Adipose-derived stem cells (AdSCs) have recently been shown to differentiate into cardiovascular lineage cells. The cell density and proliferation activity of cardiac-derived AdSCs were significantly increased compared with the other adipose tissue-derived AdSCs. Cardiac adipose tissue could be an ideal source for isolation of therapeutically effective AdSCs for cardiac regeneration in ischemic heart diseases.
Keywords: Cardiac, Differentiation, Adipose stem cells, Stem cell transplantation
Abstract
Adipose-derived stem cells (AdSCs) have recently been shown to differentiate into cardiovascular lineage cells. However, little is known about the fat tissue origin-dependent differences in AdSC function and differentiation potential. AdSC-rich cells were isolated from subcutaneous, visceral, cardiac (CA), and subscapular adipose tissue from mice and their characteristics analyzed. After four different AdSC types were cultured with specific differentiation medium, immunocytochemical analysis was performed for the assessment of differentiation into cardiovascular cells. We then examined the in vitro differentiation capacity and therapeutic potential of AdSCs in ischemic myocardium using a mouse myocardial infarction model. The cell density and proliferation activity of CA-derived AdSCs were significantly increased compared with the other adipose tissue-derived AdSCs. Immunocytochemistry showed that CA-derived AdSCs had the highest appearance rates of markers for endothelial cells, vascular smooth muscle cells, and cardiomyocytes among the AdSCs. Systemic transfusion of CA-derived AdSCs exhibited the highest cardiac functional recovery after myocardial infarction and the high frequency of the recruitment to ischemic myocardium. Moreover, long-term follow-up of the recruited CA-derived AdSCs frequently expressed cardiovascular cell markers compared with the other adipose tissue-derived AdSCs. Cardiac adipose tissue could be an ideal source for isolation of therapeutically effective AdSCs for cardiac regeneration in ischemic heart diseases.
Significance
The present study found that cardiac adipose-derived stem cells have a high potential to differentiate into cardiovascular lineage cells (i.e., cardiomyocytes, endothelial cells, and vascular smooth muscle cells) compared with stem cells derived from other adipose tissue such as subcutaneous, visceral, and subscapular adipose tissue. Notably, only a small number of supracardiac adipose-derived stem cells that were systemically transplanted sufficiently improved cardiac functional recovery after myocardial infarction, differentiating into cardiovascular cells in the ischemic myocardium. These findings suggest a new autologous stem cell therapy for patients with myocardial ischemia, especially those with secondary myocardial ischemia after cardiovascular open chest surgery.
Introduction
Heart failure resulting from ischemic heart disease is one of the leading causes of hospitalization and reduced quality of life for patients worldwide [1]. Although advances in the treatment of patients with myocardial infarction (MI), such as the development of devices for catheterization, including drug-eluting stents, have significantly improved the outcomes, a need for novel therapies remains to reduce mortality further and prevent the deleterious consequences that result from myocardial damage. At present, the only effective treatment for advanced heart failure that can fully restore cardiac function is heart transplantation. However, only a limited number of patients will be able to receive the therapy owing to the difficulty of finding appropriate donors and/or legal restrictions in each country. Recently, numerous investigators have evaluated the application of stem/progenitor cells for the treatment of ischemic heart diseases, including MI, in both animal experiments and human clinical trials, and favorable outcomes have been demonstrated. The major stem/progenitor cell sources for human autologous cell transplantation therapy have been reported to be peripheral blood [2–4] and bone marrow [4–7] in ongoing clinical trials for ischemic heart diseases. However, each of the currently used adult stem cell reservoirs has practical limitations that obstruct their widespread use in treating human disease owing to the requirement of invasive harvesting procedures or ex vivo manipulation/expansion techniques.
Adipose tissue consists of mature adipocytes and a mononuclear cell fraction termed stromal vascular fraction (SVF). The SVF is a diverse mixture of cells, including endothelial cells (ECs), vascular smooth muscle cells (VSMCs), blood cells, and mesenchymal stem cells (MSCs), that is identical to adipose tissue-derived stem cells (AdSCs). AdSCs have phenotypic and functional properties (i.e., multilineage differentiation potential) similar to those of bone marrow-derived MSCs. Of particular relevance, AdSCs have been reported to differentiate, not only into adipocytes [8], osteoblasts [8, 9], chondrocytes [10, 11], pancreatic β-cells [12], hepatocytes [13], neural cells [14, 15], and myocytes [8], but also into cardiovascular lineage cells, such as cardiomyocytes (CMs) [16], ECs [9, 17], and VSMCs [18]. Most importantly for their clinical application, AdSC-enriched SVFs can be isolated in large quantities by minimally invasive liposuction, with a significantly higher yield of progenitor cells per volume compared with bone marrow. The SVF of adipose tissue has the potential to improve cardiac function after MI by several mechanisms: delivery of cardiovascular cell replacement, salvage of host cardiomyocytes through antiapoptotic mechanisms, or stimulation of angiogenesis. Similar to bone marrow-derived MSCs, AdSC-enriched SVFs secrete a number of paracrine factors that are proangiogenic [19], anti-inflammatory [20, 21], and chemoattractive for stem/progenitor cells [19] and demonstrate their beneficial effects on jeopardized myocardium by ischemic insult. Early clinical trials of autologous AdSC-enriched SVF transplantation therapy for MI patients are now ongoing in Europe and have shown significant improvement in cardiac function [22]. However, the favorable effect of transplanted SVF has been attributed to the paracrine effect rather than a direct contribution of AdSCs to tissue regeneration via transdifferentiation into cardiovascular lineage cells, as we reported previously [19]. To use AdSCs as a tool for myocardial tissue regeneration therapy, the frequency of AdSC transdifferentiation into cardiovascular cells should be increased either by manipulation of the inducible culture method or selection of appropriate adipose tissue to isolate AdSCs that include a cell population with a high differentiation potential for cardiovascular cells.
In clinical settings, autologous AdSC-enriched SVF transplantation has already been performed in mammoplasty after mastectomy to treat breast cancer [23, 24] and in breast implants in plastic surgery [25], with favorable outcomes. Because of the simple surgical procedure, subcutaneous adipose tissue collected by liposuction has been frequently used for AdSC isolation in most cases. However, no investigation has proved that, of the different organ-derived adipose tissues, subcutaneous adipose tissue is the best source of AdSCs for the treatment of cardiovascular diseases. We therefore tested the hypothesis that AdSCs derived from cardiac adipose tissue might exhibit high transdifferentiation potential to cardiovascular lineage cells compared with a variety of organ-derived adipose tissues. In the present study, mouse AdSCs were isolated from four different adipose tissues of subcutaneous, visceral, subscapular, and cardiac fat tissues and their stem/progenitor characteristics as a source of cardiovascular tissue regeneration in vitro and in vivo were examined.
Materials and Methods
Adipose Tissue Harvesting and AdSC Isolation
The institutional animal care and use committee of Osaka Medical College approved all the following research protocols (approval ID, 22030), including surgical procedures and animal care. C57BL/6N (Shimizu Laboratory Supplies, Kyoto, Japan, http://www.shimizu-ls.co.jp) and B6.129S7-Gt(ROSA)26Sor/J (The Jackson Laboratory, Bar Harbor, ME, http://www.jax.com) male mice (aged 16–20 weeks) were sacrificed under anesthesia with pentobarbital (200 mg/kg i.p.). Adipose tissue was harvested from inguinal, abdominal, supracardiac (para-aortic root), and subscapular regions and used in all experiments as subcutaneous white adipose tissue (SC), visceral white adipose tissue (VL), cardiac brown adipose tissue (CA), and subscapular brown adipose tissue (SS), respectively.
