Abstract
Hematopoietic stem cell (HSC) self-renewal is regulated by osteoblast and/or endothelial cells within the hematopoietic niche. However, the true identity of the supporting cells and the nature of the secreted factors remain uncertain. We developed a novel mouse model and analyzed whether circulating human peripheral hematopoietic lineage negative/AP+ (lin−/AP+) cells support hematopoiesis in vivo. Thus, immunocompromised (Rag) mice expressing thymidine kinase (Tk) under the control of the 3.6Col1α1 promoter (Tk-Rag) were treated with ganciclovir, resulting in osteoblast progenitor cell ablation and subsequent loss of hematopoiesis (evaluated by measuring mouse Ter119+ erythroid cells). Following hematopoietic cell depletion, human bone marrow-derived marrow stromal cells (MSCs) or lin−/AP+ cells were infused into Tk-Rag mice and compared with saline infusions. Ganciclovir significantly reduced (7.4-fold) Ter119+cells in the bone marrow of Tk-Rag mice compared to saline injections. Infusion of either MSCs or lin−/AP+ cells into ganciclovir-treated mice resulted in a 3.3-fold and 2.7-fold increase (P<0.01), respectively, in Ter119+ cells compared to mice receiving saline. Relative to lin−/AP− cells, lin−/AP+ cells expressed high levels of mesenchymal, endothelial, and hematopoiesis supporting genes. Thus, human peripheral blood lin−/AP+ cells represent a novel cell type capable of supporting hematopoiesis in a manner comparable to MSCs. J. Cell. Biochem. 116: 58–66, 2015.
Keywords: HEMATOPOIESIS, LIN/AP+ CELLS, MESENCHYMAL CELLS, ENDOTHELIAL CELLS, MOUSE MODEL
Hematopoietic stem cells (HSCs) are maintained in specialized microenvironments, termed niches, in which supporting cells that promote their proliferation and differentiation are located. Several studies have documented the regulatory role of osteoblasts in the fate of HSCs [Calvi et al., 2003; Zhang et al., 2003; Arai et al., 2004; Mayack and Wagers, 2008], while other studies have shown that HSCs can be observed adjacent to the vasculature in bone marrow [Kiel et al., 2005]. Indeed, recent data have indicated an important role of the endothelial and perivascular stromal cells in supporting HSC cells, with these cells being characterized by the production of high levels of CXCL12, alkaline phosphatase (AP), vascular cell adhesion molecule 1 (Vcam1), platelet-derived growth factor receptor α and β and stem cell factor (SCF; also known as KITL) which are necessary to maintain HSCs [Ding et al., 2012]. Nevertheless, at present the true identity of the supporting cells and the nature of the supporting factors remain uncertain.
Previous studies demonstrated that conditional ablation of osteoblasts in transgenic mice expressing thymidine kinase (Tk) under the control of the 2.3 kb rat collagen 1 alpha 1 promoter (2.3Col1α1) resulted in a marked decrease in bone marrow cells (BMCs). In these mice, the depletion of BMCs was parallel to that of osteoblasts, suggesting a supportive role of osteoblasts in hematopoiesis [Visnjic et al., 2004]. Ganciclovir (GCV) treatment induced ablation of replicating osteoblast progenitors in mice expressing Tk under the control of the 3.6 kb rat collagen 1 alpha 1 promoter (3.6Col1α1) [Jilka et al., 2009]. GCV treatment induced depletion of hematopoietic cells which was evident after 2 weeks of treatment but only in 1–2-month-old animals, again suggesting that the self-renewal of HSCs, the cells that generate a lifelong supply of all blood cell types, might be partly regulated by osteoblastic cells within the hematopoietic niche.
Bone marrow stromal cells (MSCs) have the capacity to differentiate into different mesodermal lineages, including osteoblasts and adipocytes, constitute an essential part of the bone marrow microenvironment, and have the ability to support hematopoiesis. Infusion of MSCs in humans has been associated with a rapid recovery of hematopoiesis after bone marrow transplantation for hematological diseases as well as after chemotherapy for breast cancer [Koc et al., 2000; Meuleman et al., 2009]. Recent experimental studies have shown a recovery of hematopoiesis after MSC infusion in mice exposed to severe radiation [Lange et al., 2011]. Other studies demonstrated the reconstitution of a functional hematopoietic microenvironment through the infusion of human MSCs in the murine bone marrow compartment [Muguruma et al., 2006]. One of the principal ways by which MSCs support hematopoiesis is by supplying HSCs and their progeny with signals for survival, proliferation and differentiation through direct cell-to-cell contact and/or production of cytokines, adhesion molecules, and extracellular matrix proteins. It has been reported that MSCs produce SCF, Flt-3 ligand, thrombopoietin, leukemia-inhibiting factor (LIF), TGF-β, interleukin (IL)-6, IL-7, IL-8, IL-11, IL-12, IL-14, IL-15, granulocyte-macrophage (GM) and macrophage colony stimulating factor (M-CSF) as well as intercellular adhesion molecule-1 and VCAM-1 [Prockop, 1997; Majumdar et al., 2000; Di Nicola et al., 2002].
