A T cell-associated protective effect of IFNAR activation is revealed using a novel transgenic mouse model with functional IFNAR expression.
Keywords: cytokine receptors, T cells, MS, inflammation, transgenic mice
Abstract
Although interferon-β is used as first-line therapy for multiple sclerosis, the cell type-specific activity of type I interferons in multiple sclerosis and its animal model, experimental autoimmune encephalomyelitis, remains obscure. In this study, we have elucidated the in vivo immunomodulatory role of type I interferon signaling in T cells during experimental autoimmune encephalomyelitis by use of a novel transgenic mouse, carrying a cd2–ifnar1 transgene on a interferon-α/β receptor 1 null genetic background, thus allowing expression of the interferon-α/β receptor 1 and hence, a functional type I interferon receptor exclusively on T cells. These transgenic mice exhibited milder experimental autoimmune encephalomyelitis with reduced T cell infiltration, demyelination, and axonal damage in the central nervous system. It is noteworthy that interferon-β administration in transgenic mice generated a more pronounced, protective effect against experimental autoimmune encephalomyelitis compared with untreated littermates. In vivo studies demonstrated that before experimental autoimmune encephalomyelitis onset, endogenous type I interferon receptor signaling in T cells led to impaired T-helper 17 responses, with a reduced fraction of CCR6+ CD4+ T cells in the periphery. At the acute phase, an increased proportion of interleukin-10- and interferon-γ-producing CD4+ T cells was detected in the periphery of the transgenic mice, accompanied by up-regulation of the interferon-γ-induced gene Irgm1 in peripheral T cells. Together, these results reveal a hitherto unknown T cell-associated protective role of type I interferon in experimental autoimmune encephalomyelitis that may provide valuable clues for designing novel therapeutic strategies for multiple sclerosis.
Introduction
IFN-Is are widely expressed cytokines that possess strong antiviral and immunomodulatory properties [1] and under physiologic conditions, regulate homeostatic processes [2, 3]. The most well defined IFN-Is, IFN-α and IFN-β, are expressed ubiquitously [4]. Both IFN-I subtypes signal through a heterodimeric receptor, the IFNAR, composed of 2 subunits, IFNAR1 and IFNAR2, which are expressed in almost every cell type [5]. Pathogens and other environmental stimuli cross-regulate IFNAR-induced signaling cascades, which integrate at the gene level to control the transcription of numerous ISGs that contribute to a pro- and/or anti-inflammatory outcome [5–7]. IFN-Is are used for treatment of a wide range of diseases, with IFN-β as a first-line intervention for relapsing-remitting MS [8].
MS is a demyelinating disease of the CNS, with unknown etiology, traditionally considered to be an autoimmune inflammatory disorder, mediated by an aberrant T cell attack against CNS elements, particularly myelin [9]. EAE is the mouse model that simulates well the immune features of the human disease; it is induced by immunization with encephalitogenic antigens derived from CNS proteins [10]. The disease course of EAE can be considered to occur in 2 stages: a priming phase, in which autoreactive T cells are activated and expanded in the periphery, and an effector phase, in which infiltrating inflammatory cells cause CNS inflammation, demyelination, and axonal damage [11]. Several studies implicate Th17 and Th1 cells as the main cell subsets involved in the pathogenesis of MS and EAE; however, the contribution of each subset remains contentious [12, 13]. How these T cell subsets interact in vivo among themselves and with the other Treg and effector T cell subsets remains to be fully elucidated [11, 12, 14–16]. In addition, recent findings suggest that Th17 cells can switch to other Th cell subsets depending on the cytokine environment, thereby affecting autoimmune disorders, including EAE [17–19].
Although, to date, no curative therapy for MS is available, treatment with IFN-β is commonly used for controlling exacerbations and progression in MS [8]. Cumulative findings obtained from various in vitro and in vivo systems indicate that IFN-β treatment generates broad immunomodulatory effects and exerts beneficial effects on MS patients by acting on different levels of the immune response, although the precise, underlying mechanisms remain uncertain [20–22]. Thus, it has been reported that IFN-β increases anti-inflammatory cytokine production, such as IL-4 and IL-10; decreases proinflammatory cytokines production, such as IL-17, IL-23, osteopontin, IFN-γ, and TNF-α; induces apoptosis of autoreactive T cells; decreases leukocyte migration across the BBB; and modulates function of Tregs, while inducing expansion of naïve Treg populations [20–22]. Consistent with the beneficial effect of IFN-β in MS, EAE can be suppressed by injection of rIFN-β [23, 24]. Furthermore, the immune-suppressive activity of endogenously expressed IFN-β in EAE has also been shown recently, as Ifnb gene deletion strongly enhances the course of EAE [24, 25]. However, IFN-β therapy has been proven only partially effective, as often, patients do not respond to therapy, whereas IFN-β can even exacerbate clinical symptoms in some individuals [26]. Interestingly, recent studies show that IFN-β is a double-edged sword in autoimmune diseases; it alleviates symptoms in conditions with Th1 bias, whereas it promotes pathology in Th17-mediated diseases [23, 27]. Therefore, to improve therapeutic approaches, it is imperative to understand the mechanisms by which IFN-β exerts its pro- and anti-inflammatory functions. In this direction, an important task is to delineate the direct in vivo effects of IFN-I on different cell types. This task is largely complicated by the fact that almost all cell types respond to IFN-I.
In this study, we used a newly generated transgenic mouse strain, expressing functional IFNAR selectively on T lymphocytes, to investigate the direct role of IFN-Is on this cell type during EAE development. We show herein that T cell-targeted endogenous and exogenous IFN-I signaling is crucial for the initiation phase of EAE, resulting in delayed onset and reduced severity of the disease at the acute phase. Importantly, IFN-β administration in IFNAR1Texcl mice generated a more pronounced, protective effect during EAE compared with untreated littermates. This attenuated EAE course was accompanied by decreased infiltration of immune cells into the CNS, as well as reduced demyelination and axonal loss. IFNAR signaling in T cells was associated with a reduced Th17 profile of peripheral T cells before EAE onset and increased proportion of CD4+ IFN-γ+ and CD4+ IL-10+ T cells at the acute phase of EAE. Moreover, the expression of IFN-γ-induced gene Irgm1 was up-regulated in peripheral T cells and down-regulated in the spinal cord of IFNAR1Texcl EAE mice. Collectively, these data indicate that IFN-I signaling in T cells is an important regulator of EAE development, suggesting that T cell-targeted IFN-β therapy might be beneficial in MS.
MATERIALS AND METHODS
Generation of CD2–IFNAR1 transgenic mice in the Ifnar1−/− background
mIFNAR1 cDNA was inserted in a hcd2 promoter cassette (provided by Dr. D. Kioussis, National Institute for Medical Research, London, United Kingdom) [28], containing a FLAG tag and hcd2 LCR. The 13.4 kb KpnI-XbaI fragment was microinjected into pronuclei of F1 (CBA/CaOla × C57BL/6OlaHsd)-fertilized oocytes, as described previously [29]. Five transgenic lines were produced (TgIFNAR1) that transmitted the transgene in a Mendelian way. To obtain mice with specific expression of IFNAR1 in T cells (cd2−Ifnar1+/−/Ifnar1−/−), the transgenic mice were backcrossed to the Ifnar1−/− C57BL/6 background for at least 12 generations [30]. To identify transgenic mice, genomic DNA was amplified with primers specific for hcd2: forward, 5′-CCA AAG GTA AGC ATA AGA G-3′, and mifnar1: reverse, 5′-AGA TTC GTA TGG TGT AAC TG-3′. Mice were maintained under specific pathogen-free conditions in the facilities of the Department of Experimental Animal Models for Biomedical Research of Hellenic Pasteur Institute (Facilities License Numbers: ELBIO13 and ELBIO12). All animal procedures were approved by Institutional Animal Care and Use Committee, and licenses were issued by national authorities, according to the Greek Law 56/2013, in conformity with European Union guidelines.
Primary cell cultures and CD3+–T cell enrichment
LNs, spleens, and thymi were harvested and single-cell suspensions prepared by standard procedures. For studies of T cells, cell suspensions were negatively selected by use of mCD3+–T cell enrichment columns (R&D Systems, Minneapolis, MN, USA), according to the manufacturer’s protocol, achieving >90% purity of CD3+ T cells, as determined by flow cytometry. Cells were cultured at 37°C and 5% CO2 in RPMI 1640 medium (Invitrogen Life Technologies, Gaithersburg, MD, USA), supplemented with 10% heat-inactivated FBS, 50 mM 2-ME, and antibiotics (penicillin, streptomycin).
T Cell priming and proliferation assays
Isolated splenocytes and LN cells were stimulated in triplicates at 2 × 106 cells/ml in flat-bottom, 96-well plates, coated with 0.5–2.5 μg/ml anti-CD3 mAb (clone 145-2C11; BD Pharmingen, San Diego, CA, USA) and 1 μg/ml anti-CD28 mAb (clone 37.51; BD Pharmingen) for 72 h. Cells were pulsed with 1 μCi/2 × 105 cells of [3H]thymidine (PerkinElmer, Waltham, MA, USA) for the last 16 h of culture, and [3H]thymidine incorporation was measured by liquid scintillation counting (Wallac, Turku, Finland). Alternatively, T cells were primed in vivo by immunizing mice subcutaneously with 50 μg MOG35–55 (Biotrend, Germany), emulsified in CFA (Sigma-Aldrich, St. Louis, MO, USA), supplemented with 400 μg Mycobacterium tuberculosis H37Ra (Difco, Detroit, MI, USA). dLNs and spleen were collected on d 10 after immunization, and isolated cells were cultured for 72 h in 96-well plates with increasing concentrations of MOG35–55. Alternatively, CD3+-enriched T cells were cocultured with irradiated splenocytes in the presence of MOG35–55. Cell proliferation was measured, as described above. Results are expressed as the stimulation index (ratio between radioactivity counts of cells cultured in the presence of antigen and cells cultured with medium alone). In all cases, mitogenic stimulation with Con A served as an internal assay control.
Qualitative and quantitative RT-PCR
Total RNA was extracted from selected tissues with TRIzol (Invitrogen Life Technologies), according to the manufacturer’s instructions. For qualitative RT-PCR, DNase-treated (Promega, Madison, WI, USA) RNA was reverse transcribed with Moloney murine leukemia virus RT (Promega) and random hexamers (Roche, Indianapolis, IN, USA). For the detection of transgenic Ifnar1 mRNA, cDNA was amplified with primers specific for IFNAR1: forward, 5′-GAA GAG TGT CTT GAT GAA GA-3′; and the FLAG sequence of the transgenic cassette: reverse, 5′-GAA AAG CTG GAT ATG ATA GC-3′. The specific PCR product was 488 bp. Mouse actin PCR served as control for reverse transcription. For quantitative analysis of specific gene expression, quantitative RT-PCR was performed by use of the QuantiFast SYBR Green RT-PCR kit (Qiagen, Germantown, MD, USA), according to the manufacturer’s instructions. At the end of each PCR run, melting curve analysis was performed to verify the integrity and homogeneity of PCR products. QuantiTect Primer Assays (Qiagen) were used for Ifnar1, Irgm1, Stap1, Slfn8, Gapdh, and Gusb. All reactions were performed by use of the LightCycler 2.0 Instrument (Roche). Gene-expression levels were calculated by use of standard curves for each gene. These standard curves were created by plotting CT values versus the logarithm of serial-diluted RNA concentrations. Least-squares methods were used for the determination of A and B values in the equation, CT = A ⋅ log (CRNA) + B. R2 > 0.99. Gene-expression levels were normalized to the internal control genes Gapdh and Gusb.
