Abstract
A plasmin inhibitor, named tenerplasminin-1 (TP1), was isolated from Micrurus tener tener (Mtt) venom. It showed a molecular mass of 6542 Da, similarly to Kunitz-type serine peptidase inhibitors. The amidolytic activity of plasmin (0.5 nM) on synthetic substrate S-2251 was inhibited by 91% following the incubation with TP1 (1 nM). Aprotinin (2 nM) used as the positive control of inhibition, reduced the plasmin amidolytic activity by 71%. Plasmin fibrinolytic activity (0.05 nM) was inhibited by 67% following incubation with TP1 (0.1 nM). The degradation of fibrinogen chains induced by plasmin, trypsin or elastase was inhibited by TP1 at a 1:2, 1:4 and 1:20 enzyme:inhibitor ratio, respectively. On the other hand, the proteolytic activity of crude Mtt venom on fibrinogen chains, previously attributed to metallopeptidases, was not abolished by TP1. The tPA-clot lysis assay showed that TP1 (0.2 nM) acts like aprotinin (0.4 nM) inducing a delay in lysis time and lysis rate which may be associated with the inhibition of plasmin generated from the endogenous plasminogen activation. TP1 is the first serine protease plasmin-like inhibitor isolated from Mtt snake venom which has been characterized in relation to its mechanism of action, formation of a plasmin:TP1 complex and therapeutic potential as anti-fibrinolytic agent, a biological characteristic of great interest in the field of biomedical research. They could be used to regulate the fibrinolytic system in pathologies such as metastatic cancer, parasitic infections, hemophilia and other hemorrhagic syndromes, in which an intense fibrinolytic activity is observed.
Keywords: Fibrinolytic system, Hemostasis, Micrurus tener tener, Plasmin inhibitor, Snake venom
1. Introduction
Hemostasis is a complex physiological process that, under normal conditions, balances the formation and dissolution of fibrin clots to prevent hemorrhagic or thrombotic clinical disorders (Colman et al., 2006; van Geffen and van Heerde, 2012). The fibrinolytic system removes fibrin from the circulation in a controlled way and, therefore, prevents excessive fibrin accumulation. This system constitutes a tissue repair mechanism composed of plasminogen, activators, inhibitors, receptors and modulators, which may be activated at the site of fibrin formation and is involved in several processes, such as hemostatic balance, tissue remodeling, tumor invasion, angiogenesis and reproduction. Excessive local or systemic fibrinolysis activity can result in bleeding, as the weakened plug is dissolved. Plasmin has trypsin-like specificity in vitro, cleaving peptides on the C-terminal side of lysine and arginine residues; it plays a major role in the modulation of hemostasis, thrombosis, fibrinolysis, immune response, inflammation, apoptosis, and complement cascade (Lijnen, 2001; Hoover-Plow, 2010; Tsurupa et al., 2010; Jennewein et al., 2011). The main physiological inhibitor of plasmin is α2-antiplasmin. However, other plasma peptidase inhibitors, such as α2-macroglobulin, also show antiplasmin activity (Stassen et al., 2004; Colman et al., 2006).
In several venomous animals, there have been described hemostatically active molecules. (e.g., snakes, leeches, scorpions, caterpillars, ticks and spiders). They may show procoagulant (thrombin-like enzymes and prothrombin or factor X activators) or anticoagulant activities (protein C activators, serine peptidase inhibitors, anti-factor Xa, anti-thrombin), platelet-activating or anti-platelet function (metallopeptidases, disintegrins, C-type lectin-like proteins), fibrinolytic or hemorrhagic activities (Marsh, 1994; Markland, 1998; Sajevic et al., 2011). Several inhibitors of serine peptidases have also been identified in a number of animal venoms. They modulate the enzymatic activity of serine peptidases involved in hemostasis, such as plasmin, and show high pharmaceutical potential (Masci et al., 2000; Flight et al., 2005, 2009; Qiu et al., 2013; Wan et al., 2013).
Several elapid snake venoms with neurotoxic activity (e.g., cobra venom) also contain proteins that activate or inhibit the hemostatic system (Utkin and Osipov, 2007; Osipov et al., 2010; McCleary and Kini, 2013). Elapid venom from Australia and India presents several components which are active on platelets, thrombin and fibrinogen (Gerads et al., 1992; Jagadeesha et al., 2002; Rao et al., 2004; Banerjee et al., 2005; Osipov et al., 2010; Skejić and Hodgson, 2013). Numerous components with myotoxic, cardiotoxic, hemolytic and edematogenic activities have been isolated from Micrurus snake venoms (Weis and McIsaac, 1971; Aird and da Silva, 1991; Tan and Ponnudurai, 1992; Alape-Girón et al., 1994; Barros et al., 1994). Recently, Salazar et al. (2011) described fibrinolytic and anti-fibrinolytic activities in Micrurus tener tener venom. In this study the isolation and characterization of a component with anti-fibrinolytic activity present in M. tener tener venom was described, which could have potential applications in the control of bleeding disorders associated with hyper-fibrinolysis syndromes.
2. Materials and methods
2.1. Reagents
Molecular exclusion column Superdex-200 (10 × 300 mm) was purchased from GE Healthcare (USA). Reverse phase chromatography column C18 Vydac (250 × 4.6 mm) from Alltech Grade Division (USA). Chromogenic substrates (S-2251, S-2222, S-2238 and S-2288) and plasmin from Chromogenix AB (Italy). Human fibrinogen, factor Xa and double chain tPA (tcu-PA) from American Diagnostic (Greenwich, USA). ADP from Chrono-log (USA). Molecular mass markers for SDS-PAGE from Invitrogen Corporation (USA). Trifluoroacetic acid from Riedel-de Haën (Germany). Spectra Multicolor Broad Range Protein Ladder (Thermo Scientific, USA). Ammonium acetate, hydrochloric acid and acetic acid from Merck (Germany). Ethylenediaminetetraacetic acid (EDTA), ε-aminocaproic acid (EACA), bovine thrombin, dithiothreitol (DTT), rabbit anti plasmin, goat anti-rabbit IgG peroxidase-conjugated and other reagents were purchased from Sigma (Sigma Chemical Co., USA).
2.2. Venom
Lyophilized venom of M. tener tener snakes was purchased from the National Natural Toxins Research Center, Texas A&M University-Kingsville, Texas, USA. The venom was stored at −80 °C until used.
2.3. Plasma
Platelet-rich plasma (PRP) was obtained from healthy blood donors with their previous consent. All selected donors had not used any drugs known to interfere with platelet function during the previous 14 days, as approved by the IVIC Bioethics Committee. The blood was mixed with 3.8% sodium citrate in a 9:1 volume ratio, followed by centrifugation at 190 ×g for 20 min at 24 °C. The platelet-poor plasma (PPP) was obtained from the remaining blood by re-centrifuging at 2000 ×g for 15 min at 4 °C.
2.4. Protein concentration determination
Protein concentration was spectrophotometrically determined assuming that 1 unit of absorbance at 280 nm corresponds to 1 mg protein/mL (Simonian and Smith, 2006).
2.5. Polyacrylamide–SDS–tricine gel electrophoresis
Protein samples were run on 7, 8, 9 and 10% polyacrylamide–SDS–tricine gels using the Schägger and von Jagow (1987) method.
2.6. Isolation of TP1
Plasmin inhibitor was isolated from M. tener tener venom after fractionation on a high performance liquid chromatography system. Five milligrams of crude venom was diluted in 50 mM ammonium acetate, pH 6.9 (equilibrium buffer) and then applied to a Superdex-200 (10 × 300 mm) column, equilibrated with the same buffer at room temperature. Protein elution was performed at 0.5 mL/min under isocratic conditions. The fraction showing antiplasmin amidolytic activity was applied to a C-18 column (250 × 4.6 mm, Vydac) equilibrated with 0.12% trifluoroacetic acid (TFA) in water at 1 mL/min flow rate. Protein elution was performed at 1 mL/min with a 0–50% acetonitrile gradient in 0.12% TFA over 30 min. The active fraction was re-chromatographed on the same column and the elution was performed at 1 mL/min using the same acetonitrile gradient over 60 min. The absorbance was monitored at 280 nm. Active fraction named tenerplasminin 1 (TP1) was lyophilized and stored at −80 °C before further biochemical and biological characterization.
