Abstract
The mucosa is the primary point of entry for pathogens making it an important vaccination site to produce a protective mucosal immune response. While the sublingual (SL) mucosa presents several barriers to vaccine penetration, its unique anatomy and physiology makes it one of the best options for mucosal vaccination. Efficient and directed delivery of adjuvants and antigens to appropriate immune mediators in the SL tissue will aid in development of effective SL vaccines against infectious diseases. Herein we demonstrate a robust immune response against influenza antigens co-delivered sublingually with engineered liposomes carrying the synthetic toll like receptor-4 agonist, CRX-601. Liposome modification with PEG copolymers (Pluronics), phospholipid- PEG conjugates and chitosan were evaluated for their ability to generate an immune response in a SL murine influenza vaccine model. Phospholipid-PEG conjugates were more effective than Pluronic copolymers in generating stable, surface neutral liposomes. SL vaccination with surface modified liposomes carrying CRX-601 adjuvant generated significant improvements in flu-specific responses compared to unmodified liposomes. Furthermore, the coating of modified liposomes with methylglycol chitosan produced the most effective flu-specific immune response. These results demonstrate efficient SL vaccine delivery utilizing a combination of a mucoadhesive and surface neutral liposomes to achieve a robust mucosal and systemic immune response.
Keywords: sublingual, mucosal, vaccine, liposomes, influenza, TLR4, chitosan
Graphical abstract
Introduction
Advances in immunology, coupled with recent technological developments have transformed the field of vaccines with safer subunit vaccines, novel delivery systems, and rationally designed adjuvants and immunomodulators [1]. Further advancements have in part focused on exploiting non-invasive routes of vaccination, particularly the mucosal routes. Amongst the various mucosal sites, the sublingual (SL) surface is well suited for vaccination due to its unique anatomy and physiology. The SL mucosa has low enzyme activity, easy access to lymphatic system (membrane is only 8 – 12 cells thick), and the human SL mucosa is covered by a non-keratinized epithelium, rendering it relatively pervious to vaccine delivery [2]. Previous reports have demonstrated the rapid transport of antigens across the SL mucosa [2, 3]. Moreover, the tissue is rich in Langerhans cells, as well as other APCs such as the myeloid and the plasmacytoid dendritic cells, which are important targets for the induction of a protective immune response. With the correct co-stimulatory environment and antigen uptake these APCs are capable of entering the lymphatic system and migrating to local and distant lymph nodes generating both systemic and mucosal immunity [4].
A number of sublingual immunotherapy (SLIT) vaccines, such as SLITone®, Sublivac®, Grazax®, Oralair®, and AllerSlit®forte, are approved for treatment of type I allergies [2]. Considerably less attention has been directed towards SL vaccines for infectious diseases, with no approved products and relatively few investigations reported in literature [5]. The lack of successful SL vaccine candidates against infectious diseases is indicative of the underlying challenge of generating a robust immune response via the SL route of administration. The focus has begun to shift towards finding appropriate SL adjuvants and delivery methods to direct potent immune responses to co-delivered antigens and avoid immune tolerance more commonly observed for antigens delivered via this route [6, 7]. In particular efficient delivery of adjuvants and antigens to appropriate immune mediators in the SL tissue is a key requirement for development of a successful SL vaccine.
In the present study, we aim to address the challenges to effective SL vaccination using a synthetic toll like receptor 4 (TLR4) adjuvant, CRX-601, delivered through liposome vehicles engineered for efficient transport across the SL mucosa. CRX-601, a synthetic aminoalkyl glucosaminide 4-phosphate (AGP), is an effective intranasal mucosal adjuvant with the ability to improve humoral and cell-mediated immune responses to influenza [8]. Being amphiphillic, CRX-601 is rapidly incorporated into liposomes, which greatly reduces its pyrogenicity in rabbits and may translate to improved safety in humans [8]. Considering the additional barriers to delivery of liposomal CRX-601 through the SL surface and our preliminary studies indicating low immunological activity, modification of the vehicle for improved delivery was considered necessary.
To further improve the properties of liposomes for SL delivery of CRX-601, we evaluated two contrasting modification strategies to improve the mucus- penetrating or adhesive properties of CRX-601 liposomes. The first approach evaluated here was based on reports that nanoparticles with a hydrophilic and an electrically neutral surface can rapidly penetrate the mucus layers and achieve efficient delivery across a variety of mucosal surfaces [9]. Such particles, termed mucoinert or mucus penetrating by the authors, were achieved by chemical coupling of low–molecular weight poly(ethylene glycol) (PEG) at high densities on solid nanoparticles [10, 11] or self-assembly of amphiphilic PEG copolymers [12]. Others have described Pluronic F127 coated mucus penetrating liposomes for delivery across the intestinal mucus [13, 14]. However, the effectiveness of mucus penetration, at least for PLGA and polystyrene based systems, has been reported to be highly dependent on the PEG chain length and density [11]. It has also been noted that Pluronic copolymers can cause substantial membrane destabilization upon addition of even small amounts (∼ 2 mol%) [15-18]. A more innocuous way of achieving liposome PEG coating is using PEG lipid conjugates with long acyl chain lipid anchors, such as DSPE [19]. These conjugates are efficiently incorporated into liposome membranes with long term retention in the membrane [19]. Herein, we describe the development and optimization of CRX-601 liposomes for SL vaccination by evaluating liposome modification with 1) Pluronic copolymers which vary in lengths of their hydrophilic and hydrophobic blocks, 2) phospholipid-PEG conjugates, and 3) varying extent of Pluronic/phospholipid-PEG modification.
Alternatively, mucoadhesive liposomes are employed for increasing the retention time at the delivery site [20]. The widely used mucoadhesive chitosan and its derivatives are a popular choice for preparation of chitosan coated liposomes [21, 22]. An additional benefit to using chitosan is its ability to act as a permeation enhancer by opening epithelial tight junctions [23]. Coating liposome carriers with the chitosan derivatives was also evaluated in this study. While there is considerable interest in employing either muco- penetration or adhesion for mucosal delivery, few studies provide a comparative evaluation of both approaches to optimize mucosal delivery [13]. Furthermore, we evaluated combination approaches with chitosan derivatives to capitalize on the benefits of muco-penetration via liposome modification and the tight junction opening properties of chitosan. We provide a comparative and combinatorial evaluation of mucus- penetrating and -adhesive strategies in the context of SL immunity using a mouse model of influenza vaccination.
Materials and methods
Materials
The phospholipid 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), and the phospholipid-PEG conjugates, [N-(carbonyl-methoxypolyethylenglycol-2000)-1,2-distearoyl-sn-glycero-3-phosphoethanolamine sodium salt (PE-PEG2K) and N-(carbonyl-methoxypolyethylenglycol-5000)-1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine sodium salt (PE-PEG5K) were from Lipoid (Lipoid GmbH, Ludwigshafen, Germany). The block copolymers of PEG and propylene oxide (PO) (Pluronics, Table 1), F127, F68, and L64 were from Sigma-Aldrich (St Louis, MO, USA), BASF (Florham Park, New Jersey), and Spectrum chemicals (New Brunswick, NJ), respectively. Methylglycol chitosan (chitosan glycol trimethyl ammonium iodide, MGC), glycol chitosan (GC), chitosan oligosaccharide lactate (CO) and cholesterol were from Sigma-Aldrich (St Louis, MO, USA), 4-(2-hydroxyethyl)-1-piperazine-ethanesulfonic acid (HEPES) was from Acros Organics (Waltham, MA, USA), Millex-GV 0.22 μm PVDF syringe filters were from EMD Millipore (Billerica, MA, USA). All organic solvents used for RP-HPLC and otherwise were of HPLC grade. The aminoalkyl glucosaminide 4-phosphate (AGP) CRX-601 was synthesized as described previously [24] and purified by flash chromatography on silica gel (to >95% purity) as a monoethanolamine salt.
Table 1. Structures and composition of the studied copolymers.
| Polymer series | Phospholipid – PEG | Pluronic | |||
|---|---|---|---|---|---|
|
|
|
||||
| Composition/abbreviation | DSPE-PEG2K | DPPE-PEG5K | L64 | F68 | F127 |
| Hydrophobic repeat units, y | 17 | 15 | 30 | 30 | 56 |
| Hydrophilic repeat units, x | 45 | 113 | 26 | 152 | 101 |
| Average MW | 2803 | 5797 | 2900 | 8400 | 12600 |
| CMC (M) [25] | 1.2 × 10-5 | 1.4 × 10-5 | 1.6 × 10-3 | 1.6 × 10-4 | 6.9 × 10-5 |
| HLB values [26] | n.a. | n.a. | 12 – 18 | >24 | 18 – 23 |
Abbreviations: PEG, polyethylene glycol; PPO, poly (propylene oxide); DSPE, distearoyl-phosphoethanolamine; DPPE, dipalmitoyl-phosphoethanolamine; MW, molecular weight; CMC, critical micelle concentration; HLB, hydrophilic-lipophilic balance
Preparation of liposomes
Unmodified liposomes
The CRX-601, DOPC, and cholesterol were dissolved in tetrahydrofuran (THF) in a round bottom flask. The organic solvent removed by evaporation, first on a rotary evaporator to obtain a thin film and further with high vaccum for 12 hrs to remove residual solvent. The film was dispersed in 10 mM HEPES-saline buffer pH 7.0 using sonication on a water bath (20 – 30 °C) with intermittent vortexing until all the film was completely dispersed into solution (30 min – 1.5 h). The emulsion was then extruded successively through polycarbonate filters, with the final pass through 200 nm membranes, using a lipid miniextruder (Lipex™ extruder, Northern Lipids Inc., Canada) under N2 to form unilamellar liposomes. The liposome composition was then aseptically filtered using a 0.22 μm filter into a sterile depyrogenated container and stored at 2 – 8°C until further use. The amounts of individual ingredients were chosen so as to obtain final target concentrations of 2 mg/mL CRX- 601, 10 mg/mL cholesterol, and 40 mg/mL phospholipid. Blank liposomes were prepared using a similar procedure but without CRX-601.