Western blot analysis
For assessment of p-STAT1 and ISG15 expression, thymocytes were incubated with rmIFN-β (250 U/ml; PBL Assay Science, Piscataway, NJ, USA) at 37°C, 5% CO2, for the indicated time and lysed in 50 mM Tris-HCl (pH 7.4), 100 mM NaCl, 2 mM EDTA, 0.1% Triton X-100, 0.1% SDS, 1 mM NaF, 1 mM benzamidine, 10 μg/ml aprotinin, 1 mM sodium orthovanadate, and 0.2 mM PMSF. Western blot was performed by use of antibodies against p-STAT1 (Y701; BD Transduction Laboratories, Lexington, KY, USA) and ISG15 (Cell Signaling Technology, Danvers, MA, USA). HRP-conjugated anti-mIgG and anti-rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, CA, USA) were used. Antibody binding was detected by use of the ECL Plus Detection System (GE Healthcare, Buckinghamshire, United Kingdom). To normalize for protein content, membranes were stripped and reprobed with a pan-actin antibody (clone C4; Chemicon International, Temecula, CA, USA).
Flow cytometry analysis
Cell staining and flow cytometric analysis were performed according to standard procedures by use of a FACSCalibur (BD Biosciences, San Jose, CA, USA). For the detection of cell-surface markers, cells were stained with FITC-labeled antibodies against CD3e, CD4, CD8, CD19, and CD11b, and allophycocyanin-labeled antibodies against CD4 and CCR6 (BD Biosciences). For the detection of intracellular p-STAT1, splenocytes and thioglycollate-activated peritoneal macrophages were incubated with rmIFN-β (250 U/ml) for 15 min. Upon staining against cell-surface markers, cells were fixed in 2% PFA for 15 min at room temperature, permeabilized by use of ice-cold methanol for 20 min, and stained by use of PE-labeled anti-p-STAT1 antibody (Y701, clone 4a; BD PharMingen). Cells were then washed with FACS buffer (PBS, supplemented with 3% FBS). Tregs were labeled by use of mouse regulatory T cell staining kit 1 (eBioscience, San Diego, CA, USA). Data acquisition was done with CellQuest software (BD Biosciences). For the neutralization assay, thymocytes were incubated with 3 μg/ml anti-IFNAR1 neutralizing antibody (MAR1-5A3; Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 30 min at 37°C, 5% CO2, before culture with rmIFN-β (500 U/ml), and p-STAT1 expression was measured by flow cytometry, as described above.
Cytokine measurements
The mTh1/Th2/Th17 cytokine Cytometric Bead Array kit (BD Biosciences) was used to measure cytokine levels in lymphocyte cell-culture supernatants, according to the manufacturer’s instructions. The cytokines measured were IL-2, IL-4, IL-6, IFN-γ, TNF-α, IL-10, and IL-17. Data acquisition was done with a FACSCalibur cytometer and CellQuest software (BD Biosciences) and data analysis with FCAP Array 3.0 software (Soft Flow, St. Louis Park, MN, USA).
In vitro Th cell differentiation and intracellular cytokine staining
CD3+-enriched T cells were cultured under nonpolarizing, as well as Th1- and Th17-polarizing, conditions in the presence of anti-CD3 mAb (1 μg/ml) and anti-CD28 mAb (1 μg/ml). Cytokines added for Th1 polarization were IL-12 (10 ng/ml) plus anti-IL-4 (10 μg/ml) and for Th17 polarization were IL-6 (20 ng/ml), TGF-β (10 ng/ml; Peprotech, London, United Kingdom), IL-23 (20 ng/ml; eBioscience), plus anti-IFN-γ (10 μg/ml; R&D Systems). Cells were cultured for 4 d and then restimulated for 3 h with 1 μg/ml ionomycin and 10 ng/ml PMA in the presence of 5 μg/ml brefeldin A (all purchased from Sigma-Aldrich). Cells were stained with anti-CD4-allophycocyanin antibody and then fixed with 2% PFA in PBS for 10 min at room temperature. After being fixed, the cells were made permeable for 20 min at room temperature with permeabilization buffer (PBS, 3% FBS, 0.5% w/v saponin) before staining with anti-IFN-γ and anti-IL-17-PE-conjugated antibodies (BD Biosciences).
EAE induction and evaluation and IFN-β administration
Experiments were performed in female C57BL/6 WT, Ifnar1−/−, and IFNAR1Texcl mice of 8–12 wk of age. EAE was induced by subcutaneous tail-base injection of 50 μg MOG35–55, dissolved in 100 μl saline, and emulsified in 100 μl CFA (Sigma-Aldrich), supplemented with 400 μg M tuberculosis H37Ra (Difco). Mice also received an intraperitoneal injection of 200 ng PTx (Sigma-Aldrich) on d 0 and 2. In EAE experiments with IFN-β administration, mice were injected intraperitoneally with rmIFN-β (104 U/100 μl/mouse), 16 h before the induction of EAE and 16 h before the PTx boost on d 2 [24]. Control mice were injected intraperitoneally with PBS. Mice were assessed daily for clinical signs, according to the following scale: 0, normal; 1, limp tail; 2, hindlimb weakness; 3, hindlimb paralysis; 4, forelimb paralysis; and 5, moribund or dead (0.5 gradation represents intermediate scores). Moribund animals were euthanized. Mice were allowed free access to food and water (ad libitum) throughout the experiment.
Histopathological analysis
Mice were transcardially perfused with ice-cold 4% PFA in PBS under deep anesthesia. Spine was postfixed in the same fixative overnight at 4°C. Spinal cord was isolated and processed for standard histopathological analysis. Inflammation was visualized by H&E, demyelination by Klüver-Barrera (Luxol fast blue), and axonal damage by Bielschowsky’s silver staining. Immunohistochemistry was performed, as described previously [31], by use of rabbit anti-CD3 antibody (1/300; Biodynamics, Shoreline, WA, USA), appropriate biotinylated secondary antibodies, avidin-biotin complex, and 3,3′-diaminobenzidine (Vector Laboratories, Burlingame, CA, USA). Antigen retrieval was performed in 10 mM EDTA buffer (pH 8.5) for 1 h at 95–100°C in a household food steamer.
cDNA microarray hybridizations and analysis
For all experiments, RNA from 3–5 animals/group was pooled and investigated. Total RNA was extracted from CD3+-enriched splenic T cells and homogenized spinal cords, separately from each individual animal, by use of TRIzol (Invitrogen Life Technologies), according to the manufacturer’s instructions. RNA concentrations were determined by use of NanoDrop 2000 spectrophotometer, and equal amounts of RNA from animals of the same group were pooled. Hybridizations for each experimental condition were repeated twice by use of samples from 2 independent experiments. For genome-wide expression, 100 ng total RNA was processed by use of the Ambion WT Expression Kit (Life Technologies, Carlsbad, CA, USA), according to the manufacturer's recommended protocols. The resultant sscDNA was fragmented labeled and hybridized to the GeneChip Mouse Gene ST 1.0 array (Affymetrix, Santa Clara, CA, USA). The arrays were washed and stained by use of the Affymetrix Hybridization, Wash, and Stain Kit on a Model 450 Fluidics Station and scanned by use of Affymetrix Model 3000 scanner, according to the manufacturer's instructions. Expression values were generated by use of the Microarray Expression console software (Affymetrix). The hybridizations were normalized with the robust multichip average algorithm to obtain summary expression values for each probe set. Normalized values were imported in Excel to calculate fold change, and a >1.5-fold change in gene expression was used to extract the significant data. Microarrays complied with the Minimum Information about a Microarray Experiment and are available at ArrayExpress (http://www.ebi.ac.uk/arrayexpress; Accession Number E-MTAB-2989). When there were discrepancies in the direction of expression between multiple probe sets, the gene was not included. Significant over-representation of 5th-level gene ontology terms describing “biological process” annotation (GOTERM_BP_5) was identified with the National Institute of Allergy and Infectious Diseases DAVID website (http://david.abcc.ncifcrf.gov/).
Statistical analysis
All statistical analyses were performed with SigmaPlot 11.0 software. Data were evaluated by use of nonparametric Mann-Whitney rank-sum test and are given as mean values ± sem. Differences with P < 0.05 were considered statistically significant.
RESULTS
Generation and characterization of transgenic mice expressing IFNAR1 exclusively on T cells
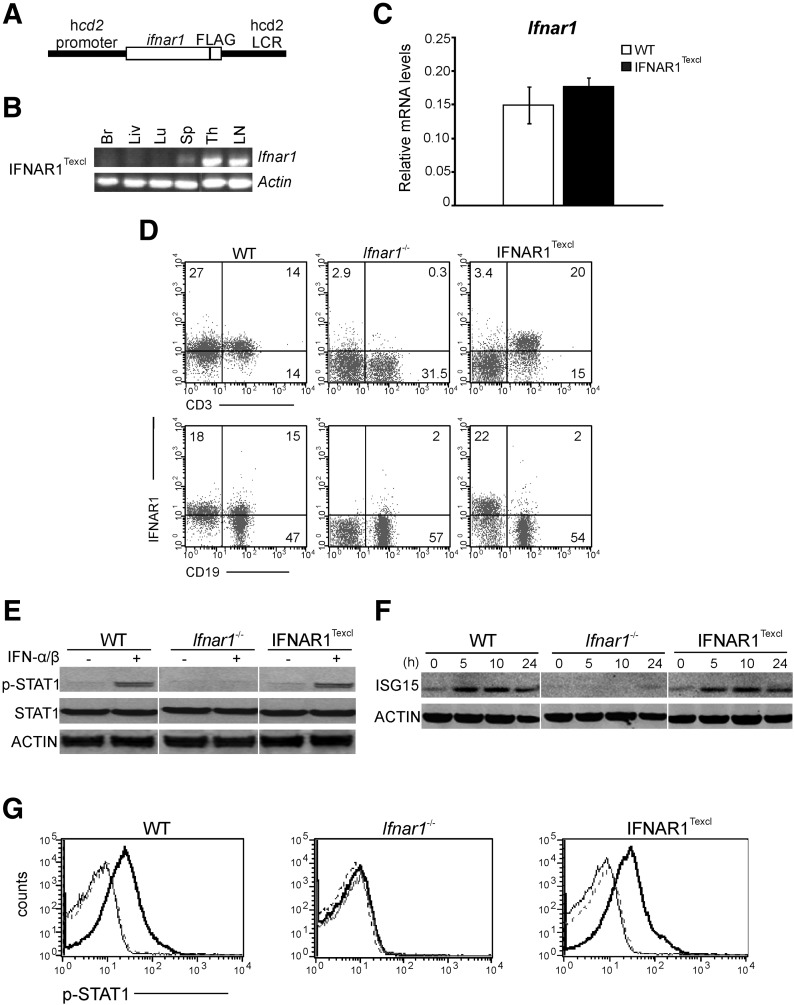
To achieve IFNAR1 signaling exclusively in T lymphocytes, we first generated transgenic mice expressing IFNAR1 under the control of the hcd2 promoter and enhancer [28] (Fig. 1A), which were backcrossed further to IFNAR1-deficient mice (Ifnar1−/−) and analyzed for re-expression and functionality of IFNAR1 on T cells (cd2–ifnar1+/−/ifnar1−/−). Five transgenic lines were identified and characterized; mice from all lines developed normally, with no obvious phenotypic abnormalities. Two transgenic lines, Tg9393 and Tg9446, were analyzed further, whereas the Tg9393 line was selected as representative in this study. As a result of the exclusive expression of IFNAR1 on T cells, these transgenic mice, from now on, will be referred as IFNAR1Texcl. The architecture of lymphoid organs and the percentages of T lymphocytes and CD4+ and CD8+ T cell subpopulations in the thymus, LNs, and spleen were normal, as determined by histologic and flow cytometry analysis, respectively (data not shown). Transcripts of ifnar1 were detected specifically in lymphoid tissues of IFNAR1Texcl mice but not in brain, liver, and lung (Fig. 1B). The levels of ifnar1 mRNA expression in IFNAR1Texcl CD3+-enriched T cells were found similar to that of WT, as measured by quantitative RT-PCR (Fig. 1C), whereas IFNAR1 was detected specifically on the surface of splenic T but not B lymphocytes, verifying T cell-specific expression of the transgene (Fig. 1D). Similar results were obtained from the analysis of the Tg9446 line (data not shown). To assess the integrity of IFN-I signaling in IFNAR1Texcl T lymphocytes, thymocytes from all groups were treated with IFN-α/β (250 U/ml) for indicated time points, and IFN activity was evaluated by detection of p-STAT1 and ISG15. Both proteins were detected only in IFN-α/β-treated WT and IFNAR1Texcl cells (Fig. 1E and F). A neutralizing antibody against IFNAR1 completely abrogated IFN-α/β-induced p-STAT1, confirming normal IFN-I signaling in our setting (Fig. 1G).