2.7. Mass analysis (MALDI-TOF-MS) of TP1
The molecular mass of TP1 was evaluated by MALDI-TOF MS on the AB SCIEX TOF/TOF™ 5800 system in positive linear mode, described by Magalhães et al. (2013). Briefly, TP1 was resuspended in 0.1% TFA and further spotted (0.3 μL) on the target MALDI plate, followed by immediate addition of an equal volume of a saturated matrix solution (10 mg/mL of α-cyano-4-hydroxycinnamic acid, in 50% acetonitrile/0.1% TFA). External calibration was performed with aprotinin (6.5 kDa).
2.8. Effect of TP1 on platelet aggregation
Platelet aggregation was determined by turbidimetry using a dual channel Chrono-log model 560 CA aggregometer (USA). To evaluate the effect on platelet aggregation, 10 μL of TP1 (150 nM final concentration), crude venom (10 μg) or Tyrode's buffer (aggregation control) were added to 490 μL of PRP (platelet count was adjusted to 3.0 × 105 platelets/mL with platelet-poor plasma). The mixtures were incubated for 4 min at 37 °C in silicone-treated glass cuvettes containing a stir bar. Aggregation was induced by 5 μL of ADP (10 μM final concentration) and changes in light transmittance were continuously registered for 8 min. The aggregation response induced by ADP/Tyrode's buffer was used as a reference of 100% aggregation. The percentage of inhibition induced by crude venom or TP1 was calculated by comparing the light transmittance of ADP-induced aggregation in the presence or in the absence of venom or TP1 (Born and Cross, 1963). The results were expressed as % inhibition of platelet aggregation.
2.9. Effect of TP1 on the amidolytic activity of thrombin, factor Xa and tPA
The effect of TP1 on the amidolytic activity of thrombin, factor Xa (FXa) and tissue plasminogen activator (tPA) was assayed upon the chromogenic substrates S-2238 (6 mM), S-2222 (4 mM) and S-2288 (12 mM), respectively. Thrombin (0.1 IU/mL), FXa (0.05 IU/mL) or tPA (0.1 ng/μL) were incubated for 30 min at 37 °C with crude venom (0.5 μg), with the chromatographic fractions (10 μg) or with TP1 (2 nM) in 10 μL 0.05 M Tris–HCl buffer, pH 7.4. Briefly, the samples were mixed on polystyrene plates of 96 wells with 10 μL of the enzymes for 30 min at 37 °C, followed by the incubation with 10 μL of the chromogenic substrate and 70 μL of buffer solution, as recommended by the manufacturer. After incubation at 37 °C for 15 min, the absorbance at 405 nm was determined. Enzymes incubated with only Tris–HCl buffer were used as positive controls. Results were expressed as inhibition percentage, considering the enzyme activity in Tris–HCl buffer as 100% activity. Soybean trypsin inhibitor (SBTI) (50 μg/mL) and hirudin (0.1 IU/mL) were used as inhibition controls of FXa and thrombin, respectively. Benzamidine (10 nM) was used as inhibition control of tPA.
2.10. Effect of TP1 on fibrinogenolytic activity of Mtt venom
The effect of TP1 on the fibrinogenolytic activity of Mtt crude venom or its F2 fraction (obtained through Superdex 200 chromatography) highly active on fibrinogen was evaluated as described by Salazar et al. (2007). After incubation for 30 min at 37 °C with TP1, the residual fibrinogenolytic activity of the crude venom or its F2 fraction was evaluated by SDS-PAGE–tricine.
2.11. Effect of TP1 on plasmin activity
-
a)
The amidolytic activity of plasmin was evaluated on S-2251 substrate. Plasmin (0.5 nM) was incubated for 30 min at 37 °C with crude venom (10 μg) or with TP1 (1 nM). Plasmin incubated only with 0.05 M Tris–HCl buffer, pH 7.4 was used as the positive control of hydrolysis. The percentage of inhibition was calculated comparing the enzymatic activity of plasmin with and without TP1. Aprotinin (2 nM) was used as the plasmin inhibition control.
-
b)
The fibrinolytic activity of plasmin was assayed following the method described by Marsh and Arocha-Pinango (1972), with modifications. Briefly, fibrin films which were formed on 96-well polystyrene plates (200 μL of 0.3% fibrinogen in the presence of plasminogen) were mixed with 6 μL of bovine thrombin (10 IU/mL, in 0.025 M CaCl2), and incubated at 22 °C for 60 min. Plasmin (0.05 nM) pre-treated with TP1 (0.1 nM) or buffer for 30 min at 37 °C was applied on the fibrin gel. The plates were incubated for 8 h at 37 °C and the absorbance at 405 nm was measured. The results were expressed as percentage of lysis, assuming the fibrin absorbance in the presence of buffer as 0% and as 100% the fibrin absorbance in the presence of plasmin (control of activity). Tenerplasminin-1 inhibition was determined by comparing the residual activity of plasmin with the control activity. Aprotinin (0.2 nM) was used as the plasmin inhibition control.
-
c)
The fibrinogenolytic activity of plasmin, trypsin, chymotrypsin and elastase was evaluated by the Gaffney and Dobos (1971) method. Briefly, plasmin (0.05 nM), trypsin (0.05 nM), elastase (0.1 nM) or chymotrypsin (0.05 nM) were mixed with buffer or TP1 at a 1:2, 1:4, 1:20 and 1:40 enzyme:inhibitor ratio, respectively. After 30 min incubation at 37 °C, the residual enzymatic activity of plasmin, trypsin, chymotrypsin or elastase on fibrinogen were evaluated following increasing hydrolysis intervals at 37 °C. The degradation of fibrinogen chains was visualized by SDS-PAGE–tricine. Aprotinin was used as the plasmin inhibition control.
Samples of plasmin incubated with TP1 or aprotinin were subjected to 9% SDS-PAGE under native conditions and Western blot analysis carried out following instructions of the Mini-Trans-Blot of Bio-RAD Laboratories Ltd., using a rabbit anti plasmin antiserum (Sigma) at a dilution of 1:1000, followed by a goat anti-rabbit IgG, peroxidase conjugated, at a dilution of 1:3000.
-
d)
Fibrin lysis: tPA-mediated fibrinolysis was evaluated with a slightly modified method of Marchi et al. (2006). Human plasma (50 μL) was diluted (1:9) with 50 mM Tris–HCl, 0.15 M NaCl, buffer, pH 7.4 (TBS). Then, tPA (0.5 μg/mL), thrombin (1 IU/mL), CaCl2 (10 mM), and aprotinin (0.4 nM) or EACA (1 nM) or TP1 (0.2 nM), were added. The optical density (OD) was recorded at 350 nm every 30 s in a Genesys 2 spectrophotometer (Spectronic Instruments, USA), until the clot was completely dissolved. In order to evaluate the internal fibrin lysis process, two parameters were measured: Lysis time (LT) expressed in seconds (time needed for complete fibrin lysis) and lysis rate (LR), measured as the slope in the linear part of the descending curve after the plateau expressed as OD units/s.
2.12. Statistical analysis
Data were expressed as mean ± standard deviation (SD), considering n ≥ 3. To compare the differences among the experimental groups, the GraphPad PRISM statistical software® was used to perform the analysis of variance (ANOVA), followed by Tukey's multiple comparison test. P values < 0.05 were considered to be statistically significant.
