An important stage in the development of any new therapeutic agent is establishment of the optimal dosage and route of administration. Inconsistent findings have been reported regarding the relationship between the cell dose and clinical benefit. The present study summarizes the data regarding the optimal cell dosage and route of administration from studies of stem cell therapy for heart disease and offers a perspective on future directions.
Keywords: Stem cell, Cardiovascular disease, Cell dosage, Route of administration
Abstract
An important stage in the development of any new therapeutic agent is establishment of the optimal dosage and route of administration. This can be particularly challenging when the treatment is a biologic agent that might exert its therapeutic effects via complex or poorly understood mechanisms. Multiple preclinical and clinical studies have shown paradoxical results, with inconsistent findings regarding the relationship between the cell dose and clinical benefit. Such phenomena can, at least in part, be attributed to variations in cell dosing or concentration and the route of administration (ROA). Although clinical trials of cell-based therapy for cardiovascular disease began more than a decade ago, specification of the optimal dosage and ROA has not been established. The present review summarizes what has been learned regarding the optimal cell dosage and ROA from preclinical and clinical studies of stem cell therapy for heart disease and offers a perspective on future directions.
Significance
Preclinical and clinical studies on cell-based therapy for cardiovascular disease have shown inconsistent results, in part because of variations in study-specific dosages and/or routes of administration (ROA). Future preclinical studies and smaller clinical trials implementing cell-dose and ROA comparisons are warranted before proceeding to pivotal trials.
Introduction
A critical step in the development of any new therapeutic agent is establishment of the optimal dosage and route of administration (ROA). This can be especially challenging when the treatment is a biologic agent that might exert its therapeutic effects via complex or poorly understood mechanisms. The Food and Drug Administration Center for Biologics Evaluation and Research Guidance for Industry: Preclinical Assessment of Investigational Cellular and Gene Therapy Products, November 2013, has recommended preclinical proof of concept studies that include (a) determination of the pharmacologically effective dose range (defined as the minimally effective and optimal doses); (b) optimization of the ROA with confirmation that the product reaches the target anatomic site; (c) optimization of the timing of administration relative to disease onset; (d) optimization of the dosing schedule; and (e) characterization of the putative mechanism of action. Additional studies to determine potential toxicity in animals and in vitro assays to evaluate biologic activity and potential safety issues are also strongly encouraged.
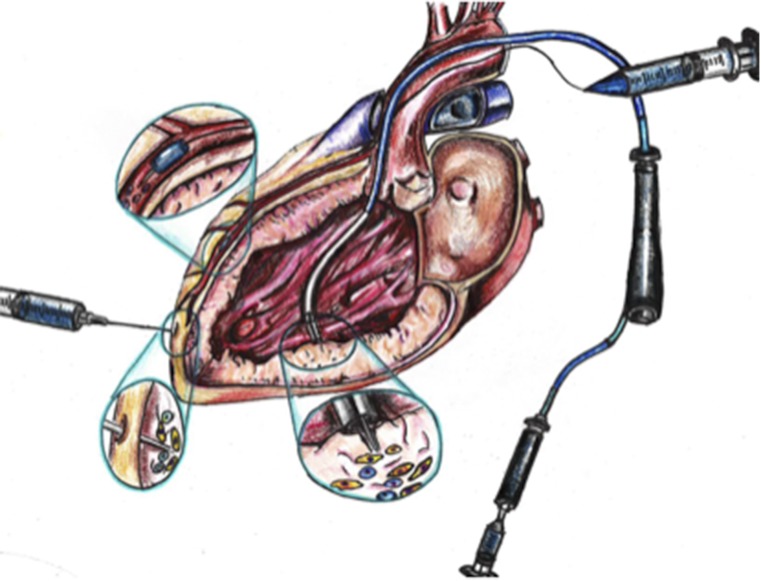
The purpose of the present review is to summarize what has been learned regarding the optimal cell dosage and ROA from preclinical and clinical studies of stem cell therapy for heart disease and to offer a perspective on future directions. Although it might seem reasonable to expect that the number of cells administered would be proportionate to the observed clinical effect, the data that has arisen from a relatively small number of studies has yielded conflicting and paradoxical results (Fig. 1). Importantly, the expected direct relationship between cell dose and clinical effect has not been consistently observed and, in fact, some studies have shown inverse dose-response effects. These findings raise challenges regarding planning increasingly complex clinical trials.
