The reparative capacity of endothelial progenitor cells appears to be limited by their poor survival when injected directly into ischemic tissue. Human endothelial colony-forming cells (ECFCs) were transduced using a lentiviral vector encoding integrin β1 (ITGB1) or enhanced green fluorescent protein. Blood perfusion of the ischemic limb was significantly augmented only in the ITGB1-ECFC group. Intravenous administration of ECFCs engineered to home to ischemic tissue appears to efficiently mediate therapeutic angiogenesis.
Keywords: Angiogenesis, Endothelial cell, Integrins, Progenitor cells, Transplantation
Abstract
When injected directly into ischemic tissue in patients with peripheral artery disease, the reparative capacity of endothelial progenitor cells (EPCs) appears to be limited by their poor survival. We, therefore, attempted to improve the survival of transplanted EPCs through intravenous injection and gene modification. We anticipated that overexpression of integrin β1 will enable injected EPCs to home to ischemic tissue, which abundantly express extracellular matrix proteins, the ligands for integrins. In addition, integrin β1 has an independent angiogenesis-stimulating function. Human endothelial colony-forming cells (ECFCs; late-outgrowth EPCs) were transduced using a lentiviral vector encoding integrin β1 (ITGB1) or enhanced green fluorescent protein (GFP). We then locally or systemically injected phosphate-buffered saline or the genetically modified ECFCs (GFP-ECFCs or ITGB1-ECFCs; 1 × 105 cells each) into NOD/Shi-scid, IL-2Rγnull mice whose right femoral arteries had been occluded 24 hours earlier. Upregulation of extracellular matrix proteins, including fibronectin, was apparent in the ischemic legs. Four weeks later, blood perfusion of the ischemic limb was significantly augmented only in the ITGB1-ECFC group. Scanning electron microscopy of vascular casts revealed increases in the perfused blood vessels in the ischemic legs of mice in the ITGB1-ECFC group and significant increases in the density of both capillaries and arterioles. Transplanted ECFC-derived vessels accounted for 28% ± 4.2% of the vessels in the ITGB1-ECFC group, with no cell fusion. Intravenous administration of ECFCs engineered to home to ischemic tissue appears to efficiently mediate therapeutic angiogenesis in a mouse model of peripheral artery disease.
Significance
The intravenous administration of endothelial colony-forming cells (ECFCs) genetically modified to overexpress integrin β1 effectively stimulated angiogenesis in ischemic mouse hindlimbs. Transplanted ECFCs were observed in the ischemic leg tissue, even at the chronic stage. Moreover, the cells appeared functional, as evidenced by the improved blood flow. The cell type used (ECFCs), the route of administration (intravenous, not directly injected into the affected area), and the use of ligand-receptor interactions (extracellular matrix and integrins) for homing represent substantial advantages over previously reported cell therapies for the treatment of peripheral artery disease.
Introduction
Peripheral artery disease (PAD) is most commonly caused by arteriosclerosis obliterans. The incidence of PAD has been increasing in recent years, in large part because of the growing prevalence of diabetes mellitus and hypertension and the overall aging of the population [1]. The current treatments of PAD include anticoagulant agents and antiplatelet drugs, percutaneous transluminal angioplasty, and bypass surgery. However, the prognosis for PAD patients remains poor, and amputation of the lower extremities is often required [2]. One promising new therapeutic strategy for PAD is enhancement of angiogenesis and collateral arterial growth. Angiogenesis can be achieved through the use of growth factors or the genes encoding them. The limited clinical data available from protein and gene delivery trials suggest that both approaches are generally safe, although additional experience is needed to resolve remaining safety concerns about the possible potentiation of pathological angiogenesis (e.g., malignancy) and the so-called bystander effects of the delivered factors (e.g., effects on the kidneys or atheroma) [3].
Regenerative medicine using stem or progenitor cells is a potential alternative to growth factors or their genes for the treatment of PAD [4, 5]. The most important issues with such cell therapies are the choice of an “optimal” cell type and the “best” mode of delivery [6]. For example, cells that are too immature can differentiate into unwanted cell types or become malignant. Endothelial progenitor cells (EPCs) in the CD34+ stem cell fraction of adult human peripheral blood participate in postnatal neovascularization after mobilization from bone marrow [7, 8]. EPCs consist of diverse progenitor-like cell populations, including early-outgrowth EPCs and late-outgrowth endothelial colony-forming cells (ECFCs). Unlike early EPCs, ECFCs have an endothelial morphology, express endothelial markers, are highly proliferative, and exhibit a progenitor-like capacity for self-renewal [7–10].
Progenitor cells are frequently delivered by direct injection into the injured tissue. However, direct delivery is probably not the best approach for patients with PAD. It seems unlikely that cells injected into uniformly ischemic tissue, which lacks the blood flow necessary to deliver oxygen and nutrients, would survive long enough for engraftment. Also, an earlier study showed that most cells will simply die if injected directly into ischemic tissue [11]. In that context, we hypothesized that noninvasive incorporation of circulating progenitor cells into the ischemic tissue through a homing mechanism would be an effective alternative to direct injection. Therefore, our aim in the present study was to develop a therapeutic angiogenesis protocol for treating ischemic legs using genetically engineered ECFCs that, after intravenous administration, would efficiently home to the injured tissue where they could be noninvasively incorporated. To accomplish this, we genetically modified ECFCs to overexpress integrin β1. We surmised that while circulating in the blood, integrin β1-expressing cells would naturally home to ischemic tissue, because it abundantly expresses extracellular matrix (ECM) proteins, the ligands for integrins [12–14]. In addition, integrin β1 has been reported to have an independent angiogenesis stimulating function [15, 16], which we expected could also result in beneficial effects on ischemic legs.
Materials and Methods
Endothelial Progenitor Cell Preparation
Late-outgrowth ECFCs were purchased from Lonza Biologics (CL00189423; Lonza Biologics, Portsmouth, NH, http://www.lonza.com). The ECFCs were cultured on type I collagen (354236; BD Biosciences, San Diego, CA, http://www.bdbiosciences.com) in endothelial growth medium 2 (CC-3162; EGM2 Bullet Kit; Lonza Biologics) containing 10% ECFCs Serum Supplement (00190284; Lonza Biologics). The cells were passaged every 2–3 days using trypsin/EDTA solution (CC-5012; Lonza Biologics) and were maintained in a humidified incubator at 37°C in a 5% CO2 atmosphere. The medium was changed every other day.
Lentiviral Vectors
Human β1 integrin (ITGB1) vector was purchased from Addgene (pRK5 beta1, plasmid 16042; Addgene, Cambridge, MA, https://www.addgene.org) [17]. The lentiviral vector pLV.CMV-ITGB1 was constructed by inserting the ITGB1 gene at the BamHI and XhoI sites of the pLenti6.3/V5-DEST plasmid (Invitrogen, Carlsbad, CA, http://www.invitrogen.com). Lentiviruses were produced by transfecting pLV.CMV-ITGB1 expression vectors and Virapower packing mix (Invitrogen) into 293FT cells, as instructed by the manufacturer. Twenty-four hours before transfection, sixth-passage ECFCs were seeded at 1 × 106 cells per dish onto 100-mm dishes coated with collagen I in EGM2, as described above. At 70%–80% confluence, the ECFCs were exposed to 5 ml of virus solution in 5 ml of complete medium for 24 hours and were harvested on day 2 after infection. Overexpression of ITGB1 was confirmed by Western blot analysis (ITGB1-ECFCs). pLV.CMV-enhanced green fluorescent protein (GFP) was used as a control vector to generate control cells (GFP-ECFCs).
