Corneal endothelial transplantation is successful but limited worldwide because of lower donor corneal supply. Alternatives to corneal tissue for endothelial transplantation therefore require immediate attention. This manuscript provides an update regarding developments in the field of human corneal endothelial culture for transplantation, which is rapidly emerging as a possible viable option.
Keywords: Cornea, Corneal endothelial cells, Cell culture, Transplantation
Abstract
The cornea forms the front window of the eye, enabling the transmission of light to the retina through a crystalline lens. Many disorders of the cornea lead to partial or total blindness, and therefore corneal transplantation becomes mandatory. Recently, selective corneal layer (as opposed to full thickness) transplantation has become popular because this leads to earlier rehabilitation and visual outcomes. Corneal endothelial disorders are a common cause of corneal disease and transplantation. Corneal endothelial transplantation is successful but limited worldwide because of lower donor corneal supply. Alternatives to corneal tissue for endothelial transplantation therefore require immediate attention. The field of human corneal endothelial culture for transplantation is rapidly emerging as a possible viable option. This manuscript provides an update regarding these developments.
Significance
The cornea is the front clear window of the eye. It needs to be kept transparent for normal vision. It is formed of various layers of which the posterior layer (the endothelium) is responsible for the transparency of the cornea because it allows the transport of ions and solutes to and from the other layers of the cornea. Corneal blindness that results from the corneal endothelial dysfunction can be treated using healthy donor tissues. There is a huge demand for human donor corneas but limited supply, and therefore there is a need to identify alternatives that would reduce this demand. Research is underway to understand the isolation techniques for corneal endothelial cells, culturing these cells in the laboratory, and finding possible options to transplant these cells in the patients. This review article is an update on the recent developments in this field.
Introduction
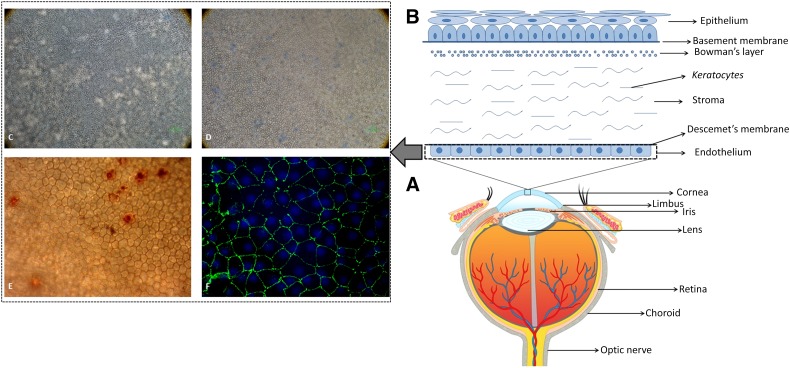
The human cornea is a transparent avascular tissue in the front part of the eye. The cornea transmits and focuses light to the retina to generate vision. Its transparency needs to be maintained for optimal vision. The cornea is structured into well-organized layers, and each layer has its own importance in maintaining the viability and transparency of the tissue. From the anterior to the posterior cornea, the human corneal tissue consists of a stratified epithelium, Bowman’s layer, the stroma, Descemet’s membrane, and a monolayered endothelium [1]. Figure 1 represents different parts of the human eye showing various compartments of the cornea including the corneal endothelium. The epithelium is a self-renewing layer and harbors a resident stem cell population at its periphery. These cells are well characterized and have been applied therapeutically. However, the stroma and endothelium are usually quiescent and so far have not been considered to regenerate [2].
Figure 1.
Human eye, cornea, and endothelium. (A): Anatomy of human eye globe showing different parts of the eye. (B): Schematic representation and structure of human cornea showing specific layers of the tissue. (C): A normal human corneal endothelium seen under an inverted microscope at ×100 magnification. (D): Human corneal endothelium with high mortality rate observed using trypan blue staining at ×100 magnification. (E): Human corneal endothelium observed using alizarin red staining to check the hexagonality of the cells at ×200 magnification. (F): Human corneal endothelium expressing zonula occludens 1 marker observed under oil immersion magnification.
Although the other layers are also important in the maintenance of appropriate corneal function, the endothelium plays a significant role in maintaining the corneal clarity. Transparency of the cornea is maintained by the crystalline organization and critical spacing of collagen fibrils. The endothelium controls hydration (maintain stromal deturgescence), and it is permeable to nutrients and other molecules that are passed on from the aqueous humor, hence behaving as a partial or a leaky barrier. These barriers help in the fluid movement into the cornea using active pump function that moves ions and draws water osmotically from the stroma into the aqueous humor. The endothelium has metabolically active cells comprising of incomplete zonula occludens, which accounts for the weak endothelial barrier function allowing nutrients and other molecules to enter the stroma. This further helps in maintaining the thickness, transparency, and active mechanism required for the normal corneal function [3, 4].
Because the human corneal endothelium is presumed to lack the ability to regenerate like the corneal epithelium, its maintenance is always a concern. Corneal endothelial dysfunction leads to stromal edema, loss of transparency, and hence compromised vision. The primary treatment for corneal endothelial dysfunction is replacement of the diseased recipient tissue with healthy donor tissue. Endothelial damage or poor initial viable endothelial cell counts are assumed to be responsible for the majority of corneal transplant failures. Selective endothelial transplantation of the cornea (as opposed to full thickness corneal transplantation) has been carried out for a decade. Such endothelial corneal transplants include Descemet’s stripping automated endothelial keratoplasty (DSAEK) and Descemet’s membrane endothelial keratoplasty, which are now getting popular for their advantages over full thickness penetrating keratoplasty [5]. Compared with full thickness corneal transplantation, specific corneal endothelial transplantation is less traumatic for the eye, and the recovery is much quicker. Regarding corneal transplantation in general, the global availability of corneal tissue for transplantation of corneal diseases is becoming an increasing challenge because of limitations in donor corneal supply [1].
