Platelet transfusion has been widely used in patients undergoing chemotherapy or radiotherapy; however, the shortage of the platelet supply limits the care of patients. A rotary cell culture system (RCCS) was evaluated to potentiate megakaryopoiesis and significantly improve the efficiency of platelet generation. The cost-effective and highly controllable strategy and methodology represent an important step toward large-scale platelet production for future biomedical and clinical applications.
Keywords: Rotary suspension culture, Megakaryopoiesis, Thrombopoiesis, CD34+ cell
Abstract
Platelet transfusion has been widely used in patients undergoing chemotherapy or radiotherapy; however, the shortage of the platelet supply limits the care of patients. Although derivation of clinical-scale platelets in vitro could provide a new source for transfusion, the devices and procedures for deriving scalable platelets for clinical applications have not been established. In the present study, we found that a rotary cell culture system (RCCS) can potentiate megakaryopoiesis and significantly improve the efficiency of platelet generation. When used with chemical compounds and growth factors identified via small-scale screening, the RCCS improved platelet generation efficiency by as much as ∼3.7-fold compared with static conditions. Shear force, simulated microgravity, and better diffusion of nutrients and oxygen from the RCCS, altogether, might account for the improved efficient platelet generation. The cost-effective and highly controllable strategy and methodology represent an important step toward large-scale platelet production for future biomedical and clinical applications.
Significance
Platelet transfusion has been widely used in patients undergoing chemotherapy or radiotherapy; however, the shortage of platelet supply limits the care of patients. Thus, derivation of clinical-scale platelets in vitro would provide a new source for transfusion. The present study evaluated a rotary suspension cell culture system that was able to potentiate megakaryopoiesis and significantly improved the efficiency of platelet generation. When used with chemical compounds and growth factors identified via small-scale screening, the three-dimensional system improved platelet generation efficiency compared with the static condition. The three-dimensional device and the strategy developed in the present study should markedly improve the generation of large-scale platelets for use in future biomedical and clinical settings.
Introduction
Platelets, progeny of adult megakaryocytes (MKs), play essential roles in hemostasis and thrombosis. Platelet transfusion is often required to prevent and treat thrombocytopenia occurring during hematological diseases, chemotherapy, and/or radiotherapy. The platelets used for transfusion are all donated by volunteers, need to be stored at room temperature when isolated, and have a shelf life varying from 3 to 7 days [1]. These factors, together, cause a frequently insufficient supply of platelets with otherwise increasing demands. Thus, the production of functional platelets in clinical-scale quantity in vitro could be an alternative strategy to resolve this issue. The generation of platelets in vitro has been described as three major sequential steps: cultivation of hematopoietic stem cells (self-renewal), amplification and differentiation of megakaryocyte progenitor cells (megakaryopoiesis), and the generation and release of platelets (thrombopoiesis) [2]. Accordingly, optimization of each of these steps might eventually increase the efficiency of platelet generation and the final yield of platelets.
Megakaryopoiesis and thrombopoiesis have been shown to be governed by a complex network of growth factors and cytokines, including thrombopoietin (TPO), stem cell factor (SCF), interleukin-3 (IL-3), interleukin-11 (IL-11), human growth hormone, and others. These extracellular factors exert their effects by regulating multiple signaling pathways [3–5]. As such, the proper use of biochemical stimuli should enable platelet generation in vitro. Recent studies have demonstrated that biophysical force that better mimics in vivo physical conditions can also enhance these processes, especially for thrombopoiesis. Dunois-Lardé et al. demonstrated that a particular type of biomechanical force, namely fluid shear stress, has the capability to accelerate platelet production [6]. CD34+ cord blood cells grown in surgical grade woven polyester fabric or three-dimensional (3D) hydrogel scaffolds can increase platelet output [7]. Pallotta et al. developed a 3D system based on silk-based vascular tubes that models the microenvironment of bone marrow and found that it can enhance platelet generation from human pluripotent stem cells (hPSCs) [8]. Two flows in different directions can promote platelet production by as much as 3.6-fold compared with static cultures [9]. The results from all these studies suggest the feasibility of establishing a highly efficient culture system by integrating multiple biochemical and biophysical signals to generate platelets on a large scale. Despite the feasibility, reliable devices and procedures to integrate those factors to produce platelets efficiently and on a large scale for clinical purposes have not yet been established.
The rotary cell culture system (RCCS) has been recommended by the National Aeronautics and Space Administration as an effective tool to simulate microgravity. This 3D dynamic culture system has a number of advantages over classic static cultures, including prevention of sedimentation, promotion of cell-cell interactions, and better diffusion of nutrients and oxygen, which might improve cell viability and proliferation [10, 11]. Furthermore, the fluid shear stresses and hydrodynamic force generated might strengthen tissue development and organogenesis [12]. In keeping with these results, we recently reported that the RCCS can stimulate the proliferation of human epidermal stem cells and subsequent formation of 3D structures. We have also demonstrated that the RCCS enhances mesendoderm differentiation of mouse embryonic stem cells by modulation of Wnt/β-catenin signaling [13, 14].
Megakaryopoiesis occurs in a 3D microenvironment in bone marrow, and thrombopoiesis can be largely boosted by mechanical force [15]. These facts led us to hypothesize that the RCCS might affect the generation of megakaryocytes and platelets in vitro. In the present study, we tested the effect of using the RCCS on megakaryopoiesis and thrombopoiesis of hematopoietic progenitor cells (HPCs) and discovered that it can enhance both processes, albeit with minimal effect on cell proliferation. More importantly, simultaneous exposure of cells to a subset of chemical compounds and growth factors synergistically promoted the generation of functional platelets in vitro. Because of its simplicity and capacity for large-scale production, our method should have the potential to generate platelets in large numbers for clinical applications.
Materials and Methods
CD34+ Cell Preparation
Umbilical cord blood (CB) units were obtained from healthy full-term neonates with informed consent from the parents and approved by the ethics committee of the Institute of Hematology and Blood Diseases Hospital, Chinese Academy of Medical Sciences. CD34+ cells were isolated from CB using Ficoll-Hypaque density centrifugation medium according to the manufacturer’s instructions (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com). In brief, the mononuclear selection fraction was subject to two cycles of immunomagnetic bead separation using a MiniMACS CD34+ isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany, http://www.miltenyibiotec.com). The percentage of CD34+ cells was higher than 95% when verified by flow cytometry (FACS Canto II, BD Biosciences, San Diego, CA, http://www.bdbiosciences.com).
Cell Culture
Enriched CD34+ cells (cell density, 1 × 105 cells per milliliter) were cultured in serum-free medium (StemSpan SFEM, Stem Cell Technologies, Vancouver, BC, Canada, http://www.stemcell.com) containing 1% penicillin/streptomycin, TPO (50 ng/ml), SCF (20 ng/ml), and IL-3 (20 ng/ml). After 6 days of expansion, the cells were cultured using StemSpan medium (Stem Cell Technologies) supplemented with TPO (50 ng/ml) and IL-11 (20 ng/ml) for the next 9 days. The medium was replaced with fresh medium every 3 days. Different chemical compounds and growth factors and/or cytokines (supplemental online Table 1) were added at different times according to the requirements of the experiment.
For rotary suspension culture, the cells were inoculated into 10 ml of the culture vessel of RCCS (supplemental online Video 1; Synthecon Inc., Houston, TX, http://www.synthecon.com) at the same density as for the static culture in 12-well culture plates (Corning, Corning, NY, http://www.corning.com). The vessel was installed onto the RCCS, rotating in a clockwise direction at a speed of 20 rpm.
May-Grünwald-Giemsa Staining and Cell Size Measurement
For staining, 2 × 104 cells were centrifuged onto slides using cytospin (Thermo Shandon Inc., Pittsburgh, PA, http://www.thermo.com) before staining with May-Grünwald-Giemsa (MGG) staining solution (Beyotime Institute of Biotechnology, Shanghai, China, http://www.beyotime.com). The morphology was observed by light microscopy. Cell size was measured using an in-house image processing program (NIS-Elements BR) or analyzed by flow cytometry (FACS Canto II, BD Biosciences).