3. Results
3.1. Isolation and physicochemical characterization of TP1
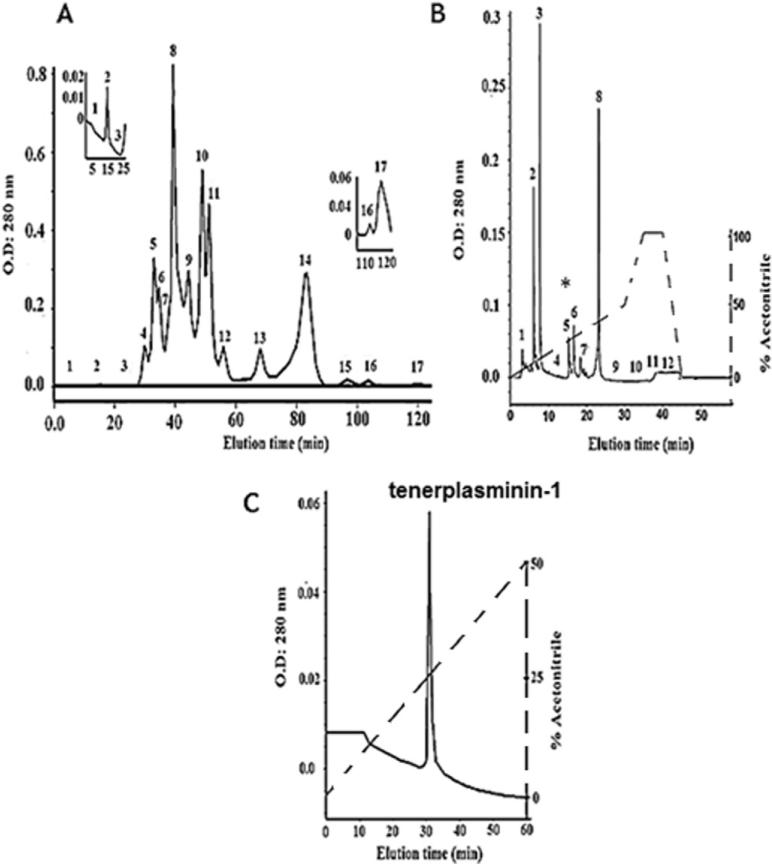
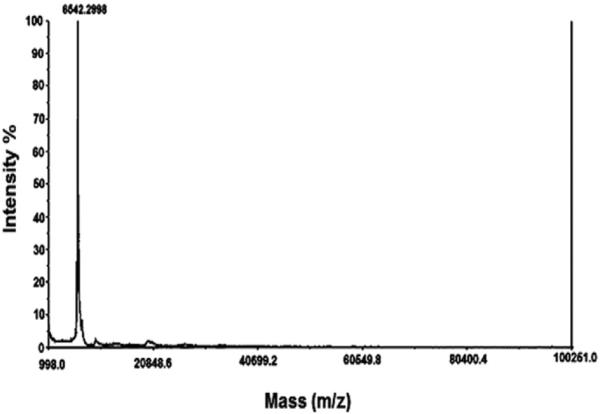
The plasmin inhibitor TP1 was isolated from Mtt crude venom by molecular exclusion chromatography on a Superdex-200 column, yielding 17 fractions named F1 to F17 (Fig. 1A). Fraction F-17 presented the highest antiplasmin amidolytic activity and was further purified by reverse phase chromatography on a C-18 column, using a 0–50% acetonitrile gradient. Twelve fractions were obtained within 30 min, with retention times varying between 5 and 45 min (Fig. 1B). Fraction 5, identified as F17-5, contained the antiplasmin amidolytic activity and was re-chromatographed on the same column, using a similar gradient elution for 60 min, yielding the active fraction named tenerplasminin-1 (Fig. 1C). This procedure was performed 20 times (5 mg venom/Superdex run). Table 1 summarizes the purification scheme of TP1: from 100 mg of crude venom, 1 mg of homogeneous TP1 was obtained. The inhibitor showed a single peak eluting at 34 min on the reverse-phase column. Mass spectrometry analysis (matrix-assisted laser desorption ionization time of flight-MALDI-TOF-TOF) showed a molecular mass of 6542 Da for TP1 (Fig. 2).
Fig. 1.
Isolation of TP1. A) Molecular exclusion chromatography of Mtt venom on a Superdex-200 column. The inserts show zoomed images of fractions 2 and 17, which present fibrinogenolytic and antiplasmin activities, respectively; B) fraction F17 was re-chromatographed on a reverse phase C-18 column; C) fraction F17-5 (*) was re-chromatographed on the same reverse phase system but using a lower gradient. The solid lines indicate the absorbance at 280 nm and the dashed lines show the acetonitrile gradient.
Table 1.
Tenerplasminin-1 purification scheme.
| Purification steps | Active fraction | Total protein (mg) | Recovery (%) |
|---|---|---|---|
| Crude venom | 100 | – | |
| Superdex-200 | F17 | 4.4 | 4.4 |
| F17 (4.4 mg) C-18 column (I) | F17-5 | 1.2 | 27.27 (a1.2%) |
| F17-5 (1.2 mg) C-18 column (II) | F17-5-I (tenerplasminin-1) | 1 | 83.33 (a1%) |
% recovery in relation to total venom. Each run was carried out with 5 mg of venom. This procedure was performed 20 times. The percent of recovery was determined by assuming the total amount of the fraction in the previous step as 100%. Antiplasmin activity was evaluated by the amidolytic activity of plasmin on the S-2251 chromogenic substrate.
Fig. 2.
Linear mode MALDI-TOF spectrum of TP1 showing a molecular mass of 6542 Da.
3.2. Functional characterization of TP1
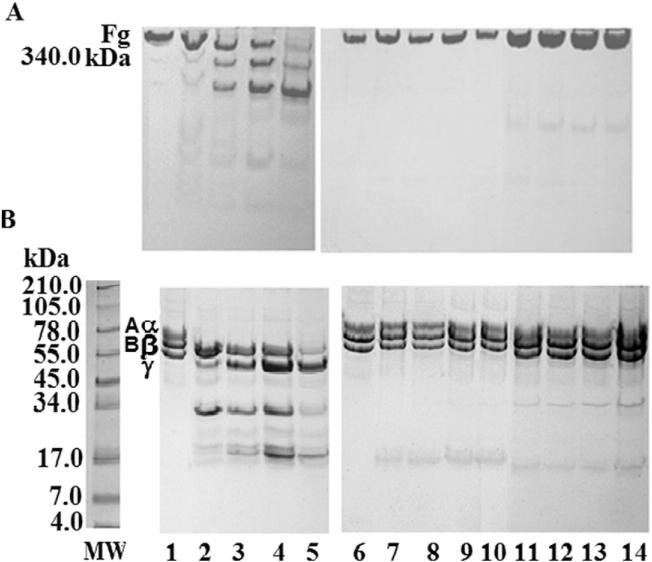
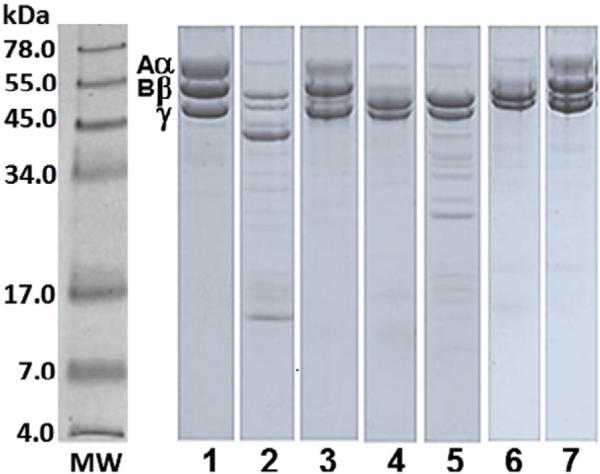
Fig. 3 shows the degradation of fibrinogen chains induced by Mtt venom or F2 fraction (Superdex-200 column) following incubation for 24 h at 37 °C in the absence or the presence of TP1. The results indicated that the plasmin inhibitor did not alter the fibrinogen degradation pattern (lanes 4 and 7) induced by this venom or its active fibrinogenolytic fraction. On the other hand, EDTA significantly inhibited the hydrolysis of fibrinogen by the crude venom or its active fraction (lanes 3 and 6).
Fig. 3.
Effect of TP1 on the fibrinogenolytic activity of Mtt venom and its F2 Superdex-200 fraction. SDS-PAGE (7% T) under reducing conditions stained with Coomassie blue. Mtt venom or its F2 fraction were preincubated for 30 min at 37 °C with EDTA (10 nM) or TP1 (10 nM). Fibrinogen (Fg) was incubated with Mtt venom at 25 μg:2.5 μg ratio for 2 h at 37 °C. MW: protein molecular weight markers; lanes: 1) Fg chains (25 μg); 2) Fg + Mtt venom; 3) Fg + (Mtt venom + EDTA); 4) Fg + (Mtt venom + TP1). Fibrinogen incubated with F2 fraction at 25 μg:2.5 μg ratio for 2 h at 37 °C; 5) Fg + F2; 6) Fg + (F2 + EDTA); 7) Fg + (F2 + TP1).
