Abstract
This study aimed to investigate the independent and interactive influences of apolipoprotein E (APOE) ε4 and beta-amyloid (Aβ) on multiple cognitive domains in a large group of cognitively normal (CN) individuals and patients with mild cognitive impairment (MCI) and Alzheimer’s disease (AD). Participants were included if clinical and cognitive assessments, amyloid imaging, and APOE genotype were all available from the Alzheimer’s Disease Neuroimaging Initiative database (CN = 324, MCI = 502, AD = 182). Individuals with one or two copies of ε4 were designated as APOE ε4 carriers (ε4+); individuals with no ε4 were designated as APOE ε4 non-carriers (ε4−). Based on mean florbetapir standard uptake value ratios, participants were classified as Aβ burden-positive (Aβ+) or Aβ burden-negative (Aβ−). In MCI, APOE ε4 effects were predominantly observed on frontal executive function, with ε4+ participants exhibiting poorer performances; Aβ positivity had no influence on this effect. Aβ effects were observed on global cognition, memory, and visuospatial ability, with Aβ+ participants exhibiting poorer performances. Measures of frontal executive function were not influenced by Aβ. Interactive effects of APOE ε4+ and Aβ were observed on global cognition and verbal recognition memory. Aβ, not APOE ε4+, influenced clinical severity and functional status. The influences of APOE ε4+ and Aβ on cognitive function were minimal in CN and AD. In conclusion, we provide further evidence of both independent and interactive influences of APOE ε4+ and Aβ on cognitive function in MCI, with APOE ε4+ and Aβ showing dissociable effects on executive and non-executive functions, respectively.
Keywords: Alzheimer Disease, Mild Cognitive Impairment, APOE ε4+, Beta-amyloid Burden, Neuropsychology
INTRODUCTION
Alzheimer’s disease (AD) is characterized by progressive cognitive decline. Mild cognitive impairment (MCI) is known as an intermediate stage between healthy aging and clinical dementia. In particular, amnestic MCI (aMCI) is considered a prodromal stage of AD dementia (1,2,3); however, the characteristics of aMCI are both pathologically and clinically heterogeneous. Approximately 40% of individuals with MCI show very low levels of cerebral beta-amyloid (Aβ) deposition that are not sufficient to represent a prodromal stage of AD (4,5). There are various clinical phenotypes of MCI, and cognitive deficits can occur in single or multiple domains (2).
The apolipoprotein E (APOE) ε4 allele is a major genetic risk factor for the development of late-onset AD dementia (6). Much evidence supports the association between APOE ε4 and cognitive decline in non-demented individuals (7,8). Nevertheless, there is disagreement regarding the specific cognitive domain affected by APOE ε4 status. APOE ε4 carriers show impairments compared to non-carriers in various cognitive domains, including episodic memory (8,9,10), executive function (7,11), language, and spatial ability (12).
Senile plaques containing Aβ are a hallmark of AD pathology. No associations between Aβ burden and cognitive function have been found in individuals with AD dementia (13,14). Some studies report a significant detrimental effect of Aβ on memory function in non-demented individuals (14,15), while others find no association (16,17). Most studies that assess non-memory cognitive domains report non-significant effects of Aβ (16), although some studies demonstrate a significant effect (15,17).
APOE ε4 is involved in Aβ binding and clearance during AD pathogenesis (18); therefore, an inextricable link between APOE ε4 and Aβ burden likely plays a role in the process of cognitive decline in AD patients. Contradictions in previous studies mentioned above might be partially explained by a failure to consider both APOE ε4 status and Aβ burden. Recent investigations of both factors report interactions in cross-sectional studies (15), suggesting that APOE ε4 status modulates the effects of Aβ on cognition. However, it is also possible that both biomarkers independently influence cognitive function. APOE ε4 is associated with a decline in executive function in subjects between the ages of 35 and 44 years who are unlikely to have significant Aβ burdens (7). Other studies have observed independent effects of the two factors (19,20). Understanding the influences of Aβ burden and APOE ε4 on cognitive function, particularly during the MCI stage, could support early detection and intervention in AD dementia. However, it remains unclear whether these factors influence cognitive function independently or interactively and which cognitive domains are affected.
The majority of previous studies have evaluated APOE ε4- or Aβ-associated cognitive characteristics using brief cognitive measures. Some studies have used only the global cognitive or episodic memory tests (11,14) or the single executive function test (7). Executive function test was not included in some studies (19). More comprehensive neuropsychological measures that assess multiple cognitive domains should be utilized in order to clarify the specific cognitive domains affected by APOE ε4 status and Aβ burden. Furthermore, although cognitive function consequently influences clinical severity and everyday function, the effects of APOE ε4 status or Aβ burden on clinical severity or functional status are rarely examined.
We aimed to investigate the independent and interactive influences of APOE ε4 status and Aβ burden on multiple cognitive domains in a large group of individuals with MCI and AD, as well as cognitively normal (CN) participants. We also examined the influences of these factors on clinical severity and functional status.
MATERIALS AND METHODS
Participants
Data were collected from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). For a detailed explanation and up-to-date information on ADNI, please see http://www.adni-info.org. We included participants from all phases of ADNI only if [18F] florbetapir positron emission tomography (PET) had been conducted within 3 months of clinical and cognitive assessment visits and APOE genotype was available. Initially, 1,030 subjects were selected. Subjects of the APOE 2/4 genotype (n = 22) were excluded due to the unclear effects of these alleles. The final analysis included 324 CN elderly participants, 502 individuals with MCI, and 182 with AD dementia who had undergone clinical evaluations and florbetapir PET scans between April 2010 and December 2013 (Table 1). Detailed eligibility criteria for the diagnostic groups are described elsewhere (21). Briefly, CN subjects had a Clinical Dementia Rating (CDR) of 0 and Mini-Mental State Examination (MMSE) scores between 24 and 30, were non-depressed and non-demented, and had not been diagnosed with MCI. Subjects with MCI had a CDR of 0.5 and MMSE scores between 24 and 30, complained of objective memory loss but showed no impairment in other cognitive domains, demonstrated preserved activities of daily living, and were non-demented. AD dementia subjects had a CDR of 0.5 or 1.0 and MMSE scores between 20 and 26 and met the National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria for probable AD (22). Participants with any significant neurological disease other than suspected incipient AD, such as Parkinson’s disease, multi-infarct dementia, Huntington’s disease, normal pressure hydrocephalus, brain tumor, progressive supranuclear palsy, seizure disorders, subdural hematoma, multiple sclerosis, or a history of significant head trauma, were excluded. In addition, participants with MRI evidence of brain infection, infarction or other focal lesions, multiple lacunes, or lacunes in a critical memory structure were also excluded.
