Abstract
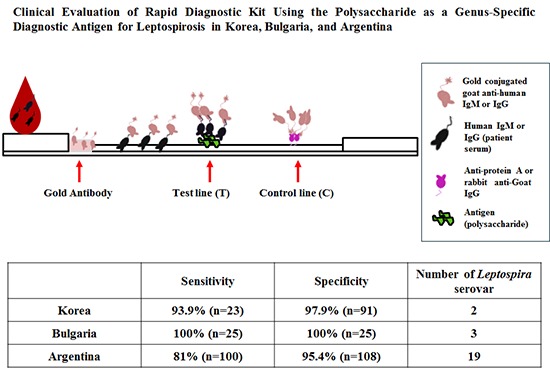
Leptospirosis, a zoonotic disease that is caused by many serovars which are more than 200 in the world, is an emerging worldwide disease. Accurate and rapid diagnostic tests for leptospirosis are a critical step to diagnose the disease. There are some commercial kits available for diagnosis of leptospirosis, but the obscurity of a species- or genus-specific antigen of pathogenic Leptospira interrogans causes the reduced sensitivity and specificity. In this study, the polysaccharide derived from lipopolysaccharide (LPS) of nonpathogenic Leptospira biflexa serovar patoc was prepared, and the antigenicity was confirmed by immunoblot and enzyme linked immunosorbent assay (ELISA). The performance of the rapid diagnostic test (RDT) kit using the polysaccharide as a diagnostic antigen was evaluated in Korea, Bulgaria and Argentina. The sensitivity was 93.9%, 100%, and 81.0% and the specificity was 97.9%, 100%, and 95.4% in Korea (which is a rare region occurring with 2 serovars mostly), Bulgaria (epidemic region with 3 serovars chiefly) and Argentina (endemic region with 19 serovars mainly) respectively. These results indicate that this RDT is applicable for global diagnosis of leptospirosis. This rapid and effective diagnosis will be helpful for diagnosis and manage of leptospirosis to use and the polysaccharide of Leptospira may be called as genus specific antigen for diagnosis.
Keywords: Leptospirosis, Genus-specific Diagnostic Antigen, Rapid Diagnosis
Graphical Abstract
INTRODUCTION
Leptospirosis has emerged globally as a widespread acute febrile disease within the past decade. It occurs worldwide in rural areas, as well as in urban regions of industrialized countries (1). Pathogenic leptospire, Leptospira interrogans lives in the proximal renal tubule of the kidney of infected animal, and it is excreted intermittently or regularly in urine and so contaminates soil, water, streams and rivers (2). Humans become accidental hosts infected through contact with contaminated environment to leptospires (3,4). The clinical symptoms of human leptospirosis vary from an asymptomatic flu-like illness to a severe systemic disease with renal and hepatic failure, extensive vasculitis, pulmonary hemorrhage, and death (5). The mortality rate in severe leptospirosis is as high as 20% (6).
As one of the most common zoonosis in the world, leptospirosis has re-emerged in Southeast Asia, North America, Europe, India, and Brazil (7,8,9). Global warming increases the risks of the emergence, and annual incidence of leptospirosis is estimated from 1 per 100,000 in temperate climates, to 10-100 per 100,000 in the humid tropics (10). The high humidity and warm temperature of tropical and subtropical countries are suitable for leptospires to survive for long periods in the environment (6).
Leptospirosis is an easily curable disease using antibiotic therapy if early diagnosis is done, but if left untreated, this disease may become aggravated. However, leptospirosis is often misdiagnosed since it is difficult to distinguish from dengue fever, malaria, influenza and other infectious diseases characterized by fever, headache, and myalgia (11). So, it is necessary for an easy, convenient and rapid diagnosis tool to initiate proper and timely management (4). The standard method of leptospirosis diagnosis is microscopic agglutination test (MAT), detecting antibody serologically against the Leptospira of suspicious serovars (12). Because MAT is a complicated test that requires all of each alive Leptospira serovar existing in each local area, therefore, enzyme-linked immunosorbent assay (ELISA), dipstick assay, indirect hemagglutination assay and rapid diagnostic test (RDT) kit as alternatives have been developed for diagnosis of leptospirosis (13,14). The performance of these serologic assays has been reported to compare the MAT, showing inaccuracy (15).
Until now, RDT relied on intra-genus cross reactivity to detect antigenically diverse pathogens, and the most commonly utilizing antigen is lipopolysaccharide (LPS) obtained from nonpathogenic Leptospira biflexa serovar patoc (6). LPS has been known as a serovar specific and some genus related antigen (2). In our study, the polysaccharide derived from LPS was rationally selected as a diagnostic antigen in order to evaluate the potential possibility to diagnose leptospirosis in Korea and two other geographic regions, including Bulgaria and Argentina.