In Vitro Homing Experiment
As an in vitro homing experiment, we studied the ability of the ECFCs to stick to the bottom of the dishes. GFP-ECFCs or ITGB1-ECFCs (5 × 105 cells each) were placed on 35-mm dishes coated with fibronectin and incubated for 15 minutes at 37°C. The dishes were then gently washed with phosphate-buffered saline (PBS) three times, and the cells attaching to the bottom were counted.
Animal Experimental Protocols
Our institutional animal research committee approved the present study, which conformed to the U.S. NIH Guide for the Care and Use of Laboratory Animals (NIH publication no. 85–23, revised 1996). Male 9-week-old NOD/Shi-scid, IL-2Rγnull mice were purchased from CLEA Japan Inc. (Tokyo, Japan, http://www.clea-japan.com). This strain is an excellent recipient mouse model for the engraftment of human cells; when human CD34+ cells from umbilical cord blood were transplanted into this strain, the engraftment rate in the peripheral circulation, spleen, and bone marrow was significantly higher than that in other established immunodeficiency strains [18]. Under sufficient anesthesia with ketamine HCl (100 mg/kg) and xylazine HCl (10 mg/kg), the local fur was removed using depilatory cream. Hindlimb ischemia was then induced by complete ligation of the right femoral artery at a point just below the inguinal ligament, as described previously [19]. In the sham-operated mice, the suture was passed through but not tied. We first assessed the expression of several ECM proteins, including fibronectin, laminin, collagen type I, and collagen type IV, in the hindlimb muscles of untreated mice at 1, 3, 7, 14, and 28 days after surgery (n = 3 each).
In the experiment, the mice were randomly assigned to a control group that received a PBS injection, a second control group that received GFP-transfected ECFCs (GFP-ECFCs), a third control group that received local treatment of integrin β1-transfected ECFCs (ITGB1-ECFCs) by intramuscular injection, or a group that received a systemic treatment of ITGB1-ECFCs by intravenous injection. PBS (500 μl) or ECFCs (1 × 105 cells dissolved in 500 μl of PBS) were administered via the tail vein 24 hours after ligation of the femoral artery. In the local treatment group, 100 μl of ITGB1-ECFCs was injected intramuscularly into five points of the ischemic hindlimb. At the times indicated, the hindlimb tissues were harvested for histological examination, immunohistochemistry, and Western blotting. In addition, three mice from each group were used for observation of blood vascular casts.
Laser Doppler Imaging
Under anesthesia, the hair was removed from both legs using a depilatory cream, after which the mice were placed on a heating plate at 37°C for 10 minutes to minimize temperature variations. The ischemic limb (right)-to-nonischemic limb (left) blood flow ratio was measured using a laser Doppler perfusion imager (Moor Instruments, Wilmington, DE, http://www.us.moor.co.uk), which provides noninvasive measurement of the blood flow by determining the Doppler frequency shift for light reflected off the moving red blood cells. These data were acquired using a method similar to that described by Rivard et al. [20]. In brief, the sedated mice were secured on a monochromatic surface, and an area of 11 × 11 cm was scanned from the lower abdomen to the end of the toes. Color images were obtained, and the hindlimb perfusion ratios were determined by comparing the perfusion of the hindlimbs before surgery and 1, 3, 7, 14, and 28 days after surgery. Ischemic Doppler ratios were determined for all the groups and compared.
Histological Examination and Immunohistochemistry
The expression of CD34 and integrin β1 in ECFCs was assessed immunohistochemically using anti-human CD34 (14486-1; Proteintech Group, Inc., Chicago, IL, http://www.ptglab.com) and anti-integrin β1 antibodies (MAB1965; EMD Millipore, Billerica, MA, http://www.emdmillipore.com) as the primary antibodies and Alexa Fluor 568 anti-mouse IgG and Alexa Fluor 488 anti-rabbit IgG antibody (Invitrogen) as the respective secondary antibodies. The nuclei were stained with Hoechst 33354. The cells were observed under a laser scanning confocal microscope (C2; Nikon, Tokyo, Japan, http://www.nikon.com).
For histological examination of the ischemic and nonischemic tissue samples, the lower calf muscles were fixed with 4% paraformaldehyde, embedded in OCT compound (Miles Scientific, Jacksonville, FL) and snap-frozen in liquid nitrogen or embedded in paraffin. The tissue sections (10 μm thick in frozen sections and 4 μm thick in paraffin-embedded sections) were incubated with primary antibodies against fibronectin (ab23750) and Flk-1, cross-reacting with human but not mouse endothelial cells (ab38464; both from Abcam); CD31, cross-reacting with both mouse and human endothelial cells (sc-1506; Santa Cruz Biotechnology Inc., Santa Cruz, CA, http://www.scbt.com); CD31, cross-reacting with mouse but not human endothelial cells (DIA310; Dianova, Hamburg, Germany, http://www.dianova.com); and α-smooth muscle actin (α-SMA; M0851; Dako, Glostrup, Denmark, http://www.dako.com). An ABC kit (Vector Laboratories, Burlingame, CA, http://www.vectorlabs.com) was then used for immunostaining, with diaminobenzidine HCl serving as the chromogen. The nuclei were counterstained with hematoxylin. For immunofluorescence, the secondary antibodies used were Alexa Fluor 488 and 568, and the nuclei were stained with Hoechst 33342. Texas Red Lycopersicon esculentum lectin (TL-1176; Vector Laboratories) was used to stain both human and mouse endothelial cells [21]. Quantitative assessments, including the number or area of immunopositive cells, were made in 20 randomly chosen high-power fields (HPFs; ×400) using a multipurpose color image processor (Win ROOF, Mitani Corporation, Tokyo, Japan, http://www.mitani-corp.co.jp). Other main organs, including the heart, lungs, liver, and spleen, were excised and examined histologically and immunohistochemically.
Scanning Electron Microscopy of Blood Vascular Casts
The three-dimensional vascular structure of the ischemic hindlimbs was visualized using scanning electron microscopy of the blood vascular casts [22, 23]. In brief, the abdominal aorta was cannulated with a 24-gauge intravenous catheter (SURFLO; Terumo Medical Products, Tokyo, Japan, http://www.terumomedical.com), after which the limbs were antegradely irrigated with saline. Mercox II Resin (Ladd Research Industries, Willington, VT, http://www.laddresearch.com), a plastic resin supplemented with the catalyst, was then injected through the aorta under moderate pressure (10 ml/min) until the hindlimbs were filled with the injected resin, approximately 5 minutes. The hindlimbs were then macerated completely using 20% sodium hydroxide in an ultrasonic generator, washed in water, and dried. Once dry, the casts were conventionally prepared for scanning electron microscopy (Hitachi S-450; Hitachi, Tokyo, Japan, http://www.hitachi.com).