AdSCs were isolated from each adipose tissue as previously described with minor modifications [26]. In brief, adipose tissue was washed in phosphate-buffered saline (PBS) and minced, followed by digestion in 5 ml of type I collagenase (1 mg/ml in 1% bovine serum albumin [BSA]/Hanks’ balanced saline solution; Life Technologies Japan, Tokyo, Japan, http://www.lifetechnologies.com) for 40 minutes at 37°C using a gentleMACS Dissociator (Miltenyi Biotec K.K., Tokyo, Japan, http://www.miltenyibiotec.com) according to the manufacturer’s instructions. The digested tissue was filtered through a 40-μm cell strainer (BD Falcon, Tokyo, Japan, http://www.bdbiosciences.com) and centrifuged at 450g for 10 minutes. The supernatant containing adipocytes and debris was discarded. Pelleted cells were suspended with 5 mmol/l EDTA/PBS and layered over an equal volume of 1.083 g/ml Histopaque 1083 solution (Sigma-Aldrich Japan K.K., Tokyo, Japan, http://www.sigmaaldrich.com). After centrifugation at 900g for 30 minutes, mononuclear cells (MNCs) were collected from the gradient interface, and the number of trypan blue-unstained cells sized 5–30 µm was measured by a conventional cytometer (LUNA; Logos Biosystems, Inc., Annandale, VA). The MNCs were used as a freshly isolated AdSC-containing SVF for the experiments. Because the number of MNCs varies depending on the tissue volume, the density of MNCs in each adipose tissue was calculated by dividing the absolute number of MNCs by the weight of the tissues, and the AdSC-rich cellularity was assessed.
AdSC Culture for Differentiation to Cardiovascular Cells
Fleshly isolated AdSCs were cultured in 10% fetal bovine serum (FBS)/Dulbecco’s modified Eagle’s medium (DMEM)-F12 containing antibiotics on plastic dishes at a density of 104/cm2 under conditions of 5% CO2 and 37°C. After 7 days in culture, adherent cells (AdSCs) were harvested by trypsinization for 5 minutes at 37°C and pipetting. For expansion, the cells were further cultured in MesenPRO RS medium (Life Technologies Japan) at a density of 5 × 103 per cm2 under 5% O2 and 37°C conditions for 5 days. The adherent AdSCs were then cultured for cardiovascular differentiation under specific culture conditions, as previously described, with minor modifications. In brief, the adherent AdSCs were cultured under conditions of 5% CO2 and 37°C in (a) 10% FBS/DMEM supplemented with transforming growth factor-β (2 ng/ml) for vascular smooth muscle cell differentiation [18, 27]; (b) 2% FBS/DMEM supplemented with EGM-2 BulletKit containing human fibroblast growth factor, human vascular endothelial growth factor, human insulin-like growth factor, ascorbic acid, human epidermal growth factor, heparin, and insulin transferrin for endothelial differentiation [17, 28]; and (c) 10% FBS/DMEM-F12 supplemented with phorbol myristate acetate (2 nmol/l) for 24 hours, followed by MethoCult medium (StemCell Technologies Inc., Vancouver, BC, Canada, http://www.stemcell.com) for cardiomyocyte differentiation for 7 days [16, 29]. The cells were fixed with 2% paraformaldehyde (PFA)/PBS for 10 minutes at room temperature (RT), followed by PBS washing, and examined under a fluorescence microscope (model BZ-8000; Keyence, Osaka, Japan, http://www.keyence.com) after immunofluorescent staining.
Cell Proliferation Assay
The adherent AdSCs (5 × 104 cells per well) were seeded on 8-well chamber glass slides (Nalgene Nunc, Rochester, NY, http://www.thermoscientific.com) cultured in MesenPRO RS medium (Life Technologies Japan) in the presence of 5-bromo-2′-deoxyuridine (BrdU; 10 µmol/l; Sigma-Aldrich Japan K.K.) for 24 hours at 37°C under a 5% O2 condition. After immunocytostaining with anti-BrdU antibody (1:100; BD Pharmingen, San Diego, CA, http://www.bdbiosciences.com) as described below, the BrdU-positive cells in each chamber were counted at five different high power fields (HPFs; ×200). Proliferation activity was evaluated using the BrdU labeling index calculated as a BrdU-positive percentage to the total cell number.
Fluorescent Immunocytochemistry for AdSC Differentiation Assay
The adherent cells were fixed with 2% PFA/PBS for 10 minutes at RT, followed by PBS washing, and permeabilized by incubation with 0.1% Triton X-100/PBS solution for 5 minutes at RT. The samples were blocked in antibody dilution buffer, 2% BSA/PBS, for 1 hour at RT. After removal of the blocking solution, primary antibodies/markers were added: anti-CD31 (1:100; Abcam, Cambridge, MA, http://www.abcam.com) and fluorescein-labeled griffonia simplicifolia lectin 1, isolectin B4 (Vector Laboratories, Burlingame, CA, http://www.vectorlabs.com) for ECs; anti-SM22 antibody (1:100; Abcam) and anti-calponin (1:200; Abcam) for VSMCs; and GATA4 (Santa Cruz Biotechnology, Santa Cruz, CA, http://www.scbt.com) and cardiac troponin T (Thermo Fisher Scientific, Fremont, CA, http://www.thermofisher.com) for CMs in antibody dilution buffer at 4°C overnight. After washing with PBS, the cells were incubated with secondary antibodies prepared at 1:500 in antibody dilution buffer: Alexa Fluor 488 donkey anti-goat IgG, Alexa Fluor 488 goat anti-rabbit IgG, and Alexa Fluor 488 goat anti-rat IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, http://www.jacksonimmuno.com) for 30 minutes at RT. After the secondary antibodies were removed and the cells had been washed with PBS, nuclear counter staining was performed by incubation with 4′,6-diamidino-2-phenylindole (DAPI) solution (1 µg/ml in PBS; Sigma-Aldrich Japan K.K.) for 10 minutes at RT. The sample slides were covered by a coverslip with mounting medium (ImmunoBioScience, Mukilteo, WA, http://www.immunobioscience.com), followed by sealing with nail varnish before evaluation under a fluorescence microscope (model BZ8000; Keyence). The antigen (marker for differentiation)-positive cells in each chamber were counted in five different HPFs (×200), and differentiation activity was evaluated by comparing the antigen-positive cell percentage to the total DAPI-positive cell number.
Fluorescence-Activated Cell Sorting Analysis
Fleshly isolated AdSCs were incubated with 0.5% BSA in PBS containing fluorescence (allophycocyanin [APC]/fluorescein isothiocyanate [FITC]/phycoerythrin [PE])-conjugated monoclonal antibodies against CD90 (Abcam), CD44 (eBioscience, San Diego, CA, http://www.ebioscience.com), Sca-1, platelet-derived growth factor receptor-β (PDGFR-β; BioLegend, San Diego, CA, http://www.biolegend.com), c-kit, CD45, CD34, CD31, and CD105 (BD Pharmingen) (1:100 for each antibody) for 30 minutes on ice. The cells stained with APC/FITC/PE-conjugated rat anti-mouse IgG 2cκ, IgG2a, and IgG2bκ (BioLegend) were used as negative controls. The cells were washed with PBS and fixed with 0.5% PFA/PBS for 15 minutes at RT. The samples were analyzed using a fluorescence-activated cell sorting (FACS) system (Cell Analyzer EC800, Sony, Tokyo, Japan) according to the manufacturer’s instructions. The analyses were run in triplicate, and the representative data are presented in the results section. The density of MNCs in each adipose tissue was also calculated by dividing the number of MNCs by the weight of tissues and considered as AdSC density.
Surgical Procedure and AdSC Transfusion Study
Male mice (C57BL6/N, 12–16 weeks old) were anesthetized with an intraperitoneal injection of 400 mg/kg 2,2,2-tribromoethanol (Avertin; Sigma-Aldrich Japan K.K.). MI was induced by ligating the left anterior descending (LAD) coronary artery at a distal site to achieve a 100% survival rate after surgery, and AdSC transfusion was performed as described previously [30]. In brief, the mice were splenectomized to prevent homing of the transfused AdSCs to the spleen. Seven days later, the MI induction procedure was performed, as described previously [31].
For assessment of SC-, VL-, CA-, and SS-derived AdSC recruitment to ischemic myocardium, 104 of DiI-labeled freshly isolated MNCs were injected to wild-type (WT) mice via a tail vein 3 days after MI surgery. The mice were sacrificed 7 days after cell injection, and the hearts were harvested for histological analysis. Transfused AdSCs were visualized in red and examined in the sectioned heart samples using fluorescent microscopy.
