Abstract
Background
Tuberculosis (TB) represents a major global health problem. The prognosis of clinically active tuberculosis depends on the complex interactions between Mycobacterium tuberculosis (Mtb) and its host. In recent years, autophagy receives particular attention for its role in host defense against intracellular pathogens, including Mtb. In present study, we aim to investigate the relationship of autophagy induction by clinical isolates of Mtb with the clinical outcomes in patients with TB.
Methodology/Principal Findings
We collected 185 clinical isolates of Mtb, and determined the effect of these Mtb isolates on autophagy induction in macrophages. It was found that most of clinical isolates of Mtb were able to induce autophagosome formation in macrophages, however, the autophagy-inducing ability varied significantly among different isolates. Of importance, our results revealed that patients infected by Mtb with poor autophagy-inducing ability displayed more severe radiographic extent of disease (p<0.001), and were more likely to have unfavorable treatment outcomes (p<0.001). No significant association was observed between the extent of Mtb-induced autophagy with some socio-demographic characteristics (such as gender, age and tobacco consumption), and some laboratory tests (such as hemoglobin, leukocyte count and erythrocyte sedimentation rate). Furthermore, results from logistic regression analysis demonstrated that the defect in autophagy induction by clinical isolates of Mtb was an independent risk factor for far-advanced radiographic disease (aOR 4.710 [1.93–11.50]) and unfavorable treatment outcomes (aOR 8.309 [2.22–28.97]) in TB.
Conclusion/Significance
These data indicated that the defect in autophagy induction by Mtb isolates increased the risk of poor clinical outcomes in TB patients, and detection of clinical isolates-induced autophagosome formation might help evaluate the TB outcomes.
Introduction
Tuberculosis (TB), caused by the bacillus Mycobacterium tuberculosis (Mtb), is a major public health problem worldwide with up to 10 million new cases each year, leading to 1.5 million deaths annually. China occupies second place, behind India, among the top five high-burden countries for the last decade [1, 2]. The application of BCG vaccine and anti-TB antibiotics has been effective in preventing and controlling TB. However, the high rates of latent tuberculosis infection (LTBI), emergence of drug-resistant Mtb and HIV co-infection, etc., have made the control and treatment of TB become difficult in recent years [3–5]. Novel and effective therapeutic strategies against TB are therefore urgently needed. Accumulating evidence indicates that the interaction between Mtb and the host is of great importance in determining the outcome of TB [6–8]. Of those strategies targeting Mtb-host interaction, autophagy receives particular attention in recent years [9–12].
Autophagy is an evolutionarily conserved process in which organelles and proteins are sequestered into a double-membrane-bound autophagosome, and delivered to the lysosome for degradation. Recent reports reveal that autophagy is involved in diverse pathophysiological processes, including cell survival, aging, neurodegeneration, cancer and the clearance of intracellular pathogen [13–15]. Accumulating evidence indicates that autophagy may function as a crucial anti-TB strategy of the host, although these data are mainly obtained from the investigation on BCG or standard H37Rv strain. On the other hand, it is also suggested that through the intimate and persistent interaction with its human host, Mtb may have evolved strategies to counter the antibacterial effect of autophagy [9, 10, 12, 16–18]. The pathogenesis of TB has been thought to be mainly related to host factors in earlier investigations, however, it appears clear now that bacterial factors also play crucial roles [7]. Reports indicate that clinical isolates of Mtb display different characteristics from those of BCG or H37Rv [19, 20]. Different clinical isolates of Mtb are also found to induce different host immune responses [21, 22]. It is therefore of interest to further investigate the role of autophagy in TB pathogenesis using clinical isolates of Mtb.
In this study, we collected 185 clinical isolates of Mtb from Zunyi, one of the highest-incidence-rate areas with TB in China [23], and investigated the effect of these clinical isolates on autophagosome formation in macrophages, and its associated clinical significance. Our data showed that most clinical isolates of Mtb were able to induce autophagosome formation in macrophages, however different clinical isolates of Mtb differed in their ability to induce autophagosome formation. Of importance, it was found that the extent of clinical isolates of Mtb-induced autophagy was negatively correlated with the clinical outcomes in patients with TB.
Materials and Methods
Mycobacterial specimens
A total of 185 Mtb isolates were obtained from clinical patients, from 2011 to 2014 in Zunyi, Guizhou Province, one of the highest-incidence-rate areas with TB in China. Of these samples, 177 specimens were from sputum, 6 from urine, 1 from the cerebrospinal fluid, and 1 from the scrotum. Mtb isolates were grown in 7H11 medium (Difco BD, NY) supplemented with 0.05% Tween 80 and 10% oleic albumin dextrose catalase enrichment (Difco, Detroit, MI), and the identification of all these isolates were performed according to the TB diagnosis bacteriology test criteria of the China Antituberculosis Association. Single-cell suspensions of mycobacteria at a concentration of 107 CFU/ml were prepared and used to infect cells.