Western Blot Analysis
Lysates from the ECFCs collected 3 days after gene transfection and from homogenates of hindlimb muscle tissue collected 1, 3, 7, 14, or 28 days after surgery were used for Western blot analysis. Proteins (10 μg) were separated and transferred to membranes using standard protocols, after which they were probed with antibodies against integrin α5 (AB1928; EMD Millipore), integrin β1 (MAB1965; EMD Millipore), fibronectin (ab23750; Abcam), laminin (ab11575; Abcam), collagen type I (ab21286; Abcam), and collagen type IV (ab6586; Abcam). Three to five specimens from each muscle sample from the ischemic and nonischemic hindlimbs were subjected to Western blotting. The blots were visualized using chemiluminescence (Amersham Biosciences, Piscataway, NJ, http://www.amersham.com), and the signals were quantified by densitometry. Glyceraldehyde-3-phosphate dehydrogenase (sc32233; Santa Cruz Biotechnology) served as the loading control.
Statistical Analysis
Values are shown as the mean ± SEM. The significance of the differences between groups was evaluated using one-way analysis of variance (ANOVA), followed by the Newman-Keuls multiple comparison test. For statistical analysis of the blood flow ratios, we used repeated-measures ANOVA. Values of p < .05 were considered significant.
Results
Integrin Expression in Genetically Modified ECFCs
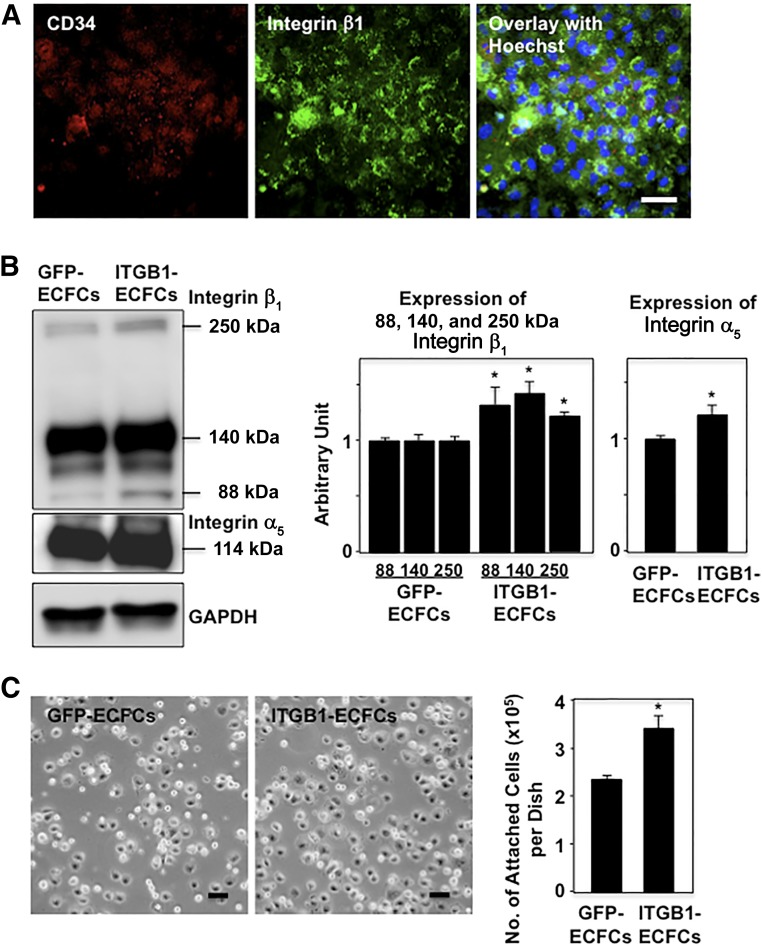
Confocal microscopic observation revealed that human ECFCs transfected with mouse integrin β1-harboring lentivirus (ITGB1-ECFCs) express both human CD34 and mouse integrin β1 (Fig. 1A). Western blotting revealed a significantly stronger expression of integrin β1 with molecular weights of 88 and 140 kDa in ITGB1-ECFCs than in ECFCs transfected with GFP (GFP-ECFCs; Fig. 1B). Such multiple molecular weights might reflect either splicing variants or sugar remodeling (sialylation) of integrin β1 [24]. We also noted an increase of 250-kDa integrin β1 on the blots. The expression of integrin α5 with 114 kDa was also more increased in the integrin β1 transfectants than in the GFP transfectants. The simultaneous upregulation of integrin α5 and integrin β1 in ITGB1-ECFCs suggests increased expression of integrin α5β1, a fibronectin receptor. Consistent with that idea, the molecular weights for integrin β1 (140 kDa) and integrin α5 (114 kDa) in our Western blots suggest the possible formation of an integrin α5β1 dimer (250 kDa).
Figure 1.
Genetically modified human ECFCs. (A): Confocal micrographs showing CD34 (red) and integrin β1 (green) immunostaining in human ECFCs transfected with integrin β1. Integrin β1 localized strongly at the cell surface. Scale bar = 50 μm. (B): Western blotting for integrins. In addition to integrin β1 (88 and 140 kDa), integrin α5 (114 kDa) was more strongly coexpressed in ITGB1-ECFCs than in GFP-ECFCs. The band at approximately 250 kDa suggests possible dimer formation between integrins β1 and α5 to form integrin α5β1, a fibronectin receptor. Graphs show densitometry for integrin β1 and integrin α5 (n = 3 each). (C): In vitro homing experiment for ECFCs. A significantly greater number of the cells from the ITGB1-ECFC group attached to the bottom of fibronectin-coated dishes compared with those from the GFP-ECFC group. Scale bars = 50 μm; ∗, p < .05 versus the GFP-ECFC group. Abbreviations: ECFCs, endothelial colony-forming cells; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GFP, green fluorescent protein; ITGB1, lentiviral vector encoding integrin β1.
We cultured the ECFCs on fibronectin-coated dishes for 15 minutes to examine the homing capacity of the ECFCs in the in vitro setting. The number of ITGB1-ECFCs attaching to the bottom of the dishes was significantly greater than that of the GFP-ECFCs (GFP, 2.3 ± 0.07 × 105 cells per dish vs. ITGB1, 3.3 ± 0.24 × 105 cells per dish; p < .05; Fig. 1C).
