Abstract
Steroid abuse is a growing problem among amateur and professional athletes. Because of an inundation of newly and illegally synthesized steroids with minor structural modifications and other designer steroid receptor modulators, there is a need to develop new methods of detection which do not require prior knowledge of the abused steroid structure. The number of designer steroids currently being abused is unknown because detection methods in general are only identifying substances with a known structure. The detection of doping is moving away from merely checking for exposure to prohibited substance toward detecting an effect of prohibited substances, as biological assays can do. Cell-based biological assays are the next generation of assays which should be utilized by antidoping laboratories; they can detect androgenic anabolic steroid and other human androgen receptor (hAR) ligand presence without knowledge of their structure and assess the relative biological activity of these compounds. This review summarizes the hAR and its action and discusses its relevance to sports doping and its use in biological assays.
Introduction
The impetus to gain an edge in competitive sporting events has existed for as long as the sports themselves. Today, not only do athletes strive to be the best in their chosen sports, but there are also large financial incentives and outside pressures to succeed associated with the international sporting industry; these reasons have lead to a constant increase in the use of performance enhancing drugs (1). Despite centuries of reports of using substances to enhance athletic performance, systematic testing of athletes for the use of performance enhancing drugs began only in 1968 (1,2). Since that time, a list of banned substances and procedures has been maintained and constantly updated by the International Olympic Committee (IOC) and the World Anti-doping Agency (WADA). The compounds and methods included on the list are those that can be used by athletes to provide an unfair advantage (3). Substances on the prohibited list include anabolic androgenic steroids, glucocorticosteroids, peptide hormones and their modulators, hormone antagonists and their modulators, stimulants, β2agonists, narcotics, alcohol, β-blockers, cannabinoids, and diuretics and masking agents (3).
Anabolic androgenic steroids (AAS) and other anabolic agents are by far the most widely abused substances included on the prohibited substances list, accounting for approximately 65% of all positive samples (both adverse and atypical findings) in 2009 (the most recent year for which official data are available) (4). The current regulations, instead of curtailing the use of AAS, have led to their clandestine production and the black market synthesis and sale of structurally unique synthetic steroids as well as other nonsteroidal compounds that modulate steroid receptors to increase endogenous anabolic processes. These compounds are produced so abusers can evade detection and identification of these substances with current analytical procedures.
The term AAS refers to testosterone and its derivatives and analogues and SARMS which bind to the human androgen receptor (hAR). Endogenous AAS primary role is the maintenance of male sexual organs (androgenic effects); activation of the hAR by AAS may also result in an increase in muscle mass and strength (anabolic effects). Clinically, AAS are used for the treatment hypogonadism, impotence, and muscle wasting disorders; they are also abused by athletes for their anabolic properties. Major problems with the abuse of endogenous AAS, such as testosterone or dihydrotestosterone, are their high metabolism and serious side effects (5–7). Synthetic AAS are manufactured to reduce metabolism and increase potency (6,8). AAS are also synthesized to circumvent typical detection methods such as mass spectrometry (MS). Minor structural modifications of a steroid can render it undetectable via conventional means yet allow it to maintain its anabolic potential, as was the case with tetrahydrogestrinone (THG) (9).The 2004 scandal in which a supposed “undetectable steroid”, later identified as THG, was discovered has brought the problem of detecting AAS and other steroid abuse to light (9–13). The number of designer steroids currently being abused is unknown because detection methods are only identifying substances with a known structure.
Current techniques for the detection of sports doping, such as gas chromatography (GC)–MS, rely on prior knowledge of the structure of the steroid. These target methods are used in anti-doping laboratories to detect the presence of low concentrations of known prohibited substances. However, because new steroids and synthetic compounds are made to evade conventional testing methods while retaining desired anabolic activity, new assays need to be developed to detect excess levels of these substances (11,12). Some research developments have recently been made to overcome some of the pitfalls of known target analysis; these methods involve more sophisticated use of MS technology, including full-scan liquid chromatography (LC)– and GC–electrospray ionization orthogonal acceleration time-of-flight MS, full scan LC–time-of-flight MS, and precursor ion scanning after LC–electrospray-tandem MS (14–16). Although very beneficial, it is still possible that these methods may miss newly developed compounds. The next generation of detection methods, as the field moves away from checking for exposure to prohibited substance toward detecting an effect of prohibited substances, will not require knowledge of the exact structure of the compound and will employ biologically based assays utilizing the hAR and other steroid receptors. Biological assays also have other applications beyond the identification of steroid receptor ligands for antidoping laboratories: they can be used alongside MS to determine the structure of new ligands, they can be utilized to determine the relative biological activity of steroid receptor ligands, and they can be coupled with microsomal metabolism studies to assess the biological activity of compound metabolites. The objective of this paper is to briefly review the androgen receptor and its action and discuss current assays being developed using the hAR.
Steroid Hormone Receptors
Steroid hormone receptors (SHRs) are members of the steroid and nuclear receptor superfamily (17). This superfamily has over 100 members, only 5 of which are SHRs: estrogen, androgen, glucocorticoid, progesterone, and mineralocorticoid (18). SHRs are located in the cytosol and in the nucleus of target cells and also on the plasma membrane. They are typically cytoplasmic and nuclear transcription factors and after ligand binding initiate signal transduction which leads to changes in gene expression.
Androgen Receptor
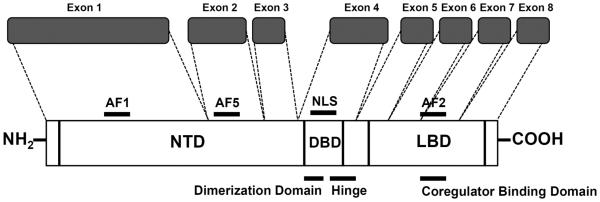
Similar to the other SHRs, the hAR functions as a transcription factor and is typically regulated by specific steroid ligands, such androgens and selective androgen receptor modulators (SARMs). The hAR cDNA (GenBank ID NM_000044) is approximately 2.8 kilobases and the eight exons code for 919 amino acids (approximately 112 kDa) (19–21). The hAR has a characteristic structure consisting of several domains: two activation functions (AFl and AF5) in the N-terminal domain (NTD), DNA-binding domain (DBD) which contains the dimerization domain, a nuclear localization signal (NLS), hinge region, and a carboxy-terminal ligand-binding domain (LBD) which contains a third activation function domain (AF2) (17,21–22) (Figure 1). AFl and AF2 are modulatory regions that are involved in accessory protein binding dependent on the conformation of the receptor after ligand binding. AF5 operates in a ligand-independent manner (22). Claessens et al. (23) review and describe the current knowledge on the structurefunction relationships within the domains of the hAR.
Figure 1.
Structure of hAR gene. Domains of the gene and receptor; NTD, N-terminal domain; AF1/2/5, activation functions 1, 2, and 5; NLS, nuclear localization signal; DBD, DNA binding domain; and LBD, ligand binding domain. Modified from Gao et al. (21).
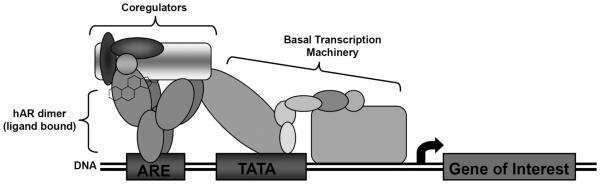
The hAR undergoes what has been called the two-step model of steroid action. In this model, inactive steroid receptors (referred to as untransformed) are associated with several chaperone proteins (including the heat shock proteins hsp90, hsp70, and hsp53, as well as p23 and immunophilins) via interactions in the LBD (24–25). The dissociation of some of these chaperone proteins results in a transformed steroid receptor with exposed DBD (which contains the dimerization domain) and LBD. Transformed receptors remain bound to hsp90, as hsp90 is required for proper LBD conformation for ligand binding. Transformed steroid receptors become activated when ligands bind and cause the loss of hsp90. Additionally, the structure of the NTD changes upon ligand binding (26) exposing a flexible region for the recruitment and assembly of coregulator proteins and transcriptional machinery. The conformational changes which occur in this region of the hAR may serve as the primary mediator of the cell and gene specific effects of androgens (26). With the loss of hsp90, the ligand-dependent NLS is exposed (note that steroid receptors can dimerize while transformed or activated) (24–25,27); the ligand-dependent NLS causes translocation of the receptor to the nucleus. Ligand-dependent NLSs of SHRs have varying strengths and cause differential nuclear translocation. Once at the nucleus, import is facilitated at the nuclear pore complex via the RanGTP-dependent importin α/β system (28–29). When inside the nucleus, translocated receptor dimers recruit transcriptional binding partners (coregulators). This receptor dimer-coregulator complex binds to hormone promoters in DNA, associates with basal transcriptional machinery, and initiates transcription of various genes involved in anabolic processes (Figure 2) (21).
Figure 2.
Structure of hAR protein. Activated hAR dimer initiating transcription via an ARE with coregulator proteins bound; ARE, androgen response element. Modified from Nettles and Greene (49).
The hAR associates with a specific DNA sequences termed androgen response elements (AREs). AREs are characterized by six-nucleotide half-site consensus sequences spaced by three random nucleotides in the promoter region of target genes: 5'-TGTTCT-3' (21,23,30–32). There are also several hormone response elements (HREs) which can bind to several (or all) steroid receptors, including the mouse mammary tumor virus promoter (MMTV), and some glucocorticoid response elements (GREs) (31,33). Steroid receptor export from the nucleus occurs at the nuclear pore complex and is facilitated via the RanGTP-dependent CRMl system (28,29).
It should be noted that in the absence of ligand, steroid receptors are in a steady state between the untransformed and transformed (transformed receptors may or may not be active and associated with hsp90) states and constantly shuttling into and out of the nucleus (though, in the absence a ligand, hAR remains in a steady state in which most of the receptor is cytosolic) (24,34). Transformation can be reversed by the chaperone system’s ATP-dependent reassociation with the steroid receptor. Association of hsp90 is also reversible via an ATP-dependent mechanism (24).
The hAR is expressed at varying levels in all major physiological systems including the central nervous, endocrine, metabolic, gastrointestinal, immune, reproductive, cardiovascular, and respiratory systems. Most tissues have low-to-moderate expression levels; however, there are high expression levels in the epididymis, ovary, uterus, prostate, vas deferens, adrenal gland, kidney, and skeletal muscle (35). Several different cancers, cardiovascular defects, neurological conditions, immune diseases, reproductive conditions, and psychiatric disorders have been associated with hAR dysfunction.