Figure 1.
Different doses and/or concentrations and routes of administration have been used in various preclinical and clinical studies for ischemic cardiomyopathy, which have led to inconsistent findings.
Preclinical Studies
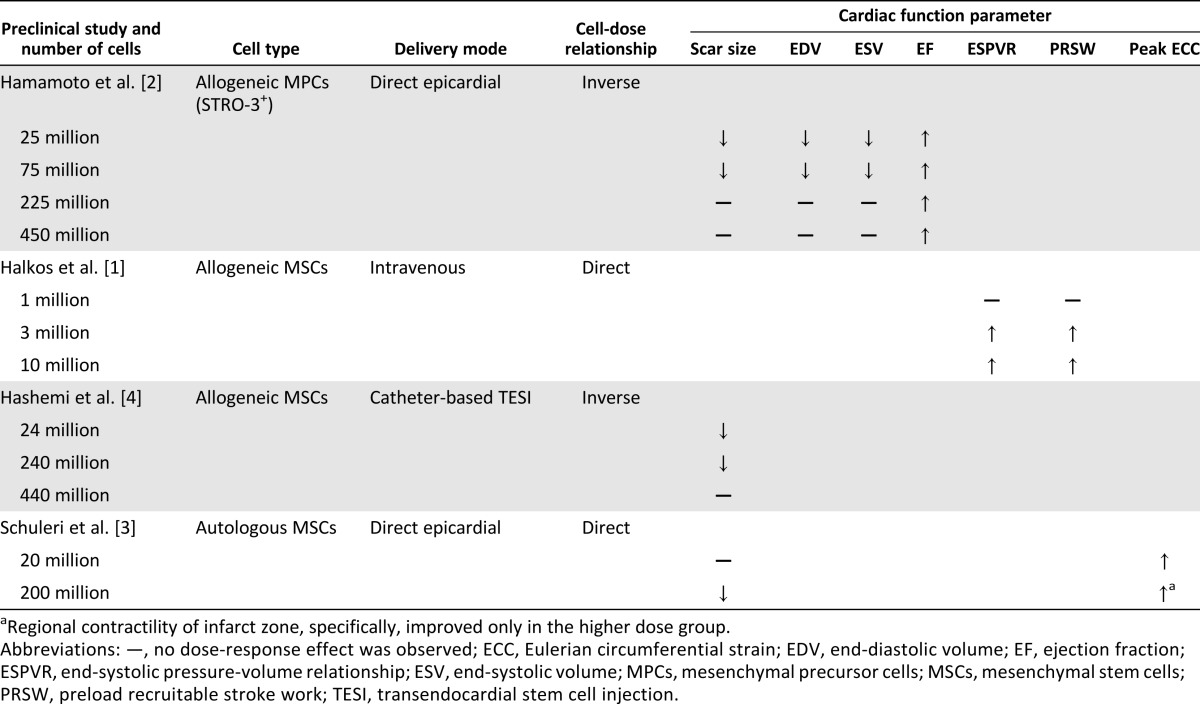
Preclinical studies addressing the dose range for cell therapy have yielded paradoxical findings. Halkos et al. [1] studied swine treated with three intravenous doses (1, 3, or 10 million) of allogeneic mesenchymal stem cells (MSCs) after a 75-minute left anterior descending coronary artery occlusion and found that the higher dose groups (3 and 10 million cells) had significantly improved left ventricular systolic function and preload-recruitable stroke work compared with the control group. In contrast, Hamamoto et al. [2] performed a dose-escalation study of sheep using four different doses (25, 75, 225, or 450 million allogeneic STRO-3-positive mesenchymal precursor cells) vs. cell media, administered intramyocardially at the infarct border zone, 1 hour after experimental acute myocardial infarction (AMI). Compared with the control group, only the lower (25 and 75 million) cell doses significantly attenuated infarct expansion and remodeling, reducing the left ventricular end-diastolic volume (LVEDV) and left ventricular end-systolic volume (LVESV) and improving the left ventricular ejection fraction (LVEF) at all cell doses (Table 1). Interestingly, the dose ranges used in the two studies did not overlap. It is also significant that the ROAs were different (intravenous vs. intramyocardial), and it is reasonable to surmise that this would influence the effects of the cell dose.