Tenerplasminin-1 neither inhibited ADP-induced platelet aggregation nor showed any effect upon the amidolytic activity of FXa, thrombin or tPA. M. tener tener venom showed an inhibition of platelet aggregation induced by ADP and an inhibitory effect on amidolytic activity of FXa and plasmin (Salazar et al., 2011).
3.3. Effect of TP1 on plasmin activity
-
a)
The amidolytic activity of plasmin (0.5 nM) upon the synthetic substrate S-2251 was 91% reduced, following the treatment with TP1 (1 nM) for 30 min at 37 °C. Aprotinin (2 nM), used as the plasmin inhibitor control, inhibited the plasmin activity by 71% (data not shown).
-
b)
Table 2 shows the fibrinolytic activity of plasmin (0.05 nM) in the presence of TP1 (0.1 nM). In the presence of plasminogen, TP1 induced a 67% decrease in the fibrin lysis; under the same conditions, aprotinin (0.2 nM) induced a 77% inhibition and EACA (1 nM) lightly modified the plasmin activity. Additionally, when TP1 was incorporated into the fibrin network, there was a 72% inhibition of the fibrin lysis. Using fibrin in the absence of plasminogen, TP1 and aprotinin induced a lysis inhibition of 73 and 81% respectively.
-
c)
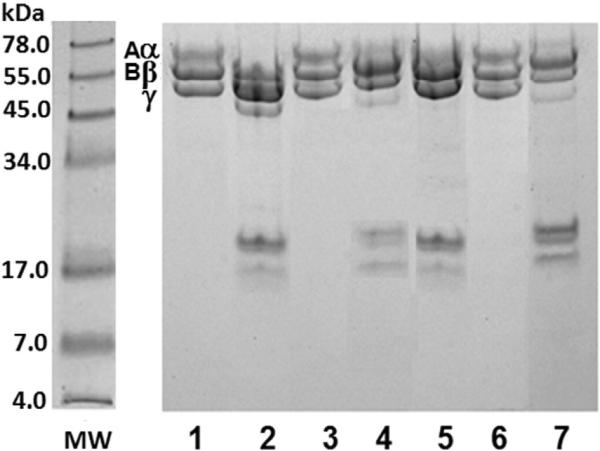
The plasmin fibrinogenolytic activity is shown in Fig. 4. Electrophoresis under native conditions showed that, at a 100:0.12 Fg:plasmin ratio, the fibrinogen molecule was degraded into fragments of different sizes following increasing incubation times (lanes 2-5). This proteolytic activity was inhibited when plasmin was pre-treated with TP1 (1:2 ratio) (lanes 7-10). Aprotinin also completely inhibited the fibrinogenolytic activity of plasmin (lanes 11-14).
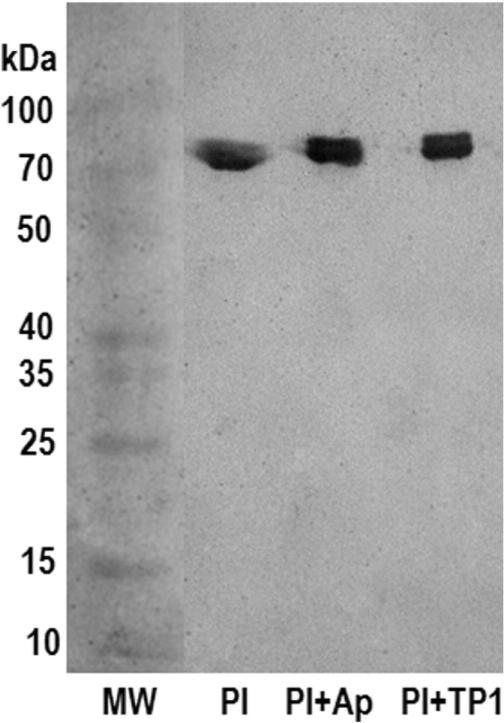
To study the mechanism by which TP strongly inhibits plasmin, we evaluated the formation of plasmin–TP1 complexes using native gel electrophoresis followed by Western blotting. The results on electrophoretic mobility showed that TP1 bound to plasmin, indicating the formation of a plasmin–TP1 complex, similar to plasmin–aprotinin complex (Fig. 5).
-
d)
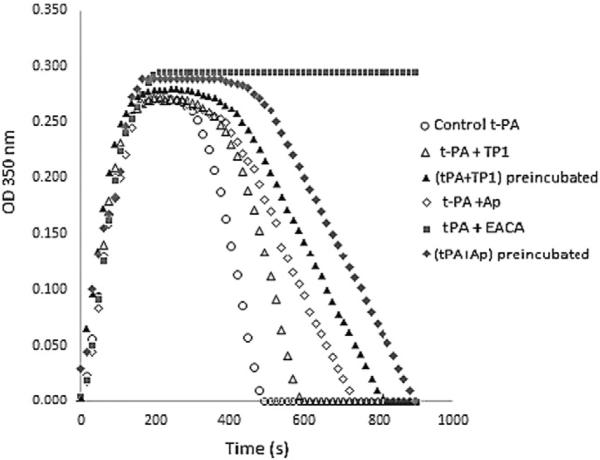
Fig. 6 shows the clot fibrin lysis induced by tPA in the presence of TP1 (0.2 nM) or aprotinin (0.4 nM) or EACA (1 nM). In the presence of EACA no lysis was observed. Tenerplasminin-1 and aprotinin, added simultaneously with the tPA, induced a delay in the clot lysis evidenced by a prolongation of both parameters, TL and VL (Table 3). Additionally, when TP1 or aprotinin were preincubated with the tPA for 30 min at 37 °C, was observed a upper fibrinolysis inhibition.
Table 2.
Effect of TP1 on the plasmin fibrinolytic activity.
| Fibrin/Plg+ + | O.D. (405 nm) | % lysisa | % inhibitionb |
|---|---|---|---|
| Buffer | 0.462 ± 0.015 | 0 | 0 |
| Plasmin (0.05 nM) | 0.187 ± 0.009 | 60 | 0 |
| Plasmin (0.05 nM) + TP1 (0.1 nM) | 0.370 ± 0.013 | 20 | 67* |
| Plasmin (0.05 nM) + TP1 (0.1 nM) included into fibrin | 0.383 ± 0.014 | 17 | 72* |
| Plasmin (0.05 nM) + Aprotinin (0.2 nM) | 0.397 ± 0.015 | 14 | 77** |
| Plasmin (0.05 nM) + EACA (1 nM) included into fibrin | 0.249 ± 0.011 | 46 | 23 |
| Plasmin (0.05 nM) + EACA (1 nM) | 0.204 ± 0.010 | 56 | 7 |
| TP1 (0.1 nM) | 0.454 ± 0.015 | NL | 0 |
| Fibrin/Plg– + | O.D. (405 nm) | % lysisa | % inhibitionb |
|---|---|---|---|
| Buffer | 0.459 ± 0.014 | 0 | 0 |
| Plasmin (0.05 nM) | 0.174 ± 0.010 | 62 | 0 |
| Plasmin (0.05 nM) + TP1 (0.1 nM) | 0.380 ± 0.011 | 17 | 73** |
| Plasmin (0.05 nM) + Aprotinin (0.2 nM) | 0.403 ± 0.016 | 12 | 81** |
| TP1 (0.1 nM) | 0.463 ± 0.013 | NL | 0 |
% fibrin lysis, assuming the absorbance of fibrin in the presence of buffer as 0% activity.
% inhibition of plasmin activity, assuming the plasmin-induced lysis as 100%.
Results represent the mean ± SD expressed as % fibrin lysis. PL: plasmin. TP1: tenerplasminin-1. n = 6. Pg+ results of fibrin lysis in the presence of plasminogen: Pg– results of fibrin lysis in the absence of plasminogen. NL: No lysis.
p < 0.01.
p < 0.001.
Fig. 4.
Effect of TP1 on fibrinogen degradation by plasmin. SDS-PAGE (Tris–tricine system). Coomassie blue staining. Fibrinogen — Fg (25 μg) incubated with plasmin — PL (0.03 μg) in the presence TBS or TP1 (preincubated with PL for 30 min × 37 ° C, at a 1:2 ratio) or aprotinin (preincubated with PL for 30 min × 37 ° C, at a 1:4 ratio). A) Gel 7% T, under native conditions. B) Gel 10% T, under reducing conditions. MW: protein molecular weight markers; lanes: 1: Fg control; 2–5: Fg + PL (1, 2, 4 and 8 h of incubation time); 6: Fg control; 7–10: Fg + (PL + TP1) (1, 2, 4 and 8 h of incubation time); 11–14: Fg + (PL + aprotinin) (1, 2, 4 and 8 h of incubation time).
