Abstract
Paroxysmal nocturnal hemoglobinuria (PNH) is a progressive, systemic, life-threatening disease, characterized by chronic uncontrolled complement activation. A retrospective analysis of 301 Korean PNH patients who had not received eculizumab was performed to systematically identify the clinical symptoms and signs predictive of mortality. PNH patients with hemolysis (lactate dehydrogenase [LDH] ≥ 1.5 × the upper limit of normal [ULN]) have a 4.8-fold higher mortality rate compared with the age- and sex-matched general population (P < 0.001). In contrast, patients with LDH < 1.5 × ULN have a similar mortality rate as the general population (P = 0.824). Thromboembolism (TE) (odds ratio [OR] 7.11; 95% confidence interval [CI] (3.052-16.562), renal impairment (OR, 2.953; 95% CI, 1.116-7.818) and PNH-cytopenia (OR, 2.547; 95% CI, 1.159-5.597) are independent risk factors for mortality, with mortality rates 14-fold (P < 0.001), 8-fold (P < 0.001), and 6.2-fold (P < 0.001) greater than that of the age- and sex-matched general population, respectively. The combination of hemolysis and 1 or more of the clinical symptoms such as abdominal pain, chest pain, or dyspnea, resulted in a much greater increased mortality rate when compared with patients with just the individual symptom alone or just hemolysis. Early identification of risk factors related to mortality is crucial for the management of PNH. This trial was registered at www.clinicaltrials.gov as NCT01224483.
Keywords: Paroxysmal Nocturnal Hemoglobinuria, PNH, Risk Factors, Mortality
Graphical Abstract
INTRODUCTION
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare acquired hematopoietic stem cell disorder whose clinical manifestations include intravascular hemolysis, thromboembolism (TE) and bone marrow failure (1). Despite best supportive care 5 years mortality was 35% (1). The development of TE is the major cause of morbidity and mortality among patients with PNH (2).
Pathophysiology of thrombosis is multifactorial and includes platelet activation, toxicity of free hemoglobin, nitric oxide depletion, absence of other glycosylphosphatidylinositol-linked proteins such as urokinase-type plasminogen activator receptor and endothelial dysfunction (3). We have previously reported that elevated hemolysis is associated with a significantly increased risk for thrombosis and that this risk is further increased by the presence of PNH-related symptoms such as abdominal pain, chest pain, dyspnea, or hemoglobinuria (4). There are only a few reports of risk factors associated with mortality in PNH patients in the study with Caucasian population (1,2).
Eculizumab improves survival and effects a sustained improvement in symptoms in PNH patients (5,6,7,8), and anti-complement therapy need to be clarified based on the risk factors of PNH. To better understanding the risk factors associated with mortality in PNH, a retrospective analysis was performed on data from 301 Korean PNH patients enrolled with pre-eculizumab era in a nationwide registry. The aim of this study was to describe the burden of disease in PNH patients with pre-eculizumab era and to systematically identify the risk factors associated with mortality in these PNH patients. In addition, this study is ever the first-time analysis of demonstrating standard mortality ratio (SMR) in PNH patients.
MATERIALS AND METHODS
The Aplastic Anemia Working Party of The Korean Society of Hematology has established a web-based nationwide Korean National PNH Registry that covers 41 years of PNH disease data. There are 9 institutions participating in the Korean National PNH Registry, which covers 96% of the Korean PNH population. A retrospective chart review was performed on 301 patients identified with the diagnosis of PNH. Although the patients were enrolled into the registry between 2009 and 2010, the chart review included medical history data prior to registry enrollment. Patient data included in this Korean Registry were captured using an electronic case report form that collected patient demographics and medical history and PNH-specific information, including erythrocyte and granulocyte clone size, symptoms and complications, laboratory values, treatment, and where applicable, cause of death.
PNH was confirmed using flow cytometry. In patients diagnosed before the establishment of flow cytometry, a positive Ham or sucrose-lysis test was used. The PNH granulocyte and erythrocyte clone sizes were based on available data from chart reviews and were not limited to minimum clone size or flow methodology.
Elevated hemolysis was defined as a lactate dehydrogenase (LDH) level ≥1.5 times the upper limit of normal (ULN) (6,9,10,11). LDH at diagnosis of PNH was chosen as a consistent point of reference for most analyses. In patients with elevated LDH at diagnosis, the occurrence of TE within 6 months of diagnosis was analyzed to determine the temporal relationship between LDH level and TE. Multiple TE events were defined in patients having more than 1 TE event. PNH-cytopenia was defined as PNH in the setting of other specified bone marrow disorder (8).