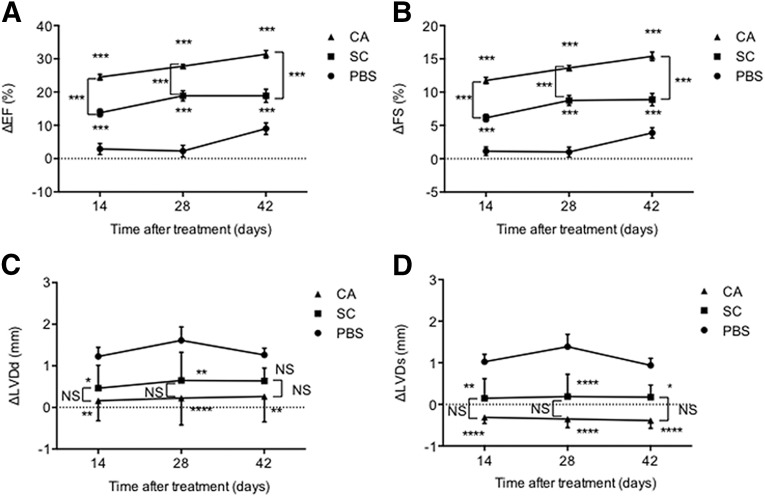
For assessment of the therapeutic effect of AdSCs on MI and long-term follow-up of recruited AdSCs to the ischemic myocardium, SC- or CA-derived AdSCs (5 × 104) of genetically labeled with lacZ transgene isolated from wild-type and B6.129S7-Gt(ROSA)26Sor/J mice (The Jackson Laboratories), respectively, were injected into WT mice via a tail vein 3 days after MI surgery. Cardiac function was sequentially evaluated by echocardiography (Nemio 30; Toshiba Medical Systems, Tochigi, Japan, http://www.toshiba-medical.co.jp) with the following parameters in the left ventricle (LV): changes in ejection fraction (∆EF), fractional shortening (∆FS), left ventricular end-systolic dimension (∆LVD), and left ventricular end-diastolic dimension (∆LVDd) before, 1 day, and 2, 4, or 6 weeks after MI induction. The mice were sacrificed 6 weeks after MI surgery, and the hearts were harvested for histological analysis.
Morphometric Evaluation of Capillary Density and Infarct Size
The vascularity of the ischemic myocardium was assessed by in situ fluorescent staining using the endothelial cell-specific marker FITC-conjugated BS1-lectin (Vector Laboratories) 6 weeks after MI, as described previously [32]. In brief, after anesthesia, BS1-lectin (0.1 mg per mouse) was injected by direct cardiac puncture systemically. Ten minutes later, the mice were sacrificed, and the hearts were removed and perfused with PBS, followed by 4% PFA/PBS through the right carotid artery retrogradely. The hearts were fixed for 6 hours in 4% PFA/PBS followed by overnight incubation in 20% sucrose/PBS. The tissues were embedded in O.C.T. compound (Sakura FineTek, Tokyo, Japan, http://www.sakura.com) and sectioned at 0.5 mm just below the LAD ligation level at a 5-µm thickness, as described previously [31].
For capillary density measurement, the capillaries were recognized under a fluorescence microscope as tubular structures positive for FITC-BS1-lectin in green. They were evaluated by morphometric examination of three randomly selected fields in the bilateral ischemic border zone from segments of the LV myocardium survived following LAD occlusion. To assess the severity of myocardial fibrosis/damage, Masson trichrome staining was performed with frozen sections in each tissue block. The stained sections were measured and calculated for the average ratio of fibrosis area (blue) to the entire LV area (percentage of fibrosis area), average ratio of scarred perimeter to the entire LV circumference (percentage of scar length), and the average ratio of the reduced LV wall thickness in the scarred area to the intact LV wall thickness from three different sites in each wall (percentage of LV wall thinning) using NIH ImageJ, version 1.42q, software (NIH, Bethesda, MD, http://www.imagej.nih.gov) and Adobe Photoshop CS4 (Adobe Systems, San Jose, CA, http://www.adobe.com) software.
Fluorescent Immunohistochemistry
The hearts of the MI-induced mice were harvested at a predetermined time point after surgery and prepared for frozen tissue sectioning after fixation with 4% PFA/PBS. Double fluorescent immunostaining was performed with an antibody against β-galactosidase (β-gal; 1:500; MP Biomedicals, Santa Ana, CA, http://www.mpbio.com) to detect LacZ gene expressing exogenously infused AdSCs and FITC-isolectin-B4 (ILB4; 1:100; Vector Laboratories) for the detection of endothelial cells and with an antibody against vascular smooth muscle (SM) α-actin (1:500; Abcam, Tokyo, Japan) for detection of VSMCs or α-sarcomeric actinin (1:200; Abcam, Tokyo, Japan)/cardiac troponin T (1:200; Thermo Fisher Scientific K.K., Yokohama, Japan) for the detection of cardiomyocytes. Normal mouse IgG or rabbit IgG were served as negative controls. Nuclei were counterstained with DAPI (Sigma-Aldrich Japan K.K.), and the sections were mounted in aqueous mounting medium. The images were examined under a fluorescent microscope (BZ8000; Keyence, Osaka, Japan). The number of β-gal/ILB4-positive capillaries, β-gal/SM α-actin-positive VSMCs, and β-gal/α-sarcomeric actinin or cardiac troponin-positive cardiomyocytes were counted in bilateral peri-infarct areas in HPF (×200) and averaged for the assessment of AdSC cardiovascular transdifferentiation frequency in ischemic myocardium.
Statistical Analysis
All values are presented as the mean ± SEM. Statistical analyses were performed with commercially available software (GraphPad Prism; MDF, Co., Inc., Tokyo, Japan, http://www.mdf-soft.com/english). A comparison between two groups was tested using the Mann-Whitney U test, and those among multiple groups were tested for significance via analysis of variance followed by post hoc testing with a Tukey procedure; p < .05 was considered statistically significant.
Results
Cardiac Adipose Tissue Contains Increased Numbers of AdSCs With High Proliferation Activity
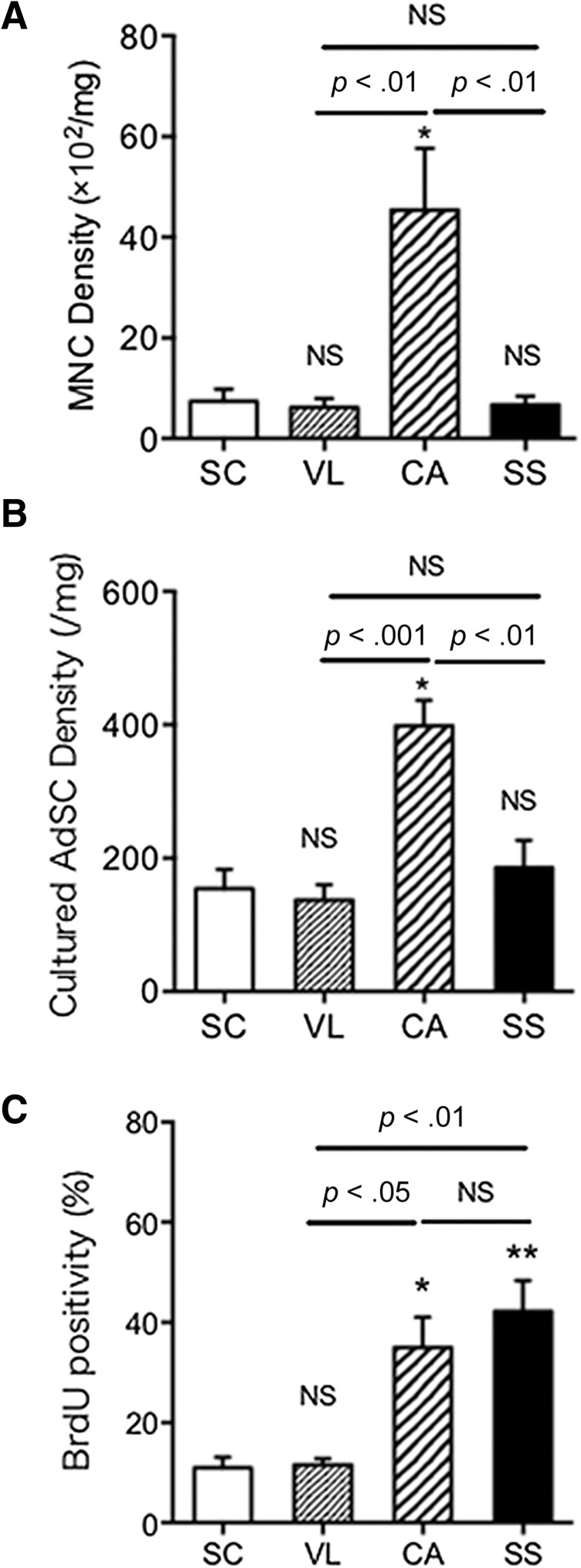
The MNCs were isolated from four different adipose tissues followed by 7 days of culture to obtain adherent AdSCs. We assessed the cell density by counting the number of MNCs/AdSCs with tissue weights in each adipose tissue (supplemental online Table 4). The cell density of the freshly isolated MNCs was significantly higher in the CA adipose tissue than that in the SC, VL, and SS adipose tissues (Fig. 1A). As expected, the number/density in unit tissue weight of adherent AdSCs derived from CA adipose tissue was also significantly higher than that in SC, VL, and SS adipose tissues (Fig. 1B). We next evaluated the proliferation activity of adherent AdSCs using BrdU incorporation assay. The positivity of BrdU in the CA and SS adipose tissue (supplemental online Fig. 2) was significantly greater than that in the SC and VL adipose tissue. No significant difference was seen between the SC and VL adipose tissue or between the CA and SS adipose tissue (Fig. 1C). These findings suggest that AdSCs are enriched in cardiac adipose tissue exhibiting high proliferation activity.