DNA Content Analysis
For DNA content analysis, 5 × 105 cells were collected and resuspended with 0.1% bovine serum albumin (BSA; Invitrogen, Carlsbad, CA, http://www.invitrogen.com) and labeled with APC-CD41a for 30 minutes in dark. Next, the cells were fixed in 4% paraformaldehyde (PFA) for 20 minutes at room temperature and permeabilized with 0.2% Triton X-100 (Sigma-Aldrich) for 15 minutes. After treatment with 100 μg/ml RNase A for 30 minutes at 37°C, the cells were incubated with 40 μg/ml propidium iodide (Sigma-Aldrich) for 10 minutes in the dark. The cells in the CD41a+ gate were analyzed for ploidy.
Quantitative Real-Time Polymerase Chain Reaction
Total RNA was isolated using an HP Total RNA Kit (Omega Bio-Tek, Norcross, GA, http://www/omegabiotek.com) according to the manufacturer’s instructions. First-strand cDNA was reverse transcribed with oligo-dT as primer using a reverse transcription system (Promega, Madison, WI, http://www.promega.com). Real-time polymerase chain reaction (PCR) was performed using QuantiTech SYBR Green PCR kit (Qiagen, Hilden, Germany, http://www.qiagen.com). The primers specific for glyceraldehyde-3-phosphate dehydrogenase, GATA1, RUNX-1, Fli-1, FOG-1, NF-E2, and ITGB3 genes are listed in supplemental online Table 2.
Flow Cytometry Analysis
The cells were collected and labeled with APC-CD34, APC-CD41a, and phycoerythrin (PE)-CD42b antibodies (BD Biosciences) for 30 minutes at room temperature in dark and then analyzed using a flow cytometer. The platelets were collected from culture supernatant (1 ml) and stained with APC-CD41a and PE-CD42b or PE-CD62P antibodies (BD Biosciences) at room temperature for 30 minutes. For α-granule release analysis, the platelets were treated with 2 U/ml of thrombin (Sigma-Aldrich) for 20 minutes at 37°C before incubation with APC-CD41a and PE-CD62P antibodies. The treatment in the control group was the same, except for the use of thrombin.
Immunofluorescence of MKs
After being centrifuged onto the slides, the cells were fixed with 4% PFA for 15 minutes and permeabilized with 0.1% Triton X-100. After being blocked with 1% BSA at 37°C for 1 hour, the cells were incubated with primary antibodies at 4°C overnight. Fluorescence-labeled secondary antibodies were applied for 1 hour at room temperature. After washing in phosphate-buffered saline (PBS) three times, the cells were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Fluorescent images were visualized using the confocal microscopy (LSM710; Carl Zeiss, Jena, Germany, http://www.zeiss.com). The antibodies used in the present study are listed in supplemental online Table 3.
Measurements of MKs and Platelets
Quantification of MKs and Platelets was conducted as described in a previous study [16]. In brief, the total nucleated cells (TNCs) produced per seeded CD34+ cell were calculated as follows: cell density at the day of analysis divided by the cell density at day 0 multiplied by all cell dilutions. The MKs generated from single CD34+ cells were calculated as follows: percentage of CD41a+CD42b+ MKs multiplied by TNCs. The platelets produced per CD34+ cell were calculated as follows: platelet-to-cell ratio multiplied by the percentage of CD41a+CD42b+ platelets multiplied by TNCs. The platelet-to-cell ratio was derived using cytometry data as the number of events belonging to the platelet region divided by the number of events belonging to the cell region. The platelets produced per MK were calculated according to the amounts of MKs and platelets: platelet/cell ratio × percentage of CD41a+CD42b+ platelets/percentage of CD41a+CD42b+ MKs.
Purification of Platelet-Like Particles
Purification was performed by performing a spin (800g for 10 minutes). Platelet-like particles (PLPs) were resuspended in 2 ml of CGS buffer (10 mM sodium citrate, 30 mM d-glucose, and 120 mM NaCl, adjusted to pH 6.5 with citric acid) in the presence of 1 μM prostaglandin E1 (PGE1; Sigma-Aldrich) and spun (80g for 10 minutes) over a BSA gradient (2%–12% prepared in PBS). PLPs were collected in the upper layers (≥5%) and mixed with an equal volume of 1% BSA in PBS and 1 μM PGE1. After being centrifuged at 800g for 10 minutes, the sample was suspended in the culture medium and maintained at room temperature before morphologic and functional analysis.
Electron Microscopy
MKs and platelets were collected and fixed with 2.5% glutaraldehyde in PBS for 1 hour at 4°C. After washing with PBS, the samples were fixed with 1% osmium tetroxide for 1 hour on ice. After dehydration, the samples were embedded in epoxy resin. Approximately 60–80-nM ultrathin sections were stained with 2% uranyl acetate in 70% methanol and observed under a transmission electron microscope (H600; Hitachi, Tokyo, Japan, http://www.hitachi.com) operating at 80 kV.
Platelet Characterization
Peripheral blood or culture-derived platelets were plated onto poly-l-lysine (100 μg/ml)-coated coverslips at 37°C for 1 hour. After washing with PBS, the platelets were fixed with 4% PFA, permeabilized with 0.2% Triton X-100 for 15 minutes at room temperature, and blocked with 1% BSA at 37°C for 30 minutes. Next, they were incubated with mouse anti-β1-tubulin for 2 hours at 37°C. After washing, the Alexa Fluor 488-conjugated goat anti-mouse IgG was applied for 1 hour at room temperature. Fluorescent images were visualized using the confocal microscopy (LSM710, Carl Zeiss, Jena, Germany, http://www.zeiss.com). Plot profiles were generated using Velocity Software, version 4.0 (Velocity Software, Mountain View, CA, http://www.velocitysoftware.com).
Using β-tubulin staining, high-content diameter measurements of the platelets were performed in ImageJ using the linescan function. All samples were manually inspected, and platelets outside the threshold were excluded.
Adhesion and Aggregation Test of Platelets
To measure the adhesion potential of the platelets, peripheral blood or culture-derived PLPs suspended in the culture medium were plated onto 100 μg/ml fibrinogen-coated coverslips in the absence or presence of 2 U/ml thrombin at 37°C for 30 minutes. The platelets were stained with mouse anti-CD41a and Alexa Fluor 594-conjugated goat anti-mouse IgG. For F-actin components, the platelets were incubated with fluorescein isothiocyanate 488-conjugated phalloidin. Fluorescent images were visualized using a confocal laser scanning microscope.
To explore the aggregation potential of the platelets, human blood platelets (2 × 107) were mixed with 2 mg/ml Calcein AM (Invitrogen)-labeled blood platelets or PLPs (2 × 105) before the assay. The mixed platelets and PLPs were plated onto fibrinogen-coated coverslips in 24-well plates and centrifuged for 5 minutes at 80g. After washing with PBS, 300 μl of culture medium in the presence of fibrinogen (300 μg/ml), ADP (20 μM), and thrombin (0.5 U/ml) were added to each microwell. The plates were incubated at 37°C for 15 minutes with shaking. Next, the aggregates were stained and observed with phalloidin.
Statistical Analysis
Unless stated otherwise, the mean values ± SEM are presented. Statistical analysis was performed using SPSS, and significance levels were determined using Student t tests. Differences were regarded as significant when p < .05.