Four serovars, including lai, canicola, yeonchon and hongchon have been isolated in Korea (16). Current incidence of leptospirosis has been about 50 patients annually as referred to a nonepidemic area from Korea Centers for Disease Control & Prevention. Bulgaria serogroups, including 10 different serovars, have caused infection during the past 13-years study. Two serogroups, icterohaemorrhagiae and pomona, have accounted for more than 87% of all leptospirosis cases. This incidence is relatively low, meaning that Bulgaria belongs to an epidemic area (17). In Argentina, occasional outbreaks have been characterized by mild leptospirosis, rendering it an endemic area (18).
Herein, we evaluated the diagnostic accuracy of the ImmuneMed Leptospira Rapid (ILR) diagnostic kit which is used polysaccharide as antigen for genus specificity. The performance was studied in Korea, Bulgaria and Argentina to determine whether ILR is applicable as a global diagnosis of human leptospirosis and the polysaccharide is concluded as genus-specific antigen for leptospirosis diagnosis.
MATERIALS AND METHODS
Leptospirosis serum samples
Thirty three Korean specimens were collected at various hospitals of Hallym University Medical Center located in Chuncheon, Kangnam, Kangdong, and Hangang from patients showing leptospirosis-like symptoms. These patients had at least two various symptoms of leptospirosis (fever, chills, headache, muscle aches, vomiting, diarrhea, jaundice, pulmonary hemorrhage, or renal failure). MAT was performed by Professor YW Kim, Department of Microbiology, School of Medicine, Hallym University, using a panel of alive Leptospira pathogens representing locally prevalent serovars, lai, and canicola. A positive MAT was taken as a single titer of ≥1:80 or a 4-fold rise in titer between acute and convalescence samples taken up to 29 days after the onset of symptoms (Table 1). Every patient sample was positive to one or two serovars, lai and canocola in MAT (≥1:80) (Table 2).
Table 1. Comparison of MAT and ELISA from cases of Korea.
| Reactions | MAT* | ELISA (lai) | ELISA (patoc) | |||
|---|---|---|---|---|---|---|
| Case | Control | Case | Control | Case | Control | |
| Positive | 20 | 0 | 19 | 1 | 19 | 1 |
| Negative | 0 | 20 | 1 | 19 | 1 | 19 |
| Total | 20 | 20 | 20 | 20 | 20 | 20 |
| Sensitivity (%) (95% CI) |
95.0 (73.0-99.7) |
95.0 (73.0-99.7) |
||||
| Specificity (%) (95% CI) |
95.0 (73.0-99.7) |
95.0 (73.0-99.7) |
||||
| PPV (%) (95% CI) |
95.0 (73.0-99.7) |
95.0 (73.0-99.7) |
||||
| NPV (%) (95% CI) |
95.0 (73.0-99.7) |
95.0 (73.0-99.7) |
||||
| Accuracy | 0.95 | 0.95 | ||||
| Reliability | 0.9 | 0.9 | ||||
*MAT was considered as standard method for diagnosis of leptospirosis.
Table 2. Reference serovars used for MAT.
| Korea (titer ≥ 1:80)* | Bulgaria (titer ≥ 1:200)* | Argentina (titer ≥ 1:800)* |
|---|---|---|
| lai | icterohaemorrhagiae | castellonis |
| canicola | pomona | canicola |
| bratislava | grippothyphosa | |
| copenhageni | ||
| icterohaemorrhagiae | ||
| pomona | ||
| pyrogenes | ||
| tarassovi | ||
| wolffi | ||
| hardjo | ||
| bataviae | ||
| patoc | ||
| australis | ||
| autumnalis | ||
| cynopteri | ||
| hebdomadis | ||
| javanica | ||
| panama | ||
| sejroe |
*Positive cutoff titer at MAT in each country at single serum.
Twenty-five specimens in Bulgaria were selected for this study from serum samples submitted to the National Reference Vector-borne Infections Laboratory of the National Center of Infectious and Parasitic Diseases. Every patient had at least two symptoms of typical leptospirosis and in every case MAT titer of ≥ 1:200 was observed, using 3 serovars (Table 2). Samples from one-hundred leptospirosis patients in Argentina were randomly selected from the Instituto Nacional de Enfermedades Respiratorias. Each case was considered epidemiologically and clinically compatible with the disease and a four-fold or greater rise in MAT titers at second or third serum sample from different stage of the illness or one titer ≥1:800 in one sample (Table 2). Every sample used in this evaluation is the first sample which is mostly taken in acute phase. Mean annual incidence rate is 0.1, 4.2, and 25 cases per 100,000 resident in Korea, Bulgaria and Argentina respectively.
Control serum samples
In Korea, the serum samples from healthy donors (n=23) and patients (n=71) with three acute febrile diseases other than leptospirosis, which included scrub typhus (n=25), hemorrhagic fever with renal syndrome (n=21), and murine typhus (n=25), were provided by Hallym University Hospital (n=70) and Korea University Hospital (n=24). These patients were confirmed by indirect fluorescent antibody-IgM (IFA-IgM) and -IgG test for each disease, and as negative by MAT.