Clinical PNH symptoms, including abdominal pain, chest pain, dyspnea, and hemoglobinuria, were based on physician reporting in medical charts and did not necessarily include the symptom onset date. However, dates of diagnosis, flow cytometric assessments, bone marrow transplant (BMT), TE, and death were accurately recorded. TE was calculated 2 ways, either as the occurrence at a specific time point or as the cumulative incidence over a specific time period. The cumulative incidence of TE was collected for the time periods prior to diagnosis and post-diagnosis of PNH. Transfusion requirements were categorized as 0 units, 1 to 4 units, or ≥5 units within the last 12 months, where documented, prior to data collection.
Statistical analysis
Binary variables were analyzed using univariate and multivariate logistic regression. Potential risk factors included LDH ≥ 1.5× ULN at the time of diagnosis, TE, impaired renal function (IRF), abdominal pain, chest pain, dyspnea, hemoglobinuria, granulocyte clone size and PNH-cytopenia. The percentage of glycosylphosphatidylinositol anchor protein-deficient granulocytes was determined from flow cytometry assessments at diagnosis or conducted at the closest time point to diagnosis. LDH values at diagnosis were used in all analyses in an attempt to investigate their association with clinical outcomes. The null hypothesis of no association between mortality and a specific risk factor was tested using the log-likelihood ratio statistic, which follows a chi-square distribution. Results are presented as the odds ratio (OR) and the 95% confidence interval (CI). In the presence of a zero frequency, it is not strictly possible to estimate the ORs and the 95% CIs; thus, in a sensitivity analysis any zero values were imputed as 0.5.
Time-to-event variables, such as overall survival, were estimated by the Kaplan-Meier method. The effects of prognostic factors on overall survival were analyzed using univariate or multivariate Cox proportional hazards models. Prognostic factors were similar to those stated above for the logistic regression analyses. The null hypothesis of equal hazards for death across the levels of the prognostic factor of interest was tested using the chi-square distribution. Relative risks are presented as hazard ratios (HR) and 95% CIs. We analyzed each LDH cutoff values of 1.5×, 3.0×, and 5.0× ULN by sensitivity, specificity and positive and negative likelihood ratios with 95% CIs. The Receiver Operating Characteristic Curve (ROC) plotted sensitivity (y-axis) against 1 minus the specificity (x-axis).
The Standard Mortality Ratio (SMR) is the ratio of the observed mortality rate in the PNH population to the expected mortality rate of the age- and sex-matched subjects in the national population. The Korean National Mortality rates were provided for each year from 1983 to 2009 and for 5-year age groups: 5-9, 10-14, 75-79, and ≥80 years (www.kostat.go.kr). The expected number of deaths for males and females were calculated separately for purposes of comparison with the general population and then summed to obtain the total number of expected deaths. The Statistics Korea (KOSTAT, www.kostat.go.kr) provided cause of death for any patient in the study who was lost to follow up at the hospital where they were treated.
Ethics statement
This study was conducted in accordance with the Declaration of Helsinki and was reviewed and approved by the institutional review board of the participating hospitals including Seoul St. Mary's Hospital (No. KC10RSME0270). Informed consent was exempted by the board.
RESULTS
Patient characteristics
Patients (n=301) from the Korean PNH Registry were evaluated for signs, symptoms, and outcomes. A summary of patient demographics, medical history, and PNH-specific information for the 301 PNH patients is provided in Table 1. The median age at diagnosis was 37 years (range: 8-88 years), median follow-up time from diagnosis was 6.6 years (range: 0-28.8 years), and there was an approximately equal numbers of male and female patients. Based on physician-recorded medical charts, 42% of patients had a history of aplastic anemia (AA) and 6.3% had a history of myelodysplastic syndrome (MDS) either before or after diagnosis of PNH from the medical chart written by physicians. Overall, 46.5% of patients (n=140) were categorized as having PNH-cytopenia. Diagnosis of PNH by flow cytometry was reported for 236 patients (78.4%), Ham's and sucrose tests for 56 patients (18.6%), Ham's test only for seven patients (2.3%), and sucrose test only for two patients (<1%). Further analysis showed that there were no significant differences in age, prevalence of TE, or early mortality between patients diagnosed using the different methods (data not shown). The median granulocyte and erythrocyte clone sizes were 48.8% and 28.1%, respectively. At diagnosis, 76.3% of the patients with recorded LDH levels (171/224) had elevated hemolysis. The most frequently reported clinical symptoms were hemoglobinuria (56.1% of patients) and abdominal pain (46.8% of patients). Cases of hemoglobinuria were probably self-reported macroscopic hemoglobinuria as it was not objectively assessed. TE was reported in 54 (17.9%) patients and IRF was reported in 17% of patients. Fifty-one patients (16.9%) reported IRF, defined as a history of acute renal failure (ARF) or estimated glomerular filtration rate (eGFR) of <60 mL/min/1.73 m2 captured before or after diagnosis of PNH. Forty-four patients (14.6%) had a recorded history of ARF, and 26 (8.6%) had chronic eGFR<60 mL/min/1.73 m2. One-third of patients who reported IRF had both history of ARF at diagnosis and eGFR <60 mL/min/1.73 m2. Corticosteroids were the most frequently used treatment in this patient population. Of the 39 patients treated with opioids, 29 (74.4%) had abdominal pain. Forty-one percent of patients had no documentation of transfusion. Overall, 37 patients (12.3%) received BMT from related or unrelated donors. None of patients received eculizumab.