Results
3D Rotation Suspension Culture Potentiates Megakaryopoiesis
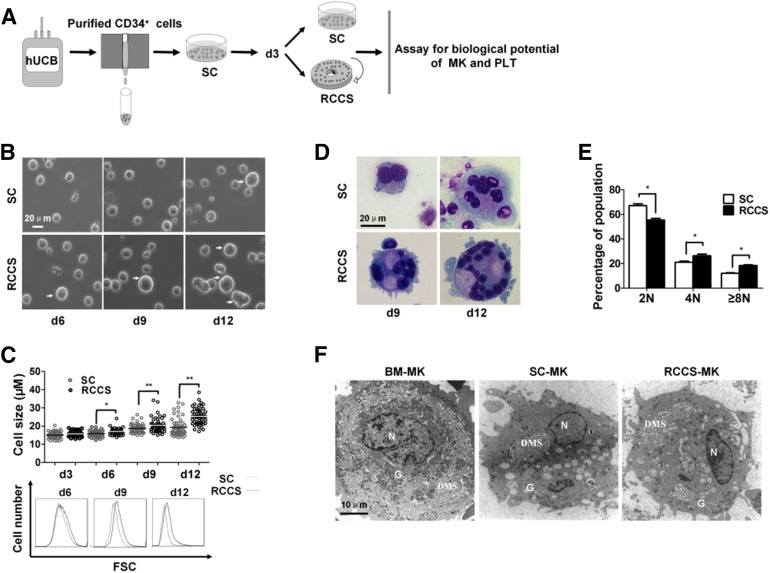
In previous studies, we have shown that the RCCS can provide a beneficial biophysical environment to enhance the proliferation of human epidermal stem cells and mesendoderm differentiation of mouse embryonic stem cells [13, 14]. Because megakaryopoiesis and thrombopoiesis occur in a 3D environment and respond favorably to stress stimulations, we hypothesized that the RCCS might exert a positive effect on those differentiation processes. To test this, we took advantage of the rotary suspension culture device described in our previous study and developed a stepwise strategy (Fig. 1A). The culture medium and cytokines were added at different time points to promote CD34+ progenitor cell proliferation or megakaryocytic differentiation and maturation, as described earlier, with modifications [2]. Hematopoietic progenitor cells cultured in the static culture (SC) condition were used in parallel as a control. Compared with the SC group, the cells in the RCCS group appeared larger on day 6, and the differences became more evident at later time points (Fig. 1B). Quantitative analysis with flow cytometry confirmed the significant increase in cells with larger sizes in the RCCS group (Fig. 1C). MGG staining indicated that the larger cells were MKs at various stages of maturation (Fig. 1D). Thin-section electron microscopy showed that culture-derived MKs were slightly smaller than bone marrow-derived MKs. However, the ultrastructure was quite similar to that of MKs derived in vivo, containing granules, a demarcation membrane system, and lobulated nuclei (Fig. 1F). We further determined the DNA content of the cells cultured under different conditions and found that more MKs with a high level of polyploidy were generated using the RCCS (MKs ≥8 N, 18.40% ± 0.50% vs. 11.92% ± 0.59%, p < .05; MKs ≥4 N, 45.13% ± 0.92% vs. 32.55% ± 1.40%, p < .05). In keeping with this, fewer diploid MKs were discovered in cultures using the RCCS (Fig. 1E; supplemental online Fig. 1). Together, the changes in morphology, cell size, and DNA content led us to conclude that the RCCS remarkably potentiates megakaryopoiesis of CD34+ progenitor cells derived from cord blood.
Figure 1.
Three-dimensional rotation suspension culture potentiates megakaryopoiesis. (A): Schematic representation of the experimental procedure from hUCB-CD34+ cells to MKs and platelets. (B): Representative cell morphology at various stages of MK lineage-specific differentiation (scale bar = 20 μm). Large MKs indicated by white arrows in both groups. (C): Size distribution of MKs formed under SC and RCCS measured by microscopy (top) and flow cytometry (bottom). (D): Morphology analysis of MKs at days 9 and 12 by May-Grünwald-Giemsa staining (scale bar = 20 μm). (E): Ploidy distribution of MKs in both groups at day 12 analyzed by staining cellular DNA with propidium iodide (mean ± SEM, n = 3). Asterisks represent significant differences between SC and RCCS groups: ∗, p < .05; ∗∗, p < .01. (F): Thin-section electron micrographs of bone marrow-derived MK (left) and culture-derived MK in SC (middle) and RCCS (right). Abbreviations: BM, bone marrow; d, day; DMS, demarcation membrane system; FSC, fetal stem cell; G, granules; hUCB, human umbilical cord blood; MK, megakaryocyte; N, nuclei; PLT, platelet; RCCS, rotary cell culture system; SC, static culture.
Rotary Suspension Culture Enhances Megakaryocyte-Associated Gene Expression
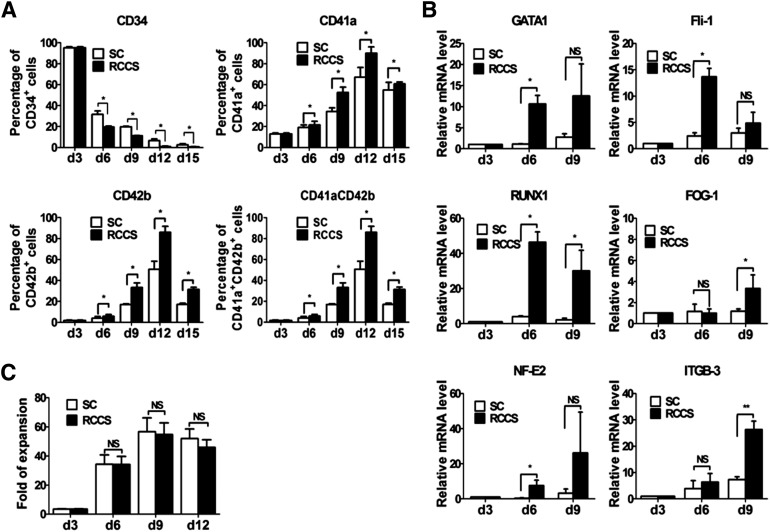
To further explore whether the RCCS could enhance megakaryopoiesis at the molecular level, we examined the dynamic expression of megakaryocyte-associated genes during cultivation. We found that the percentage of CD34+ cells decreased much faster using the RCCS than with the control (Fig. 2A), indicating that the hematopoietic progenitor cells differentiated more quickly in the rotation suspension culture than in the static condition. Many more CD41a+, CD42b+, and CD41a+CD42b+ cells had been produced with the RCCS at day 12 (CD41a+ cells, 89.97% ± 6.05% vs. 67.23% ± 9.27%, p < .05; CD42b+ cells, 85.93% ± 5.75% vs. 50.75% ± 7.59%, p < .05; CD41a+ CD42b+ cells, 85.93% ± 5.75% vs. 50.75% ± 7.59%, p < .05; Fig. 2A). In addition to the surface markers, we also measured the expression of several transcription factors associated with megakaryopoiesis with real-time PCR analysis. As expected, the levels of megakaryocytic-associated transcription factors GATA1, RUNX1, Fli1, FOG1, and NF-E2 and the surface marker CD61 (ITGB3) were much higher in the cells cultured in the RCCS throughout differentiation (Fig. 2B). These results further confirmed that the rotary suspension culture cannot only significantly accelerate the megakaryocytic differentiation process but also increase the efficiency of megakaryocytic differentiation.
Figure 2.
Rotary suspension culture promotes megakaryocytic gene expression. (A): Cell-surface marker (CD34, CD41a, and CD42b) analysis at various stages of MK differentiation culture (mean ± SEM, n = 3). (B): mRNA levels of megakaryocytic-associated transcription factors (GATA1, Fli-1, RUNX1, NF-E2, ITGB3, and glycoprotein Ib/α) was assessed by real-time polymerase chain reaction at days 3, 6, and 9 (mean ± SEM, n = 3). Glyceraldehyde-3-phosphate dehydrogenase was used as an internal control. All values were normalized to the level (= 1) of mRNA in the cells at day 3. (C): Cell counting was performed with a hemocytometer under the microscope (mean ± SEM, n = 3). Asterisks represent significant differences between SC and RCCS groups: ∗, p < .05; ∗∗, p < .01. Abbreviations: d, day; MK, megakaryocyte; RCCS, rotary cell culture system; SC, static culture.