In Bulgaria, 25 control specimens were collected from five other febrile diseases which included rheumatoid arthritis (n=5), EBV infectious mononucleosis (n=5), multiple sclerosis (n=5), Lyme disease (n=5), and Syphilis (n=5) and all patients were confirmed by indirect fluorescent antibody (IFA) test and MAT. In Argentina, 108 specimens with healthy controls (n=85) and three other acute febrile diseases, including dengue fever (n=13), Argentina hemorrhagic fever (n=5), and hemorrhagic fever with renal syndrome (n=5), were used as control serum samples. Healthy controls were obtained from endemic areas and all patients were confirmed by IFA and MAT.
In Bulgaria, 25 control specimens were collected from five other febrile diseases which included rheumatoid arthritis (n=5), EBV infectious mononucleosis (n=5), multiple sclerosis (n=5), Lyme disease (n=5), and Syphilis (n=5) and all patients were confirmed by indirect fluorescent antibody (IFA) test and MAT. In Argentina, 108 specimens with healthy controls (n=85) and three other acute febrile diseases, including dengue fever (n=13), Argentina hemorrhagic fever (n=5), and hemorrhagic fever with renal syndrome (n=5), were used as control serum samples. Healthy controls were obtained from endemic areas and all patients were confirmed by IFA and MAT.
Microscopic agglutination test
The MAT was performed using the standard procedure, with minor modifications (19). Alive Leptospira bacteria suspension was used to determine the titer under dark field microscope.
Preparation of polysaccharide as diagnostic antigen
Leptospira biflexa serovar patoc or L. interrogans serovar lai was used for production of diagnostic antigen. Leptospire pellet was heated in boiling water and treated with proteinase K. EGTA was added to the solution and centrifuged. The precipitate LPS was treated with 1% acetic acid in boiling water and centrifuged to remove lipid A. The prepared polysaccharide was quantified, using a standard polysaccharide which had been prepared from LPS purified from Escherichia coli (Sigma) in the same manner as described above.
Antigenicity analysis of leptospire polysaccharide
Antigenicity was confirmed by Western blotting and ELISA as described in the ImmuneMed patent (20).
SDS-PAGE and Western blotting
Briefly, polysaccharide isolated from Leptospira was subjected to SDS-PAGE and silver-staining. Another gel was transferred to a PVDF membrane (Millipore, Billerica, MA, USA) for further analysis. The membrane was reacted with rabbit immunoserum to L. interrogans, and then reacted with a horse-radish peroxidase conjugated anti-rabbit IgG (Bio-Rad Laboratories, Richmond, CA, USA). The immunoblots were developed with an ECL plus Kit (Rockford, IL, USA).
Enzyme linked immunosorbent assay
ELISA for polysaccharide was performed on the basis of the method described in the ImmuneMed patent (20). Test was performed in a 96-well flat-bottom plate (BD, San Diego, CA, USA), which was coated with poly-L-lysine (Sigma Aldrich, St. Louis, MO, USA). The polysaccharide was suspended in phosphate buffered saline (PBS) and incubated. After the plate was blocked with goat serum, serum samples from patients with leptospirosis and healthy persons were used as primary antibodies and a horse-radish peroxidase conjugated anti-human IgG (Bio-Rad Laboratories, Richmond, CA) was used as a secondary antibody. Each well was treated with a TMB single substrate solution (Life Technologies, Frederick, MD, USA) to develop, and the absorbance was measured using an ELISA reader (Spectra Max 250, Molecular Devices, Sunnyvale, CA, USA) at 490 nm. After completing the reaction, the result was compared with MAT.
Rapid diagnostic test
Polysaccharide (1.6 µg/strip) of L. biflexa patoc was applied to RDT kit and this RDT kit manufactured by ImmuneMed (20). After 3 µL of serum were applied to the sample port of the device, 300 µL of sample diluent were added. The qualitative result was read between 10 and 15 minutes. The red color appearing concurrently on the control line (C) and test line (T) was regarded as positive. The test was considered as negative when only the control line appeared and it was invalid if there was no detection of control line. RDT kit was set up as a positive in IgM or IgG at MAT 1:100.
To evaluate the performance of RDT in Bulgaria and Argentina, ImmuneMed Leptospira Rapid (ILR) kits were mailed to Dr. Christova in Bulgaria and Dr. Vanasco in Argentina, respectively.
Data analysis
Variables indicate the number of true positives (TP), true negatives (TN), false positives (FP), and false negatives (FN). Calculation of accuracy and reliability were conducted by (TP+TN)/number of all tests and ([TPxTN]-[FPxFN])/([TP+FN][TN+FP]) respectively (21). Positive predictive value (PPV) indicates the value of TP/(TP+FP) and negative predictive value (NPV) is TN/ (TN+FN).