In recent years, corneal tissue engineering has evolved dramatically from culturing cells in vitro to developing synthetic scaffolds and artificial corneas. Isolation, culturing, and bioengineering have been studied to a limited extent, and further research is ongoing. Attempts have already been discussed in the literature for exploiting the power of native cells for the manufacturing of stroma-like extracellular matrix and for the production of cell sheets, whether it be the epithelium with amniotic membrane/fibrin or endothelial cells with intact basement membranes [6]. It has also been found that collagen-based engineered matrices support cell growth and demonstrate appropriate optical and mechanical properties. Current research shows promising results in growing corneal constructs. The next challenge is to ensure that these constructs show appropriate thickness, transparency, and strength in vivo. However, issues like cellular responses, wound healing, and inflammatory responses need to be studied in detail clinically before any conclusions can be drawn [6]. This paper highlights the new insights in the corneal endothelial cell culture and transplantation.
Evidence for Corneal Endothelial Stem Cells
Both in vitro and in vivo studies have been carried out that indicate the presence of corneal endothelial progenitors. Some of these studies are listed below.
BrdU
Studies carried out using alkaline phosphatase (AP)-bromodeoxyuridine (BrdU) have suggested the presence of corneal endothelial progenitors. In these studies, the limbus was intensely stained with AP-BrdU [7, 8]. This experimental method was used to detect the presence of dividing cells. BrdU retention was identified by alkaline phosphatase activity. Although this technique indicates dividing cells, it may also identify the presence of native, cellular alkaline phosphatase activity, which is typically observed in both intracellular and cellular plasma membrane isoforms of this enzyme and particularly in stem cells. These studies also showed that human corneal endothelial cells (HCECs) from the corneal periphery increased in number, suggesting the presence of progenitor cells.
Telomerase
In the telomerase-based studies, corneas were divided into three sections: (a) central, (b) middle, and (c) peripheral. The authors observed telomerase activity in the middle and the peripheral sections, whereas the central section did not show the presence of telomerase activity [9–12]. It was also observed that donor age may have a potential limitation to the human wounding response or cell division in the periphery [9–12].
Schwalbe’s Line Cells
A novel cell type (named Schwalbe’s line cells) was identified in 1982 by Raviola et al. [13]. These cells form a discontinuous cord that is usually found in the circumference beneath the Schwalbe’s ring (transition region between the corneal endothelium and the anterior extension of the trabecular meshwork), which is also marked in Figure 1 [13]. An increase in cell division by the Schwalbe’s ring cells after laser trabeculoplasty has been observed. This indicates that Schwalbe’s line cells may hold progenitor cell-like properties. A fourfold increase in cell division in human laser-treated explants was also confirmed by Acott et al. [14]. It was observed that more than 60% of the cell division was initially localized to the anterior nonfiltering region of the trabecular meshwork, and they migrated toward the burn [15–18].
Corneal Endothelial Regeneration In Vivo
It has been hypothesized that a slow regeneration of HCECs starting from the corneal periphery can occur [19]. The microanatomy of the endothelium showed anatomic organization in the periphery of the human corneal endothelium, which also suggested a continuous slow centripetal migration throughout life of HCECs from specific niches. This further led to the understanding that the corneal endothelium can be regenerated if cells are excised from the peripheral region [19].
Isolation of Human Corneal Endothelial Progenitors
Single Cell Culture
There are several methods used for HCEC isolation. Initially, collagenase or trypsin was used on whole corneas, but because it inevitably caused contamination of corneal fibroblasts, a selective l-valine-free medium was used. Because this medium had to be used for several passages to completely abolish the presence of fibroblast growth, l-valine-free selection medium was assumed to have acted by arresting the growth of fibroblast-like cells rather than by killing them.
The peel-and-digest method by Peh et al. [20] has resulted in successful cultures. Other methods include stripping or bubble techniques to separate the Descemet’s membrane from the stroma and then digestion of the cells using trypsin for cell culture. Stripping of HCECs can also be performed with the aid of a vacuum suction holder [18]. After peeling, the Descemet’s membrane and endothelial layer are finally digested enzymatically using dispase or EDTA, followed by gentle pipetting. However, enzymatic digestion requires a prolonged incubation time to detach cells from the matrix, subsequently also leading to higher cellular degeneration. Thus, although several potentially successful methods do exist in the literature, it is evident that there is still the need to standardize a specific and reproducible method to isolate and culture HCECs from donor corneas.
Explant Culture Method
A recent study showed remarkable growth that was achieved using a serial explant culture technique. The explant was transferred to 7 new plates over a period of 6 months, generating sheets of small, primitive cells in each plate. The findings are consistent with the theory that progenitor cells for the corneal endothelium reside within the limbus and provide new insights to HCECs culture [21].