Human satellite cells are stem cells involved in the repair and maintenance of skeletal muscle and have been proposed as the primary site of the anabolic action of AAS (36–37). However, the exact mechanism of action of AAS in these cells is still poorly understood. It has been proposed that AAS act through an hAR-mediated mechanism and induce the commitment of these cells into a myogenic lineage. The results of this mechanism in skeletal muscle are hypertrophy of both type I and type II muscle fibers (but not an increase in the number of fibers) (36,38–43), an increase in the number of myonuclei and satellite cells resulting in an increase in the number available for conversion to skeletal muscle fibers (38,44–46), and an upregulation of the number of hARs in the cells (39,44–47).
Nuclear Receptor Coregulators
Coregulators of hAR are proteins that are recruited by the receptor and either enhance (coactivators) or reduce (corepressors) hAR mediated transactivation. Coregulators act at DNA response elements in the promoter region of target genes to facilitate DNA binding, chromatin remodeling, or the recruitment of general transcription factors (48). AF2 in the LBD (Figure 1) of SHRs contains a surface-exposed hydrophobic pocket that provides a docking site for coregulator proteins (21,49). The hAR has 12 conserved alpha helices (26); helix 12 of SHRs, also in the LBD, acts as a molecular switch and aids in the docking of coregulators. The position of helix 12 changes depending on which ligand is bound to the SHR. In the case of antagonist binding, corepressor proteins can bind and in the case of agonist binding, coactivator proteins can bind (49–52). The "LXXLL" motif present on coregulators specifically binds to a hydrophobic pocket-helix 12 region in AF2 of SHRs (21,53,54).
Coactivator recruitment is required for ligand activated SHR mediated transactivation (50). Changes in the amount of free coactivator available for binding, expression level of coactivators, and tissue availability of coactivators can affect transcriptional activity of receptors (48). Several families and types of coactivators and corepressors exist; there are specific coregulator proteins for each SHR as well as several that are shared by some or all SHRs (55,56). Numerous families of coactivator proteins have been described and several coactivators have been identified as binding to and enhancing the ligand-inducing transcriptional activation of hAR (48). Heemers and Tindall (48) extensively review the large number of hAR coregulator proteins. Coregulators indeed play a significant role in activation of hAR, and the interplay of coactivators and coregulators contribute to the fine-tuning of hAR activity.
Anabolic-Androgenic Steroids
The primary role of androgen mediated hAR signaling is the proper development and function of male reproductive organs as well as muscle maintenance. The physiologic effects of endogenous androgens are both androgenic and anabolic; AAS vary in their balance of androgenic to anabolic effects. Androgenic effects include the growth, development, and maintenance of primary (genitalia and genital tract) and secondary sexual characteristics in men, the early stages of breast and pubertal development in girls (adrenarche), spermatogenesis promotion, neuroendocrine regulation of gonadotropin secretion, and libido stimulation. Anabolic effects include stimulation of nitrogen retention, increased protein synthesis and production, increased lean body mass, increased body and muscle growth, skeletal growth, and epiphysis closure of long bones during puberty. There are also some metabolic and hematologic actions of AAS including erythropoiesis, decreased synthesis of several clotting factors, increased sebum production in skin, decreased synthesis of HDL cholesterol, increased synthesis of LDL cholesterol, androgenic alopecia (male pattern baldness), and increased bone density (57,58).
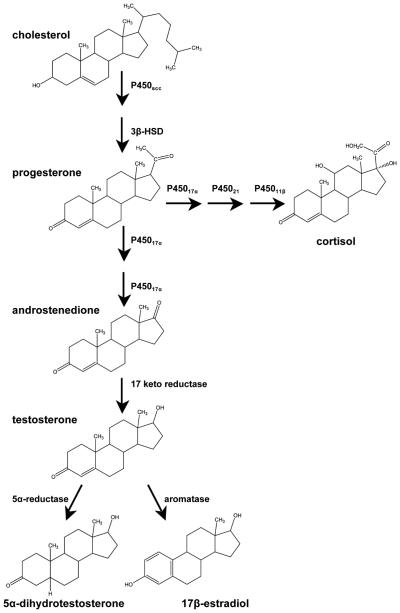
Endogenous hAR ligands include testosterone and its active metabolite 5α-dihydrotestosterone (DHT), in addition to several others. Testosterone is primarily synthesized in Leydig cells of the testes in men and the adrenal cortex, liver, and ovary in women. Although some of the precursors in the synthetic pathway of testosterone, such as androstenedione, have weak agonist activity, testosterone and DHT are the primary endogenous androgens (Figure 3). Testosterone binds with high affinity to hAR and also cross-reacts with the progesterone receptor (PR) and the estrogen receptor (ER), and DHT binds more specifically to the hAR (21,57–58).
Figure 3.
Abridged pathway of AAS and cortisol biosynthesis. Steroid synthesis begins with cholesterol. CYP11A1, cholesterol side-chain cleavage enzyme; 3β-HSD, 3β-hydroxysteroid dehydrogenase; CYPl7, steroid 17α-hydroxylase; CYP21, steroid 21-hydroxylase; CYP11B1, steroid 11β-hydroxylase. Modified from Schimmer and Parker (135).
Testosterone is metabolized in target tissues and in the liver. In target tissues, most testosterone is metabolized by 5α-reductase to DHT (the most potent endogenous AAS). Testosterone can also be metabolized by aromatase enzymes to estradiol (Figure 3). Both 5α-reduction and aromatization are irreversible. In addition to these metabolic pathways, TEST can be inactivated in the liver through reduction and oxidation followed by glucuronidation and renal excretion (21,57–59).
In addition to the endogenous AAS, there are several synthetic testosterone derivatives which are used clinically. The therapeutic uses of androgens include hormone replacement therapy (HRT) in primary or secondary hypogonadism in men, induction of puberty in delayed sexual maturation of boys, osteoporosis in males, HRT in female menopause, endometriosis, treatment of anemia, treatment of hereditary angioedema, and the stimulation weight gain after surgery, infection, and AIDS. Because of aromatization, AAS also possess some estrogenic effects such as gynecomastia. Common adverse effects include acne, scalp hair loss, obstructive sleep apnea, hirsutism, mild voice deepening, and edema. Serious adverse effects of AAS use and abuse are both physiological and psychiatric and include hepatotoxicity (cholestatic hepatitis and jaundice, adenomas, carcinoma, peliosis hepatis), premature bone maturation and epiphyseal closure (in adolescents), increased risk of cardiovascular disease, testicular atrophy, oligospermia, and prostatic hyperplasia or carcinoma (43,57,58).
A healthy adult male has circulating androgen concentrations ranging from 300 to 700 ng/dL (approximately 11–25 nM); endogenous testosterone secretion is pulsatile and diurnal with the highest concentration occurring in the morning and lowest concentration at night (58). The hAR in skeletal muscle is typically saturated with physiologic concentrations of circulating testosterone (6,8); however, those who use anabolic agents administer supraphysiologic doses (100 times the physiological dose or more) and multiple steroids on a daily basis. Evidence suggests that at the supraphysiologic concentrations some steroids are competitive antagonists at the human glucocorticoid receptor (hGR) complementary to their agonist activity at hAR (60–62). These actions can lead to increased nitrogen retention and protein production mediated by hAR, decreased protein catabolism mediated by the hGR (8), and ultimately a net gain in muscle mass.
Because of their anabolic effects, AAS are commonly abused by athletes (5,6,8,63). Many synthetic AAS have been developed in an attempt to alleviate some of the adverse androgenic and estrogenic effects while enhancing the anabolic properties. Most designer androgens originate from 1960–1970s pharmaceutical industry androgen discovery and synthesis programs largely geared at identifying a purely anabolic steroid and oral contraceptives (64). In 1969, Julius A. Vida published a comprehensive book which discusses metabolic factors, structure activity relationships, and the therapeutic action of androgens. He compiles approximately 650 androgens and anabolic agents and documents the relatively small changes in chemical structure which can bring about sharp changes in potency (65). Recently, this textbook has re-emerged in bodybuilding circles and is discussed on website forums. AAS abusers are looking for new compounds to use as doping agents that are not included in traditional screens (66,67).
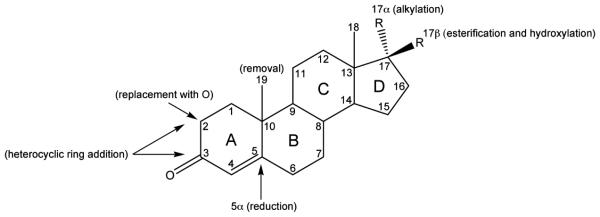
Testosterone is prototype for the development of new androgen receptor agonists; the goals of the development of synthetic AAS are to improve the pharmacologic properties of the compounds (63). For example, carbon 17-β esters of TEST have been manufactured; esterification of this site makes the steroid more fat soluble for parental use and delays its absorption. Testosterone alkyl derivatives at the carbon 17-α position resist metabolism in the liver and are orally active, but are also associated with severe liver toxicities. Modification of the A, B, or C rings of the steroid have several goals including slowing the metabolism (and decreasing affinity for metabolizing enzymes), enhancing affinity for the hAR and hGR, and causing resistance to aromatization (6,8,63). For example, the removal of carbon 19, reduction at the 5α carbon position, the replacement of carbon 2 with oxygen, and the addition of a heterocyclic ring to carbons 2 and 3 all increase anabolic activity. Figure 4 summarizes the common modifications made to the steroid backbone to develop synthetic AAS. Several AAS have been developed for clinical use [extensively reviewed by Kiernan (68)] by utilizing the modifications listed. However, many of the AAS that have been developed for therapeutic use have also been abused, and the development of designer AAS to circumvent doping tests is a common problem (64).
Figure 4.
Steroid backbone labeled with the most common modifications of synthetic AAS.
Selective Androgen Receptor Modulators (SARMs)
Steroid replacement therapy using synthetic AAS has been employed since the structure of testosterone was identified. Testosterone has drawbacks including limited oral bioavailability, inability to dissociate the anabolic and androgenic effects, and severe side effects (43,57,58,69). In an attempt to diminish these drawbacks, thousands of potential AAS have been proposed and developed (65,68). Only a few are approved for pharmaceutical applications, and none of them are considered acceptable for long-term therapy due to adverse side effects. The task to develop the perfect anabolic steroid has proven difficult.