Table 1. Patient characteristics.
| Patient demographics | n = 301 |
|---|---|
| Age, yr | |
| Median (range) | 37 (8-88) |
| Mean (SD) | 39.3 (15.4) |
| Patients < 40 yr, No. (%) | 172 (57.1) |
| Gender, female, No. (%) | 149 (49.5) |
| Additional bone marrow disorder, No. (%) | |
| Aplastic anemia | 121 (40.2) |
| Myelodysplastic syndrome | 19 (6.3) |
| PNH granulocyte clone size, % (n = 195) | |
| Median (range) | 48.8 (0-100) |
| Mean (SD) | 49.5 (30.8) |
| PNH RBC clone size, % (n = 199) | |
| Median | 28.1 (0-99.8) |
| Mean (SD) | 33.2 (27.8) |
| LDH, fold above ULN (n = 224) | |
| Median (range) | 4.10 (0.2-36.3) |
| Mean (SD) | 5.6 (5.5) |
| ≥ 1.5 × ULN, No. (%) | 171 (76.3) |
| Follow-up since diagnosis, yr | |
| Median (range) | 6.6 (0-28.8) |
| Mean (SD) | 7.8 (6.0) |
Risk factors for mortality
During the median follow-up period (6.6 years; range 0-28.8), 43 of the patients (14.3%) died. TE, IRF, and PNH-cytopenia were reported in 23 (53.5%), 7 (16.3%), and 27 (63%) of these patients, respectively, although these symptoms were not necessarily reported as the cause of death. There were significantly more patient deaths reported in PNH patients with LDH ≥1.5×ULN at diagnosis (n=28; 16.4 %) compared with patients with LDH <1.5×ULN (n=2; 3.7%; P=0.009). Of the patients with LDH ≥1.5×ULN, 7 (4.1%) died within 1 year compared with none of the patients with LDH <1.5×ULN.
Twenty-three out of 54 patients (46.2%) with a history of TE died after a median time of 8 months (range: 1-17 years) as a direct result of a TE. Multiple TE events were reported in 10 of these patients (43.5%) with the remaining 13 patients experiencing a single TE event. The multivariate Cox proportional hazards model showed a statistically significant association for mortality with both multiple (HR, 6.3; 95% CI, 2.96-13.55; P<0.001) and one TE event also had a significantly increased risk of mortality (HR, 4.5; 95% CI, 2.3-9.1; P<0.001) when compared with patients without TE.
Univariate analyses showed that significant predictors of mortality were TE (P<0.001), IRF (P=0.001), LDH ≥1.5× ULN (P=0.009), PNH-cytopenia (P=0.023), abdominal pain (P=0.026), and dyspnea/chest pain (P=0.026) (Table 2). Multivariate analyses indicated that only the first of these predictors (i.e. TE, IRF, and PNH-cytopenia) were significantly associated with an increased risk of mortality (P<0.001, P=0.029, and P=0.020, respectively) (Table 2). Elevated levels of LDH and abdominal pain were not independent risk factors for mortality in the multivariate regression analysis (P=0.159 and P=0.162, respectively) although hemoglobinuria approached significance (P=0.073).
Table 2. Univariate and multivariate analysis of risk factors of mortality.
| Risk factors for mortality | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| P value | Odds ratio (95% CI) | P value | Odds ratio (95% CI) | |
| TE | < 0.001 | 8.42 (4.15-17.08) | < 0.001 | 7.110 (3.052-16.562) |
| IRF | 0.001 | 3.41 (1.66-7.02) | 0.029 | 2.953 (1.116-7.818) |
| PNH-cytopenia | 0.023 | 2.17 ( 1.11-4.21) | 0.020 | 2.547 (1.159- 5.597) |
| LDH ≥ 1.5 × ULN | 0.009 | 4.99 (1.15-21.70) | 0.159 | 3.204 (0.633-16.230) |
| Abdominal pain | 0.026 | 2.10 (1.08-4.08) | 0.162 | 1.828 (0.785-4.256) |
| Dyspnea/Chest pain | 0.026 | 2.09 (1.086-4.024) | 0.855 | 1.077 (0.487-2.381) |
| Hemoglobinuria | 0.636 | 0.86 (0.45-1.63) | 0.073 | 0.449 (0.187-1.077) |
| Clone size | 0.391 | 1.01 (0.99-1.02) | 0.744 | 0.995 (0.967-1.024) |
CI, confidence interval; IRF, impaired renal function; LDH, lactate dehydrogenase; TE, thromboembolism; ULN, upper limit of normal.