Figure 1. Transgenic IFNAR1 is expressed specifically on T cells of IFNAR1Texcl mice and is functionally active.
(A) Construct used to generate cd2–ifnar1 transgenic mice. (B) RT-PCR demonstrated lymphoid tissue-specific expression of transgenic ifnar1. With the use of a reverse primer for FLAG, this PCR reaction amplified the ifnar1 transcript only from transgenic mice. Br, Brain; Liv, liver; Lu, lung; Sp, spleen; Th, thymus. (C) RNA was extracted from CD3+-enriched T cells, and ifnar1 mRNA was amplified by real-time RT-PCR. (D) Splenocytes from WT, Ifnar1−/−, and IFNAR1Texcl mice were stained for CD3, CD19, and IFNAR1. (E and F) Splenocytes were stimulated by IFN-α/β (250 U/ml) for 30 min (E) or for the indicated time (F) and then analyzed by Western blot. STAT1 and p-STAT1, 89/91 kDa; ISG15, 15 kDa; actin, 42 kDa. The images were spliced and joined, as indicated by white lines for the sake of the presentation. (G) Thymocytes were incubated with anti-IFNAR1-neutralizing antibody before IFN-α/β treatment and stained with a p-STAT1 antibody. The histogram plots show the p-STAT1-positive cells in the total live-cell gate (IFN treatment, bold, black line; antibody + IFN treatment, black line; no treatment, dotted line).
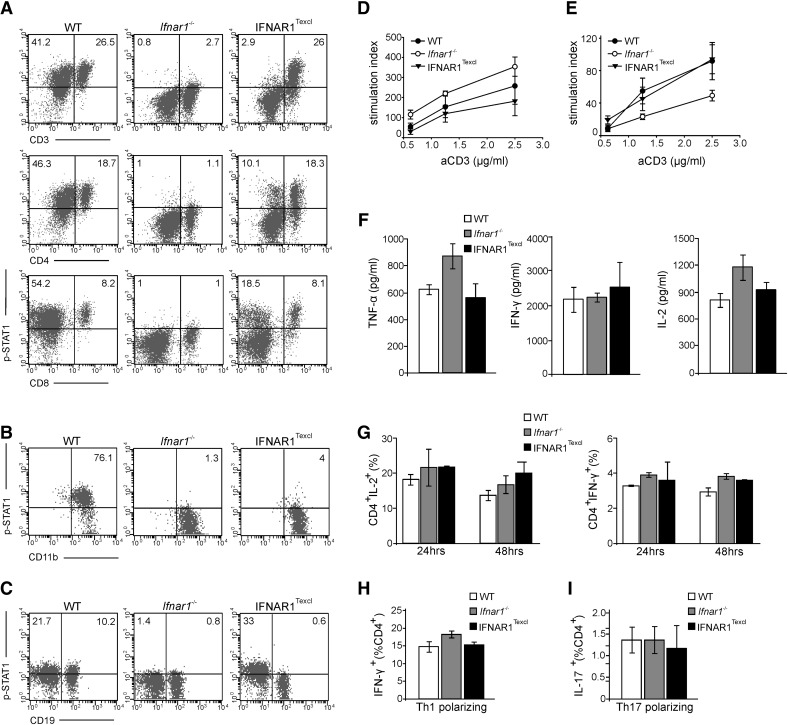
To ascertain T cell specificity of IFNAR signaling, p-STAT1 levels in various immune cell types were measured by flow cytometry in response to IFN-α/β (250 U/ml) for 15 min (Fig. 2A–C). p-STAT1 was detected specifically in the IFNAR1Texcl splenic T lymphocytes and their major subsets, CD4+ and CD8+ T cells (Fig. 2A), as well as in thymocytes (data not shown) at approximately similar levels to that of WT, but not in B cells or peritoneal macrophages (Fig. 2B and C), verifying T cell-specific IFNAR signaling. As expected, p-STAT1 was detected in all different cell types from WT mice but not from Ifnar1−/−mice. As previous studies demonstrated that IFN-I signaling can influence TCR-mediated T cell activation, proliferation, and cytokine profile [2, 32, 33], splenocytes and LN cells from all groups were stimulated by use of anti-CD3 and anti-CD28 antibodies. IFNAR1Texcl lymphoid cells exhibited equivalent responses compared with that of WT and Ifnar1−/− cells, showing a similar proliferation index (Fig. 2D and E) and similar production of TNF-α, IFN-γ, and IL-2 (Fig. 2F and G). Finally, to ensure that IFNAR1Texcl Th cells preserve their differentiation capacity, we compared cytokine production by CD3+-enriched T cells under Th1- and Th17-polarizing conditions. FACS analysis showed similar frequencies of CD4+ IFN-γ+ and CD4+ IL-17+ T cells in all groups, indicating that IFNAR1Texcl T cells differentiate normally into Th1 (Fig. 2H) and Th17 cells in vitro (Fig. 2I). Collectively, these results show that transgenic IFNAR1 is expressed specifically on T lymphocytes and signals properly without altering T cell capacity to proliferate, differentiate, and produce cytokines upon TCR engagement.
Figure 2. Transgenic IFNAR1 mediates IFN-α/β signaling exclusively in T lymphocytes without altering their functionality upon TCR stimulation.
(A–C) Splenocytes (A), peritoneal macrophages (B), and LN cells (C) were isolated from WT, Ifnar1−/−, and IFNAR1Texcl mice. Cells were treated with IFN-α/β (250 U/ml) for 15 min and double stained for CD3, CD4, CD8, CD11b, CD19, and p-STAT1. Dot plots show the double-positive cells in the total live-cell gate, and a representative dot plot/group is shown. (D and E) Thymidine uptake after anti (a)-CD3/anti-CD28 stimulation of splenocytes (D) and LN cells (E). Results shown are presented as mean values ± sem. (F) Cytokine production in splenocyte supernatants following stimulation with anti-CD3/anti-CD28. (G) Intracellular staining of splenocytes for IL-2 and IFN-γ after stimulation with anti-CD3/anti-CD28, followed by PMA/ionomycin/brefeldin A incubation. (H and I) CD3+-enriched T cells were stimulated under Th1- and Th17-polarizing conditions and stained for IFN-γ (H) and IL-17A (I). The bars represent the double-positive (CD4+ cytokine+) cells in the total live-cell gate. All data shown are representative of at least 2 independent experiments (n = 5 mice/genotype/experiment).
T Cell-targeted IFN-I signaling attenuates EAE
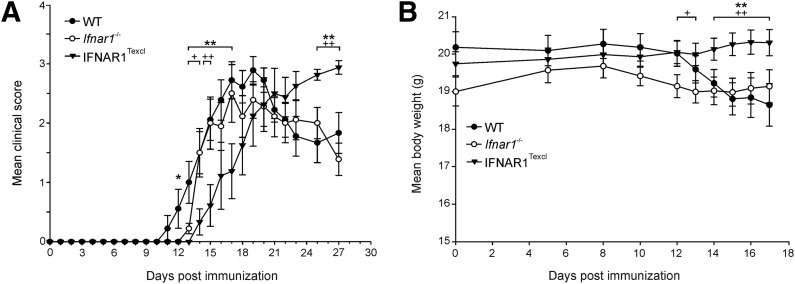
To study the in vivo effect of endogenous IFN-I signaling selectively in T lymphocytes during EAE, WT, Ifnar1−/−, and IFNAR1Texcl mice were immunized with MOG35–55 peptide, and the clinical manifestations and body weight were assessed daily until d 27. IFNAR1Texcl mice exhibited a significantly delayed onset and milder development of EAE compared with WT and Ifnar1−/− mice (Fig. 3 and Table 1). Milder disease development was also observed by use of the 2nd transgenic line, Tg9446 (data not shown). It is noteworthy that during the chronic phase of the disease, IFNAR1Texcl mice fared worse than those from both control groups (Fig. 3A), although the accumulative score of EAE in these mice was reduced (Table 1). Overall, these data show that endogenous T cell-specific IFN-I signaling modulates onset and progression of EAE.
Figure 3. Endogenous IFN-I signaling selectively in T cells modulates onset and progression of EAE.
(A) Mean clinical scores for WT, Ifnar1−/−, and IFNAR1Texcl mice (n = 9 mice/genotype) after immunization with MOG35–55 peptide. Results shown are representative of 3 independent experiments and are presented as mean values ± sem. (B) Mean body weight of WT (n = 30), Ifnar1−/− (n = 28), and IFNAR1Texcl (n = 32) mice after EAE induction, pooled from 3 independent experiments. Results shown are presented as mean values ± sem.*P < 0.05, **P < 0.01: IFNAR1Texcl versus WT mice; and +P < 0.05, ++P < 0.01: IFNAR1Texcl versus Ifnar1−/− mice.
TABLE 1.