The HCEC Sphere-Forming Assay
It is believed that the corneal endothelium is derived from neural crest cells during embryonic development but also has a mesenchymal origin [22, 23]. Sphere colonies from corneal endothelial cells express neural and mesenchymal proteins and have been shown to have the potential to differentiate into neuronal lineages [24, 25]. The sphere-forming method has also been used to investigate cultivated HCECs with differences in telomere length, telomerase activity, and characteristics reflecting senescence. The sphere-forming assay was performed to obtain precursors from cultured sixth passage (p6) HCECs. p6 and p7 cultured HCECs were used as the controls. It was observed that precursors obtained from the spheres had longer telomeres and higher telomerase activity than cultured p6 cells. Strong positive staining for senescence-associated β-galactosidase activity was detected in p6 and p7 cultured CECs, whereas little or no staining was detected in the precursors within spheres obtained from p6-cultured CECs or their progeny. The progeny of spheres derived from cultured HCECs were small regular cells that grew at a higher density and contained more 5-bromo-2′-deoxyuridine-incorporating cells compared with the parental cultured cells. These findings indicate that the sphere-forming assay enriches precursors with longer telomeres, higher telomerase activity, and younger progeny than the original cells. Thus, the sphere-forming assay may contribute to obtaining the young HCECs needed for regenerative medicine [24].
In another study, the cultured cells partially retained the properties of neural crest and periocular mesenchyme that are believed to be the source of origination of corneal endothelium via the neural crest using serum free media. The progenitors have a high proliferative potency and possess endothelial function that was checked by Ussing chamber and transplantation in the rabbit cornea. Human corneal endothelial progenitor cells can be used for tissue regenerative medicine, and therefore they are further exploited [23].
The Culture of Human Corneal Endothelial Cells Using Conditioned Medium
Many human corneal endothelial cell culture media have been described earlier with a combination of endothelial growth factors, base medium, serum, growth factors, insulin, vitamins, etc. There have been several studies using conditioned medium from other cell types to promote HCEC proliferation. These include conditioned medium from mouse embryonic stem cells, human bone marrow-derived mesenchymal stem cells, and human amniotic fluid.
Mouse Embryonic Stem Cell-Conditioned Medium
Studies were performed to determine whether mouse embryonic stem cell-conditioned medium had any effect on the proliferative capacity of HCECs in vitro. Primary HCECs were cultured in human corneal endothelium medium (CEM) containing 25% embryonic stem cell-conditioned medium (ESC-CM) for the experimental group and CEM alone for the control group. The results showed that HCECs in the 25% ESC-CM group resulted in polygonal cells on day 2, whereas those in the CEM group showed slightly larger cells during days 3 and 4. HCECs in the 25% ESC-CM group could be subcultured until 6th passage without increasing in cell volume, whereas those in the CEM group were cultured and lost their polygonal appearance by passage 2. Cells in both the groups expressed zonula occludens 1 (ZO-1), Na+-K+ ATPase, VDAC3, SLC4A4, and CLCN3 (described further in the article). Ki67-positive cells and the percentage of cells entering the S and G2 phases were higher in the 25% ESC-CM group than in the CEM group. The 25% ESC-CM group showed a decrease in apoptotic cells and p21 protein expression. Furthermore, it was also reported that the cells cultured in 25% ESC-CM had enhanced HCEC proliferation and promoted HCECs into the cell cycle [26–30]. To summarize, in these studies, primary cultures of HCECs were cultured using 25% mouse embryonic stem cell-conditioned medium. Compared with control cultures, these cultures showed cells with increasing polygonal morphology (characteristic of healthy HCECs); the cultures could be subcultured further (until the sixth passage), had a higher number of cells expressing Ki-67, and had a fewer apoptotic cells.
Human Bone Marrow-Derived Mesenchymal Stem Cell-Conditioned Medium
Human bone-marrow-derived mesenchymal stem cell-derived conditioned medium (MSC-CM) use has also been suggested. It has been observed that cells cultured in MSC-CM showed regular morphology, functional phenotypes of intracellular junctions, and pump functions as compared with those cells that were cultured without MSC-CM. An increase in cellular proliferation was also noted with double the amount of positive cells in MSC-CM cultures. Pump proteins such as VDAC3, CLCN3, SLC4A4, and p120 were expressed in the MSC-CM-cultured cells. It also regulated the G1 proteins of the cell cycle, which are required for endothelial functioning [31].
Human Amniotic Fluid
Another study was designed to evaluate the effects of human amniotic fluid (HAF) on the growth of HCECs and to establish an in vitro method for expanding HCECs. This showed that 20% HAF-containing medium exhibited a greater stimulatory effect on HCEC growth and could represent a potential enriched supplement for HCEC regeneration studies [32].
The conditioned media that have been discussed so far either have an animal origin or an animal-derived component, mainly serum. However, a xeno-free medium would be more suitable to reduce the chances of transmission of prions or any potential disease from the animal origin. Moreover, it is always required by the regulatory authorities to show the minimal risks of using animal-derived components for human use or use a complete synthetic medium. This will further be challenging but required in terms of both health care and regulatory issues.
Cultivation of the Primary Human Donor Corneal Cells Using a Dual-Media Approach
A study by Peh et al. [33] demonstrated that the outcome of culturing primary HCECs and proliferative potential can be successful if negatively impacted by lower, suboptimal plating density. The study showed that it was possible to obtain the hexagonal morphology of the cells at the end of third passage and the count of up to 2.5 × 107 cells with a seeding density of greater than or equal to 1 × 104 cells per cm2 [33]. However, after defining the seeding density, the same group also described a novel dual-media approach for the expansion of primary HCECs in vitro. Analysis of growth dynamics of the cells in proliferative or maintenance media was carried out. At the third passage, homogeneous appearance of the cells and polygonal morphology was observed using the dual-media approach. The cells also expressed endothelium-associated markers. Using this method, 7-day exposure to maintenance medium showed differential gene expression associated with cell proliferation and wound healing, which eventually confirmed that this is a reproducible and consistent method for the culture of HCECs [34].