Table 1.
Summary of preclinical studies on stem cell therapy dosing
Schuleri et al. [3] in a study delivering cells via direct injection in open chest pigs reported a significant reduction in infarct size with “high dose” (200 million) autologous MSCs compared with “low dose” (20 million) autologous MSCs in post-AMI swine. Regional contractility, as assessed by tagged magnetic resonance imaging-derived circumferential shortening, improved in both groups, although the contractility of the infarct zone improved only in the higher dose group. In contrast to these findings, Hashemi et al. [4], using endomyocardial delivery, found that the lower dose MSC groups (24 and 240 million) exhibited a significant decrease in infarct size, but the higher dose group of 440 million MSCs did not.
Summarizing, the foregoing preclinical studies varied in design, ROA, and the results related to cell dose. The range of total cell numbers used in each study differed significantly, and the definitions of “low” versus “high dose” were inconsistent. Of particular importance, the effects of cell concentration and total injection volume also remain to be elucidated as they relate to the various routes of administration being used (discussed below). Thus, whether a “low” or “high” dose is most efficacious in reducing the infarct size and improving cardiac structure and function remains unknown.
Clinical Studies
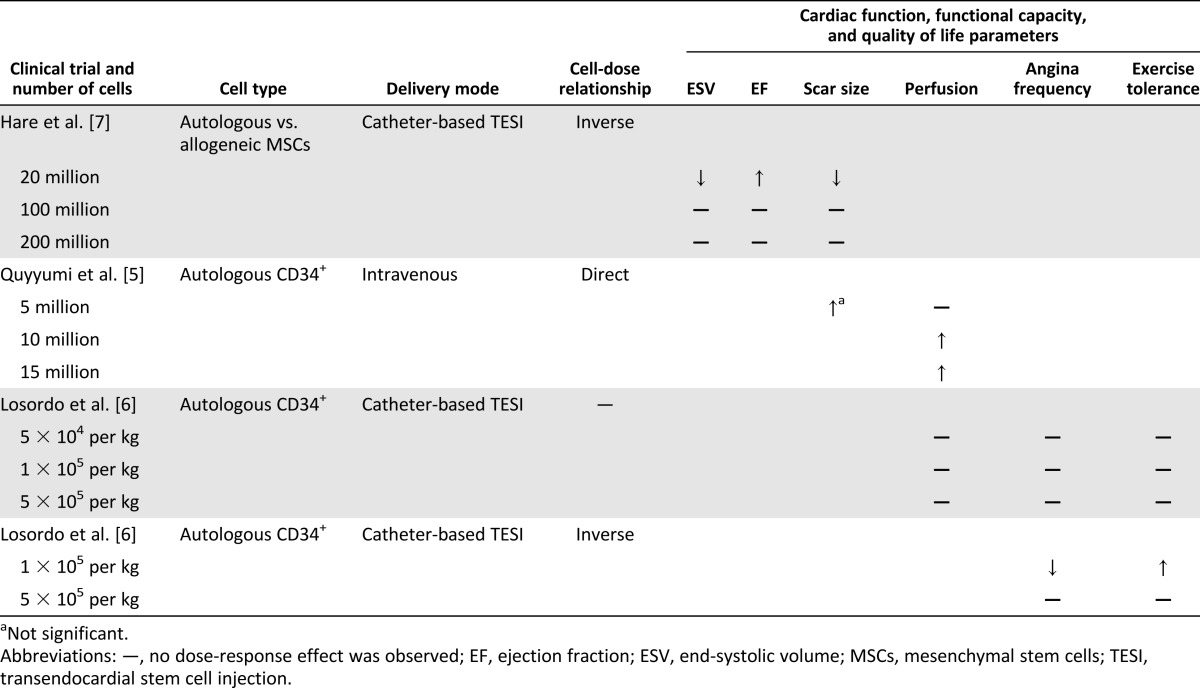
Three clinical trials have evaluated the relationship between cell dose and clinical benefit. Quyyumi et al. [5] administered autologous bone marrow CD34+ cells to patients with an ST-elevation MI. The cells were infused by an intracoronary route into the infarct-related artery 8 days after stenting and three different cell doses (5, 10, or 15 million) were studied. Patients who received ≥10 million CD34+ cells demonstrated a significant improvement in perfusion, as measured by single-photon emission computed tomography, and a trend toward improved LVEF in those receiving 5 million CD34+ cells compared with the control. In contrast, Losordo et al. [6] evaluated 24 patients who had received one of three dose cohorts (5 × 104, 1 × 105, or 5 × 105 per kilogram autologous CD34+ cells) in a phase I to IIa, double-blinded, placebo-controlled, randomized clinical trial, and no dose-response relationship was observed. However, in a larger trial [6] by the same group, 167 patients were randomized to one of two doses (1 × 105 per kilogram or 5 × 105 per kilogram autologous CD34+ cells or an equal volume of placebo), and the low-dose group experienced a significant improvement in angina frequency and exercise tolerance. Finally, in the PercutaneOus StEm Cell Injection Delivery