Phospholipid-PEG modified liposomes
The procedure was similar to preparation of unmodified liposomes except that the conjugate PE-PEG2K or PE-PEG5K was co-dissolved with CRX-601, DOPC, and cholesterol during thin film preparation. By varying the amount of PE-PEG added, liposomes with 1 – 25 mole % PE-PEG (wrt DOPC) substitution were obtained.
Pluronic modified liposomes
The procedure was similar to preparation of conventional liposomes except that the individual Pluronic was co-dissolved with CRX-601 and DOPC during thin film preparation. No cholesterol was included in these preparations as it was reported to reduce incorporation of Pluronics into the phospholipid bilayer [17]. By varying the amount of Pluronic added, liposomes with 1 – 25 mole % Pluronic (wrt DOPC) addition were obtained.
Chitosan coated liposomes
For preparation of chitosan coated liposomes, MGC was dissolved in 10 mM HEPES-saline buffer (pH 7.0), sterile filtered using a 0.22 μm filter and admixed with CRX-601 liposomes at varying ratios.
Characterization of formulations
Liposomes were characterized by dynamic light scattering (DLS) using a Malvern Zetasizer Nano ZS (Malvern Instruments, USA) for zeta (ζ)-potential, particle size, and polydispersity indices (PDI) using standard analytical technique. Samples (8 μL) were diluted with 800 μL ultrapure water before measurement. The average values given are calculated with intensity distribution data and are the means ± standard deviation of three independent experiments.
The concentration of CRX-601 in formulations was determined by ion-pair reverse phase high-performance liquid chromatography (RP-HPLC, Waters Alliance 2690/2695, Milford, MA) on a C8 column (Ace 3, 3 μm, 50 mm × 3.0 mm; Mac-Mod Analytical, Chadds Ford, PA) and UV detection at 210 nm (Waters model 2487 or 996 PDA detector). Elution consisted of a linear gradient at 0.8 mL/min from 50% to 100% B over 10 min and 100% B for 5 min. Solvent A consisted of 8% ACN, 2% buffer and 90% water. Solvent B consisted of 2% buffer in ACN. Buffer was prepared from 62.5 mL of 0.4 M tetrabutylammonium hydroxide in water with pH adjustment to 6.0 with 15 M phosphoric acid and a final volume of 100 mL. Samples were diluted in THF (1:1 v/v) and analyzed against a five-point standard curve with system suitability injections at the start and the end of the run.
Stability testing
Formulations were stored at 2 – 8 °C, 25 °C, and 40 °C without humidity control. At predetermined time intervals, the formulations were observed for possible signs of agglomeration/settling. Detailed analysis of size, PDI and ζ-potential was also performed.
Rabbit pyrogen test
The pyrogen test is used here as a surrogate measure of CRX-601 incorporation into liposomes and as a measure of their stability in biological milieu. The test was performed at Pacific Biolabs (Hercules, CA) following the procedures outlined in USP<151>. Briefly, naïve female New Zealand White rabbits (n = 3), weighing at least 2 Kg, were administered the test article in a 3 mL/Kg dose via the ear vein. Body temperature was measured using rectal temperature-measuring probe 30 min prior to administration and thereafter at 30 minute intervals between 1 and 3 hours following administration. The test article was considered pyrogen free if none of the rabbits showed an individual temperature rise of 0.5 °C or more above its respective control temperature at any point.
Sublingual vaccination of mice
For vaccination, female BALB/c mice (6 to 8 weeks of age) from Charles River Laboratories (Wilmington, MA) were anesthetized by intraperitoneal (IP) administration of ketamine (100 mg/kg) and xylazine (10 mg/kg), and vaccinated by SL (6 μL/mouse) or intramuscular (IM, 50 μL/mouse) administration on days 0, 21 and 42. All animals in the SL treatment groups received 3.0 μg HA (monovalent detergent-split influenza (A/Victoria/210/2009 H3N2)) in combination with blank liposome, various CRX-601 loaded liposomes, or CRX-601 loaded liposomes in combination with MGC. Animals in the IM group received 1.5 or 3.0 μg HA in combination with CRX-601. The CRX-601, when present, was dosed at 5 μg/animal in SL groups and 1 μg/animal in the IM group. All vaccine dilutions were made in 10 mM HEPES-saline. Serum was harvested from mice under anesthesia on day 36 (14 days post-secondary immunization, 14dp2). On day 56 (14dp3) mice were sacrificed, and vaginal wash, tracheal wash and serum samples were collected as described previously [8]. All samples were stored at − 70 °C prior to analysis. All animals were used in accordance with guidelines established by the U.S. Department of Health and Human Services Office of Laboratory Animal Welfare and the Institutional Animal Care and Use Committee at GSK Vaccines, Hamilton, Montana.
Determination of specific antibody responses
Antibody responses specific to flu were measured by two independent immunoassays, the influenza hemagglutination inhibition (HI) assay and the enzyme linked immunosorbent assay (ELISA).
Quantitative analysis of the functional antibody response in mice vaccinated with influenza vaccines was performed using a standard HI assay. Briefly, chicken red blood cells (RBCs) were washed once with Alsever's solution (Sigma) and two times with Dulbecco's phosphate buffered saline (DPBS, Sigma). Serum samples (14dp3) from vaccinated mice were treated with a 1.6% solution of receptor destroying enzyme (RDE, Sigma), to eliminate non-specific inhibitors of hemagglutination (18 h at 37 °C), prior to addition of sodium citrate solution (Sigma) at 0.75% final concentration for 30 min at 56 °C. RDE-treated serum samples were incubated with a 2.5% solution of washed-RBCs for 60 min at 4 °C, to eliminate non-specific hemagglutination by endogenous serum constituents (supernatants were collected and background hemagglutination was evaluated by incubation with 0.5% RBC suspension for 45 min at room temperature [RT]). Whole inactivated influenza virus (H3N2, A/Victoria/210/2009) was back-titrated to ensure 8 HA units/50 μL immediately prior to and after performing the HI assay. RDE/RBC-treated serum samples (1:20 dilution during pretreatment) were added to wells of a 96-well microtiter plate and serially diluted by two-fold dilutions down the plate. Diluted influenza virus (8 HA units/50 μL) was added to serum samples and incubated for 45 min at RT. 0.5% RBC suspension was added to each well and incubated for 45 min at RT. Plates were immediately evaluated for hemagglutination inhibition (tear-drop pattern). The functional antibody (HI) titer represents the reciprocal of the last sample dilution that does not produce a complete or partial agglutination of RBCs.
Anti-flu IgG titers in mouse serum samples (14dp2 and 14dp3) and IgA levels in both mucosal vaginal and tracheal wash samples were analyzed by ELISA as previously described [8], with the following modifications. 96-well nunc MaxiSorp plates (Thermo Fisher Scientific, Rochester, NY) were coated overnight with 2.0 μg/mL of monovalent detergent-split flu antigen (H3N2, A/Victoria/210/2009) in DPBS. For standard curves, wells were coated with goat anti-mouse IgG or IgA (1 μg/mL or 4 μg/mL, respectively). Plates were washed and blocked with Super Blok (ScyTek, Logan, UT) for 1 h at 37 °C. Serum, mucosal wash samples were added in diluting buffer (DPBS, 1% bovine serum albumin, 0.1%Tween 20, 5% heat inactivated fetal bovine serum) and titrated in two-fold serial dilutions down the assay plate. For standard curves, murine IgG (Sigma), or IgA (Thermo Fisher Scientific) were added in dilution buffer (at 200 ng/mL, or 125 ng/mL, respectively) and similarly titrated in two-fold serial dilutions down the assay plate. Following 1 h of incubation at 37 °C, bound antibody was detected with peroxidase goat anti-mouse IgG or IgA (Southern Biotech, Birmingham, AL, 1 h at 37 °C) and 3,3′,5,5′-tetramethylbenzidine substrate. The optical density was read at 450 nm and antibody titers were extrapolated from the corresponding standard curve.
Statistics
Statistical comparisons for liposome size and ζ-potential were performed using Student t-test (two-tails, equal variance) with Microsoft Excel 2007 (Microsoft Corporation, Redmond, WA). Titers from mouse SL studies were analyzed using one way analysis of variance (ANOVA) with Tukey's multiple comparisons test using GraphPad Prism version 6.01 for Windows (GraphPad Software, San Diego, CA). P values less than 0.05 were considered significant.
Results
Preparation and characterization of liposomal formulations with CRX-601 adjuvant
(Table 2 shows the particle size, PDI and ζ-potential of unmodified, phospholipid-PEG modified, and Pluronic modified CRX-601 liposomes).
Table 2. Particle size, polydispersity, and ζ-potential of phospholipid-PEG and Pluronic modified liposomes.
| Formulation | Diameter (nm) | PDI | ζ-potential (mV) |
|---|---|---|---|
| CRX-601/unmodified liposome | 112 ± 34 | 0.19 ± 0.03 | -37 ± 18 |
|
| |||
| Phospholipid-PEG modified liposomes | |||
|
| |||
| CRX-601/1 mol% PE-PEG2K liposome | 104 ± 23 | 0.21 ± 0.03 | -13.5 ± 10* |
| CRX-601/5 mol% PE-PEG2K liposome | 102 ± 15 | 0.23 ± 0.03 | -15 ± 10* |
| CRX-601/25 mol% PE-PEG2K liposome | 96 ± 14 | 0.19 ± 0.03 | -8 ± 6* |
| CRX-601/1 mol% PE-PEG5K liposome | 100 ± 8 | 0.22 ± 0.02 | -9 ± 10* |
| CRX-601/5 mol% PE-PEG5K liposome | 105 ± 15 | 0.20 ± 0.03 | -10 ± 7* |
| CRX-601/25 mol% PE-PEG5K liposome | 87 ± 13 | 0.22 ± 0.02 | -5 ± 3* |
|
| |||
| Pluronic modified liposomes | |||
|
| |||
| CRX-601 /5 mol% L64 liposomes | 108 ± 25 | 0.24 ± 0.05 | -37 ± 18 |
| CRX-601 /15 mol% L64 liposomes | 99 ± 27 | 0.23 ± 0.02 | -30 ± 20 |
| CRX-601 /25 mol% L64 liposomes | 120 ± 28 | 0.18 ± 0.06 | -15 ± 14 |
| CRX-601/ 5 mol% F68 liposomes | 155 ± 26 | 0.16 ± 0.07 | -47 ± 7 |
| CRX-601/ 15 mol% F68 liposomes | 173 ± 17* | 0.12 ± 0.03 | -34 ± 8 |
| CRX-601 /25 mol% F68 liposomes | 174 ± 9* | 0.12 ± 0.04 | -23 ± 2 |
| CRX-601/5 mol% F127 liposome | 125 ± 1 | 0.17 ± 0.02 | -22 ± 3* |
| CRX-601/15 mol% F127 liposome | 115 ± 23 | 0.18 ± 0.05 | -19 ± 16 |
| CRX-601/25 mol% F127 liposomes | 135 ± 13 | 0.16 ± 0.05 | -7 ± 1* |
Data expressed as mean ± standard deviation (n = 3). P values less than 0.05 were set as the level of significance.