Clinical severity, day of onset, and peak of MOG35–55-induced EAE in IFNAR1Texcl and control mice
| Genotype of mice | Day of onset (mean ± sem) | Maximum score until d 27 (mean ± sem) | Time to peak (d; mean ± sem) | Accumulative score until d 27 (mean ± sem) |
|---|---|---|---|---|
| WT | 13.1 ± 0.6 | 3.3 ± 0.1 | 16.9 ± 0.8 | 31.7 ± 2.6 |
| Ifnar1−/− | 14.1 ± 0.6 | 3.3 ± 0.1 | 16.6 ± 0.6 | 35.8 ± 2.4 |
| IFNAR1Texcl | 16.6 ± 0.8*,† | 3.5 ± 0.2 | 20.8 ± 1.2‡,§ | 25.3 ± 4.7† |
P < 0.01, ‡P < 0.05, comparisons between IFNAR1Texcl mice versus WT mice. †P < 0.05, §P < 0.01, comparisons between IFNAR1Texcl mice versus Ifnar1−/− mice. n = 9 Mice/genotype.
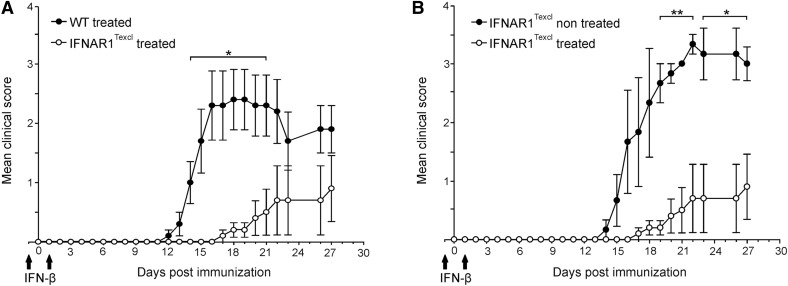
Given the beneficial effect of endogenous IFN-I in IFNAR1Texcl mice during EAE and as EAE can be suppressed by administration of rIFN-β [23, 24], we explored the effect of exogenous IFN-β in the same disease model. WT and IFNAR1Texcl mice received 2 doses of IFN-β, on d −1 and +1 relative to EAE induction. As expected, IFN-β treatment reduced the severity of the disease in WT mice [23, 24] (Supplemental Fig. 1). It is important to note that the mean maximum and accumulative score of IFN-β-treated IFNAR1Texcl mice was several-fold lower than that of untreated littermates and of treated WT animals (Fig. 4 and Table 2). These results further strengthen our initial finding that T cell-restricted IFNAR signaling is critical during the initial phase of EAE and collectively reveal for the first time that T cells are direct targets of IFN-β in EAE.
Figure 4. Exogenous IFN-β signaling selectively in T cells attenuates EAE.
WT and IFNAR1Texcl mice were treated or not with IFN-β (104 U/mouse) on d −1 and +1 relative to EAE induction, as indicated by arrows. (A) Comparison of WT and IFNAR1Texcl mice upon treatment with IFN-β and (B) comparison of untreated IFNAR1Texcl mice with IFN-β-treated littermates. Results shown are presented as mean values ± sem and are representative of 2 independent experiments (n = 5 mice/genotype/experiment). *P < 0.05, **P < 0.01.
TABLE 2.
Clinical severity of WT and IFNAR1Texcl mice upon PBS or IFN-β administration
| Genotype of mice | Treatment | Maximum score (mean ± sem) | Accumulative score (mean ± sem) |
|---|---|---|---|
| IFNAR1Texcl | PBS | 3.7 ± 0.2 | 27.8 ± 2.5 |
| IFNAR1Texcl | rIFN-β | 0.9 ± 0.1*,† | 4.4 ± 3.1†,‡ |
| WT | rIFN-β | 2.5 ± 0.5 | 24.8 ± 5.5 |
P < 0.05, ‡P < 0.01 compared with PBS-treated IFNAR1Texcl mice. †P <0.001 compared with IFN-β-treated WT mice. n = 5 Mice/genotype.
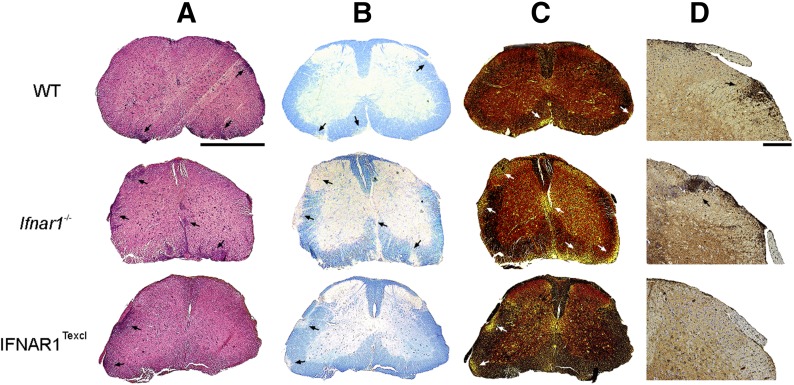
Fewer T cell infiltrates in the CNS and less severe neuropathology in IFNAR1Texcl EAE mice
A hallmark of EAE pathology is leukocyte infiltration into the CNS, which typically correlates with the severity of clinical signs [34]. To investigate whether the milder EAE phenotype in IFNAR1Texcl mice was reflected by differences in neuropathology, we examined spinal cord from mice of all groups collected at d 17 when the mean clinical score of IFNAR1Texcl mice was statistically lower than that of WT and Ifnar1−/−mice (Fig. 3A). Infiltration by immune cells, demyelination, and axonal damage were evaluated in spinal cord sections by H&E, Luxol fast blue, and Bielschowsky's silver stainings, respectively (Fig. 5A–C). In accordance with the attenuated EAE phenotype, spinal cord sections from IFNAR1Texcl mice exhibited less inflammatory infiltrates, as well as less demyelinated foci and axonal damage, compared with both control groups (Fig. 5A–C). Most interestingly, fewer CD3+ T cells were observed in spinal cord sections of IFNAR1Texcl mice relative to WT and Ifnar1−/− mice (Fig. 5D). During the chronic phase of the disease (d 26), IFNAR1Texcl mice demonstrated enhanced clinical symptoms of EAE compared with both control groups (Fig. 3A), and this was associated with severe neuropathology (Supplemental Fig. 2).
Figure 5. Neuropathology in IFNAR1Texcl mice during EAE.
(A–D) Spinal cord sections from WT, Ifnar1−/−, and IFNAR1Texcl mice (n = 3 mice/genotype) were prepared 17 d upon EAE induction and stained with H&E (A), Luxol fast blue (B), and Bielschowsky's silver stain (C). Immunohistochemistry for CD3+ T cells was also performed (D). Arrows indicate immune cell infiltration (A), demyelination (B), axonal damage (C), and CD3+ T cell infiltration in the CNS parenchyma. Scale bars, 1 mm (A–C); 200 μm (D).
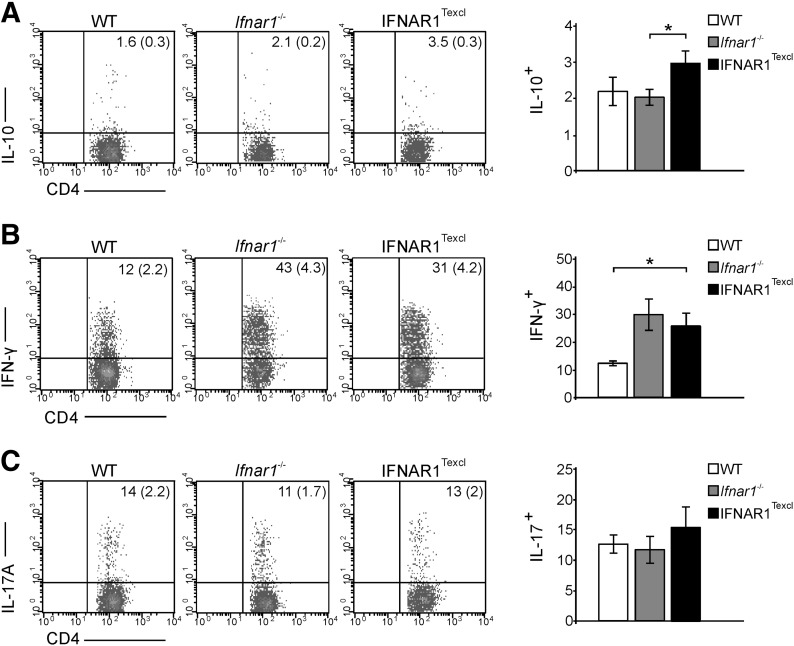
Increased frequency of IL-10+ and IFN-γ+ CD4+ T cells in the periphery of IFNAR1Texcl mice at the acute phase of EAE
As the Th profile of encephalitogenic T cells greatly influences the course of EAE, we next examined whether the observed differences between the groups reflected distinct T cell-associated cytokine profiles in the periphery and in the CNS. For this purpose, the proportion of cytokine-producing cells, known to be associated with EAE, was measured in dLNs and splenocytes from all groups of mice on d 17 of EAE. Intracellular staining revealed a significant increase in the frequency of CD4+ IL-10+ spleen cells in IFNAR1Texcl compared with WT and Ifnar1−/− mice (Fig. 6A and Table 3). Surprisingly, a higher frequency of CD4+ IFN-γ+ spleen cells was detected in IFNAR1Texcl compared with WT mice (Fig. 6B and Table 3). No differences were observed in the proportion of CD4+ IL-17+ spleen cells among the groups (Fig. 6C and Table 3) or in any cytokine-producing CD4+ subset in the dLNs (Table 3). The profile of cytokine-producing mononuclear cells could not be determined in the CNS of IFNAR1Texcl mice, as cell infiltrates were almost undetectable in the spinal cord of these mice (Table 3).
Figure 6. Frequency of cytokine-producing splenic T cells at the acute phase of EAE.
Mice were immunized with MOG35–55 peptide, and on d 17, splenic cells were stimulated with PMA/ionomycin/brefeldin A, stained for intracellular cytokines, and analyzed by flow cytometry. The dot plots and bars represent the IL-10 (A)-, IFN-γ (B)-, and IL-17A (C)-producing cells gated on the CD4+ population (values in the parentheses show percentage in the total live-cell gate). A representative dot plot per genotype is shown. Results are presented as means ± sem and are representative from 2 independent experiments with similar results (n = 5 mice/genotype/experiment). *P < 0.05.
TABLE 3.