Table 1. Demographic and clinical characteristics by patient groups.
| Characteristics | Patient groups | ||
|---|---|---|---|
| CN (n = 324) | MCI (n = 502) | AD (n = 182) | |
| Age (SD), yr | 74.6 (6.5) | 72.5 (7.8)† | 75.0 (7.8)* |
| Education (SD), yr | 16.5 (2.6) | 16.1 (2.7) | 15.9 (2.7)b |
| Female, n (%) | 173 (53.4) | 219 (43.6) | 75 (41.2) |
| APOE ε4 carriers, n (%) | 85 (26.2) | 228 (45.4) | 120 (65.9) |
| Positive Aβ status, n (%) | 101 (31.2) | 269 (53.6) | 153 (84.1) |
| CDR-SOB | 0.06 (0.24) | 1.48 (0.62)† | 4.95 (2.23)*,† |
| FAQ | 0.35 (1.29) | 2.68 (3.75)† | 14.05 (7.04)*,† |
| Global cognition | |||
| MMSE | 28.99 (1.28) | 28.05 (1.73)† | 22.49 (3.20)*,† |
| ADAS-cog11 | 5.73 (3.09) | 9.15 (4.39)† | 21.12 (8.23)*,† |
| ADAS-cog13 | 9.06 (4.63) | 14.75 (6.70)† | 31.59 (9.72)*,† |
| MoCA | 25.58 (2.53) | 23.41 (3.15)† | 17.01 (4.63)*,† |
| Memory | |||
| ADNI_Mem | 0.92 (0.53) | 0.36 (0.55)† | -0.65 (0.54)*,† |
| RAVLT_imm | 45.85 (10.43) | 36.94 (11.04)† | 22.13 (7.41)*,† |
| RAVLT_delayed | 7.62 (4.03) | 4.73 (4.08)† | 0.71 (1.70)*,† |
| RAVLT_recog | 12.73 (2.65) | 11.35 (3.13)† | 6.64 (3.96)*,† |
| LM_imm | 14.64 (1.9) | 9.78 (3.55)† | 4.19 (2.78)*,† |
| LM_delayed | 13.73 (3.33) | 7.37 (3.42)† | 1.59 (2.16)*,† |
| Frontal executive function | |||
| ADNI_EF | 0.81 (0.74) | 0.34 (0.79)† | -0.77 (0.83)*,† |
| TMT A | 33.77 (12.72) | 39.00 (17.06)† | 61.98(35.80)*,† |
| TMT B | 81.18 (38.46) | 107.55 (60.27)† | 189.38 (86.31)*,† |
| Animal fluency | 20.98 (5.43) | 17.92 (5.06)† | 12.00 (5.01)*,† |
| BNT | 28.04 (2.38) | 26.40 (3.49)† | 22.04 (6.25)*,† |
| Visuospatial ability | |||
| Clock drawing | 4.70 (0.54) | 4.47 (0.81)† | 3.38 (1.44)*,† |
| Clock copying | 4.87 (0.35) | 4.73 (0.59)† | 4.34 (1.02)*,† |
CN, cognitively normal; MCI, mild cognitive impairment; AD, Alzheimer’s disease; APOE, apolipoprotein E; Aβ, average florbetapir mean standard uptake value ratio of frontal, anterior cingulate, and parietal cortices and precuneus relative to the cerebellum; CDR-SOB, sum of boxes of the Clinical Dementia Rating scale; FAQ, Functional Assessment Questionnaire; MMSE, Mini-Mental State Examination; ADAS-cog11, Alzheimer’s Disease Assessment Scale-cognitive subscale, consisting of 11 items; ADAS-cog13, Alzheimer’s Disease Assessment Scale-cognitive subscale, consisting of 13 items; MoCA, Montreal Cognitive Assessment; ADNI_Mem, Alzheimer’s Disease Neuroimaging Initiative composite score for memory; RAVLT_imm, Rey Auditory Verbal Learning test, immediate recall score; RAVLT_delayed, RAVLT, delayed recall score; RAVLT_recog, RAVLT, recognition score; LM_imm, Logical Memory test, immediate recall score; LM_delayed, LM, delayed recall score; ADNI_EF, Alzheimer’s Disease Neuroimaging Initiative composite score for executive functioning; TMT, Trail Making Test; BNT, Boston Naming Test.
Data are presented as mean (standard deviation) or number (percentage).
*Significant compared to MCI (P < 0.05); †Significant compared to CN (P< 0.05).
Cognitive, clinical, and functional measures
We selected cognitive testing data from ADNI participants. Four tests were selected to evaluate global cognition, including the MMSE; the Alzheimer’s Disease Assessment Scale-cognitive subscale, consisting of 11 (ADAS-cog11) and 13 items (ADAS-cog13); and the Montreal Cognitive Assessment (MoCA) (23). We included each of these global cognitive measures in the analysis because each measure has specific characteristics and clinical usefulness. For example, a delayed recall task and number cancellation item were added to the ADAS-cog13. The MoCA was designed for MCI screening. These two measures have additional executive function and attention components compared to the MMSE and ADAS-cog11. Six measures were selected to assess memory, including the Rey Auditory Verbal Learning Test (RAVLT) trials 1-5 total recall as immediate recall (RAVLT_imm), 30-minute delayed recall (RAVLT_delayed), and yes-no recognition (RAVLT_recog); Logical Memory immediate recall (LM_imm) and 30-minute delayed recall (LM_delayed) from the Wechsler Memory Scale-Revised; and the ADNI composite scores for memory (ADNI_Mem) (24). Four measures were selected to assess frontal executive function, including the Trail Making Test (TMT) parts A and B, Animal fluency, and ADNI composite scores for executive functioning (ADNI_EF) (25). The Boston Naming Test (BNT) was included as a measure of language. Clock drawing and copying tests were included to assess visuospatial ability.