Effects On Neomyogenesis (POSEIDON) trial [7], Hare et al. randomized 30 patients to six subgroups according to the source of the cells (allogeneic vs. autologous) and the dose (20 million, 100 million, or 200 million cells). All patients received ten 0.5-ml injections transendocardially via catheter. Compared with the 200 million cell dose group, the patients who received 20 million cells were found to have significantly greater LVEF, improvement in LVESV, and a reduction in scar size, as measured by early enhancement (Table 2).
Table 2.
Summary of clinical studies of stem cell therapy dosing
Thus, just as noted for the preclinical studies, clinical trials have reported inconsistent and conflicting results regarding the relationship between the cell dose and clinical benefit. It is also important to note that it might not be appropriate to make comparisons between cell dosing studies that have used different routes of administration. Furthermore, although many preclinical studies [8–11] and clinical trials [12–20] have examined other cell types, such as bone marrow-derived mononuclear cells (BMMNCs), c-kit+ cardiac stem cells, and cardiac CD105+ culture-expanded MSCs (cardiosphere-derived cells), they did not compare the cell doses but, rather, single doses with different cell products or placebo.
Route of Administration
Studies evaluating both the cell dose and the ROA or compared intravenous administration directly with other routes have not yet been reported. We have summarized the three studies comparing intracoronary and transendocardial delivery.
Perin et al. compared intracoronary and transendocardial delivery of allogeneic MSCs in a canine model of AMI [21] and found that transendocardial injection improved LVEF, LVEDV, LVESV, and capillary density. However, intracoronary infusion did not [21]. In addition, transendocardial injection was associated with a greater reduction of myocardial ischemia. When both delivery techniques were assessed for cell retention, transendocardial injection yielded a higher MSC concentration per µm2 than did intracoronary infusion. Vrtovec et al. reported similar findings in patients with nonischemic cardiomyopathy, notable for increased myocardial cellular retention and improvements in short- and long-term ventricular function with transendocardial versus intracoronary delivery of CD34+ cells [22, 23]. In contrast, Rigol et al. compared intracoronary versus transendocardial administration of adipose tissue-derived stem cells in a porcine model of AMI and found that the intracoronary route significantly increased neovascularization compared with the transendocardial route, although both delivery modes resulted in similar rates of engraftment [24].