Figure 1.
Comparison of cell density, proliferation, and surface markers among AdSCs from four different adipose tissues. (A, B): MNCs were isolated from four different adipose tissues (SC, VL, CA, SS), followed by 7 days of culture to obtain adherent AdSCs. The densities in freshly isolated MNCs (A) and cultured AdSCs (B) from each source were measured and compared among the AdSCs from the four different adipose tissues. (C): The positivity of BrdU in AdSCs was assessed by immunocytochemistry. ∗, p < .05; ∗∗, p < .01; NS, not significant vs. SC. All experiments were performed in triplicate and statistically analyzed. Abbreviations: AdSCs, adipose-derived stem cells; BrdU, 5-bromo-2′-deoxyuridine; CA, cardiac brown adipose tissue; MNCs, mononuclear cells; NS, not significant; SC, subcutaneous white adipose tissue; SS, subscapular brown adipose tissue; VL, visceral white adipose tissue.
Immunophenotypic Characterization of AdSCs Isolated From Four Different Adipose Tissues
We assessed the expression of cell surface markers for stem cell (Sca-1 and c-Kit), endothelial (CD31), vascular smooth muscle cell (PDGFR-β), hematopoietic (CD45 and CD34), and mesenchymal (CD90, CD44, and CD105) lineages in freshly isolated MNCs from four different adipose tissues harvested from three mice per each adipose tissue and the 7-day-cultured AdSCs by FACS analysis. Most of all MNCs from all four adipose tissues expressed CD45 but not CD105, CD34, c-Kit, or PDGFR-β, regardless of the adipose tissue origin. A relatively greater number of CD44- and Sca-1-positive cells were observed in VL and SC, respectively. Only CA-derived AdSCs included fewer numbers of CD31-positive cells compared with the other three adipose tissues, suggesting that vascularity is low in CA compared with the other tissues (supplemental online Table 1; supplemental online Fig. 3). In cultured AdSCs, only VL-derived AdSCs had a low percentage of Sca-1 and a high percentage of CD45, exhibiting characteristics unlike those of MSCs. The other three AdSCs demonstrated a similar expression pattern (high rates of CD44 and Sca-1). However, unlike in human AdSCs, CD90- or CD105-positive cells were few or none, respectively (supplemental online Table 2; supplemental online Fig. 3).
Cardiac AdSCs Exhibit High Differentiation Capacity for Cardiovascular Cells
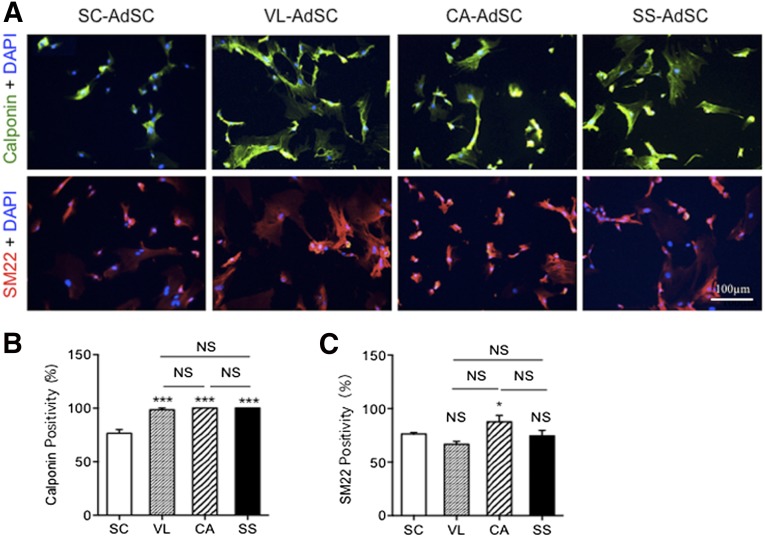
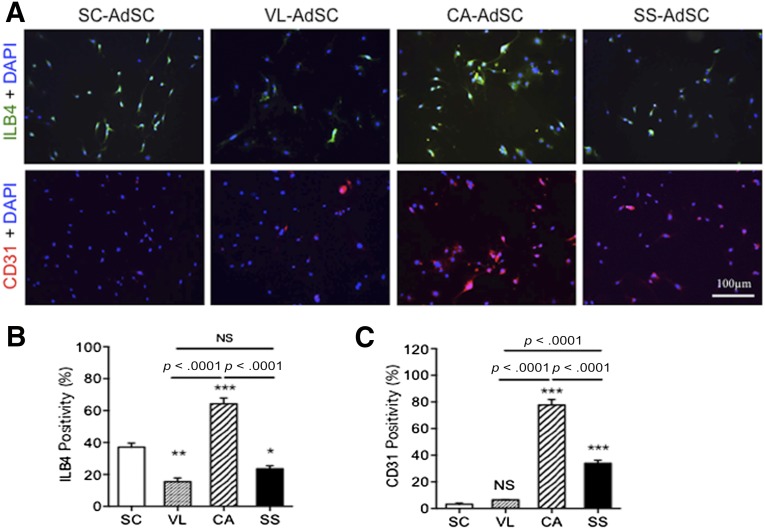
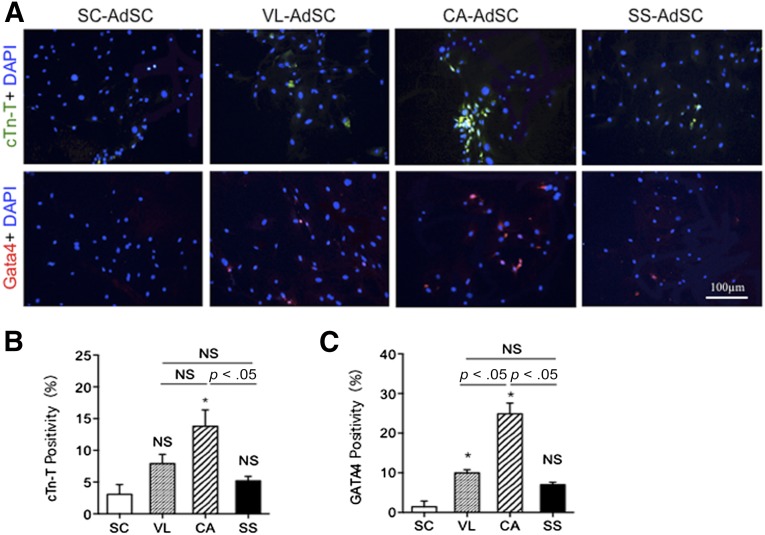
We assessed the transdifferentiation capacity of AdSCs toward cardiovascular lineage cells using markers of ILB4/CD31 for ECs, markers of calponin/SM22 for VSMCs, and markers of cardiac troponin T (cTnT)/GATA4 for CMs. Although no significant difference was found in the percentages of SM22- and calponin-positive cells (Fig. 2), which was attributed to VSMC differentiation from AdSCs among the four adipose tissues, the cardiac AdSCs exhibited a significant increase in the percentages of ILB4- and CD31-positive cells (Fig. 3) and cTnT- and GATA4-positive cells (Fig. 4), attributed to the endothelial and cardiac differentiation, respectively.