Ethics statement
The study protocol was approved by the institutional review board of Hallym University (IRB, 2013-65). Informed consent was exempted by the board.
RESULTS
Purification and characterization of polysaccharide as an antigen
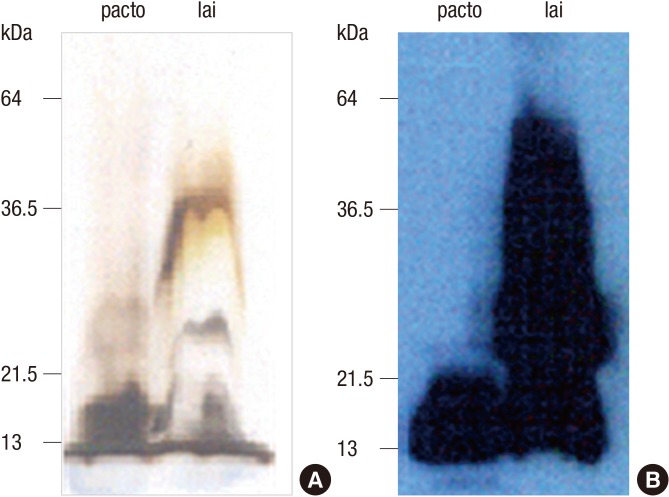
Silver staining for polysaccharide derived from LPS of patoc and lai revealed the common band about 14-30 kDa in SDS-PAGE, corresponding to the previous report (2). After reaction with pooled serum samples of leptospirosis patients, the strong reactivities were detected at 14 kDa band in both patoc and lai, meaning that polysaccharide of nonpathogenic serovar patoc has the common epitope to react with antibodies to pathogenic Leptospira interrogans (Fig. 1).
Fig. 1. Examination of polysaccharide as common epitope for Leptospira. (A) Polysaccharide prepared from LPS of L. biflexa serovar patoc or L. interrogans serovar lai was analyzed by SDS-PAGE and silver-staining. (B) Purified antigens were transferred onto nitrocellulose (NC) membrane and 12-20 kDa of polysaccharide was detected by human pooled serum of leptospirosis patients as common antigen to both patoc and lai.
In order to determine the antigenicity of polysaccharide derived from LPS, ELISA which was coated with polysaccharide derived from Leptospira interrogans serovar lai or L. biflexa serovar patoc, was performed with 20 cases of leptospirosis in Hallym University Medical Center to compare with MAT. The cutoff value of ELISA between leptospirosis and healthy serum specimens was set to 0.2 through adjustment of ELISA conditions and in this evaluation, no serum samples from a healthy control had OD greater than the cutoff value.
In Table 1, when 20 leptospirosis and 20 control specimens were applied to MAT, all leptospirosis specimens were confirmed 100% positive and all controls were 100% negative. When 20 cases and 20 healthy control specimens were tested with polysaccharides derived from lai and patoc in ELISA, there were the same results between lai and patoc with 95% sensitivity (19/20) and 95% specificity (19/20). The significant correlation was obtained between the lai and patoc polysaccharide-coated ELISAs (Table 1). Accordingly, as a candidate antigen for diagnosis of leptospirosis, the polysaccharides from lai or patoc LPS were considered to be suitable.
Leptospirosis and control specimen's characteristics
Acute serum samples from mild leptospirosis-patients were collected in Korea to evaluate the performance of RDT because early diagnosis is critically important in the diagnostic test of leptospirosis to prevent aggravation and death.
A total of 33 leptospirosis cases in Korea, 25 in Bulgaria and 108 in Argentina were taken for evaluation in double-blind tests at each designated sites. The ages of Korea, Bulgaria, and Argentina's patients are 50.0±11.3 (mean±SD), 50.5 ±14.3, and 29.4±14.8 yr, respectively. Days after onset of symptoms are 9.0±4.0 (Korea), 15.6±3.3 (Bulgaria), and 8.9±8.3 (Argentina), and the cases number of male leptospirosis is higher than those of female, and each ratio of male versus female is 2.0:1 (Korea), 2.1:1 (Bulgaria), and 14.7:1 (Argentina). All specimens were collected from more than five different districts of each country to obtain the serum samples infected with various serovars. According to the information provided by each institute, positive cut-off value of MAT antibody titer is 1:80 (Korea), 1:200 (Bulgaria), and 1:800 (Argentina).
The major or important serovars at MAT in each country are shown in Table 2. Two or three serovars were taken in Korea and Bulgaria, but in Argentina 19 serovars were in this study (Table 2). All patients with other acute febrile diseases were confirmed by IFA in each country (data not shown).