Cytokines producing T cells on different time points upon MOG35–55- induced EAE
| d 10 |
d 17 |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| WT | Ifnar1−/− | IFNAR1Texcl | P | WT | Ifnar1−/− | IFNAR1Texcl | P | ||
| dLNs | |||||||||
| CD4+ IFN-γ+ | ND | ND | ND | ND | 0.64 ± 0.1 | 0.93 ± 0.2 | 0.98 ± 0.2 | 0.25a | |
| ND | 0.79b | ||||||||
| CD4+ IL-17+ | ND | ND | ND | ND | 0.48 ± 0.1 | 0.8 ± 0.2 | 0.6 ± 0.1 | 0.29a | |
| ND | 0.46b | ||||||||
| CD4+ IL-10+ | ND | ND | ND | ND | 0.24 ± 0.02 | 0.28 ± 0.04 | 0.32 ± 0.1 | 0.34a | |
| ND | 0.60b | ||||||||
| Spleen | |||||||||
| CD4+ IFN-γ+ | 1.6 ± 0.2 | 1.8 ± 0.4 | 1.8 ± 0.5 | 0.63a | 1.75 ± 0.3 | 3.2 ± 0.9 | 3.2 ± 0.5 | 0.027a | |
| 0.90b | 0.97b | ||||||||
| CD4+ IL-17+ | 0.7 ± 0.1 | 0.8 ± 0.1 | 0.2 ± 0.04 | 0.003a | 1.62 ± 0.5 | 1.93 ± 0.3 | 1.9 ± 0.2 | 0.37a | |
| 0.016b | 0.89b | ||||||||
| CD4+ IL-10+ | ND | ND | ND | ND | 0.3 ± 0.05 | 0.19 ± 0.02 | 0.28 ± 0.03 | 0.59a | |
| ND | 0.026b | ||||||||
| Spinal cord | |||||||||
| CD4+ IFN-γ+ | ND | ND | ND | ND | 13.44 | 8.7 | NAc | NA | |
| CD4+ IL-17+ | ND | ND | ND | ND | 6.6 | 2.8 | NAc | NA | |
The data presented are from 2 independent experiments/time point (n = 5 mice/genotype/experiment). Measurement of cytokines in cells isolated from spinal cord was performed once. All percentages are expressed as means ± sem. The percentages represent the cytokine-positive cells gated on total live cells. ND, not determined.
Comparisons between IFNAR1Texcl and WT mice; bcomparisons between IFNAR1Texcl and Ifnar1−/− mice; cvery few mononuclear cells were detected; thus, flow cytometry and statistical analysis were not applicable (NA). Boldface indicates statistically significant difference.
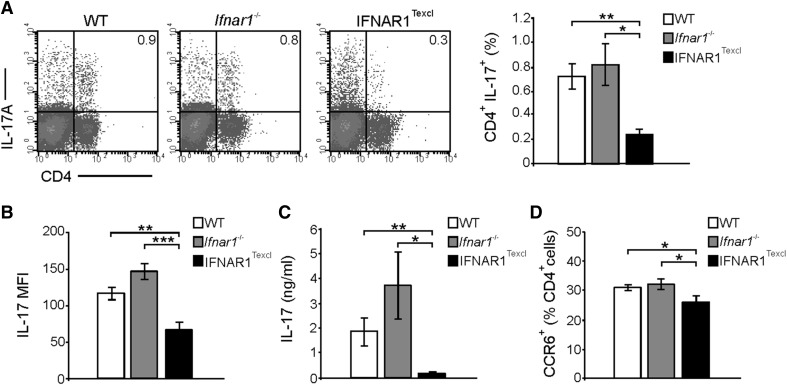
Impaired Th17 responses in the periphery of IFNAR1Texcl mice at the presymptomatic phase of EAE
During T cell priming, CD4+ T cells differentiate to distinct cytokine-expression profiles, depending on the cytokine environment. Moreover, in EAE, autoreactive T cells are detected in the periphery several days before entering the CNS parenchyma [10, 35]. To determine the phenotype of T cells at the preclinical stage of the disease, we isolated splenocytes on d 10 postimmunization and measured the frequency of cytokine-producing CD4+ T cells. Interestingly, a decrease in the proportion of CD4+ T cells was observed in the spleen of IFNAR1Texcl mice compared with WT and Ifnar1−/− mice, although this was not statistically significant (data not shown). We observed a significantly lower proportion of CD4+ IL-17+ T cells (Fig. 7A and Table 3) and lower expression levels of IL-17 (Fig. 7B), as evaluated by the MFI in IFNAR1Texcl compared with WT and Ifnar1−/− mice. No difference was detected in the proportion of CD4+ IFN-γ+ T cells (Supplemental Fig. 3A and Table 3), whereas variable results were obtained between experiments, when measuring CD4+ IL-10+ Τ cells (data not shown). Moreover, lower secreted levels of IL-17 in the supernatant of IFNAR1Texcl splenocytes were detected after ex vivo restimulation with the MOG35–55 peptide (Fig. 7C), indicating a reduced Th17 profile in IFNAR1Texcl mice. Notably, the secreted levels of other cytokines, i.e., IFN-γ, TNF-α, IL-2, IL-4, IL-10, and IL-6, were not different among the groups (data not shown). In agreement with the reduced Th17 response, CCR6, a marker of Th17 cells, was found to be expressed in a significantly lower proportion of IFNAR1Texcl CD4+ splenocytes (Fig. 7D). Tregs play a pronounced role in the suppression of autoimmune inflammatory responses [36], and IFN-β is reported to modulate frequency and suppressive capacity of Tregs in MS [37, 38]. However, no differences in CD25+Forkhead box p3+ Tregs were detected between IFNAR1Texcl mice and the control groups, as well as between the WT and Ifnar1−/− mice, in agreement with previous studies [39] (Supplemental Fig. 3B). Collectively, these data suggest that T cell-specific IFNAR signaling leads to impaired Th17 immune response, thus contributing to a milder course of EAE.
Figure 7. Selective T cell IFNAR signaling leads to a decreased Th17 response at the presymptomatic phase of EAE.
Splenocytes were isolated from WT, Ifnar1−/−, and IFNAR1Texcl mice, 10 d upon EAE induction. (A) Cells were stimulated with PMA/ionomycin/brefeldin A, and the frequency of IL- 17A-producing CD4+ T cells in the spleen was determined. The dot plots and bars represent IL-17A+ cells gated on total splenocytes. A representative dot plot per genotype is shown. (B) Expression levels of IL-17A by CD4+ T cells were evaluated by measuring MFI from (A) dot plots. (C) Splenocytes from all groups were restimulated ex vivo with MOG35–55 for 72 h, and the levels of secreted IL-17A were measured. (D) Frequency of CCR6+ splenocytes was determined (gated on the CD4+ compartment). All results are shown as means ± sem (n = 5 mice/genotype) and are representative from 2 independent experiments with similar results. *P < 0.05, **P < 0.01, ***P < 0.001.
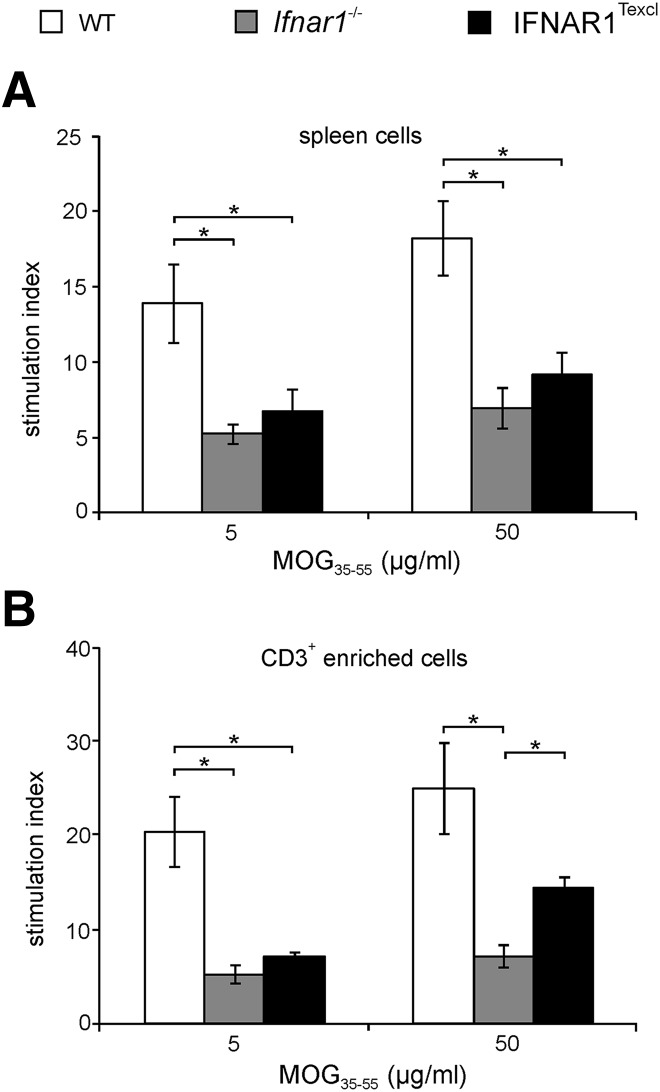
Diminished ex vivo proliferative capacity of IFNAR1Texcl T cells in response to MOG35–55 peptide
There are data suggesting that IFN-I affects T cell expansion and proliferation by acting directly on them or indirectly by modulating the antigen-presenting capacity of APCs [33, 40]. As a lower proportion of splenic CD4+ T cells was observed in IFNAR1Texcl mice on d 10 of EAE, we analyzed T cell-proliferative capacity in our setting. Splenic and dLN cells from immunized mice were restimulated with MOG35–55 ex vivo. dLN cells derived from IFNAR1Texcl mice exhibited an equivalent proliferative recall response upon antigen restimulation ex vivo compared with WT and Ifnar1−/− mice (Supplemental Fig. 4). The proliferative capacity of IFNAR1Texcl splenocytes was impaired compared with that of WT but similar to Ifnar1−/− cells (Fig. 8A). Similar results were obtained when we cocultured CD3+-enriched T cells with irradiated splenocytes in the presence of MOG35–55 peptide (Fig. 8B). As differences in proliferation were observed between IFNAR1Texcl and WT mice, but not between IFNAR1Texcl and Ifnar1−/− mice, we propose that variation in T cell proliferation is not a contributing factor for distinct EAE profiles among the mouse strains.
Figure 8. Ex vivo proliferation of T cells from mice immunized with MOG35–55.
Mice were immunized with MOG35–55/CFA, and spleen cells were isolated on d 10. Thymidine incorporation was measured 72 h upon culture of (A) spleen cells and (B) CD3+-enriched T cells cocultured with irradiated APCs in the presence of MOG35–55. Data are shown as means ± sem and are representative of 2 independent experiments with similar results (n = 5 mice/genotype/experiment). *P < 0.05.