Our group has previously identified circulating cells in humans expressing the osteoblast markers, osteocalcin (OCN), or AP. These cells have the ability to mineralize in vitro and to induce bone formation in vivo [Eghbali-Fatourechi et al., 2005]. In subsequent studies we further enriched cells expressing mesenchymal markers by first depleting hematopoietic cells, resulting in a hematopoietic negative fraction (lin−) which was then stained with antibodies to AP or Stro1. The gene expression data of these cells suggested that they were quiescent cells that could be involved in supporting hematopoiesis, with nearly 40% of these cells expressing the hematopoietic/endothelial marker CD34 [Eghbali-Fatourechi et al., 2007; Undale et al., 2010]. Recent work has further demonstrated that these cells also contained a significant population of CD31 cells (marker for mature endothelial cells) [Modder et al., 2012]. Although several lines of evidence have suggested a regulatory role for mesenchymal/osteoblastic cells in regulating hematopoiesis, at present the role of these circulating cells expressing osteoblastic and endothelial markers remains unknown.
A significant proportion of HSCs are localized adjacent to sinusoidal blood vessels in the bone marrow [Kiel et al., 2005; Kiel et al., 2007]. Administration of antibodies against endothelial cells in vivo impairs HSC engraftment and transformed endothelial cells promote HSC expansion in culture [Butler et al., 2010]. Co-culture of progenitor cells with endothelial cells increased their capacity to repopulate the bone marrow of SCID mice [Chute et al., 2002]. In addition, the exposure of HSCs to endothelial cells isolated from different tissues resulted in varying HSC growth and repopulation ability [Li et al., 2004]. Endothelial cells play an important role in HSC maintenance; however, it is now also accepted that factors secreted by these cells may have a beneficial effect. Recently, Ding et al. (2012) demonstrated that endothelial and perivascular cells are major sources of SCF. In their study conditional deletion of Scf from endothelial cells resulted in a significant reduction of HSCs in the bone marrow of transgenic mice compared to controls. The authors showed that endothelial cells and especially perivascular stromal cells were the principal source of SCF for HSC maintenance. Nevertheless, perivascular stromal cells are probably heterogeneous and may include multiple cell types that contribute to HSC maintenance through additional mechanisms other than SCF secretion [Ding et al., 2012].
The aim of this study was to generate mice which express Tk under the control of the 3.6Col1α1 promoter in an immunocompromised (Rag) background in order to evaluate the ability of circulating human peripheral hematopoietic lineage negative/AP+ (lin−/AP+) cells to support hematopoiesis in vivo and to compare these results with the effect of human MSCs.
MATERIALS AND METHODS
MICE
The generation of 3.6Col1α1 Tk mice has been described before [Jilka et al., 2009]. The female 3.6Col1α1 Tk mice were bred to male immunocompromised B6.129S7-Rag1tm1Mom/J mice (Jackson Laboratory 002216). 3.6Col1α1Tk-Rag mice were generated in order to infuse them with human MSCs and lin−/AP+ cells. The animals were housed in sterile microisolator boxes with ad libitum mouse chow and 12 h light/dark cycles. PCR analysis on extracted tissue DNA was performed to confirm the correct genotype. All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at the Mayo Clinic.
PRE-EXPERIMENTS AND STUDY DESIGN
The GCV concentration for bone marrow ablation, the injection schedule and time point for analyzing bone marrow components were established in a pre-experiment. Different doses of GCV (200 μg/day versus 300μg/day, once or twice a day) and different periods of treatment duration (14, 21, or 30 days) were compared with respect to the total number of BMCs and of CD3 (T-cell marker), CD11b (myeloid marker), CD45 (hematopoietic cell marker), CD220 (B-cell marker), and Ter119 (erythroid marker) cells. Flow cytometry analysis for CD3, CD11b, CD220, and Ter119 cells in peripheral blood and bone marrow was performed; however, since Rag mice are immunodeficient with a marked decrease in B and T cells, we observed that measurement of the Ter119+ cells was the most sensitive marker for assessing the decrease in the bone marrow components [Mombaerts et al., 1992]. It should be noted that Ter119 antibody is specific for mouse protein with no cross reactivity to human cells [Kina et al., 2000]. Concentration and duration of GCV treatment showing a significant reduction of BMCs and Ter119 were used in the present study.
In a second pre-experiment we infused different cell numbers (50,000 or 100,000) at multiple time points (at days 18, 21, and 25) into the femurs to be able to choose the most efficacious schedule (optimal recovery with the fewest infusions) for BMC recovery. Human MSCs (Poietics™, Lonza) were used to establish the optimal conditions, since it has been shown that MSCs are associated with recovery of hematopoiesis and present a good control for setting up the experiments [Lange et al., 2011]. For localization experiments, hTERT–GFP stably transfected MSCs, using a lentiviral construct (Biogenova, Catalog ID: LG508), were used. hTERT is driven by a CMV promoter and the GFP is separately driven by a EF1a promoter. The construct does not contain any antibiotic resistance gene.
To explore the ability of peripheral lin−/AP+ cells to support hematopoiesis, thirty-two 4-week-old 3.6Col1a1 Tk-Rag male mice were divided into four treatment groups: The control group was treated with saline and saline was infused into their femoral cavities. Three groups were treated with GCV (300 μg/day, one injection) and infused with either saline, human MSCs or human peripheral blood lin−/AP+ cells. Mice were treated with 300μg/day of GCV intraperitoneally for 30 days. On day 18, hMSCs (~105 cells in 10μl saline), human lin−/AP+ cells (~105 cells in 10μl saline), or saline (10 μl) were infused into both femoral bone marrow cavities, and at day 30 (12 days following cell or saline infusion) mice were sacrificed and the total number of bone marrow cells (BMCs) and Ter119+ (mouse-specific erythroid marker) cells in the femurs and tibias were analyzed.
