–3774. doi: 10.2337/db13-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rigby MR, DiMeglio LA, Rendell MS, Felner EI, Dostou JM, Gitelman SE, Patel CM, Griffin KJ, Tsalikian E, Gottlieb PA, Greenbaum CJ, Sherry NA, Moore WV, Monzavi R, Willi SM, Raskin P, Moran A, Russell WE, Pinckney A, Keyes-Elstein L, Howell M, Aggarwal S, Lim N, Phippard D, Nepom GT, McNamara J, Ehlers MR T1DAL Study Team. Targeting of memory T cells with alefacept in new-onset type 1 diabetes (T1DAL study): 12 Month results of a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Diabetes Endocrinol. 2013;1:284–294. doi: 10.1016/S2213-8587(13)70111-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haller MJ, Gitelman SE, Gottlieb PA, Michels AW, Rosenthal SM, Shuster JJ, Zou B, Brusko TM, Hulme MA, Wasserfall CH, Mathews CE, Atkinson MA, Schatz DA. Anti- thymocyte globulin/G-CSF treatment preserves b cell function in patients with established type 1 diabetes. J Clin Invest. 2015;125:448–455. doi: 10.1172/JCI78492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voltarelli JC, Couri CEB, Stracieri ABPL, Oliveira MC, Moraes DA, Pieroni F, Coutinho M, Malmegrim KCR, Foss-Freitas MC, Simões BP, Foss MC, Squiers E, Burt RK. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2007;297:1568–1576. doi: 10.1001/jama.297.14.1568. [DOI] [PubMed] [Google Scholar]
- 8.Tang Q, Bluestone JA. Regulatory T-cell therapy in transplantation: Moving to the clinic. Cold Spring Harb Perspect Med. 2013;3:a015552. doi: 10.1101/cshperspect.a015552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall BM, Pearce NW, Gurley KE, Dorsch SE. Specific unresponsiveness in rats with prolonged cardiac allograft survival after treatment with cyclosporine. III. Further characterization of the CD4+ suppressor cell and its mechanisms of action. J Exp Med. 1990;171:141–157. doi: 10.1084/jem.171.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor a-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 11.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: A jack of all trades, master of regulation. Nat Immunol. 2008;9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bacchetta R, Passerini L, Gambineri E, Dai M, Allan SE, Perroni L, Dagna-Bricarelli F, Sartirana C, Matthes-Martin S, Lawitschka A, Azzari C, Ziegler SF, Levings MK, Roncarolo MG. Defective regulatory and effector T cell functions in patients with FOXP3 mutations. J Clin Invest. 2006;116:1713–1722. doi: 10.1172/JCI25112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green EA, Gorelik L, McGregor CM, Tran EH, Flavell RA. CD4 CD25 T regulatory cells control anti-islet CD8+ T cells through TGF-b-TGF-b receptor interactions in type 1 diabetes. Proc Natl Acad Sci USA. 2003;100:10878–10883. doi: 10.1073/pnas.1834400100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waldmann H, Hilbrands R, Howie D, Cobbold SJ. Harnessing FOXP3+ regulatory T cells for transplantation tolerance. J Clin Invest. 2014;124:1439–1445. doi: 10.1172/JCI67226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bluestone JA, Trotta E, Xu D. The therapeutic potential of regulatory T cells for the treatment of autoimmune disease. Expert Opin Ther Targets. 2015;19:1091–1103. doi: 10.1517/14728222.2015.1037282. [DOI] [PubMed] [Google Scholar]
- 16.Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, Masteller EL, McDevitt H, Bonyhadi M, Bluestone JA. In vitro–expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang Q, Adams JY, Penaranda C, Melli K, Piaggio E, Sgouroudis E, Piccirillo CA, Salomon BL, Bluestone JA. Central role of defective interleukin-2 production in the triggering of islet auto- immune destruction. Immunity. 2008;28:687–697. doi: 10.1016/j.immuni.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Long SA, Cerosaletti K, Bollyky PL, Tatum M, Shilling H, Zhang S, Zhang Z-Y, Pihoker C, Sanda S, Greenbaum C, Buckner JH. Defects in IL-2R signaling contribute to diminished maintenance of FOXP3 expression in CD4+CD25+ regulatory T-cells of type 1 diabetic subjects. Diabetes. 2010;59:407–415. doi: 10.2337/db09-0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Putnam AL, Brusko TM, Lee MR, Liu W, Szot GL, Ghosh T, Atkinson MA, Bluestone JA. Expansion of human regulatory T-cells from patients with type 1 diabetes. Diabetes. 2009;58:652–662. doi: 10.2337/db08-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nascimbeni M, Shin E-C, Chiriboga L, Kleiner DE, Rehermann B. Peripheral CD4+CD8+ T cells are differentiated effector memory cells with antiviral functions. Blood. 2004;104:478–486. doi: 10.1182/blood-2003-12-4395. [DOI] [PubMed] [Google Scholar]