DEGs in IFNAR1Texcl mice at the presymptomatic and acute phase of EAE
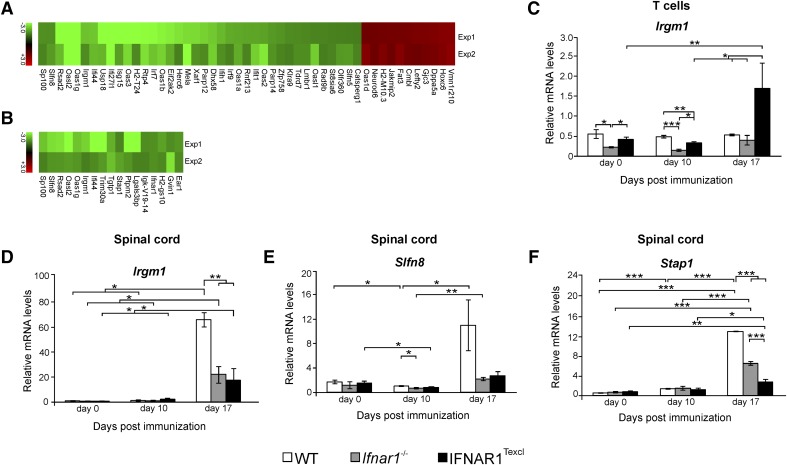
To track down genes that could possibly account for the altered cytokine profile of IFNAR1Texcl T cells associated with the delayed EAE course demonstrated above, we performed transcriptome analysis on splenic CD3+-enriched T cells (thereafter referred to as T cells) and spinal cord tissues isolated on d 10 of EAE. Genes having at least 1.5-fold increased or decreased expression in 2 independent experiments, in IFNAR1Texcl versus WT mice, were considered DEGs, and the magnitude of up- or down-regulation was depicted in heatmaps, separately for T cell (Fig. 9A) and spinal cord (Fig. 9B) samples. Genes were then tabulated according to their biologic function (Table 4). Among the DEGs in T cells of IFNAR1Texcl mice, there are genes involved in immune responses (Parp14, Usp18, Ifit1, Slfn8, etc.), apoptosis/survival (Xaf1, Eif2ak2, Ifih1), IFN-I biosynthetic process (Irf7, Irf9), anti-viral responses (Oas1a, Oas1b, Isg15, Klra9, Rsad2), or response to stress (Irgm1, Dhx58). Among the DEGs in spinal cord tissues, there are genes involved in immune response (Irgm1, Oasl2, Rsad2, Oas1g), cell differentiation (Stap1), or cell adhesion (Lglas3bp). The differential expression of selected genes was validated by quantitative RT-PCR in T cell and spinal cord samples from individual mice from 2 independent experiments. To explore how the expression profile of the selected genes changes with respect to disease progression, we performed quantitative RT-PCR in the same tissues from mice of all groups, euthanized on d 17, at the acute phase of the disease (Fig. 9C–F). Samples from Ifnar1−/− mice euthanized at the same time points were also included to identify genes differentially regulated by IFN-I signaling. Expression levels of Irgm1 mRNA, an IFN-γ-inducible gene, were lower in IFNAR1Texcl T cells on d 10 of EAE compared with WT samples but higher compared with Ifnar1−/− samples (Fig. 9C), although this was reversed on d 17, with significant up-regulation in IFNAR1Texcl-derived T cells versus both control groups. mRNA levels of Irgm1 and the ISG Slfn8 were lower in the IFNAR1Texcl spinal cord at d 17 of EAE compared with WT but not Ifnar1−/− mice (Fig. 9D and E). Moreover, the expression levels of Stap1 mRNA, a neurotoxicity gene, were significantly lower in transgenic spinal cord samples compared with both control groups at d 17 (Fig. 9F). In conclusion, transcriptome analysis in combination with quantitative RT-PCR revealed the differential expression of several genes in IFNAR1Texcl mice during EAE. In addition, it is the 1st time that Slfn8 and Stap1 are reported to be associated with EAE.
Figure 9. Microarray data analysis and quantitative RT-PCR of CD3+-enriched T cells and spinal cord.
Microarray analysis was performed on RNA from splenic, CD3+-enriched T cells and spinal cords from WT, Ifnar1−/−, and IFNAR1Texcl mice on d 0, 10, and 17 relative to EAE induction. (A and B) Heat map depicting the relative expression of selected genes that are different in expression between IFNAR1Texcl and WT T cells (A) and spinal cord (B) samples and were common in 2 independent experiments. Table 4 shows the function and number of molecules/category, as assessed by DAVID microarray software. The levels of mRNA transcripts for selected genes were measured in T cells (C) and spinal cords (D–F) by quantitative RT-PCR by use of single samples from each group at each time point (d 0: n = 3–5 mice/genotype; d 10: n = 3–4 mice/genotype; d 17: n = 2–3 mice/genotype). The results shown are the means ± sem of samples from 1 representative of 2 independent EAE experiments with similar results.*P < 0.05, **P < 0.01, ***P < 0.001.
TABLE 4.
Function and number of molecules/category, as assessed by DAVID microarray software
| Sample type | Function annotation | No. of genes |
|---|---|---|
| Spinal cord | Immune system process | 8 |
| Response to virus | 2 | |
| T cells | Immune system process | 13 |
| Response to virus | 8 | |
| Type I IFN production | 2 | |
| Innate immune response | 3 | |
| Multicellular organismal development | 7 |
DISCUSSION
In this paper, we addressed how IFNAR signaling directly modulates the activity of T cells in EAE and subsequently impinges on the outcome of the disease. For this purpose, we generated mice that express IFNAR1 exclusively on T cells, denominated IFNAR1Texcl. Transgenic T cells presented a normal capacity to proliferate, differentiate, and produce cytokines upon TCR engagement. This newly generated transgenic mouse strain provides a unique in vivo system for studying the direct activities of IFN-I on T cells in various autoimmune, infectious, or neoplastic inflammatory conditions. IFNAR1Texcl mice represent a valuable tool for understanding how IFN-β therapy affects T cell biology, in particular, for EAE, in which T cells are considered the main pathogenic population.
When IFNAR1Texcl animals were immunized with MOG35–55 peptide, a significantly delayed onset and a milder progression of EAE were observed compared with Ifnar1−/− mice. This finding unravels a hitherto unknown, in vivo, protective effect of endogenous IFN-I when acting directly on T cells at the early phase of EAE. This beneficial effect observed in IFNAR1Texcl mice was abrogated in IFNAR-sufficient animals, suggesting that counterbalancing effects of IFN-I signaling on non–T cell compartments obscure the protective effect of IFN-I on T cells. This interpretation was further supported by the observation that IFNAR1Texcl animals demonstrated an impressive attenuated disease course, when exogenous IFN-β was administered prophylactically.
The beneficial effect of IFN-I in IFNAR1Texcl EAE mice was found to be associated with down-regulation of the Th17 response at the presymptomatic phase of EAE in the periphery. Previous in vitro and in vivo studies have shown that differentiation of Th17 cells is inhibited by IFN-β [23, 41–44]. However, this is the first study showing that IFN-I acts directly on T cells to reduce the percentage of CD4+ IL-17+ cells and the secreted IL-17A in EAE, as illustrated in Fig. 7. In addition to this, the frequency of CD4+ T cells expressing CCR6, a marker of Th17 cells and migrating T cells in the CNS in EAE [45–47], was found significantly decreased in IFNAR1Texcl mice. This finding indicates a lower migratory capacity of these CD4+ T cells at the priming phase of EAE that explains the low number of infiltrating T cells in the spinal cord of these animals and the milder neuropathology at the acute phase.
Overall, convergent findings in our study advocate in favor of a direct IFN-I activity on T cells that restrains the proinflammatory Th17 response at the priming phase of EAE, leading to delayed onset of clinical manifestations in IFNAR1Texcl mice.
At the acute phase of the disease, Th17 differences among the groups were subdued, whereas IFNAR1Texcl mice showed an increased proportion of IL-10-producing CD4+ T cells in the periphery compared with the control groups. Previous studies showed that increased, IFN-β-induced IL-10 production has a beneficial effect in EAE [48, 49], which is consistent with our results. It is intriguing that in parallel to increased IL-10, an up-regulation of the Th1 pattern was observed in the spleen of IFNAR1Texcl mice compared with WT. Although Th1 cells were initially thought to be the main pathogenic T cell subset in EAE and MS [50, 51], subsequent studies revealed that the biologic significance of IFN-γ in EAE is ambiguous [52]. A crosstalk between IFN-I and -II pathways has been reported recently, which seems to determine the effect of IFN-γ itself in EAE. Thus, IFN-γ treatment at the effector phase of EAE attenuated disease in IFNAR-sufficient mice but had the opposite effect in Ifnar1−/− mice [53]. This may explain why Ifnar1−/− mice in this study do not benefit from the elevated Th1 pattern, and in contrast, their disease evolution is similar to WT mice that present low IFN-γ production. An alternative explanation is that IFN-γ acts beneficially in synergy with IL-10 when the production of IL-10 reaches a certain threshold. This condition is met in IFNAR1Texcl but not in Ifnar1−/− mice that demonstrate low IL-10 production.
Congruent with the enhanced Th1 response in IFNAR1Texcl mice was the expression of the Irgm1 gene, which encodes immunity-related GTPase family M member 1 and is induced mainly by IFN-γ. Recent studies revealed that Irgm1 exacerbates EAE by promoting BBB disruption, whereas Irgm1−/− mice are resistant to EAE [54, 55]. In our study, at the acute phase of the disease, Irgm1 demonstrated higher expression in splenic T cells of IFNAR1Texcl mice, in line with the increased IFN-γ production. However, at the same time, Irgm1 expression in the spinal cord of IFNAR1Texcl mice was significantly lower compared with WT mice. These results, taken together, concur with a high Th1 profile in the periphery of IFNAR1Texcl mice, while implying lower BBB disruption. This suggests that BBB of IFNAR1Texcl mice restricts crossing of T cells into the CNS, which is in line with the milder pathology observed. In addition, preliminary experiments performed at the acute phase to examine the migration of T cells showed an enhanced proportion of CD69+ and CCR5+ T cells in the peripheral blood of IFNAR1Texcl mice (unpublished data) that possibly represent activated Th1 cells [56, 57] and at the same time, a lower frequency of VLA4+ peripheral T cells in IFNAR1Texcl mice (unpublished data), implying that fewer T cells have the capacity to adhere to BBB endothelial cells and transmigrate into the CNS [58]. This hypothesis may explain why fewer cell infiltrates were detected in the spinal cord of IFNAR1Texcl mice and is consistent with the lower EAE score at the acute phase of the disease. Additional studies are needed to confirm this hypothesis.
Taking into account our results and the aforementioned studies, a challenging hypothesis is that in the IFNAR1Texcl mice, IFN-I differentially affects T cell behavior at discrete stages of EAE. At the priming phase, it suppresses Th17 responses, thus delaying onset of clinical manifestations, whereas at the acute phase, it stimulates IL-10 expression and regulates Th1 cell transmigration into CNS, eventually leading to milder development and reduced CNS inflammation.
Another interesting finding of the present study is that among the DEGs detected in the microarray experiments, 2 genes, stap1, and the IFN-inducible gene slfn8 [59], never reported before to be associated with EAE, were up-regulated in the spinal cord in EAE mice of all groups. It is interesting to note that expression of stap1, which was recently linked to neuronal apoptosis and degeneration [60], was decreased in the IFNAR1Texcl spinal cord relative to control groups, in line with the less severe neuropathology observed. These 2 genes may constitute new markers for monitoring the evolution of EAE, but further studies are needed to confirm these observations.
Previous studies have addressed the question of the role of endogenous IFN-I on different cell types and its tissue-specific activity in EAE. Teige et al. [25, 61] examined Ifnβ−/− mice and concluded that the anti-inflammatory activity of endogenous IFN-β is mainly exerted at the effector phase of the disease in the inflamed CNS by down-regulating microglia activation. In agreement with our results, the authors observed that proliferation and cytokine production of LN T cells was not altered in the absence of IFN-β signaling. Based on these results, the authors concluded that in EAE, IFN-β does not impact T cell behavior in the periphery. However, splenic T cells in the present study demonstrated IFNAR-associated skewing in the cytokine response. Two conclusions can be drawn from this observation: first, the IFN-I-induced cytokine profile of Th subsets in different peripheral lymphoid organs is not necessarily the same, and second, not only T cells derived from dLNs but also from other peripheral lymphoid organs, for instance, the spleen, may play an important role in the evolution of EAE pathogenesis.