Human Corneal Endothelial Cell Markers
Structural markers such as ZO-1, which is a tight junctional protein, have been used widely to study the junctions of in vitro expanded HCECs. Figure 1F shows the expression of ZO-1 in a normal human cornea. Because the major function of corneal endothelia is to prevent corneal swelling by the activity of membrane pumps, they are also used as functional markers for cultivated HCECs. These pumps include Na+-K+ ATPase, VDAC3, CLCN3, SLC4A4, and p120.
Leucine-rich repeat-containing G protein-coupled receptor 5 (LGR5), a target of Wnt signaling, has also been identified as a potential biomarker for HCECs. LGR5 has been widely used as a marker of intestine, stomach, and hair follicle stem cells in mice models; however, human corneal tissues were used to study functional gain and loss. The peripheral region of corneal endothelial cells shows a high expression of LGR5, and the cells that express this protein have also been shown to have stem/progenitor cell characteristics. It has been observed that LGR5 is the target molecule of the Hedgehog (HH) signaling pathway in the human corneal endothelium. More importantly, this study showed a repeatable expression of LGR5 that helped to maintain endothelial cell phenotype and the potential to inhibit mesenchymal transformation through the Wnt pathway. Thus, this study highlights new findings that underline homeostatic regulation of human corneal endothelial stem/progenitor cells by LGR5 through the HH and Wnt pathways that are important for corneal endothelial cell functioning [35].
Another study reported the characterization of HCECs in developmental stages. mRNA transcriptomes in human fetal and adult corneal endothelium were studied. High expression signature genes were identified for both fetal (245) and adult (284) HCECs. Many of these genes were identified as disease target genes in hereditary corneal dystrophies, consistent with their functional significance in HCEC physiology. Immunohistochemistry showed localization of four novel markers for fetal and adult HCECs such as Wnt5a, S100A4, S100A6, and IER3. This can further be used to characterize HCECs derived from stem cells or ex vivo expanded cells [36].
Because the currently used markers are not highly satisfactory because of their nonspecific or coexpression in other cell types, a published RNA-seq data of CECs, and the FANTOM5 atlas representing diverse range of cell types based on expression patterns was studied. Five genes CLRN1, MRGPRX3, HTR1D, and ZP4 were identified as novel markers for CECs. The specificities of these genes were confirmed at RNA and protein levels. These markers could further be used for purification of actual CECs and to evaluate the products derived from other cell types [37].
Wound Healing of CECs
ρ-associated protein kinase (ROCK) inhibitor helps in regulating the shape and movement of cells by acting on their cytoskeleton. It has been reported that ROCK inhibitor Y-27632 promotes adhesion, inhibits apoptosis, increases monkey CECs in vitro, and has also been suggested as enhancing corneal endothelial wound healing in vivo in animal models. HCECs did not show any toxicity or cell alterations when treated with ROCK inhibitor. Although it did not show any cell proliferation, ROCK inhibitor significantly enhanced cell adhesion and wound healing. Thus, because ROCK inhibitor did not show any toxicity, its benefits in corneal wound healing and adhesion may be relevant for therapeutic purposes [38].
Tissue Engineering HCEC Sheets
Scaffolds
Once the cells are cultured, it should be seeded on a transportation unit. The scaffolds play an important role in keeping the cells viable and transplanting the same being a biomimetic or as synthetic material. Earlier reports have shown that the cultured CECs have been seeded on the denuded Descemet membrane scaffolds or stromal discs [39]. Human amniotic membranes have been used as a carrier for cultured CECs [40], but because of its translucent nature, it is not highly recommended for clinical applications. Gospodarowicz et al. [41–43] tried to combine two species by seeding bovine CECs onto rabbit corneas denuded of their endothelium. It was reported that when corneas were transplanted back into rabbits, the corneal buttons showed full transparency without any edema [41–43].
Recently, many methods for culturing CECs on synthetic or biological grafts have been established. Mimura et al. [44] used a network of loosely cross-linked type I collagen fibers for CECs. Corneal edema decreased rapidly after transplantation in DSAEK groups [44]. Of the many scaffolds that are described in the literature and have proven its efficiency in vitro or in animal models, Koizumi et al. [45] cultured monkey endothelial cell sheets on collagen type I carriers into monkey eyes; however, the corneas recovered their clarity only 6 months after transplantation. The scaffolds for CECs transplantation can be biologic or synthetic, permanent or biodegradable. Common biological materials in scaffolds reported so far are collagen, fibronectin, and hyaluronan. However, the next step forward would be to check the feasibility of the use of these scaffolds in vivo.