The intracoronary infusion of stem cells has certain logistical benefits, including its relatively less complex technique. However, it is limited by the inaccessibility of some myocardial distributions in many patients with advanced coronary artery disease. Perhaps most importantly, the potential for microvascular obstruction by the infused cells, which can result in myocardial necrosis, could limit the applicability of this technique for certain cell types. Nevertheless, to date, this is the most studied technique for cell delivery during the time of percutaneous coronary intervention after AMI [25]. Moreover, the SCIPIO (Cardiac Stem Cell Infusion in Patients With Ischemic Cardiomyopathy) trial demonstrated in patients with ischemic cardiomyopathy that intracoronary infusion of 1 million c-kit+ cardiac stem cells is safe and effective in improving left ventricular systolic function and reducing infarct size [12]. However, whether higher doses are safe or exert greater effectiveness is unknown. To this end, Keith et al. investigated the safety of delivering higher doses of human c-kit+ cardiac stem cells by intracoronary infusion in a porcine model. The investigators found that infusion of 20 million human c-kit+ cardiac stem cells does not lead to acute cardiac injury, impairment of cardiac function, or end organ damage [26].
Catheter-based transendocardial injection and direct surgical intramyocardial injection of MSCs have been investigated in various preclinical and clinical studies and were shown to be safe and effective [2, 3, 7, 27–36]. In an analysis from the POSEIDON clinical trial, transendocardial injection of MSCs reduced the scar size in both injected and noninjected myocardial segments; however, segmental contractility improved only in the injected scar segments. The increase in segmental contractility was greatest in those territories with severe baseline dysfunction [35].
Conclusion
An importnt issue defining any new effective therapy is to establish the optimal dose and delivery method. The use of living cells as therapeutic agents differs in many important ways from traditional pharmacology, for which well-established principles of pharmacokinetics and pharmacodynamics are operative. However, in the field of cell therapy for cardiovascular disease, these issues remain to be defined. For cell therapy, the appropriate quantity and/or concentration of the transplanted cells and the ROA are important; however, very different principles and assumptions might be involved in assessing the correct dosing regimens. Although it might seem intuitive that the raw number of cells administered would be proportionately related to their clinical effect, using the cardiac structure, functional capacity, and quality of life measurements as clinical parameters, this concept has not been established conclusively.
Despite an extensive body of data since the publication of the first clinical trials of stem cell therapy for heart disease in 2002 to 2003 [37–39], the specification of an optimal dosage and ROA for the various stem cell preparations remains an elusive goal. The factors contributing to this include (a) no rational basis for standardizing the broad variety of stem cell sources and production methods; (b) inadequate methods for determining the quality and potency or biologic activity of stem cell preparations; (c) a lack of studies comparing both cell dose and ROA; and (d) the heterogeneity of target indications and patients. Furthermore, to our knowledge, no systematic studies have been performed of the potential sources of error or variability, including (a) concentration-dependent cell aggregation, which might affect transendothelial migration and/or homing to injured myocardium; (b) vehicle-dependent effects on exposure of receptor or effector sites; and (c) needle bore-dependent effects on cell integrity resulting from excessive shear forces.
As noted, the available preclinical and clinical evidence is conflicting, with some studies reporting that a lower cell dosage and/or infusion cell concentration would provide the most benefit [2, 4, 7], and others finding either an inverse or nonlinear relationship [3, 40]. To our knowledge, no studies have evaluated both the cell dose and the ROA. Also, and perhaps importantly, no clinical trials have evaluated the cell dose for BMMNCs, cardiosphere-derived cells, or c-kit+ cardiac stem cells. Of note, a flat dose-response relationship for intracoronary c-kit+ cardiac stem cells was recently reported in a rat model of acute myocardial infarction [41]. It is also important to highlight that immune status, in relation to whether autologous or allogeneic stem cells are administered, might play a larger role than expected in the dose response [42]. A recent study by Premer et al. showed that allogeneic, but not autologous, MSCs increased endothelial progenitor cell colonies and restored flow-mediated vasodilation in patients with ischemic and nonischemic cardiomyopathy. The inconclusiveness of the published data on the optimal cell type, together with the potentially paradoxical effects of autologous versus allogeneic cell sources, further complicates matters, necessitating additional studies on these relationships before advancing to larger dose and ROA comparative trials.
Thus, the field of cell therapy for cardiovascular disease still lacks consistent and reliable dosage and ROA data that would inform safety and efficacy considerations. We encourage the scientific community to consider cell comparisons, dose-response assessments, and comparative ROA evaluations in their preclinical and clinical study designs.