Expression of ECM Proteins in the Ischemic Legs
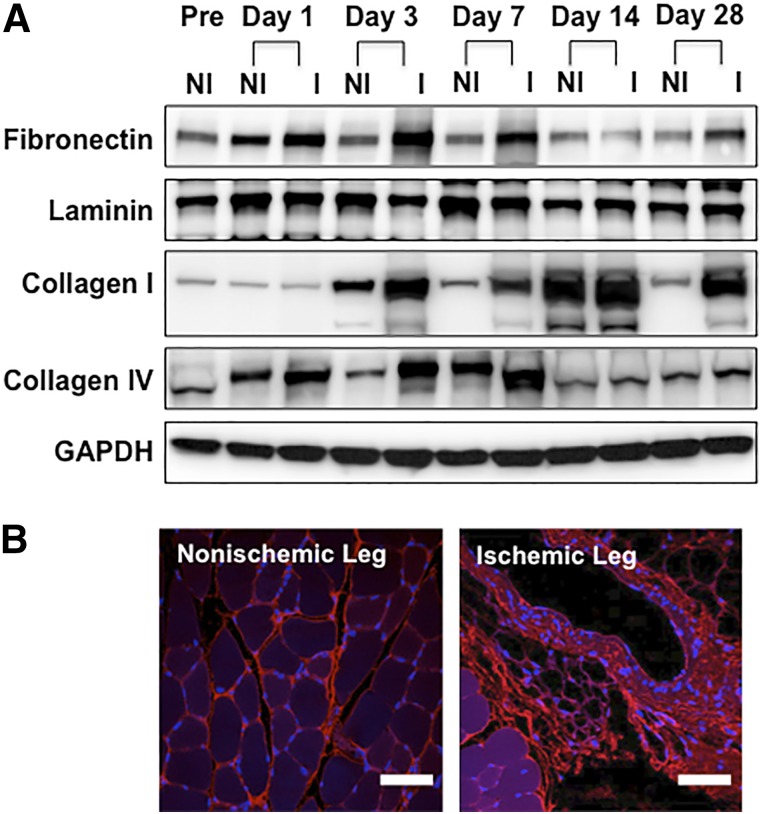
We examined the expression of four ECM proteins (fibronectin, laminin, collagen type I, and collagen type IV) in mouse hindlimbs after occlusion of the femoral artery. Western blotting showed augmented expression of all four proteins in the ischemic legs after surgery (Fig. 2A), and the enhanced expression of fibronectin and collagen type I persisted for the entire 28-day observation period. Confocal microscopy revealed strong fibronectin immunofluorescence in the interstitium of the ischemic tissue 28 days after surgery (Fig. 2B).
Figure 2.
Expression of extracellular matrix proteins in hindlimb tissues after ligation of the femoral artery. (A): Western blotting for fibronectin, laminin, collagen types I and IV, and GAPDH (control) in the nonischemic and ischemic legs of mice before surgery and 1, 3, 7, 14 and 28 days after surgery. (B): Immunofluorescence showing strong expression of fibronectin (red) in the ischemic hindlimbs at the chronic stage after femoral artery ligation (28 days after surgery). Scale bars = 50 μm. Abbreviations: GAPDH, glyceraldehyde-3-phosphate dehydrogenase; I, ischemic; NI, nonischemic; Pre, preoperatively.
Blood Flow in the Ischemic Legs
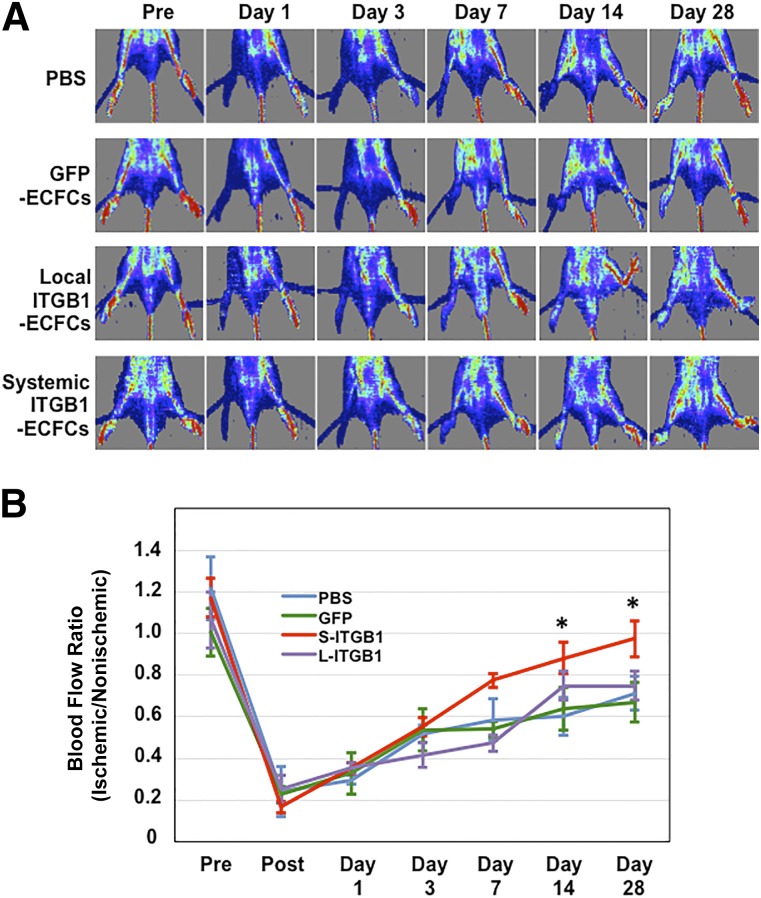
Laser Doppler imaging showed that blood flow in the ischemic hindlimbs was reduced equally in all groups immediately after femoral artery ligation. Although blood perfusion of the ischemic limb progressively increased after ligation in all four groups, the perfusion was significantly greater in the group administered ITGB1-ECFCs (ischemic-to-nonischemic limb flow ratio, 0.97 ± 0.09) than in any of the three control groups (Fig. 3).
Figure 3.
Blood flow in the ischemic hindlimbs. (A): Laser Doppler blood perfusion images. (B): Blood perfusion in ischemic hindlimbs was measured before and 1, 3, 7, 14, and 28 days after right femoral artery ligation (n = 6 in each group). Results are expressed as the ratio of the right (ischemic) to the left (nonischemic) limb perfusion. ∗, p < .05 versus the PBS, GFP-ECFC, and local ITGB1-ECFC groups. Abbreviations: ECFC, endothelial colony-forming cell; GFP, green fluorescent protein; ITGB1, lentiviral vector encoding integrin β1; L, local; PBS, phosphate-buffered saline; Pre, preoperatively; S, systemic.