CELL SORTING
To isolate human lin−/AP+ cells, peripheral blood mononuclear cells (MNC) obtained from normal human donors were isolated by Ficoll density centrifugation. After incubation of the MNC fraction with a StemSep Human Progenitor enrichment cocktail (StemCell Technologies) containing antibodies to CD2, CD3, CD14, CD16, CD19, CD24, CD56, CD66b, and glycophorin A, depletion of hematopoietic cells was achieved by passage over a magnetic column (autoMACS, Miltenyi Biotec); this essentially removed T cells, B cells, neutrophils and erythroid cells. Subsequent enrichment for lin−/AP+ cells was performed by magnetic activated cell sorting (MACS) using biotinylated AP antibody (R&D systems) followed by staining with antibiotin beads (Miltenyi Biotec). Flow cytometry characterization for CD31, CD34 and CD45 including the appropriate isotype controls was performed on these cells (all antibodies purchased from BioLegend). Furthermore, Lin−/AP+ and lin−/AP− cells were cultured in medium containing an additional 5 mmol Ca2+ for 5 days, since we have previously described that brief exposure to calcium results in plastic adherence and upregulation of osteoblastic genes [Gossl et al., 2008]. Following culture, lin−/AP+ cells were infused into the femoral cavities of recipient mice. Remaining cells and lin−/AP− cells, as control, were stored at −70°C in RLT buffer for gene expression analysis.
GENE EXPRESSION ANALYSIS
Total RNA from lin−/AP− and Lin−/AP+ cells were isolated. Amplified cDNA was used in QPCR analyses to evaluate expression of endothelial marker genes (CD31, CD34, Tie-2, SCF [KILT]), genes that support the hematopoietic niche (Agrin, N-Cadherin, Osteoporotin, CXCL12/SDF1), as well as mesenchymal marker genes (CD71, CD105, CD146, VCAM1).
Total RNA was isolated using spin columns (Micro columns, Qiagen) followed by DNase digestion in solution using Turbo RNA-free DNase (Ambion). We used the WT-Ovation™ Pico RNA linear amplification system (NuGEN, Technologies, Inc, San Carlos, CA) to synthesize microgram quantities of amplified cDNA starting with total RNA input amounts of 50 ng for all the samples. The amplified cDNA was then used in quantitative polymerase chain reaction (QPCR) analyses. Analysis was undertaken by calculating the differences in cycle threshold (dCT) values between the sample and the geometric mean cycle threshold of the housekeeping gene. A panel of ten reference genes using the geNorm algorithm was used to select the three most stable reference genes [Vandesompele et al., 2002; Radonic et al., 2004]. All primer sequences can be provided on request. The 2−dCT method was used to determine the expression level of each individual gene.
FLOW CYTOMETRY
Bone marrow cells obtained from tibias and femurs were isolated and stained using the following fluorescent conjugated antibodies: PE anti-mouse CD3, PE anti-mouse CD11b, PE anti-mouse CD45R/B220, PE anti-mouse CD45, PE anti-mouse Ter119 (mouse specific, no cross reactivity with human cells); appropriate isotype control antibodies were also used (all antibodies purchased from BioLegend). Cell fluorescence was measured immediately after staining (Becton Dickinson, FACS Calibur) and data were analyzed using the CellQuest software (Becton Dickinson). A maximum of 150,000 events were counted and final data were obtained within the lymphocyte gate.
LOCATION OF THE INFUSED CELLS
In order to identify the location of the infused cells, three additional mice were infused with 100,000 hTERT–GFP stably transfected MSCs (GFP-expressing MSCs), and tissues were dissected 5 days later for immunohistochemical. Femurs, tibias, and livers from each animal were harvested, fixed in 4% formalin and bones were decalcified in 5% formic acid. Decalcified bones, as well as livers, were paraffin embedded and sectioned (5 μm) for immunohistochemistry. The sections were stained with GFP antibody (1:100 GFP mouse antibody, Cell Signaling Technology) using a modified protocol to the Innovex Biosciences STAT-Q IHC Staining system for human and animal tissues (Richmond, CA). As a control, sequential sections were incubated with PBS. Sections were incubated with the secondary linking antibody, peroxidase-streptavidin label, DAB substrate and counterstained in hematoxylin.
STATISTICAL ANALYSES
Statistical analysis was performed using the JMP® Statistical Discovery Software (SAS Institute Inc). Comparison of two groups was done using two-sample t-tests. ANOVA was used to compare larger number of groups and if it was significant the Fisher protected least significant difference test was used for pairwise comparisons of the groups. All data are presented as mean ± SEM, with the exception of the gene expression data.