Data collection
Following verbal and written consent, socio-demographic, clinical, radiographic and laboratory data were obtained from patients' medical record, and the data were analyzed anonymously. The Ethics Committee of Fudan University and Affiliated Hospital of Zunyi Medical College specifically approved this study, and this work was also performed in compliance with the Helsinki Declaration. The radiographic extent of disease was categorized to be "minimal", "moderately advanced", or "far advanced" according to the classification of the National Tuberculosis and Respiratory Disease Association [24]. The TB treatment outcomes were defined by WHO criteria as "favourable" (cured and treatment completed) and "unfavourable" (defaulted, failed and died) [25]. Retreatment cases were those having history of previous TB treatment of more than one month.
Macrophage stimulation with mycobacterial strains
The murine macrophage cell line RAW264.7 (ATCC number: TIB-71) was maintained at 37°C in DMEM (Invitrogen, Carlsbad, CA, USA) supplemented with 10% FBS (HyClone, Logan, UT, USA) and antibiotics in a 5% CO2 atmosphere. The human monocyte cell line THP-1 (ATCC number: TIB-202) was cultured in RPMI1640 (Invitrogen) supplemented with 10% FBS (HyClone). Prior to Mtb infection, THP-1 cells were treated with 50 ng/ml Phorbol 12-myristate 13-acetate (PMA) for 24 hours to allow differentiation into macrophages. BMDM of > 95% purity were obtained from BALB/c as described previously [26]. Macrophage stimulation with Mtb strains was performed according to previous reports [16, 27]. Briefly, macrophages were infected with clinical isolates of Mtb or H37Rv at multiplicity of infection (MOI) of 10:1. Four hours after infection, macrophages were washed twice with prewarmed serum-free RPMI1640 or DMEM to remove unbound bacilli, and were further cultured in serum-supplemented RPMI1640 or DMEM for another 4 hours. No toxicity was observed in Mtb-infected macrophages.
Western blot analysis
Western blot was performed as described previously [28]. Antibody against LC3 was obtained from Sigma (St. Louis, Mo, USA), and anti-GAPDH was from CWBio (Beijing, China).
Immunofluorescence
THP-1 cells were washed twice with PBS, fixed with 4% paraformaldehyde in PBS for 10 min, permeabilized with 0.25% Triton X-100 in PBS for 10 min. Cells were then incubated sequentially with rabbit anti-LC3 antibody and tetramethyl rhodamine isothiocyanate (TRITC)-conjugated goat anti-rabbit IgG (red), followed by the staining with 4',6-diamidino-2-phenylindole (DAPI) to visualize the nuclei (blue). Fluorescence images were acquired with a confocal laser-scanning microscope.
Statistical analysis
Categorical variables were compared using pearson chi-square test, and the difference in continuous variables was analyzed by one-way analysis of variance (One-way ANOVA). Variables with a p-value < 0.2 in univariate logistic regression analyses were further subjected to multivariate logistic regression analysis to identify independent variables that evaluated the role of autophagy in the pathogenesis of TB. A p-value <0.05 was considered to be statistically significant. Data were entered and analyzed using a statistical software package (SPSS18.0).
Results
Clinical isolates of Mtb were able to induce autophagosome formation in macrophages
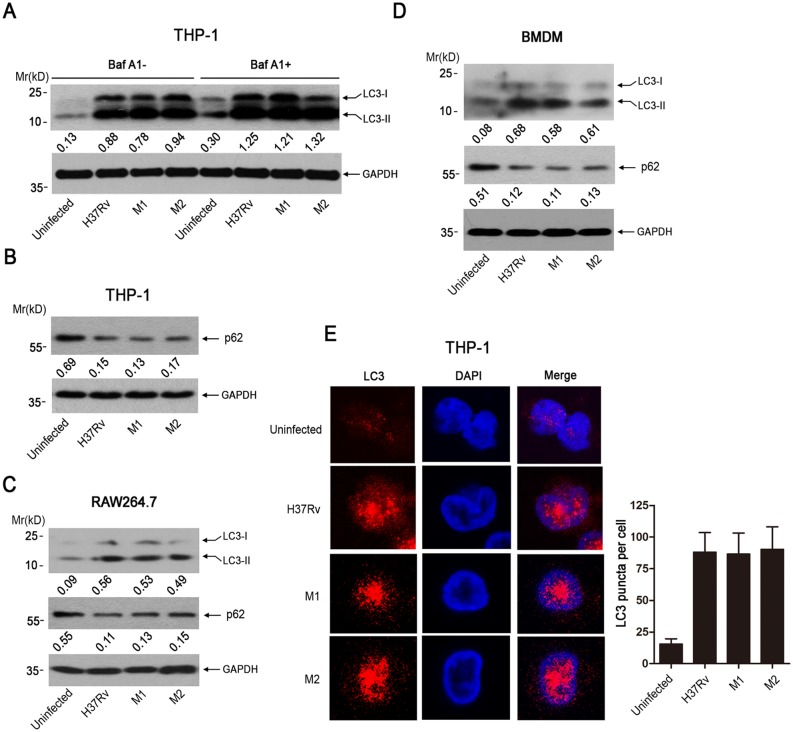
To investigate the effect of Mtb on autophagy induction, we infected human THP-1 macrophages with two clinical isolates of Mtb or H37Rv for 4 hr, followed by detecting the autophagosome formation using western blot. Results showed that, similar to H37Rv, two clinical isolates of Mtb (M1, M2) were able to significantly increase the level of LC3 (microtubule-associated protein light chain 3)-II, a hallmark of autophagy induction (Fig 1A). As the the increase in LC3II level could mean that there is an increase in autophagy induction or there is an inhibition of flux, we examined the effect of Bafilomycin A1 (Baf A1) treatment on Mtbs-induced autophagy. It was found that Baf A1 treatment could further increase the LC3II level in Mtbs-infected THP1 cells (Fig 1A). We have also investigated the effect of Mtbs infection on the expression level of p62, which is incorporated into autophagosomes and degraded along with other substrates by lysosomal hydrolyses. Our results showed that infection with clinical isolates (M1, M2) or H37Rv led to the downregulation of p62 significantly in THP1 cells at 4 hours postinfection (Fig 1B). Together, these data indicated that these Mtbs-induced autophagy was functional rather than blocking autophagical flux at 4 hours postinfection. We also examined the effect of clinical isolates of Mtb on autophagy induction in mouse macrophage RAW264.7 cells and mouse bone marrow-derived macrophages (BMDM). Similar to the observation in THP-1 cells, infection with clinical isolates (M1, M2) or H37Rv led to the increase in LC3II level while downregulated p62 expression in RAW264.7 cells (Fig 1C) and BMDM cells (Fig 1D). We further determined the effect of clinical Mtb isolate on autophagosome formation using the immunofluorescence technique. The confocal result showed that clinical Mtb isolates (M1, M2) or H37Rv stimulated the formation of LC3 punctuate in THP-1 cells significantly (Fig 1E).