Vasculature in the Ischemic Legs
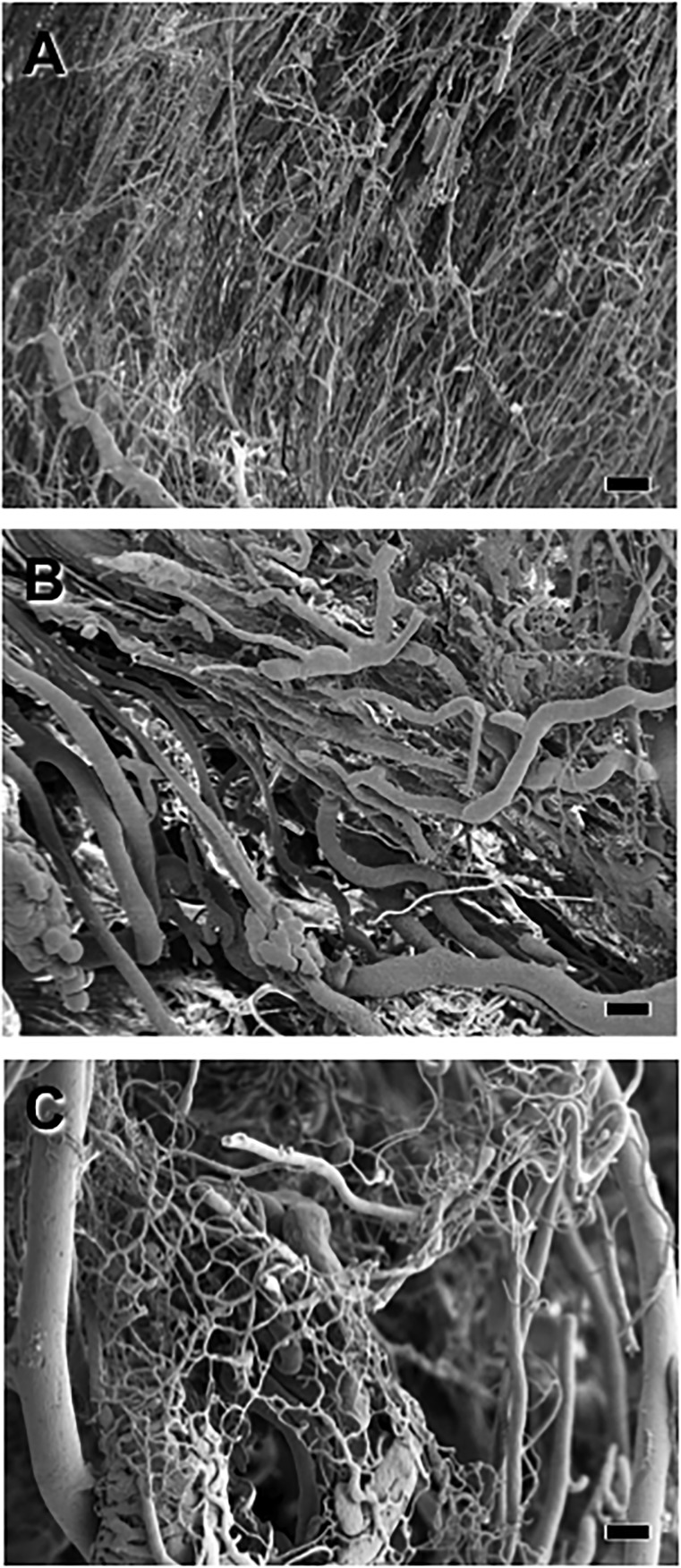
Casts of the hindlimb vascular beds in the sham-operated mice revealed an elaborate vascular plexus containing arteries, arterioles, capillaries, veins, and venules (Fig. 4A). Twenty-eight days after surgery, examination of the ischemic hindlimb revealed that occlusion of the femoral artery had greatly diminished the vascular beds in the mice treated with PBS (Fig. 4B) or GFP-ECFCs (data not shown). In contrast, in the mice administered ITGB1-ECFCs, the vascular beds of all types were substantially restored 28 days after surgery (Fig. 4C).
Figure 4.
Scanning electron photomicrographs of vascular casts of the hindlimbs 28 days after femoral artery ligation. (A): Vascular casts from a sham-operated mouse showing normal vascular profiles. (B): Vascular casts showing the less dense vasculature in the ischemic leg of a phosphate-buffered saline-treated mouse. (C): Vascular casts from the ischemic hindlimb of a mouse systemically treated with endothelial colony-forming cells overexpressing integrin β1. Note that the vasculature was substantially restored in this mouse. Scale bars = 10 μm.
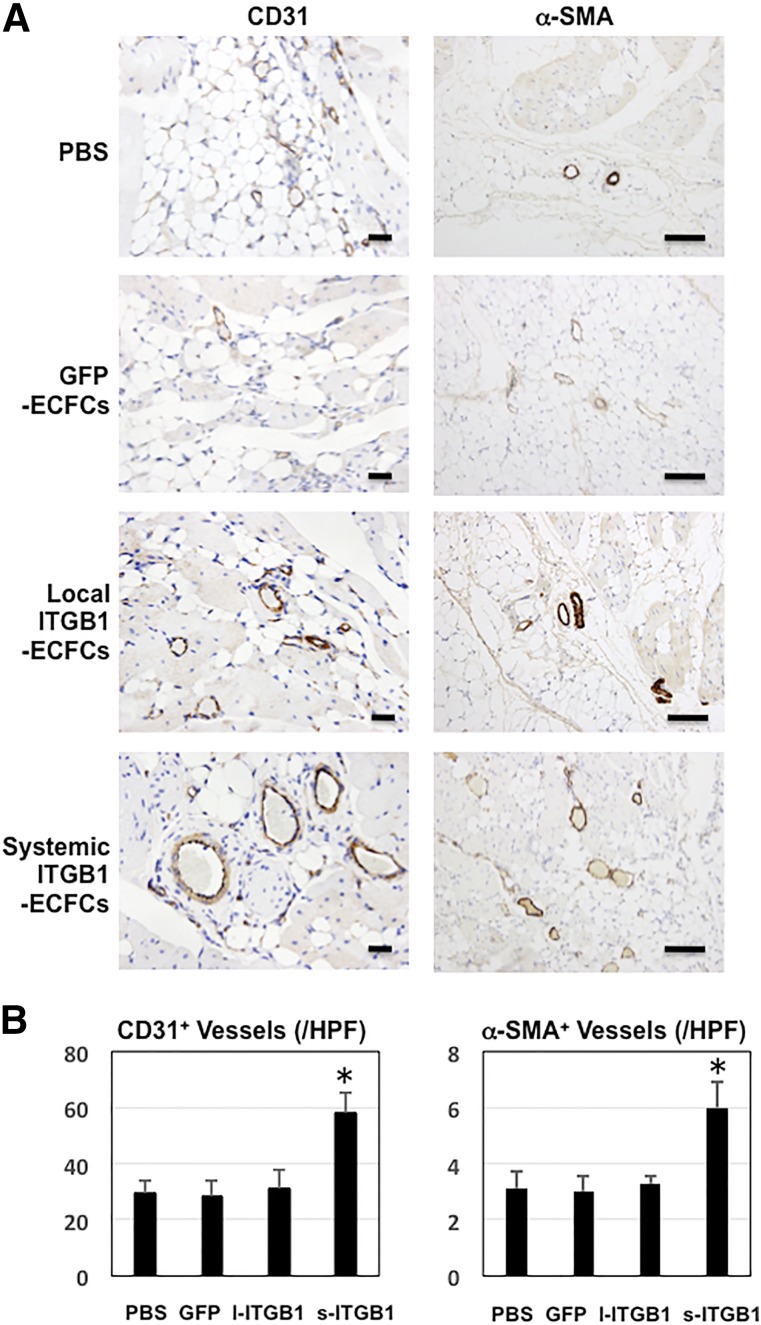
Immunohistochemical detection of CD31 (mouse and human), an endothelial cell marker, and of α-SMA, an arteriole marker, showed that both the overall vessel (mainly capillary) density and the density of the arterioles were greater in the ischemic legs of mice treated with ITGB1-ECFCs than in those treated otherwise. As shown in Figure 5, vessel formation was enhanced to a greater degree in the systemic ITGB1-ECFC group (58 ± 6.8 vessels per HPF) than in the control groups (PBS group, 29 ± 4.9 vessels per HPF; GFP-ECFC group, 28 ± 5.8 vessels per HPF; and local ITGB1-ECFC group, 31 ± 6.5 vessels per HPF; p < .05). The systemic ITGB1-ECFC group also exhibited a greater density of α-SMA-positive arterioles (6.0 ± 0.9 arterioles per HPF) than any of the control groups (PBS group, 3.1 ± 0.6 arterioles per HPF; GFP-ECFC group, 3.0 ± 0.6 arterioles per HPF; and local ITGB1-ECFC group, 3.3 ± 0.3 arterioles per HPF; p < .05). It is noteworthy that not only the capillary density, but also the density of α-SMA-positive arterioles, were increased by the systemic treatment with ECFCs overexpressing integrin β1. Perhaps the ECFC-derived capillaries were able to mature into arterioles. In the nonischemic legs, no significant difference was seen in the overall vessel or arteriole densities among the three groups and those densities were all similar to the vascular density in the sham-operated group, indicating no effect of treatment on the vessels in the nonischemic legs.