RESULTS
CHARACTERIZATION OF HUMAN LIN−/AP+
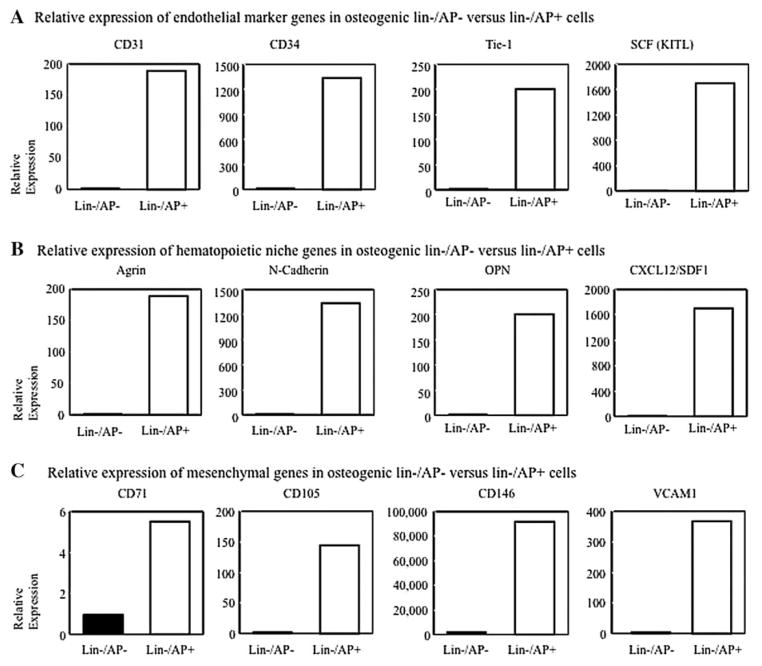
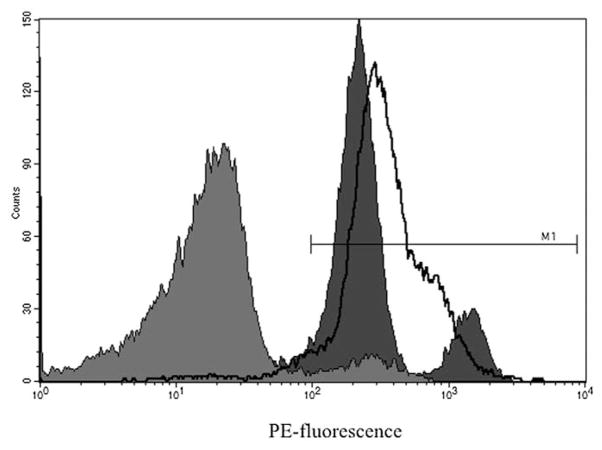
Lin−/AP+ cells expressed higher levels of CD31, CD34, and Tie-2 as well as SCF (endothelial marker genes, Fig. 1A) as compared to lin−/AP− cells. These cells were also enriched for expression of a number of hematopoiesis supporting genes such as Agrin, N-Cadherin, Osteopontin, and SDF-1 (Fig. 1B). Moreover, mesenchymal genes, including CD71, CD105, CD146, and VCAM1 (Fig. 1C) were also expressed more abundantly in lin−/AP+ cells compared to lin−/AP− cells. Further characterization by flow cytometry revealed that lin−/AP+ cells were enriched for CD31 (80.75%), CD34 (7.10%), and CD45 (95.86%), respectively (Fig. 2).
Fig. 1.
Gene expression analysis of osteogenic lin−/AP− cells and lin−/AP+ cells from human peripheral blood (after culture in 5mMCa2+ for 5 days). Endothelial marker (A), supporting hematopoietic niche genes (B), and mesenchymal stem cell genes (C) are upregulated in lin−/AP+ cells. Results are expressed as a relative expression.
Fig. 2.
Flow cytometry of lin−/AP+ cells showing the enrichment for CD31 (dark gray), CD34 (light gray) and CD 45 (line) cells.
GANCICLOVIR TREATMENT RESULTS IN THE ABLATION OF HEMATOPOIESIS IN Tk-Rag MICE
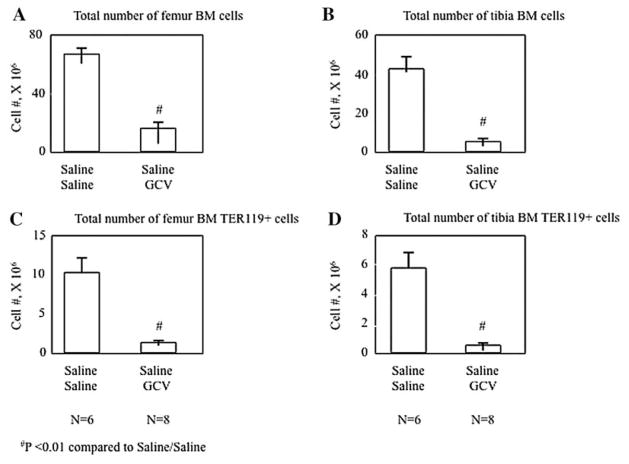
As shown in Figure 3A, the total number of BMCs collected from both femurs was significantly reduced in mice treated with GCV and infused with saline compared with controls (saline/saline) (66.8 ± 4.8 × 106 vs. 17 ± 3.4 × 106, P<0.01). A similar change was observed in the total number of BMCs (43 ± 6.5 × 106 vs. 5.6 ± 1.6 × 106, P<0.01) isolated from both tibias (Fig. 3B). The number of erythroid precursors were detected by measuring cells positive for the mouse-specific cell surface marker, Ter119, using flow cytometry. Ter119+ cells identified in BMCs from the femurs were significantly decreased following GCV treatment and saline infusion (10.4 ± 1.7 × 106 vs. 1.4 ± 0.1 × 106, P<0.01, Fig. 3C) compared to controls. Similar results were obtained measuring Ter119+ cells in BMCs isolated from the tibias (5.9 ± 0.9 × 106 vs. 0.6 ± 0.1 × 106, P<0.01, Fig. 3D).
Fig. 3.