Although the cell type, dosage, concentration, and delivery modalities are important considerations for regenerative cell therapy clinical trials, our survey of the published studies suggests that the available data are inconclusive and additional early phase studies could be needed before proceeding to pivotal clinical trials. At a minimum, investigators undertaking phase III trials should be mindful of any assumptions determined from studies of other cell types and/or ROAs and ensure that adequate attention has been given to these as yet incompletely understood variables.
Acknowledgments
This work was supported by the NIH (Grants UM1 HL113460, R01 HL084275, and R01 HL110737 and cooperative agreement Grant 5 UM1 HL087318). The views expressed in the present report are those of the authors and do not necessarily reflect those of the National Heart, Lung, and Blood Institute, the NIH, or the U.S. federal government.
Author Contributions
S.G., I.H.S., and J.M.H.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; R.F.E.: collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; A.W.H., D.L.D., P.C.Y., J.C.W., R.B., E.C.P., L.M., and R.D.S.: manuscript writing, final approval of manuscript; A.W.: production of central illustration, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
A.W.H. is listed on a patent for cardiac cell-based therapy; receives research support from Biocardia; and is a board member, is a consultant, and reports an equity interest in Vestion Inc. D.L.D. is a compensated consultant. E.C.P. is a compensated consultant for Biosense-Webster. J.M.H. is listed on a patent for cardiac cell-based therapy and reports an equity interest in Vestion, Inc. The other authors indicated no potential conflicts of interest.
References
- 1.Halkos ME, Zhao ZQ, Kerendi F, et al. Intravenous infusion of mesenchymal stem cells enhances regional perfusion and improves ventricular function in a porcine model of myocardial infarction. Basic Res Cardiol. 2008;103:525–536. doi: 10.1007/s00395-008-0741-0. [DOI] [PubMed] [Google Scholar]
- 2.Hamamoto H, Gorman JH, 3rd, Ryan LP, et al. Allogeneic mesenchymal precursor cell therapy to limit remodeling after myocardial infarction: The effect of cell dosage. Ann Thorac Surg. 2009;87:794–801. doi: 10.1016/j.athoracsur.2008.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuleri KH, Feigenbaum GS, Centola M, et al. Autologous mesenchymal stem cells produce reverse remodelling in chronic ischaemic cardiomyopathy. Eur Heart J. 2009;30:2722–2732. doi: 10.1093/eurheartj/ehp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hashemi SM, Ghods S, Kolodgie FD, et al. A placebo controlled, dose-ranging, safety study of allogenic mesenchymal stem cells injected by endomyocardial delivery after an acute myocardial infarction. Eur Heart J. 2008;29:251–259. doi: 10.1093/eurheartj/ehm559. [DOI] [PubMed] [Google Scholar]
- 5.Quyyumi AA, Waller EK, Murrow J, et al. CD34(+) cell infusion after ST elevation myocardial infarction is associated with improved perfusion and is dose dependent. Am Heart J. 2011;161:98–105. doi: 10.1016/j.ahj.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 6.Losordo DW, Henry TD, Davidson C, et al. Intramyocardial, autologous CD34+ cell therapy for refractory angina. Circ Res. 2011;109:428–436. doi: 10.1161/CIRCRESAHA.111.245993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hare JM, Fishman JE, Gerstenblith G, et al. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: The POSEIDON randomized trial. JAMA. 2012;308:2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee ST, White AJ, Matsushita S, et al. Intramyocardial injection of autologous cardiospheres or cardiosphere-derived cells preserves function and minimizes adverse ventricular remodeling in pigs with heart failure post-myocardial infarction. J Am Coll Cardiol. 2011;57:455–465. doi: 10.1016/j.jacc.2010.07.049. [DOI] [PubMed] [Google Scholar]
- 9.Bolli R, Tang XL, Sanganalmath SK, et al. Intracoronary delivery of autologous cardiac stem cells improves cardiac function in a porcine model of chronic ischemic cardiomyopathy. Circulation. 2013;128:122–131. doi: 10.1161/CIRCULATIONAHA.112.001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li CJ, Gao RL, Yang YJ, et al. [Effect of intracoronary transplantation of autologous bone marrow mononuclear cells on myocardial ischemia reperfusion injury in mini-swine model] Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2008;30:86–90. [PubMed] [Google Scholar]