We also questioned whether the RCCS affected cell proliferation. We observed little difference in cell growth under these two culture conditions (Fig. 2C). Furthermore, the percentage of cells positive for Ki-67, a widely used marker for cell proliferation, exhibited little difference between the two conditions (supplemental online Fig. 2A, 2B). Thus, we concluded that RCCS potentiates megakaryopoiesis without causing effects on hematopoietic progenitor cell proliferation.
Rotary Suspension Culture Enhances Thrombopoiesis
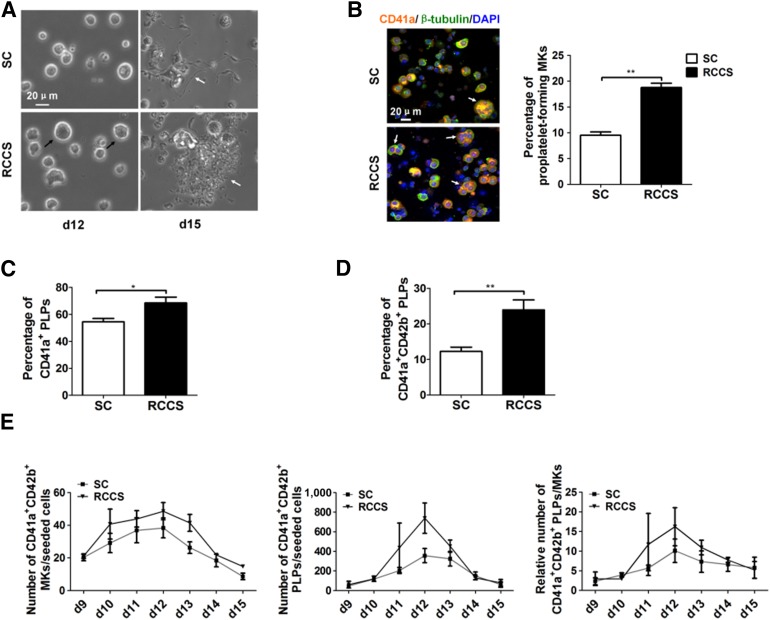
To test whether the MKs generated via the 3D rotation culture system were able to produce functional platelets, we documented the behavior of the MKs during the late stage of cultivation. Although the larger size MKs appeared in both the control and the RCCS groups at day 12, the number of pro-platelets, formed through profound rearrangement of the actin and tubulin cytoskeleton before genuine platelets can be released, was much higher in the 3D rotation culture (Fig. 3A). Furthermore, we used immunofluorescence of β-tubulin and CD41 and DAPI nuclear staining to classify the various populations of MKs. Among them, CD41 was expressed in almost all MKs. We defined the CD41 and β-tubulin-positive polyploid cells (determined by DAPI staining) as pro-platelet-forming MKs (Fig. 3B, left panel). Strikingly more pro-platelet-forming MKs were found in the rotary suspension culture (18.75% ± 0.85% vs. 9.50% ± 0.65%, p < .05; Fig. 3B, right panel). Thus, the rotary suspension culture significantly increased the number of platelet-producing cells.
Figure 3.
Rotary suspension culture enhances thrombopoiesis. (A): Representative cell morphology of large MKs (black arrows) at day 12 and pro-platelets (white arrows) at day 15 during MK differentiation (scale bar = 20 μm). (B): Immunolocalization of CD41 (orange), β-tubulin (green), and F-actin (green) in differentiated MKs was assessed by immunofluorescence staining at day 12 (scale bar = 20 μm; left). Cell nuclei were stained with DAPI (blue). Polyploid and pro-platelet-forming MKs indicated by white arrows. Quantification of polyploid and pro-platelet-forming MKs per 100 cells performed by immunofluorescence staining of CD41, β-tubulin, and DAPI (right). (C): Percentage of CD41a+ PLPs identified by flow cytometry (right; mean ± SEM; n = 3). (D): Percentage of CD41a+CD42b+ PLPs identified by flow cytometry (right; mean ± SEM; n = 3). (E): Comparative kinetics of CD41a+CD42b+ MKs (left) and platelets (middle) per stem cell and platelets per MK (right; mean ± SEM; n = 3). Asterisks represent significant differences between SC and RCCS groups: ∗, p < .05; ∗∗, p < .01. Abbreviations: d, day; DAPI, 4′,6-diamidino-2-phenylindole; MKs, megakaryocytes; PLP, platelet-like particle; RCCS, rotary cell culture system; SC, static culture.
To directly assess platelet production, we initially determined the percentages of platelet-sized particles in culture supernatant at day 12. To exclude the potential interference of cell debris in the culture, platelets from human peripheral blood were used to establish proper gating (supplemental online Fig. 3A, left top panels). Much higher percentages of CD41a+ (68.46% ± 4.32% vs. 54.39% ± 2.55%; p < .05) and CD41a+CD42b+ PLPs (23.95% ± 2.79% vs. 12.24% ± 1.23%; p < .01) were generated in the suspension culture compared with the percentage in the control (Fig. 3C, 3D). We also measured the dynamics of CD41a+CD42b+ MKs and platelets during the late stage of cultivation (supplemental online Fig. 3B) and determined the number of MKs and platelets generated from a single CD34+ progenitor cell using previously described methods [17, 18]. More mature CD41a+CD42b+ MKs were produced in the rotary suspension culture (Fig. 3E, left panel). The platelet yields in the RCCS group from days 11 to 13 were also significantly higher than those in the static condition (Fig. 3E, middle panel). Platelet generation increased gradually from day 10 and had peaked at day 12 (Fig. 3E, right panel). Together, these results have convincingly shown that many more mature MKs and more platelets were produced per MK, leading to much higher platelet yield in the RCCS.
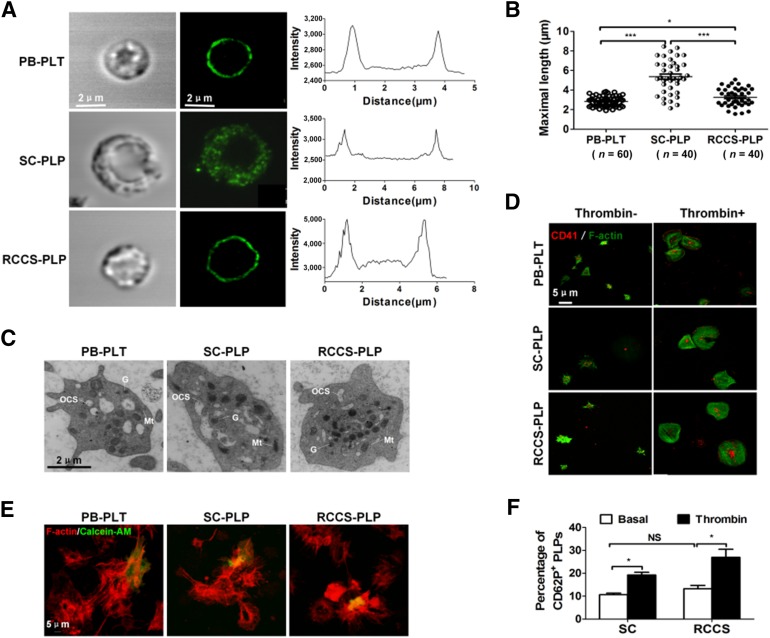
To further explore whether differences were present between the platelets generated from the RCCS and those generated in vivo, we further characterized the structure of the platelets derived from the RCCS. A typical discoid shape and a smooth contour were observed (Fig. 4A, left panel). Normal microtubule coils, which were larger than blood platelets on average, were also observed (Fig. 4A, middle and right panels). Moreover, the sizes of the platelets generated with the RCCS were more homogeneous. In contrast to the platelets generated under the static condition, they were highly reminiscent of the platelets derived in vivo (peripheral blood, 2.84 ± 0.07 μm, n = 60; SC, 5.37 ± 0.27 μm, n = 40; RCCS, 3.25 ± 0.15 μm, n = 40; Fig. 4B). A significant proportion of PLPs contained a normal distribution of the open canalicular system and α-granules, dense granules, and mitochondria, nearly indistinguishable from human blood platelets (Fig. 4C). Taken together, these analyses confirmed that platelets cultured in the RCCS displayed the ultrastructural and morphological characteristics of blood platelets.