Fig. 5.
Western blot of plasmin–TP1 complex. Proteins were separated by SDS-PAGE (9% T) under native conditions, transferred onto nitrocellulose membrane and revealed with a rabbit anti plasmin antiserum at a dilution of 1:1000, followed by a goat anti-rabbit IgG, peroxidase conjugated, at a dilution of 1:3000. MW: protein molecular weight markers (spectra multicolor broad range protein ladder); Pl: plasmin; Ap (aprotinin); TP1 (tenerplasmin 1). Pl + Ap (1:4), Pl + TP1 (1:2).
Fig. 6.
Effect of TP1 on fibrin lysis induced by tPA. Human plasma (50 μL, diluted 1:9) was coagulated with thrombin (1 IU/mL) and CaCl2 (10 mM) in the presence of tPA (0.5 μg/mL), and TBS or aprotinin (0.4 nM) or EACA (1 nM) or TP1 (0.2 nM), final concentrations. Curves were recorded continuously using a spectrophotometer (Genesys 2, New York, USA) at 37 °C and with stirring, measuring absorbance changes at 350 nm which were registered in time function.
Table 3.
Effect of TP1 on clot lysis induced by tPA
| LR (–OD/seg × 10–3) | LT (s) | LT 50 (s) | |
|---|---|---|---|
| Control clot + tPA (0.5 μg/mL) | 1.76 | 480 | 210 |
| tPA + TP1 (0.2 nM) | 1.34 | 620* | 350* |
| tPA + Ap (0.4 nM) | 0.74 | 720* | 375* |
| tPA + EACA (1 nM) | 0 | >900** | >900** |
| (tPA + TP1 0.2 nM preincubated) | 0.68 | 865** | 475* |
| (tPA + Ap 0.4 nM preincubated) | 0.65 | 885** | 520* |
TP1: tenerplasminin-1; Ap: aprotinin; LR: lysis rate; LT: lysis time.
p < 0.01.
p < 0.001.
3.4. Effect of TP1 on trypsin, chymotrypsin and elastase fibrinogenolytic activity
The effect of TP1 on the fibrinogenolytic activity of trypsin, chymotrypsin and elastase is shown in Fig. 7. SDS-PAGE under reducing conditions showed that fibrinogen Aα and Bβ chains were degraded into fragments of different sizes by trypsin, elastase and chymotrypsin following increasing incubation times. Trypsin and elastase proteolytic activity was significantly reduced when these enzymes were pre-treated with TP1 at a 1:4 and 1:20 enzyme/inhibitor ratio. However, the proteolytic activity of chymotrypsin was not inhibited in the presence of TP1.
Fig. 7.
Effect of TP1 on the fibrinogenolytic activity of trypsin, chymotrypsin and elastase. SDS-PAGE (10%) under reducing conditions. Coomassie blue staining. Trypsin (Try, 0.05 nM), chymotrypsin (QTry, 0.05 nM), elastase (Elas, 0.1 nM). Fibrinogen (Fg, 25 μg) and TP1 (at a 1:4, 1:40 and 1:20 enzyme:inhibitor ratio, preincubated for 30 min at 37 °C). MW: protein molecular weight markers. Lanes: 1) Fg control; 2) Fg + Try; 3) Fg + (Try + TP1); 4) Fg + QTry; 5) Fg + (QTry + TP1); 6) Fg + Elas; 7) Fg + (Elas + TP1).
4. Discussion
Under physiologic conditions, the hemostatic system restores the lesions on blood vessels and eliminates blood clots following wound healing, with successive restitution of normal blood flow. Fibrinolysis is a physiologic pathway that dissolves the clot in the circulation by the plasmin enzyme. Premature fibrinolysis or hyper-fibrinolysis will dissolve the fibrin deposits and may increase the bleeding tendency. Bleeding is a leading complication of surgery, and has been shown to be associated with increased mortality (Lijnen, 2001; Karkouti et al., 2004). Plasmin is involved in various pathological processes, including thrombolysis, tumor progression, hyper-fibrinolysis states, and other diseases (Kummer et al., 1992; Judex and Mueller, 2005). Physiopathological stimuli, such as stress and exhaustive exercise, or pathological conditions, such as dengue infection, hypotension, surgical trauma, tumors, heatstroke, deficiencies of inhibitors or negative modulators of fibrinolysis system, coagulation factor deficiencies, such as factor VIII or factor IX, associated to a low thrombin generation, may induce excessive fibrinolysis activation, which result in primary hyper-fibrinolysis and bleeding (Karkouti et al., 2004; Perel et al., 2013).
Two categories of therapeutic agents are usually used to reduce the bleeding condition in clinical situations associated with fibrinolysis activation dysregulation: aprotinin, a serine peptidase inhibitor, and lysine analogs, such as ε-aminocaproic acid (EACA) and tranexamic acids (Ortmann et al., 2013). Aprotinin is a Kunitz-type peptidase inhibitor with a molecular mass of ~6.5 kDa, which operates over the reversible binding in the active site of several enzymes, including plasmin, trypsin, kallikrein, urokinase and thrombin (Fritz and Wunderer, 1983). Aprotinin has been the antifibrinolytic drug of choice for some time. EACA with a molecular weight of 131 Da and tranexamic acid, are synthetic inhibitors of the plasmin–plasminogen system. Both agents interact with plasminogen lysine-binding sites and competitively inhibit the binding of plasmin(ogen) to lysine residues on fibrin(ogen) and have been successfully used in a wide range of medical conditions, including cardiac surgery, liver transplantation, hemophilia, von Willebrand factor disease and menorrhagia (McNicol et al., 1966; Eaton, 2008).
Previous studies (Salazar et al., 2011) provided the first evidence of a fibrinolytic activity in Mtt venom related to metallopeptidases. This activity was assayed on fibrin plates in the presence of plasminogen. In addition, during the fibrin lysis, the authors have observed the presence of an inhibitor, which was thought to be associated with the plasmin generated from the endogenous plasminogen activation. These findings guided the isolation of the anti-fibrinolytic compound termed TP1, with a molecular mass of 6542 Da, similarly to other plasmin inhibitors isolated from different animal venoms (Masci et al., 2000; Flight et al., 2005; Brazón et al., 2009; Choo et al., 2012; Cheng and Tsai, 2013; Wan et al., 2013). During that time, the small amount of venom that adult coral snakes produce did not allow having availability of sufficient Mtt venom to purify TP1 to achieve the N-terminal sequence or internal sequences of this peptide.
Peptidase inhibitors are grouped primarily as serine, cysteine, aspartic or metallopeptidase inhibitors. They are found in diverse organisms (Laskowski and Kato, 1980; Wan et al., 2013) including snake venoms from Viperidae and Elapidae families (Takahashi et al., 1972; Masci et al., 2000; Inagaki et al., 2012). Serine peptidase inhibitors target serine peptidases such as plasmin, which plays major roles in the modulation of thrombosis, fibrinolysis, inflammation and apoptosis, thus representing interesting candidates for drug development (van Gent et al., 2003).
Tenerplasminin-1 may be similar to other peptidase inhibitors identified in elapid venoms, which have been associated with Kunitz-type inhibitors, and are also usually found in numerous tissues and organisms, including animals, plants, and microbes (Masci et al., 2000; Cheng et al., 2005; He et al., 2008; Earl et al., 2012; Inagaki et al., 2012). Functionally, Kunitz-type serine peptidase inhibitors show inhibitory activity against trypsin (Peigneur et al., 2011; Choo et al., 2012), chymotrypsin (Zhou et al., 2004), or both (He et al., 2008). For instance, textilinin-1, a 6.7 kDa serine peptidase inhibitor from Pseudonaja textilis venom has been found to inhibit trypsin and plasmin activities, exhibiting a three-dimensional structure typical of Kunitz/BPTI-type inhibitors, with 45% similarity with aprotinin (Masci et al., 2000; Flight et al., 2005). Recently, another Kunitz-type inhibitor, the DrKIn-II from Daboia russelii venom was isolated. It inhibits the amidolytic activity of factor Xa and plasmin amidolytic and fibrinolytic activities on fibrin plates acts by forming a DrKIn-II–plasmin complex (Cheng and Tsai, 2013).