Figure 2.
Smooth muscle cell differentiation in AdSCs from four different adipose tissues by fluorescent immunocytochemistry. (A): To assess which source for AdSCs could differentiate to smooth muscle cells, the cells were stained with anti-calponin (green) and anti-SM22 (red) antibodies. Nuclei were stained with DAPI (blue). (B, C): The rate of calponin-positive (B) and SM22-positive (C) cells was compared among AdSCs from four different adipose tissues. ∗, p < .05; ∗∗∗, p < .001; NS, not significant vs. SC. All experiments were performed in triplicate and statistically analyzed. Abbreviations: AdSCs, adipose-derived stem cells; CA, cardiac brown adipose tissue; DAPI, 4′,6-diamidino-2-phenylindole; NS, not significant; SC, subcutaneous white adipose tissue; SS, subscapular brown adipose tissue; VL, visceral white adipose tissue.
Figure 3.
Endothelial differentiation in AdSCs from four different adipose tissues by fluorescent immunocytochemistry. (A): To assess which source of AdSCs could differentiate into endothelial cells, the cells were stained with anti-ILB4 (green) and anti-CD31 (red) antibodies. Nuclei were stained with DAPI (blue). (B, C): The rate of ILB4-positive (B) and CD31-positive (C) cells was compared among the AdSCs from four different adipose tissues. ∗, p < .05; ∗∗, p < .01; ∗∗∗, p < .001; NS, not significant vs. SC. All experiments were performed in triplicate and statistically analyzed. Abbreviations: AdSCs, adipose-derived stem cells; CA, cardiac brown adipose tissue; DAPI, 4′,6-diamidino-2-phenylindole; ILB4, isolectin-B4; NS, not significant; SC, subcutaneous white adipose tissue; SS, subscapular brown adipose tissue; VL, visceral white adipose tissue.
Figure 4.
Cardiomyocyte differentiation in AdSCs from four different adipose tissues by fluorescent immunocytochemistry. (A): To assess which source for AdSCs could differentiate into cardiomyocytes, the cells were stained with anti-cTnT (green) and anti-GATA4 (red) antibodies. Nuclei were stained with DAPI (blue). (B, C): The rate of cTnT-positive (B) and GATA4-positive (C) cells was compared among AdSCs from four different adipose tissues. ∗, p < .05; NS, not significant vs. SC. All experiments were performed in triplicate and statistically analyzed. Abbreviations: AdSCs, adipose-derived stem cells; CA, cardiac brown adipose tissue; cTn-T, cardiac troponin T; DAPI, 4′,6-diamidino-2-phenylindole; NS, not significant; SC, subcutaneous white adipose tissue; SS, subscapular brown adipose tissue; VL, visceral white adipose tissue.
Cardiac AdSC Transplantation Exhibited Therapeutic Efficacy for Myocardial Infarction
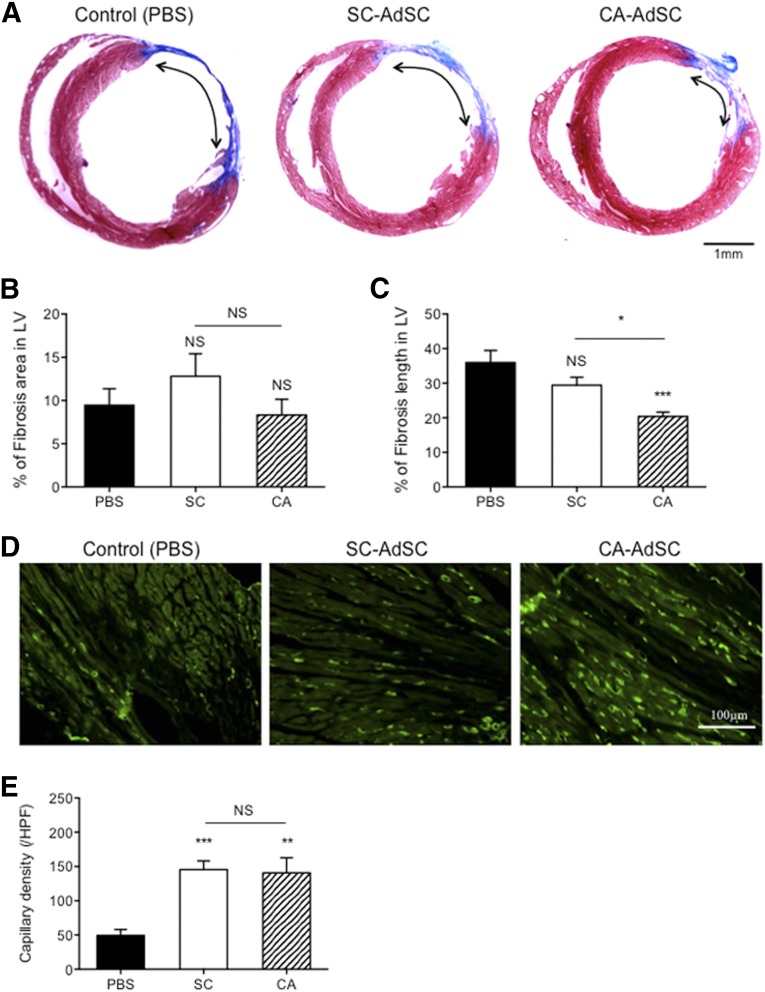
First, we evaluated the recruitment potential of freshly isolated DiI-labeled AdSC-rich MNCs (AdSCs, 104 per mouse, i.v.) to ischemic myocardium (supplemental online Fig. 4A). The frequencies of SC-, VL-, and CA-derived AdSC recruitment were significantly greater than the frequency of SS-derived AdSC recruitment (supplemental online Fig. 4B). Double immunofluorescent staining demonstrated that both recruited SC- and CA-AdSCs differentiated into ILB4-/SM-α-actin-positive vascular lineage cells (supplemental online Fig. 4C, 4D), and only CA-AdSCs could differentiate into cTnT-positive cardiomyocytes by day 7 after cell transfusion (supplemental online Fig. 4E). The quantitative analysis for the cardiovascular differentiation (supplemental online Fig. 4F) also allowed us to focus on CA- and SC-AdSCs, excluding VL- and SS-AdSCs in the next series of experiments. Next, we compared the therapeutic efficacy of CA-AdSCs with that of SC-AdSCs, both are easy to obtain compared with VL- and SS-AdSCs and commonly used for autologous AdSC transplantation in clinical settings. Consistent with the results in previous studies, SC-AdSC transplantation significantly improved cardiac functions, which was assessed by the changes in the echocardiographic parameters, ΔEF, ΔFS, ΔLVDd, and ΔLVDs. Notably, further significant cardiac functional recovery by CA-AdSC transplantation was observed compared with that by SC-AdSC transplantation (Fig. 5). The histological analysis also revealed significant reductions in the infarct area, assessed by the fibrosis length in the cross-sectional LV area treated with CA-AdSC transplantation compared with PBS (control) and SC-AdSC transplantation (Fig. 6A–6C). The immunofluorescent staining (Fig. 6D) exhibited a significant increase in capillary density in the ischemic border zone of the LV in both the SC- and CA-AdSC groups compared with the control group; however, no significant difference was found in the SC- and CA-AdSC groups (Fig. 6E).
Figure 5.