Figure 4.
Ultrastructural and functional characterization of culture-derived platelets. (A): Light (left) and immunofluorescence (middle) micrographs of human blood and cultured platelets. Platelets were probed for β-tubulin (microtubule cytoskeleton, green). Representative line function for highlighted platelet shown (inset). Scale bar = 2 μm. (B): Distribution of the maximal longitudinal length of blood platelets and PLPs quantified on immunofluorescence micrographs. The mean sizes of the platelets are presented by a bar. ∗, p < .05; ∗∗∗, p < .001, unpaired t test with Welch’s correction. (C): Thin-section electron micrographs of blood (left) and culture-derived platelets in SC (middle) and RCCS (right). (D): CD41 (red) and F-actin filament (phalloidin A488; green) staining of blood platelets (top) or PLPs in SC (middle) and RCCS (bottom) bound to immobilized fibrinogen in the absence or presence of 1 U/ml thrombin (scale bar = 5 μm). (E): Aggregates of a mixture of 2 × 105 calcein-AM (green)-labeled blood (left) or cultured platelets (middle and right) and 2 × 107 blood platelets. In red, F-actin staining of both populations. Scale bar = 5 μm. (F): Percentage of P-selectin (CD62P)-positive events in gated SC-PLPs and RCCS-PLPs (mean ± SEM; n = 3). Asterisks represent significant differences between SC and RCCS groups: ∗, p < .05. Abbreviations: G, granules; Mt, mitochondria; OCS, open canalicular system; PB, peripheral blood; PLP, platelet-like particle; PLT, platelet; RCCS, rotary cell culture system; SC, static culture.
We also determined the function of platelets by measuring their capability of shape change and motility. We found that PLPs generated in the suspension culture were able to undergo normal shape change and to spread out as platelets from peripheral blood (Fig. 4D). Once incubated with thrombin, the platelets extended lamellipodia, as demonstrated by F-actin staining. Thus, the suspension-cultured PLPs responded properly to the αIIbβ3 signaling induced by glycoprotein Ib–IX, as previously described [19]. In addition, the capacity of PLPs to aggregate was also evaluated. F-actin staining of aggregates showed that blood and culture-derived PLPs can interact and form large aggregates with one another after stimulation by agonist (Fig. 4E). Induction of CD62P expression after agonist stimulation is another well-recognized parameter for the functional integrity of platelets. The rotary suspension culture elevated the expression of CD62P from α-granules to the surface of PLPs in response to thrombin (Fig. 4F; supplemental online Fig. 4A). Thus, the rotary suspension culture not only promotes megakaryocytic differentiation and maturation but also augments the production of viable and functional intact platelets. Because this device is cost-effective and is easy to manipulate compared with previously developed devices, it is highly suitable for large-scale in vitro platelet generation under clinical settings.
Pilot Screening to Identify Factors Improving Platelet Generation
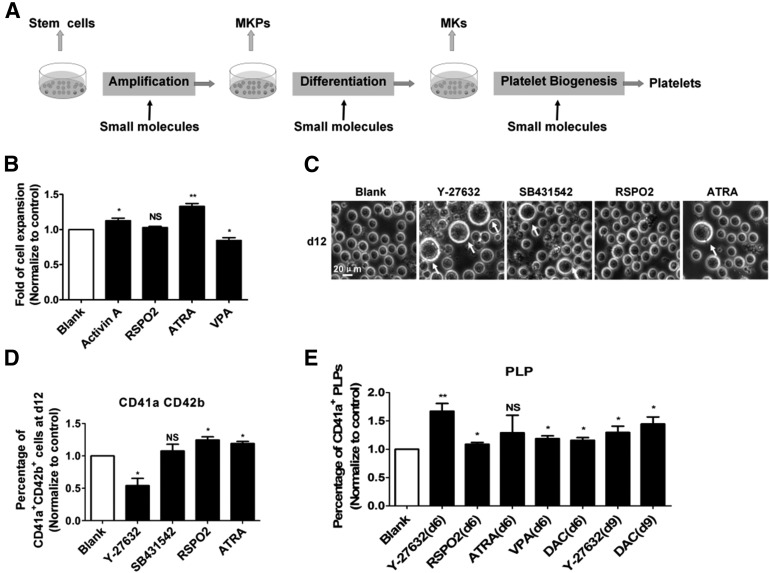
Chemical approaches are extremely powerful for modulating cell fate and have been widely applied in regenerative medicine [20, 21]. To identify the chemical compounds and/or soluble factors that can be used to further augment platelet generation from HPCs in combination with the rotary suspension culture system, we conducted a small-scale screen of compounds and growth factors/cytokines (supplemental online Table 1) during various stages, including HPC proliferation, MK differentiation, and platelet generation/release. Some of the molecules were chosen because of their known effects on megakaryocytic cells [22–26]. To assess the stage-specific effects, the compounds and factors were provided at the stage of HPC proliferation (days 3–6), megakaryocytic differentiation (days 6–9), or platelet generation/release (days 9–12; Fig. 5A).
Figure 5.
Stage-specific pilot screening for factors to increase PLP generation. (A): Schematic illustration of stepwise screening of extrinsic factors during MK differentiation. The complete list of factors examined is shown in supplemental online Table 1. (B): Fold of cell expansion at day 6 counted by hemocytometer with addition of extrinsic factors at day 3 (mean ± SEM; n = 4). All values were normalized to the level (= 1) of blank. Student t tests were used to compare data between cells treated with extrinsic factors versus control cells. (C): Morphology of large MKs (white arrows) at day 12 among every group (scale bar = 20 μm). Chemical compounds and growth factors/cytokines were added from day 6. (D): Cell-surface marker (CD41a and CD42b) analysis at day 12 of MK differentiation culture among every group (mean ± SEM; n = 5). All values were normalized to the level (= 1) of blank. Student t tests were used to compare data between cells treated with extrinsic factors versus control cells. (E): Quantification of CD41a+ PLPs among every group identified by flow cytometry (mean ± SEM; n = 6). Extrinsic factors were added from day 6 or day 9. All values were normalized to the level (= 1) of blank. Student t tests were used to compare data between cells treated with extrinsic factors versus control cells. Asterisks represent significant differences between SC and RCCS groups: ∗, p < .05; ∗∗, p < .01. Abbreviations: ATRA, all trans retinoic acid; d, day; DAC, decitabine; MKP, megakaryocyte progenitor; MKs, megakaryocytes; PLP, platelet-like particle; RSPO2, R-spondin2; VPA, valproic acid.
We found that among all chemical compounds and growth factors/cytokines tested, activin A and all trans retinoic acid (ATRA) consistently enhanced the proliferation of HPCs (1.15 ± 0.08 fold for activin A, p < .05; 1.35 ± 0.13 fold for ATRA, p < .01; Fig. 5B). In contrast, valproic acid (VPA) did not enhance but instead suppressed HPC proliferation (0.87 ± 0.06 fold, p < .05; Fig. 4B). All the other factors tested exerted minimal effects on cell expansion (Fig. 5B; supplemental online Fig. 5A). When applied on day 6, Y-27632 significantly increased the cell size, as described previously [25, 26], confirming the reliability of our strategy. In addition to Y-27632, we found that two chemical compounds, SB431542 and ATRA, were able to increase the cell size (Fig. 5C). The percentage of CD41a+CD42b+ cells was higher after treatment with RSPO2 or ATRA at day 12 (Fig. 5D). Although RSPO2 treatment increased the expression of the megakaryocyte-associated surface markers, it had little effect on cell size (Fig. 5C). The MKs produced via Y-27632 treatment showed giant cell bodies, but the percentage of CD41a+CD42b+ cells was actually lower than that in the control group (Fig. 5D). We speculated that although the MKs induced with Y-27632 treatment exhibited giant and hyperploid nuclei (Fig. 5C), the morphological changes caused by endomitosis might not necessarily lead to stable MK differentiation.