In their seminal study, Prinz et al. [62] generated several conditional knockout mouse lines, in which IFNAR expression was ablated in different cell populations, to address the question of cell-specific activity of IFN-I. With the use of mice with T cell-restricted IFNAR1 deletion, they concluded that IFNAR triggering on T cells had no impact on EAE induction and progression. Nonetheless, the authors showed that during the priming phase of the disease, these mice demonstrated a statistically higher clinical score, but this phenomenon was not investigated further in their study [62]. This observation indicates a nontrivial, protective T cell-associated role of IFN-I at the priming phase of EAE, a suggestion that is substantiated by our findings that use IFNAR1Texcl mice. It is of note that this benefit is reversed at the later stages of the disease, implying a T cell-independent role of IFN-I in the chronic phase of EAE. Indeed, Prinz et al. [62] have shown that at this phase, IFN-I acts beneficially on myeloid cells. Based on their study and our results, one could speculate that IFN-I signaling may exert cell- and disease stage-specific, protective activity in EAE.
The present study contributes to our understanding of how the protective effect of endogenous IFN-I extends beyond the inflamed CNS, acting on splenic T lymphocytes to modify their behavior during the priming phase of EAE. The finding, with the most far-reaching consequences, is the observation that administration of exogenous IFN-β almost abrogated EAE in IFNAR1Texcl mice.
These results were obtained by use of a genetically engineered animal model, which is useful in further understanding the direct activities of IFN-I on T cells in EAE in vivo. However, to develop and test preclinical therapeutics, T cell-targeted, IFN-β-based protocols should be tested in intact animals.
The effectiveness of IFN-β therapy in MS patients seems to depend on several factors, including the profile of encephalitogenic Th cells, levels of endogenous IFN-I, as well as other factors [23, 63]. Based on the finding of the present study and the assumption that EAE recapitulates well the immune components of MS, we propose that in addition to the factors that have emerged from the above studies, the responsiveness of MS patients to IFN-β treatment may depend on the integration of positive and negative effects that IFN-β exerts on different cell types, in particular, on the T cell and the non-T cell compartment, respectively.
Thus, the investigation of underlying mechanisms of IFN-I activity on T cells would give useful information on whether T cell-targeted, IFN-β-based therapies may improve existing therapeutic interventions in MS.
AUTHORSHIP
G.T. and S.H. designed the study. N.K., G.T., and S.H. designed experiments and wrote the manuscript. N.K. and M.M. performed experiments and analyzed the data. N.K. performed statistical analyses and prepared the figures. M.E. advised with immunological techniques and discussed the data. M.T. provided key reagents. G.T. prepared the cd2–ifnar1 transgenic construct. N.K. and S.H. generated and analyzed the IFNAR1Texcl mouse strain. M.E. and M.T. provided valuable comments on the manuscript.
ACKNOWLEDGMENTS
This work was performed in the context of the InfeNeuTra project, part of the KRIPIS action, with Code Number MIS 450598, funded by the General Secretariat of Research & Technology (GSRT); the entire action was co-funded by Greece and the European Regional Development Fund of the European Union through Operational Programme Competitiveness and Entrepreneurship of the National Strategic Reference Framework (NSRF) 2007–2013. The project was also supported by the Transgenic Technology Unit (TTU) of Hellenic Pasteur Institute. N.K. was a fellow of the Hellenic Pasteur Institute and Ph.D. candidate in the Department of Biological Applications and Technology, University of Ioannina. The authors thank the Microarray Facility of the Institute of Molecular Biology and Biotechnology, Foundation for Research and Technology-Hellas, for performing microarray experiments and Dr. D. Kazazi, Laboratory of Virology, Hellenic Pasteur Institute, for assisting on analysis of microarray experiments and submission of the results to the ArrayExpress Database. The authors also thank Drs. E. Douni and T. Michaelidis for valuable comments on the manuscript and colleagues I. J. Grivas and E. Malaktari for technical assistance during the course of this work.
Glossary
- −/−
deficient
- BBB
blood brain barrier
- CT
threshold cycle
- DAVID
Database for Annotation, Visualization and Integrated Discovery
- DEG
differentially expressed gene
- dLN
draining lymph node
- EAE
experimental autoimmune encephalomyelitis
- h
human
- IFN-I
type I IFN
- IFNAR1
IFN-α/β receptor 1
- IFNAR1Texcl
IFNAR1 expression exclusively on T cells
- ISG15
IFN-stimulated gene 15
- LCR
locus control region
- LN
lymph node
- m
mouse
- MFI
mean fluorescence intensity
- MOG35–55
myelin oligodendrocyte glycoprotein 35–55 peptide
- MS
multiple sclerosis
- p-STAT1
phosphorylated STAT1
- PFA
paraformaldehyde
- PTx
pertussis toxin
- Treg
regulatory T cell
- WT
wild-type
Footnotes
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
DISCLOSURES
The authors declare no conflict of interest.
REFERENCES
- 1.Gresser I. (1990) Biologic effects of interferons. J. Invest. Dermatol. 95 (6 Suppl) 66S–71S. [DOI] [PubMed] [Google Scholar]
- 2.Dondi E., Rogge L., Lutfalla G., Uzé G., Pellegrini S. (2003) Down-modulation of responses to type I IFN upon T cell activation. J. Immunol. 170, 749–756. [DOI] [PubMed] [Google Scholar]
- 3.Gough D. J., Messina N. L., Clarke C. J., Johnstone R. W., Levy D. E. (2012) Constitutive type I interferon modulates homeostatic balance through tonic signaling. Immunity 36, 166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uematsu S., Akira S. (2007) Toll-like receptors and type I interferons. J. Biol. Chem. 282, 15319–15323. [DOI] [PubMed] [Google Scholar]
- 5.Platanias L. C. (2005) Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 5, 375–386. [DOI] [PubMed] [Google Scholar]
- 6.González-Navajas J. M., Lee J., David M., Raz E. (2012) Immunomodulatory functions of type I interferons. Nat. Rev. Immunol. 12, 125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ivashkiv L. B., Donlin L. T. (2014) Regulation of type I interferon responses. Nat. Rev. Immunol. 14, 36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.IFNB Multiple Sclerosis Study Group (2001) Interferon beta-lb is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. 1993 [classical article]. Neurology 57 (12 Suppl 5) S3–S9. [PubMed] [Google Scholar]
- 9.Sospedra M., Martin R. (2005) Immunology of multiple sclerosis. Annu. Rev. Immunol. 23, 683–747. [DOI] [PubMed] [Google Scholar]
- 10.Kuerten S., Lehmann P. V. (2011) The immune pathogenesis of experimental autoimmune encephalomyelitis: lessons learned for multiple sclerosis? J. Interferon Cytokine Res. 31, 907–916. [DOI] [PubMed] [Google Scholar]
- 11.Fletcher J. M., Lalor S. J., Sweeney C. M., Tubridy N., Mills K. H. (2010) T Cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin. Exp. Immunol. 162, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-behi M., Rostami A., Ciric B. (2010) Current views on the roles of Th1 and Th17 cells in experimental autoimmune encephalomyelitis. J. Neuroimmune Pharmacol. 5, 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korn T., Bettelli E., Oukka M., Kuchroo V. K. (2009) IL-17 and Th17 cells. Annu. Rev. Immunol. 27, 485–517. [DOI] [PubMed] [Google Scholar]
- 14.Domingues H. S., Mues M., Lassmann H., Wekerle H., Krishnamoorthy G. (2010) Functional and pathogenic differences of Th1 and Th17 cells in experimental autoimmune encephalomyelitis. PLoS One 5, e15531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy A. C., Lalor S. J., Lynch M. A., Mills K. H. (2010) Infiltration of Th1 and Th17 cells and activation of microglia in the CNS during the course of experimental autoimmune encephalomyelitis. Brain Behav. Immun. 24, 641–651. [DOI] [PubMed] [Google Scholar]
- 16.Stromnes I. M., Cerretti L. M., Liggitt D., Harris R. A., Goverman J. M. (2008) Differential regulation of central nervous system autoimmunity by T(H)1 and T(H)17 cells. Nat. Med. 14, 337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bailey-Bucktrout S. L., Martinez-Llordella M., Zhou X., Anthony B., Rosenthal W., Luche H., Fehling H. J., Bluestone J. A. (2013) Self-antigen-driven activation induces instability of regulatory T cells during an inflammatory autoimmune response. Immunity 39, 949–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirota K., Duarte J. H., Veldhoen M., Hornsby E., Li Y., Cua D. J., Ahlfors H., Wilhelm C., Tolaini M., Menzel U., Garefalaki A., Potocnik A. J., Stockinger B. (2011) Fate mapping of IL-17-producing T cells in inflammatory responses. Nat. Immunol. 12, 255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou L., Chong M. M., Littman D. R. (2009) Plasticity of CD4+ T cell lineage differentiation. Immunity 30, 646–655. [DOI] [PubMed] [Google Scholar]
- 20.Dhib-Jalbut S., Marks S. (2010) Interferon-beta mechanisms of action in multiple sclerosis. Neurology 74 (Suppl 1), S17–S24. [DOI] [PubMed] [Google Scholar]
- 21.Kieseier B. C. (2011) The mechanism of action of interferon-β in relapsing multiple sclerosis. CNS Drugs 25, 491–502. [DOI] [PubMed] [Google Scholar]
- 22.Markowitz C. E. (2007) Interferon-beta: mechanism of action and dosing issues. Neurology 68 (24 Suppl 4) S8–S11. [DOI] [PubMed] [Google Scholar]
- 23.Axtell R. C., de Jong B. A., Boniface K., van der Voort L. F., Bhat R., De Sarno P., Naves R., Han M., Zhong F., Castellanos J. G., Mair R., Christakos A., Kolkowitz I., Katz L., Killestein J., Polman C. H., de Waal Malefyt R., Steinman L., Raman C. (2010) T Helper type 1 and 17 cells determine efficacy of interferon-beta in multiple sclerosis and experimental encephalomyelitis. Nat. Med. 16, 406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galligan C. L., Pennell L. M., Murooka T. T., Baig E., Majchrzak-Kita B., Rahbar R., Fish E. N. (2010) Interferon-beta is a key regulator of proinflammatory events in experimental autoimmune encephalomyelitis. Mult. Scler. 16, 1458–1473. [DOI] [PubMed] [Google Scholar]