Nanotopography
It has been reported that in the native environment, corneal endothelial cells interact with the nanotopography of the underlying Descemet’s membrane. The study showed that nanotopography enhanced bovine corneal endothelial cell (BCEC) responses and created a monolayer that resembles the healthy corneal endothelium. Topographies of different geometries were first tested to identify those that would elicit the most significant responses. A BCEC monolayer was generated on both micro and nanoscale pillars and wells, and these topographies showed polygonal geometries with well-developed tight junction proteins. Scanning electron microscopy revealed that cells on pillars showed a higher density of microvilli, which was similar to native corneal endothelium. BCECs on nanopillars displayed a lower coefficient of variation of area that was within the range of healthy corneal endothelium. More importantly, a BCEC monolayer cultured on nanopillars also had enhanced Na+/K+ ATPase immunofluorescence expression and mRNA upregulation and a higher Na+/K+ ATPase activity. These results suggest that nanopillar substrate topography may provide the relevant topographical cues, which could significantly enhance the formation and function of the corneal endothelium [46].
Hydrogels
In a recent study, fabrication of biocompatible and biodegradable polyethylene glycol-based hydrogel films (PHFs) for the regeneration and transplantation of CECs has been described. 50-μm thin hydrogel films have similar or greater tensile strengths to human corneal tissue. Light transmission studies revealed that the films were >98% optically transparent, whereas in vitro degradation studies demonstrate their biodegradation characteristics. Cell culture studies demonstrate the regeneration of sheep corneal endothelium on the PHFs. Although sheep CECs do not regenerate in vivo, these cells proliferate on the films with natural morphology and become 100% confluent within 7 days. Implantation of the PHFs into live sheep corneas demonstrates the robustness of the films for surgical purposes. Regular slit lamp examinations and histology of the cornea 28 days after surgery revealed minimal inflammatory responses and no toxicity, indicating that the films were benign. The results of this study suggest that PHFs are excellent candidates as platforms for the regeneration and transplantation of CECs as a result of their favorable biocompatibility, degradability, mechanical, and optical properties; however, in vivo trials will show its real potential in the future [47].
Thermoresponsive Plates
Cellular organization of foreign grafts constructed from cultivated cells is critical to successful graft-host integration and tissue repair. This study described a novel HCEC therapeutic method, in which cultivated adult HCEC sheet with uniform orientation was prepared and transplanted to the rabbit cornea. Having a correct morphology and intact barriers, the HCEC sheet was made by the temperature-modulated detachment of monolayered HCECs from thermoresponsive poly-N-isopropylacrylamide-grafted surfaces and was delivered with proper polarity to the corneal posterior surface by a bioadhesive gelatin disc. The results of the in vivo studies, including the follow-up clinical observations and histological examinations, showed the laminated CEC sheet successfully integrated into the rabbit corneas denuded of their endothelial layer after the biodegradation of gelatin carrier. More importantly, the feasibility of handling and delivery of a monosingular layer of cell sheet in a clinical setting was a major advantage. These data indicate the feasibility of the proposed procedure in cell therapy for corneal endothelial cell loss [48].
HCEC Transplantation
Multiple studies have demonstrated the possibility of culturing HCECs, and the current prospects are to study the transplantation potential of cultured cells in human and animal models. In one study of note, collagen sheets were used as substitute carriers for cultured HCECs. Pump functions showed 76%–95% of those of natural human donor corneas. Thickness was significantly less, and no stromal edema was found postsurgery in a rabbit model [44]. In another study, the cells were injected in the anterior chamber with face down position in the rabbit models. Very early studies on cultured human corneal endothelial cells have been recently carried out on monkey models. Recently, 11 human patients (in an unpublished clinical trial) with bullous keratopathy or Fuch’s dystrophy were treated using the same technique, and 20/20 vision was attained postoperatively. Early stage experiments using Rho kinase inhibitor drops have also showed promising results in terms of visual recovery, thickness, and maintenance of intraocular pressure as reported during the American Society for Cataract and Refractive Surgeons 2015 symposium.
Immunosuppression
Although there have been studies carried out on transplanting the cultured corneal endothelial cells in human recipients, it should not be left out of consideration that immunological responses may hinder the outcomes. The corneal graft has a huge immune privilege as compared with other transplants because of the absence of blood and lymph vessels in the transplantable corneal button, the absence of MHC class II+ antigen-presenting cells in the graft, reduced expression of MHC-encoded alloantigens on graft cells, constitutive expression of T cell-deleting CD95 ligand on the endothelium, the presence of an immunosuppressive local microenvironment (aqueous humor), and the capability of the graft to induce anterior chamber associated immune deviation. Corneal endothelial cells have a distinct molecular strategy to reduce their antigenic visibility to CD4+ and CD8+ effector T cells and to alter the functional program of responding T cells. Although this may be advantageous in terms of donor corneal transplantation, the immunological reactions of the cultured and transplanted HCECs are still being studied [49].
Differentiation of Other Cell Types Into HCEC
Studies have also shown the potential to derive CEC-like cells from human embryonic stem cells (hESCs). The differentiation potential of hESCs to HCECs through the periocular mesenchymal precursor (POMP) phase has been shown using the Transwell coculture system of hESCs with differentiated human corneal stromal cells. CEC-like cells were derived from POMPs using lens epithelial cell-conditioned medium. Corneal endothelial differentiation marker N-cadherin and transcription factors FOXC1 and PitX2 were expressed within 1 week of culture. The isolated cells were seeded onto posterior acellular porcine corneal matrix lamellae to construct the CEC-like cell sheets. These cell sheets were transplanted into rabbit eyes, and the transparency was found to have restored gradually. The cells derived from this source displayed characteristics of native human CECs [50].