Echocardiographic assessment of cardiac function after myocardial infarction with AdSC transfusion. The cardiac functional parameters in mice were assessed by echocardiography. The EF (A), FS (B), LVDd (C), and LVD (D) before and after treatment (14, 28, and 42 days) were measured, and the changes in each parameter (ΔEF, ΔFS, ΔLVDd, and ΔLVD) were statistically analyzed. ∗, p < .05; ∗∗, p < .01; ∗∗∗, p < .001; ∗∗∗∗, p < .0001; NS, not significant vs. PBS. Abbreviations: Δ, change; AdSCs, adipose-derived stem cells; CA, cardiac brown adipose tissue; EF, ejection fraction; FS, fractional shortening; LVD, left ventricular end-systolic dimension; LVDd, left ventricular end-diastolic dimension; NS, not significant; PBS, phosphate-buffered saline; SC, subcutaneous white adipose tissue.
Figure 6.
Cardiac morphometric analysis after myocardial infarction with AdSC transfusion. (A): Mouse cardiac cross-sections of control group (PBS), SC-AdSC-treated group, and CA-AdSC-treated group through infarcted myocardium 42 days after surgery were assessed by Masson’s trichrome staining. The percentage of the mouse LV fibrotic area (B) and LV fibrosis length (C) were measured. (D, E): Mouse cardiac cross-sections were stained with an anti-BS-1 lectin antibody and the endothelial cell marker (green), and the capillary densities were compared among the PBS, SC-AdSC, and CA-AdSC groups. ∗, p < .05; ∗∗, p < .01; ∗∗∗, p < .001; NS, not significant vs. PBS. Abbreviations: AdSCs, adipose-derived stem cells; CA, cardiac brown adipose tissue and CA-AdSC group; HPF, high power field; LV, left ventricle; NS, not significant; PBS, phosphate-buffered saline; SC, subcutaneous white adipose tissue and SC-AdSC group.
Recruited Cardiac AdSCs Differentiated Into Cardiovascular Cells in Ischemic Myocardium
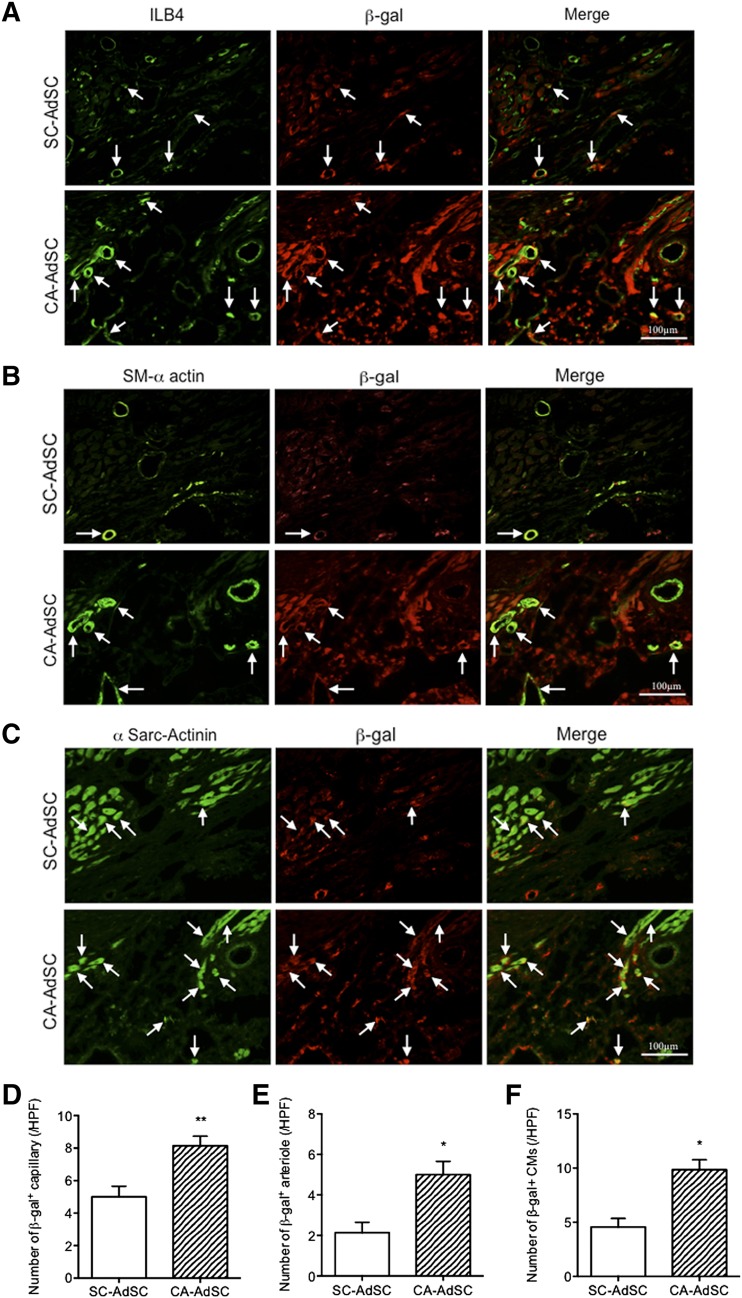
Finally, we examined the infarct heart samples transplanted with SC- versus CA-AdSCs isolated from mice constitutively expressing the lacZ gene in whole cells to assess whether the transplanted AdSCs contributed to cardiac tissue regeneration. The cardiovascular cell differentiation of the transplanted AdSCs in the ischemic myocardium was evaluated using double immunofluorescent staining for β-gal, a transcript of the lacZ gene, and ILB4 for ECs (Fig. 7A), SM-α-actin for VSMCs (Fig. 7B), and α-sarcomeric actinin for CM (Fig. 7C). The numbers of the ILB4/β-gal, SM-α-actin/β-gal, and α-sarcomeric actinin/β-gal double-positive cells were significantly greater in the CA-AdSC group than in the SC-AdSC group (Fig. 7D–7F), suggesting that the recruited CA-AdSCs frequently differentiate into cardiovascular cells in ischemic myocardium compared with the recruited SC-AdSCs. Because we did not perform sex-mismatched fluorescent in situ hybridization analysis, we could not distinguish cell fusion and transdifferentiation of the recruited AdSCs scientifically; however, a recent report did not find that the recruited human adipose-derived MNCs and AdSCs fused with host mouse cells [26].
Figure 7.
Immunohistological assessment of ischemic myocardium with recruited β-gal expressing AdSCs. Mouse cardiac cross-sections of SC-AdSC-treated group and CA-AdSC-treated group through infarcted myocardium 42 days after surgery were stained with β-gal costained with ILB4, an endothelial cell marker (green) (A), SM-α-actin, a smooth muscle cell marker (green) (B), and a cardiac myocyte marker (green) (C). Arrows indicate the positive cells, where ILB4 (A), SM-α actin (B), and α-Sarc-actinin (C) was costained with β-gal. The double-positive cells for β-gal and ILB4, β-gal and SM-α actin, and β-gal and α-Sarc-actinin were quantified and expressed as the number of β-gal+ capillary (D), number of β-gal+ arteriole (E), and number of β-gal+ CMs (F), respectively. ∗, p < .05; ∗∗, p < .01 vs. SC-AdSCs. Abbreviations: α-Sarc-Actinin, α-sarcomeric actinin; AdSCs, adipose-derived stem cells; β-gal, β-galactosidase; CA, cardiac brown adipose tissue; HPF, high power field; ILB4, isolectin-B4; SC, subcutaneous white adipose tissue; SM-α actin, smooth muscle α-actin.
Discussion
In the present study, we have demonstrated that both MNCs and AdSCs isolated from cardiac adipose tissue (CA-AdSCs) have a prominent potential for differentiation into cardiovascular cells, in particular, ECs and CMs, compared with those isolated from adipose tissue in other organs. ECs are a major component of the capillaries for angiogenesis, and CMs are essential in the myocardium for cardiac functionality. These cellular differentiation potentials of AdSCs are therefore important for cardiac tissue regeneration. Our in vivo data have indicated the superior therapeutic efficacy of CA-AdSCs in myocardial infarction, contributing to cardiovascular regeneration by transdifferentiation, but, probably, not by cell fusion [26], into ECs, VSMCs, and CMs in ischemic myocardium compared with SC-AdSCs. In recent ongoing clinical studies, freshly isolated MNCs (SVF) from subcutaneous adipose tissue have been frequently used as adipose-derived regenerative cells, exhibiting favorable outcomes in ischemic cardiovascular diseases. Nevertheless, both freshly isolated MNCs (SVF) and cultured AdSCs have been shown to have a similar therapeutic potential in acute myocardial infarction [26].