Fig 1. Clinical isolates of Mtb could induce autophagy in macrophages.
(A) THP-1 were infected with H37Rv and two clinical isolates of Mtb (M1 and M2) (MOI = 10) for 4 hr in the absence or presence of Baf A1. Cells were harvested and subjected to western blot analysis using anti-LC3. The expression of GAPDH was used as a loading control. The immunoblots were scanned and subjected to densitometric analysis. LC3-II/GAPDH ratio was calculated, and the mean value of at least five samples from three independent experiments was shown at the bottom of each lane. (B) THP-1 were infected with clinical Mtb isolates (M1, M2) or H37Rv (MOI = 10) for 4 hr. Cells were harvested and subjected to western blot analysis using antibodies against p62 and GAPDH. Protein was quantitified by densitometry. P62/GAPDH ratio was calculated, and the mean value of at least five samples from three independent experiments was shown at the bottom of each lane. (C and D) RAW264.7 and BMDMs macrophages were treated as in B. Cells were harvested and subjected to western blot analysis using antibodies against p62 and GAPDH. Protein was quantitified by densitometry. LC3-II/GAPDH or p62/GAPDH ratio was calculated, respectively, and the mean value of at least five samples from three independent experiments was shown at the bottom of each lane. (E) THP-1 macrophages were infected with a clinical isolates of Mtb (MOI = 10) for 4 h. Cells were then incubated sequentially with anti-LC3B antibody and TRITC goat anti-rabbit IgG (red), followed by the staining with DAPI to visualize the nuclei (blue), the right panel was the quantification of LC3 punctuate per cell. The data shown represent mean ± SE from three independent experiments.
Different clinical isolates of Mtb induced autophagosome formation in macrophages to different extent
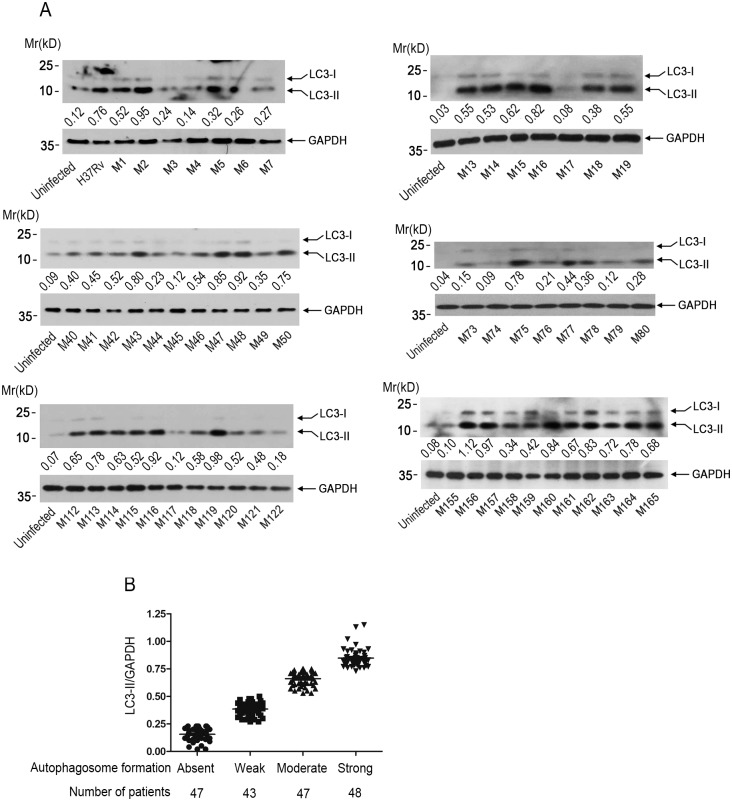
Above data showed that two clinical isolates of Mtb were able to induce autophagosome formation in macrophages, we therefore further collected more clinical isolates of Mtb to a total of 185 during the period from 2011 to 2014, and examined the effect of these clinical isolates of Mtb on autophagosome formation in THP-1 macrophages using western blot. It was found that most of these clinical isolates of Mtb could induce autophagosome formation, however, the autophagy-inducing ability appeared to vary greatly among different isolates (Fig 2A). To better define the clinical isolates of Mtb-induced autophagosome formation, the extent of autophagosome formation was graded on LC3-II/GAPDH ratio into four classes: absent (0–0.25), weak (0.26–0.50), moderate (0.51–0.75), and strong (>0.75) (Fig 2B).