Umbilical cord blood mesenchymal stem cells have also been differentiated into HCECs and to determine whether these MSCs can “home” to sites of corneal endothelial cell injury using an ex vivo corneal wound model. RNA was isolated and purified from umbilical cord blood (UCB) MSCs and HCECs. The ability of different culture media was determined, and the morphology, immunolocalization, and gene expression were determined in both tissue culture and ex vivo corneal endothelial wound models. MSCs attached to damaged, but not intact, corneal endothelium in ex vivo corneal wounds. The results have indicated the potential differentiation of cord blood-derived MSCs toward HCEC-like cells [31]. However, further studies will be required to identify the specific microenvironmental conditions that would permit tissue engineering of UCB MSCs to replace damaged or diseased corneal endothelium.
ESCs and induced pluripotent stem cells (iPSCs) have an extensive self-renewal capacity and the potential to differentiate into any tissue specific cell lineages. A study carried out using trans-retinoic acid (RA) treatment in the embryoid body (EB) on mouse ESCs and iPSCs showed the promotion of neural crest cells. These cells were further differentiated into CEC-like cells using lens epithelial cell-conditioned media. For the initial differentiation into neural crest cells, dose of 1 μM RA on day 4 of EB formation showed neural crest differentiation. Plating these cells on gelatin-coated plates further led to cell migration out of EBs. Further, LEC-CM enhanced the differentiation of neural crest into CEC-like cells, which was confirmed using immunocytochemistry and quantitative reverse-transcription polymerase chain reaction. This study showed a potential of two-step inducement procedure for treatment of corneal endothelial failure [51].
Conclusion
Dysfunction in the corneal endothelium, which controls the hydration and transparency of the cornea, is one of the most common reasons of corneal transplantation. The current primary treatment to cure the endothelial failure is the replacement of the diseased corneas with a healthy donor tissue. However, globally the donor tissue supply is too low as compared with the requests, and therefore there is an enormous interest in the development of alternative treatment options or therapeutic strategies to lower the current requirement of human donor corneas. A tissue-engineered corneal endothelium is of much interest because it would allow culturing the corneal endothelial cells harvested from a donor followed by transplantation to many recipients. Recently, research has focused on the challenges of culturing human corneal endothelial cells successfully and on the generation of new biomembranes to be used as cell scaffolds in surgical procedures. However, a standard culture method has not been well established yet for clinical purposes. The studies using telomerase activity and Schwalbe’s line progenitor cells indicated the presence of potential stem-like or progenitor cells in the periphery of the corneal endothelium, but no clear evidence of corneal endothelial stem cells has been reported so far. One step ahead toward a better characterization of endothelial cells might come from the identification of novel cell markers. Although a few patients have already been treated using the injection method, the results are not yet very clear, and doubts about their clinical validity remain. The lack of real alternatives is therefore a great challenge for those working on the replacement of the endothelium through cell culture- and tissue engineering-based techniques.
Author Contributions
M.P., S.F., C.S., S.K., and S.A.: manuscript writing, final approval of the manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Nishida T. Cornea. In: Krachmer J, Mannis M, Holland E, editors. Cornea: Fundamentals, Diagnosis and Management. Vol. 1. Philadelphia, PA: : Elsevier-Mosby; 20053–26. [Google Scholar]
- 2.Polisetti N, Joyce NC. The culture of limbal stromal cells and corneal endothelial cells Methods Mol Biol 2013;1014:131–139. [DOI] [PubMed]
- 3.Maurice DM. Davson H, editor. The cornea and sclera. In: Davson H, ed. The Eye. Orlando, FL: Academic Press, 1984:85.
- 4.Bourne WM. Biology of the corneal endothelium in health and disease. Eye (Lond) 2003;17:912–918. doi: 10.1038/sj.eye.6700559. [DOI] [PubMed] [Google Scholar]
- 5.Parekh M, Salvalaio G, Ruzza A, et al. Posterior lamellar graft preparation: A prospective review from an eye bank on current and future aspects. J Ophthalmol. 2013:769860. doi: 10.1155/2013/769860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah A, Brugnano J, Sun S, et al. The development of a tissue-engineered cornea: Biomaterials and culture methods. Pediatr Res. 2008;63:535–544. doi: 10.1203/PDR.0b013e31816bdf54. [DOI] [PubMed] [Google Scholar]
- 7.Moss DW. Perspectives in alkaline phosphatase research. Clin Chem. 1992;38:2486–2492. [PubMed] [Google Scholar]
- 8.Park JH, Kim SJ, Lee JB, et al. Establishment of a human embryonic germ cell line and comparison with mouse and human embryonic stem cells. Mol Cells. 2004;17:309–315. [PubMed] [Google Scholar]