Total number of BMCs in the femurs (A) and tibias (B) and the total number of Ter119+ cells in femurs (C) and tibias (D) in saline infused mice at day 30. The control group was treated with saline and on day 18 were infused with saline. The treated mice received GCV (300μg/day) and were infused with saline on day 18. All animals were sacrificed on day 30. Values are expressed as mean ± SEM.
INFUSION OF LIN−/AP+ CELLS RESCUES HEMATOPOIESIS
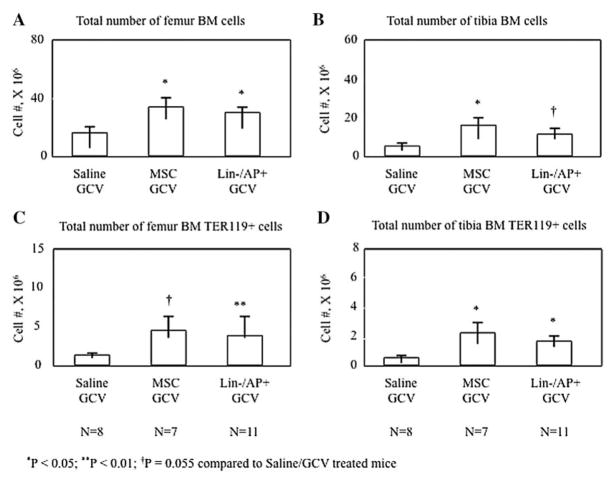
Since we had established a mouse model which showed a significant decrease in hematopoiesis following GCV treatment, most likely through the targeted loss of osteoblastic cells on the bone surface, we next tested whether human MSCs or peripheral blood lin−/AP+cells could rescue hematopoiesis in these mice. 1 × 105 MSCs or lin−/AP+ cells were infused into GCV-treated mice and total BMCs and Ter119+ cells were analyzed. Mice infused with human peripheral blood lin−/AP+ cells as well as those receiving human MSCs maintained a significantly higher number of BMCs compared to the saline/GCV treated mice not only in the infused bones, the femurs (Fig. 4A), but also in the tibias (Fig. 4B). Moreover, the number of BMCs recovered from mice infused with MSCs or lin−/AP+ cells reached almost 50% of that in the femurs of control mice (MSCs: 34.1 ± 5.5 × 106; lin−/AP+: 30.3 ± 3.2 × 106 vs 66.8 ± 4.8 × 106). Similar results were observed analyzing Ter 119+ cells. Whereas a significant decrease was observed in the total number of Ter 119+ cells after GCV treatment compared to control mice (P<0.01), infusion with lin−/AP+ or MSCs cells resulted in a significant increase in Ter119+ cells compared to saline/GCV treated mice, with changes being observed in femurs (MSCs: 4.6 ± 1.8 × 106; lin−/AP+: 3.9 ± 0.5 × 106 vs 10.4 ± 1.7 × 106, Fig. 4C) and tibias (MSCs: 2.2 ± 0.8 × 106; lin−/AP+: 1.7 ± 0.4 × 106 vs 5.9 ± 0.9 × 106, Fig. 4D).
Fig. 4.
Total number of BMCs in the femurs (A) and tibias (B) and the total number of Ter119+ cells in femurs (C) and tibias (D) of the additional treatment groups in direct comparison to GCV/saline mice. The treated mice received GCV (300μg/day) and on day 18 were infused with 1 × 105 MSCs and lin−/AP+ cells into each femoral cavity. All animals were sacrificed on day 30. Values are expressed as mean ± SEM.
INFUSED CELLS LOCATE TO ENDOCORTICAL SURFACES
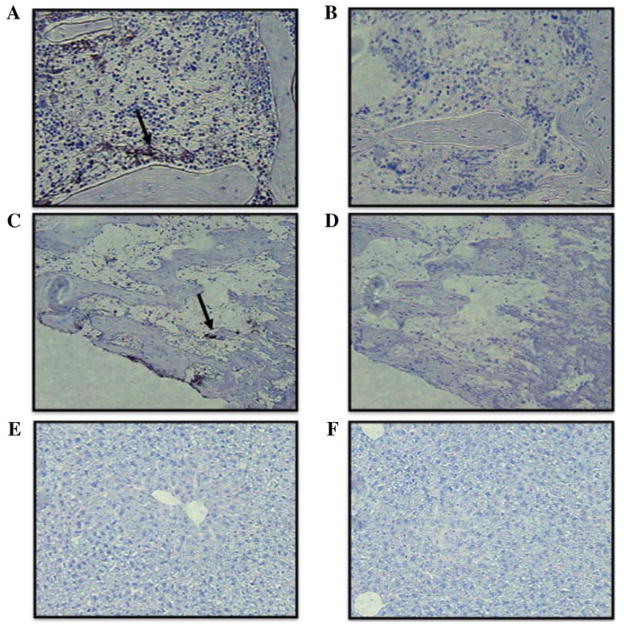
Femurs, tibias, and liver from Tk-Rag mice 5 days following infusion of GFP-expressing MSCs were analyzed to explore cell distribution following infusion in the bone cavities and other tissues. As shown in Figure 5, the infused GFP-expressing MSCs were located in the bone marrow of the femurs, especially on the endocortical surface (Fig. 5A). GFP-expressing MSCs were also identified in the tibias (particularly in the distal epiphysis), albeit to a lesser extent compared to the femurs (Fig. 5B). Immumohistochemical analysis of the liver did not reveal the presence of infused GFP–MSCs in this tissue (Fig. 5C).