- 11.Silva GV, Fernandes MR, Cardoso CO, et al. A dosing study of bone marrow mononuclear cells for transendocardial injection in a pig model of chronic ischemic heart disease. Tex Heart Inst J. 2011;38:219–224. [PMC free article] [PubMed] [Google Scholar]
- 12.Bolli R, Chugh AR, D’Amario D, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): Initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Makkar RR, Smith RR, Cheng K, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): A prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perin EC, Willerson JT, Pepine CJ, et al. Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: The FOCUS-CCTRN trial. JAMA. 2012;307:1717–1726. doi: 10.1001/jama.2012.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perin EC, Silva GV, Zheng Y, et al. Randomized, double-blind pilot study of transendocardial injection of autologous aldehyde dehydrogenase-bright stem cells in patients with ischemic heart failure. Am Heart J. 2012;163:415–421. doi: 10.1016/j.ahj.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 16.Assmus B, Leistner DM, Schächinger V, et al. Long-term clinical outcome after intracoronary application of bone marrow-derived mononuclear cells for acute myocardial infarction: Migratory capacity of administered cells determines event-free survival. Eur Heart J. 2014;35:1275–1283. doi: 10.1093/eurheartj/ehu062. [DOI] [PubMed] [Google Scholar]
- 17.Williams AR, Trachtenberg B, Velazquez DL, et al. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: Functional recovery and reverse remodeling. Circ Res. 2011;108:792–796. doi: 10.1161/CIRCRESAHA.111.242610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blatt A, Cotter G, Leitman M, et al. Intracoronary administration of autologous bone marrow mononuclear cells after induction of short ischemia is safe and may improve hibernation and ischemia in patients with ischemic cardiomyopathy. Am Heart J. 2005;150:986. doi: 10.1016/j.ahj.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 19.Santoso T, Siu CW, Irawan C, et al. Endomyocardial implantation of autologous bone marrow mononuclear cells in advanced ischemic heart failure: A randomized placebo-controlled trial (END-HF) J Cardiovasc Transl Res. 2014;7:545–552. doi: 10.1007/s12265-014-9580-6. [DOI] [PubMed] [Google Scholar]
- 20.Perin EC, Sanz-Ruiz R, Sanchez PL, et al. Adipose-derived regenerative cells in patients with ischemic cardiomyopathy: The PRECISE trial. Am Heart J. 2014;168:88–95.e2. doi: 10.1016/j.ahj.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 21.Perin EC, Silva GV, Assad JA, et al. Comparison of intracoronary and transendocardial delivery of allogeneic mesenchymal cells in a canine model of acute myocardial infarction. J Mol Cell Cardiol. 2008;44:486–495. doi: 10.1016/j.yjmcc.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 22.Vrtovec B, Poglajen G, Lezaic L, et al. Comparison of transendocardial and intracoronary CD34+ cell transplantation in patients with nonischemic dilated cardiomyopathy. Circulation. 2013;128(suppl 1):S42–S49. doi: 10.1161/CIRCULATIONAHA.112.000230. [DOI] [PubMed] [Google Scholar]
- 23.Vrtovec B, Poglajen G, Lezaic L, et al. Effects of intracoronary Cd34+ stem cell transplantation in non-ischemic dilated cardiomyopathy patients: 5-Year follow up. Circ Res. 2013;112:165–173. doi: 10.1161/CIRCRESAHA.112.276519. [DOI] [PubMed] [Google Scholar]
- 24.Rigol M, Solanes N, Farré J, et al. Effects of adipose tissue-derived stem cell therapy after myocardial infarction: Impact of the route of administration. J Card Fail. 2010;16:357–366. doi: 10.1016/j.cardfail.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Sherman W, Martens TP, Viles-Gonzalez JF, et al. Catheter-based delivery of cells to the heart. Nat Clin Pract Cardiovasc Med. 2006;3(suppl 1):S57–S64. doi: 10.1038/ncpcardio0446. [DOI] [PubMed] [Google Scholar]