In 1998, the first nonsteroidal hAR agonist was developed (70). Following this discovery, several more nonsteroidal selective androgen receptor modulators (SARMs) were developed; all lacking a core steroid structure (69,71). SARMs have tissue selective activation of the hAR; they can be strong agonists in one tissue and antagonists or weak agonist in another (63). The ideal SARM has been described as a compound which exerts tissue-specific anabolic effects in vivo, with anabolic effects in muscle and bone but lesser effects in the prostate and seminal vesicles (72). Additionally, SARMs bind to the hAR but are resistant to metabolism by 5α-reductases and aromatases (73), and therefore, the unwanted adverse effects due to estrogenic and amplified androgenic signaling do not occur (74).
Most SARMs were developed for the treatment of specific diseases such as sarcopenia and osteoporosis. SARMs illicit varying conformations of the hAR which enables interactions with differing coregulators; this association with varying coregulators causes tissue specific actions of SARMs (69,75). Tissue selectivity is useful for medical treatment of muscle wasting diseases and is very tantalizing for athletes looking to enhance their performance and evade detection in doping tests (74).
Currently, there are three SARMs in clinical trials and large numbers of compounds claiming to be SARMs being sold on the internet. SARMs are included on the prohibited list of the WADA (76). There are MS methods for the detection of known intact SARMs, their known metabolites and degradation products (74,77–79). However, the complexity and structural heterogeneity of SARMs is a challenge for detection methods (77). There is the possibility of newly synthesized and modified SARMs emerging in attempts to evade detection in antidoping tests.
Biological Androgen Receptor Assays
The number of abused compounds and methods used by doping athletes is constantly changing (80). Sports testing procedures need to be continuously updated to keep up with the abusers. Previously, doping control focused on approved therapeutics, but athletes misuse compounds that are not tested and not clinically approved (some synthesized solely for doping purposes, e.g., THG). The current challenge for antidoping laboratories is to identify these compounds with new, more comprehensive technologies (64,81,82) such as biological assays which do not require the knowledge of the structure of the compound for detection.
Current analytical methods for the detection of AAS, SARMs, and other steroids and compounds of abuse include GC and LC coupled to MS detectors. MS-based methods are powerful and very useful for use in sports doping laboratories because they are specific and sensitive detection methods. They, however, have the major limitation that they cannot identify compounds of unknown structure and rely on prior knowledge of the structure of the steroid (64,83–86).
There is a high risk of overlooking compounds with similar biological activity, but differing structures, such as designer steroids. An alternative to chromatography and MS methods are biologically based assays. These include yeast and mammalian cell reporter gene assays involving steroid receptors. They have been developed to amplify and measure biological activity and can be sensitive and provide information on the presence of steroid receptor activating compounds independently of knowing the structure. Table I compares some of the properties of bioassays with those of common chromatography–MS methods.
Table I.
Comparison of Biological hAR Assays and Chromatography–MS Methods
| Bioassay | Chromatography–MS | |
|---|---|---|
| Endogenous steroids detected | Yes | Yes |
| Unknown steroids detected | Yes | No |
| Supraphysiological levels of steroids detected |
Yes | Yes |
| Detection of multi-drug abuse | Yes | Yes (if all are known) |
| Chemical structure determination | No | Yes |
| Metabolites (active) | Yes (known or unknown) | Yes (if known) |
| Metabolites (inactive) | No | Yes (if known) |
| Sensitivity | Excellent | Excellent only with last generation instrumentation |
| Time for completion of assay | 6 h up to 2 days | < 6 h |
| Cost | Economical | Expensive |
| Drug-drug interaction assessment |
Yes, with the use of competitive binders and antagonists |
No |
Biological hAR assays exploit the natural signaling pathway of androgens and compounds that bind to the hAR. Bio-asays measure the relative activity of a substance (or a mixture of substances) to achieve an intended biological effect without the requirement of information about the chemical structure of the ligand (64,87). As detailed earlier, AAS exert their effects in cells by binding the hAR. Binding triggers several cellular events including the recruitment of coregulator proteins and ends with the receptors translocating to the nucleus and binding to AREs or HREs (21,23,30–32). We have previously detailed the kinetics of hAR and hGR translocation by observing, in real-time, the ligand-dependent transport of the receptors to the nucleus (34), which could be a factor in the resulting bioactivity. The DNA-bound receptor-ligand complexes then initiate the transcription of target genes and a protein is produced. In biologically assays, host cells (yeast or mammalian) are chosen to exploit and monitor this pathway. DNA vectors coding for the hAR are introduced into the cells along with vectors containing AREs or HREs which are linked to a reporter gene. The reporter genes encode a protein that can be easily measured such as luciferase, β-galactosidase, or green fluorescent protein. When ligands are added to the system, the constitutively expressed receptors are activated, bind to the response elements, and produce the protein of the reporter, which can then be measured. Figure 5 is an illustration which shows the process of the biological assay. These bioassays directly measure the bioactivity of compounds which bind to the hAR without prior knowledge of the structure of the compound.
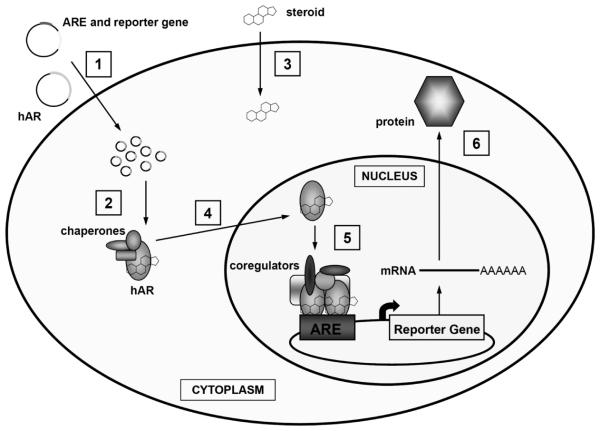
Figure 5.
The process of the biological assay. 1. DNA vectors encoding the hAR and an ARE linked to a reporter gene are transfected into cells. The hAR is under the control of a constitutive promoter and is therefore constantly expressed. 2. After the vectors are transfected into the cell, the hAR is produced and the ARE-reporter gene plasmids are present. 3. After the addition of an hAR ligand (steroid), the ligand will bind to the receptor. 4. Upon ligand binding, the hAR will translocate to the nucleus. 5. Once in the nucleus, hAR receptor dimers will bind to the AREs on the vectors and the reporter gene will be transcribed and a protein produced. 6. The reporter protein will be collected and measured.
There are two main varieties of biological assays: those which utilize the laboratory yeast strain, Saccharomyces cerevisiae (88–100), and those which utilize mammalian cells (101–115). Table II summarizes the reported bioassays. The first bioassays were developed in yeast because they grow rapidly and are economical (compared to mammalian cell culture). Typically, yeast cells are transformed with hAR cDNA and a reporter vector containing an ARE and a reporter gene. Yeast assays are robust, reliable, and reproducible. They do have some problems, however. Yeast assays can require laborious pre-assay cell preparation, long incubation times, and complex cell lysis steps. Using yeast systems to express mammalian proteins can pose problems such as incorrect phosphorylation, glycosylation, folding, or other post-translational modification. Also, there is a chance of causing toxicity to the yeast with high levels of steroids or the possibility of steroid permeability issues (87). Additionally, yeast systems lack the appropriate chaperone and coregulator proteins which are necessary for proper hAR mediated transactivation.
Table II.
Summary of hAR Biological Assays
| Author(s) (Reference) | Cell Type | Reporter | Promoter | Receptor Type | Transfection Method |
|---|---|---|---|---|---|
| Yeast | |||||
| Beck et al. (100) | 188R1 | GFP | ARE2 | Full length hAR | |
| Bovee et al. (88–90) | K20 | yEGFP | ARE2 | Full length hAR | |
| Gaido et al. (91) | BJ3505 | β-gal | ARE2 | Full length hAR | |
| Lee et al. (92) | EGY48 | β-gal | ACS-1 | hAR LBD | |
| Leskinen et al. (93) and Michilini et al. (95) |
BMA64-1A | Luciferase | ARE2 | Full length hAR | |
| Mak et al. (94) | BJ2168 strain | β-gal | GRE/PRE | Full length mAR | |
| Purvis et al. (97) | BJ1991 (turned PGKhAR) |
β-gal | PGK (MMTV) | Full length hAR | |
| Sohoni & Sumpter (98) | PGKhAR | β-gal | PGK (MMTV) | Full length hAR | |
| Zierau et al. (99,133) | β-gal | PGK (MMTV) | |||
| Mammalian | |||||
| Blankvoort et al. (101) | T47D | Luciferase | PB-ARE-2 | Endogenous AR | Stable (AR-LUX) |
| Cadwallader (this article) | COS-7 | Luciferase | PB-ARE-2 (119,121) | Full length hAR | Transient (Lipofectamine) |
| Chen et al. (102) | HEK 293 | Luciferase | MMTV | Full length hAR | Stable |
| de Gooyer et al. (103) | CHO-K1 | Luciferase | MMTV | Full length hAR | Stable |
| Paris et al. (106,108) | CHO | Luciferase | MMTV | Full length hAR | Stable |
| Raivio et al. (109) | COS-1 | Luciferase | Complex Yeast Gal4 system |
hAR LBD + mAR NTD |
Transient (FuGene) |
| Roy et al. (110,111) | CHO K1 and HEK 293 |
Luciferase | MMTV | Full length hAR | Stable |
| Shen et al. (129) | MDA-kb2 | Luciferase | MMTV | Endogenous AR | Stable |
| Sonneveld et al. (112,113) and Houtman (104) |
U2-OS | Luciferase | MMTV and 3xHRE-TATA |
Full length hAR | Stable (CALUX) |
| Terouanne et al. (114,134) and Mnif et al. (105) |
CV-1 and PC-3 | Luciferase | MMTV | hAR LBD Full length hAR |
Transient (Calcium phosphate) Stable (PALM) |
Mammalian cell-based bioassays are typically developed in immortalized cells lines which are relatively easy to culture. The hAR and ARE-reporter gene vectors are introduced into the cells via transfection. Some bioassays utilize transient transfection of cells prior to each assay while others have developed stably transfected cell lines constantly expressing the vectors (Table II). Mammalian cell bioassays have been reported to have a higher sensitivity than yeast assays (64,108,112). Stable cell lines have the possibility of vector loss and degradation over time. The use of transient transfection methods does not risk this vector loss and degradation. It could, however, lead to inconsistent results, but this can be corrected with the use of control vectors to measure basal levels of activity and serve as an internal control of transfection efficiency. Cell choice is an important consideration for mammalian-based assays. The presence of steroid metabolizing enzymes can give inaccurate results; however, typically, immortalized cell lines which are not of liver origin lack most metabolizing enzymes. The presence of other steroid receptors in the cells can cause some cross-reactivity (87); this makes the choice of ARE/HRE important. Later in this paper, we will detail a robust mammalian assay we have developed and have in use in our laboratories.