P < 0.05 compared to unmodified liposomes.
Phospholipid-PEG modified liposomes
Modification with 1, 5 and 25 mol% PE-PEG2K or PE-PEG5K did not alter the size or PDI compared to unmodified liposomes, but significantly (P < 0.05) changed the negative ζ-potential to a more neutral value. For example, the average ζ-potential for liposomes with 1% PE-PEG5K modification was – 9 mV, significantly (P < 0.05) more neutral compared to unmodified liposomes with a potential of – 37 mV. Such an effect is characteristic of PEG mediated shielding of surface charge [27, 28] and indicates an efficient incorporation of phospholipid-PEG conjugates into the liposome membrane.
Pluronic modified liposomes
Physiochemical assessments for 5, 15 and 25 mol% modification with Pluronics L64, F68 and F127 are shown in Table 2. Modification with Pluronic F68 led to a significant increase (P < 0.05) in hydrodynamic diameter by ∼ 60 nm at both 15 and 25% modification when compared to unmodified liposomes. No significant changes in diameter were measured with L64 or F127 modification. Modification with Pluronic copolymers led to a stoichiometry dependent partial reduction in net negative surface charge of the liposomes, with 25% modification having the largest reduction (by –15 to –30 mV) for all three Pluronics. The reduction in surface charge was however not significantly different from unmodified liposomes, except in the case of liposomes with 5% and 25% F127 modification (P < 0.05). This indicates only a partial shielding of surface charge with Pluronic modification, especially with L64 and F68 modification, and is likely due to their limited incorporation into liposomes, as reported previously [29].
Chitosan coated liposomes
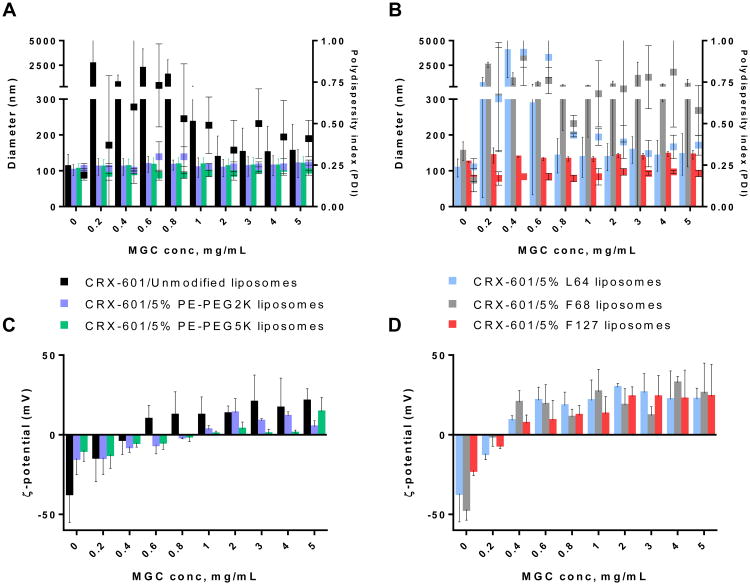
The commonly used chitosan mucoadhesive, N-deacytelated chitosan (pKa of primary amines is ∼ 6.5 [30]), has limited utility under physiological conditions due to its poor solubility (< 1mg/mL) and reduced activity at neutral and alkaline pH [31]. Thus, chitosan derivatives: MGC, GC and CO with increased solubility (> 10 mg/mL) at neutral pH were chosen for evaluation in this study. Chitosan coated liposome formulations were prepared by admixing unmodified, phospholipid-PEG modified, or Pluronic modified CRX-601 liposomes with the chitosan derivative and evaluated for changes in size and ζ-potential. When combined with MGC, unmodified liposomes exhibited aggregation, leading to precipitation, at 0.2 – 1 mg/mL MGC, indicated in Figure 1A as particles exhibiting size in the μm range. At MGC concentrations exceeding 1 mg/mL, the formulations appeared colloidally stable initially but tended to aggregate over 1 – 4 days (Figure S1). PE-PEG2K and PE-PEG5K modified liposomes with 5 mol% modification were colloidally stable in presence of MGC with no major change in size at any of concentrations tested (Figure 1A). Reversal in ζ-potential from a negative potential to positive, occurred at approximately 0.5 mg/ml MGC with unmodified liposomes and 1 mg/mL with 5% PE-PEG2K or PE-PEG5K modification. Modification with as little as 1 mol% PE-PEG2K or PE-PEG5K was demonstrated to provide sufficient protection against MGC induced aggregation at most concentrations (Figure S2A). At 1% modification, PE-PEG5K liposomes were more resistant to MGC induced aggregation than PE-PEG2K modified liposomes (Figure S2A). At 0.4 – 0.6 mg/mL MGC, no major change in particle size or PDI were observed with 1% PE-PEG5K modification but an increase in size to the μm range were observed with 1% PE-PEG2K modification. Modification of up to 25% did not result in any destabilization/aggregation in presence of MGC (Figure S2B).
Figure 1. Stability of adjuvant-liposomes in presence methylglycol chitosan (MGC).
Size/PDI and ζ-potential values with increasing concentration of MGC for adjuvant-loaded unmodified, PE-PEG2K and PE-PEG5K modified liposomes (A) & (C); and Pluronic L64, F68 and F127 modified liposomes (B) & (D). For (A) & (B), sizes are plotted as bars on the left Y-axis and PDI values as dot plot on right Y-axis. Data are expressed as mean ± SD, (n = 3). Particles in the μm size range tended to precipitate over time.
Amongst Pluronic modified liposomes, F127 modified liposomes were the most stable, exhibiting no visible aggregation or increase in polydispersity over the complete range of MGC concentrations evaluated (Figure 1B). Increase in particle size was about 10 – 30 nm, and reversal of ζ-potential from a net negative to positive potential occurred at MGC concentrations ≥ 0.4 mg/mL. F127 modified liposomes at 15 and 25 % modification were similarly stable in presence of MGC (Figure S3A), but at 1% modification indicated aggregation (data not shown). Liposomes with 5 mol% L64 modification when combined with MGC, showed an increase in size and polydispersity at 0.2 – 0.8 mg/mL MGC (Figure 1B), corresponding to complete neutralization of liposome surface charge. At ≥ 1 mg/mL MGC, similar to unmodified liposomes, the L64 modified liposomes appeared stable initially but tended to aggregate and precipitate over time (Figure 1B, S1). F68 modified liposomes were the least stable, and caused instantaneous precipitation with MGC at all tested concentrations. Similar trends were observed with liposomes with higher Pluronic modification of 15 and 25% (Figure S3). Hence, the order of stability of Pluronic modified liposomes in presence of MGC was F127>L64>F68.
The summary of stability evaluation for these liposomal formulations in the presence of chitosan derivatives, MGC, GC and CO, is shown in Table 3. Overall, amongst all tested chitosan derivatives, least aggregation was observed with MGC. Phospholipid-PEG modified liposomes were more stable against chitosan induced aggregation than Pluronic liposomes. PE-PEG5K liposomes were more stable than PE-PEG2K liposomes, as evident by lack of any change in size/PDI in presence of MGC at 1% modification and improved stability in the presence of GC and CO. This could be due to a more efficient steric stabilization by the relatively longer PEG5K chain compared to the PEG2K chain.
Table 3. Stability summary of unmodified, phosholipid-PEG, and Pluronic liposomes with varying degree of modification against chitosan derivative induced aggregationa.
| Formulation | + MGCb | + GCc | + COd |
|---|---|---|---|
| CRX-601 unmodified liposomes | Aggregation | Aggregation | Aggregation |
|
| |||
| Phospholipid-PEG modified liposomes | |||
|
| |||
| CRX-601/PE-PEG2K liposome | Stable at ≥ 1% modificatione | Aggregation | Stable at ≥ 5% modification |
| CRX-601/PE-PEG5K liposome | Stable at ≥ 1% modification | Stable at ≥ 5% modification | Stable at ≥ 1% modification |
|
| |||
| Pluronic modified liposomes | |||
|
| |||
| CRX-601 /L64 liposomes | Aggregation | Aggregation | Aggregation |
| CRX-601/F68 liposomes | Aggregation | Aggregation | Aggregation |
| CRX-601/F127 liposome | Stable at ≥ 5% modification | Aggregation | Stable at ≥ 5% modification |
The lowest modification tested was 1 mol%
Partial aggregation 0.2 – 0.6 mg/mL
MGC: Methylglycol chitosan
GC: Glycol chitosan
CO: Chitosan oligosaccharide lactate
Preliminary stability testing
Phospholipid-PEG modified liposomes with 1, 5, and 25 mol% modification and Pluronic modified liposomes with 5, 15, and 25 mol% modifications were stored at 2-8, 25 °C, and 40 °C and observed for possible signs of agglomeration/settling and changes in size, PDI and ζ-potential. Phospholipid-PEG liposomes, except with 25 % PE-PEG5K modification, did not show any settling, phase separation, creaming or any significant changes in particle size and ζ-potential for up to 4 weeks at 40 °C (data not shown). The 25 mol% PE-PEG5K liposomes formed gels in about 2 weeks on storage at 40 °C. No changes were observed at the other storage temperature of 2 – 8 °C and 25 °C. Amongst Pluronic modified liposomes, predominantly F68 liposomes and to some extent L64 modified liposomes within 1 – 4 weeks showed a creaming-like behavior with settling of particles and appearance of a clear continuous phase on top or bottom at all storage temperatures. This was not accompanied by any changes in particle size or ζ-potential (data not shown). The 25% F127 modified liposomes stored at 40 °C also formed gels, in agreement with the known thermogelling behavior of F127 [26, 32]. The gelling behavior for 25% PE-PEG5K and F127 is consistent with decreased solubility of PEG chains at higher temperatures.