- 9.Blake DA, Yu H, Young DL, et al. Matrix stimulates the proliferation of human corneal endothelial cells in culture. Invest Ophthalmol Vis Sci. 1997;38:1119–1129. [PubMed] [Google Scholar]
- 10.Wilson SE, Lloyd SA, He YG, et al. Extended life of human corneal endothelial cells transfected with the SV40 large T antigen. Invest Ophthalmol Vis Sci. 1993;34:2112–2123. [PubMed] [Google Scholar]
- 11.Zhu C, Joyce NC. Proliferative response of corneal endothelial cells from young and older donors. Invest Ophthalmol Vis Sci. 2004;45:1743–1751. doi: 10.1167/iovs.03-0814. [DOI] [PubMed] [Google Scholar]
- 12.Joyce NC, Harris DL, Mello DM. Mechanisms of mitotic inhibition in corneal endothelium: Contact inhibition and TGF-beta2. Invest Ophthalmol Vis Sci. 2002;43:2152–2159. [PubMed] [Google Scholar]
- 13.Raviola G. Schwalbe line’s cells: A new cell type in the trabecular meshwork of Macaca mulatta. Invest Ophthalmol Vis Sci. 1982;22:45–56. [PubMed] [Google Scholar]
- 14.Acott TS, Samples JR, Bradley JM, et al. Trabecular repopulation by anterior trabecular meshwork cells after laser trabeculoplasty. Am J Ophthalmol. 1989;107:1–6. doi: 10.1016/0002-9394(89)90805-2. [DOI] [PubMed] [Google Scholar]
- 15.Dueker DK, Norberg M, Johnson DH, et al. Stimulation of cell division by argon and Nd:YAG laser trabeculoplasty in cynomolgus monkeys. Invest Ophthalmol Vis Sci. 1990;31:115–124. [PubMed] [Google Scholar]
- 16.Kelley MJ, Rose AY, Keller KE, et al. Stem cells in the trabecular meshwork: Present and future promises. Exp Eye Res. 2009;88:747–751. doi: 10.1016/j.exer.2008.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodrigues MM, Spaeth GL, Donohoo P. Electron microscopy of argon laser therapy in phakic open-angle glaucoma. Ophthalmology. 1982;89:198–210. doi: 10.1016/s0161-6420(82)34806-x. [DOI] [PubMed] [Google Scholar]
- 18.Alexander RA, Grierson I, Church WH. The effect of argon laser trabeculoplasty upon the normal human trabecular meshwork. Graefes Arch Clin Exp Ophthalmol. 1989;227:72–77. doi: 10.1007/BF02169830. [DOI] [PubMed] [Google Scholar]
- 19.He Z, Campolmi N, Gain P, et al. Revisited microanatomy of the corneal endothelial periphery: New evidence for continuous centripetal migration of endothelial cells in humans. Stem Cells. 2012;30:2523–2534. doi: 10.1002/stem.1212. [DOI] [PubMed] [Google Scholar]
- 20.Peh GS, Toh KP, Wu FY, et al. Cultivation of human corneal endothelial cells isolated from paired donor corneas. PLoS One. 2011;6:e28310. doi: 10.1371/journal.pone.0028310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walshe J, Harkin D. Serial explant culture provides novel insights into the potential location and phenotype of corneal endothelial progenitor cells. Exp Eye Res. 2014;127:9e13. doi: 10.1016/j.exer.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Yokoo S, Yamagami S, Yanagi Y, et al. Human corneal endothelial cell precursors isolated by sphere-forming assay. Invest Ophthalmol Vis Sci. 2005;46:1626–1631. doi: 10.1167/iovs.04-1263. [DOI] [PubMed] [Google Scholar]
- 23.Susumu H, Ryuhei H, Takeshi S, et al. Identification and potential application of human corneal endothelial progenitor cells. Stem Cells Dev. 2014;23:2190–2201. doi: 10.1089/scd.2013.0387. [DOI] [PubMed] [Google Scholar]
- 24.Mimura T, Yamagami S, Yokoo S, et al. Selective isolation of young cells from human corneal endothelium by the sphere-forming assay. Tissue Eng Part C Methods. 2010;16:803–812. doi: 10.1089/ten.TEC.2009.0608. [DOI] [PubMed] [Google Scholar]
- 25.Yu WY, Sheridan C, Grierson I, et al. Progenitors for the corneal endothelium and trabecular meshwork: A potential source for personalized stem cell therapy in corneal endothelial diseases and glaucoma. J Biomed Biotechnol. 2011;2011:412743. doi: 10.1155/2011/412743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chou YF, Chen HH, Eijpe M, et al. The growth factor environment defines distinct pluripotent ground states in novel blastocyst-derived stem cells. Cell. 2008;135:449–461. doi: 10.1016/j.cell.2008.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo Y, Graham-Evans B, Broxmeyer HE. Murine embryonic stem cells secrete cytokines/growth modulators that enhance cell survival/anti-apoptosis and stimulate colony formation of murine hematopoietic progenitor cells. Stem Cells. 2006;24:850–856. doi: 10.1634/stemcells.2005-0457. [DOI] [PubMed] [Google Scholar]
- 28.Smith AG, Heath JK, Donaldson DD, et al. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 1988;336:688–690. doi: 10.1038/336688a0. [DOI] [PubMed] [Google Scholar]
- 29.Singla DK, Singla RD, McDonald DE. Factors released from embryonic stem cells inhibit apoptosis in H9c2 cells through PI3K/Akt but not ERK pathway. Am J Physiol Heart Circ Physiol. 2008;295:H907–H913. doi: 10.1152/ajpheart.00279.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo Y, Hangoc G, Bian H, et al. SDF-1/CXCL12 enhances survival and chemotaxis of murine embryonic stem cells and production of primitive and definitive hematopoietic progenitor cells. Stem Cells. 2005;23:1324–1332. doi: 10.1634/stemcells.2005-0085. [DOI] [PubMed] [Google Scholar]