Investigators have shown that androgens have distinct expression profiles using the promoters ARE2-TATA, MMTV, and GRE-OCT (31). However, because of SHR promiscuity at HREs, it is possible that both the hAR and the other SHRs activate these promoters and the results could be due to cross-reactivity of ligands binding to other SHRs and general HREs (116–118). If testing samples from humans containing endogenous steroids, this lack of cross-reactivity becomes important. A selective HRE, PB-ARE-2, has been described for the hAR (23,119–123); however, most of the biological assays using mammalian cells have utilized the MMTV promoter, which has shown cross-reactivity with other receptors and ligands (108,110,111).
Additionally, the use of full length or truncated (only LBD) hAR is an issue for consideration. Using full length hAR will provide a more accurate assessment of steroid bioactivity, as there are ligand-dependent conformational changes of the hAR resulting in differential coregulator recruitment, differential DNA binding, and transactivation (49,51,53,56,124–128).
A Newly Developed hAR Biological Assay
Figures 6–8 show data from an hAR bioassay developed in our laboratory. Transactivation (biological activity) mediated through the human hAR after the binding of abused steroids was assessed. A firefly luciferase assay that uses the specific hAR promoter PB-ARE-2 and full length hAR was developed using COS-7 cells and transient transfection. This assay uses the PBARE-2 promoter which is specific for the hAR (119, 121), full length hAR to assure proper funtion of the receptor, and COS-7 cells, which are derived from CV-1 cells that have been shown to be devoid of AR, ER, and TR (129) to minimize cross-reactivity in the assay.
Figure 6.
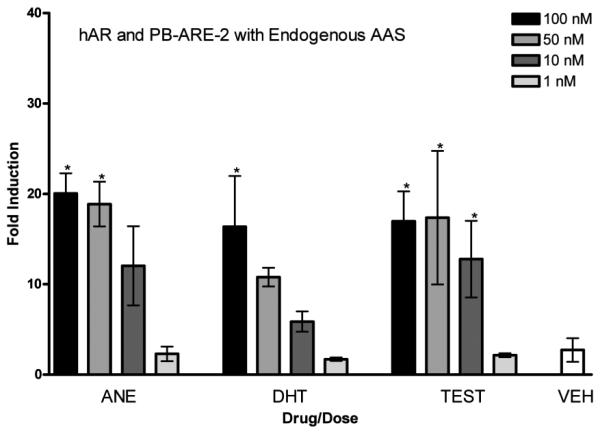
hAR and PB-ARE-2 with endogenous AAS. Activity of the PB-ARE-2 promoter (luc) driven by hAR in COS-7 cells transfected with the promoter PB-ARE-2 and hAR after 24-h treatment with the endogenous AAS androstenedione (ANE), dihydrotestosterone (DHT), testosterone (TEST) [4 doses, 100 nM (black bars), 50 nM (medium grey bars), 10 nM (dark grey bars), 1 nM (light grey bars)], and ethanol vehicle (VEH). Columns represent the mean fold induction of three independent experiments run in triplicate. Error bars represent ± S.D. Significant difference from vehicle indicated with *, p < 0.05.
Assay methods
Briefly, a full length hAR plasmid (from A. Saporita, North-western University) (130) and a PB-ARE-2 plasmid (containing the probasin element ARE-2 promoter, which selectively interacts with the DBD of the hAR) (from F. Claessens, University of Leuven, Belgium) (119,121) were transfected into COS-7 cells using Lipofectamine 2000 CD™ (Invitrogen). After transfection and a recovery period of 24 h, RPMI-1640 containing 10% charcoal stripped fetal bovine serum (FBS) was added to the COS-7 cells. The COS-7 cells were then treated with steroids at concentrations of 1, 10, 50, and 100 nM or vehicle (ethanol). After 24 h of steroid treatment, cells were harvested and subjected to two freeze-thaw cycles to lyse cells for the luci ferase assay.
The Dual Luciferase Reporter Assay System® (Promega, Sydney, Australia), which performs two reporter assays sequentially from a single sample, was used to determine firefly luciferase activity (to measure hAR transactivation via pPBARE-2-luciferase) and renilla luciferase activity (to measure basal levels of activity via the SV40 promoter and serve as an internal control of transfection efficiency) according to manufacturer's instructions. Luciferase activity was quantified using a luminometer (Stratec PlateLumino, Birkenfeld, Germany). Three independent reporter gene assays were performed per steroid concentration for each receptor. Each replicate was run in the luciferase assay three times.
The values [in response units (RUs)] obtained from luminometer measurement of firefly luciferase were normalized to values obtained from luminometer measurement of renilla luciferase in the same sample and expressed as fold induction of luciferase produced. Steroid treated samples were also normalized to vehicle. The assays were averaged and standard deviation calculated. Data was analyzed with one-way ANOVA with Dunnett's post test, p < 0.05 considered significant (GraphPad Prism version 4.00 for Windows).
Assay results
Endogenous anabolic-androgenic steroids
Figure 6 shows the activity of endogenous steroids androstenedione, DHT, and testosterone in this assay. There is a dose-dependent increase in fold induction over vehicle for these endogenous steroids. A maximum fold induction of approximately 20 was observed for these endogenous anabolic-androgenic steroids.
Synthetic anabolic-androgenic steroids
The fold induction observed after treatment with the synthetic steroids nandrolone, oxandrolone, and methyltrienelone is shown in Figure 7. A significant difference from vehicle was observed for all 4 doses of methyltrienelone and nandrolone and for the 10, 50, and 100 nM doses of oxandrolone. The maximum fold induction seen for these synthetic steroids was approximately 30.
Figure 7.
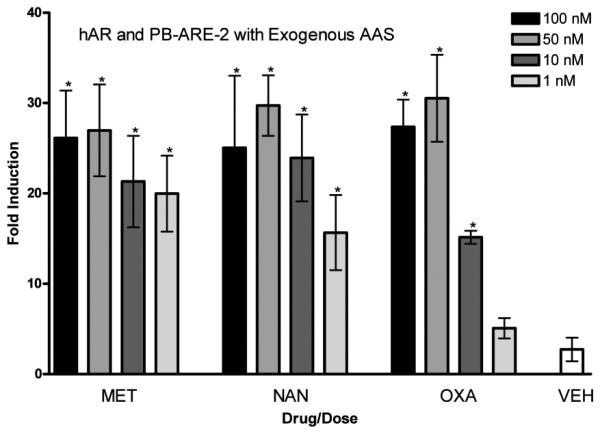
hAR and PB-ARE-2 with exogenous AAS. Activity of the PB-ARE-2 promoter (luc) driven by hAR in COS-7 cells transfected with the promoter PB-ARE-2 and hAR after 24-h treatment with the synthetic AAS methyhrienelone (MET), nandrolone (NAN), oxandrolone (OXA) [4 doses, 100 nM (black bars), 50 nM (medium grey bars), 10 nM (dark grey bars), and 1 nM (light grey bars)], and ethanol vehicle (VEH). Columns represent the mean fold induction of three independent experiments run in triplicate. Error bars represent ± S.D. Significant difference from vehicle indicated with *, p < 0.05.
Glucocorficosteroids
Glucocorticoid ligands cortisol and dexamethasone were tested in the assay after transfection of COS-7 cells with hAR and PB-ARE-2. Figure 8 shows there was no significant induction of hAR promoter driven luciferase production mediated via these ligands after a 24-h treatment, as expected.
Figure 8.
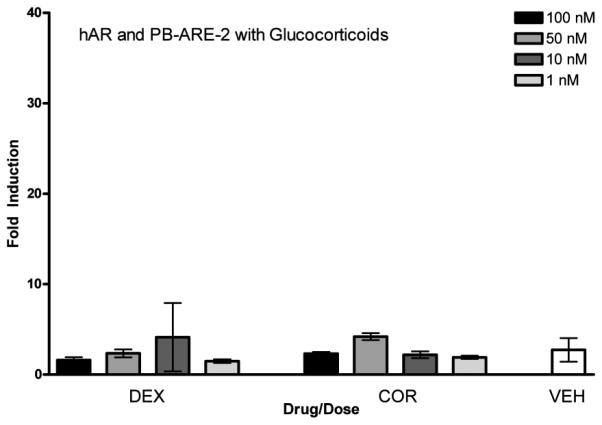
hAR and PB-ARE-2 with glucocorticosteroids. Activity of the PB-ARE-2 promoter (luc) driven by hAR in COS-7 cells transfected with the promoter PB-ARE-2 and hAR after 24-h treatment with the glucocorticosteroids dexamethasone (DEX) and cortisol (COR) [4 doses, 100 nM (black bars), 50 nM (medium grey bars), 10 nM (dark grey bars), and 1 nM (light grey bars)], and ethanol vehicle (VEH). Columns represent the mean fold induction of three independent experiments run in triplicateError bars represent ± S.D. Significant difference from vehicle indicated with *, p < 0.05.
The data presented here show a dose-dependent response for all steroids studied in this reproducible biological assay. In a healthy adult male, the approximate physiologic range of endogenous androgens is 300–700 ng/dL (approximately 11–25 nM) (58). Many steroid dosing regimens followed by athletes who are doping use much more than this, up to 100 times the physiologic range (and multiple steroids), on a daily basis. Four concentrations of steroids (1, 10, 50, and 100 nM), adjusted to the volume of the cell culture wells, were tested ranging from very low to superraphysiological and encompassing these normal physiological levels. With this assay, synthetic steroids (nandrolone and oxandrolone) have a higher activity (as indicated by fold induction in the luciferase assay) than endogenous steroids (androstenedione, DHT, and TEST). The higher activity is indicated by the greater production and measurement of luciferase by the synthetic steroids at the same dose as the endogenous steroids. This higher activity seen in the assay is parallel to the effect seen in vivo; that is, the synthetic steroids are reported to have a higher efficacy because of their structural modifications (5,6). It is also worth noting the general trend that statistical significance (compared to vehicle) was seen when doses of steroid in the assay exceeded the normal physiological range of endogenous steroids. Previously, we have shown that increasing concentrations of steroids cause an increase in the rate of transport of the hAR to the nucleus. It is likely that this increase in translocation correlates to an increase in biological activity (34). This has significance when attempting to detect steroid abuse since the amount of steroid abused is typically in excess (nearly 100-fold) of physiologic levels.