Fig 2. Different clinical isolates of Mtb induced autophagy in THP-1 cells to a different extent.
(A) THP-1 cells were infected with clinical isolates of Mtb for 4 hr. Cells were harvested and subjected to western blot analysis using anti-LC3. GAPDH was used as a loading control. Representative immunoblots (sample 1–7, 13–19, 40–50, 73–80, 112–122, 155–165) were shown. The immunoblots were scanned and subjected to densitometric analysis. LC3-II/GAPDH ratio was calculated, and the mean value of at least five samples from three independent experiments was shown at the bottom of each lane. (B) The extent of autophagosome formation was graded on LC3-II/GAPDH ratio into four classes: absent (0–0.25), weak (0.26–0.50), moderate (0.51–0.75), and strong (>0.75).
Characteristics of patients harbouring clinical isolates of Mtb with different autophagy-inducing ability
As above data demonstrated that clinical isolates of Mtb differed in their ability to induce autophagosome formation in THP-1 macrophages and autophagy were reported to be crucial for Mtb clearance, we would like to know the relationship between the extent of Mtb-induced autophagosome formation and the clinical outcomes of TB patients. The clinicopathological comparisons among patients harbouring clinical isolates of Mtb with different autophagy-inducing ability were shown in Table 1. It was found that patients infected by Mtb with poor autophagy-inducing ability displayed more severe radiographic extent of disease (p<0.001) and were more likely to have unfavorable treatment outcomes (p<0.001). We also observed that re-treatment TB cases were more likely to harbour isolates with poor autophagy-inducing ability (p<0.001). There was no significant association of the extent of Mtb-induced autophagy with some socio-demographic characteristics (including gender, age, tobacco smoking and alcohol consumption), the coexistence of pulmonary and extra pulmonary tuberculosis (PTB+EPTB), and some laboratory tests [such as hemoglobin, leukocyte count, erythrocyte sedimentation rate (ESR), and carbon dioxide combining power (CO2CP)].
Table 1. Characteristics of patients harboring clinical Mtb isolates with different autophagy-inducing ability.
| Variable | Autophagosome formation | |||||
|---|---|---|---|---|---|---|
| Total | Absent | Weak | Moderate | Strong | P-valuea | |
| N = 185 n (%) | N = 47 n (%) | N = 43 n (%) | N = 47 n (%) | N = 48 n (%) | ||
| Gender | 0.098 | |||||
| Male | 106 (57.3) | 21 (44.7) | 25 (58.1) | 33 (70.0) | 27 (57.3) | |
| Female | 79 (42.7) | 26 (55.3) | 18 (61.9) | 14 (30.0) | 21 (42.7) | |
| Age (years) | 0.334 | |||||
| <30 | 40 (21.6) | 11 (23.4) | 14 (32.6) | 8 (17.0) | 7 (14.6) | |
| 30–50 | 88 (47.6) | 21 (44.7) | 16 (37.2) | 27 (57.4) | 24 (50.0) | |
| >50 | 57 (30.8) | 15 (31.9) | 13 (30.2) | 12 (25.6) | 17 (35.4) | |
| Tobacco | 0.161 | |||||
| Yes | 86 (46.5) | 27 (57.4) | 22 (51.2) | 20 (42.6) | 17 (35.4) | |
| No | 99 (53.5) | 20 (42.6) | 21 (48.8) | 27 (57.4) | 31 (64.6) | |
| Alcohol | 0.561 | |||||
| Yes | 91 (49.2) | 25 (53.2) | 18 (41.9) | 24 (51.1) | 27 (56.3) | |
| No | 94 (50.8) | 22 (46.8) | 25 (58.1) | 23 (48.9) | 21 (43.7) | |
| TB form | 0.889 | |||||
| PTB alone | 131 (70.8) | 35 (74.5) | 28 (65.1) | 32 (68.1) | 36 (75.0) | |
| EPTB alone | 8 (4.3) | 1 (2.1) | 2 (4.7) | 3 (6.4) | 2 (4.2) | |
| PTB+EPTB | 46 (24.9) | 11 (23.4) | 13 (30.2) | 12 (25.5) | 10 (20.8) | |
| Radiographic extent of disease | 0.001 | |||||
| Minimal | 53 (28.6) | 7 (14.9) | 9 (20.9) | 16 (34.0) | 21 (43.8) | |
| Moderate-advanced | 70 (37.8) | 14 (29.8) | 16 (37.2) | 21 (44.7) | 19 (39.6) | |
| Far-advanced | 62 (33.6) | 26 (55.3) | 18 (41.9) | 10 (21.3) | 8 (16.6) | |
| Laboratory testings | ||||||
| Hemoglobin (g/L) | 129.4±18.3 | 128.2±16.3 | 132.6±18.4 | 126.8±17.9 | 130.3±20.5 | 0.463b |