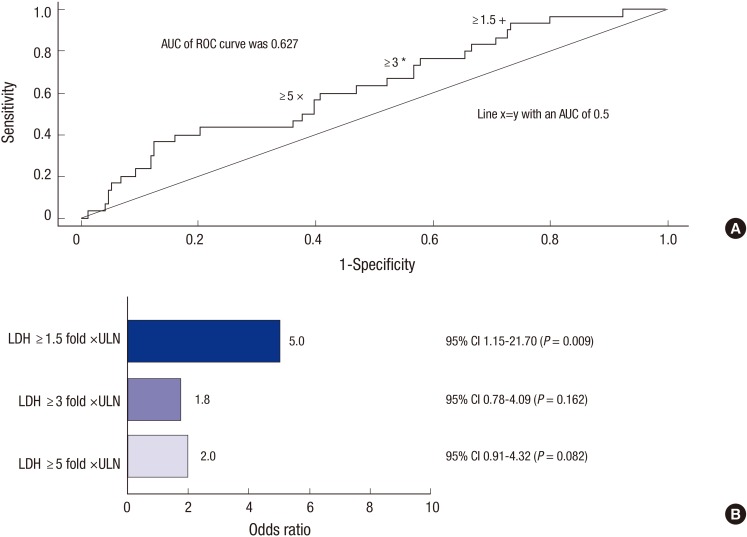
A ROC analysis was performed as a sensitivity analysis comparing LDH concentrations at diagnosis of ≥1.5 vs.<1.5× ULN, ≥3.0 vs.<3.0× ULN, and ≥5.0 vs.<5.0× ULN at diagnosis and compared the mortality of patients with LDH<1.5,<3.0× or<5.0× ULN. The results showed that the sensitivity, specificity, and negative positive value (NPV) of elevated LDH at baseline as a predictor of mortality was 93.3% (28/30), 26.3%, and 96.2% (51/53), respectively, for LDH ≥1.5× ULN; 70.0% (21/30), 43.3%, and 90.3% (84/93), respectively, for LDH ≥3.0× ULN; and 56.7% (17/30), 60.3%, and 90.0% (106/130), respectively, for LDH ≥5.0× ULN. The analysis showed that neither LDH >3.0× ULN nor LDH >5.0× ULN, were associated with a significant increase in mortality (P=0.162 and P=0.082, respectively; Fig. 1A). The relationship between mortality and LDH cutoff value was also analyzed by logistic regression. The univariate OR was 4.99, 95% CI (1.15-21.70), P=0.009 for patients with LDH cutoff value ≥1.5× ULN, but was non-significant with multivariate analysis (OR, 5.71; 95% CI, 0.67-47.22; P=0.122). For LDH cutoff values of ≥3×, and >5× ULN the association with mortality was not statistically significant. The univariate OR for both of these groups was 1.78 (P=0.162) and 1.99 (P=0.082), respectively (Fig. 1B).
Fig. 1. Impact of LDH in PNH patients. (A) Receiver operating characteristic curve of LDH cutoff for mortality, (B) Logistic regression analysis of the association of LDH ≥ 1.5, ≥ 3.0× and ≥ 5.0× ULN at diagnosis and mortality.
Mortality according to supportive treatment
Overall, 13 of the 137 patients (9.5%) who remained transfusion free from the time of diagnosis prior to entry in the registry died compared with 8 of the 44 patients (18.2%) who had received 1 to 4 units of packed red blood cells and 22 of the 120 patients (18.3%) who had received more than 5 units. The association between number of transfusions and mortality was not statistically significant (P=0.088). The overall median duration of corticosteroid use for patients included in this study was 5.42 years. A Kaplan-Meier analysis showed that there was no difference in mortality between the 104 patients who either received corticosteroids for >5.4 years (13%) compared with the 197 patients who either received corticosteroids for <5.4 years or had not received corticosteroids at all (15%; P=0.520). Eleven out of the 44 patients (25.0%) with a history of anticoagulant use during the course of disease died, with 10 of these patients (90.9%) having a history of TE. The hazard ratio of death for patients with a history of anticoagulant compared with those without a history of anticoagulant use was 2.34 times (95% CI, 1.078-5.094, P=0.036).