Although there are other systems available to detect steroid bioactivity, our system more closely mimics endogenous receptor activity and function because it uses full length receptors (instead of just ligand binding domains) as well as a mammalian system (as opposed to yeast). Additionally, a very specific hAR promoter (PB-ARE-2) was used in an attempt to make a receptor specific assay to determine the presence of hAR agonists without cross reactivity with other endogenous steroids or steroid receptors. In addition to the hAR biological assay presented, we have also developed an hGR assay for the detection of glucocorticosteroids and selective glucocorticoid receptor modulators. Similar to the hAR assay presented, this assay utilizes full length hGR, a promoter specific for the hGR, the cytosolic aspartate amino transferase glucocorticoid response element (cAspAT-GRE) (131), and COS-7 cells. Experiments are underway to optimize both receptor assays for use in antidoping laboratories and to assess urine samples from both steroid abusers and non-abusers. Current experiments being performed are attempting to distinguish endogenous steroids in biological samples from exogenous steroids in the samples. The current hypothesis uses an idea similar to the testosterone/epitestosterone ratio, that there is a normal range of steroids (and therefore expected response) using the biological assay. Data are being collected to determine this normal range for both males and females. Once a normal range is determined, it will be used to identify irregular and possibly positive samples.
The only requirement to obtain a positive response and assess transcriptional activity in these assays is the presence and ability of an agonist to bind to the hAR or hGR. No prior knowledge of the structure of the compound is required. After the addition of a sample containing steroids to the cells in these biological assays, the amount of exogenous protein produced can be measured. This amount of protein corresponds to the activity and amount of steroid present in the sample. Once the presence of an agonist is determined, further studies can be pursued to identify the compound. These biological assays may also, along with determining the presence of a ligand, be used to predict the anabolic potential of existing and newly synthesized compounds, be utilized in tandem with MS technology for structure identification, and, when coupled with microsomal metabolism studies, metabolites of compounds can be assessed (132).
Conclusions
The biological assays discussed here exploit the transcriptional activation mediated by SHRs, specifically the hAR, in an attempt to directly determine the presence of and biological activity of hAR ligands (agonists and antagonist when used as competitive assays). The biological assay data presented here and similar biological assays may be adapted to screen urine samples for steroid abuse. In the event of a positive response, the compound in the samples can then be determined using typical structure identification methods such as mass spectrometry. With the application of this assay to evaluate urine samples, it may be possible to determine the presence of previously non-detectable and designer steroids as well as SARMs in an easy and reliable way. In addition to this assay’s ability to detect the abuse of steroids, it may also be used to determine the relative anabolic potential of AAS as well as newly synthesized steroids and other new non-steroidal compounds. If coupled with microsomal metabolism experiments, the bioactivity of metabolites can also be assessed. These assays are the beginning of the next generation of detection methods in the antidoping field; the antidoping field is moving away from merely checking for exposure to prohibited substances to detecting an actual and measureable effect of prohibited substances with possibly unknown structures. Biological assays allow for the determination of an overall hAR mediated effect instead of the measuring of single receptor ligands. With further experimentation, biological assays can be adapted for routine use in antidoping laboratories and become a valuable tool.
Acknowledgments
The new data presented here were superported by NIH NRSA grant F31DA022809 from the National Institute on Drug Abuse, NIH grants DA07820 and DK070060, and a University of Utah Funding Incentive Seed Grant. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health.
Footnotes
There are no conflicts of interest associated with this publication.
References
- 1.Barroso O, Mazzoni I, Rabin O. Hormone abuse in sports: the antidoping perspective. Asian J. Androl. 2008;10:391–402. doi: 10.1111/j.1745-7262.2008.00402.x. [DOI] [PubMed] [Google Scholar]
- 2.Botrè F. New and old challenges of sports drug testing. J. Mass. Spectrom. 2008;43:903–907. doi: 10.1002/jms.1455. [DOI] [PubMed] [Google Scholar]
- 3.WADA The 2009 WADA Prohibited List. http://www.wada-ama.org/rtecontent/document/2008_List_En.pdf (accessed April 2011)
- 4.WADA The 2009 WADA Laboratory Statistics. http://www.wada-ama.org/Documents/Science_Medicine/Anti-Doping_Laboratories/Lab_Statistics/WADA_2009_LaboratoryStatisticsReport_Final.pdf (accessed April 2011)
- 5.Hartgens F, Kuipers H. Effects of androgenic-anabolic steroids in athletes. Sports Med. 2004;34:513–554. doi: 10.2165/00007256-200434080-00003. [DOI] [PubMed] [Google Scholar]
- 6.Kuhn C. Anabolic steroids. Recent Prog. Horm. Res. 2002;57:411–434. doi: 10.1210/rp.57.1.411. [DOI] [PubMed] [Google Scholar]
- 7.Thiblin I, Petersson A. Pharmacoepidemiology of anabolic androgenic steroids: a review. Fundam. Clin. Pharmacol. 2005;19:27–44. doi: 10.1111/j.1472-8206.2004.00298.x. [DOI] [PubMed] [Google Scholar]
- 8.Shahidi NT. A review of the chemistry, biological action, and clinical applications of anabolic-androgenic steroids. Clin. Ther. 2001;23:1355–1390. doi: 10.1016/s0149-2918(01)80114-4. [DOI] [PubMed] [Google Scholar]
- 9.. Catlin D, Sekera MH, Ahrens BD, Starcevic B, Chang YC, Hatton CK. Tetrahydrogestrinone: discovery, synthesis, and detection in urine. Rapid Commun. Mass Spectrom. 2004;18:1245–1049. doi: 10.1002/rcm.1495. [DOI] [PubMed] [Google Scholar]
- 10.FDA warning on unapproved performance enhancer FDA Consum. 2004;38:4. [PubMed] [Google Scholar]
- 11.Ashley S. Doping by design. Sci. Am. 2004;290:22–23. doi: 10.1038/scientificamerican0204-22. [DOI] [PubMed] [Google Scholar]
- 12.Knight J. Drugs in sport: no dope. Nature. 2003;426:114–115. doi: 10.1038/426114a. [DOI] [PubMed] [Google Scholar]
- 13.Kondro W. Athletes’ “designer steroid” leads to widening scandal. Lancet. 2003;362:1466. doi: 10.1016/S0140-6736(03)14733-2. [DOI] [PubMed] [Google Scholar]
- 14.. Georgakopoulos C, Vonaparti A, Stamou M, Kiousi P, Lyris E, Angelis YS, Tsoupras G, Wuest B, Nielen MW, Panderi I, Koupparis M. Preventive doping control analysis: liquid and gas chromatography time-of-flight mass spectrometry for detection of designer steroids. Rapid Commun. Mass Spectrom. 2007;21:2439–2446. doi: 10.1002/rcm.3103. [DOI] [PubMed] [Google Scholar]
- 15.Vonaparti A, Lyris E, Angelis YS, Panderi I, Koupparis M, Tsantili-Kakoulidou A, Peters RJ, Nielen MW, Georgakopoulos C. Preventive doping control screening analysis of prohibited substances in human urine using rapid-resolution liquid chromatography/high-resolution time-of-flight mass spectrometry. JRapid Commun. Mass Spectrom. 2010;24:1595–1609. doi: 10.1002/rcm.4554. [DOI] [PubMed] [Google Scholar]
- 16.. Pozo O, Deventer K, VanEenoo P, Delbeke FT. Efficient approach for the comprehensive detection of unknown anabolic steroids and metabolites in human urine by liquid chromatography–electrospray-tandem mass spectrometry. Anal. Chem. 2008;80:709–720. doi: 10.1021/ac7020757. [DOI] [PubMed] [Google Scholar]
- 17.Tsai MJ, O’Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu. Rev. Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 18.Freedman LP. Molecular Biology of Steroid and Nuclear Hormone Receptors. Birkhäuser; Boston, MA: 1998. [Google Scholar]
- 19.. Chang C, Kokontis J, Liao ST. Structural analysis of complementary DNA and amino acid sequences of human and rat androgen receptors. Proc. Natl. Acad. Sci. USA. 1988;85:7211–7215. doi: 10.1073/pnas.85.19.7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang CS, Kokontis J, Liao ST. Molecular cloning of human and rat complementary DNA encoding androgen receptors. Science. 1988;240:324–326. doi: 10.1126/science.3353726. [DOI] [PubMed] [Google Scholar]
- 21.Gao W, Bohl CE, Dalton JT. Chemistry and structural biology of androgen receptor. Chem. Rev. 2005;105:3352–3370. doi: 10.1021/cr020456u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brinkmann AO. Lessons to be learned from the androgen receptor. Eur. J. Dermatol. 2001;11:301–303. [PubMed] [Google Scholar]
- 23.Claessens F, Denayer S, Van Tilborgh N, Kerkhofs S, Helsen C, Haelens A. Diverse roles of androgen receptor (AR) domains in AR-mediated signaling. Nucl. Recept. Signal. 2008;6:e008. doi: 10.1621/nrs.06008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pratt WB, Toft DO. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr. Rev. 1997;18:306–360. doi: 10.1210/edrv.18.3.0303. [DOI] [PubMed] [Google Scholar]