Kunitz inhibitors consist of about 60 amino acids with six conserved cysteine residues that form three disulfide bridges. These inhibitors have a molecular mass of 6–7 kDa, such as TP1 (Laskowski and Kato, 1980; Yang et al., 2009). Many Kunitz family components are inhibitors of serine peptidases with important activities, such as blood coagulation, tissue remodeling and hemostasis, in which the magnitude of the proteolytic function must be wisely restricted (Laskowski, 1986).
In order to determine the specificity of TP1, the fibrinogenolytic activity of plasmin, trypsin, chymotrypsin, elastase and Mtt crude venom, and the amidolytic activity of serine peptidases involved in coagulation and fibrinolysis were evaluated in the presence of this molecule. Tenerplasmin-1 inhibited the amidolytic and the fibrino(geno)lytic activity of plasmin at a 1:2 ratio. Tenerplasminin-1 at a 1:4 and 1:20 ratio also inhibited the fibrinogenolytic activity of trypsin and elastase, but did not inhibit the fibrinogenolytic activity of chymotrypsin. There are six residues identified as P3, P2, P1, P1′, P2′, and P3′, which could interact with the peptidases (Schechter and Berger, 1967). The specificity of Kunitz-type peptidase inhibitor toward serine peptidases is closely associated with the amino acid residues of the main peptidase contact site (P1) position and around P1 position (Inagaki et al., 2012) and small sequence differences in the region that interacts with the peptidase. According to Laskowski and Kato (1980), peptidase inhibitors with P1 Lys and Arg tend to inhibit trypsin and those with P1 Leu, Met, Phe, Tyr and Trp tend to inhibit chymotrypsin. However, in addition to residues surrounding the reactive site, residues present in the weak contact loop are also important for different interactions with various serine peptidases (Cheng et al., 2005). Other snake venom Kunitz-type peptidase inhibitors have been demonstrated to specifically inhibit the proteolytic activity of trypsin (Ritonja et al., 1983; Shafqat et al., 1990; Chou et al., 2010). A new Kunitz-type serine peptidase inhibitor named PIVL, isolated from the Tunisian viper Macrovipera lebetina transmediterranea venom was able to selectively inactivate trypsin but not chymotrypsin (Morjen et al., 2013). The intermolecular interactions of subsites resulting in the elongation on both sides of the scissile bond, which are very important for the mechanism of inhibition of serine peptidases by peptidase inhibitors, providing the base for the inhibitory specificity (Bode et al., 1990).
In contrast, TP1 showed no effect on the amidolytic activity of factor Xa, thrombin or tPA and showed no inhibition of platelet aggregation induced by ADP. Additionally, TP1 did not inhibit the fibrinogenolytic activity of Mtt crude venom or its fibrinolytic fraction F2 (Fig. 3), whose activity has been associated with metallopeptidases and cysteine peptidases (Salazar et al., 2011). Our data evidence that TP1 acts as a strong plasmin inhibitor. A possible mechanism for the anti-fibrinolytic activity of TP1 may be the formation of a plasmin–TP1 complex (Fig. 5).
The effect of the TP1, aprotinin and EACA on fibrin clot lysis by tPA was also evaluated. The results showed that TP1 acts as aprotinin and discreplasminin (Brazón et al., 2009), inducing a delay in lysis time and lysis rate which may be associated with the inhibition of plasmin generated from the endogenous plasminogen activation. In contrast, EACA presented a total inhibitory effect on fibrin lysis (Fig. 6).
In summary, to our knowledge, we have isolated the first peptidase inhibitor derived from Mtt coral snake venom, the TP1. The results suggested that the TP1 induces an anti-fibrinolytic mechanism similar to aprotinin, a plasmin non-competitive inhibitor and a plasminogen activation competitive inhibitor, however, aprotinin is associated with acute renal failure, myocardial infarction, stroke, encephalopathy and increased risk of death (Longstaff, 1994; Karkouti, et al., 2004; Levy and Sypniewski, 2004; Royston, 2015). Its mechanism of action may be through the formation of stable complex made of TP1:enzyme (Fig. 5), as it has already been described for Kunitz inhibitors acting on these enzymes and has also been demonstrated for other serine peptidase inhibitors (Wiman, 1977; Choo et al., 2012; Cheng and Tsai, 2013; Wan et al., 2013).
The inhibition of plasmin fibrinogenolytic activity makes TP1 a potential antifibrinolytic agent, which could be developed for use in patients with bleeding disorders associated with the activation of the endogenous fibrinolytic system.
Acknowledgments
Funding for the research was provided by grants from the Science and Technology Fund (FONACIT) programs (PG-2005000400 grant); Instituto Venezolano de Investigaciones Científicas, Caracas, Venezuela; Consejo de Desarrollo Científico y Humanístico de la Universidad Central de Venezuela PG-09-8760-2013/1; NCRR/BMRG, Viper Resource Grants 8P40OD01960-10 and 3P40OD01096-10S1 (NNTRC, Texas A&M University-Kingsville Texas, USA).
Footnotes
Conflict of interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- Aird SD, Da Silva NJ., Jr. Comparative enzymatic composition of Brazilian coral snake (Micrurus) venoms. Comp. Biochem. Physiol. B. 1991;99:287–294. doi: 10.1016/0305-0491(91)90043-d. [DOI] [PubMed] [Google Scholar]
- Alape-Girón A, Lomonte B, Gustafsson B, Da Silva NJ, Thelestam M. Electrophoretic and immunochemical studies of Micrurus snake venoms. Toxicon. 1994;32:713–723. doi: 10.1016/0041-0101(94)90340-9. [DOI] [PubMed] [Google Scholar]
- Banerjee Y, Mizuguchi J, Iwanaga S, Kini M. Hemextin AB complex — a snake venom anticoagulant protein complex that inhibits factor VIIa activity. Pathophysiol. Haemost. Thromb. 2005;34:184–187. doi: 10.1159/000092420. [DOI] [PubMed] [Google Scholar]
- Barros ACS, Fernandes DP, Ferreira LCL, Santos MC. Local effects induced by venoms from five species of genus Micrurus sp. coral snakes. Toxicon. 1994;32:445–452. doi: 10.1016/0041-0101(94)90296-8. [DOI] [PubMed] [Google Scholar]
- Bode W, Engh R, Musil D, Laber B, Stubbs M, Huber R, Turk V. Mechanism of interaction of cysteine proteinases and their protein inhibitors as compared to the serine proteinase–inhibitor interaction. Biol. Chem. Hoppe Seyler. 1990;371(Suppl.):111–118. [PubMed] [Google Scholar]
- Born G, Cross M. The aggregation of blood platelets. J. Physiol. 1963;168:178–195. doi: 10.1113/jphysiol.1963.sp007185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazón J, D'Suze G, D'Errico ML, Arocha-Piñango CL, Guerrero B. Discreplasminin, a plasmin inhibitor isolated from Tityus discrepans scorpion venom. Arch. Toxicol. 2009;83:669–678. doi: 10.1007/s00204-008-0377-8. [DOI] [PubMed] [Google Scholar]
- Cheng AC, Tsai IH. Functional characterization of a slow and tight-binding inhibitor of plasmin isolated from Russell's viper venom. Biochim. Biophys. Acta. 2013;1840:153–159. doi: 10.1016/j.bbagen.2013.08.019. [DOI] [PubMed] [Google Scholar]