Fig. 5.
Immunochemical analysis of infused MSC–GFP (stained in black) (A) Distribution of infused GFP-expressing MSC in the bone marrow of the femur (arrow, 10x), and (B) control without antibody; (C) Distribution of infused GFP-expressing MSC in the bone marrow of the tibia (arrow, 10x), and (D) control without antibody; (E) Absence of GFPexpressing MSC in the liver (10x), and (F) control without antibody.
DISCUSSION
In the present study, we developed a novel mouse model which is useful to test the capacity of human primary cells and cell lines to support hematopoiesis. Our results demonstrate that human circulating lin−/AP+ cells, which expressed endothelial and mesenchymal markers, supported and maintained hematopoiesis in 3.6Col1α1 Tk-Rag mice during treatment with GCV and were as potent as MSCs.
The current results provide additional information on the potential role of circulating lin−/AP+ cells. These cells may not only participate in long bone development and fracture healing, as suggested in previous studies, but may also play a role in supporting hematopoiesis [Garrett and Emerson, 2009; Wu et al., 2009; Pignolo and Kassem, 2011]. Thus, we observed that infusion of peripheral lin−/AP+cells into femoral cavities of transgenic 3.6Col1α1 Tk-Rag mice partially prevented BMCs depletion after GCV treatment compared to the control group receiving saline, a finding that was also evident after evaluation of the erythroid marker, Ter119. This latter finding further confirmed the supportive role of lin−/AP+ cell in hematopoiesis even considering potential continued support of hematopoiesis by 3.6Col1α1-negative immature endogenous cells. The antibody used to detect Ter119 cells is mouse specific, having no cross-reactivity with human cells, therefore indicating that the increase in BMCs observed after cell infusion was due to bone marrow recovery and not due to the infusion of contaminating human erythroid cells itself.
Previous studies have shown that MSC infusion into mice exposed to severe radiation has been associated with a recovery of hematopoiesis [Lange et al., 2011]. MSC infusion in humans has been associated with a rapid generation of new blood cells from haematopoietic stem cells after bone marrow transplantation as well as after chemotherapy confirming their important role and representing a known model for hematopoiesis recovery [Koc et al., 2000; Meuleman et al., 2009]. In our study, infusion of both peripheral lin−/AP+ cells and MSCs supported hematopoiesis to a similar degree. Total recovery of BMCs after either MSC or lin−/AP+ cell infusion was ~50%, a finding that was attributed to either insufficient cell dose infusion or insufficient long-term follow-up.
In our study, the precise mechanism related to BMC recovery through lin−/AP+cells and MSCs has not been elucidated. However, flow cytometry analysis of isolated lin−/AP+ cells showed expression of CD31, CD34, and CD45. Furthermore, gene expression of CD31, CD34, and Tie-2 was markedly increased in lin−/AP+ cells used for the infusion experiments. The data suggest a mixed mesenchymal/endothelial phenotype of these cells, which support hematopoiesis. Interestingly the expression of the regulatory signal SCF was highly increased in lin−/AP+ cells. SCF is essential for the generation of new blood cells and is mainly secreted by endothelial cells, osteoblasts, and nestin-expressing stromal cells [Heinrich et al., 1993; Mendez-Ferrer et al., 2010]. Ding et al. [2012] demonstrated that particularly endothelial and mesenchymal cells play an important role in HSC niche maintenance through their production of SCF. Furthermore, the expression of genes such as N-cadherin, osteopontin, and SDF-1/CXCL12, which are important microenvironmental factors for HSC regulation, were highly expressed in lin−/AP+ cells [Askmyr et al., 2009]. Indeed, the CXCL12/CXCR4 axis seems to be an essential pathway for mobilization of HSCs from bone marrow, with CXCL12 having a major role in chemotaxis, homing, survival and retention of HSCs in the bone marrow niche [Askmyr et al., 2009; Lo Celso et al., 2009]. Moreover, the cell adhesion molecule, N-cadherin, seems to play a critical role in the regulation of HSC engraftment, being functionally required for the establishment of hematopoiesis in the bone marrow niche after bone marrow transplantation [Hosokawa et al., 2010; Arai et al., 2012]. Other factors such as agrin, an extracellular matrix protein, were also upregulated in lin−/AP+ cells. Interestingly, this factor has recently been related to hematopoietic cell development, with recent work attributing agrin with a crucial role in the hematopoietic niches and in the cross-talk between stromal and HSCs [Mazzon et al., 2012]. Furthermore, lin−/AP+ cells also showed a consistent upregulation of several mesenchymal genes with a high expression of VCAM1, CD71, CD146, and CD105 (Endoglin); the latter is also related to the recruitment of blood vessels and the generation of the bone marrow cavity for the HSC niche [Chan et al., 2009].
Although, lin−/AP+ cells express markers of endothelial cells, it is, however, not clear whether these cells include endothelial cells or whether they are a different subpopulation of osteoblastic/mesenchymal cells. Nonetheless, similar to recent studies, these cells seem to support hematopoiesis, producing several necessary soluble and membrane-associated factors. Most likely, several different cell types directly or indirectly contribute to HSC maintenance through different mechanisms. Further studies, therefore, are needed to define the precise phenotype of lin−/AP+ cells, particularly in direct comparison with MSCs, endothelial and HSC niche cells, and it needs to be determined whether lin−/AP+ cells change their phenotype following infusion into the bone marrow cavity.