| Leukocytes (109/L) | 7.8±2.7 | 7.1±2.4 | 8.15±2.5 | 8.3±3.1 | 7.6±2.6 | 0.607b |
| ESR (mm) | 48.2±21.4 | 48.5±20.8 | 49.0±20.4 | 49.3±21.4 | 45.9±23.1 | 0.865b |
| CO2CP (mmol/L) | 24.9±3.5 | 25.2±3.3 | 24.8±3.7 | 24.6±3.5 | 25.3±3.7 | 0.720b |
| Type of cases | <0.001 | |||||
| New | 99 (53.5) | 12 (25.5) | 17(39.5) | 31 (66.0) | 37 (77.1 | |
| Re-treatment | 86 (46.5) | 35 (74.5) | 26(60.5) | 16 (34.0) | 11 (22.9) | |
| Treatment outcomes | <0.001 | |||||
| Favourable (cured, completed) | 145 (78.4) | 29 (61.7) | 30 (69.8) | 40 (85.1) | 46 (95.8) | |
| Unfavourable (failed,defaulted,died) | 40 (21.6) | 18 (38.3) | 13 (30.2) | 7 (14.9) | 2 (4.2) | |
Abbreviation: PTB, pulmonary tuberculosis; EPTB, extra-pulmonary tuberculosis; ESR, erythrocyte sedimentation rate; CO2CP, Carbon Dioxide Combining Power
aChi-square test
bOne way ANOVA.
The defect in autophagy induction by Mtb increased the risk of far-advanced radiographic disease and unfavorable treatment outcomes in TB patients
Results from Table 1 showed that there was a higher portion of patients with far-advanced radiographic disease in the poor autophagy-inducing group as compared with the strong autophagy-inducing group. We further determined the association between them by logistic regression analysis. The univariate analysis showed that the defect in Mtb isolates-induced autophagosome formation was positively correlated with the far-advanced radiographic disease [odd ration (OR), 4.574; 95% confidence interval (CI) 1.93–10.86; p = 0.001]. When controlling for age, tobacco consumption and ESR, the poor autophagy-inducing ability by Mtb remained as a significant risk factor for the far-advanced radiographic disease [adjusted OR (aOR), 4.710; 95% CI 1.93–11.50; p = 0.001]. Besides, over 50 years of age was also revealed to be associated with the far-advanced radiographic disease (aOR, 2.915; 95% CI 1.12–7.58; p = 0.028) in the final regression model. Details are showed in Table 2.
Table 2. Univariate and multivariate logistic regression analysis of far-advanced radiographic disease in TB patients*.
| Variable | Radiographic finding | ||||
|---|---|---|---|---|---|
| Far-advanced* | Univariate analysis | Multivariate Analysis | |||
| All | n | % | OR (95%CI), P-value | aOR (95%CI), P-value | |
| Autophagosome formation | |||||
| Strong | 48 | 8 | 16.7 | Reference | Reference |
| Moderate | 47 | 10 | 21.3 | 1.351 (0.48–3.79), 0.567 | 1.303 (0.46–3.72), 0.621 |
| Absent-Low | 90 | 43 | 47.8 | 4.574 (1.93–10.86), 0.001 | 4.710 (1.93–11.50), 0.001 |
| Gender | |||||
| Female | 79 | 25 | 31.7 | Reference | |
| Male | 106 | 36 | 34 | 1.005 (0.54–1.87), 0.988 | |
| Age | |||||
| <30 | 40 | 10 | 25 | Reference | Reference |
| 30–50 | 88 | 28 | 31.8 | 1.400 (0.60–3.26), 0.435 | 2.089 (0.85–5.16), 0.110 |
| >50 | 57 | 23 | 40.4 | 2.029 (0.83–4.94), 0.119 | 2.915(1.12–7.58), 0.028 |
| Alcohol | |||||
| No | 94 | 33 | 35.1 | Reference | |
| Yes | 91 | 28 | 30.8 | 0.822 (0.44–1.52), 0.531 | |
| Tobacco | |||||
| No | 99 | 28 | 28.3 | Reference | Reference |
| Yes | 86 | 33 | 38.4 | 1.579 (0.85–2.93), 0.147 | 1.381 (0.71–2.69), 0.344 |
| TB form | |||||
| PTB alone | 131 | 45 | 34.4 | Reference | |
| PTB+EPTB | 46 | 16 | 34.8 | 1.019 (0.50–2.07), 0.958 | |
| Leukocytes (109/L) | |||||
| ≤10.0 | 152 | 50 | 32.9 | Reference | |
| >10.0 | 33 | 11 | 33.3 | 0.980 (0.44–2.18), 0.961 | |
| ESR (mm) | |||||
| ≤40 | 77 | 21 | 27.3 | Reference | Reference |
| >40 | 108 | 40 | 37 | 1.569 (0.83–2.96), 0.165 | 1.609 (0.81–3.19), 0.174 |
Abbreviation: OR, odd ratio; aOR, adjusted OR; CI, confidence interval
*For the regression analysis of radiographic findings, the radiographic extent of disease was categorized into two group: Minimal to moderate-advanced group and Far-advanced group.