- 25.Teige I., Treschow A., Teige A., Mattsson R., Navikas V., Leanderson T., Holmdahl R., Issazadeh-Navikas S. (2003) IFN-beta gene deletion leads to augmented and chronic demyelinating experimental autoimmune encephalomyelitis. J. Immunol. 170, 4776–4784. [DOI] [PubMed] [Google Scholar]
- 26.Warabi Y., Matsumoto Y., Hayashi H. (2007) Interferon beta-1b exacerbates multiple sclerosis with severe optic nerve and spinal cord demyelination. J. Neurol. Sci. 252, 57–61. [DOI] [PubMed] [Google Scholar]
- 27.Axtell R. C., Raman C., Steinman L. (2013) Type I interferons: beneficial in Th1 and detrimental in Th17 autoimmunity. Clin. Rev. Allergy Immunol. 44, 114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhumabekov T., Corbella P., Tolaini M., Kioussis D. (1995) Improved version of a human CD2 minigene based vector for T cell-specific expression in transgenic mice. J. Immunol. Methods 185, 133–140. [DOI] [PubMed] [Google Scholar]
- 29.Ittner L. M., Götz J. (2007) Pronuclear injection for the production of transgenic mice. Nat. Protoc. 2, 1206–1215. [DOI] [PubMed] [Google Scholar]
- 30.Müller U., Steinhoff U., Reis L. F., Hemmi S., Pavlovic J., Zinkernagel R. M., Aguet M. (1994) Functional role of type I and type II interferons in antiviral defense. Science 264, 1918–1921. [DOI] [PubMed] [Google Scholar]
- 31.Nicolussi E. M., Huck S., Lassmann H., Bradl M. (2009) The cholinergic anti-inflammatory system limits T cell infiltration into the neurodegenerative CNS, but cannot counteract complex CNS inflammation. Neurobiol. Dis. 35, 24–31. [DOI] [PubMed] [Google Scholar]
- 32.Deonarain R., Verma A., Porter A. C., Gewert D. R., Platanias L. C., Fish E. N. (2003) Critical roles for IFN-beta in lymphoid development, myelopoiesis, and tumor development: links to tumor necrosis factor alpha. Proc. Natl. Acad. Sci. USA 100, 13453–13458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zietara N., Łyszkiewicz M., Gekara N., Puchałka J., Dos Santos V. A., Hunt C. R., Pandita T. K., Lienenklaus S., Weiss S. (2009) Absence of IFN-beta impairs antigen presentation capacity of splenic dendritic cells via down-regulation of heat shock protein 70. J. Immunol. 183, 1099–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rangachari M., Kuchroo V. K. (2013) Using EAE to better understand principles of immune function and autoimmune pathology. J. Autoimmun. 45, 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Targoni O. S., Baus J., Hofstetter H. H., Hesse M. D., Karulin A. Y., Boehm B. O., Forsthuber T. G., Lehmann P. V. (2001) Frequencies of neuroantigen-specific T cells in the central nervous system versus the immune periphery during the course of experimental allergic encephalomyelitis. J. Immunol. 166, 4757–4764. [DOI] [PubMed] [Google Scholar]
- 36.Zozulya A. L., Wiendl H. (2008) The role of regulatory T cells in multiple sclerosis. Nat. Clin. Pract. Neurol. 4, 384–398. [DOI] [PubMed] [Google Scholar]
- 37.Graber J. J., McGraw C. A., Kimbrough D., Dhib-Jalbut S. (2010) Overlapping and distinct mechanisms of action of multiple sclerosis therapies. Clin. Neurol. Neurosurg. 112, 583–591. [DOI] [PubMed] [Google Scholar]
- 38.de Andrés C., Aristimuño C., de Las Heras V., Martínez-Ginés M. L., Bartolomé M., Arroyo R., Navarro J., Giménez-Roldán S., Fernández-Cruz E., Sánchez-Ramón S. (2007) Interferon beta-1a therapy enhances CD4+ regulatory T-cell function: an ex vivo and in vitro longitudinal study in relapsing-remitting multiple sclerosis. J. Neuroimmunol. 182, 204–211. [DOI] [PubMed] [Google Scholar]
- 39.Liu Y., Carlsson R., Comabella M., Wang J., Kosicki M., Carrion B., Hasan M., Wu X., Montalban X., Dziegiel M. H., Sellebjerg F., Sørensen P. S., Helin K., Issazadeh-Navikas S. (2014) FoxA1 directs the lineage and immunosuppressive properties of a novel regulatory T cell population in EAE and MS. Nat. Med. 20, 272–282. [DOI] [PubMed] [Google Scholar]
- 40.Shinohara M. L., Kim J. H., Garcia V. A., Cantor H. (2008) Engagement of the type I interferon receptor on dendritic cells inhibits T helper 17 cell development: role of intracellular osteopontin. Immunity 29, 68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Durelli L., Conti L., Clerico M., Boselli D., Contessa G., Ripellino P., Ferrero B., Eid P., Novelli F. (2009) T-Helper 17 cells expand in multiple sclerosis and are inhibited by interferon-beta. Ann. Neurol. 65, 499–509. [DOI] [PubMed] [Google Scholar]
- 42.Guo B., Chang E. Y., Cheng G. (2008) The type I IFN induction pathway constrains Th17-mediated autoimmune inflammation in mice. J. Clin. Invest. 118, 1680–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martín-Saavedra F. M., González-García C., Bravo B., Ballester S. (2008) Beta interferon restricts the inflammatory potential of CD4+ cells through the boost of the Th2 phenotype, the inhibition of Th17 response and the prevalence of naturally occurring T regulatory cells. Mol. Immunol. 45, 4008–4019. [DOI] [PubMed] [Google Scholar]
- 44.Ramgolam V. S., Sha Y., Jin J., Zhang X., Markovic-Plese S. (2009) IFN-beta inhibits human Th17 cell differentiation. J. Immunol. 183, 5418–5427. [DOI] [PubMed] [Google Scholar]
- 45.Liston A., Kohler R. E., Townley S., Haylock-Jacobs S., Comerford I., Caon A. C., Webster J., Harrison J. M., Swann J., Clark-Lewis I., Korner H., McColl S. R. (2009) Inhibition of CCR6 function reduces the severity of experimental autoimmune encephalomyelitis via effects on the priming phase of the immune response. J. Immunol. 182, 3121–3130. [DOI] [PubMed] [Google Scholar]
- 46.Reboldi A., Coisne C., Baumjohann D., Benvenuto F., Bottinelli D., Lira S., Uccelli A., Lanzavecchia A., Engelhardt B., Sallusto F. (2009) C-C Chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat. Immunol. 10, 514–523. [DOI] [PubMed] [Google Scholar]
- 47.Yamazaki T., Yang X. O., Chung Y., Fukunaga A., Nurieva R., Pappu B., Martin-Orozco N., Kang H. S., Ma L., Panopoulos A. D., Craig S., Watowich S. S., Jetten A. M., Tian Q., Dong C. (2008) CCR6 regulates the migration of inflammatory and regulatory T cells. J. Immunol. 181, 8391–8401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hesse D., Krakauer M., Lund H., Søndergaard H. B., Limborg S. J., Sørensen P. S., Sellebjerg F. (2011) Disease protection and interleukin-10 induction by endogenous interferon-β in multiple sclerosis? Eur. J. Neurol. 18, 266–272. [DOI] [PubMed] [Google Scholar]
- 49.Zhang L., Yuan S., Cheng G., Guo B. (2011) Type I IFN promotes IL-10 production from T cells to suppress Th17 cells and Th17-associated autoimmune inflammation. PLoS One 6, e28432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kennedy M. K., Torrance D. S., Picha K. S., Mohler K. M. (1992) Analysis of cytokine mRNA expression in the central nervous system of mice with experimental autoimmune encephalomyelitis reveals that IL-10 mRNA expression correlates with recovery. J. Immunol. 149, 2496–2505. [PubMed] [Google Scholar]
- 51.Renno T., Lin J. Y., Piccirillo C., Antel J., Owens T. (1994) Cytokine production by cells in cerebrospinal fluid during experimental allergic encephalomyelitis in SJL/J mice. J. Neuroimmunol. 49, 1–7. [DOI] [PubMed] [Google Scholar]
- 52.Steinman L. (2007) A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat. Med. 13, 139–145. [DOI] [PubMed] [Google Scholar]
- 53.Naves R., Singh S. P., Cashman K. S., Rowse A. L., Axtell R. C., Steinman L., Mountz J. D., Steele C., De Sarno P., Raman C. (2013) The interdependent, overlapping, and differential roles of type I and II IFNs in the pathogenesis of experimental autoimmune encephalomyelitis. J. Immunol. 191, 2967–2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang C., Wang C., Dong H., Wu X. M., Wang C., Xia F., Li G., Jia X., He S., Jiang X., Li H., Xu H. (2013) Immune-related GTPase Irgm1 exacerbates experimental auto-immune encephalomyelitis by promoting the disruption of blood-brain barrier and blood-cerebrospinal fluid barrier. Mol. Immunol. 53, 43–51. [DOI] [PubMed] [Google Scholar]
- 55.Xu H., Wu Z. Y., Fang F., Guo L., Chen D., Chen J. X., Stern D., Taylor G. A., Jiang H., Yan S. S. (2010) Genetic deficiency of Irgm1 (LRG-47) suppresses induction of experimental autoimmune encephalomyelitis by promoting apoptosis of activated CD4+ T cells. FASEB J. 24, 1583–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheng W., Chen G. (2014) Chemokines and chemokine receptors in multiple sclerosis. Mediators Inflamm. 2014, 659206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hamann J., Fiebig H., Strauss M. (1993) Expression cloning of the early activation antigen CD69, a type II integral membrane protein with a C-type lectin domain. J. Immunol. 150, 4920–4927. [PubMed] [Google Scholar]
- 58.Glatigny S., Duhen R., Oukka M., Bettelli E. (2011) Cutting edge: loss of α4 integrin expression differentially affects the homing of Th1 and Th17 cells. J. Immunol. 187, 6176–6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mavrommatis E., Fish E. N., Platanias L. C. (2013) The schlafen family of proteins and their regulation by interferons. J. Interferon Cytokine Res. 33, 206–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stoecker K., Weigelt K., Ebert S., Karlstetter M., Walczak Y., Langmann T. (2009) Induction of STAP-1 promotes neurotoxic activation of microglia. Biochem. Biophys. Res. Commun. 379, 121–126. [DOI] [PubMed] [Google Scholar]
- 61.Teige I., Liu Y., Issazadeh-Navikas S. (2006) IFN-beta inhibits T cell activation capacity of central nervous system APCs. J. Immunol. 177, 3542–3553. [DOI] [PubMed] [Google Scholar]
- 62.Prinz M., Schmidt H., Mildner A., Knobeloch K. P., Hanisch U. K., Raasch J., Merkler D., Detje C., Gutcher I., Mages J., Lang R., Martin R., Gold R., Becher B., Brück W., Kalinke U. (2008) Distinct and nonredundant in vivo functions of IFNAR on myeloid cells limit autoimmunity in the central nervous system. Immunity 28, 675–686. [DOI] [PubMed] [Google Scholar]
- 63.Comabella M., Lünemann J. D., Río J., Sánchez A., López C., Julià E., Fernández M., Nonell L., Camiña-Tato M., Deisenhammer F., Caballero E., Tortola M. T., Prinz M., Montalban X., Martin R. (2009) A type I interferon signature in monocytes is associated with poor response to interferon-beta in multiple sclerosis. Brain 132, 3353–3365. [DOI] [PubMed] [Google Scholar]