BMCs and Ter119 cell recovery was not only observed in the infused bones, the femurs, but also in the non-infused bones, the tibias. Several possible hypotheses may be postulated, such as the systemic circulation of the infused cells or a local circulation of the infused cells through the synovial and metaphyseal vessels, among others. The absence of detection of the infused MSCs-GFP in the immunochemical study of the other tissues analyzed including the liver suggests that the second hypothesis is more likely. In addition, the identification of these cells, mainly in the proximal epiphysis of the tibia near the cell infusion site, further supports the latter possibility. In this study, GFP-expressing MSCs were used for the localization experiment, since peripheral lin−/AP+ cells are relatively sparse and present a challenge to be labeled permanently for in vivo work.
We recognize several potential limitations of our study, including the small number of cells used for the infusions and the lack of knowledge of the precise mechanisms related to the recovery of hematopoiesis after lin−/AP+ cell infusion.
In conclusion, in this study we developed a mouse model that can be used to identify human cells that have the capacity to support hematopoiesis. In addition, our results indicate that similar to MSCs, human peripheral lin−/AP+ cells support and maintain hematopoiesis in 3.6Col1α1 Tk-Rag mice during treatment with GCV and that these cells express mesenchymal and endothelial genes and are enriched for hematopoietic niche genes. Our findings not only provide further information about the roles of circulating lin−/AP+ cells but may also have potential implications in cell therapy protocols to enhance BMC recovery after chemotherapy and bone marrow transplantation. Nevertheless, further studies are needed to determine whether the infused cells home to the hematopoietic niche itself and how long they reside there.
Acknowledgments
Grant sponsor: NIH; Grant numbers: AG004875, UL1TR000135 (Center for translational Science Activities).
We would like to thank James Peterson for technical support. This work was supported by NIH Grants AG004875, UL1TR000135 (Center for translational Science Activities).
Footnotes
All authors state that they have no conflicts of interest.
References
- Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Arai F, Hosokawa K, Toyama H, Matsumoto Y, Suda T. Role of N-cadherin in the regulation of hematopoietic stem cells in the bone marrow niche. Ann N Y Acad Sci. 2012;1266:72–77. doi: 10.1111/j.1749-6632.2012.06576.x. [DOI] [PubMed] [Google Scholar]
- Askmyr M, Sims NA, Martin TJ, Purton LE. What is the true nature of the osteoblastic hematopoietic stem cell niche? Trends Endocrinol Metab. 2009;20:303–309. doi: 10.1016/j.tem.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Butler JM, Nolan DJ, Vertes EL, Varnum-Finney B, Kobayashi H, Hooper AT, Seandel M, Shido K, White IA, Kobayashi M, Witte L, May C, Shawber C, Kimura Y, Kitajewski J, Rosenwaks Z, Bernstein ID, Rafii S. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell. 2010;6:251–264. doi: 10.1016/j.stem.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- Chan CK, Chen CC, Luppen CA, Kim JB, DeBoer AT, Wei K, Helms JA, Kuo CJ, Kraft DL, Weissman IL. Endochondral ossification is required for haematopoietic stem-cell niche formation. Nature. 2009;457:490–494. doi: 10.1038/nature07547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chute JP, Saini AA, Chute DJ, Wells MR, Clark WB, Harlan DM, Park J, Stull MK, Civin C, Davis TA. Ex vivo culture with human brain endothelial cells increases the SCID-repopulating capacity of adult human bone marrow. Blood. 2002;100:4433–4439. doi: 10.1182/blood-2002-04-1238. [DOI] [PubMed] [Google Scholar]
- Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eghbali-Fatourechi GZ, Lamsam J, Fraser D, Nagel D, Riggs BL, Khosla S. Circulating osteoblast-lineage cells in humans. N Engl J Med. 2005;352:1959–1966. doi: 10.1056/NEJMoa044264. [DOI] [PubMed] [Google Scholar]
- Eghbali-Fatourechi GZ, Modder UI, Charatcharoenwitthaya N, Sanyal A, Undale AH, Clowes JA, Tarara JE, Khosla S. Characterization of circulating osteoblast lineage cells in humans. Bone. 2007;40:1370–1377. doi: 10.1016/j.bone.2006.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett RW, Emerson SG. Bone and blood vessels: The hard and the soft of hematopoietic stem cell niches. Cell Stem Cell. 2009;4:503–506. doi: 10.1016/j.stem.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Gossl M, Modder UI, Atkinson EJ, Lerman A, Khosla S. Osteocalcin expression by circulating endothelial progenitor cells in patients with coronary atherosclerosis. J Am Coll Cardiol. 2008;52:1314–1325. doi: 10.1016/j.jacc.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich MC, Dooley DC, Freed AC, Band L, Hoatlin ME, Keeble WW, Peters ST, Silvey KV, Ey FS, Kabat D, et al. Constitutive expression of steel factor gene by human stromal cells. Blood. 1993;82:771–783. [PubMed] [Google Scholar]