In humans, mesenchymal stem cells originate from a variety of organs (i.e., bone marrow, adipose tissue, placenta, amnion, amniotic fluid, and cord blood). AdSCs have been characterized with positive cell surface markers of CD90, CD44, CD29, and CD105 and negative cell surface markers of CD45 and CD31 [33]. Regarding CD34 expression in human AdSCs, it has been reported to vary, depending on the isolation or culture method [34]. In addition to the marker expression pattern in human AdSCs, Sca-1 has been described as an additional marker in mouse AdSCs [35, 36]. Consistent with previous reports, our data have indicated an increased percentage of Sca-1-positive cells in mouse AdSCs after culturing. However, we detected only a reduced percentage of CD90-positive (<10%) and a few CD105-positive (<1%) cells even in culture conditions (supplemental online Tables 1, 2). The discrepancy between the previous reports and our data might have been because of the following reasons: (a) CD90 expression generally increases after successive passages [35] and is high when AdSCs are isolated from lymph node-containing adipose tissue [36]; (b) CD105 expression can be affected by trypsin, depending on the type and/or activity [37] in trypsinized AdSCs for FACS analysis; and (c) the variability of antibody specificity/function to recognize cell surface antigen in each report.
Regardless of the variation in cell surface antigen expression to identify or characterize AdSCs, SC-AdSCs, specifically, have been shown to have a therapeutic effect on cardiac functional recovery after myocardial infarction in experimental animal models. Previous studies have demonstrated a favorable effect of SC-AdSCs, along with an indirect paracrine mechanism [19, 38, 39] by which angiogenesis/neovascularization including bone marrow-derived endothelial progenitor cell recruitment to ischemic myocardium [19] is promoted, on improved cardiac functional recovery, rather than a direct contribution by the transplanted SC-AdSCs to tissue regeneration. In contrast, our study has demonstrated a therapeutic effect of CA-AdSCs on MI via a direct contribution to the ischemic myocardium and transdifferentiation to, at least in part, CMs, ECs, and VSMCs, in addition to the paracrine effect (Fig. 7). Although a very recent similar study [40] also demonstrated the superior therapeutic effect of pericardiac AdSCs on cardiac functional recovery and tissue regeneration compared with that of subcutaneous AdSCs in a rat MI model, only a few transplanted or intramuscularly injected pericardiac AdSCs (5 × 105 at the center of infarcted wall) were engrafted in the ischemic myocardium 28 days after surgery. This suggests that the transplanted cardiac AdSCs could not survive in acute ischemic and inflammatory conditions without differentiation into cardiovascular cells. In order to overcome the problems in the previous study, we systemically injected CA-AdSCs via a tail vein in mice 3 days after MI induction. In this setting, the systemically infused cells were recruited to the myocardial ischemic border zone, where ischemia and inflammation is not severe. These AdSCs could survive even 28 days after surgery.
Most of the previous mouse studies applied SC-AdSCs at doses of 5 × 105 to 5 × 106 in the coronary artery ligation-induced MI model in a cardiac intramuscular fashion (supplemental online Table 3). In contrast, our approach of only 104 of CA-AdSCs with intravenous infusion significantly improved cardiac functional recovery after MI, which might be attributed to (a) prevention of the injected AdSCs homing to the spleen and (b) altered postinfarction myocardial inflammation by the splenectomy. This is a study limitation and a potential bias of our study. Another distinct point of our study was the source of AdSCs from supracardiac brown adipose tissue (supplemental online Fig. 1) but not from pericardial white adipose tissue [41]. Although the differences and similarity of white and brown adipose tissue-derived stem cells has been investigated [42] (supplemental online Table 5), not only the difference in adipose tissue type (white vs. brown) but also the origin of fat tissue (i.e., cardiac origin) might be a critical factor in determining the therapeutic effect on MI and cell fate in ischemic myocardium.
Conclusion
CA-AdSCs tend to differentiate into cardiovascular lineage cells (i.e., cardiomyocytes, endothelial cells, and vascular smooth muscle cells) compared with other (SC, VL, and SS) adipose tissue-derived stem cells. A small number of systemically transplanted CA-AdSCs sufficiently improved cardiac functional recovery after MI by differentiating into cardiovascular cells in ischemic myocardium. Because supracardiac brown adipose tissue also exists below the thymus in a region free of major vessels in human [43], the strategy for cardiac regeneration with CA-AdSCs might give rise to a new autologous stem cell therapy for patients with myocardial ischemia. Cardiac adipose tissue can be easily harvested during open chest coronary bypass graft surgery or other cardiovascular procedures, and patients will be able to use their own AdSCs with the cell bank system after cell culture expansion to treat future recurrent ischemic heart disease.
Supplementary Material
Author Contributions
H.N.: collection and/or assembly of data, manuscript writing; M.I.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; E.K.: collection and/or assembly of data; M.H. and T.H.: financial support; M.A.: manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Guilbert JJ. The world health report 2002—Reducing risks, promoting healthy life. Educ Health (Abingdon) 2003;16:230. doi: 10.1080/1357628031000116808. [DOI] [PubMed] [Google Scholar]
- 2.Oguz E, Ayik F, Ozturk P, et al. Long-term results of autologous stem cell transplantation in the treatment of patients with congestive heart failure. Transplant Proc. 2011;43:931–934. doi: 10.1016/j.transproceed.2011.01.115. [DOI] [PubMed] [Google Scholar]
- 3.Losordo DW, Schatz RA, White CJ, et al. Intramyocardial transplantation of autologous CD34+ stem cells for intractable angina: A phase I/IIa double-blind, randomized controlled trial. Circulation. 2007;115:3165–3172. doi: 10.1161/CIRCULATIONAHA.106.687376. [DOI] [PubMed] [Google Scholar]
- 4.Leistner DM, Fischer-Rasokat U, Honold J, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI): Final 5-year results suggest long-term safety and efficacy. Clin Res Cardiol. 2011;100:925–934. doi: 10.1007/s00392-011-0327-y. [DOI] [PubMed] [Google Scholar]
- 5.Wollert KC, Meyer GP, Lotz J, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: The BOOST randomised controlled clinical trial. Lancet. 2004;364:141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 6.Amado LC, Saliaris AP, Schuleri KH, et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci USA. 2005;102:11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Döbert N, Britten M, Assmus B, et al. Transplantation of progenitor cells after reperfused acute myocardial infarction: Evaluation of perfusion and myocardial viability with FDG-PET and thallium SPECT. Eur J Nucl Med Mol Imaging. 2004;31:1146–1151. doi: 10.1007/s00259-004-1490-4. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez AM, Pisani D, Dechesne CA, et al. Transplantation of a multipotent cell population from human adipose tissue induces dystrophin expression in the immunocompetent mdx mouse. J Exp Med. 2005;201:1397–1405. doi: 10.1084/jem.20042224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shoji T, Ii M, Mifune Y, et al. Local transplantation of human multipotent adipose-derived stem cells accelerates fracture healing via enhanced osteogenesis and angiogenesis. Lab Invest. 2010;90:637–649. doi: 10.1038/labinvest.2010.39. [DOI] [PubMed] [Google Scholar]
- 10.Huang JI, Beanes SR, Zhu M, et al. Rat extramedullary adipose tissue as a source of osteochondrogenic progenitor cells. Plast Reconstr Surg. 2002;109:1033–1042. doi: 10.1097/00006534-200203000-00037. [DOI] [PubMed] [Google Scholar]