Figure 5.
Vascular immunohistochemistry. (A): Photomicrographs showing immunohistochemical staining of CD31- and α-SMA-positive lumens revealing total vessels (mainly capillaries) (left) and small arteries (right) in ischemic hindlimb tissues 28 days after surgery (n = 6 in each group), respectively. Scale bars = 50 μm. (B): Graphs showing densities of total vessels (left) and small arteries (right) in each group (n = 6 in each group). ∗, p < .05 versus the PBS, GFP-ECFC, and local ITGB1-ECFC groups. Abbreviations: α-SMA, α-smooth muscle actin; ECFC, endothelial colony-forming cell; GFP, green fluorescent protein; HPF, high-power fields; l, local; ITGB1, lentiviral vector encoding integrin β1; s, systemic.
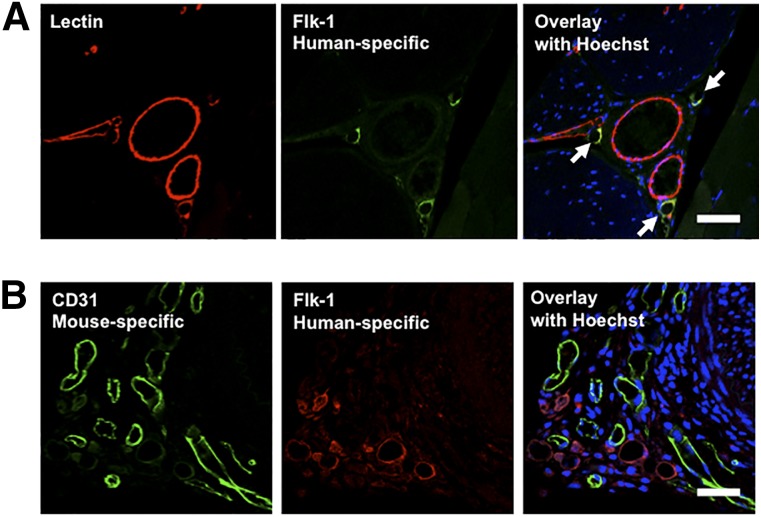
Using immunohistochemistry with an anti-human Flk-1 antibody, we next studied the origin of vessels in the ischemic hindlimb tissue. This antibody does not react with mouse Flk-1. As shown in Figure 6A, human Flk-1-positive, and thus ECFC-derived, vessels were frequently found in the ischemic hindlimbs of mice systemically administered ITGB1-ECFCs, accounting for 28% ± 4.2% of the total vessels. In contrast, human Flk-1-positive vessels were never found in any of the control groups (0% ± 0%; p < .05 vs. the systemic ITGB1-ECFC group). No endothelial cells immunopositive for the human Flk-1 were observed in the other main organs (i.e., heart, lungs, liver, and spleen) of any groups.
Figure 6.
Vascular immunofluorescence of the ischemic hindlimb of a mouse systemically treated with ECFCs overexpressing integrin β1. (A): Origin of the detected endothelial cells. Detection of total vessels using Lycopersicon esculentum lectin and human vessels (arrows) using anti-human Flk-1 antibody. Scale bars = 50 μm. (B): Testing for cell fusion by double immunostaining for human- and mouse-derived endothelial cells using anti-human Flk-1 (cross-reacting with human but not with mouse endothelial cells) and anti-mouse CD31 (cross-reacting with mouse but not with human endothelial cells) antibodies. No detected endothelial cells were positive for both antibodies, indicating no cell fusion. Scale bar = 50 μm.
To determine whether fusion had occurred between the transplanted ECFCs and the host endothelial cells, we double immunostained for human and murine endothelial cells using anti-human Flk-1 and anti-mouse CD31 antibodies. We found no endothelial cells that were positive for both antibodies, indicating no cell fusion (Fig. 6B).
Discussion
The utility of stem cells derives in part from their pluripotency, which enables them to differentiate into a variety of cell types. However, this feature can also be problematical in cell therapies, because a risk exists that stem cells will differentiate into unwanted cell types or even become malignant. ECFCs, however, are well-characterized progenitor cells that contribute to postnatal neovascularization after mobilization from the bone marrow [5, 6]. The CD34+ stem cell fraction of adult human peripheral blood has already been used in two preclinical trials of therapeutic angiogenesis for PAD. One trial showed significant improvements in the efficacy scores (toe-brachial pressure index, pain scale, and total walking distance); however, the other study showed no effect on amputation rates [25, 26]. Neither trial reported any treatment-associated adverse events; thus, the safety and feasibility of ECFC-based therapy can be considered established.
In both of these trials, the ECFCs were delivered through intramuscular injection into the ischemic legs. We suggest this delivery method would not allow the injected cells to receive sufficient oxygen and nutrients for engraftment. In the present study, however, the ECFCs were intravenously administered and were expected to reach the injured tissue through the circulation. In addition, the cells were genetically modified to overexpress integrin β1. Integrins serve as receptors for various ECM proteins (e.g., fibronectin, laminin, and collagen, among others) [12–14]. Integrin β1 was chosen for use in the present study in an effort to facilitate homing of the ECFCs to the ischemic tissue, which would be expected to abundantly express ECM. Although ECFCs have been genetically modified in a number of earlier studies, most of those involved overexpression of angiogenic factors (e.g., vascular endothelial growth factor, hypoxia inducible factor-1α, hepatocyte growth factor, or endothelial nitric oxide synthase) in an effort to strengthen or accelerate the angiogenic function of ECFCs, not to facilitate homing [27–32]. Our approach might be unique in that context. We found that integrin α5 was also overexpressed in ECFCs transfected with integrin β1. Furthermore, our results suggest that integrins α5 and β1 dimerized to form integrin α5β1, a ligand for fibronectin. This result is not unprecedented. Coinduction of endogenous integrin monomers is known to occur with integrin transfection, although the precise mechanism remains unclear [12, 13].