In addition to HPC expansion and megakaryocytic differentiation, we also tested whether those chemical compounds and growth factors/cytokines affected platelet generation/release. PLPs were collected and analyzed at day 14, after the cells had been treated with chemical compounds or growth factors added at day 6 or day 9. Flow cytometry analysis revealed that both RSPO2 and VPA improved the generation of CD41a+ PLPs when added at day 6 (Fig. 5E). As expected, Y-27632 treatment also improved platelet generation. In contrast, no effect was detected with ATRA (Fig. 5E), and decitabine (DAC) at high concentrations (>0.1 μM) caused severe cell death (data not shown). We next measured the effect of treatment at day 9, using Y-27632 as a control. We found that both DAC and VPA improved the generation of platelets (Fig. 5E). Consistently, we also observed more pro-platelet formation after RSPO2, DAC, and VPA treatment (supplemental online Fig. 5C). In particular, RSPO2 not only enhanced megakaryocytic differentiation, but also increased platelet generation/release at the late stage. By exploiting the strategy of stage-specific screening, we identified chemical compounds and growth factors capable of augmenting HPC expansion, megakaryocytic differentiation, and platelet generation/release (supplemental online Fig. 5D).
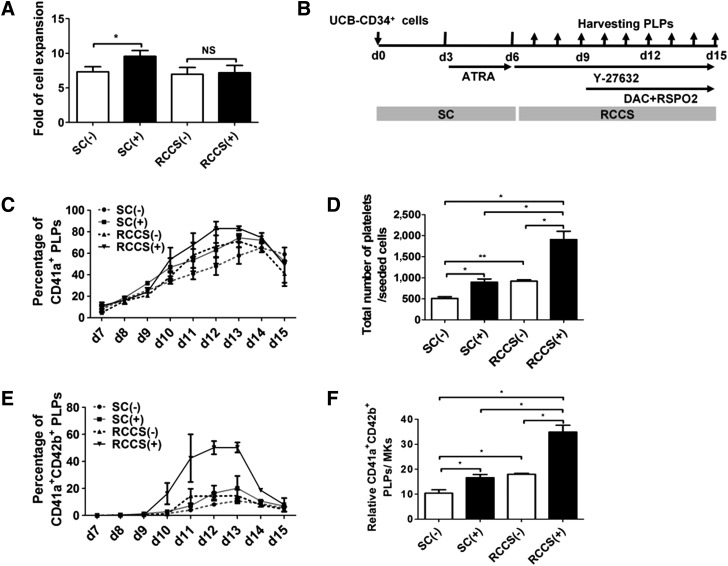
Stepwise Strategy for Highly Efficient Generation of Functional Platelets
We next asked whether the factors screened out could further enhance platelet generation in conjunction with the 3D rotary culture system, leading to an improved method for high-efficiency and large-scale generation of platelets in vitro. We first tested whether ATRA was able to enhance HPC proliferation in rotary suspension culture. Surprisingly, ATRA failed to enhance cell proliferation in the rotary suspension culture (Fig. 6A). As described, no difference was observed in static or rotary suspension culture (Fig. 2C; supplemental online Fig. 2). We thus inferred that the major contribution from this rotary suspension culture might be initiating and/or enhancing the megakaryocyte differentiation/maturation or platelet generation/release without exerting an effect on cell proliferation. Thus, we began to apply the suspension culture only from day 6, and HPC expansion was still conducted under the static culture condition with the addition of ATRA up to day 6, when the rotary suspension culture was initiated (Fig. 6B). Y-27632, DAC, and RSPO2 were added to the culture at day 6 or day 9 to maximize generation according to our previous screening findings (Fig. 6B). As a control, we also included the same chemical compounds and growth factors in the static culture. To monitor the dynamics of thrombopoiesis, PLPs were collected daily from days 7 to 15. We found that CD41a+ PLPs could be detected as early as day 7. However, a drastic upregulation only occurred from day 11 and had generally peaked at day 13 (Fig. 6C). Although more PLPs were produced in both the suspension and the static culture after the addition of chemical compounds and growth factors, more PLPs were produced, as expected, in the suspension culture than the static condition, with or without the addition of chemical compounds. The highest yield of CD41a+ PLPs was produced in the rotary suspension culture using the stepwise strategy (Fig. 6B). Furthermore, we also measured the percentage of CD41a+CD42b+ double-positive PLPs, which have been recognized as a more stringent standard for functional platelets than CD41a alone [18]. A much higher percentage of CD41a+CD42b+ platelets was generated with the addition of chemical compounds and growth factors (Fig. 6D). Consistently, the rotary suspension culture condition generated the highest percentage. Interestingly, the critical window for generating CD42b+ platelets was from days 10 to 14, and a minimal amount of CD41a+CD42b+ platelets was produced after day 14. This was different from the production of CD41a+ single-positive platelets (Fig. 6C, 6D). These results indicated that subtle temporal windows exist for the generation of platelets of the highest quantity and quality. The underlying mechanism remains to be determined. Consistent with previous findings [18], the platelets with the highest quality (i.e., positive for CD41a+/CD42b+ expression) had the greatest potential to induce α-granule release after thrombin stimulation (supplemental online Fig. 6A). Thus, to further measure the efficiency of platelet generation in our system, we quantified the total amount of platelets generated from single CD34+ cells and found that our method produced ∼1,907.67 ± 196.90 platelets per CD34+ cell (Fig. 6E), which was substantially higher than that with previously described methods (∼40–180) [18]. Furthermore, with the combination of the rotary suspension culture and chemical addition, both megakaryocytic differentiation efficiency and platelet generation were enhanced (Fig. 6F; supplemental online Fig. 6B) and thus together resulted in an increase in total platelet yield. Interestingly, with the addition of chemical compounds, the rotary suspension culture itself had a minimal effect on megakaryocytic differentiation compared with the static condition (55.79 ± 0.90 vs. 53.23 ± 1.52, p = .24; supplemental online Fig. 6B). This indicates that compensation might exist between chemical compound treatment and rotary suspension cultivation in promoting megakaryocyte differentiation. However, the detailed mechanism remains to be determined.
Figure 6.
Stepwise strategy for highly efficient generation of functional platelets. (A): Fold of cell expansion at day 6 counted by hemocytometer with addition of ATRA at day 3 (mean ± SEM; n = 3). Student t tests were used to compare data between cells treated with small molecules versus control cells. (B): Schematic illustration of stepwise differentiation under biochemical and biophysical stimulus. (C): Kinetics of CD41a+ platelet production monitored by flow cytometry. (D): Kinetics of CD41a+CD42b+ platelet production monitored by flow cytometry. (E): Total yield of functional platelets (CD41a+CD42b+) among each group, calculated as described previously [16] (mean ± SEM; n = 3). (F): Quantitative results of platelet production per mature MK (CD41a+CD42b+) in each group (mean ± SEM; n = 3). Paired t tests were used to compare data between treatment and control groups. Asterisks represent significant differences among groups: ∗, p < .05; ∗∗, p < .01. Abbreviations: ATRA, all trans retinoic acid; d, day; DAC, decitabine; MKs, megakaryocytes; PLPs, platelet-like particles; RCCS(−), rotation suspension culture without addition of extrinsic factors; RCCS(+), rotation suspension culture with addition of extrinsic factors; RSPO2, R-spondin2; SC(−), static culture without addition of extrinsic factors; SC(+), static culture with addition of extrinsic factors; UCB, umbilical cord blood.