Sensitivity and specificity of ImmuneMed RDT
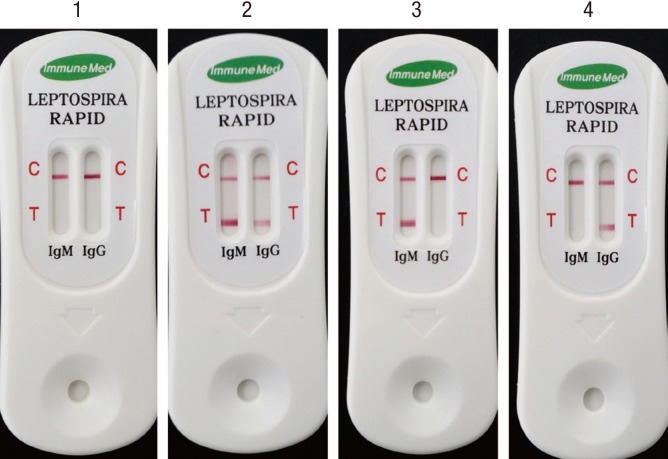
Positive or negative was determined by subjective visual interpretation (Fig. 2). Control line (C) represents the validation of test results. Red colorization of test line (T) indicates the presence of human antibody against Leptospira.
Fig. 2. Representative pictures for RDT indicating negative and positive results. Red colorization of the test line (T) indicates the presence of human antibody against Leptospira and the red control line (C) represents the valid test result. Demonstration is negative (kit 1), both positive in IgM and IgG (kit 2), IgM positive (kit 3), and IgG positive (kit 4) in RDT results.
Table 3 shows the sensitivity and specificity performed in Korea, Bulgaria, and Argentina. Of the 33 serum samples from Korea, 31 were positive in RDT assay with 93.9% sensitivity. Two of the 94 controls were found false positive in Korea. Thus, specificity of RDT was 97.9%.
Table 3. Sensitivity and specificity of the ImmuneMed RDT in different geographic countries.
| Reactions | No. in Argentina | No. in Argentina | No. in Argentina | |||
|---|---|---|---|---|---|---|
| Cases | Controls | Cases | Controls | Cases | Controls | |
| RDT positive | 31 | 2 | 25 | 0 | 81 | 5 |
| RDT negative | 2 | 92 | 0 | 25 | 19 | 103 |
| Total | 33 | 94 | 25 | 25 | 100 | 108 |
| Sensitivity (%) (95% CI) |
93.9 (80.4-98.3) |
100 (83.4-100) |
81.0 (72.2-87.5) |
|||
| Specificity (%) (95% CI) |
97.9 (92.6-99.4) |
100 (83.4-100) |
95.4 (89.6-98.0) |
|||
| PPV (%) (95% CI) |
93.9 (80.4-98.3) |
100 (83.4-100) |
94.2 (87.1-97.5) |
|||
| NPV (%) (95% CI) |
97.9 (92.6-99.4) |
100 (83.4-100) |
84.4 (77.0-89.8) |
|||
| Accuracy | 0.97 | 1.00 | 0.88 | |||
| Reliability | 0.92 | 1.00 | 0.76 | |||
In Bulgaria, 25 serum samples confirmed by L. interrogans serovar pomona (MAT titer, 1:400-25,600), icterohaemorrhagiae (MAT titer, 1:400-25,600), and bratislava (MAT titer, 1:200-400) were evaluated for the performance of RDT kit and their results showed 100% sensitivity. A total of 25 MAT negative were evaluated as all negative (100% specificity).
In Argentina, out of 100 leptospirosis evaluated, 81 cases were identified as test positive (81.0% sensitivity). Argentina's MAT reference panel included 19 serovars having MAT titers ≥1:800. Among 108 control specimens, only 5 healthy controls were considered as the false positive, indicating 95.4% specificity.
DISCUSSION
For diagnosis of leptospirosis, MAT is a gold standard with high sensitivity and specificity. However, it does not represent perfect sensitivity due to the requirement of all of each alive serovar existing in each local region. Because a better diagnostic method is not available at present, and leptospira is widespread as pathogens having over 200 serovars, species or genus specific, a simple, rapid and accurate diagnostic method is necessary. Therefore, we evaluated the performance of Leptospira rapid diagnostic test (RDT) comparing with MAT. We used various panels of positive controls in different geographic countries representing a wide range of serovars and negative controls including both healthy controls and disease controls with similar clinical manifestation or potentially cross-reactivity.
Since the antigenic epitopes of L. biflexa LPS has been identified as disaccharide unit (22), polysaccharide derived from LPS may be a strong candidate antigen for serodiagnosis of Leptospirosis. The polysaccharide of Leptospira consists of O-specific polysaccharide chain which displayed in various serovars and core polysaccharide which was speculated with genus-specific antigen due to coexisting diversity and conserved region (23). The Lipid A of LPS components may interfere with the binding of core polysaccharide to it's antibody, so it was removed for better interaction of antigen-antibody by the ImmuneMed. The excellent sensitivity of the RDT showed that core polysaccharide is the good antigen which is target of common recognition by antibody from various leptospirosis patients.