- 22.Chauhan SK, Saban DR, Dohlman TH, Dana R. CCL-21 conditioned regulatory T cells induce allotolerance through enhanced homing to lymphoid tissue. J Immunol. 2014;192:817–823. doi: 10.4049/jimmunol.1203469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J, Chen Y-G, Reifsnyder PC, Schott WH, Lee C-H, Osborne M, Scheuplein F, Haag F, Koch-Nolte F, Serreze DV, Leiter EH. Targeted disruption of CD38 accelerates autoimmune diabetes in NOD/Lt mice by enhancing autoimmunity in an ADP-ribosyltransferase 2- dependent fashion. J Immunol. 2006;176:4590–4599. doi: 10.4049/jimmunol.176.8.4590. [DOI] [PubMed] [Google Scholar]
- 24.Baron U, Floess S, Wieczorek G, Baumann K, Grützkau A, Dong J, Thiel A, Boeld TJ, Hoffmann P, Edinger M, Türbachova I, Hamann A, Olek S, Huehn J. DNA demethylation in the human FOXP3 locus discriminates regulatory T cells from activated FOXP3+ conventional T cells. Eur J Immunol. 2007;37:2378–2389. doi: 10.1002/eji.200737594. [DOI] [PubMed] [Google Scholar]
- 25.Long SA, Rieck M, Sanda S, Bollyky JB, Samuels PL, Goland R, Ahmann A, Rabinovitch A, Aggarwal S, Phippard D, Bianchine PJ, Boyle KD, Adah SA, Bluestone JA, Buckner JH, Greenbaum CJ Diabetes TrialNet and the Immune Tolerance Network. Rapamycin/IL-2 combination therapy in patients with type 1 diabetes augments Tregs yet transiently im- pairs b-cell function. Diabetes. 2012;61:2340–2348. doi: 10.2337/db12-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McClymont SA, Putnam AL, Lee MR, Esensten JH, Liu W, Hulme MA, Hoffmüller U, Baron U, Olek S, Bluestone JA, Brusko TM. Plasticity of human regulatory T cells in healthy subjects and patients with type 1 diabetes. J Immunol. 2011;186:3918–3926. doi: 10.4049/jimmunol.1003099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hansmann L, Schmidl C, Kett J, Steger L, Andreesen R, Hoffmann P, Rehli M, Edinger M. Dominant Th2 differentiation of human regulatory T cells upon loss of FOXP3 expression. J Immunol. 2012;188:1275–1282. doi: 10.4049/jimmunol.1102288. [DOI] [PubMed] [Google Scholar]
- 28.Hippen KL, Merkel SC, Schirm DK, Sieben CM, Sumstad D, Kadidlo DM, McKenna DH, Bromberg JS, Levine BL, Riley JL, June CH, Scheinberg P, Douek DC, Miller JS, Wagner JE, Blazar BR. Massive ex vivo expansion of human natural regulatory T cells (Tregs) with minimal loss of in vivo functional activity. Sci Transl Med. 2011;3:83ra41. doi: 10.1126/scitranslmed.3001809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bluestone JA, Bour-Jordan H, Cheng M, Anderson M. T cells in the control of organ- specific autoimmunity. J Clin Invest. 2015;125:2250–2260. doi: 10.1172/JCI78089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greenbaum CJ, Beam CA, Boulware D, Gitelman SE, Gottlieb PA, Herold KC, Lachin JM, McGee P, Palmer JP, Pescovitz MD, Krause-Steinrauf H, Skyler JS, Sosenko JM Type 1 Diabetes TrialNet Study Group. Fall in C-peptide during first 2 years from diagnosis: Evidence of at least two distinct phases from composite Type 1 Diabetes TrialNet data. Diabetes. 2012;61:2066–2073. doi: 10.2337/db11-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Milush JM, López-Vergès S, York VA, Deeks SG, Martin JN, Hecht FM, Lanier LL, Nixon DF. CD56negCD16+ NK cells are activated mature NK cells with impaired effector function during HIV-1 infection. Retrovirology. 2013;10:158. doi: 10.1186/1742-4690-10-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brunstein CG, Miller JS, Cao Q, McKenna DH, Hippen KL, Curtsinger J, Defor T, Levine BL, June CH, Rubinstein P, McGlave PB, Blazar BR, Wagner JE. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: Safety profile and detection kinetics. Blood. 2011;117:1061–1070. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maecker HT, McCoy JP, Nussenblatt R. Standardizing immunophenotyping for the human immunology project. Nat Rev Immunol. 2012;12:191–200. doi: 10.1038/nri3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.