- 25.Warren BS, Kusk P, . Wolford R, Hager GL. Purification and stabilization of transcriptionally active glucocorticoid receptor. J. Biol. Chem. 1996;271:11434–11440. doi: 10.1074/jbc.271.19.11434. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Al-Azzawi F. Mechanism of androgen receptor action. Maturitas. 2009;63:142–148. doi: 10.1016/j.maturitas.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 27.Dahmer MK, Quasney MW, Bissen ST, Pratt WB. Molybdate permits resolution of untransformed glucocorticoid receptors from the transformed state. J. Biol. Chem. 1981;256:9401–9405. [PubMed] [Google Scholar]
- 28.Davis JR, Kakar M, Lim CS. Controlling protein compartmentalization to overcome disease. Pharm. Res. 2007;24:17–27. doi: 10.1007/s11095-006-9133-z. [DOI] [PubMed] [Google Scholar]
- 29.Gorlich D, Kutay U. Transport between the cell nucleus and the cytoplasm. Annu. Rev. Cell Dev. Biol. 1999;15:607–660. doi: 10.1146/annurev.cellbio.15.1.607. [DOI] [PubMed] [Google Scholar]
- 30.Jenster G, Spencer TE, Burcin MM, Tsai SY, Tsai M-J, O’Malley BW. Steroid receptor induction of gene transcription: a two-step model. Proc. Natl. Acad. Sci. USA. 1997;94:7879–7884. doi: 10.1073/pnas.94.15.7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holterhus PM, Piefke S, Hiort O. Anabolic steroids, testosterone-precursors and virilizing androgens induce distinct activation profiles of androgen responsive promoter constructs. J. Steroid Biochem. Mol. Biol. 2002;82:269–275. doi: 10.1016/s0960-0760(02)00220-0. [DOI] [PubMed] [Google Scholar]
- 32.Prefontaine GG, Lemieux ME, Giffin W, Schild-Poulter C, Pope L, LaCasse E, Walker P, Hache RJG. Recruitment of octamer transcription factors to DNA by glucocorticoid receptor. Mol. Cell. Biol. 1998;18:3416–3430. doi: 10.1128/mcb.18.6.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Truss M, Beato M. Steroid hormone receptors: interaction with deoxyribonucleic acid and transcription factors. Endocr. Rev. 1993;14:459–479. doi: 10.1210/edrv-14-4-459. [DOI] [PubMed] [Google Scholar]
- 34.Cadwallader AB, Rollins DE, Lim CS. Effect of anabolic-androgenic steroids and glucocorticoids on the kinetics of hAR and hGR nucleocytoplasmic translocation. Mol. Pharm. 2010;7:689–698. doi: 10.1021/mp900259w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.NURSA. Tissue-specific expression patterns of nuclear receptors, Androgen Receptor http://www.nursa.org/10.1621/datasets.02001 (accessed April 2011)
- 36.Bhasin S, Taylor WE, Singh R, Artaza J, Sinha-Hikim I, Jasuja R, Choi H, Gonzalez-Cadavid NF. The mechanisms of androgen effects on body composition: mesenchymal pluripotent cell as the target of androgen action. J. Gerontol. A Biol. Sci. Med. Sci. 2003;58:M1103–1110. doi: 10.1093/gerona/58.12.m1103. [DOI] [PubMed] [Google Scholar]
- 37.Chen JC, Goldhamer DJ. Skeletal muscle stem cells. Reprod. Biol. Endocrinol. 2003;1:101. doi: 10.1186/1477-7827-1-101. http://www.rbej.com/content/1/1/101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herbst KL, Bhasin S. Testosterone action on skeletal muscle. Curr. Opin. Clin. Nutr. Metab. Care. 2004;7:271–277. doi: 10.1097/00075197-200405000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Sinha-Hikim I, Taylor WE, Gonzalez-Cadavid NF, Zheng W, Bhasin S. Androgen receptor in human skeletal muscle and cultured muscle satellite cells: up-regulation by androgen treatment. J. Clin. Endocrinol. Metab. 2004;89:5245–5255. doi: 10.1210/jc.2004-0084. [DOI] [PubMed] [Google Scholar]
- 40.Sinha-Hikim I, Artaza J, Woodhouse L, Gonzalez-Cadavid N, Singh AB, Lee MI, Storer TW, Casaburi R, Shen R, Bhasin S. Testosterone-induced increase in muscle size in healthy young men is associated with muscle fiber hypertrophy. Am. J. Physiol. Endocrinol. Metab. 2002;283:E154–164. doi: 10.1152/ajpendo.00502.2001. [DOI] [PubMed] [Google Scholar]
- 41.Singh R, Artaza JN, Taylor WE, Braga M, Yuan X, Gonzalez-Cadavid NF, Bhasin S. Testosterone inhibits adipogenic differentiation in 3T3-L1 cells: nuclear translocation of androgen receptor complex with beta-catenin and T-cell factor 4 may bypass canonical Wnt signaling to down-regulate adipogenic transcription factors. Endocrinology. 2006;147:141–154. doi: 10.1210/en.2004-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singh R, Artaza JN, Taylor WE, Gonzalez-Cadavid NF, Bhasin S. Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor-mediated pathway. Endocrinology. 2003;144:5081–5088. doi: 10.1210/en.2003-0741. [DOI] [PubMed] [Google Scholar]
- 43.Choong K, Lakshman KM, Bhasin S. The physiological and pharmacological basis for the ergogenic effects of androgens in elite sports. Asian J. Androl. 2008;10:351–363. doi: 10.1111/j.1745-7262.2008.00407.x. [DOI] [PubMed] [Google Scholar]
- 44.Chen Y, Zajac JD, MacLean HE. Androgen regulation of satellite cell function. J. Endocrinol. 2005;186:21–31. doi: 10.1677/joe.1.05976. [DOI] [PubMed] [Google Scholar]
- 45.Joubert Y, Tobin C. Satellite cell proliferation and increase in the number of myonuclei induced by testosterone in the levator ani muscle of the adult female rat. Dev. Biol. 1989;131:550–557. doi: 10.1016/s0012-1606(89)80025-9. [DOI] [PubMed] [Google Scholar]
- 46.Sinha-Hikim I, Roth SM, Lee MI, Bhasin S. Testosterone-induced muscle hypertrophy is associated with an increase in satellite cell number in healthy, young men. Am. J. Physiol. Endocrinol. Metab. 2003;285:E197–E205. doi: 10.1152/ajpendo.00370.2002. [DOI] [PubMed] [Google Scholar]
- 47.Doumit M, Cook DR, Merkel RA. Testosterone up-regulates androgen receptors and decreases differentiation of porcine myogenic satellite cells in vitro. Endocrinology. 1996;137:1385–1394. doi: 10.1210/endo.137.4.8625915. [DOI] [PubMed] [Google Scholar]
- 48.Heemers HV, Tindall DJ. Androgen receptor (AR) coregulators: a diversity of functions converging on and regulating the AR transcriptional complex. Endocr. Rev. 2007;28:778–808. doi: 10.1210/er.2007-0019. [DOI] [PubMed] [Google Scholar]
- 49.Nettles KW, Greene GL. Ligand control of coregulator recruitment to nuclear receptors. Annu. Rev. Physiol. 2005;67:309–333. doi: 10.1146/annurev.physiol.66.032802.154710. [DOI] [PubMed] [Google Scholar]
- 50.Gao X, Loggie BW, Nawaz Z. The roles of sex steroid receptor coregulators in cancer. Mol. Cancer. 2002;1:1–7. doi: 10.1186/1476-4598-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pereira de Jesus-Tran K, Cote PL, Cantin L, Blanchet J, Labrie F, Breton R. Comparison of crystal structures of human androgen receptor ligand-binding domain complexed with various agonists reveals molecular determinants responsible for binding affinity. Protein Sci. 2006;15:987–999. doi: 10.1110/ps.051905906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stevens A, Garside H, Berry A, Waters C, White A, Ray D. Dissociation of steroid receptor coactivator 1 and nuclear receptor corepressor recruitment to the human glucocorticoid receptor by modification of the ligand-receptor interface: the role of tyrosine 735. Mol. Endocrinol. 2003;17:845–859. doi: 10.1210/me.2002-0320. [DOI] [PubMed] [Google Scholar]
- 53.Darimont BD, Wagner RL, Apriletti JW, Stallcup MR, Kushner PJ, Baxter JD, Fletterick RJ, Yamamoto KR. Structure and specificity of nuclear receptor-coactivator interactions. Genes Dev. 1998;12:3343–3356. doi: 10.1101/gad.12.21.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Estebanez-Perpina E, Moore JM, Mar E, Delgado-Rodrigues E, Nguyen P, Baxter JD, . Buehrer B, Webb P, Fletterick RJ, Guy RK. The molecular mechanisms of coactivator utilization in ligand-dependent transactivation by the androgen receptor. J. Biol. Chem. 2005;280:8060–8068. doi: 10.1074/jbc.M407046200. [DOI] [PubMed] [Google Scholar]
- 55.Hur E, Pfaff SJ, Payne ES, Grøn H, Buehrer BM, Fletterick RJ. Recognition and accommodation at the androgen receptor coactivator binding interface. PLoS Biol. 2004;2:E274. doi: 10.1371/journal.pbio.0020274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heinlein CA, Chang C. Androgen receptor (AR) coregulators: an overview. Endocr. Rev. 2002;23:175–200. doi: 10.1210/edrv.23.2.0460. [DOI] [PubMed] [Google Scholar]
- 57.Nussey SS, Whitehead SA. BIOS Scientific Publishers; Oxford, U.K.: 2001. Endocrinology: An Integrated Approach. [PubMed] [Google Scholar]
- 58.Snyder P, Androgens . In: Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 10th Hardman JG, Limbird LE, Gilman AG, editors. McGraw-Hill; New York, NY: 2001. pp. pp 1635–1648. [Google Scholar]
- 59.Schanzer W. Metabolism of anabolic androgenic steroids. Clin. Chem. 1996;42:1001–1020. [PubMed] [Google Scholar]
- 60.Hickson R, Czerwinski S, Falduto M, Young A. Glucocorticoid antagonism by exercise and androgenic-anabolic steroids. Med. Sci. Sports Exerc. 1990;22:331–340. [PubMed] [Google Scholar]
- 61.Hickson RC, Marone JR. Exercise and inhibition of glucocorticoid-induced muscle atrophy. Exerc. Sport Sci. Rev. 1993;21:135–167. [PubMed] [Google Scholar]
- 62.Mayer M, Rosen F. Interaction of anabolic steroids with glucocorticoid receptor sites in rat muscle cytosol. Am. J. Physiol. 1975;229:1381–1386. doi: 10.1152/ajplegacy.1975.229.5.1381. [DOI] [PubMed] [Google Scholar]
- 63.Brueggemeier RW. Sex Hormones (Male): Analogs and Antagonists. Wiley; Weinheim, Germany: 2006. [Google Scholar]
- 64.Handelsman DJ, Heather A. Androgen abuse in sports. Asian J. Androl. 2008;10:403–415. doi: 10.1111/j.1745-7262.2008.00406.x. [DOI] [PubMed] [Google Scholar]
- 65.Vida JA. Androgens and Anabolic Agents: Chemistry and Pharmacology. Academic Press; Nework, NY: 1969. [Google Scholar]