- 31.Joyce NC, Harris DL, Markov V, et al. Potential of human umbilical cord blood mesenchymal stem cells to heal damaged corneal endothelium. Mol Vis. 2012;18:547–564. [PMC free article] [PubMed] [Google Scholar]
- 32.Feizi S, Soheili Z-S, Bagheri A, et al. Effect of amniotic fluid on the in vitro culture of human corneal endothelial cells. Exp Eye Res. 2014;122:132–140. doi: 10.1016/j.exer.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Peh GS, Toh KP, Ang HP, et al. Optimization of human corneal endothelial cell culture: Density dependency of successful cultures in vitro. BMC Res Notes. 2013;6:176. doi: 10.1186/1756-0500-6-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peh GS, Chng Z, Ang HP, et al. Propagation of human corneal endothelial cells: A novel dual media approach. Cell Transplant. 2015;24:287–304. doi: 10.3727/096368913X675719. [DOI] [PubMed] [Google Scholar]
- 35.Hirata-Tominaga K, Nakamura T, Okumura N, et al. Corneal endothelial cell fate is maintained by LGR5 through the regulation of hedgehog and Wnt pathway. Stem Cells. 2013;31:1396–1407. doi: 10.1002/stem.1390. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y, Huang K, Nakatsu MN, et al. Identification of novel molecular markers through transcriptomic analysis in human fetal and adult corneal endothelial cells. Hum Mol Genet. 2013;22:1271–1279. doi: 10.1093/hmg/dds527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshihara M, Ohmiya H, Hara S, et al. Discovery of molecular markers to discriminate corneal endothelial cells in the human body. PLoS One. 2015;10:e0117581. doi: 10.1371/journal.pone.0117581. published correction appears in: PLoS One 2015;10:e0129412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pipparelli A, Arsenijevic Y, Thuret G, et al. ROCK inhibitor enhances adhesion and wound healing of human corneal endothelial cells. PLoS One. 2013;8:e62095. doi: 10.1371/journal.pone.0062095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi JS, Williams JK, Greven M, et al. Bioengineering endothelialized neo-corneas using donor-derived corneal endothelial cells and decellularized corneal stroma. Biomaterials. 2010;31:6738–6745. doi: 10.1016/j.biomaterials.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 40.Honda N, Mimura T, Usui T, et al. Descemet stripping automated endothelial keratoplasty using cultured corneal endothelial cells in a rabbit model. Arch Ophthalmol. 2009;127:1321–1326. doi: 10.1001/archophthalmol.2009.253. [DOI] [PubMed] [Google Scholar]
- 41.Gospodarowicz D, Greenburg G, Alvarado J. Transplantation of cultured bovine corneal endothelial cells to species with nonregenerative endothelium: The cat as an experimental model. Arch Ophthalmol. 1979;97:2163–2169. doi: 10.1001/archopht.1979.01020020481016. [DOI] [PubMed] [Google Scholar]
- 42.Gospodarowicz D, Greenburg G, Alvarado J. Transplantation of cultured bovine corneal endothelial cells to rabbit cornea: Clinical implications for human studies. Proc Natl Acad Sci USA. 1979;76:464–468. doi: 10.1073/pnas.76.1.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gospodarowicz D, Greenburg G. The coating of bovine and rabbit corneas denuded of their endothelium with bovine corneal endothelial cells. Exp Eye Res. 1979;28:249–265. doi: 10.1016/0014-4835(79)90087-3. [DOI] [PubMed] [Google Scholar]
- 44.Mimura T, Yamagami S, Yokoo S, et al. Cultured human corneal endothelial cell transplantation with a collagen sheet in a rabbit model. Invest Ophthalmol Vis Sci. 2004;45:2992–2997. doi: 10.1167/iovs.03-1174. [DOI] [PubMed] [Google Scholar]
- 45.Koizumi N, Sakamoto Y, Okumura N, et al. Cultivated corneal endothelial cell sheet transplantation in a primate model. Invest Ophthalmol Vis Sci. 2007;48:4519–4526. doi: 10.1167/iovs.07-0567. [DOI] [PubMed] [Google Scholar]
- 46.Teo BK, Goh KJ, Ng ZJ, et al. Functional reconstruction of corneal endothelium using nanotopography for tissue-engineering applications. Acta Biomater. 2012;8:2941–2952. doi: 10.1016/j.actbio.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 47.Ozcelik B, Brown KD, Blencowe A, et al. Biodegradable and biocompatible poly(ethylene glycol)-based hydrogel films for the regeneration of corneal endothelium. Adv Healthc Mater. 2014;3:1496–1507. doi: 10.1002/adhm.201400045. [DOI] [PubMed] [Google Scholar]
- 48.Hsiue GH, Lai JY, Chen KH, et al. A novel strategy for corneal endothelial reconstruction with a bioengineered cell sheet. Transplantation. 2006;81:473–476. doi: 10.1097/01.tp.0000194864.13539.2c. [DOI] [PubMed] [Google Scholar]
- 49.Streilein JW. New thoughts on the immunology of corneal transplantation. Eye (Lond) 2003;17:943–948. doi: 10.1038/sj.eye.6700615. [DOI] [PubMed] [Google Scholar]
- 50.Zhang K, Pang K, Wu X. Isolation and transplantation of corneal endothelial cell-like cells derived from in-vitro-differentiated human embryonic stem cells. Stem Cells Dev. 2014;23:1340–1354. doi: 10.1089/scd.2013.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen P, Chen J-Z, Shao C-Y, et al. Treatment with retinoic acid and lens epithelial cell-conditioned medium in vitro directed the differentiation of pluripotent stem cells towards corneal endothelial cell-like cells. Exp Ther Med. 2015;9:351–360. doi: 10.3892/etm.2014.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]