- Cheng YC, Yan FJ, Chang LS. Taiwan cobra chymotrypsin inhibitor, cloning, functional expression and gene organization. Biochim. Biophys. Acta. 2005;1747:213–220. doi: 10.1016/j.bbapap.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Choo YM, Lee KS, Yoon HJ, Qiu Y, Wan H, Sohn MR, Sohn HD, Jin BR. Antifibrinolytic role of a bee venom serine protease inhibitor that acts as a plasmin inhibitor. PLoS One. 2012;7:e32269. doi: 10.1371/journal.pone.0032269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou WM, Liu WH, Chen KC, Chang LS. Structure–function studies on inhibitory activity of Bungarus multicinctus protease inhibitor-like protein on matrix metalloprotease-2, and invasion and migration of human neuroblastoma SK-N-SH cells. Toxicon. 2010;55:353–360. doi: 10.1016/j.toxicon.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Colman RW, Marder VJ, Clowes AW, George JN, Goldhaber SZ. Hemostasis and Thrombosis. In: Colman R, editor. Basic Principles and Clinical Practice. Lippincott Williams & Wilkins; London: 2006. pp. 335–364. [Google Scholar]
- Earl ST, Richards R, Johnson LA, Flight S, Anderson S, Liao A, de Jersey J, Masci PP, Lavin MF. Identification and characterization of Kunitz-type plasma kallikrein inhibitors unique to Oxyuranus sp. snake venoms. Biochimie. 2012;94:365–973. doi: 10.1016/j.biochi.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Eaton MP. Antifibrinolytic therapy in surgery for congenital heart disease. Anesth. Analg. 2008;106:1087–1100. doi: 10.1213/ane.0b013e3181679555. [DOI] [PubMed] [Google Scholar]
- Flight S, Johnson L, Trabi M, Gaffney P, Lavin M, de Jersey J, Masci P. Comparison of textilinin-1 with aprotinin as serine protease inhibitors and as antifibrinolytic agents. Pathophysiol. Haemost. Thromb. 2005;34:188–193. doi: 10.1159/000092421. [DOI] [PubMed] [Google Scholar]
- Flight SM, Johnson LA, Du QS, Warner RL, Trabi M, Gaffney PJ, Lavin MF, de Jersey J, Masci PP. Textilinin-1, an alternative anti-bleeding agent to aprotinin: importance of plasmin inhibition in controlling blood loss. Br. J. Haematol. 2009;145:207–211. doi: 10.1111/j.1365-2141.2009.07605.x. [DOI] [PubMed] [Google Scholar]
- Fritz H, Wunderer G. Biochemistry and applications of aprotinin, the kallikrein inhibitor from bovine organs. Arzneimittelforschung. 1983;33:479–494. [PubMed] [Google Scholar]
- Gaffney PJ, Dobos P. A structural aspect of human fibrinogen suggested by its plasmin degradation. FEBS Lett. 1971;15:13–16. doi: 10.1016/0014-5793(71)80067-4. [DOI] [PubMed] [Google Scholar]
- Gerads I, Tans G, Yukelson LY, Zwaal RF, Rosing J. Activation of bovine factor V by an activator purified from the venom of Naja naja oxiana. Toxicon. 1992;30:1065–1079. doi: 10.1016/0041-0101(92)90052-7. [DOI] [PubMed] [Google Scholar]
- He YY, Liu SB, Lee WH, Qian JQ, Zhang Y. Isolation, expression and characterization of a novel dual serine protease inhibitor, OH-TCI, from king cobra venom. Peptides. 2008;29:1692–1699. doi: 10.1016/j.peptides.2008.05.025. [DOI] [PubMed] [Google Scholar]
- Hoover-Plow J. Does plasmin have anticoagulant activity? Vasc. Health Risk Manag. 2010;6:199–205. doi: 10.2147/vhrm.s9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki H, Kimoto H, Yamauchi Y, Toriba M, Kubo T. Functional characterization of Kunitz-type protease inhibitor Pr-mulgins identified from New Guinean Pseudechis australis. Toxicon. 2012;59:74–80. doi: 10.1016/j.toxicon.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Jagadeesha DK, Shashidharamurthy R, Girish KS, Kemparaju K. A non-toxic anticoagulant metalloprotease, purification and characterization from Indian cobra Naja naja naja venom. Toxicon. 2002;40:667–675. doi: 10.1016/s0041-0101(01)00216-1. [DOI] [PubMed] [Google Scholar]
- Jennewein C, Tran N, Paulus P, Ellinghaus P, Eble JA, Zacharowski K. Novel aspects of fibrinogen fragments during inflammation. Mol. Med. 2011;17:568–573. doi: 10.2119/molmed.2010.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judex MO, Mueller BM. Plasminogen activation/plasmin in rheumatoid arthritis, matrix degradation and more. Am. J. Pathol. 2005;166:645–647. doi: 10.1016/S0002-9440(10)62285-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkouti K, Wijeysundera DN, Yau TM, Beattie WS, Abdelnaem E, McCluskey SA, Ghannam M, Yeo E, Djaiani G, Karski J. The independent association of massive blood loss with mortality in cardiac surgery. Transfusion. 2004;44:1453–1462. doi: 10.1111/j.1537-2995.2004.04144.x. [DOI] [PubMed] [Google Scholar]
- Kummer JA, Abbink JJ, de Boer JP, Roem D, Nieuwenhuys EJ, Kamp AM, Swaak TJ, Hack CE. Analysis of intraarticular fibrinolytic pathways in patients with inflammatory and noninflammatory joint diseases. Arthritis Rheum. 1992;35:884–893. doi: 10.1002/art.1780350806. [DOI] [PubMed] [Google Scholar]
- Laskowski M., Jr. Protein inhibitors of serine proteinases — mechanism and classification. Adv. Exp. Med. Biol. 1986;199:1–17. doi: 10.1007/978-1-4757-0022-0_1. [DOI] [PubMed] [Google Scholar]
- Laskowski M, Jr., Kato I. Protein inhibitors of proteinases. Annu. Rev. Biochem. 1980;49:593–626. doi: 10.1146/annurev.bi.49.070180.003113. [DOI] [PubMed] [Google Scholar]
- Levy JH, Sypniewski E. Aprotinin: a pharmacologic overview. Orthopedics. 2004;27(6 Suppl.):S653–S658. doi: 10.3928/0147-7447-20040602-05. [DOI] [PubMed] [Google Scholar]
- Lijnen HR. Plasmin and matrix metalloproteinases in vascular remodeling. Thromb. Haemost. 2001;86:324–333. [PubMed] [Google Scholar]
- Longstaff C. Studies on the mechanisms of action of aprotinin and tranexamic acid as plasmin inhibitors and antifibrinolytic agents. Blood Coagul. Fibrinolysis. 1994;5:537–542. [PubMed] [Google Scholar]
- Magalhães GS, Caporrino MC, Della-Casa MS, Kimura LF, Prezotto-Neto JP, Fukuda DA, Portes-Junior JA, Neves-Ferreira AG, Santoro M, Barbaro KC. Cloning, expression and characterization of a phospholipase D from Loxosceles gaucho venom gland. Biochimie. 2013;95:1773–1783. doi: 10.1016/j.biochi.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Marchi R, Carvajal Z, Meyer M, Soria J, Ruiz-Saez A, Arocha-Piñango CL, Weisel J. Fibrinogen Guarenas, an abnormal fibrinogen with an Aα-chain truncation due to a nonsense mutation at Aα 467 (GAA) → stop (TAA). Thromb. Res. 2006;118:637–650. doi: 10.1016/j.thromres.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Markland FS. Snake venoms and the hemostatic system. Toxicon. 1998;36:1749–1800. doi: 10.1016/s0041-0101(98)00126-3. [DOI] [PubMed] [Google Scholar]
- Marsh NA. Snake venoms affecting the haemostatic mechanism — a consideration of their mechanisms, practical applications and biological significance. Blood Coagul. Fibrinolysis. 1994;5:399–410. [PubMed] [Google Scholar]
- Marsh NA, Arocha-Pinango CL. Evaluation of the fibrin plate method for estimating plasminogen activators. Thromb. Diath. Haemorrh. 1972;28:75–88. [PubMed] [Google Scholar]
- Masci PP, Whitaker AN, Sparrow LG, de Jersey J, Winzor DJ, Watters DJ, Lavin MF, Gaffney PJ. Textilinins from Pseudonaja textilis textilis. Characterization of two plasmin inhibitors that reduce bleeding in an animal model. Blood Coagul. Fibrinolysis. 2000;11:385–393. doi: 10.1097/00001721-200006000-00011. [DOI] [PubMed] [Google Scholar]
- McCleary RJ, Kini RM. Snake bites and hemostasis/thrombosis. Thromb. Res. 2013;132:642–646. doi: 10.1016/j.thromres.2013.09.031. [DOI] [PubMed] [Google Scholar]