Improved safety profile of CRX-601 with liposomes
The maximum intravenous non-pyrogenic dose of aqueous CRX-601 in New Zealand white rabbits is 1 ng/kg. Incorporation of CRX-601 into unmodified liposomes (CRX-601/DOPC = 1/20) improves the safety profile by reducing the pyrogenicity >500-fold (Table 4). Similar improvements in terms of reduced pyrogenicity were observed with PE-PEG2K and PE-PEG5K modified liposomes. This improvement in non-pyrogenic dose was reduced with Pluronic modified liposomes compared to unmodified or PE-PEG modified liposomes. F68 and F127 modified liposomes were non-pyrogenic at 250 ng/kg (250-fold improvement over aqueous CRX-601). With the exception of 25% F127 modified liposomes, all Pluronic modified liposome formulations were pyrogenic at 500 ng/kg. This indicates possibly a lower encapsulation or faster release of CRX-601 compared to unmodified or phospholipid-PEG modified liposomes. L64 modified liposomes were pyrogenic at 250 ng/kg, suggesting the lowest degree of CRX-601 encapsulation or fastest release rate amongst all Pluronic modified liposomes tested.
Table 4. Results of USP rabbit pyrogen test on adjuvant loaded unmodified, phospholipid-PEG and Pluronic modified liposomes.
| Formulation | Assay outcome NPa or Pb (Individual temperature change values for three rabbits) | Fold improvement over aqueous CRX-601c | |
|---|---|---|---|
| Dose of 250 ng/kg | Dose of 500 ng/kg | ||
| CRX-601/Unmodified liposome | NP (0.0 °C, 0.0 °C, 0.0 °C) | NP (0.0 °C, 0.3 °C, 0.0 °C) | 500 |
|
| |||
| Phospholipid-PEG modified liposomes | |||
|
| |||
| CRX-601/5 mol% PE-PEG2K liposome | NP (0.0 °C, 0.1 °C, 0.0 °C) | NP (0.0 °C, 0.0 °C, 0.2 °C) | 500 |
| CRX-601/25 mol% PE-PEG2K liposome | NP (0.1 °C, 0.0 °C, 0.0 °C) | NP (0.1 °C, 0.3 °C, 0.0 °C) | 500 |
| CRX-601/5 mol% PE-PEG5K liposome | NP (0.3 °C, 0.2 °C, 0.0 °C) | NP (0.0 °C, 0.0 °C, 0.4 °C) | 500 |
| CRX-601/25 mol% PE-PEG5K liposome | NP (0.3 °C, 0.0 °C, 0.3 °C) | NP (0.3 °C, 0.0 °C, 0.0 °C) | 500 |
|
| |||
| Pluronic modified liposomes | |||
|
| |||
| CRX-601 /15 mol% L64 liposomes | P (0.3 °C, 0.6 °C, 0.7 °C) | P (0.8 °C, 0.8 °C, 0.4 °C) | 125d |
| CRX-601 /25 mol% L64 liposomes | P (0.9 °C, 0.5 °C, 0.9 °C) | P (0.5 °C, 0.6 °C, 0.9 °C) | 125d |
| CRX-601/ 15 mol% F68 liposomes | NP (0.0 °C, 0.0 °C, 0.4 °C) | P (0.5 °C, 0.4 °C, 0.8 °C) | 250 |
| CRX-601 /25 mol% F68 liposomes | NP (0.0 °C, 0.1 °C, 0.0 °C) | P (0.3 °C, 0.7 °C, 0.4 °C) | 250 |
| CRX-601/15 mol% F127 liposome | NP (0.2 °C, 0.0 °C, 0.1 °C) | NP (0.0 °C, 0.3 °C, 0.0 °C) | 500 |
| CRX-601/25 mol% F127 liposomes | NP (0.4 °C, 0.2 °C, 0.4 °C) | P (0.3 °C, 0.3 °C, 0.5 °C) | 250 |
NP: Non-pyrogenic response
P: Pyrogenic response. The test is considered NP if none of the rabbits shows an individual temperature rise of 0.5 °C or more above its respective control temperature at any point.
Maximum non-pyrogenic dose of aqueous CRX-601 is 1 ng/kg.
The formulations were non-pyrogenic at 125 ng/Kg (individual temperature changes not shown).
Sublingual vaccination of mice with PEG modified liposome formulations
The effectiveness of CRX-601 loaded phospholipid-PEG and Pluronic liposome formulations for SL vaccination against split-flu was evaluated in a murine model. Mice were vaccinated SL on days 0, 21 and 42 with 3 μg (HA) of detergent split influenza antigen in combination with either blank liposomes, CRX-601 loaded PE-PEG liposomes (Figure 2), or CRX-601 loaded Pluronic liposomes (Figure 3). Serum and mucosal wash samples collected on day 56 (14dp3) were evaluated for flu specific immune responses by influenza hemagglutination inhibition (HI) assay, and anti-flu IgG and IgA by ELISA.
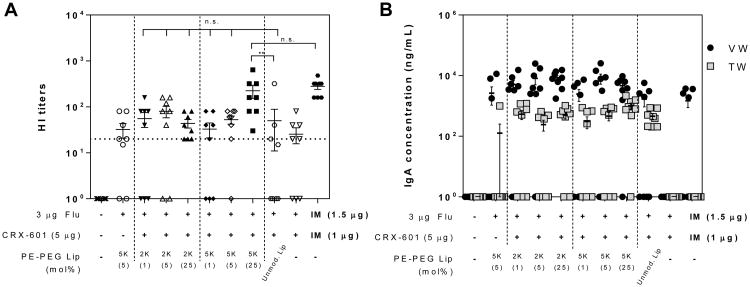
Figure 2. Flu specific HI titers and mucosal IgA following SL vaccination with phospholipid-PEG modified liposomes.
(A) Serum HI titers and (B) mucosal wash anti-flu IgA concentration from mice vaccinated with the indicated formulations. Serum, tracheal wash, and vaginal wash samples were collected from mice on day 56 following vaccination on days 0, 21 and 42. The horizontal dashed line represents the titer necessary for seroconversion. Anti-flu IgA concentration in vaginal wash (VW) and tracheal wash (TW) samples are indicated as black circles (●) and grey squares (■), respectively. Values less than the lower limit of quantitation (LLOQ) at 1:20 sample dilution for HI titers and 1:100 dilution for IgA are represented as a value of 1. Data expressed as mean ± standard error (n = 8). **, P < 0.005 compared to unmodified liposome group; n.s., not significant (P > 0.05) compared to IM group.
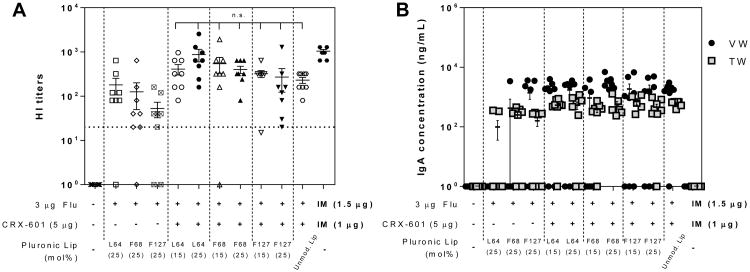
Figure 3. Flu specific HI titers and mucosal IgA responses following SL vaccination with Pluronic modified liposomes.
(A) Serum HI titers and (B) mucosal wash anti-flu IgA concentration from mice vaccinated with the indicated formulations. Serum, tracheal wash, and vaginal wash samples were collected from mice on day 56 following vaccination on days 0, 21 and 42. The horizontal dashed line represents the titer necessary for seroconversion. Anti-flu IgA concentration in vaginal wash and tracheal wash samples are indicated as black circles (●) and grey squares (■), respectively. Values less than the LLOQ at 1:20 sample dilution for HI titers and 1:100 dilution for IgA are represented as a value of 1. Data expressed as mean ± standard error (n = 8). n.s., not significant (P > 0.05) compared to unmodified liposome treatment group.
Amongst the phospholipid-PEG modified liposomes tested, only the CRX-601/25% PE-PEG5K modified liposomes induced functional HI titers and serum IgG levels significantly higher (P < 0.05) than unmodified liposomes and equivalent to that of IM vaccinated controls (Figure 2A, S4). None of the other adjuvanted PE-PEG2K or PE-PEK5K modified CRX-601 liposome groups demonstrated improvement over unmodified CRX-601 liposomes. Functional serum HI titers correlated with flu-specific IgG concentrations (Figure S4). Additionally, anti-flu IgA antibodies were readily detected at both local (tracheal wash, TW) and distal (vaginal wash, VW) mucosal sites in all mice vaccinated SL (Figure 2B), but were below the level of detection in the IM vaccinated and naïve groups. VW IgA levels were generally higher than TW IgA levels.
No significant differences in IgA levels were noted between the various CRX-601 liposome groups or antigen alone vaccination, although mice vaccinated with 25% PE-PEG2K or PE-PEK5K modified CRX-601 liposomes exhibited higher numbers of mice with measurable TW and VW IgA responses.