We further investigated the correlation between the extents of Mtb-induced autophagosome formation with the treatment outcome of TB patient. Result from the univariate analysis showed that the defect in Mtb-induced autophagosome formation was positively correlated with the unfavorable treatment outcomes of TB patients (OR, 7.881; 95% CI 2.27–27.43; p = 0.001). In further multivariate analysis adjusted for age, tobacco smoking and leucocyte count, the poor autophagy-inducing ability by Mtb remained as a significant risk factor for unfavorable outcomes in TB patients (aOR, 8.310; 95% CI 2.22–28.97; p = 0.001). Of the covariates included in the final model, over 50 years of age (aOR, 4.274; 95% CI 1.32–13.86; p = 0.015) and PTB+EPTB (aOR, 2.504; 95% CI 1.18–5.33; p = 0.031) were also revealed as the risk factors for unfavorable treatment outcomes in TB patients. Details are presented in Table 3.
Table 3. Univariate and multivariate logistic regression analysis of treatment outcomes in TB patients.
| Variable | Treatment outcome | ||||
|---|---|---|---|---|---|
| Unfavorable | Univariate analysis | Multivariate Analysis | |||
| All | n | % | OR (95%CI), P-value | aOR (95%CI), P-value | |
| Autophagosome formation | |||||
| Strong | 48 | 3 | 6.3 | Reference | Reference |
| Moderate | 47 | 7 | 14.9 | 2.625 (0.64–10.84), 0.182 | 2.412 (0.57–10.30), 0.235 |
| Absent-Low | 90 | 31 | 34.4 | 7.881 (2.27–27.43), 0.001 | 8.024 (2.22–28.97), 0.001 |
| Gender | |||||
| Female | 79 | 15 | 19 | Reference | |
| Male | 106 | 26 | 24.5 | 1.387 (0.68–2.84), 0.371 | |
| Age | |||||
| <30 | 40 | 5 | 12.5 | Reference | Reference |
| 30–50 | 88 | 19 | 21.6 | 1.928 (0.66–5.60), 0.228 | 2.730 (0.88–8.48), 0.083 |
| >50 | 57 | 14 | 24.6 | 2.975 (0.99–8.90), 0.051 | 4.274 (1.32–13.86), 0.015 |
| Alcohol | |||||
| No | 94 | 19 | 20.2 | Reference | |
| Yes | 91 | 22 | 24.2 | 1.259 (0.63–2.52), 0.517 | |
| Tobacco | |||||
| No | 99 | 18 | 18.2 | Reference | Reference |
| Yes | 86 | 23 | 26.7 | 1.643 (0.82–3.31), 0.164 | 1.616 (0.73–3.57), 0.318 |
| TB form | |||||
| PTB alone | 131 | 22 | 16.8 | Reference | Reference |
| EPTB alone | 8 | 2 | 25 | 1.585 (0.30–8.25), 0.597 | 2.003 (0.29–12.85), 0.494 |
| PTB+EPTB | 46 | 16 | 34.8 | 2.504 (1.18–5.33), 0.017 | 2.525 (1.09–5.85), 0.031 |
| Leukocytes (109/L) | |||||
| ≤10.0 | 152 | 32 | 21.1 | Reference | |
| >10.0 | 33 | 9 | 27.3 | 1.406 (0.60–3.32), 0.437 | |
| ESR (mm) | |||||
| ≤40 | 77 | 12 | 15.6 | Reference | Reference |
| >40 | 108 | 29 | 26.9 | 1.99 (0.94–4.20), 0.072 | 2.104 (0.92–4.80), 0.077 |
Abbreviation: PTB, pulmonary tuberculosis; EPTB, extra-pulmonary tuberculosis; OR, odd ratio; aOR, adjusted OR; CI, confidence interval.
Together, these data indicated that the defect in autophagy induction by Mtb posed as an independent risk factor for poor clinical outcomes in patients with TB.
Discussion
The current study revealed that most of clinical isolates of Mtb was able to induce autophagosome formation in macrophages. The results also revealed that clinical isolates of Mtb differed significantly in their ability to induce autophagosome formation. Furthermore, our data revealed that the defect in autophagy induction by clinical isolates was positively correlated with the poor clinical outcomes in TB patients.
Autophagy is revealed to play a crucial role in host defense against Mtb by both in vitro and in vivo investigation [9, 12, 16]. Results from a genome-wide analysis of the host intracellular network also indicate that autophagy is implicated in the regulation of Mtb survival [29]. Autophagy may contribute to the elimination of Mtb through the fusion of Mtb-containing autophagosomes with lysosomes, leading to the xenophagic degradation of Mtb, and/or the enhancement of antigen presentation, and the consequent activation of adaptive immunity. On the other hand, through long battles with the host, Mtb may have developed various strategies to evade the autophagy-mediated antibacterial activities [11, 14]. In present investigation, we focus on the effect of clinical isolates of Mtb on autophagy induction and its possible association with clinical outcomes in TB patients.