Stromal cell-derived factor (SDF)-1 is an important chemoattractant for circulating CXCR4-positive cells, including CD34-positive EPCs, which is expressed mainly by vascular endothelial and smooth muscle cells within damaged tissues [33]. For example, SDF-1 appears to play a critical role in the mobilization of CXCR4+ cells into myocardial tissues after infarction [33–35]. However, in animal models of myocardial infarction, SDF-1 expression in the infarcted myocardium was enhanced on day 3 after infarction, but the upregulation had disappeared by day 7 [33, 36, 37]. This relatively short interval of SDF-1 expression in ischemic tissue suggests the SDF-1/CXCR4 axis would not be suitable for use as a homing mechanism under conditions of chronic ischemia. In contrast, ECM proteins such as fibronectin and collagens are persistently expressed, even in scar tissue. In addition, mesenchymal stem cells reportedly use integrin β1, not CXCR4, for migration and engraftment into myocardial tissue [38]. Taken together, these data suggest integrin β1 would be an appropriate homing molecule for use in cases of chronic ischemia such as PAD.
We confirmed in vitro the homing capacity of the ITGB1-ECFCs to fibronectin and the in vivo homing and differentiation into endothelial cells by immunohistochemistry. We furthermore confirmed the absence of the cell fusion phenomenon between the ECFC-derived human and recipient mouse endothelial cells. However, this does not deny the possibility that human ECFC-derived vasculature connect to constitute the branches of mice vasculature to improve blood flow. However, integrin β1 has been reported to have an independent angiogenesis-stimulating function, and one of the principle downstream effector molecules for integrin β1 is vascular endothelial growth factor (VEGF) [15, 16, 39, 40]. The excreted VEGF from exogenous ECFCs could stimulate new blood vessel formation, not only from exogenous human ECFCs, but also from endogenous mouse ECFCs. Therefore, the improved therapeutic effect observed in the present study might not be entirely concluded as a response to integrin β1-overexpressing exogenous ECFCs only. It remains to be determined which of the functions of ITGB1-ECFCs, homing or angiogenesis, is more important for the benefits, although both would be desirable in clinical situations.
The protocol used in the present study had several limitations. First, it entailed simultaneous cell and gene therapy, and no consensus has yet been reached on the safety of virus-mediated gene therapy. Second, the treatment was started 1 day after the onset of ischemia in our protocol. Clinically, however, patients typically do not consult a physician until the chronic stage, after they have experienced ischemia for a considerable period. We have confirmed that at least two ECM proteins, including fibronectin, are strongly expressed during the chronic stage, but we have not yet confirmed the effectiveness of our treatment when it is started during the chronic stage. Nevertheless, we believe that the present protocol provides an important hint to a less-invasive and more effective cell therapy for PAD than previously reported. Particularly important is the intravenous route of administration. Our systemic treatment with ITGB1-ECFCs resulted in a significantly better therapeutic advantage compared with local treatment. The former might have homed the progenitor cells to the injured tissue where they could have been noninvasively incorporated without being exposed to lethal ischemia. In leukemia patients, bone marrow cell transplantation is performed intravenously through drip infusion, which enables the cells to be effectively conveyed to the patients’ bone marrow. Mimicking that approach, intravenous administration of integrin-expressing ECFCs could prove to be an effective therapeutic strategy for the future treatment of PAD.
Conclusion
The major conclusion of the present study is that intravenous administration of ECFCs genetically modified to overexpress integrin β1 effectively stimulated angiogenesis in ischemic mouse hindlimbs. Transplanted ECFCs were observed in the ischemic leg tissue, even at the chronic stage; moreover, the cells appeared functional, as evidenced by the improved blood flow. We suggest that the cell type used (ECFCs), the route of administration (intravenous, not directly injected into the affected area), and our use of ligand-receptor interactions (ECM and integrins) for homing represent substantial advantages over previously reported cell therapies for the treatment of PAD.
Acknowledgments
The present study was supported in part by a Grant in Aid from the Ministry of Education, Science, and Culture of Japan (Grant 24591093 to G.T.). We thank Natsuko Ishigami, Chika Ogawa, and Megumi Minagawa of Gifu University and Rieko Hori and Norie Soga of Asahi University for secretarial assistance. We also thank Filippo Giancotti, who provided the ITGB1 plasmid.
Author Contributions
K.G.: conception and design, collection and/or assembly of data, data analysis and interpretation; G.T.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; T.T. and K.-i.K.: provision of study material or patients; H.O., H.K., I.K., T.W., K.M., A.T., N.M., H.U., M.K., and A.M.: collection and/or assembly of data, data analysis and interpretation; S.M.: final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Second European Consensus Document on chronic critical leg ischemia. Circulation. 1991;84(suppl):IV1–IV26. [PubMed] [Google Scholar]
- 2.Dormandy JA, Rutherford RB. Management of peripheral arterial disease (PAD). TASC Working Group. TransAtlantic Inter-Society Consensus (TASC) J Vasc Surg. 2000;31:S1–S296. [PubMed] [Google Scholar]
- 3.Simons M, Bonow RO, Chronos NA, et al. Clinical trials in coronary angiogenesis: Issues, problems, consensus: An expert panel summary. Circulation. 2000;102:E73–E86. doi: 10.1161/01.cir.102.11.e73. [DOI] [PubMed] [Google Scholar]
- 4.Rinkevich Y, Lindau P, Ueno H, et al. Germ-layer and lineage-restricted stem/progenitors regenerate the mouse digit tip. Nature. 2011;476:409–413. doi: 10.1038/nature10346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang S, Wei J, Pentinmikko N, et al. Generation of functional blood vessels from a single c-kit+ adult vascular endothelial stem cell. PLoS Biol. 2012;10:e1001407. doi: 10.1371/journal.pbio.1001407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dimmeler S, Zeiher AM, Schneider MD. Unchain my heart: The scientific foundations of cardiac repair. J Clin Invest. 2005;115:572–583. doi: 10.1172/JCI24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 8.Asahara T, Masuda H, Takahashi T, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 9.Baker CD, Ryan SL, Ingram DA, et al. Endothelial colony-forming cells from preterm infants are increased and more susceptible to hyperoxia. Am J Respir Crit Care Med. 2009;180:454–461. doi: 10.1164/rccm.200901-0115OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ingram DA, Mead LE, Tanaka H, et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 11.Beauchamp JR, Morgan JE, Pagel CN, et al. Dynamics of myoblast transplantation reveal a discrete minority of precursors with stem cell-like properties as the myogenic source. J Cell Biol. 1999;144:1113–1122. doi: 10.1083/jcb.144.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hynes RO. Integrins: Versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 13.Stupack DG, Cheresh DA. Get a ligand, get a life: Integrins, signaling and cell survival. J Cell Sci. 2002;115:3729–3738. doi: 10.1242/jcs.00071. [DOI] [PubMed] [Google Scholar]