We selected the CDR Sum of Boxes (CDR-SOB) score as a clinical measure. This scale is a useful tool for staging clinical severity. It evaluates six domains of cognitive and daily functioning, with possible scores ranging from 0 to 18. We included the Functional Assessment Questionnaire (FAQ) to assess everyday functioning. This tool assesses instrumental activities of daily living, with scores ranging from 0 to 30, and is useful for distinguishing MCI from very mild AD as well as for monitoring functional changes (26).
APOE genotyping
APOE genotyping was performed at the time of participant enrollment in the ADNI study. APOE genotypes were determined using standard polymerase chain reaction methods, which have been described previously (27). Individuals with one or two copies of allele 4 were designated as APOE ε4 carriers (ε4+); individuals with no allele 4 were designated as APOE ε4 non-carriers (ε4−).
Florbetapir PET
We obtained the mean florbetapir standard uptake value ratio (SUVR) for each participant from the ADNI database. A detailed description of florbetapir PET acquisition and processing can be found on the ADNI website (http://adni.loni.usc.edu/wp-content/uploads/2010/05/ADNI2_PET_Tech_Manual_14201.pdf) or as previously published (14). Briefly, the subject’s first florbetapir image was coregistered to their MR image and segmented into cortical regions (frontal, anterior/posterior cingulate, lateral parietal, and lateral temporal) using FreeSurfer (version 4.5.0). The mean florbetapir uptake from these gray matter regions was then extracted and normalized to uptake in the whole cerebellum. Participants were classified as Aβ burden-positive (Aβ+) or Aβ burden-negative (Aβ−) according to the SUVR cutoff of 1.11 for amyloid positivity (14).
Statistical analysis
Demographic and clinical data were compared between study groups using one-way analysis of variance (ANOVA) and χ2 tests for continuous and categorical variables, respectively. Scores on neuropsychological, clinical, and functional measures were compared between groups using analysis of covariance (ANCOVA). To determine the main effects and interactive effects of APOE ε4 and Aβ burden on these scores, a series of 2 × 2 ANCOVAs was performed. We corrected P values for multiple comparisons using false discovery rate (FDR) correction. Post hoc pairwise comparisons were performed using a general linear model. The effects of age, gender, and education were adjusted for all ANCOVAs and pairwise comparisons. Cohen’s d was used to calculate the effect size between ε4+ and ε4− and between Aβ+ and Aβ− participants for each cognitive score. Statistical analyses were performed using SPSS (version 21.0) for Windows.
Ethics statement
Study procedures were approved by the institutional review boards of 55 research centers in the United States and Canada participating in ADNI. Written informed consent to share data for scientific research purposes was obtained from each participant.
RESULTS
Participant characteristics
The demographic and clinical characteristics of 1,008 subjects are presented in Table 1. Participants with MCI were, on average, younger than CN and AD dementia participants, while participants with AD dementia had received fewer years of education than those in the CN and MCI groups (P < 0.001). The CN group included more women than the other two study groups (χ2 [2, n = 1,008] = 9.90, P = 0.067). The frequencies of APOE ε4+ and Aβ+ statuses increased with increasing diagnostic severity (χ2 [2, n = 1,008] = 77.43, P < 0.001 for APOE ε4; χ2 [2, n = 1,008] = 131.76, P < 0.001 for Aβ). As expected, subsequent comparisons of cognitive test scores, clinical severity, and functional status revealed significant differences among groups after controlling for demographic variables. Post hoc pairwise comparisons showed multiple significant differences between groups (Table 1).
Effects of APOE ε4 status and Aβ positivity on cognitive function
There were no significant main effects or interactive effects of APOE ε4 status and Aβ positivity on any neuropsychological scores in the CN group, with the exception of LM_imm scores. The significant main effect of APOE ε4 status on LM_imm test scores (F[1, 317] = 6.84, FDR-corrected P < 0.001) indicated poorer performances by ε4+ compared to ε4− participants.
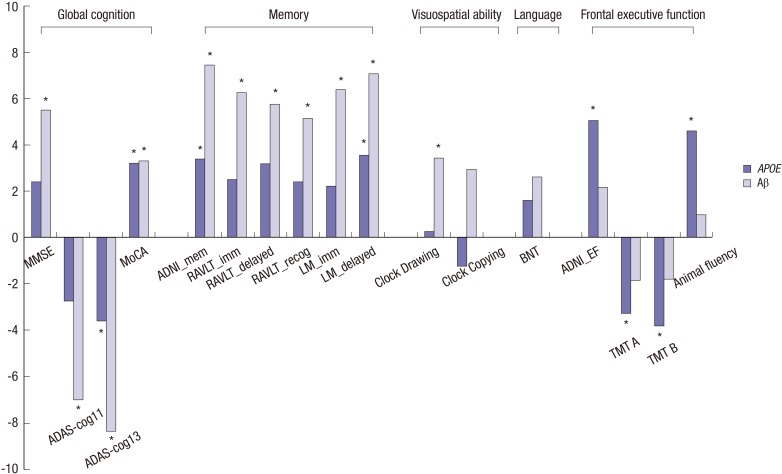
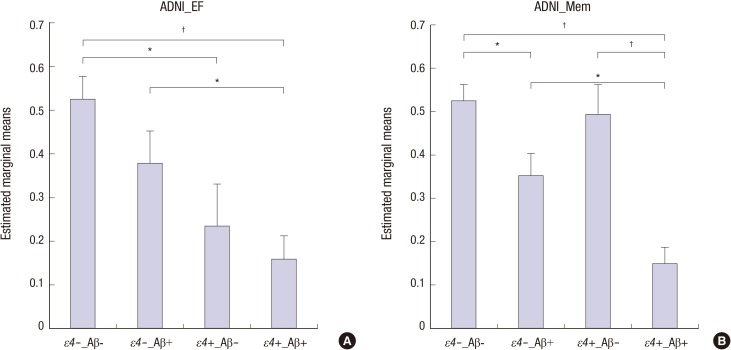
There were significant main effects of APOE ε4 status on scores on all measures of frontal executive function in the MCI group (Table 2). ADNI_EF scores were lower in ε4+ compared to ε4− participants. Significant main effects of APOE ε4 status were also observed on ADNI_Mem, LM_delayed, ADAS-cog13, and MoCA scores. In contrast, no significant effects of APOE ε4 status were found in the visuospatial and language domains. The magnitudes of the differences between ε4+ and ε4− participants, averaged for Aβ positivity, indicated that ε4+ participants showed poorer performances compared to ε4− participants on all measures of frontal executive function and on several measures of global cognitive and memory (Fig. 1). There were significant main effects of Aβ positivity on all tests of global cognition, memory, and visuospatial ability. Conversely, none of the measures of frontal executive function or language showed Aβ-related effects. The magnitude of the differences between Aβ+ and Aβ− participants, averaged for APOE ε4 status, indicated that Aβ+ participants showed poorer performances on global cognition, memory, and visuospatial tests compared to Aβ− participants (Fig. 1). Furthermore, pairwise comparisons between four subgroups (ε4−Aβ−, ε4−Aβ+, ε4+Aβ−, and ε4+Aβ+) of representative scores on the ADNI_EF and ADNI_Mem tests, which measure frontal executive function and memory, respectively, showed different patterns; APOE ε4 status and Aβ positivity predominantly affected scores on the ADNI_EF and ADNI_Mem tests, respectively (Fig. 2).