- Hosokawa K, Arai F, Yoshihara H, Iwasaki H, Nakamura Y, Gomei Y, Suda T. Knockdown of N-cadherin suppresses the long-term engraftment of hematopoietic stem cells. Blood. 2010;116:554–563. doi: 10.1182/blood-2009-05-224857. [DOI] [PubMed] [Google Scholar]
- Jilka RL, O’Brien CA, Ali AA, Roberson PK, Weinstein RS, Manolagas SC. Intermittent PTH stimulates periosteal bone formation by actions on post-mitotic preosteoblasts. Bone. 2009;44:275–286. doi: 10.1016/j.bone.2008.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel MJ, Radice GL, Morrison SJ. Lack of evidence that hematopoietic stem cells depend on N-cadherin-mediated adhesion to osteoblasts for their maintenance. Cell Stem Cell. 2007;1:204–217. doi: 10.1016/j.stem.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Kina T, Ikuta K, Takayama E, Wada K, Majumdar AS, Weissman IL, Katsura Y. The monoclonal antibody TER-119 recognizes a molecule associated with glycophorin A and specifically marks the late stages of murine erythroid lineage. Br J Haematol. 2000;109:280–287. doi: 10.1046/j.1365-2141.2000.02037.x. [DOI] [PubMed] [Google Scholar]
- Koc ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI, Lazarus HM. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 2000;18:307–316. doi: 10.1200/JCO.2000.18.2.307. [DOI] [PubMed] [Google Scholar]
- Lange C, Brunswig-Spickenheier B, Cappallo-Obermann H, Eggert K, Gehling UM, Rudolph C, Schlegelberger B, Cornils K, Zustin J, Spiess AN, Zander AR. Radiation rescue: Mesenchymal stromal cells protect from lethal irradiation. PLoS One. 2011;6:14486. doi: 10.1371/journal.pone.0014486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Johnson SA, Shelley WC, Yoder MC. Hematopoietic stem cell repopulating ability can be maintained in vitro by some primary endothelial cells. Exp Hematol. 2004;32:1226–1237. doi: 10.1016/j.exphem.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Lo Celso, Wu JW, Lin CP. In vivo imaging of hematopoietic stem cells and their microenvironment. J Biophotonics. 2009;2:619–631. doi: 10.1002/jbio.200910072. [DOI] [PubMed] [Google Scholar]
- Majumdar MK, Thiede MA, Haynesworth SE, Bruder SP, Gerson SL. Humanmarrow-derived mesenchymal stemcells(MSCs)express hematopoietic cytokines and support long-term hematopoiesis when differentiated toward stromal and osteogenic lineages. J Hematother Stem Cell Res. 2000;9:841–848. doi: 10.1089/152581600750062264. [DOI] [PubMed] [Google Scholar]
- Mayack SR, Wagers AJ. Osteolineage niche cells initiate hematopoietic stem cell mobilization. Blood. 2008;112:519–531. doi: 10.1182/blood-2008-01-133710. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Mazzon C, Anselmo A, Soldani C, Cibella J, Ploia C, Moalli F, Burden SJ, Dustin ML, Sarukhan A, Viola A. Agrin is required for survival and function of monocytic cells. Blood. 2012;119:5502–5511. doi: 10.1182/blood-2011-09-382812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuleman N, Tondreau T, Ahmad I, Kwan J, Crokaert F, Delforge A, Dorval C, Martiat P, Lewalle P, Lagneaux L, Bron D. Infusion of mesenchymal stromal cells can aid hematopoietic recovery following allogeneic hematopoietic stem cell myeloablative transplant: A pilot study. Stem Cells Dev. 2009;18:1247–1252. doi: 10.1089/scd.2009.0029. [DOI] [PubMed] [Google Scholar]
- Modder UI, Roforth MM, Nicks KM, Peterson JM, McCready LK, Monroe DG, Khosla S. Characterization of mesenchymal progenitor cells isolated from human bone marrow by negative selection. Bone. 2012;50:804– 810. doi: 10.1016/j.bone.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- Muguruma Y, Yahata T, Miyatake H, Sato T, Uno T, Itoh J, Kato S, Ito M, Hotta T, Ando K. Reconstitution of the functional human hematopoietic microenvironment derived from human mesenchymal stem cells in the murine bone marrow compartment. Blood. 2006;107:1878–1887. doi: 10.1182/blood-2005-06-2211. [DOI] [PubMed] [Google Scholar]
- Pignolo RJ, Kassem M. Circulating osteogenic cells: implications for injury, repair, and regeneration. J Bone Miner Res. 2011;26:1685–1693. doi: 10.1002/jbmr.370. [DOI] [PubMed] [Google Scholar]
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- Radonic A, Thulke S, Mackay IM, Landt O, Siegert W, Nitsche A. Guideline to reference gene selection for quantitative real-time PCR. Biochem Biophys Res Commun. 2004;313:856–862. doi: 10.1016/j.bbrc.2003.11.177. [DOI] [PubMed] [Google Scholar]
- Undale A, Srinivasan B, Drake M, McCready L, Atkinson E, Peterson J, Riggs BL, Amin S, Modder UI, Khosla S. Circulating osteogenic cells: Characterization and relationship to rates of bone loss in postmenopausal women. Bone. 2010;47:83–92. doi: 10.1016/j.bone.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002:RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visnjic D, Kalajzic Z, Rowe DW, Katavic V, Lorenzo J, Aguila HL. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004;103:3258–3264. doi: 10.1182/blood-2003-11-4011. [DOI] [PubMed] [Google Scholar]
- Wu JY, Scadden DT, Kronenberg HM. Role of the osteoblast lineage in the bone marrow hematopoietic niches. J Bone Miner Res. 2009;24:759–764. doi: 10.1359/jbmr.090225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, Mishina Y, Li L. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]