Each leptospirosis patient made and developed different kinds of antibodies for various polysaccharides according to serovar or serogroup. Because the standard diagnostic method, MAT, detects IgM originated from O-specific polysaccharide, it is specific to serovar or serogroup, and ImmuneMed diagnostic kit does not represent the same result of MAT due to antigen originated from core polysaccharide.
To evaluate the antigenicity of polysaccharide derived from L. biflexa serovar patoc and L. interrogans lai, immunoblotting to these antigens was performed with pooled serum samples of leptospirosis. The result was corresponding to the previous study, showing 14-20 kDa mass on membrane (2). In this result, there are suggested common epitopes of polysaccharides from pathogenic L. interrogans and nonpathogenic L. biflexa. Additionally, ELISA supported that both polysaccharides derived from pathogenic and nonpathogenic serovars can react with most leptospirosis serum samples, which is suggesting that polysaccharide was genus-specific antigen.
In this study, the RDT, using polysaccharide derived from nonpathogenic L. biflexa patoc, was successfully applied to the different geographic regions in the world, including areas with different serovars and a higher epidemicity of leptospirosis than Korea (see Table 2 and 3). Korean two patients who showed false negative in sensitivity got MAT titer 1:160 and 1:80. These titer values are very low in the MAT positive criterion and do not indicate enough antibody in these patients' serum samples to react the core polysaccharide. Therefore, the kit may not be reacting with patients' serum.
False positive in specificity rarely appeared in healthy controls and other disease controls. However, this false positive may not be nonspecific reaction. Hence we cannot exclude the possibility that patients have residual antibody reacting with core polysaccharide from a past infection, an inapparent infection, or an infection with other serovar which is not used at MAT.
Three serovars in Bulgaria and 19 serovars in Argentina have been used as reference panel of MAT and therefore, Table 3 shows that ImmuneMed Leptospira RDT is possible for serological diagnosis of various serovars. Because leptospirosis is treatable with use of penicillin or doxycycline, early, simple and accurate diagnosis is very important. Hence, the discovery of genus specific antigen is very essential for diagnosis. The sensitivity and specificity of this RDT in three different regions were reliable to diagnose leptospirosis (sensitivity; 81%-100%, specificity; 95.4%-100%).
False positive RDT reaction in controls could be explained by the hypothesis that after removing lipid A, the exposed core polysaccharide was strongly bound to residual antibody which were obtained from an infection and targeted to the epitope in the core polysaccharide but not enough to show adequate MAT titer. In addition, higher cutoff, to increase the specificity of the MAT in endemic areas may cause a false positive. When a patient infected with a rare or new serovar has not been diagnosed as leptospirosis in MAT, RDT is a real positive but it might be interpreted as a false positive. Seroconversion usually takes place 6 to 10 days after infection, so the RDT as well as MAT may have low sensitivity in the early phase of infection. Thus, if suspicious samples are collected early in the disease, it is possible to be negative in RDT (24). In these cases, it is advised that RDT assay be repeated with a sample collected a few days later (13,25).
Although genes-specific antigen is used in ImmuneMed RDT for detection of the antibody generated by various serovar, this genus-specific antigen purified from one serovar may have the limitation to detect all serovars. For this reason, despite the more than 19 serovars in Argentina, the sensitivity is to 81% (Table 3). And then, we cannot exclude the possibility of false positive in MAT from Argentine 19 specimens which is negative in RDT (Table 3). The possibility of false positive in MAT could be to longer culture at microbe than the appropriate period of time, quite a less number of microbe, characteristics of strain with autoagglutination tendency, longer incubation time before reading, lower incubation temperature, or error and lack of skill especially usage of many strains for MAT.
Among Argentine 81 positive specimens in ImmuneMed RDT, 13 specimens show IgG negative. Leptospirosis standard diagnosis assay MAT recognizes mainly IgM among the antibodies, even if IgG exists or not. As the ImmuneMed RDT is a qualitative diagnostic kit, it cannot analyze below the minimum detection limit. The remaining 68 specimens are positive to both IgM and IgG in ImmuneMed RDT. We consider that IgM positive is similar to MAT results, and IgG positive is more than two weeks after infection or at reinfection. Leptospirosis outbreaks in humans and animals has been regularly reported in Argentina recently, and main exposure occurs during floods in the warm and rainy season (26). In our study, sensitivity (81%) in Argentina was relatively lower than sensitivity in Korea and Bulgaria, but it is still acceptable to use. Additionally, negative predictive value (84.4%) was still high in Argentina, meaning that RDT can be used as a screening test in Argentina.