CRX-601 loaded Pluronic liposomes induced functional HI titers that trended higher than the unmodified liposome group, but did not provide any statistically significant improvement over unmodified CRX-601 liposomes (Figure 3, S5). HI titers were highest for the CRX-601 adjuvanted 25% L64 liposome groups, not significantly different from that of IM vaccinated controls (870 ± 274 compared to 1040 ± 100, respectively), and were closely followed by those from F68, and F127 liposome treatment groups. Between the liposomes groups with 15 and 25% modification, no significant improvement in titers were noted with increasing PEG concentrations. HI titers were higher for each of the 25% Pluronic modified liposome groups containing CRX-601 adjuvant compared to respective vehicle controls. However, these differences were not statistically significant (P > 0.05) and the high titers observed in the absence of CRX-601 could be due to the previously reported adjuvant effect of Pluronics [33, 34]. IgG responses largely correlated with HI titers, except for CRX-601 adjuvanted 25% L64 and 25% F68 liposome groups where IgG concentrations were significantly higher compared to the respective vehicle controls (Figure S5). All SL groups elicited strong mucosal IgA responses, with higher levels in VW compared to TW (Figure 3B).
Sublingual vaccination with chitosan coated liposome formulations
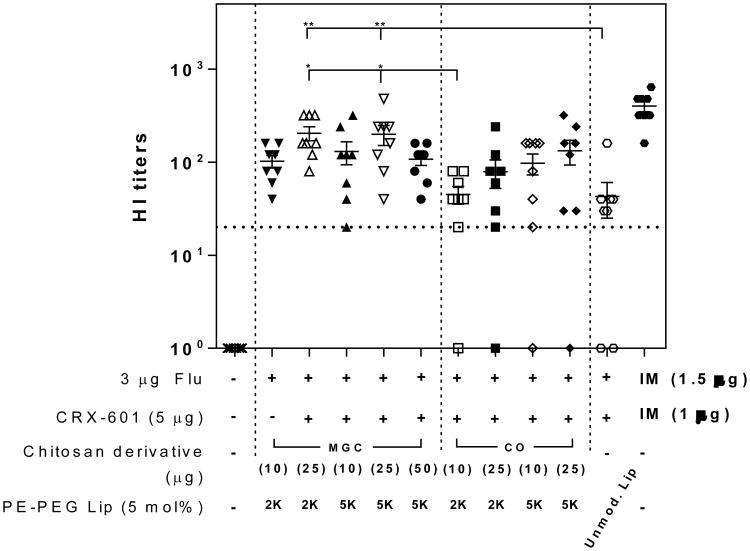
To determine the potential added value of chitosan coated liposomes, PE-PEG5K (5 mol%) modified CRX-601 liposomes were coated with MGC or CO, admixed with split-flu antigen and evaluated following SL vaccination of mice (Figure 4). All groups vaccinated SL with CRX-601 adjuvanted formulations exhibited strong functional HI titers with at least 6 of 8 mice seroconverting in each group (compared to naïve group for which none of the mice had detectable HI titers). When evaluated in combination with 25 μg MGC, both PE-PEG2K and PE-PEG5K modified liposome groups induced serum HI titers significantly higher than unmodified liposome treatment group (P < 0.005). CO appeared to have a weaker affect than MGC with lower overall titers when combined with liposomes with equivalent modification (e.g. for PE-PEG5K liposomes, 200 ± 48 with MGC compared to 133 ± 40 with CO) and did not provide a significant improvement over the unmodified liposome group. Notably, all mice seroconverted in the vehicle control group (PE-PEG2K liposomes) with 10 μg MGC, which is in agreement with the known adjuvant effect of chitosan [35]. Antigen specific mucosal IgA was readily detected, with vaginal wash IgA concentrations generally higher than tracheal wash IgA (Figure S6). Two vaccine groups with all mice (8/8) demonstrating measurable IgA levels were the PE-PEG2K and PE-PEG5K modified CRX-601 liposomes with 25 μg MGC; the same groups also exhibited the highest HI titers.
Figure 4. Flu specific titers following SL vaccination with MGC or CO coated phospholipid-PEG modified liposomes.
Serum HI titers from mice vaccinated with the indicated formulations. Serum samples were collected from mice on day 56 following vaccination on days 0, 21 and 42. The horizontal dashed line represents the titer necessary for seroconversion. Values less than the LLOQ at 1:20 sample dilution are represented as a value of 1. Data expressed as mean ± standard error (n = 8). **, P < 0.005, compared to unmodified liposome group; *, P <0.05, compared to 10 μg CO + PE-PEG2K liposome group.
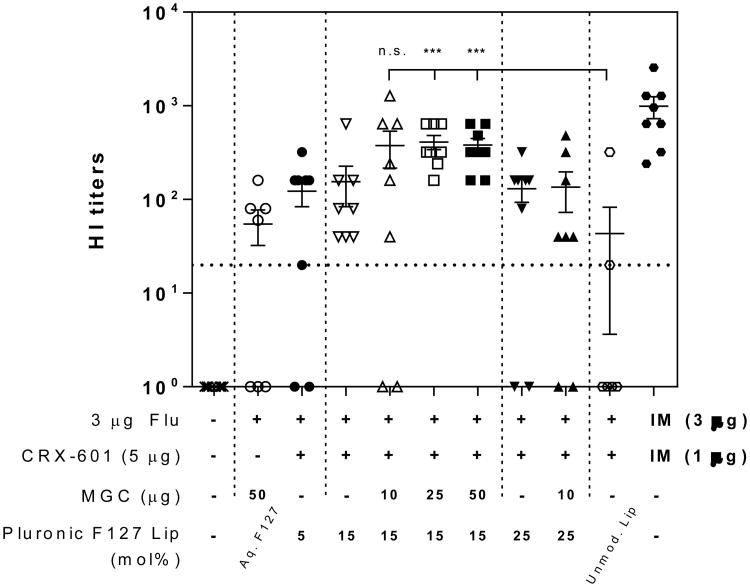
For in vivo evaluation of Pluronic liposomes co-formulated with chitosan, only F127 modified liposomes were tested (colloidally stable in presence of MGC and other derivatives) as L64 or F68 modified liposomes tended to aggregate and precipitate in presence of chitosan derivatives (Figure 1, 1S, Table 3). Functional HI titers for Pluronic F127 modified liposomes with 15 and 25 mol% modifications in combination with MGC are shown in Figure 5. MGC coated CRX-601/15% F127 Pluronic liposomes with 25 or 50 μg MGC elicited significantly higher HI titers compared to unmodified liposomes (P < 0.0005). Addition of MGC to CRX-601/15% F127 liposomes group led to increase in HI titers in each case, but this increase was not significant over CRX-601/15% F127 liposomes without MGC. No significant differences were noted amongst the three different amounts of MGC tested although titers with either 25 or 50 μg MGC demonstrated lower variability and were not significantly different from the IM positive control. Mucosal anti-flu IgA responses were detected in the tracheal and vaginal wash samples for some mice in all CRX-601 adjuvanted groups (Figure S7). Unlike the PE-PEG modified liposomes, none of the groups had 100% of mice with positive IgA levels (Figures S6 and S7). Thus, the combination of CRX-601 adjuvanted PEG modified (PE-PEG5K or Pluronic F127) liposomes with MGC resulted in highest functional antibody titers amongst the formulations evaluated within the scope of this work while PE-PEG5K plus MGC provided the most consistent mucosal IgA responses.
Figure 5. Flu specific titers following SL vaccination with MGC coated Pluronic F127 liposomes.
Serum functional antibody HI titers from mice vaccinated with the indicated formulations. Serum samples were collected from mice on day 56 following vaccination on days 0, 21 and 42. The horizontal dashed line represents the titer necessary for seroconversion. Values less than the LLOQ at 1:20 sample dilution are represented as a value of 1. Data expressed as mean ± standard error (n = 8). ***, P < 0.0005 compared to unmodified liposome group; n.s., not significant (P > 0.05) compared to unmodified liposome treatment group.
Discussion
While most vaccines continue to be administered intramuscularly or subcutaneously, further advancements in vaccines have focused on exploiting non-invasive routes of administration. Amongst the various mucosal routes, the SL surface in particular offers unique opportunities and challenges to vaccination. The relative thinness of SL mucosa allows rapid access to the underlying immune mediators, the effectiveness of which is evident by the success of SLIT [36]. Yet, the lack of successful SL vaccines against infectious diseases is indicative of the underlying challenge of overcoming the physical and immuno-tolerogenic barriers to delivery of such antigens. It has been envisioned that these limitations can be overcome using carrier mediated delivery of adjuvants and antigens across the SL mucosa. We have actively pursued the design of receptor specific chemically synthetic adjuvants and immunomodulators to trigger a specific immune response. CRX-601, a synthetic TLR4 adjuvant, was identified as a potent mediator of cellular and humoral immunity to a variety of antigens including detergent split influenza antigens [8]. In the present study, we have evaluated the potential of using CRX-601 as a SL adjuvant with a monovalent detergent split influenza vaccine. Engineered liposomes were used to improve CRX-601 safety and delivery across the SL mucosa.