- 26.Keith MC, Tang XL, Tokita Y, et al. Safety of intracoronary infusion of 20 million C-kit positive human cardiac stem cells in pigs. PLoS One. 2015;10:e0124227. doi: 10.1371/journal.pone.0124227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shake JG, Gruber PJ, Baumgartner WA, et al. Mesenchymal stem cell implantation in a swine myocardial infarct model: Engraftment and functional effects. Ann Thorac Surg. 2002;73:1919–1926. doi: 10.1016/s0003-4975(02)03517-8. [DOI] [PubMed] [Google Scholar]
- 28.Dixon JA, Gorman RC, Stroud RE, et al. Mesenchymal cell transplantation and myocardial remodeling after myocardial infarction. Circulation. 2009;120(suppl):S220–S229. doi: 10.1161/CIRCULATIONAHA.108.842302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quevedo HC, Hatzistergos KE, Oskouei BN, et al. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci USA. 2009;106:14022–14027. doi: 10.1073/pnas.0903201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hatzistergos KE, Quevedo H, Oskouei BN, et al. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107:913–922. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams AR, Suncion VY, McCall F, et al. Durable scar size reduction due to allogeneic mesenchymal stem cell therapy regulates whole-chamber remodeling. J Am Heart Assoc. 2013;2:e000140. doi: 10.1161/JAHA.113.000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams AR, Hatzistergos KE, Addicott B, et al. Enhanced effect of combining human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and to restore cardiac function after myocardial infarction. Circulation. 2013;127:213–223. doi: 10.1161/CIRCULATIONAHA.112.131110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heldman AW, DiFede DL, Fishman JE, et al. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: The TAC-HFT randomized trial. JAMA. 2014;311:62–73. doi: 10.1001/jama.2013.282909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karantalis V, DiFede DL, Gerstenblith G, et al. Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting: The Prospective Randomized Study of Mesenchymal Stem Cell Therapy in Patients Undergoing Cardiac Surgery (PROMETHEUS) trial. Circ Res. 2014;114:1302–1310. doi: 10.1161/CIRCRESAHA.114.303180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suncion VY, Ghersin E, Fishman JE, et al. Does transendocardial injection of mesenchymal stem cells improve myocardial function locally or globally?: An analysis from the Percutaneous Stem Cell Injection Delivery Effects on Neomyogenesis (POSEIDON) randomized trial. Circ Res. 2014;114:1292–1301. doi: 10.1161/CIRCRESAHA.114.302854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams A, Hatzistergos K, Carvalho D, et al. Synergistic effect of human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and restore cardiac function. Circulation. 2011;124:A559. doi: 10.1161/CIRCULATIONAHA.112.131110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Assmus B, Schächinger V, Teupe C, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI) Circulation. 2002;106:3009–3017. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 38.Strauer BE, Brehm M, Zeus T, et al. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002;106:1913–1918. doi: 10.1161/01.cir.0000034046.87607.1c. [DOI] [PubMed] [Google Scholar]
- 39.Perin EC, Dohmann HF, Borojevic R, et al. Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation. 2003;107:2294–2302. doi: 10.1161/01.CIR.0000070596.30552.8B. [DOI] [PubMed] [Google Scholar]
- 40.Schuster MD, Kocher AA, Seki T, et al. Myocardial neovascularization by bone marrow angioblasts results in cardiomyocyte regeneration. Am J Physiol Heart Circ Physiol. 2004;287:H525–H532. doi: 10.1152/ajpheart.00058.2004. [DOI] [PubMed] [Google Scholar]
- 41.Tang XL, Rokosh G, Sanganalmath SK, et al. Effects of intracoronary infusion of escalating doses of cardiac stem cells in rats with acute myocardial infarction. Circ Heart Fail. 2015;8:757–765. doi: 10.1161/CIRCHEARTFAILURE.115.002210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Premer C, Blum A, Bellio MA, et al. Allogeneic mesenchymal stem cells restore endothelial function in heart failure by stimulating endothelial progenitor cells. EBioMedicine. 2015;2:467–475. doi: 10.1016/j.ebiom.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]