ImmuneMed Leptospira RDT has distinguished itself in the diagnosis of leptospirosis, and it can detect IgM and IgG to assume not only in current infection but also the progress of the disease simultaneously. In spite of the superior sensitivity of the kit, there remained the possibility of false positive in the case of low antibodies to core polysaccharide even though MAT was negative at high cutoff. Especially in an endemic area, it can be positive interpretation based on inapparent infection, past infection, or infection of serovar which is not used in MAT even though MAT titer is not positive.
The clinical evaluations in this study reveal that ImmuneMed RDT assay is very consistent with MAT in different geographic locations, and polysaccharide derived from LPS is the diagnostic genus-specific antigen. Additionally, the use of ImmuneMed RDT as an initial diagnosis for leptospiral infection would ensure easy diagnosis of leptospirosis. Since leptospirosis can be treated with penicillin or doxycycline, accurate and rapid diagnosis will provide effective management of this disease (4,27). We believe that the ImmuneMed RDT may be appropriate for hospitals and other health centers owing to its accuracy, rapidity, simplicity, and low requirements for skill. Furthermore, it may improve the rapid detection of leptospirosis and be applicable for global diagnosis.
Footnotes
Funding: This research was supported by the Hallym University Research Fund, 2013 (HRF-201312-023).
DISCLOSURE: The authors declare that there is no conflict of interests regarding the publication of this paper.
AUTHOR CONTRIBUTION: Study design: Lee JW, Park S, Kim SH, Kim YW. Evaluation of Leptospira rapid kit: Lee JW, Park S, Kim SH, Christova I, Jacob P, Vanasco NB. Preparation of Leptospira antigen: Kang YM, Woo YJ. Preparation of Leptospira rapid kit: Kim MS, Kim YJ. MAT assay: Cho MK, Kim YW. Writing manuscript: Kim YW. Revision and final approval of manuscript: all authors.
References
- 1.Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, Lovett MA, Levett PN, Gilman RH, Willig MR, Gotuzzo E, et al. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis. 2003;3:757–771. doi: 10.1016/s1473-3099(03)00830-2. [DOI] [PubMed] [Google Scholar]
- 2.Barnett JK, Barnett D, Bolin CA, Summers TA, Wagar EA, Cheville NF, Hartskeerl RA, Haake DA. Expression and distribution of leptospiral outer membrane components during renal infection of hamsters. Infect Immun. 1999;67:853–861. doi: 10.1128/iai.67.2.853-861.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dey S, Mohan CM, Ramadass P, Nachimuthu K. Diagnosis of leptospirosis by recombinant antigen based single serum dilution ELISA. Indian J Med Res. 2008;128:172–177. [PubMed] [Google Scholar]
- 4.Chaudhry R, Das A, Premlatha MM, Choudhary A, Chourasia BK, Chandel DS, Dey AB. Serological & molecular approaches for diagnosis of leptospirosis in a tertiary care hospital in north India: a 10-year study. Indian J Med Res. 2013;137:785–790. [PMC free article] [PubMed] [Google Scholar]
- 5.Cinco M. New insights into the pathogenicity of leptospires: evasion of host defences. New Microbiol. 2010;33:283–292. [PubMed] [Google Scholar]
- 6.Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14:296–326. doi: 10.1128/CMR.14.2.296-326.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laras K, Cao BV, Bounlu K, Nguyen TK, Olson JG, Thongchanh S, Tran NV, Hoang KL, Punjabi N, Ha BK, et al. The importance of leptospirosis in Southeast Asia. Am J Trop Med Hyg. 2002;67:278–286. doi: 10.4269/ajtmh.2002.67.278. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization; International Leptospirosis Society. Human leptospirosis: guidance for diagnosis, surveillance and control. Geneva: World Health Organization; 2003. [Google Scholar]
- 9.Dias JP, Teixeira MG, Costa MC, Mendes CM, Guimarães P, Reis MG, Ko A, Barreto ML. Factors associated with Leptospira sp infection in a large urban center in northeastern Brazil. Rev Soc Bras Med Trop. 2007;40:499–504. doi: 10.1590/s0037-86822007000500002. [DOI] [PubMed] [Google Scholar]
- 10.Victoriano AF, Smythe LD, Gloriani-Barzaga N, Cavinta LL, Kasai T, Limpakarnjanarat K, Ong BL, Gongal G, Hall J, Coulombe CA, et al. Leptospirosis in the Asia Pacific region. BMC Infect Dis. 2009;9:147. doi: 10.1186/1471-2334-9-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tappero JW, Ashford DA, Perkins BA. Leptospira species (leptospirosis) In: Mendell GL, Bennett JE, Dolin R, editors. Mandell, Douglas, and Bennett's principles and practice of infectious diseases. 5th ed. Philadelphia, PA: Churchill Livingstone; 2000. pp. 2495–2501. [Google Scholar]