Table 2. Effects of APOE ε4 and Aβ on neuropsychological performance and clinical characteristics in participants with MCI.
| Variables | Main effect | APOE ε4 × Aβ Interaction | |
|---|---|---|---|
| APOE ε4 | Aβ | ||
| Global cognition | |||
| MMSE | 2.875 | 14.825* | 1.246 |
| ADAS-cog11 | 3.646 | 23.695* | 3.424 |
| ADAS-cog13 | 6.305* | 31.948* | 5.637* |
| MoCA | 5.021* | 5.424* | 0.154 |
| Memory | |||
| ADNI_Mem | 5.425* | 26.353* | 3.070 |
| RAVLT_imm | 2.981 | 19.013* | 1.762 |
| RAVLT_delayed | 4.884 | 16.187* | 2.430 |
| RAVLT_recog | 2.794 | 12.787* | 9.112* |
| LM_imm | 2.349 | 22.082* | 1.211 |
| LM_delayed | 6.131* | 24.250* | 2.454 |
| Executive/psychomotor speed | |||
| ADNI_EF | 12.437* | 2.331 | 0.253 |
| TMT A | 5.118* | 1.660 | 0.232 |
| TMT B | 7.039* | 1.485 | 0.175 |
| Animal fluency | 10.165* | 0.483 | 0.001 |
| Language | |||
| BNT | 1.240 | 3.402 | 0.232 |
| Visuospatial ability | |||
| Clock Drawing | 0.037 | 5.985* | 1.155 |
| Clock Copying | 0.739 | 4.305 | 0.010 |
| Clinical data | |||
| CDR-SOB | 0.146 | 9.560 * | 4.509 |
| FAQ | 0.228 | 10.458* | 2.952 |
MCI, mild cognitive impairment; APOE, apolipoprotein E; Aβ, beta-amyloid; MMSE, Mini-Mental State Examination; ADAS-cog11, Alzheimer’s Disease Assessment Scale-cognitive subscale, consisting of 11 items; ADAS-cog13, Alzheimer’s Disease Assessment Scale-cognitive subscale, consisting of 13 items; MOCA, Montreal Cognitive Assessment; ADNI_Mem, composite score for memory using Alzheimer’s Disease Neuroimaging Initiative; RAVLT_imm, Rey Auditory Verbal Learning Test, immediate recall score; RAVLT_delayed, RAVLT, delayed recall score; RAVLT_recog, RAVLT, recognition score; LM_imm, Logical Memory, immediate recall score; LM_delayed, LM, delayed recall score; ADNI_EF, Alzheimer’s Disease Neuroimaging Initiative composite score for executive functioning; TMT, Trail Making Test; BNT, Boston Naming Test; CDR-SOB, sum of boxes of the Clinical Dementia Rating scale; FAQ, Functional Assessment Questionnaire.
Data are presented as F values.
*False discovery rate (FDR)-corrected P < 0.05, using 2 × 2 analyses of covariance (ANCOVA) with age, gender, and education as covariates.
Fig. 1. Effect sizes of APOE ε4 status and Aβ positivity on neuropsychological measures in participants with MCI. Effect sizes were calculated using Cohen’s d. The magnitude of the differences in scores on each neuropsychological measure are presented according to apolipoprotein E (APOE) ε4 status (ε4 non-carriers and ε4 carriers; gray bars) and beta-amyloid positivity (Aβ negative and positive; shaded bars). Lower scores on the ADAS-cog11, ADAS-cog13, TMT A, and TMT B indicate better performances. MMSE, Mini-Mental State Examination; ADAS-cog11, Alzheimer’s Disease Assessment Scale-cognitive subscale, consisting of 11 items; ADAS-cog13, Alzheimer’s Disease Assessment Scale-cognitive subscale, consisting of 13 items; MoCA, Montreal Cognitive Assessment; ADNI_Mem, Alzheimer’s Disease Neuroimaging Initiative composite score for memory; RAVLT_imm, Rey Auditory Verbal Learning Test, immediate recall score; RAVLT_delayed, RAVLT, delayed recall score; RAVLT_recog, RAVLT, recognition score; LM_imm, Logical Memory, immediate recall score; LM_delayed, LM, delayed recall score; ADNI_EF, Alzheimer’s Disease Neuroimaging Initiative composite score for executive functioning; TMT, Trail Making Test; BNT, Boston Naming Test. *False discovery rate (FDR)-corrected P < 0.05.
Fig. 2. Frontal executive and memory performances of four subgroups of participants with MCI. ε4−, APOE ε4 non-carriers; ε4+, APOE ε4 carriers; Aβ−, beta-amyloid negative; Aβ+, beta-amyloid positive; ADNI_EF, Alzheimer’s Disease Neuroimaging Initiative composite score for executive functioning; ADNI_Mem, Alzheimer’s Disease Neuroimaging Initiative composite score for memory. *P < 0.01; †P < 0.001.