Together, our results provide convincing evidence that the combination of RCCS and biochemical factors significantly promotes platelet production from CB-derived MKs. This strategy might be highly promising for future large-scale platelet generation in vitro.
Discussion
In the present study, we have successfully applied the rotary suspension culture system to the derivation of platelets in vitro, resulting in dramatic improvement in megakaryocytic differentiation. More importantly, when used in combination with chemical compounds and growth factors identified by us, the rotary culture system significantly increased the yield of platelet generation in vitro—much higher than with the previously developed methods [16, 18]. Thus, the 3D device and the strategy we have developed in the present study should markedly improve the generation of large-scale platelets for future biomedical and clinical settings.
Why can the 3D rotary suspension culture significantly augment both megakaryopoiesis and thrombopoiesis compared with the static culture? The rotary suspension culture has been shown to be able to generate shear stress and simulate microgravity in our and other studies [13, 14, 27, 28]. We speculated that the shear stress generated in this system facilitates both platelet generation and release, as described previously, both in the bone marrow [15] and under other in vitro conditions [6–9]. However, the underlying mechanism underlying shear stress induction of pro-platelet generation and platelet release remains to be identified. In addition to shear stress, the microgravity produced in this system might also play an important role in promoting platelet generation. The microgravity generated by the rotary culture system has been described in our previous study and other studies [13, 14, 29]. Meyers et al. has demonstrated that modeled microgravity by the RCCS can suppress RhoA activity during human mesenchymal stem cell differentiation toward osteoblasts [30]. Inhibition of the ρ/ROCK pathway with Y-27632 has been shown to facilitate platelet generation by inhibiting cytokinesis and consequently promoting megakaryocyte polyploidization [25, 26]. Thus, the rotary suspension culture might also promote platelet generation by suppressing RhoA via microgravity. Furthermore, in addition to the shear force and simulated microgravity, the better diffusion of nutrients and oxygen in the rotary suspension culture than in the traditional static culture might also facilitate cell survival, differentiation, and platelet generation and release. We found that expression of pro-survival genes, such as BCL-XL and MCL-1, is much higher in the suspension culture than in the static culture (data not shown).
Chemical compounds have become powerful tools for modulating stem cell self-renewal, differentiation, and cellular reprogramming [20, 21]. In our study, we also applied chemical compounds to the rotary suspension culture system to induce maximal platelet production. By using a small-scale screen, we found that ATRA, Y-27632, RSPO2, and DAC can synergistically induce the highest efficiency of functional platelet derivation. The major role of ATRA appears to be to promote cell proliferation, consistent with the previously reported function in promoting the growth of colony-forming unit-megakaryocyte [31]. Y-27632, a ρ/ROCK pathway inhibitor, has been shown to enhance megakaryocytic polyploidization [25, 26]. In our system, the ROCK inhibitor might cooperate synergistically with the simulated microgravity generated by the rotary suspension culture, thus further suppressing the ρ/ROCK pathway and achieving better efficiency than Y-27632 alone. In addition to the ρ/ROCK pathway, canonical Wnt signaling is essential for megakaryocyte proliferation and maturation [32, 33]. Activation of canonical Wnt signaling profoundly stimulates pro-platelet formation [33]. We thus infer that the secreted activator of Wnt/β-catenin pathway RSPO2 might exert its effects in our system by activating the Wnt signaling pathway [34]. The addition of RSPO2 also enhanced the production of CD41a+CD42b+ MKs; however, the underlying mechanism needs to be explored further. We also included DAC in our system, because it has been shown to increase platelet generation both in vitro and in vivo [24, 35]. By using chemical compounds, growth factors, and the rotary suspension culture, we have established a stepwise strategy to generate the highest platelet yield in vitro. This suspension culture leads to ∼3.7-fold more total platelets and, most importantly, many more CD41a+CD42b+ functional platelets than with the static condition. To date, effective strategies for platelet generation in vitro are still lacking, and, as such, a detailed comparison between different strategies was not possible. Nevertheless, our method produced ∼1,907.67 ± 196.90 PLPs, more than 10-fold higher than in a previously described study (40–180 PLPs generated from one CD34+ cell) [18].
hPSCs have theoretically unlimited self-renewal capabilities and provide a potentially inexhaustible source for generating various functional human cell types. Recently, successful derivation of large-scale platelets from hPSCs has been reported by several groups [36, 37]. Liu et al. reported efficient generation of megakaryocytes by using Food and Drug Administration-approved pharmacological reagents [38]. Thus, it will be interesting to investigate in the future whether large-scale, clinical-grade platelets can be generated from hPSCs with the use of the rotary suspension culture system.
Supplementary Material
Acknowledgments
This work was supported by National Basic Research Program of China (Grants 2012CB966403 and 2015CB964902 to J.Z., Grant 2011CB710905 to E.D.), Chinese National Natural Science Foundation (Grant 31171431 to J.Z., Grant 31471291 to L.S.), Basic Scientific Research Funds in National Nonprofit Institutes (Grant 744 to L.S.). We thank Wanzhu Yang and Wenying Yu for their technical support.
Author Contributions
Y.Y. and C.L.: collection and assembly of data, manuscript writing; X.L.: collection and assembly of data, device contribution; H.W., P.S., and Y.R.: collection and assembly of data; X.R., E.D., S.F., M.H., and Y.X.: final approval of manuscript, provision of study material or patients; L.S., E.J., and J.Z.: conception and design, data analysis and interpretation, manuscript writing.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Williamson LM, Devine DV. Challenges in the management of the blood supply. Lancet. 2013;381:1866–1875. doi: 10.1016/S0140-6736(13)60631-5. [DOI] [PubMed] [Google Scholar]
- 2.Reems JA. A journey to produce platelets in vitro. Transfusion. 2011;51(suppl 4):169S–176S. doi: 10.1111/j.1537-2995.2011.03380.x. [DOI] [PubMed] [Google Scholar]
- 3.Zheng C, Yang R, Han Z, et al. TPO-independent megakaryocytopoiesis. Crit Rev Oncol Hematol. 2008;65:212–222. doi: 10.1016/j.critrevonc.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Teramura M, Kobayashi S, Yoshinaga K, et al. Effect of interleukin 11 on normal and pathological thrombopoiesis. Cancer Chemother Pharmacol. 1996;38(suppl):S99–S102. doi: 10.1007/s002800051048. [DOI] [PubMed] [Google Scholar]
- 5.Xu Y, Wang S, Shen M, et al. hGH promotes megakaryocyte differentiation and exerts a complementary effect with c-Mpl ligands on thrombopoiesis. Blood. 2014;123:2250–2260. doi: 10.1182/blood-2013-09-525402. [DOI] [PubMed] [Google Scholar]
- 6.Dunois-Lardé C, Capron C, Fichelson S, et al. Exposure of human megakaryocytes to high shear rates accelerates platelet production. Blood. 2009;114:1875–1883. doi: 10.1182/blood-2009-03-209205. [DOI] [PubMed] [Google Scholar]