The primary approach evaluated here relied on achieving dense PEG coating on liposome surface to achieve surface neutral liposomes. Such surface neutral nanoparticles [9-11, 28] and Pluronic F127 modified liposomes [13, 14] have previously been reported to be muco-inert/mucus penetrating, allowing rapid diffusion through the mucus to the underlying epithelium. Our evaluation of various PEG polymers indicates that phospholipid-PEG conjugates (PE-PEG2K/PE-PEG5K) were more efficient in achieving surface charge neutralization on CRX-601 loaded liposomes compared to Pluronic copolymers (Table 2). Liposome with as little as 1% PE-PEG modification had a significant reduction in surface charge which could only be achieved effectively with Pluronic F127 modification and to a lesser extent with L64 at 25% modification. While Pluronic copolymers have previously been reported to have limited incorporation into unilamelar vesicles [29], it is also a possibility that the presence of CRX-601, which likely resides in the lipid bilayer, further reduced the incorporation of Pluronic copolymers into the liposome membrane. Indeed, Pluronic modified liposomes prepared without CRX-601 (not shown) had a lower surface potential than those prepared with CRX-601. The stability profiles of Pluronic liposomes was also reduced compared to phospholipid-PEG modified liposomes with a tendency to phase separate over time (1 week – month) at all storage temperatures for all Pluronic modified liposomes. Phospholipid-PEG liposomes were stable at all time-points and temperatures of 2 – 8 °C and 25 °C for up to 4 weeks tested. The gelation phenomenon observed with F127 and PE-PEG5K liposomes at 40 °C relates to the decreased solubility of PEG chains at higher temperatures and has been reported previously [26, 32]. Furthermore, the destabilizing effect of Pluronics on liposomes was evident from evaluation of CRX-601 associated pyrogenicity in rabbits. While incorporation of CRX-601 into unmodified liposomes reduced its pyrogenicity 500-fold (Table 4), this reduction in pyrogenicity with Pluronic liposomes was 250-fold or less. Modification with L64, the most hydrophobic of the polymers tested, had the most destabilizing effect on liposomes and were pyrogenic at the lowest dose evaluated (250 ng/kg). Such membrane permeabilizing effect has previously been demonstrated with biological membranes by L64 [37] and with phosphatidylcholine liposomes by F127 [17]. Interestingly, F68 has widely been studied for injured membrane stabilization [38], but in our system did not show such an effect. This could be due to the high copolymer concentrations employed in our studies compared to the ones which reported a sealing effect [38]. No destabilizing effect was noted with phospholipid-PEGs, even at 25% modification. We therefore conclude that phospholipid-PEG conjugates are better suited for achieving dense PEG coating on the liposomal surface (with CRX-601) compared to Pluronic copolymers.
Furthermore, the steric stabilization effect achieved with phospholipid-PEGs and Pluronic F127 was instrumental in preparation of stable, sub-200 nm chitosan coated liposomes. Chitosan, a polysaccharide mucoadhesive, has extensively been employed for preparation of mucoadhesive-liposome systems [39-42]. These preparations rely on interaction of polycation charges on chitosan with the oppositely charged groups on the liposome surface to form polyelectrolyte complexes, yielding particles in the size range of 0.5 – 5 μm [42], [41]. However, the usefulness of these systems for SL delivery of an entrapped adjuvant (CRX-601) is limited by the fact that the average pore spacing for various mucus secretions has been reported to be less than 500 nm and is known to entrap particles in the micrometer range [43, 44]. In fact, our unmodified liposomes exhibited precipitation in presence of chitosan derivatives (Figure 1A, S1, and Table 3) and were therefore not suitable for evaluation with chitosan derivatives. L64 and F68 modified liposomes formed aggregates in presence of chitosan derivatives as well (Figure 1, S1, S2, and Table 3). Only the formulations which exhibited a significant reduction in charge (phospholipid-PEG or Pluronic F127 modified liposomes) were resistant to chitosan induced aggregation (Figure 1, S1, S2, and Table 3). Thus it appears that the more neutral formulations are sterically stabilized and are resistant to chitosan induced aggregation whereas the unmodified, L64 and F68 modified liposomes are charge stabilized and form aggregates upon chitosan induced neutralization of their repulsive charge. Of note, the interaction of these relatively surface neutral liposomes (phospholipid-PEG or Pluronic F127 modified liposomes) with chitosan derivatives, as shown for MGC (Figure 1, S2, S3), albeit reduced, was not completely abolished due to steric effects. This is evidenced by a small increase in hydrodynamic diameter and reversal of surface charge in each of the preparations. To our knowledge, this represents the first report utilizing sterically stabilized liposomes for preparation of mucoadhesive chitosan formulations in the sub-200 nm range. We envision this approach could be useful for mucosal delivery of complex bioactive payloads like CRX-601.
In addition to the physiochemical and analytical assessments of these promising mucosal adjuvant delivery systems, the comparative and combinatorial evaluation of the contrasting muco- penetrating and adhesive formulation strategies were evaluated for SL vaccine delivery in combination with a detergent split influenza vaccine. Overall, strong mucosal IgA responses were detected at both local (tracheal wash) and distal (vaginal wash) mucosal sites with all CRX-601 adjuvanted liposome formulations delivered SL. Some of the PEG modified CRX-601 liposome formulations matched the benchmark IM control for serum IgG and HI titers. Notably, the IM benchmark control used in these studies was adjuvanted with CRX-601 which is not a component of flu vaccine approved for human use, thereby increasing serum titers from what would be expected from the vaccine without CRX-601 (data not shown). In general, a boost in flu-specific serum IgG concentration between 2nd and 3rd vaccinations was observed for most formulations with a good correlation amongst individual groups (data not shown). The average improvement in IgG levels between 2nd and 3rd vaccination was 3.5 fold for SL groups and 2.3 fold for IM groups. High variability in SL responses was observed for our unmodified liposome formulation, which fluctuated greatly both within and amongst multiple studies (Figure 2A vs. Figure 3A). A beneficial aspect of modified liposomes in this regard was a more consistent immune response between multiple studies. With regards to the liposome modification with PEG polymers, an increase in serum anti-flu response was detected with some of our phospholipid-PEG and Pluronic modified liposome formulations compared to unmodified liposomes. In the absence of chitosan derivatives, a significant improvement could only be detected for CRX-601/25% PE-PEG5K modified liposomes compared to unmodified liposomes (Figure 2). It is interesting to note that 25 mol% PE-PEG5K modified liposomes were the most surface neutral and their enhanced activity could relate to the reduced ζ-potential as suggested previously to be crucial for muco-inertness [45]. Amongst the Pluronic modified liposomes, surprisingly titers were highest for the L64 liposome groups rather than the more surface neutral F127 liposomes (Figure 3). Considering the limited incorporation and destabilization of liposomes observed with L64, this effect could be mediated by processes other than increased diffusion of modified liposomes through the unaltered mucus. Surfactants can interact with biological membranes as well as alter the rheology of the mucus, making it less viscous and pervious to drug delivery [46]. The interaction of Pluronic copolymers, in particular L64, with biological membranes is well known and has been utilized for transfection of DNA vaccines [15]. We hypothesize that the improved mucosal delivery (in consideration of the enhanced SL effect) observed in our work could in part be due to the effects of free or desorbed Pluronic on mucus rheology and/or epithelial permeability.
Chitosan derivatives with improved solubility at neutral pH were chosen for preparation of mucoadhesive type liposome formulations. Methylglycol chitosan provided the most consistent formulations in terms of physicochemical characteristics and was also more effective than chitosan oligosaccharide lactate in its in vivo performance (Figure 4). With both PE-PEG5K and PE-PEG2K modified liposomes, a combination with MGC resulted in titers significantly higher (P < 0.05) than unmodified CRX-601 liposomes vaccinated mice (Figure 4). Similarly, addition of MGC to CRX-601/15 mol% F127 modified liposomes resulted in a significant improvement (P < 0.05) over unmodified CRX-601 liposomes (Figure 5). The top performing formulations were therefore combinations of PEG modified CRX-601 liposomes with MGC. The specific mechanisms for improved immunity are not known but could be related to retention of adjuvant-liposomes on the SL surface or more complex set of interactions involving retention and permeation of the flu antigen. It has been reported previously that interaction of chitosan with mucus can result in mucin aggregation due to suppression of its sialic acid group ionization [47]. This in principle could result in alteration of mucus barrier properties, allowing access for the muco-inert formulation to the underlying epithelium. Nonetheless, these results highlight the possibility of achieving a synergistic effect by combining novel muco-inert nanoparticles in combination with conventional mucoadhesives for sublingual delivery which bears implications for delivery through other mucosal sites as well, and thus needs further evaluation.
Conclusions
This study demonstrates that SL administration of detergent split influenza vaccine adjuvanted with CRX-601 in engineered liposomal carriers can elicit a systemic immune response comparable to or exceeding IM immunization. Moreover, SL vaccination elicits a flu-specific mucosal IgA response not produced by IM administration of the vaccine (with or without CRX-601). While modest improvement in response over unmodified CRX-601 liposomes could be observed for the surface neutral PE-PEG modified and Pluronic F127 modified liposomes carrying the adjuvant CRX-601, the combination of these formulations with the mucoadhesive MGC induced the most robust and consistent immune response. Such combination approaches could be the key to successful development of mucosal vaccines with complex payloads, and hence warrants further exploration.
Supplementary Material
Supplementary figure S1: Stability of CRX-601 liposomes in presence of MGC. Photographs of various liposome formulations (5 mol% modification) incubated with MGC (5 mg/mL) for 24 h. Unmodified, L64, and F68 modified liposomes formed visible aggregates. PE-PEG2K, PE-PEG5K, and F127 modified liposomes were stable against MGC induced aggregation.
Supplementary figure S2: Characterization of adjuvant loaded phospholipid-PEG liposomes in presence methylglycol chitosan (MGC). Size/PDI and ζ-potential values with increasing concentration of MGC for adjuvant-loaded 1% (A) and 25% (B) PE-PEG2K and PE-PEG5K modified liposomes. Sizes are plotted as bars and PDI values as dot plot. Data are expressed as mean ± SD, (n = 2) for 1 mol% modification (top row) and n = 1 for 25 mol% modification (bottom row). Particles in the μm size range tended to precipitate over time.
Supplementary figure S3: Characterization of adjuvant loaded Pluronic liposomes in presence methylglycol chitosan (MGC). Size/PDI and ζ-potential values with increasing concentration of MGC for adjuvant-loaded 15% (A) and 25% (B) Pluronic L64, F68 and F127 modified liposomes. Sizes are plotted as bars and PDI values as dot plot. Data are expressed as mean ± SD, (n = 2) for 1 mol% modification (top row) and n = 1 for 25 mol% modification (bottom row). Particles in the μm size range tended to precipitate over time.