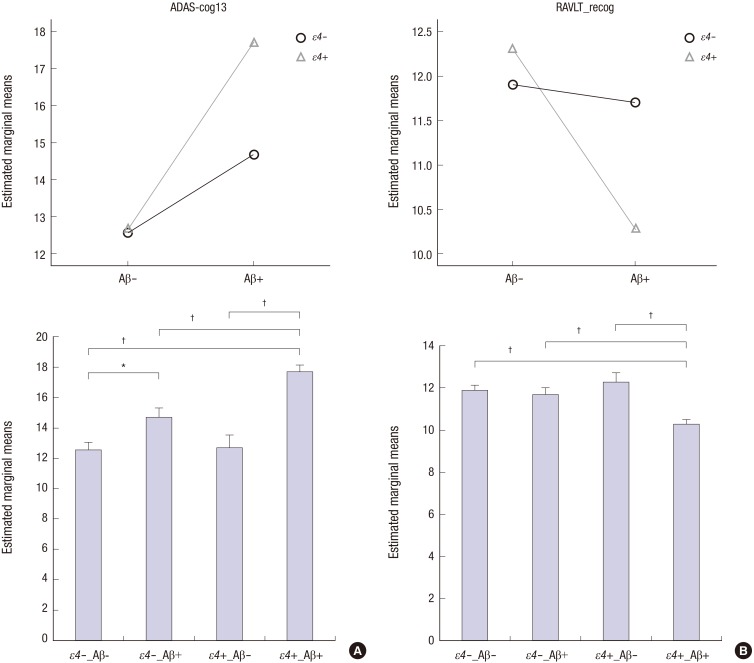
There were significant interactive effects of APOE ε4 status and Aβ positivity on ADAS-cog13 and RAVLT_recog scores in the MCI group. For both measures, ε4-related poor performance was found only among Aβ+ participants (Fig. 3A and B, upper row). To elucidate the interaction between APOE ε4 status and Aβ positivity, post hoc pairwise comparisons among the ε4−Aβ−, ε4−Aβ+, ε4+Aβ−, and ε4+Aβ+ subgroups were performed after controlling for age, gender, and education. ε4+Aβ+ individuals showed significantly poorer performances compared to participants in the other subgroups on the ADAS-cog13 (P < 0.001 for each comparison). In addition, ε4−Aβ+ individuals showed poorer performances than ε4−Aβ− individuals (P = 0.007) but showed no significant difference compared to ε4+Aβ− individuals (P = 0.067). ε4+Aβ+ individuals showed significantly poorer performances compared to the other three subgroups on the RAVLT_recog test (P < 0.001 for each comparison); there were no significant differences between performances on this measure among the other three subgroups (Fig. 3A and B, lower row).
Fig. 3. Interactive effects of APOE ε4 status and Aβ positivity on cognitive measures in participants with MCI. The upper row displays interactive effects of APOE ε4 status and Aβ positivity on the ADAS-cog13 (A) and RAVLT_recog tests (B). The lower row displays four subgroups according to APOE ε4 and Aβ status on the ADAS-cog13 (A) and RAVLT_recog tests (B). ε4−, APOE ε4 non-carriers, blue circle; ε4+, APOE ε4 carriers, green triangle; Aβ−, beta-amyloid negative; Aβ+, beta-amyloid positive; ADAS-cog13, Alzheimer’s Disease Assessment Scale-cognitive subscale, consisting of 13 items; RAVLT_recog, Rey Auditory Verbal Learning Test, recognition score. *P < 0.01; †P < 0.001.
No significant main effects or interactive effects of APOE ε4 status and Aβ positivity on any neuropsychological measure were found in the AD dementia group.
Effects of APOE ε4 status and Aβ positivity on clinical severity and functional status
No significant main effects or interactive effects of APOE ε4 status and Aβ positivity on CDR-SOB or FAQ scores were found in the CN and AD dementia groups. In contrast, there was a significant main effect of Aβ positivity on both CDR-SOB and FAQ scores in the MCI group; however, there was no significant effect of APOE ε4 status (Table 2).
DISCUSSION
Our data revealed four main findings. First, both APOE ε4 status and Aβ positivity independently influenced cognitive function in participants with MCI. Specifically, there were ε4-related performance impairments in frontal executive function tests and other tests with frontal executive components. There were Aβ-related performance impairments in global cognition, memory, and visuospatial tests, but not in tests of frontal executive function. Second, there were interactive effects of the two factors on global cognition and verbal recognition memory in the MCI group, with ε4+Aβ+ participants exhibiting the most significant impairments. Third, Aβ positivity, but not APOE ε4 status, influenced clinical severity and functional status in the MCI group. Lastly, the influences of APOE ε4status and Aβ positivity on cognitive function, clinical severity, and functional status were minimal in the CN and AD dementia groups.
Interestingly, there were dissociable influences of APOE ε4 status and Aβ positivity on cognitive performances in the MCI group. APOE ε4 status predominantly influenced scores on frontal executive function tests and other measures with frontal executive components (i.e., the ADAS-cog13, MoCA, and delayed free recall task). Aβ positivity had no significant influence on these effects. These results suggest that the contribution of APOE ε4 to AD pathogenesis may be partially independent of its role in Aβ pathology. Furthermore, the predominantly affected cognitive domain may be frontal executive function. The frontal lobe is the neural substrate for executive function. A systemic review and meta-analysis study previously revealed a robust APOE ε4-related decrease in frontal lobe metabolism in non-demented subjects (28). Additionally, Chu et al. (11) recently reported that non-demented APOE ε4 carriers showed impaired performances on frontal executive function measures, but not on memory tests. Previous reports assessing APOE ε4-related cognitive characteristics are inconsistent (29). The discrepancy among previous investigations may be attributable, at least in part, to failure to control for Aβ burden in their analyses. It is likely that Aβ burden is a confounding factor when assessing the relationship between APOE ε4 status and cognitive function, particularly in the elderly population. Aβ deposition linearly increases with age, with a high number of Aβ+ individuals aged 60 and older (17). In agreement with this result, APOE ε4 status affects performance on executive function tasks and frontal lobe thickness in younger subjects, in whom the accumulation of Aβ is unlikely to be a factor (7,30).