Standard mortality ratio
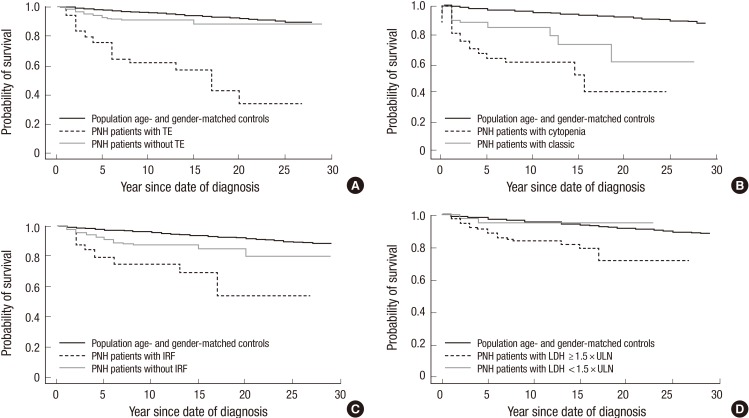
Table 3 presents the calculated SMR for this population of PNH patients compared with an age- and gender-matched general Korean population. Overall, a diagnosis of PNH increased the risk of mortality by 3.9-fold (P<0.001). The greatest risk factor for mortality was a TE, which increased mortality by 13.9-fold compared with the general population (P<0.001; Fig. 2A). Other significant risk factors, compared with an age- and gender matched population (P<0.001 in each case) included, IRF (7.8-fold increase), PNH-cytopenia (6.2-fold increase), classic PNH, i.e. patients with overt hemolysis, elevated reticulocyte count, elevated LDH, and normocellular to hypercellular bone marrow (4.4-fold increase) and LDH ≥1.5× ULN (4.8-fold increase) (Fig. 2B to D). Patients without evidence of elevated hemolysis (LDH<1.5×ULN) were the only subset of patients with a mortality rate similar to that of the age- and gender-matched population (SMR=1.17; P=0.824). Symptomatic risk factors for higher mortality rates compared with the general population included abdominal pain (4.92-fold increase; P<0.001), dyspnea (4.48-fold increase; P<0.001), and chest pain (4.29-fold increase; P<0.001). Patients with both one of these symptoms and LDH≥1.5×ULN at diagnosis had SMR values that were approximately 25% (dyspnea) or 33% (abdominal pain and chest pain) higher than for the overall subset of patients with these individual symptoms alone (Table 3).
Table 3. Standard mortality ratio according to risk factors.
| Patient population | SMR vs. age- and sex-matched general population | |
|---|---|---|
| SMR (95% CI) | P value | |
| Total PNH patients (n = 301) | 3.89 (2.73-5.05) | < 0.001 |
| No TE (n = 247) | 2.13 (1.19-3.06) | < 0.001 |
| TE (n = 54) | 13.92 (8.23-19.61) | < 0.001 |
| LDH < 1.5 × ULN (n = 53) | 1.17 (0.00-2.79) | 0.824 |
| LDH ≥ 1.5 × ULN (n = 171) | 4.81 (3.03-6.59) | < 0.001 |
| No IRF (n = 251) | 3.06 (1.93-4.20) | < 0.001 |
| IRF (n = 50) | 7.81 (3.86-11.77) | < 0.001 |
| Classic PNH (n = 157) | 4.4 (2.72-6.23) | < 0.001 |
| PNH-Cytopenia (n = 107) | 6.2 (4.71-9.34) | < 0.001 |
| No abdominal pain (n = 159) | 2.87 (1.46-4.28) | < 0.001 |
| Abdominal pain (n = 142) | 4.92 (3.06-6.77) | < 0.001 |
| LDH ≥ 1.5 × ULN + abdominal pain (n = 92) | 6.55 (3.60-9.49) | < 0.001 |
| No dyspnea (n = 189) | 3.42 (1.95-4.88) | < 0.001 |
| Dyspnea (n = 112) | 4.48 (2.61-6.35) | < 0.001 |
| LDH ≥ 1.5 × ULN + dyspnea (n = 58) | 5.58 (2.85-8.32) | < 0.001 |
| No chest pain (n = 263) | 3.82 (2.57-5.06) | < 0.001 |
| Chest pain (n = 38) | 4.29 (1.11-7.47) | < 0.001 |
| LDH ≥ 1.5 × ULN + chest pain (n = 24) | 5.72 (0.71-10.73) | < 0.001 |
SMR, standard mortality ratio; CI, confidence interval; PNH, paroxysmal nocturnal hemoglobinuria; LDH, lactate dehydrogenase; ULN, upper limit of normal; TE, thromboembolism; IRF, impaired renal function.
Fig. 2. Kaplan-Meier survival of PNH patients compared with age- and gender-matched general population. (A) Patients with TE had a 14-fold higher mortality rate compared with the general population (standard mortality ratio [SMR]=13.9; 95% confidence interval [CI], 8.2-19.6; P < 0.001). (B) Patients with cytopenia had a mortality rate 6.2-fold greater than the age- and gender-matched general population (SMR=6.2; 95% CI, 4.7-9.3; P < 0.001). (C) Patients with impaired renal function (IRF) had a mortality rate 7.8-fold greater than the age- and gender-matched general population (SMR=7.8; 95% CI, 3.9-11.8; P < 0.001). (D) PNH patients with lactate dehydrogenase (LDH) ≥1.5 times the upper limit of normal (ULN) had a 5.0-fold greater risk for mortality compared with patients with LDH <1.5×ULN (95% CI, 1.15-21.70; P = 0.009). PNH patients with LDH ≥1.5×ULN had a 4.8-fold higher mortality rate compared with the general population (SMR=4.8; 95% CI, 3.0-6.6; P < 0.001).