- 66. http://anabolicminds.com/forum/steroids/9989-vida-text-androgens.html (accessed June 2010)
- 67. http://www.demonoid.com/files/details/1643294/008522825736/ (accessed June 2010)
- 68.Kicman AT. Pharmacology of anabolic steroids. Br. J. Pharmacol. 2008;154:502–521. doi: 10.1038/bjp.2008.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mohler ML, Bohl CE, Jones A, Coss CC, Narayanan R, He Y, Hwang DJ, Dalton JT, Miller DD. Nonsteroidal selective androgen receptor modulators (SARMs): dissociating the anabolic and androgenic activities of the androgen receptor for therapeutic benefit. J. Med. Chem. 2009;52:3597–3617. doi: 10.1021/jm900280m. [DOI] [PubMed] [Google Scholar]
- 70.Dalton JT, Mukherjee A, Zhu Z, Kirkovsky L, Miller DD. Discovery of nonsteroidal androgens. Biochem. Biophys. Res. Commun. 1998;244:1–4. doi: 10.1006/bbrc.1998.8209. [DOI] [PubMed] [Google Scholar]
- 71.Narayanan R, Mohler ML, Bohl CE, Miller DD, Dalton JT. Selective androgen receptor modulators in preclinical and clinical development. Nucl. Recept. Signal. 2008;6:e010. doi: 10.1621/nrs.06010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kearbey JD, Wu D, Gao W, Miller DD, Dalton JT. Pharmacokinetics of S-3-(4-acetylamino-phenoxy)-2-hydroxy-2-methyl-N-(4-nitro-3-trifluoromethyl-phenyl)-propionamide in rats, a non-steroidal selective androgen receptor modulator. Xenobiotica. 2004;3:273–280. doi: 10.1080/0049825041008962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen J, Kim J, Dalton JT. Discovery and therapeutic promise of selective androgen receptor modulators. Mol. Interv. 2005;5:173–188. doi: 10.1124/mi.5.3.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thevis M, Schänzer W. Synthetic anabolic agents: steroids and nonsteroidal selective androgen receptor modulators. Handb. Exp. Pharmacol. 2010;195:99–126. doi: 10.1007/978-3-540-79088-4_5. [DOI] [PubMed] [Google Scholar]
- 75.Chang CY, McDonnell DP. Androgen receptor-cofactor interactions as targets for new drug discovery. Trends Pharmacol. Sci. 2005;26:225–228. doi: 10.1016/j.tips.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 76.WADA. The World Anti-Doping Code . International Standard. WADA; Montreal, QC, Canada: 2010. The 2010 Prohibited List. [Google Scholar]
- 77.Thevis M, Schanzer W. Mass spectrometry of selective androgen receptor modulators. J. Mass Spectrom. 2008;43:865–876. doi: 10.1002/jms.1438. [DOI] [PubMed] [Google Scholar]
- 78.Kuuranne T, Leinonen A, Schanzer W, Kamber M, Kostiainen R, Thevis M. Aryl-propionamide-derived selective androgen receptor modulators: liquid chromatography–tandem mass spectrometry characterization of the in vitro synthesized metabolites for doping control purposes. Drug Metab. Dispos. 2008;36:571–581. doi: 10.1124/dmd.107.017954. [DOI] [PubMed] [Google Scholar]
- 79.Thevis M, Kohler M, Thomas A, Maurer J, Schlorer N, Kamber M, Schanzer W. Determination of benzimidazole-and bicyclic hydantoin-derived selective androgen receptor antagonists and agonists in human urine using LC–MS/MS. Anal. Bioanal. Chem. 2008;391:251–261. doi: 10.1007/s00216-008-1882-6. [DOI] [PubMed] [Google Scholar]
- 80.Thevis M, Thomas A, Kohler M, Beuck S, Schanzer W. Emerging drugs: mechanism of action, mass spectrometry and doping control analysis. J. Mass Spectrom. 2009;44:442–460. doi: 10.1002/jms.1584. [DOI] [PubMed] [Google Scholar]
- 81.Thevis M, Schanzer W. Illicit organogenesis: methods and substances of doping and manipulation. Organogenesis. 2008;4:264–271. doi: 10.4161/org.4.4.7286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thevis M, Kohler M, Schanzer W. New drugs and methods of doping and manipulation. Drug Discov. Today. 2008;13:59–66. doi: 10.1016/j.drudis.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 83.Borges CR, Miller N, Shelby M, Hansen M, White C, Slawson MH, Monti K, Crouch DJ. Analysis of a challenging subset of World Anti-Doping Agency-banned steroids and antiestrogens by LC–MS–MS. J. Anal. Toxicol. 2007;31:125–131. doi: 10.1093/jat/31.3.125. [DOI] [PubMed] [Google Scholar]
- 84.Kolmonen M, Leinonen A, Pelander A, Ojanperä I. A general screening method for doping agents in human urine by solid phase extraction and liquid chromatography/time-of-flight mass spectrometry. Anal. Chim. Acta. 2007;585:94–102. doi: 10.1016/j.aca.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 85.Mazzarino M, Orengia M, Botre F. Application of fast gas chromatography/mass spectrometry for the rapid screening of synthetic anabolic steroids and other drugs in anti-doping analysis. Rapid Commun. Mass Spectrom. 2007;21:4117–4124. doi: 10.1002/rcm.3326. [DOI] [PubMed] [Google Scholar]
- 86.Pozo OJ, Van Eenoo P, Deventer K, Delbeke FT. Development and validation of a qualitative screening method for the detection of exogenous anabolic steroids in urine by liquid chromatography–tandem mass spectrometry. Anal. Bioanal. Chem. 2007;389:1209–1224. doi: 10.1007/s00216-007-1530-6. [DOI] [PubMed] [Google Scholar]
- 87.Roy P, Alevizaki M, Huhtaniemi I. In vitro bioassays for androgens and their diagnostic applications. Hum. Reprod. Update. 2008;14:73–82. doi: 10.1093/humupd/dmm038. [DOI] [PubMed] [Google Scholar]
- 88.Bovee TF, Bor G, Heskamp HH, Lasaroms JJ, Sanders MB, Nielen MW. Validation and application of a yeast bioassay for screening androgenic activity in calf urine and feed. Anal. Chim. Acta. 2009;637:225–234. doi: 10.1016/j.aca.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 89.Bovee TF, Helsdingen RJ, Hamers AR, van Duursen MB, Nielen MW, Hoogenboom RL. A new highly specific and robust yeast androgen bioassay for the detection of agonists and antagonists. Anal. Bioanal. Chem. 2007;389:1549–1558. doi: 10.1007/s00216-007-1559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bovee TF, Thevis M, Hamers AR, Peijnenburg AA, Nielen MW, Schoonen WG. SERMs and SARMs: detection of their activities with yeast based bioassays. J. Steroid Biochem. Mol. Biol. 2009;118:85–92. doi: 10.1016/j.jsbmb.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 91.Gaido KW, Leonard LS, Lovell S, Gould JC, Babai D, Portier CJ, McDonnell DP. Evaluation of chemicals with endocrine modulating activity in a yeast-based steroid hormone receptor gene transcription assay. Toxicol. Appl. Pharmacol. 1997;143:205–212. doi: 10.1006/taap.1996.8069. [DOI] [PubMed] [Google Scholar]
- 92.Lee HJ, Lee YS, Kwon HB, Lee K. Novel yeast bioassay system for detection of androgenic and antiandrogenic compounds. Toxicol. In Vitro. 2003;17:237–244. doi: 10.1016/s0887-2333(03)00009-2. [DOI] [PubMed] [Google Scholar]
- 93.Leskinen P, Michelini E, Picard D, Karp M, Virta M. Bioluminescent yeast assays for detecting estrogenic and androgenic activity in different matrices. Chemosphere. 2005;61:259–266. doi: 10.1016/j.chemosphere.2005.01.080. [DOI] [PubMed] [Google Scholar]
- 94.Mak P, Cruz FD, Chen S. A yeast screen system for aromatase inhibitors and ligands for androgen receptor: yeast cells transformed with aromatase and androgen receptor. Environ. Health Perspect. 1999;107:855–860. doi: 10.1289/ehp.99107855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Michelini E, Leskinen P, Virta M, Karp M, Roda A. A new recombinant cell-based bioluminescent assay for sensitive androgen-like compound detection. Biosens. Bioelectron. 2005;20:2261–2267. doi: 10.1016/j.bios.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 96.Michelini E, Magliulo M, Leskinen P, Virta M, Karp M, Roda A. Recombinant cell-based bioluminescence assay for androgen bioactivity determination in clinical samples. Clin. Chem. 2005;51:1995–1998. doi: 10.1373/clinchem.2005.053017. [DOI] [PubMed] [Google Scholar]
- 97.Purvis IJ, Chotai D, Dykes CW, Lubahn DB, French FS, Wilson EM, Hobden AN. An androgen-inducible expression system for Saccharomyces cerevisiae. Gene. 1991;106:35–42. doi: 10.1016/0378-1119(91)90563-q. [DOI] [PubMed] [Google Scholar]
- 98.Sohoni P, Sumpter JP. Several environmental oestrogens are also anti-androgens. J. Endocrinol. 1998;158:327–339. doi: 10.1677/joe.0.1580327. [DOI] [PubMed] [Google Scholar]
- 99.Zierau O, Lehmann S, Vollmer G, Schanzer W, Diel P. Detection of anabolic steroid abuse using a yeast transactivation system. Steroids. 2008;73:1143–1147. doi: 10.1016/j.steroids.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 100.Beck V, Reiter E, Jungbauer A. Androgen receptor transactivation assay using green fluorescent protein as a reporter. Anal. Biochem. 2008;373:263–271. doi: 10.1016/j.ab.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 101.Blankvoort BM, de Groene EM, van Meeteren-Kreikamp AP, Witkamp RF, Rodenburg RJ, Aarts JM. Development of an androgen reporter gene assay (AR-LUX) utilizing a human cell line with an endogenously regulated androgen receptor. Anal. Biochem. 2001;298:93–102. doi: 10.1006/abio.2001.5352. [DOI] [PubMed] [Google Scholar]
- 102.Chen J, Sowers MR, Moran FM, McConnell DS, Gee NA, Greendale GA, Whitehead C, Kasim-Karakas SE, Lasley BL. Circulating bioactive androgens in midlife women. J. Clin. Endocrinol. Metab. 2006;91:4387–4394. doi: 10.1210/jc.2006-0284. [DOI] [PubMed] [Google Scholar]
- 103.de Gooyer ME, Oppers-Tiemissen HM, Leysen D, Verheul HA, Kloosterboer HJ. Tibolone is not converted by human aromatase to 7alpha-methyl-17alpha-ethynylestradiol (7alpha-MEE): analyses with sensitive bioassays for estrogens and androgens and with LC–MSMS. Steroids. 2003;68:235–243. doi: 10.1016/s0039-128x(02)00184-8. [DOI] [PubMed] [Google Scholar]