We found that although most of clinical isolates of Mtb could induce autophagy in macrophages, the autophagy-inducing ability varied significantly among these clinical isolates. Reports indicate that the genotype of Mtb may have an important role in its behaviors [23, 30, 31], we thus would like to know whether the autophagy-inducing ability of these clinical isolates was related to their genetic background. We examined the genotypes of these Mtb isolates by the mycobacterial interspersed repetitive unit-variable number of tandem repeats (MIRU-VNTR) technique, and determined the possible association between the genotype and autophagy induction by cluster analysis using BioNumerics software. Our results revealed that there was no significant correlation between the clinical isolates-induced autophagosome formation and their genotypes (data not shown). We further investigated the association between the extent of autophagy induction by clinical isolates of Mtb with various clinical variables, including the socio-demographic characters, radiographic findings, laboratory tests and treatment outcomes, etc. Our results showed that that the defect in autophagy induction by clinical isolates was an independent risk factor for far-advanced radiographic disease and unfavorable treatment outcomes in TB patients. Additionally, our data also identified the Mtb-induced autophagosome formation was an independent risk factor for re-treatment TB cases (OR, 8.754; 95% CI 3.64–21.08; p<0.001) (S1 Table). Collectively, these data indicated that the autophagy-inducing ability by clinical isolates of Mtb might play a crucial role in determining the clinical outcomes in TB patients.
Given the importance of clinical isolates of Mtb-induced autophagy in TB outcomes, an interesting question was raised. Why different clinical isolates of Mtb possessed different ability in inducing autophagosome formation. This work was now undertaken in our laboratory. Reports indicated that H37Rv infection could suppress the autophagy flux in macrophages [17, 18], however, which could be rescued by Mtb-specific T cells [17]. We think that, besides the manipulation of autophagy induction, clinical isolates of Mtb may have developed other strategies for their survival. It is of interest in further investigation to determine whether clinical isolates of Mtb could impair autophagy flux, and the possible involvement of host factors, such as Mtb-specific T cells, in this process.
In conclusion, this study revealed that clinical isolates of Mtb differed in their ability to induce autophagy, which was closely correlated with clinical outcomes in TB patients, indicating the control of autophagy induction might be an important strategy manipulated by the bacteria to evade host immune responses. To our knowledge, this is the first report investigating the association between the autophagy induction and TB outcomes using clinical isolates. These data may help better understand the role of autophagy in the pathogenesis of tuberculosis, and provide important information for the better control of TB infection.
Supporting Information
(DOC)
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by grants from the National Science & Technology Key Projects during the Twelfth Five-Year Plan Period of China (2013ZX10003007), Major State Basic Research Development Program of China (2013CB530501, 2013CB531502), the National Natural Science Foundation of China (31470839, 81072428), Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), Jiangsu Provincial Innovative Research Team, and the Program for Changjiang Scholars and Innovative Research Team in University of Ministry of Education of China (PCSIRT-IRT1075). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Global Tuberculosis Report 2014. World Health Organization 2014.
- 2.Zumla A, George A, Sharma V, Herbert N, Baroness MOI. WHO's 2013 global report on tuberculosis: successes, threats, and opportunities. Lancet. 2013; 382:1765–1767. 10.1016/S0140-6736(13)62078-4 [DOI] [PubMed] [Google Scholar]
- 3.Rubin EJ. Troubles with tuberculosis prevention. N Engl J Med. 2014; 370:375–376. 10.1056/NEJMe1312301 [DOI] [PubMed] [Google Scholar]
- 4.Miotto P, Cirillo DM, Migliori GB. Drug resistance in Mycobacterium tuberculosis: molecular mechanisms challenging fluoroquinolones and pyrazinamide effectiveness. Chest. 2015; 147:1135–1143. 10.1378/chest.14-1286 [DOI] [PubMed] [Google Scholar]
- 5.Guidelines on the Management of Latent Tuberculosis Infection. World Health Organization, Geneva, Switzerland: World Health Organization, 2015. [PubMed] [Google Scholar]
- 6.Harding CV, Ramachandra L, Wick MJ. Interaction of bacteria with antigen presenting cells: influences on antigen presentation and antibacterial immunity. Curr Opin Immunol. 2003; 15:112–119. [DOI] [PubMed] [Google Scholar]
- 7.Bruns H, Stenger S. New insights into the interaction of Mycobacterium tuberculosis and human macrophages. Future Microbiol. 2014; 9:327–341. 10.2217/fmb.13.164 [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Li BX, Ge PP, Li J, Wang Q, Gao GF, et al. Mycobacterium tuberculosis suppresses innate immunity by coopting the host ubiquitin system. Nat Immunol. 2015; 16:237–245. 10.1038/ni.3096 [DOI] [PubMed] [Google Scholar]