Supplementary figure S4: Flu specific IgG concentration following SL vaccination with phospholipid-PEG modified liposomes. Serum anti-flu IgG concentration 14 days post-tertiary vaccination (day 56) from mice vaccinated with the indicated formulations. Values less than LLOQ at 1:100 sample dilutions are represented as a value of 1. Data expressed as mean ± standard error (n = 8). **, P < 0.005, compared to unmodified liposome group; n.s., not significant (P > 0.05) compared to unmodified liposome treatment group; N.S., not significant (P > 0.05) between the indicated groups. See material and methods for experimental details.
Supplementary figure S5: Flu specific IgG concentration following SL vaccination with Pluronic modified liposomes. Serum anti-flu IgG concentration 14 days post-tertiary vaccination (day 56) from mice vaccinated with the indicated formulations. Values less than LLOQ at 1:100 sample dilutions are represented as a value of 1. Data expressed as mean ± standard error (n = 8). **, P < 0.005; N.S., not significant (P > 0.05) for the indicated groups; n.s., not significant (P > 0.05) compared to unmodified liposome treatment group. See material and methods for experimental details.
Supplementary figure S6: Flu specific IgA concentration following SL vaccination with chitosan coated phospholipid-PEG modified liposomes. Mucosal wash IgA concentration from mice vaccinated with the indicated formulations. Values less than LLOQ at 1:100 sample dilutions are represented as a value of 1. Anti-flu IgA levels in vaginal wash and tracheal wash samples from mice vaccinated with the indicated formulations are shown as black circles (●) and grey squares (■), respectively. The mean and standard error are indicated. See material and methods for experimental details.
Supplementary figure S7: Flu specific IgA concentration following SL vaccination with MGC coated Pluronic F127 modified liposomes. Mucosal wash IgA concentration from mice vaccinated with the indicated formulations. Values less than LLOQ at 1:100 sample dilutions are represented as a value of 1. Anti-flu IgA levels in vaginal wash and tracheal wash samples from mice vaccinated with the indicated formulations are shown as black circles (●) and grey squares (■), respectively. The mean and standard error are indicated. See material and methods for experimental details.
Acknowledgments
This work was supported in part by the National Institute of Allergy and Infectious Diseases (NIAID) under contract HHSN272200900008C (to Corixa Corporation d/b/a GlaxoSmithKline Biologicals SA). Any opinions, findings, conclusions or recommendations expressed in this article are those of the authors and do not necessarily reflect the views of the NIAID.
We thank Jon Ward, Danielle Poirier, Margaret Whitacre, Connie Mullen and Cindy Nilles for their technical support. We thank Kendal Ryter and George Ettenger for synthesis of CRX-601, and Angela Jockheck-Clark for comments and edits on this manuscript.
Footnotes
Authorship: H.O., Y.Y., A.M., J.E., and D.B were involved in the conception of the study and critical analysis of data. All authors contributed to the design and performance of experiments. All authors wrote and edited the manuscript.
Conflict of Interest Disclosure: All authors were employees of the GSK group of companies during performance of the work reported herein.
Note on Trademarks: SLITone® and Grazax® are trademark names of ALK-Abelló, Sublivac® is a trademark name of HAL Allergy Group, Oralair®, is a trademark name of Stallergenes S.A and AllerSlit®forte is a trademark name of Allergopharma GmbH & Co. KG.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Garçon N, Van Mechelen M. Recent clinical experience with vaccines using MPL- and QS-21-containing Adjuvant Systems. Expert Rev Vaccines. 2011;10:471–486. doi: 10.1586/erv.11.29. [DOI] [PubMed] [Google Scholar]
- 2.Kraan H, Vrieling H, Czerkinsky C, Jiskoot W, Kersten G, Amorij JP. Buccal and sublingual vaccine delivery. Journal of controlled release : official journal of the Controlled Release Society. 2014;190:580–592. doi: 10.1016/j.jconrel.2014.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song JH, Kim JI, Kwon HJ, Shim DH, Parajuli N, Cuburu N, Czerkinsky C, Kweon MN. CCR7-CCL19/CCL21-regulated dendritic cells are responsible for effectiveness of sublingual vaccination. Journal of immunology (Baltimore, Md : 1950) 2009;182:6851–6860. doi: 10.4049/jimmunol.0803568. [DOI] [PubMed] [Google Scholar]
- 4.Hervouet C, Luci C, Bekri S, Juhel T, Bihl F, Braud VM, Czerkinsky C, Anjuere F. Antigen-bearing dendritic cells from the sublingual mucosa recirculate to distant systemic lymphoid organs to prime mucosal CD8 T cells. Mucosal immunology. 2014;7:280–291. doi: 10.1038/mi.2013.45. [DOI] [PubMed] [Google Scholar]
- 5.Shim BS, Choi Y, Cheon IS, Song MK. Sublingual delivery of vaccines for the induction of mucosal immunity. Immune network. 2013;13:81–85. doi: 10.4110/in.2013.13.3.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lombardi V, Van Overtvelt L, Horiot S, Moussu H, Chabre H, Louise A, Balazuc AM, Mascarell L, Moingeon P. Toll-like receptor 2 agonist Pam3CSK4 enhances the induction of antigen-specific tolerance via the sublingual route. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2008;38:1819–1829. doi: 10.1111/j.1365-2222.2008.03056.x. [DOI] [PubMed] [Google Scholar]
- 7.Sun JB, Czerkinsky C, Holmgren J. Sublingual ‘oral tolerance’ induction with antigen conjugated to cholera toxin B subunit generates regulatory T cells that induce apoptosis and depletion of effector T cells. Scand J Immunol. 2007;66:278–286. doi: 10.1111/j.1365-3083.2007.01975.x. [DOI] [PubMed] [Google Scholar]
- 8.Maroof A, Yorgensen YM, Li Y, Evans JT. Intranasal vaccination promotes detrimental Th17-mediated immunity against influenza infection. PLoS pathogens. 2014;10:e1003875. doi: 10.1371/journal.ppat.1003875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lai SK, Wang YY, Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Advanced drug delivery reviews. 2009;61:158–171. doi: 10.1016/j.addr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ensign LM, Tang BC, Wang YY, Tse TA, Hoen T, Cone R, Hanes J. Mucus-penetrating nanoparticles for vaginal drug delivery protect against herpes simplex virus. Science translational medicine. 2012;4:138ra179. doi: 10.1126/scitranslmed.3003453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lai SK, O'Hanlon DE, Harrold S, Man ST, Wang YY, Cone R, Hanes J. Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:1482–1487. doi: 10.1073/pnas.0608611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang BC, Dawson M, Lai SK, Wang YY, Suk JS, Yang M, Zeitlin P, Boyle MP, Fu J, Hanes J. Biodegradable polymer nanoparticles that rapidly penetrate the human mucus barrier. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:19268–19273. doi: 10.1073/pnas.0905998106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen D, Xia D, Li X, Zhu Q, Yu H, Zhu C, Gan Y. Comparative study of Pluronic(®) F127-modified liposomes and chitosan-modified liposomes for mucus penetration and oral absorption of cyclosporine A in rats. International journal of pharmaceutics. 2013;449:1–9. doi: 10.1016/j.ijpharm.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Chen D, Le C, Zhu C, Gan Y, Hovgaard L, Yang M. Novel mucus-penetrating liposomes as a potential oral drug delivery system: preparation, in vitro characterization, and enhanced cellular uptake. International journal of nanomedicine. 2011;6:3151–3162. doi: 10.2147/IJN.S25741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gau-Racine J, Lal J, Zeghal M, Auvray L. PEO-PPO block copolymer vectors do not interact directly with DNA but with lipid membranes. The journal of physical chemistry B. 2007;111:9900–9907. doi: 10.1021/jp0687302. [DOI] [PubMed] [Google Scholar]
- 16.Woodle MC, Newman MS, Martin FJ. Liposome leakage and blood circulation: Comparison of adsorbed block copolymers with covalent attachment of PEG. International journal of pharmaceutics. 1992;88:327–334. [Google Scholar]
- 17.Johnsson M, Silvander M, Karlsson G, Edwards K. Effect of PEO-PPO-PEO triblock copolymers on structure and stability of phosphatidylcholine liposomes. Langmuir. 1999;15:6314–6325. [Google Scholar]
- 18.Jamshaid M, Farr SJ, Kearney P, Kellaway IW. Poloxamer sorption on liposomes: comparison with polystyrene latex and influence on solute efflux. International journal of pharmaceutics. 1998;48:125–131. [Google Scholar]
- 19.Demina T, Grozdova I, Krylova O, Zhirnov A, Istratov V, Frey H, Kautz H, Melik-Nubarov N. Relationship between the structure of amphiphilic copolymers and their ability to disturb lipid bilayers. Biochemistry. 2005;44:4042–4054. doi: 10.1021/bi048373q. [DOI] [PubMed] [Google Scholar]
- 20.Takeuchi H, Yamamoto H, Kawashima Y. Mucoadhesive nanoparticulate systems for peptide drug delivery. Advanced drug delivery reviews. 2001;47:39–54. doi: 10.1016/s0169-409x(00)00120-4. [DOI] [PubMed] [Google Scholar]
- 21.Takeuchi H, Yamamoto H, Niwa T, Hino T, Kawashima Y, Henriksen I, Smistad G, Karlsen J. Enteral absorption of insulin in rats from mucoadhesive Chitosan-Coated liposomes. Pharmaceutical research. 1996;13:896–901. doi: 10.1023/a:1016009313548. [DOI] [PubMed] [Google Scholar]
- 22.Henriksen I, Smistad G, Karlsen J. Interactions between liposomes and chitosan. International journal of pharmaceutics. 1994;101:227–236. [Google Scholar]
- 23.Smith J, Wood E, Dornish M. Effect of chitosan on epithelial cell tight junctions. Pharmaceutical research. 2004;21:43–49. doi: 10.1023/b:pham.0000012150.60180.e3. [DOI] [PubMed] [Google Scholar]
- 24.Bazin HG, Murray TJ, Bowen WS, Mozaffarian A, Fling SP, Bess LS, Livesay MT, Arnold JS, Johnson CL, Ryter KT, Cluff CW, Evans JT, Johnson DA. The ‘Ethereal’ nature of TLR4 agonism and antagonism in the AGP class of lipid A mimetics. Bioorganic & medicinal chemistry letters. 2008;18:5350–5354. doi: 10.1016/j.bmcl.2008.09.060. [DOI] [PubMed] [Google Scholar]