- 104.Houtman CJ, Sterk SS, van de Heijning MP, Brouwer A, Stephany RW, van der Burg B, Sonneveld E. Detection of anabolic androgenic steroid abuse in doping control using mammalian reporter gene bioassays. Anal. Chim. Acta. 2009;637:247–258. doi: 10.1016/j.aca.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 105.Mnif W, Dagnino S, Escande A, Pillon A, Fenet H, Gomez E, Casellas C, Duchesne MJ, Hernandez-Raquet G, Cavaillès V, Balaguer P, Bartegi A. Biological analysis of endocrine-disrupting compounds in Tunisian sewage treatment plants. Arch. Environ. Contam. Toxicol. 2010;59(1):1–12. doi: 10.1007/s00244-009-9438-0. [DOI] [PubMed] [Google Scholar]
- 106.Paris F, Rabeolina F, Balaguer P, Bacquet A, Sultan C. Antiandrogenic activity of norgestimate in a human androgen-dependent stable-transfected cell line. Gynecol. Endocrinol. 2007;23:193–197. doi: 10.1080/09513590701214414. [DOI] [PubMed] [Google Scholar]
- 107.Paris F, Servant N, Terouanne B, Balaguer P, Nicolas JC, Sultan C. A new recombinant cell bioassay for ultrasensitive determination of serum estrogenic bioactivity in children. J. Clin. Endocrinol. Metab. 2002;87:791–797. doi: 10.1210/jcem.87.2.8269. [DOI] [PubMed] [Google Scholar]
- 108.Paris F, Servant N, Terouanne B, Sultan C. Evaluation of androgenic bioactivity in human serum by recombinant cell line: preliminary results. Mol. Cell Endocrinol. 2002;198:123–129. doi: 10.1016/s0303-7207(02)00375-1. [DOI] [PubMed] [Google Scholar]
- 109.Raivio T, Palvimo JJ, Dunkel L, Wickman S, Janne OA. Novel assay for determination of androgen bioactivity in human serum. J. Clin. Endocrinol. Metab. 2001;86:1539–1544. doi: 10.1210/jcem.86.4.7329. [DOI] [PubMed] [Google Scholar]
- 110.Roy P, Franks S, Read M, Huhtaniemi IT. Determination of androgen bioactivity in human serum samples using a recombinant cell based in vitro bioassay. J. Steroid Biochem. Mol. Biol. 2006;101:68–77. doi: 10.1016/j.jsbmb.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 111.Roy P, Salminen H, Koskimies P, Simola J, Smeds A, Saukko P, Huhtaniemi IT. Screening of some anti-androgenic endocrine disruptors using a recombinant cell-based in vitro bioassay. J. Steroid Biochem. Mol. Biol. 2004;88:157–166. doi: 10.1016/j.jsbmb.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 112.Sonneveld E, Jansen HJ, Riteco JA, Brouwer A, van der Burg B. Development of androgen- and estrogen-responsive bioassays, members of a panel of human cell line-based highly selective steroid-responsive bioassays. Toxicol. Sci. 2005;83:136–148. doi: 10.1093/toxsci/kfi005. [DOI] [PubMed] [Google Scholar]
- 113.Sonneveld E, Riteco JA, Jansen HJ, Pieterse B, Brouwer A, Schoonen WG, van der Burg B. Comparison of in vitro and in vivo screening models for androgenic and estrogenic activities. Toxicol. Sci. 2006;89:173–187. doi: 10.1093/toxsci/kfj009. [DOI] [PubMed] [Google Scholar]
- 114.Terouanne B, Nirdè P, Rabenoelina F, Bourguet W, Sultan C, Auzou G. Mutation of the androgen receptor at amino acid 708 (Gly-->Ala) abolishes partial agonist activity of steroidal antiandrogens. Mol. Pharmacol. 2003;63:791–798. doi: 10.1124/mol.63.4.791. [DOI] [PubMed] [Google Scholar]
- 115.Szafran AT, Szwarc M, Marcelli M, Mancini MA. Androgen receptor functional analyses by high throughput imaging: determination of ligand, cell cycle, and mutation-specific effects. PLoS One. 2008;3:e3605. doi: 10.1371/journal.pone.0003605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Denolet E, Gendt KD, Swinnen JV, Verrijdt G, Deboel L, Roskams T, Verhoeven G. Transfection with steroid-responsive reporter constructs shows glucocorticoid rather than androgen responsiveness in cultured Sertoli cells. J. Steroid Biochem. Mol. Biol. 2006;98:164–173. doi: 10.1016/j.jsbmb.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 117.Wilson VS, Bobseine K, Lambright CR, Gray LE., Jr. A novel cell line, MDA-kb2, that stably expresses an androgen- and glucocorticoid-responsive reporter for the detection of hormone receptor agonists and antagonists. Toxicol. Sci. 2002;66:69–81. doi: 10.1093/toxsci/66.1.69. [DOI] [PubMed] [Google Scholar]
- 118.Zuccarello D, Ferlin A, Vinanzi C, Prana E, Garolla A, Callewaert L, Claessens F, Brinkmann AO, Foresta C. Detailed functional studies on androgen receptor mild mutations demonstrate their association with male infertility. Clin. Endocrinol. (Oxf.) 2008;68:580–588. doi: 10.1111/j.1365-2265.2007.03069.x. [DOI] [PubMed] [Google Scholar]
- 119.Claessens F, Alen P, Devos A, Peeters B, Verhoeven G, Rombauts W. The androgen-specific probasin response element 2 interacts differentially with androgen and glucocorticoid receptors. J. Biol. Chem. 1996;271:19013–19016. doi: 10.1074/jbc.271.32.19013. [DOI] [PubMed] [Google Scholar]
- 120.Claessens F, Verrijdt G, Schoenmakers E, Haelens A, Peeters B, Verhoeven G, Rombauts W. Selective DNA binding by the androgen receptor as a mechanism for hormone-specific gene regulation. J. Steroid Biochem. Mol. Biol. 2001;76:23–30. doi: 10.1016/s0960-0760(00)00154-0. [DOI] [PubMed] [Google Scholar]
- 121.Schoenmakers E, Verrijdt G, Peeters B, Verhoeven G, Rombauts W, Claessens F. Differences in DNA binding characteristics of the androgen and glucocorticoid receptors can determine hormone-specific responses. J. Biol. Chem. 2000;275:12290–12297. doi: 10.1074/jbc.275.16.12290. [DOI] [PubMed] [Google Scholar]
- 122.Verrijdt G, Schoenmakers E, Alen P, Haelens A, Peeters B, Rombauts W, Claessens F. Androgen specificity of a response unit upstream of the human secretory component gene is mediated by differential receptor binding to an essential androgen response element. Mol. Endocrinol. 1999;13:1558–1570. doi: 10.1210/mend.13.9.0347. [DOI] [PubMed] [Google Scholar]
- 123.Verrijdt G, Schoenmakers E, Haelens A, Peeters B, Verhoeven G, Rombauts W, Claessens F. Change of specificity mutations in androgen-selective enhancers. Evidence for a role of differential DNA binding by the androgen receptor. J. Biol. Chem. 2000;275:12298–12305. doi: 10.1074/jbc.275.16.12298. [DOI] [PubMed] [Google Scholar]
- 124.Maruvada P, Baumann CT, Hager GL, Yen PM. Dynamic shuttling and intranuclear mobility of nuclear hormone receptors. J. Biol. Chem. 2003;278:12425–12432. doi: 10.1074/jbc.M202752200. [DOI] [PubMed] [Google Scholar]
- 125.Schaaf MJ, Cidlowski JA. Molecular determinants of glucocorticoid receptor mobility in living cells: the importance of ligand affinity. Mol. Cell Biol. 2003;23:1922–1934. doi: 10.1128/MCB.23.6.1922-1934.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Klinge CM, Jernigan SC, Mattingly KA, Risinger KE, Zhang J. Estrogen response element-dependent regulation of transcriptional activation of estrogen receptors alpha and beta by coactivators and corepressors. J. Mol. Endocrinol. 2004;33:387–410. doi: 10.1677/jme.1.01541. [DOI] [PubMed] [Google Scholar]
- 127.Norris JD, Paige LA, Christensen DJ, Chang CY, Huacani MR, Fan D, Hamilton PT, Fowlkes DM, McDonnell DP. Peptide antagonists of the human estrogen receptor. Science. 1999;285:744–746. doi: 10.1126/science.285.5428.744. [DOI] [PubMed] [Google Scholar]
- 128.Zhang J, Hu X, Lazar MA. A novel role for helix 12 of retinoid X receptor in regulating repression. Mol. Cell Biol. 1999;19:6448–6457. doi: 10.1128/mcb.19.9.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shen O, Du G, Sun H, Wu W, Jiang Y, Song L, Wang X. Comparison of in vitro hormone activities of selected phthalates using reporter gene assays. Toxicol. Lett. 2009;191:9–14. doi: 10.1016/j.toxlet.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 130.Saporita AJ, Zhang Q, Navai N, Dincer Z, Hahn J, Cai X, Wang Z. Identification and characterization of a ligand-regulated nuclear export signal in androgen receptor. J. Biol. Chem. 2003;278:41998–42005. doi: 10.1074/jbc.M302460200. [DOI] [PubMed] [Google Scholar]
- 131.Massaad C, Garlatti M, Wilson EM, Cadepond F, Barouki R. A natural sequence consisting of overlapping glucocorticoid-responsive elements mediates glucocorticoid, but not androgen, regulation of gene expression. Biochem. J. 350 Pt. 2000;1:123–129. [PMC free article] [PubMed] [Google Scholar]
- 132.Escande A, Servant N, Rabenoelina F, Auzou G, Kloosterboer H, Cavailles V, Balaguer P, Maudelonde T. Regulation of activities of steroid hormone receptors by tibolone and its primary metabolites. J. Steroid Biochem. Mol. Biol. 2009;116:8–14. doi: 10.1016/j.jsbmb.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 133.Zierau O, Morrissey C, Watson RW, Schwab P, Kolba S, Metz P, Vollmer G. Antiandrogenic activity of the phytoestrogens naringenin, 6-(1,1-dimethylallyl)naringenin and 8-prenylnaringenin. Planta Med. 2003;69:856–858. doi: 10.1055/s-2003-43222. [DOI] [PubMed] [Google Scholar]
- 134.Terouanne B, Tahiri B, Georget V, Belon C, Poujol N, Avances C, Orio F, Jr., Balaguer P, Sultan C. A stable prostatic bioluminescent cell line to investigate androgen and antiandrogen effects. Mol. Cell Endocrinol. 2000;160:39–49. doi: 10.1016/s0303-7207(99)00251-8. [DOI] [PubMed] [Google Scholar]
- 135.Schimmer BP, Parker KL. Adrenocorticotropic hormone; adrenocortical steroids and their synthetic analogs; inhibitors of the synthesis and actions of adrenocortical hormones. In: Hardman JG, Limbird LE, Gilman AG, editors. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 10th McGraw-Hill; New York, NY: 2001. pp. pp 1649–1677. [Google Scholar]