- McNicol GP, Browne MK, Bayley C, Douglas AS. Pathological fibrinolytic states and their treatment with epsilon aminocaproic acid EACA. Br. J. Surg. 1966;53:26–29. doi: 10.1002/bjs.1800530105. [DOI] [PubMed] [Google Scholar]
- Morjen M, Kallech-Ziri O, Bazaa A, Othman H, Mabrouk K, Zouari-Kessentini R, Sanz L, Calvete JJ, Srairi-Abid N, El Ayeb M, Luis J, Marrakchi N. A new serine protease inhibitor from Macrovipera lebetina transmediterranea venom, impairs motility of human glioblastoma cells. Matrix Biol. 2013;32:52–62. doi: 10.1016/j.matbio.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Ortmann E, Besser MW, Klei AA. Antifibrinolytic agents in current anaesthetic practice. Br. J. Anaesth. 2013;111:549–563. doi: 10.1093/bja/aet154. [DOI] [PubMed] [Google Scholar]
- Osipov AV, Filkin SY, Makarova YV, Tsetlin VI, Utkin YN. A new type of thrombin inhibitor, noncytotoxic phospholipase A2, from the Naja haje cobra venom. Toxicon. 2010;55:186–194. doi: 10.1016/j.toxicon.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Peigneur S, Billen B, Derua R, Waelkens E, Debaveye S, Béress L, Tytgat J. A bifunctional sea anemone peptide with Kunitz type protease and potassium channel inhibiting properties. Biochem. Pharmacol. 2011;82:81–90. doi: 10.1016/j.bcp.2011.03.023. [DOI] [PubMed] [Google Scholar]
- Perel P, Prieto-Merino D, Shakur H, Roberts I. Development and validation of a prognostic model to predict death in patients with traumatic bleeding, and evaluation of the effect of tranexamic acid on mortality according to baseline risk, a secondary analysis of a randomised controlled trial. Health Technol. Assess. 2013;17:1–45. doi: 10.3310/hta17240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu F, Tian H, Zhang Z, Yuan XL, Tan YF, Ning XQ. Pharmacological study on hemostasis, analgesic and anti-inflammation effects of the alcohol extract of Hibiscus tiliaceus. Zhong Yao Cai. 2013;36:1648–1651. [PubMed] [Google Scholar]
- Rao VS, Swarup S, Kini RM. The catalytic subunit of pseutarin C, a group C prothrombin activator from the venom of Pseudonaja textilis, is structurally similar to mammalian blood coagulation factor Xa. Thromb. Haemost. 2004;92:509–521. doi: 10.1160/TH04-03-0144. [DOI] [PubMed] [Google Scholar]
- Ritonja A, Turk V, Gubensek F. Serine proteinase inhibitors from Vipera ammodytes venom. Isolation and kinetic studies. Eur. J. Biochem. 1983;133:427–432. doi: 10.1111/j.1432-1033.1983.tb07481.x. [DOI] [PubMed] [Google Scholar]
- Royston D. The current place of aprotinin in the management of bleeding. Anaesthesia. 2015;70(Suppl. 1):46–49. doi: 10.1111/anae.12907. [DOI] [PubMed] [Google Scholar]
- Sajevic T, Leonardi A, Križaj I. Haemostatically active proteins in snake venoms. Toxicon. 2011;57:627–645. doi: 10.1016/j.toxicon.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Salazar AM, Rodriguez-Acosta A, Girón ME, Aguilar I, Guerrero B. A comparative analysis of the clotting and fibrinolytic activities of the snake venom Bothrops atrox Serpentes, Viperidae from different geographical areas in Venezuela. Thromb. Res. 2007;120:95–104. doi: 10.1016/j.thromres.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Salazar AM, Vivas J, Sánchez EE, Rodríguez-Acosta A, Ibarra C, Gil A, Carvajal Z, Girón ME, Estrella A, Navarrete LF, Guerrero B. Hemostatic and toxinological diversities in venom of Micrurus tener tener, Micrurus fulvius fulvius and Micrurus isozonus coral snakes. Toxicon. 2011;58:35–45. doi: 10.1016/j.toxicon.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H, von Jagow G. Tricine–sodium dodecyl sulfate–polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 1987;166:368–337. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Schechter I, Berger A. On the size of the active site in proteases. I. Papain. Biochem. Biophys. Res. Commun. 1967;27:157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- Shafqat J, Beg OU, Yin SJ, Zaidi ZH, Jörnvall H. Primary structure and functional properties of cobra (Naja naja naja) venom Kunitz-type trypsin inhibitor. Eur. J. Biochem. 1990;194:337–341. doi: 10.1111/j.1432-1033.1990.tb15622.x. [DOI] [PubMed] [Google Scholar]
- Simonian MH, Smith JA. Spectrophotometric and colorimetric determination of protein concentration. Curr. Protoc. Mol. Biol. 2006 doi: 10.1002/0471142727.mb1001as76. http://dx.doi.org/10.1002/0471142727.mb1001as76 (Chapter 10:Unit 10.1A) [DOI] [PubMed]
- Skejić J, Hodgson WC. Population divergence in venom bioactivities of elapid snake Pseudonaja textilis, role of procoagulant proteins in rapid rodent prey incapacitation. PLoS One. 2013;8:1–10. doi: 10.1371/journal.pone.0063988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stassen JM, Arnout J, Deckmyn H. The hemostatic system. Curr. Med. Chem. 2004;11:2245–2260. doi: 10.2174/0929867043364603. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Iwanaga S, Suzuki T. Isolation of a novel inhibitor of kallikrein, plasmin and trypsin from the venom of Russell's viper Vipera russelli. FEBS Lett. 1972;27:207–210. doi: 10.1016/0014-5793(72)80621-5. [DOI] [PubMed] [Google Scholar]
- Tan NH, Ponnudurai G. The biological properties of venoms of some American coral snakes genus Micrurus. Comp. Biochem. Physiol. B. 1992;101:471–474. doi: 10.1016/0305-0491(92)90029-q. [DOI] [PubMed] [Google Scholar]
- Tsurupa G, Yakovlev S, McKee P, Medved L. Noncovalent interaction of alpha2-antiplasmin with fibrinogen, localization of alpha2-antiplasmin-binding sites. Biochemistry. 2010;49:7643–7651. doi: 10.1021/bi1010317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utkin YN, Osipov AV. Non-lethal polypeptide components in cobra venom. Curr. Pharm. Des. 2007;13:2906–2915. doi: 10.2174/138161207782023757. [DOI] [PubMed] [Google Scholar]
- Van Geffen M, van Heerde WL. Global haemostasis assays, from bench to bedside. Thromb. Res. 2012;129:681–687. doi: 10.1016/j.thromres.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Van Gent D, Sharp P, Morgan K, Kalsheker N. Serpins: structure, function and molecular evolution. Int. J. Biochem. Cell Biol. 2003;35:1536–1547. doi: 10.1016/s1357-2725(03)00134-1. [DOI] [PubMed] [Google Scholar]
- Wan H, Lee KS, Kim BY, Zou FM, Yoon HJ, Je YH, Li J, Jin BR. A spider-derived Kunitz-type serine protease inhibitor that acts as a plasmin inhibitor and an elastase inhibitor. PLoS One. 2013;8:e53343. doi: 10.1371/journal.pone.0053343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis R, McIsaac RJ. Cardiovascular and muscular effects of venom from coral snake, Micrurus fulvius. Toxicon. 1971;9:219–228. doi: 10.1016/0041-0101(71)90073-0. [DOI] [PubMed] [Google Scholar]
- Wiman B. Primary structure of the B-chain of human plasmin. Eur. J. Biochem. 1977;76:129–137. doi: 10.1111/j.1432-1033.1977.tb11578.x. [DOI] [PubMed] [Google Scholar]
- Yang X, Wang Y, Lu Z, Zhai L, Jiang J, Liu J, Yu H. A novel serine protease inhibitor from the venom of Vespa bicolor Fabricius. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2009;153:116–120. doi: 10.1016/j.cbpb.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Zhou XD, Jin Y, Lu QM, Li DS, Zhu SW, Wang WY, Xiong YL. Purification, characterization and primary structure of a chymotrypsin inhibitor from Naja atra venom. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2004;137:219–224. doi: 10.1016/j.cbpc.2003.11.007. [DOI] [PubMed] [Google Scholar]