- 14.Ross RS. Molecular and mechanical synergy: Cross-talk between integrins and growth factor receptors. Cardiovasc Res. 2004;63:381–390. doi: 10.1016/j.cardiores.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 15.Bloch W, Forsberg E, Lentini S, et al. Beta 1 integrin is essential for teratoma growth and angiogenesis. J Cell Biol. 1997;139:265–278. doi: 10.1083/jcb.139.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Renz M, Otten C, Faurobert E, et al. Regulation of β1 integrin-Klf2-mediated angiogenesis by CCM proteins. Dev Cell. 2015;32:181–190. doi: 10.1016/j.devcel.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 17.Giancotti FG, Ruoslahti E. Elevated levels of the alpha 5 beta 1 fibronectin receptor suppress the transformed phenotype of Chinese hamster ovary cells. Cell. 1990;60:849–859. doi: 10.1016/0092-8674(90)90098-y. [DOI] [PubMed] [Google Scholar]
- 18.Ito M, Hiramatsu H, Kobayashi K, et al. NOD/SCID/gamma(c)(null) mouse: An excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- 19.Li L, Okada H, Takemura G, et al. Sustained release of erythropoietin using biodegradable gelatin hydrogel microspheres persistently improves lower leg ischemia. J Am Coll Cardiol. 2009;53:2378–2388. doi: 10.1016/j.jacc.2009.02.056. [DOI] [PubMed] [Google Scholar]
- 20.Rivard A, Silver M, Chen D, et al. Rescue of diabetes-related impairment of angiogenesis by intramuscular gene therapy with adeno-VEGF. Am J Pathol. 1999;154:355–363. doi: 10.1016/S0002-9440(10)65282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McLean JW, Fox EA, Baluk P, et al. Organ-specific endothelial cell uptake of cationic liposome-DNA complexes in mice. Am J Physiol. 1997;273:H387–H404. doi: 10.1152/ajpheart.1997.273.1.H387. [DOI] [PubMed] [Google Scholar]
- 22.Murakami T. Application of the scanning electron microscope to the study of the fine distribution of the blood vessels. Arch Histol Jpn. 1971;32:445–454. doi: 10.1679/aohc1950.32.445. [DOI] [PubMed] [Google Scholar]
- 23.Lametschwandtner A, Lametschwandtner U, Weiger T. Scanning electron microscopy of vascular corrosion casts—Technique and applications. Scan Electron Microsc. 1984;((Pt 2):):663–695. [PubMed] [Google Scholar]
- 24.Seales EC, Shaikh FM, Woodard-Grice AV, et al. A protein kinase C/Ras/ERK signaling pathway activates myeloid fibronectin receptors by altering beta1 integrin sialylation. J Biol Chem. 2005;280:37610–37615. doi: 10.1074/jbc.M508476200. [DOI] [PubMed] [Google Scholar]
- 25.Kawamoto A, Katayama M, Handa N, et al. Intramuscular transplantation of G-CSF-mobilized CD34(+) cells in patients with critical limb ischemia: A phase I/IIa, multicenter, single-blinded, dose-escalation clinical trial. Stem Cells. 2009;27:2857–2864. doi: 10.1002/stem.207. [DOI] [PubMed] [Google Scholar]
- 26.Losordo DW, Kibbe MR, Mendelsohn F, et al. A randomized, controlled pilot study of autologous CD34+ cell therapy for critical limb ischemia. Circ Cardiovasc Interv. 2012;5:821–830. doi: 10.1161/CIRCINTERVENTIONS.112.968321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lavoie JR, Stewart DJ. Genetically modified endothelial progenitor cells in the therapy of cardiovascular disease and pulmonary hypertension. Curr Vasc Pharmacol. 2012;10:289–299. doi: 10.2174/157016112799959413. [DOI] [PubMed] [Google Scholar]
- 28.Iwaguro H, Yamaguchi J, Kalka C, et al. Endothelial progenitor cell vascular endothelial growth factor gene transfer for vascular regeneration. Circulation. 2002;105:732–738. doi: 10.1161/hc0602.103673. [DOI] [PubMed] [Google Scholar]
- 29.Song MB, Yu XJ, Zhu GX, et al. Transfection of HGF gene enhances endothelial progenitor cell (EPC) function and improves EPC transplant efficiency for balloon-induced arterial injury in hypercholesterolemic rats. Vascul Pharmacol. 2009;51:205–213. doi: 10.1016/j.vph.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 30.Jiang M, Wang B, Wang C, et al. Angiogenesis by transplantation of HIF-1 alpha modified EPCs into ischemic limbs. J Cell Biochem. 2008;103:321–334. doi: 10.1002/jcb.21416. [DOI] [PubMed] [Google Scholar]
- 31.Zhao YD, Courtman DW, Deng Y, et al. Rescue of monocrotaline-induced pulmonary arterial hypertension using bone marrow-derived endothelial-like progenitor cells: efficacy of combined cell and eNOS gene therapy in established disease. Circ Res. 2005;96:442–450. doi: 10.1161/01.RES.0000157672.70560.7b. [DOI] [PubMed] [Google Scholar]
- 32.Ward MR, Thompson KA, Isaac K, et al. Nitric oxide synthase gene transfer restores activity of circulating angiogenic cells from patients with coronary artery disease. Mol Ther. 2011;19:1323–1330. doi: 10.1038/mt.2011.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abbott JD, Huang Y, Liu D, et al. Stromal cell-derived factor-1α plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110:3300–3305. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- 34.Yamaguchi J, Kusano KF, Masuo O, et al. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 35.Wojakowski W, Tendera M, Michałowska A, et al. Mobilization of CD34/CXCR4+, CD34/CD117+, c-met+ stem cells, and mononuclear cells expressing early cardiac, muscle, and endothelial markers into peripheral blood in patients with acute myocardial infarction. Circulation. 2004;110:3213–3220. doi: 10.1161/01.CIR.0000147609.39780.02. [DOI] [PubMed] [Google Scholar]
- 36.Hendrix CW, Flexner C, MacFarland RT, et al. Pharmacokinetics and safety of AMD-3100, a novel antagonist of the CXCR-4 chemokine receptor, in human volunteers. Antimicrob Agents Chemother. 2000;44:1667–1673. doi: 10.1128/aac.44.6.1667-1673.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Misao Y, Takemura G, Arai M, et al. Importance of recruitment of bone marrow-derived CXCR4+ cells in post-infarct cardiac repair mediated by G-CSF. Cardiovasc Res. 2006;71:455–465. doi: 10.1016/j.cardiores.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 38.Ip JE, Wu Y, Huang J, et al. Mesenchymal stem cells use integrin beta1 not CXC chemokine receptor 4 for myocardial migration and engraftment. Mol Biol Cell. 2007;18:2873–2882. doi: 10.1091/mbc.E07-02-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eliceiri BP, Puente XS, Hood JD, et al. Src-mediated coupling of focal adhesion kinase to integrin alpha(v)beta5 in vascular endothelial growth factor signaling. J Cell Biol. 2002;157:149–160. doi: 10.1083/jcb.200109079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Serini G, Napione L, Arese M, et al. Besides adhesion: New perspectives of integrin functions in angiogenesis. Cardiovasc Res. 2008;78:213–222. doi: 10.1093/cvr/cvn045. [DOI] [PubMed] [Google Scholar]