We found significant main effects of Aβ positivity on global cognition, memory, and visuospatial ability in participants with MCI, with Aβ+ individuals exhibiting poorer performances. However, measures of frontal executive function were not influenced by Aβ positivity. Although there are conflicting investigations assessing the effects of Aβ positivity (31,32), our results are consistent with observations that high Aβ burden is associated with poor cognitive function in subjects with MCI (14). Non-significant associations between Aβ positivity and frontal executive function are rarely reported; the majority of previous studies have mainly focused on episodic memory and lack detailed tests of frontal executive function (14,33). Thus, the influence of Aβ burden on frontal executive function has not been thoroughly examined. Consistent with our results, one recent follow-up study using comprehensive neuropsychological measures demonstrated that Aβ positivity did not affect frontal executive function in subjects with MCI but was associated with declines in other cognitive domains (20). Our subgroup analyses of representative scores on frontal executive function and memory tests showed that APOE ε4 status and Aβ positivity predominantly affected frontal executive function and memory, respectively. These dissociable influences of APOE ε4 status and Aβ positivity on executive and non-executive cognitive functions, respectively, in subjects with MCI could provide new insights into the mechanisms underlying AD-related cognitive decline.
We also found interactive effects of APOE ε4 status and Aβ positivity on measures of global cognition and verbal recognition memory in the MCI group. Individuals positive for both APOE ε4 and Aβ exhibited the most significant impairments in these tests, whereas no differences were found between individuals positive for only one of these factors. This result is in line with a previous study showing that the combination of APOE ε4+ status and Aβ burden is a significant risk factor for AD, though their independent effects may not be sufficient to cause AD (34). Our results suggest that the combination of APOE ε4+ genotype and Aβ burden is associated with greater detrimental effects on cognition than each single factor.
In the current study, the influences of APOE ε4 status and Aβ positivity on cognitive characteristics were minimal in the CN group. APOE ε4 influenced verbal immediate story recall; CN ε4+ subjects exhibited significantly lower performances compared to CN ε4− participants. Furthermore, Aβ positivity did not influence this effect. This result is consistent with previous studies showing adverse APOE ε4 effects on verbal memory in a healthy normal elderly population, particularly in an episodic learning procedure similar to the test used in this study (8,35). Our findings also suggest that episodic memory, as measured by story recall, can be a sensitive tool to detect APOE ε4-related memory problems in the healthy normal elderly population. A meta-analysis of 77 studies reported that ε4+ individuals showed small but significant adverse effects not only on episodic memory, but also on global cognition, executive function, and perceptual speed (29). This discrepancy may be related to differences in study samples. Participants with MCI were not excluded in many of the studies included in the meta-analysis [e.g., (36)]. Although the meta-analysis included studies of cognitively intact subjects, it is likely that a considerable proportion of participants with MCI were also included. In contrast, MCI was not included in our CN group. We did not find any effect of Aβ positivity on cognitive performance. The relationship between Aβ burden and cognition in the healthy normal elderly is generally weak or absent (19,31). However, a recent meta-analysis investigating the relationship between Aβ and cognition reported that Aβ burden, as examined by amyloid imaging, was negatively associated with episodic memory (37). One possible reason for this discrepancy could be the study design (cross-sectional or longitudinal). The meta-analysis performed by Hedden et al. included both cross-sectional and longitudinal analyses, whereas the current study is a cross-sectional analysis (37). Effects of Aβ accumulation may be more apparent in longitudinal studies of cognitive decline rather than cross-sectional studies. A longitudinal study showed that, while effects of Aβ on cognitive function were insignificant in baseline evaluations, Aβ accumulation was significantly associated with declines in episodic memory after 18 months (19).
Neither APOE ε4 status nor Aβ positivity influenced any cognitive measures in the AD dementia group. A lack of association between Aβ burden and cognition in AD dementia patients has been consistently reported (13,31). This implies that Aβ deposition is an early event that has plateaued at the point of clinical diagnosis of AD dementia (38). In line with previous studies (39,40), we failed to find APOE ε4-related differences in cognitive function in the AD dementia group. However, one study has demonstrated an APOE ε4-associated effect on memory and frontal executive function in AD dementia (10). The age of the participants may contribute to this inconsistency; Chang et al. (39) have hypothesized that the effect of APOE ε4 on cognition may differ according to the mean age of the population being studied. Effects on cognitive function have been observed in elderly patients less than 75 years of age (10), whereas no significant effects have been found in studies of elderly patients over 80 years of age (39) or in our study, which included a wide range of ages (55 to 94 years).
We did not find any associations of APOE ε4 status or Aβ positivity with clinical or functional status in the CN and AD dementia groups. However, Aβ positivity, but not APOE ε4 status, was associated with poorer clinical and functional status in the MCI group, suggesting that a greater Aβ burden negatively influences both clinical severity and everyday functioning.
We propose several possible explanations for the strong effects of APOE ε4 and Aβ burden observed only in the MCI group. First and most importantly, AD dementia has a very long preclinical period. APOE ε4 and Aβ accumulation may negatively influence cognitive function during the prodromal stage (i.e., the MCI stage); however these effects may lessen during the clinical stage of AD dementia. Second, cognitive function in the MCI group was more heterogeneous than in the CN or AD dementia groups. CN subjects showed no cognitive impairments, raising the possibility of ceiling effects, whereas participants with AD showed significant cognitive impairments that may have been susceptible to floor effects. Third, the distribution of APOE ε4 status and Aβ positivity within each group could influence the results. The CN and AD dementia groups included high percentages of ε4−Aβ− (56%) and ε4+Aβ+ individuals (64%), respectively, whereas the MCI group showed a relatively even distribution in the cells of positive or negative for both factors. Therefore, there may be insufficient variance between the two factors and cognitive scores to detect any effects in the CN and AD groups.
Some limitations and future directions should be discussed. First, this study had a cross-sectional design, precluding an investigation of the effects of APOE ε4 and Aβ on longitudinal cognitive decline in this population. To clarify the independent effects of these two factors, this issue should be addressed through further assessment of APOE ε4- or Aβ burden-related cognitive changes, particularly in the CN group. Second, cognitive performance is closely related to brain function and structure; however, dissociable influences of APOE ε4 and Aβ burden on brain function were not examined in the current study. Future neuroimaging studies are needed to verify our results and to understand the precise role of the APOE gene in frontal executive function. Third, AD dementia diagnosis is made on a clinical rather than a pathological basis. Although the proportion of Aβ− AD dementia patients was small (16%), we cannot entirely exclude the possibility that participants with non-AD dementia were included in our study.