- 7.Sullenbarger B, Bahng JH, Gruner R, et al. Prolonged continuous in vitro human platelet production using three-dimensional scaffolds. Exp Hematol. 2009;37:101–110. doi: 10.1016/j.exphem.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pallotta I, Lovett M, Kaplan DL, et al. Three-dimensional system for the in vitro study of megakaryocytes and functional platelet production using silk-based vascular tubes. Tissue Eng Part C Methods. 2011;17:1223–1232. doi: 10.1089/ten.tec.2011.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakagawa Y, Nakamura S, Nakajima M, et al. Two differential flows in a bioreactor promoted platelet generation from human pluripotent stem cell-derived megakaryocytes. Exp Hematol. 2013;41:742–748. doi: 10.1016/j.exphem.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Li S, Ma Z, Niu Z, et al. NASA-approved rotary bioreactor enhances proliferation and osteogenesis of human periodontal ligament stem cells. Stem Cells Dev. 2009;18:1273–1282. doi: 10.1089/scd.2008.0371. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Zhang Y, Zhang S, et al. Rotating microgravity-bioreactor cultivation enhances the hepatic differentiation of mouse embryonic stem cells on biodegradable polymer scaffolds. Tissue Eng Part A. 2012;18:2376–2385. doi: 10.1089/ten.TEA.2012.0097. [DOI] [PubMed] [Google Scholar]
- 12.Marsano A, Wendt D, Raiteri R, et al. Use of hydrodynamic forces to engineer cartilaginous tissues resembling the non-uniform structure and function of meniscus. Biomaterials. 2006;27:5927–5934. doi: 10.1016/j.biomaterials.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 13.Lei XH, Ning LN, Cao YJ, et al. NASA-approved rotary bioreactor enhances proliferation of human epidermal stem cells and supports formation of 3D epidermis-like structure. PLoS One. 2011;6:e26603. doi: 10.1371/journal.pone.0026603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lei X, Deng Z, Zhang H, et al. Rotary suspension culture enhances mesendoderm differentiation of embryonic stem cells through modulation of Wnt/β-catenin pathway. Stem Cell Rev. 2014;10:526–538. doi: 10.1007/s12015-014-9511-6. [DOI] [PubMed] [Google Scholar]
- 15.Junt T, Schulze H, Chen Z, et al. Dynamic visualization of thrombopoiesis within bone marrow. Science. 2007;317:1767–1770. doi: 10.1126/science.1146304. [DOI] [PubMed] [Google Scholar]
- 16.Boyer L, Robert A, Proulx C, et al. Increased production of megakaryocytes near purity from cord blood CD34+ cells using a short two-phase culture system. J Immunol Methods. 2008;332:82–91. doi: 10.1016/j.jim.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 17.Robert A, Cortin V, Garnier A, et al. Megakaryocyte and platelet production from human cord blood stem cells. Methods Mol Biol. 2012;788:219–247. doi: 10.1007/978-1-61779-307-3_16. [DOI] [PubMed] [Google Scholar]
- 18.Robert A, Boyer L, Pineault N. Glycoprotein Ibα receptor instability is associated with loss of quality in platelets produced in culture. Stem Cells Dev. 2011;20:379–390. doi: 10.1089/scd.2010.0041. [DOI] [PubMed] [Google Scholar]
- 19.Li Z, Xi X, Du X. A mitogen-activated protein kinase-dependent signaling pathway in the activation of platelet integrin α IIbbeta3. J Biol Chem. 2001;276:42226–42232. doi: 10.1074/jbc.M106129200. [DOI] [PubMed] [Google Scholar]
- 20.Yu C, Liu K, Tang S, et al. Chemical approaches to cell reprogramming. Curr Opin Genet Dev. 2014;28:50–56. doi: 10.1016/j.gde.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griffin MF, Butler PE, Seifalian AM, et al. Control of stem cell fate by engineering their micro and nanoenvironment. World J Stem Cells. 2015;7:37–50. doi: 10.4252/wjsc.v7.i1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishimura M, Kaku K, Azuno Y, et al. Effect of erythroid differentiation factor on megakaryocytic differentiation of L8057, a murine megakaryoblastic leukemia cell line. Biochem Biophys Res Commun. 1991;181:1042–1047. doi: 10.1016/0006-291x(91)92042-i. [DOI] [PubMed] [Google Scholar]
- 23.Schweinfurth N, Hohmann S, Deuschle M, et al. Valproic acid and all trans retinoic acid differentially induce megakaryopoiesis and platelet-like particle formation from the megakaryoblastic cell line MEG-01. Platelets. 2010;21:648–657. doi: 10.3109/09537104.2010.513748. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Yi Z, Wang S, et al. The effect of decitabine on megakaryocyte maturation and platelet release. Thromb Haemost. 2011;106:337–343. doi: 10.1160/TH10-11-0744. [DOI] [PubMed] [Google Scholar]
- 25.Avanzi MP, Chen A, He W, et al. Optimizing megakaryocyte polyploidization by targeting multiple pathways of cytokinesis. Transfusion. 2012;52:2406–2413. doi: 10.1111/j.1537-2995.2012.03711.x. [DOI] [PubMed] [Google Scholar]
- 26.Avanzi MP, Goldberg F, Davila J, et al. Rho kinase inhibition drives megakaryocyte polyploidization and proplatelet formation through MYC and NFE2 downregulation. Br J Haematol. 2014;164:867–876. doi: 10.1111/bjh.12709. [DOI] [PubMed] [Google Scholar]
- 27.Mitteregger R, Vogt G, Rossmanith E, et al. Rotary cell culture system (RCCS): A new method for cultivating hepatocytes on microcarriers. Int J Artif Organs. 1999;22:816–822. [PubMed] [Google Scholar]
- 28.Morabito C, Steimberg N, Mazzoleni G, et al. RCCS bioreactor-based modelled microgravity induces significant changes on in vitro 3D neuroglial cell cultures. Biomed Res Int. 2015;2015:754283. doi: 10.1155/2015/754283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Navran S. The application of low shear modeled microgravity to 3-D cell biology and tissue engineering. Biotechnol Annu Rev. 2008;14:275–296. doi: 10.1016/S1387-2656(08)00011-2. [DOI] [PubMed] [Google Scholar]
- 30.Meyers VE, Zayzafoon M, Douglas JT, et al. RhoA and cytoskeletal disruption mediate reduced osteoblastogenesis and enhanced adipogenesis of human mesenchymal stem cells in modeled microgravity. J Bone Miner Res. 2005;20:1858–1866. doi: 10.1359/JBMR.050611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Visani G, Ottaviani E, Zauli G, et al. All-trans retinoic acid at low concentration directly stimulates normal adult megakaryocytopoiesis in the presence of thrombopoietin or combined cytokines. Eur J Haematol. 1999;63:149–153. doi: 10.1111/j.1600-0609.1999.tb01762.x. [DOI] [PubMed] [Google Scholar]
- 32.Paluru P, Hudock KM, Cheng X, et al. The negative impact of Wnt signaling on megakaryocyte and primitive erythroid progenitors derived from human embryonic stem cells. Stem Cell Res (Amst) 2014;12:441–451. doi: 10.1016/j.scr.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Macaulay IC, Thon JN, Tijssen MR, et al. Canonical Wnt signaling in megakaryocytes regulates proplatelet formation. Blood. 2013;121:188–196. doi: 10.1182/blood-2012-03-416875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kazanskaya O, Glinka A, del Barco Barrantes I, et al. R-Spondin2 is a secreted activator of Wnt/β-catenin signaling and is required for Xenopus myogenesis. Dev Cell. 2004;7:525–534. doi: 10.1016/j.devcel.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 35.Zhou H, Hou Y, Liu X, et al. Low-dose decitabine promotes megakaryocyte maturation and platelet production in healthy controls and immune thrombocytopenia. Thromb Haemost. 2015;113:1021–1034. doi: 10.1160/TH14-04-0342. [DOI] [PubMed] [Google Scholar]
- 36.Feng Q, Shabrani N, Thon JN, et al. Scalable generation of universal platelets from human induced pluripotent stem cells. Stem Cell Rep. 2014;3:817–831. doi: 10.1016/j.stemcr.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakamura S, Takayama N, Hirata S, et al. Expandable megakaryocyte cell lines enable clinically applicable generation of platelets from human induced pluripotent stem cells. Cell Stem Cell. 2014;14:535–548. doi: 10.1016/j.stem.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 38.Liu Y, Wang Y, Gao Y, et al. Efficient generation of megakaryocytes from human induced pluripotent stem cells using food and drug administration-approved pharmacological reagents. Stem Cells Translational Medicine. 2015;4:309–319. doi: 10.5966/sctm.2014-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.