Cause of death
Cause of death was attributed to septicemia/infection in 14 of the 43 patients who died (32.6%), TE in 7 patients (16.3%), bleeding in 4 patients (9.3%), clonal evolution (acute myeloid leukemia [AML] and MDS) in 3 patients (7.0%), and renal failure in 1 patient (2.3%). Mortality related to treatment with allogeneic BMT was 14% (n=6). Ten of the 14 patients (71.4%) with a cause of death of septicemia/infection and 3 of the 4 patients (75%) who died from bleeding were diagnosed with PNH-cytopenia. The remaining 8 patients (18.6%) died from causes non-related to PNH (including other malignancy and unknown origins).
DISCUSSION
Our retrospective analysis of 301 patients enrolled in the Korean National PNH Registry demonstrated the incidence of thrombosis in these patients was 17.9% (4). According to previous retrospective PNH studies, the incidence of thrombosis ranges from 6.2% to 39% (1,12,13) with a retrospective French study of 460 PNH patients demonstrating 10-year cumulative incidence of 31% (12). Recently it has been reported that the incidence of thrombosis in PNH is likely to be underestimated as a result of evidence of subclinical pulmonary embolism or myocardial ischemia observed in 6 of 10 patients in one study (3) and evidence of subclinical myocardial damage in 2 of 10 patients in another study (14). These subclinical thromboses can lead to long-term organ damage as reflected by compromised cardiac function in the majority of these patients (14). Thrombosis has been reported as the leading cause of mortality in patients with PNH with 29% to 44% of patients suffering from at least one TE event during the course of their disease (1,2,6). In our retrospective study of 301 patients, the relative risk of mortality with TE was robust and confirmed across several relevant analyses. Assessment of the medical history of our study population not only identified patients with a TE event, but showed that there was progression of existing TE events across multiple sites, despite the use of anticoagulants from which we have concluded that the administration of anticoagulation therapy to patients with PNH has little, if any effect on thrombosis management and patient survival. A prospective follow-up study from the International PNH registry including over 1,600 patients worldwide, may provide more definite information on the incidence and influence of thrombosis in PNH (15).
None of previous research showed a survival data in a large population with PNH compared to SMR. In this study, IRF is an independent risk factor for mortality among Korean patients with PNH. Patients with IRF have a mortality rate 8-fold higher than that of a general population (P<0.001). One report found that between 8% and 18% of PNH deaths are caused by renal failure (13). It has been reported that hemolytic PNH patients manifested progressive renal dysfunction over time, which suggests that IRF is a late-stage consequence of chronic hemolysis (5).
We report here PNH-cytopenia patients have a significantly greater risk of mortality compared with classic PNH patients. In contrast to classic PNH, which manifests with overt hemolysis, an elevated reticulocyte count, an elevated LDH, and a normocellular to hypercellular bone marrow (16), patients with PNH-cytopenia have a hypocellular bone marrow and seldom demonstrate signs and symptoms of hemolysis as these patients have small populations of PNH cells; symptoms of anemia, thrombocytopenia and neutropenia are usually a consequence of bone marrow failure (17). Despite these different clinical presentations, the long-term outcomes have been reported to be similar in both groups of patients (2,18). In patients with PNH-cytopenia risk factors for negative long-term outcomes have been identified as an ANC<0.5×109/L, infection, and evolution to MDS/AML (2,18). Therefore PNH-cytopenia might be a separate disease entity from classic PNH. Distinguishing between classical PNH and PNH-cytopenia is particularly important because if the PNH patients have severe cytopenia, immunosuppressive therapy or allogeneic BMT should be considered as a first-line therapy.
LDH was not found to be an independent risk factor for mortality in this study. This finding is probably because of the inclusion of TE, IRF, and history of AA or MDS in the multivariate model, which are strong predictors of mortality. LDH concentrations will fluctuate over time and as mortality can occur a significant time after PNH diagnosis, this may be why LDH was not an independent factor for mortality in our model. The ROC analysis showed that LDH ≥1.5×ULN is a more sensitive and specific predictor than LDH ≥3.0× and ≥5.0×ULN which suggests LDH≥1.5×ULN is a realistic clinical measurement for identification of hemolysis. Further evidence of this was seen from the results of the logistic regression analysis which also confirmed LDH≥1.5×ULN is a significant predictor of mortality. Taken together, these data confirm that uncontrolled complement activation leads to increase risk of mortality and demonstrates PNH patients with LDH≥1.5×ULN (a measure of complement activation) is a predictor for poor outcomes.