In conclusion, we provide further evidence that APOE ε4 and Aβ burden play both independent and interactive roles in altering cognitive function in individuals with MCI. Dissociable, independent influences of APOE ε4 and Aβ burden on executive and non-executive cognitive functions, respectively, were found in participants with MCI. Interactive effects of these two factors on global cognition and recognition memory were also observed.
ACKNOWLEDGMENT
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI; National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen Idec Inc.; Bristol-Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Medpace, Inc.; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Synarc Inc.; and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (http://www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory of Neuro Imaging at the University of Southern California.
Footnotes
Funding: This work was supported by research fund from Chosun University (K206556001-1 and K206996001-1).
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Conception and design: Seo EH, Choo IH. Analysis and interpretation of data: Seo EH, Choo IH. Writing and revision of the manuscript: Seo EH, Choo IH. Administrative, technical & material supports: Park SH, Kim SH, Kang SH. Critical revisions and approval of the manuscript: all authors.
References
- 1.Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, Berg L. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 2.Petersen RC, Caracciolo B, Brayne C, Gauthier S, Jelic V, Fratiglioni L. Mild cognitive impairment: a concept in evolution. J Intern Med. 2014;275:214–228. doi: 10.1111/joim.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sohn BK, Yi D, Seo EH, Choe YM, Kim JW, Kim SG, Choi HJ, Byun MS, Jhoo JH, Woo JI, et al. Comparison of regional gray matter atrophy, white matter alteration, and glucose metabolism as a predictor of the conversion to Alzheimer’s disease in mild cognitive impairment. J Korean Med Sci. 2015;30:779–787. doi: 10.3346/jkms.2015.30.6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nordberg A, Carter SF, Rinne J, Drzezga A, Brooks DJ, Vandenberghe R, Perani D, Forsberg A, Långström B, Scheinin N, et al. A European multicentre PET study of fibrillar amyloid in Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2013;40:104–114. doi: 10.1007/s00259-012-2237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolk DA, Price JC, Saxton JA, Snitz BE, James JA, Lopez OL, Aizenstein HJ, Cohen AD, Weissfeld LA, Mathis CA, et al. Amyloid imaging in mild cognitive impairment subtypes. Ann Neurol. 2009;65:557–568. doi: 10.1002/ana.21598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat Genet. 2007;39:17–23. doi: 10.1038/ng1934. [DOI] [PubMed] [Google Scholar]
- 7.Izaks GJ, Gansevoort RT, van der Knaap AM, Navis G, Dullaart RP, Slaets JP. The association of APOE genotype with cognitive function in persons aged 35 years or older. PLoS One. 2011;6:e27415. doi: 10.1371/journal.pone.0027415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu F, Pardo LM, Schuur M, Sanchez-Juan P, Isaacs A, Sleegers K, de Koning I, Zorkoltseva IV, Axenovich TI, Witteman JC, et al. The apolipoprotein E gene and its age-specific effects on cognitive function. Neurobiol Aging. 2010;31:1831–1833. doi: 10.1016/j.neurobiolaging.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 9.Kerchner GA, Berdnik D, Shen JC, Bernstein JD, Fenesy MC, Deutsch GK, Wyss-Coray T, Rutt BK. APOE epsilon4 worsens hippocampal CA1 apical neuropil atrophy and episodic memory. Neurology. 2014;82:691–697. doi: 10.1212/WNL.0000000000000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolk DA, Dickerson BC, Weiner M, Aiello M, Aisen P, Albert MS, Alexander G, Anderson HS, Anderson K, Apostolova L, et al. Apolipoprotein E (APOE) genotype has dissociable effects on memory and attentional-executive network function in Alzheimer’s disease. Proc Natl Acad Sci USA. 2010;107:10256–10261. doi: 10.1073/pnas.1001412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chu CS, Lu T, Tsai SJ, Hong CJ, Yeh HL, Yang AC, Liu ME. APOE varepsilon4 polymorphism and cognitive deficit among the very old Chinese veteran men without dementia. Neurosci Lett. 2014;576:17–21. doi: 10.1016/j.neulet.2014.05.046. [DOI] [PubMed] [Google Scholar]
- 12.Bretsky P, Guralnik JM, Launer L, Albert M, Seeman TE. MacArthur Studies of Successful Aging. The role of APOE-epsilon4 in longitudinal cognitive decline: MacArthur Studies of Successful Aging. Neurology. 2003;60:1077–1081. doi: 10.1212/01.wnl.0000055875.26908.24. [DOI] [PubMed] [Google Scholar]
- 13.Jack CR, Jr, Lowe VJ, Weigand SD, Wiste HJ, Senjem ML, Knopman DS, Shiung MM, Gunter JL, Boeve BF, Kemp BJ, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer’s disease: implications for sequence of pathological events in Alzheimer’s disease. Brain. 2009;132:1355–1365. doi: 10.1093/brain/awp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landau SM, Mintun MA, Joshi AD, Koeppe RA, Petersen RC, Aisen PS, Weiner MW, Jagust WJ, Alzheimer’s Disease Neuroimaging Initiative Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Ann Neurol. 2012;72:578–586. doi: 10.1002/ana.23650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kantarci K, Lowe V, Przybelski SA, Weigand SD, Senjem ML, Ivnik RJ, Preboske GM, Roberts R, Geda YE, Boeve BF, et al. APOE modifies the association between Abeta load and cognition in cognitively normal older adults. Neurology. 2012;78:232–240. doi: 10.1212/WNL.0b013e31824365ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oh H, Mormino EC, Madison C, Hayenga A, Smiljic A, Jagust WJ. β-Amyloid affects frontal and posterior brain networks in normal aging. Neuroimage. 2011;54:1887–1895. doi: 10.1016/j.neuroimage.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodrigue KM, Kennedy KM, Devous MD, Sr, Rieck JR, Hebrank AC, Diaz-Arrastia R, Mathews D, Park DC. β-Amyloid burden in healthy aging: regional distribution and cognitive consequences. Neurology. 2012;78:387–395. doi: 10.1212/WNL.0b013e318245d295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dorey E, Chang N, Liu QY, Yang Z, Zhang W. Apolipoprotein E, amyloid-beta, and neuroinflammation in Alzheimer’s disease. Neurosci Bull. 2014;30:317–330. doi: 10.1007/s12264-013-1422-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim YY, Ellis KA, Pietrzak RH, Ames D, Darby D, Harrington K, Martins RN, Masters CL, Rowe C, Savage G, et al. Stronger effect of amyloid load than APOE genotype on cognitive decline in healthy older adults. Neurology. 2012;79:1645–1652. doi: 10.1212/WNL.0b013e31826e9ae6. [DOI] [PubMed] [Google Scholar]