- 11.Lin Y, Luo E, Chen X, et al. Molecular and cellular characterization during chondrogenic differentiation of adipose tissue-derived stromal cells in vitro and cartilage formation in vivo. J Cell Mol Med. 2005;9:929–939. doi: 10.1111/j.1582-4934.2005.tb00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Timper K, Seboek D, Eberhardt M, et al. Human adipose tissue-derived mesenchymal stem cells differentiate into insulin, somatostatin, and glucagon expressing cells. Biochem Biophys Res Commun. 2006;341:1135–1140. doi: 10.1016/j.bbrc.2006.01.072. [DOI] [PubMed] [Google Scholar]
- 13.Aurich H, Sgodda M, Kaltwasser P, et al. Hepatocyte differentiation of mesenchymal stem cells from human adipose tissue in vitro promotes hepatic integration in vivo. Gut. 2009;58:570–581. doi: 10.1136/gut.2008.154880. [DOI] [PubMed] [Google Scholar]
- 14.Jang S, Cho HH, Cho YB, et al. Functional neural differentiation of human adipose tissue-derived stem cells using bFGF and forskolin. BMC Cell Biol. 2010;11:25. doi: 10.1186/1471-2121-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liqing Y, Jia G, Jiqing C, et al. Directed differentiation of motor neuron cell-like cells from human adipose-derived stem cells in vitro. Neuroreport. 2011;22:370–373. doi: 10.1097/WNR.0b013e3283469615. [DOI] [PubMed] [Google Scholar]
- 16.Planat-Bénard V, Menard C, André M, et al. Spontaneous cardiomyocyte differentiation from adipose tissue stroma cells. Circ Res. 2004;94:223–229. doi: 10.1161/01.RES.0000109792.43271.47. [DOI] [PubMed] [Google Scholar]
- 17.Fischer LJ, McIlhenny S, Tulenko T, et al. Endothelial differentiation of adipose-derived stem cells: Effects of endothelial cell growth supplement and shear force. J Surg Res. 2009;152:157–166. doi: 10.1016/j.jss.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris LJ, Abdollahi H, Zhang P, et al. Differentiation of adult stem cells into smooth muscle for vascular tissue engineering. J Surg Res. 2011;168:306–314. doi: 10.1016/j.jss.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ii M, Horii M, Yokoyama A, et al. Synergistic effect of adipose-derived stem cell therapy and bone marrow progenitor recruitment in ischemic heart. Lab Invest. 2011;91:539–552. doi: 10.1038/labinvest.2010.191. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez-Rey E, Anderson P, González MA, et al. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58:929–939. doi: 10.1136/gut.2008.168534. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez-Rey E, Gonzalez MA, Varela N, et al. Human adipose-derived mesenchymal stem cells reduce inflammatory and T cell responses and induce regulatory T cells in vitro in rheumatoid arthritis. Ann Rheum Dis. 2010;69:241–248. doi: 10.1136/ard.2008.101881. [DOI] [PubMed] [Google Scholar]
- 22.Perin EC, Sanz-Ruiz R, Sanchez PL, et al. Adipose-derived regenerative cells in patients with ischemic cardiomyopathy: The PRECISE trial. Am Heart J. 2014;168:88.e92–95.e82. doi: 10.1016/j.ahj.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 23.Perez-Cano R, Vranckx JJ, Lasso JM, et al. Prospective trial of adipose-derived regenerative cell (ADRC)-enriched fat grafting for partial mastectomy defects: The RESTORE-2 trial. Eur J Surg Oncol. 2012;38:382–389. doi: 10.1016/j.ejso.2012.02.178. [DOI] [PubMed] [Google Scholar]
- 24.Kølle SF, Fischer-Nielsen A, Mathiasen AB, et al. Enrichment of autologous fat grafts with ex-vivo expanded adipose tissue-derived stem cells for graft survival: A randomised placebo-controlled trial. Lancet. 2013;382:1113–1120. doi: 10.1016/S0140-6736(13)61410-5. [DOI] [PubMed] [Google Scholar]
- 25.Kamakura T, Ito K. Autologous cell-enriched fat grafting for breast augmentation. Aesthetic Plast Surg. 2011;35:1022–1030. doi: 10.1007/s00266-011-9727-7. [DOI] [PubMed] [Google Scholar]
- 26.Bai X, Yan Y, Song YH, et al. Both cultured and freshly isolated adipose tissue-derived stem cells enhance cardiac function after acute myocardial infarction. Eur Heart J. 2010;31:489–501. doi: 10.1093/eurheartj/ehp568. [DOI] [PubMed] [Google Scholar]
- 27.Jeon ES, Moon HJ, Lee MJ, et al. Sphingosylphosphorylcholine induces differentiation of human mesenchymal stem cells into smooth-muscle-like cells through a TGF-beta-dependent mechanism. J Cell Sci. 2006;119:4994–5005. doi: 10.1242/jcs.03281. [DOI] [PubMed] [Google Scholar]
- 28.Konno M, Hamazaki TS, Fukuda S, et al. Efficiently differentiating vascular endothelial cells from adipose tissue-derived mesenchymal stem cells in serum-free culture. Biochem Biophys Res Commun. 2010;400:461–465. doi: 10.1016/j.bbrc.2010.08.029. [DOI] [PubMed] [Google Scholar]
- 29.Park E, Patel AN. PKC-delta induces cardiomyogenic gene expression in human adipose-derived stem cells. Biochem Biophys Res Commun. 2010;393:582–586. doi: 10.1016/j.bbrc.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 30.Ii M, Takenaka H, Asai J, et al. Endothelial progenitor thrombospondin-1 mediates diabetes-induced delay in reendothelialization following arterial injury. Circ Res. 2006;98:697–704. doi: 10.1161/01.RES.0000209948.50943.ea. [DOI] [PubMed] [Google Scholar]
- 31.Ii M, Nishimura H, Iwakura A, et al. Endothelial progenitor cells are rapidly recruited to myocardium and mediate protective effect of ischemic preconditioning via “imported” nitric oxide synthase activity. Circulation. 2005;111:1114–1120. doi: 10.1161/01.CIR.0000157144.24888.7E. [DOI] [PubMed] [Google Scholar]
- 32.Ii M, Nishimura H, Kusano KF, et al. Neuronal nitric oxide synthase mediates statin-induced restoration of vasa nervorum and reversal of diabetic neuropathy. Circulation. 2005;112:93–102. doi: 10.1161/CIRCULATIONAHA.104.511964. [DOI] [PubMed] [Google Scholar]
- 33.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshimura K, Shigeura T, Matsumoto D, et al. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J Cell Physiol. 2006;208:64–76. doi: 10.1002/jcp.20636. [DOI] [PubMed] [Google Scholar]
- 35.Taha MF, Hedayati V. Isolation, identification and multipotential differentiation of mouse adipose tissue-derived stem cells. Tissue Cell. 2010;42:211–216. doi: 10.1016/j.tice.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto N, Akamatsu H, Hasegawa S, et al. Isolation of multipotent stem cells from mouse adipose tissue. J Dermatol Sci. 2007;48:43–52. doi: 10.1016/j.jdermsci.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 37.Tabatabaei M, Mosaffa N, Nikoo S, et al. Isolation and partial characterization of human amniotic epithelial cells: The effect of trypsin. Avicenna J Med Biotechnol. 2014;6:10–20. [PMC free article] [PubMed] [Google Scholar]
- 38.Rehman J, Traktuev D, Li J, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 39.Salgado AJ, Reis RL, Sousa NJ, et al. Adipose tissue derived stem cells secretome: Soluble factors and their roles in regenerative medicine. Curr Stem Cell Res Ther. 2010;5:103–110. doi: 10.2174/157488810791268564. [DOI] [PubMed] [Google Scholar]
- 40.Wang X, Zhang H, Nie L, et al. Myogenic differentiation and reparative activity of stromal cells derived from pericardial adipose in comparison to subcutaneous origin. Stem Cell Res Ther. 2014;5:92. doi: 10.1186/scrt481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamdi H, Planat-Benard V, Bel A, et al. Epicardial adipose stem cell sheets results in greater post-infarction survival than intramyocardial injections. Cardiovasc Res. 2011;91:483–491. doi: 10.1093/cvr/cvr099. [DOI] [PubMed] [Google Scholar]
- 42.Silva FJ, Holt DJ, Vargas V, et al. Metabolically active human brown adipose tissue derived stem cells. Stem Cells. 2014;32:572–581. doi: 10.1002/stem.1595. [DOI] [PubMed] [Google Scholar]
- 43.Cheung L, Gertow J, Werngren O, et al. Human mediastinal adipose tissue displays certain characteristics of brown fat. Nutr Diabetes. 2013;3:e66. doi: 10.1038/nutd.2013.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.