PNH patients with hemolysis have a mortality rate 4.8-fold higher than the age and gender-matched general population (P<0.001). In contrast, there was no difference in mortality rates between PNH patients with LDH<1.5×ULN and a healthy age- and gender-matched Korean population (P=0.824). Interestingly, the combination of hemolysis and one or more of the clinical symptoms such as abdominal pain, chest pain or dyspnea, had a much greater mortality rate when compared to patients with individual symptoms or hemolysis alone. These symptoms may be a consequence of progressive organ damage such as gastrointestinal tract, heart, and lungs, due to chronic hemolysis and complement activity. In addition to contributing to mortality, symptoms such as abdominal pain, chest pain, and dyspnea contribute to the debilitating quality of life in PNH patients (19,20). Therefore once a diagnosis of PNH has been confirmed, clinical symptoms should be carefully monitored. Early therapeutic intervention administered in order to minimize the adverse clinical effects of hemolysis and reduce mortality in all PNH patients.
In the era of eculizumab, chronic hemolysis-related to mortality in PNH can be controlled by an anti-complement inhibitor. Survival of eculizumab-treated patients has significantly improved compared with those not treated with eculizumab and was similar to an age- and gender-matched general population (7). This retrospective study reports the risk for mortality in a cohort of PNH patients who had not received eculizumab which suggests that in an era of this anti-complement inhibitor the natural course of PNH and survival of patients with this disease can be changed.
In summary, PNH patients with hemolysis (LDH ≥1.5× ULN) have a 4.8-fold increase in mortality rate compared with the general population. The combination of hemolysis and one or more of the clinical symptoms such as abdominal pain, chest pain, and dyspnea, had much greater increased mortality rate. TE, IRF, and PNH-cytopenia are the independent predictors of mortality. Early identification of risk factors related to mortality is crucial for the management of PNH.
ACKNOWLEDGMENT
The authors wish to thank Honey Cho (Handok Pharmaceutical Co.) for assistance of statistical analysis.
Footnotes
Funding: This work was supported by Korean Society of Haematology.
DISCLOSURE: The authors declare no competing financial interests.
AUTHOR CONTRIBUTION: Conception and design: Lee JW. Data collection: all authors. Statistical analysis: Jang JH. Analysis and interpretation of results: Lee JW, Jang JH, Kim JS. Preparation of manuscript: Jang JH, Kim JS. Final approval: all authors.
References
- 1.Hillmen P, Lewis SM, Bessler M, Luzzatto L, Dacie JV. Natural history of paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1995;333:1253–1259. doi: 10.1056/NEJM199511093331904. [DOI] [PubMed] [Google Scholar]
- 2.Socié G, Mary JY, de Gramont A, Rio B, Leporrier M, Rose C, Heudier P, Rochant H, Cahn JY, Gluckman E French Society of Haematology. Paroxysmal nocturnal haemoglobinuria: long-term follow-up and prognostic factors. Lancet. 1996;348:573–577. doi: 10.1016/s0140-6736(95)12360-1. [DOI] [PubMed] [Google Scholar]
- 3.Hill A, Kelly RJ, Hillmen P. Thrombosis in paroxysmal nocturnal hemoglobinuria. Blood. 2013;121:4985–4996. doi: 10.1182/blood-2012-09-311381. [DOI] [PubMed] [Google Scholar]
- 4.Lee JW, Jang JH, Kim JS, Yoon SS, Lee JH, Kim YK, Jo DY, Chung J, Sohn SK. Clinical signs and symptoms associated with increased risk for thrombosis in patients with paroxysmal nocturnal hemoglobinuria from a Korean Registry. Int J Hematol. 2013;97:749–757. doi: 10.1007/s12185-013-1346-4. [DOI] [PubMed] [Google Scholar]
- 5.Hillmen P, Elebute M, Kelly R, Urbano-Ispizua A, Hill A, Rother RP, Khursigara G, Fu CL, Omine M, Browne P, et al. Long-term effect of the complement inhibitor eculizumab on kidney function in patients with paroxysmal nocturnal hemoglobinuria. Am J Hematol. 2010;85:553–559. doi: 10.1002/ajh.21757. [DOI] [PubMed] [Google Scholar]
- 6.Hillmen P, Muus P, Dührsen U, Risitano AM, Schubert J, Luzzatto L, Schrezenmeier H, Szer J, Brodsky RA, Hill A, et al. Effect of the complement inhibitor eculizumab on thromboembolism in patients with paroxysmal nocturnal hemoglobinuria. Blood. 2007;110:4123–4128. doi: 10.1182/blood-2007-06-095646. [DOI] [PubMed] [Google Scholar]