- 20.Lim YY, Maruff P, Pietrzak RH, Ames D, Ellis KA, Harrington K, Lautenschlager NT, Szoeke C, Martins RN, Masters CL, et al. Effect of amyloid on memory and non-memory decline from preclinical to clinical Alzheimer’s disease. Brain. 2014;137:221–231. doi: 10.1093/brain/awt286. [DOI] [PubMed] [Google Scholar]
- 21.Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, Jack CR, Jr, Jagust WJ, Shaw LM, Toga AW, et al. Alzheimer’s Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010;74:201–209. doi: 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 23.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 24.Crane PK, Carle A, Gibbons LE, Insel P, Mackin RS, Gross A, Jones RN, Mukherjee S, Curtis SM, Harvey D, et al. Development and assessment of a composite score for memory in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) Brain Imaging Behav. 2012;6:502–516. doi: 10.1007/s11682-012-9186-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibbons LE, Carle AC, Mackin RS, Harvey D, Mukherjee S, Insel P, Curtis SM, Mungas D, Crane PK, Alzheimer’s Disease Neuroimaging Initiative A composite score for executive functioning, validated in Alzheimer’s Disease Neuroimaging Initiative (ADNI) participants with baseline mild cognitive impairment. Brain Imaging Behav. 2012;6:517–527. doi: 10.1007/s11682-012-9176-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teng E, Becker BW, Woo E, Knopman DS, Cummings JL, Lu PH. Utility of the functional activities questionnaire for distinguishing mild cognitive impairment from very mild Alzheimer disease. Alzheimer Dis Assoc Disord. 2010;24:348–353. doi: 10.1097/WAD.0b013e3181e2fc84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saykin AJ, Shen L, Foroud TM, Potkin SG, Swaminathan S, Kim S, Risacher SL, Nho K, Huentelman MJ, Craig DW, et al. Alzheimer’s Disease Neuroimaging Initiative biomarkers as quantitative phenotypes: genetics core aims, progress, and plans. Alzheimers Dement. 2010;6:265–273. doi: 10.1016/j.jalz.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y, Yu JT, Wang HF, Han PR, Tan CC, Wang C, Meng XF, Risacher SL, Saykin AJ, Tan L. APOE genotype and neuroimaging markers of Alzheimer’s disease: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2015;86:127–134. doi: 10.1136/jnnp-2014-307719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wisdom NM, Callahan JL, Hawkins KA. The effects of apolipoprotein E on non-impaired cognitive functioning: a meta-analysis. Neurobiol Aging. 2011;32:63–74. doi: 10.1016/j.neurobiolaging.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Fennema-Notestine C, Panizzon MS, Thompson WR, Chen CH, Eyler LT, Fischl B, Franz CE, Grant MD, Jak AJ, Jernigan TL, et al. Presence of ApoE epsilon4 allele associated with thinner frontal cortex in middle age. J Alzheimers Dis. 2011;26(Suppl 3):49–60. doi: 10.3233/JAD-2011-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hampel H. Amyloid-beta and cognition in aging and Alzheimer’s disease: molecular and neurophysiological mechanisms. J Alzheimers Dis. 2013;33(Suppl 1):S79–86. doi: 10.3233/JAD-2012-129003. [DOI] [PubMed] [Google Scholar]
- 32.Rodrigue KM, Kennedy KM, Park DC. Beta-amyloid deposition and the aging brain. Neuropsychol Rev. 2009;19:436–450. doi: 10.1007/s11065-009-9118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lim YY, Maruff P, Pietrzak RH, Ellis KA, Darby D, Ames D, Harrington K, Martins RN, Masters CL, Szoeke C, et al. Abeta and cognitive change: examining the preclinical and prodromal stages of Alzheimer’s disease. Alzheimers Dement. 2014;10:743–751.e1. doi: 10.1016/j.jalz.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 34.Knopman DS. beta-Amyloidosis and neurodegeneration in Alzheimer disease: who’s on first? Neurology. 2014;82:1756–1757. doi: 10.1212/WNL.0000000000000438. [DOI] [PubMed] [Google Scholar]
- 35.Caselli RJ, Dueck AC, Osborne D, Sabbagh MN, Connor DJ, Ahern GL, Baxter LC, Rapcsak SZ, Shi J, Woodruff BK, et al. Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. N Engl J Med. 2009;361:255–263. doi: 10.1056/NEJMoa0809437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, Small GW. Patterns of brain activation in people at risk for Alzheimer’s disease. N Engl J Med. 2000;343:450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hedden T, Oh H, Younger AP, Patel TA. Meta-analysis of amyloid-cognition relations in cognitively normal older adults. Neurology. 2013;80:1341–1348. doi: 10.1212/WNL.0b013e31828ab35d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang YL, Fennema-Notestine C, Holland D, McEvoy LK, Stricker NH, Salmon DP, Dale AM, Bondi MW, Alzheimer’s Disease Neuroimaging Initiative APOE interacts with age to modify rate of decline in cognitive and brain changes in Alzheimer’s disease. Alzheimers Dement. 2014;10:336–348. doi: 10.1016/j.jalz.2013.05.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kleiman T, Zdanys K, Black B, Rightmer T, Grey M, Garman K, Macavoy M, Gelernter J, van Dyck C. Apolipoprotein E epsilon4 allele is unrelated to cognitive or functional decline in Alzheimer’s disease: retrospective and prospective analysis. Dement Geriatr Cogn Disord. 2006;22:73–82. doi: 10.1159/000093316. [DOI] [PubMed] [Google Scholar]