- 25.Sezgin Z, Yüksel N, Baykara T. Preparation and characterization of polymeric micelles for solubilization of poorly soluble anticancer drugs. Eur J Pharm Biopharm. 2006;64:261–268. doi: 10.1016/j.ejpb.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Alexandridis P, Alan Hatton T. Poly(ethylene oxide)poly(propylene oxide)poly(ethylene oxide) block copolymer surfactants in aqueous solutions and at interfaces: thermodynamics, structure, dynamics, and modeling. Colloids Surf A Physicochem Eng Asp. 1995;96:1–46. [Google Scholar]
- 27.Immordino ML, Dosio F, Cattel L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. International journal of nanomedicine. 2006;1:297–315. [PMC free article] [PubMed] [Google Scholar]
- 28.Cu Y, Saltzman WM. Controlled surface modification with poly(ethylene)glycol enhances diffusion of PLGA nanoparticles in human cervical mucus. Mol Pharm. 2009;6:173–181. doi: 10.1021/mp8001254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moghimi SM, Porter CJH, Illum L, Davis SS. The effect of poloxamer-407 on liposome stability and targeting to bone marrow: comparison with polystyrene microspheres. International journal of pharmaceutics. 1991;68:121–126. [Google Scholar]
- 30.Liu W, Sun S, Cao Z, Zhang X, Yao K, Lu WW, Luk KD. An investigation on the physicochemical properties of chitosan/DNA polyelectrolyte complexes. Biomaterials. 2005;26:2705–2711. doi: 10.1016/j.biomaterials.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 31.Schipper NG, Olsson S, Hoogstraate JA, deBoer AG, Varum KM, Artursson P. Chitosans as absorption enhancers for poorly absorbable drugs 2: mechanism of absorption enhancement. Pharmaceutical research. 1997;14:923–929. doi: 10.1023/a:1012160102740. [DOI] [PubMed] [Google Scholar]
- 32.Bromberg LE, Ron ES. Temperature-responsive gels and thermogelling polymer matrices for protein and peptide delivery. Advanced drug delivery reviews. 1998;31:197–221. doi: 10.1016/s0169-409x(97)00121-x. [DOI] [PubMed] [Google Scholar]
- 33.Sang H, Pisarev VM, Munger C, Robinson S, Chavez J, Hatcher L, Parajuli P, Guo Y, Talmadge JE. Regional, but not systemic recruitment/expansion of dendritic cells by a pluronic-formulated Flt3-ligand plasmid with vaccine adjuvant activity. Vaccine. 2003;21:3019–3029. doi: 10.1016/s0264-410x(03)00143-9. [DOI] [PubMed] [Google Scholar]
- 34.Jain-Gupta N, Contreras-Rodriguez A, Vemulapalli R, Witonsky SG, Boyle SM, Sriranganathan N. Pluronic P85 enhances the efficacy of outer membrane vesicles as a subunit vaccine against Brucella melitensis challenge in mice. FEMS Immunol Med Microbiol. 2012;66:436–444. doi: 10.1111/1574-695X.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X, Min M, Du N, Gu Y, Hode T, Naylor M, Chen D, Nordquist RE, Chen WR. Chitin, chitosan, and glycated chitosan regulate immune responses: The novel adjuvants for cancer vaccine. Clin Dev Immunol. 2013;2013 doi: 10.1155/2013/387023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valenta R, Campana R, Marth K, van Hage M. Allergen-specific immunotherapy: from therapeutic vaccines to prophylactic approaches. Journal of internal medicine. 2012;272:144–157. doi: 10.1111/j.1365-2796.2012.02556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pembouong G, Morellet N, Kral T, Hof M, Scherman D, Bureau MF, Mignet N. A comprehensive study in triblock copolymer membrane interaction. Journal of controlled release : official journal of the Controlled Release Society. 2011;151:57–64. doi: 10.1016/j.jconrel.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 38.Moloughney JG, Weisleder N. Poloxamer 188 (p188) as a membrane resealing reagent in biomedical applications. Recent patents on biotechnology. 2012;6:200–211. doi: 10.2174/1872208311206030200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khatri K, Goyal AK, Gupta PN, Mishra N, Mehta A, Vyas SP. Surface modified liposomes for nasal delivery of DNA vaccine. Vaccine. 2008;26:2225–2233. doi: 10.1016/j.vaccine.2008.02.058. [DOI] [PubMed] [Google Scholar]
- 40.Gradauer K, Vonach C, Leitinger G, Kolb D, Frohlich E, Roblegg E, Bernkop-Schnurch A, Prassl R. Chemical coupling of thiolated chitosan to preformed liposomes improves mucoadhesive properties. International journal of nanomedicine. 2012;7:2523–2534. doi: 10.2147/IJN.S29980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Werle M, Takeuchi H. Chitosan-aprotinin coated liposomes for oral peptide delivery: Development, characterisation and in vivo evaluation. International journal of pharmaceutics. 2009;370:26–32. doi: 10.1016/j.ijpharm.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 42.Zaru M, Manca ML, Fadda AM, Antimisiaris SG. Chitosan-coated liposomes for delivery to lungs by nebulisation. Colloids and surfaces B, Biointerfaces. 2009;71:88–95. doi: 10.1016/j.colsurfb.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 43.Olmsted SS, Padgett JL, Yudin AI, Whaley KJ, Moench TR, Cone RA. Diffusion of macromolecules and virus-like particles in human cervical mucus. Biophysical journal. 2001;81:1930–1937. doi: 10.1016/S0006-3495(01)75844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cone RA. Barrier properties of mucus. Advanced drug delivery reviews. 2009;61:75–85. doi: 10.1016/j.addr.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 45.Wang YY, Lai SK, Suk JS, Pace A, Cone R, Hanes J. Addressing the PEG mucoadhesivity paradox to engineer nanoparticles that “slip” through the human mucus barrier. Angewandte Chemie (International ed in English) 2008;47:9726–9729. doi: 10.1002/anie.200803526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parthasarathy G, Bhaskar K, Jayaveera KN, Prasanth VV. Buccal mucosa a gifted choice for systemic drug delivery. International Journal of Drug Delivery. 2011;3:586–596. [Google Scholar]
- 47.Sogias IA, Williams AC, Khutoryanskiy VV. Why is chitosan mucoadhesive? Biomacromolecules. 2008;9:1837–1842. doi: 10.1021/bm800276d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure S1: Stability of CRX-601 liposomes in presence of MGC. Photographs of various liposome formulations (5 mol% modification) incubated with MGC (5 mg/mL) for 24 h. Unmodified, L64, and F68 modified liposomes formed visible aggregates. PE-PEG2K, PE-PEG5K, and F127 modified liposomes were stable against MGC induced aggregation.
Supplementary figure S2: Characterization of adjuvant loaded phospholipid-PEG liposomes in presence methylglycol chitosan (MGC). Size/PDI and ζ-potential values with increasing concentration of MGC for adjuvant-loaded 1% (A) and 25% (B) PE-PEG2K and PE-PEG5K modified liposomes. Sizes are plotted as bars and PDI values as dot plot. Data are expressed as mean ± SD, (n = 2) for 1 mol% modification (top row) and n = 1 for 25 mol% modification (bottom row). Particles in the μm size range tended to precipitate over time.
Supplementary figure S3: Characterization of adjuvant loaded Pluronic liposomes in presence methylglycol chitosan (MGC). Size/PDI and ζ-potential values with increasing concentration of MGC for adjuvant-loaded 15% (A) and 25% (B) Pluronic L64, F68 and F127 modified liposomes. Sizes are plotted as bars and PDI values as dot plot. Data are expressed as mean ± SD, (n = 2) for 1 mol% modification (top row) and n = 1 for 25 mol% modification (bottom row). Particles in the μm size range tended to precipitate over time.
Supplementary figure S4: Flu specific IgG concentration following SL vaccination with phospholipid-PEG modified liposomes. Serum anti-flu IgG concentration 14 days post-tertiary vaccination (day 56) from mice vaccinated with the indicated formulations. Values less than LLOQ at 1:100 sample dilutions are represented as a value of 1. Data expressed as mean ± standard error (n = 8). **, P < 0.005, compared to unmodified liposome group; n.s., not significant (P > 0.05) compared to unmodified liposome treatment group; N.S., not significant (P > 0.05) between the indicated groups. See material and methods for experimental details.
Supplementary figure S5: Flu specific IgG concentration following SL vaccination with Pluronic modified liposomes. Serum anti-flu IgG concentration 14 days post-tertiary vaccination (day 56) from mice vaccinated with the indicated formulations. Values less than LLOQ at 1:100 sample dilutions are represented as a value of 1. Data expressed as mean ± standard error (n = 8). **, P < 0.005; N.S., not significant (P > 0.05) for the indicated groups; n.s., not significant (P > 0.05) compared to unmodified liposome treatment group. See material and methods for experimental details.
Supplementary figure S6: Flu specific IgA concentration following SL vaccination with chitosan coated phospholipid-PEG modified liposomes. Mucosal wash IgA concentration from mice vaccinated with the indicated formulations. Values less than LLOQ at 1:100 sample dilutions are represented as a value of 1. Anti-flu IgA levels in vaginal wash and tracheal wash samples from mice vaccinated with the indicated formulations are shown as black circles (●) and grey squares (■), respectively. The mean and standard error are indicated. See material and methods for experimental details.
Supplementary figure S7: Flu specific IgA concentration following SL vaccination with MGC coated Pluronic F127 modified liposomes. Mucosal wash IgA concentration from mice vaccinated with the indicated formulations. Values less than LLOQ at 1:100 sample dilutions are represented as a value of 1. Anti-flu IgA levels in vaginal wash and tracheal wash samples from mice vaccinated with the indicated formulations are shown as black circles (●) and grey squares (■), respectively. The mean and standard error are indicated. See material and methods for experimental details.