- 7.Kelly RJ, Hill A, Arnold LM, Brooksbank GL, Richards SJ, Cullen M, Mitchell LD, Cohen DR, Gregory WM, Hillmen P. Long-term treatment with eculizumab in paroxysmal nocturnal hemoglobinuria: sustained efficacy and improved survival. Blood. 2011;117:6786–6792. doi: 10.1182/blood-2011-02-333997. [DOI] [PubMed] [Google Scholar]
- 8.Parker C, Omine M, Richards S, Nishimura J, Bessler M, Ware R, Hillmen P, Luzzatto L, Young N, Kinoshita T, et al. Diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood. 2005;106:3699–3709. doi: 10.1182/blood-2005-04-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hillmen P, Hall C, Marsh JC, Elebute M, Bombara MP, Petro BE, Cullen MJ, Richards SJ, Rollins SA, Mojcik CF, et al. Effect of eculizumab on hemolysis and transfusion requirements in patients with paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2004;350:552–559. doi: 10.1056/NEJMoa031688. [DOI] [PubMed] [Google Scholar]
- 10.Hillmen P, Young NS, Schubert J, Brodsky RA, Socié G, Muus P, Röth A, Szer J, Elebute MO, Nakamura R, et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2006;355:1233–1243. doi: 10.1056/NEJMoa061648. [DOI] [PubMed] [Google Scholar]
- 11.Brodsky RA, Young NS, Antonioli E, Risitano AM, Schrezenmeier H, Schubert J, Gaya A, Coyle L, de Castro C, Fu CL, et al. Multicenter phase 3 study of the complement inhibitor eculizumab for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Blood. 2008;111:1840–1847. doi: 10.1182/blood-2007-06-094136. [DOI] [PubMed] [Google Scholar]
- 12.de Latour RP, Mary JY, Salanoubat C, Terriou L, Etienne G, Mohty M, Roth S, de Guibert S, Maury S, Cahn JY, et al. Paroxysmal nocturnal hemoglobinuria: natural history of disease subcategories. Blood. 2008;112:3099–3106. doi: 10.1182/blood-2008-01-133918. [DOI] [PubMed] [Google Scholar]
- 13.Nishimura J, Kanakura Y, Ware RE, Shichishima T, Nakakuma H, Ninomiya H, Decastro CM, Hall S, Kanamaru A, Sullivan KM, et al. Clinical course and flow cytometric analysis of paroxysmal nocturnal hemoglobinuria in the United States and Japan. Medicine (Baltimore) 2004;83:193–207. doi: 10.1097/01.md.0000126763.68170.46. [DOI] [PubMed] [Google Scholar]
- 14.Hill A, Sapsford RJ, Scally A, Kelly R, Richards SJ, Khurisgara G, Sivananthan MU, Hillmen P. Under-recognized complications in patients with paroxysmal nocturnal haemoglobinuria: raised pulmonary pressure and reduced right ventricular function. Br J Haematol. 2012;158:409–414. doi: 10.1111/j.1365-2141.2012.09166.x. [DOI] [PubMed] [Google Scholar]
- 15.Schrezenmeier H, Muus P, Socié G, Szer J, Urbano-Ispizua A, Maciejewski JP, Brodsky RA, Bessler M, Kanakura Y, Rosse W, et al. Baseline characteristics and disease burden in patients in the International Paroxysmal Nocturnal Hemoglobinuria Registry. Haematologica. 2014;99:922–929. doi: 10.3324/haematol.2013.093161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brodsky RA. Advances in the diagnosis and therapy of paroxysmal nocturnal hemoglobinuria. Blood Rev. 2008;22:65–74. doi: 10.1016/j.blre.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukhina GL, Buckley JT, Barber JP, Jones RJ, Brodsky RA. Multilineage glycosylphosphatidylinositol anchor-deficient haematopoiesis in untreated aplastic anaemia. Br J Haematol. 2001;115:476–482. doi: 10.1046/j.1365-2141.2001.03127.x. [DOI] [PubMed] [Google Scholar]
- 18.Ge M, Li X, Shi J, Shao Y, Zheng Y. Clinical features and prognostic factors of Asian patients with paroxysmal nocturnal hemoglobinuria: results from a single center in China. Ann Hematol. 2012;91:1121–1128. doi: 10.1007/s00277-012-1413-6. [DOI] [PubMed] [Google Scholar]
- 19.Hill A, Hillmen P, Richards SJ, Elebute D, Marsh JC, Chan J, Mojcik CF, Rother RP. Sustained response and long-term safety of eculizumab in paroxysmal nocturnal hemoglobinuria. Blood. 2005;106:2559–2565. doi: 10.1182/blood-2005-02-0564. [DOI] [PubMed] [Google Scholar]
- 20.Weitz I, Meyers G, Lamy T, Cahn JY, Uranga MT, García Vela JA, Sanz MA, Severino B, Kelly RJ, Hillmen P, et al. Cross-sectional validation study of patient-reported outcomes in patients with paroxysmal nocturnal haemoglobinuria. Intern Med J. 2013;43:298–307. doi: 10.1111/j.1445-5994.2012.02924.x. [DOI] [PubMed] [Google Scholar]