- 9.Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004; 119:753–766. [DOI] [PubMed] [Google Scholar]
- 10.Songane M, Kleinnijenhuis J, Netea MG, van Crevel R. The role of autophagy in host defence against Mycobacterium tuberculosis infection. Tuberculosis (Edinb). 2012; 92:388–396. [DOI] [PubMed] [Google Scholar]
- 11.Deretic V, Saitoh T, Akira S. Autophagy in infection, inflammation and immunity. Nat Rev Immunol. 2013; 13:722–737. 10.1038/nri3532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bento CF, Empadinhas N, Mendes V. Autophagy in the Fight Against Tuberculosis. Dna Cell Biol.2015; 34:228–242. 10.1089/dna.2014.2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008; 132:27–42. 10.1016/j.cell.2007.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med. 2013; 368:1845–1846. [DOI] [PubMed] [Google Scholar]
- 15.Murrow L, Debnath J. Autophagy as a stress-response and quality-control mechanism: implications for cell injury and human disease. Annu Rev Pathol. 2013; 8:105–137. 10.1146/annurev-pathol-020712-163918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JJ, Lee HM, Shin DM, Kim W, Yuk JM, Jin HS, et al. Host cell autophagy activated by antibiotics is required for their effective antimycobacterial drug action. Cell Host Microbe. 2012; 11:457–468. 10.1016/j.chom.2012.03.008 [DOI] [PubMed] [Google Scholar]
- 17.Petruccioli E, Romagnoli A, Corazzari M, Coccia EM, Butera O, Delogu G, et al. Specific T cells restore the autophagic flux inhibited by Mycobacterium tuberculosis in human primary macrophages. J Infect Dis. 2012; 205:1425–1435. 10.1093/infdis/jis226 [DOI] [PubMed] [Google Scholar]
- 18.Romagnoli A, Etna MP, Giacomini E, Pardini M, Remoli ME, Corazzari M, et al. ESX-1 dependent impairment of autophagic flux by Mycobacterium tuberculosis in human dendritic cells. Autophagy. 2012; 8:1357–1370. 10.4161/auto.20881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sassetti CM, Boyd DH, Rubin EJ. Comprehensive identification of conditionally essential genes in mycobacteria. Proc Natl Acad Sci U S A. 2001; 98:12712–12717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vrba-Pech A, Fol M, Krawczyk M, Kowalewicz-Kulbat M, Kwiatkowska S. Clinical Mycobacterium tuberculosis isolates from the population of Lodz, Poland stimulated macrophages to the lower production of IL-12 and NO when compared to the virulent H37Rv strain. Tuberculosis (Edinb). 2014; 94:383–388. [DOI] [PubMed] [Google Scholar]
- 21.Romero MM, Balboa L, Basile JI, Lopez B, Ritacco V, de la Barrera SS, et al. Clinical isolates of Mycobacterium tuberculosis differ in their ability to induce respiratory burst and apoptosis in neutrophils as a possible mechanism of immune escape. Clin Dev Immunol. 2012; 2012:152546 10.1155/2012/152546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krishnan N, Robertson BD, Thwaites G. Pathways of IL-1beta secretion by macrophages infected with clinical Mycobacterium tuberculosis strains. Tuberculosis (Edinb). 2013; 93:538–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen L, Li N, Liu Z, Liu M, Lv B, Wang J, et al. Genetic diversity and drug susceptibility of Mycobacterium tuberculosis isolates from Zunyi, one of the highest-incidence-rate areas in China. J Clin Microbiol. 2012; 50:1043–1047. 10.1128/JCM.06095-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Falk AJ, O'Connor B, Pratt PC. Classification of pulmonary tuberculosis. 12th ed. New York: National Tuberculosis and Respiratory disease Association; 1969. [Google Scholar]
- 25.World Health Organization. Treatment of tuberculosis: guidelines for national programmes, 4th ed., Geneva, Switzerland: 2009. [Google Scholar]
- 26.Zhang W, Xu W, Xiong S. Blockade of Notch1 signaling alleviates murine lupus via blunting macrophage activation and M2b polarization. J Immunol. 2010; 184:6465–6478. 10.4049/jimmunol.0904016 [DOI] [PubMed] [Google Scholar]
- 27.Li S, Yue Y, Xu W, Xiong S. MicroRNA-146a represses mycobacteria-induced inflammatory response and facilitates bacterial replication via targeting IRAK-1 and TRAF-6. PLoS One. 2013; 8:e81438 10.1371/journal.pone.0081438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao B, Wang Y, Xu W, Li S, Li Q, Xiong S. Inhibition of histone deacetylase activity suppresses IFN-gamma induction of tripartite motif 22 via CHIP-mediated proteasomal degradation of IRF-1. J Immunol. 2013; 191:464–471. 10.4049/jimmunol.1203533 [DOI] [PubMed] [Google Scholar]
- 29.Kumar D, Nath L, Kamal MA, Varshney A, Jain A, Singh S, et al. Genome-wide analysis of the host intracellular network that regulates survival of Mycobacterium tuberculosis. Cell. 2010; 140:731–743. 10.1016/j.cell.2010.02.012 [DOI] [PubMed] [Google Scholar]
- 30.Chakraborty P, Kulkarni S, Rajan R, Sainis K. Drug resistant clinical isolates of Mycobacterium tuberculosis from different genotypes exhibit differential host responses in THP-1 cells. PLoS One. 2013; 8:e62966 10.1371/journal.pone.0062966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Srilohasin P, Chaiprasert A, Tokunaga K, Nishida N, Prammananan T, Smittipat N, et al. Genetic diversity and dynamic distribution of Mycobacterium tuberculosis isolates causing pulmonary and extrapulmonary tuberculosis in Thailand. J Clin Microbiol. 2014; 52:4267–4274. 10.1128/JCM.01467-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.