- 12.Cole JR, Jr, Sulzer CR, Pursell AR. Improved microtechnique for the leptospiral microscopic agglutination test. Appl Microbiol. 1973;25:976–980. doi: 10.1128/am.25.6.976-980.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goris MG, Leeflang MM, Loden M, Wagenaar JF, Klatser PR, Hartskeerl RA, Boer KR. Prospective evaluation of three rapid diagnostic tests for diagnosis of human leptospirosis. PLoS Negl Trop Dis. 2013;7:e2290. doi: 10.1371/journal.pntd.0002290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park KH, Choi YJ, Shin SH, Choi MK, Kim YW, Jang WJ. Identification of Leptospira species of Korean isolates using phylogenetic analysis of polymerase chain reaction-amplified 16S rDNA and LipL32 genes. J Bacteriol Virol. 2014;44:59–66. [Google Scholar]
- 15.McBride AJ, Santos BL, Queiroz A, Santos AC, Hartskeerl RA, Reis MG, Ko AI. Evaluation of four whole-cell Leptospira-based serological tests for diagnosis of urban leptospirosis. Clin Vaccine Immunol. 2007;14:1245–1248. doi: 10.1128/CVI.00217-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho MK, Lee JH, Yoon CS, Kim YW, Min CH, Kim YS, Park KS, Oh HB. Serological analysis of Leptospira interrogans isolated in Korea using monoclonal antibodies and cross-agglutinin absorption test. J Korean Soc Microbiol. 1989;24:539–548. [Google Scholar]
- 17.Christova I, Tasseva E, Manev H. Human leptospirosis in Bulgaria, 1989-2001: epidemiological, clinical, and serological features. Scand J Infect Dis. 2003;35:869–872. doi: 10.1080/00365540310016709. [DOI] [PubMed] [Google Scholar]
- 18.Seijo A, Coto H, San Juan J, Videla J, Deodato B, Cernigoi B, Messina OG, Collia O, de Bassadoni D, Schtirbu R, et al. Lethal leptospiral pulmonary hemorrhage: an emerging disease in Buenos Aires, Argentina. Emerg Infect Dis. 2002;8:1004–1005. doi: 10.3201/eid0809.010499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu D. Molecular detection of human bacterial pathogens. Boca Raton, FL: CRC press; 2011. [Google Scholar]
- 20.Byun YH, Chang IA, Cho MK, Chun JM, Kim IS, Kim WC, Kim YW, Kim YJ, Woo SD. Korean Patent, WO 2008029981 A1. Diagnostic formulation for tsutsugamushi disease. 2008 Mar 13;
- 21.Singh N, Mishra AK, Shukla MM, Chand SK, Bharti PK. Diagnostic and prognostic utility of an inexpensive rapid on site malaria diagnostic test (ParaHIT f) among ethnic tribal population in areas of high, low and no transmission in central India. BMC Infect Dis. 2005;5:50. doi: 10.1186/1471-2334-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuo K, Isogai E, Araki Y. Occurrence of [→3)-β-d-Manp-(1→4)-β-d-Manp-(1→]n units in the antigenic polysaccharides from Leptospira biflexa serovar patoc strain Patoc I. Carbohydr Res. 2000;328:517–524. doi: 10.1016/s0008-6215(00)00143-9. [DOI] [PubMed] [Google Scholar]
- 23.Yanagihara Y, Kamisango K, Takeda K, Mifuchi I, Azuma I. Identification of 4-O-methylmannose in cell wall polysaccharide of Leptospira. Microbiol Immunol. 1983;27:711–715. doi: 10.1111/j.1348-0421.1983.tb00634.x. [DOI] [PubMed] [Google Scholar]
- 24.Goris MG, Leeflang MM, Boer KR, Goeijenbier M, van Gorp EC, Wagenaar JF, Hartskeerl RA. Establishment of valid laboratory case definition for human leptospirosis. J Bacteriol Parasitol. 2012;3:1000132. [Google Scholar]
- 25.Zochowski WJ, Palmer MF, Coleman TJ. An evaluation of three commercial kits for use as screening methods for the detection of leptospiral antibodies in the UK. J Clin Pathol. 2001;54:25–30. doi: 10.1136/jcp.54.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caimi K, Varni V, Melendez Y, Koval A, Brihuega B, Ruybal P. A combined approach of VNTR and MLST analysis: improving molecular typing of Argentinean isolates of Leptospira interrogans. Mem Inst Oswaldo Cruz. 2012;107:644–651. doi: 10.1590/s0074-02762012000500011. [DOI] [PubMed] [Google Scholar]
- 27.Goarant C, Bourhy P, D'Ortenzio E, Dartevelle S, Mauron C, Soupé-Gilbert ME, Bruyère-Ostells L, Gourinat AC, Picardeau M, Nato F, et al. Sensitivity and specificity of a new vertical flow rapid diagnostic test for the serodiagnosis of human leptospirosis. PLoS Negl Trop Dis. 2013;7:e2289. doi: 10.1371/journal.pntd.0002289. [DOI] [PMC free article] [PubMed] [Google Scholar]