Abstract
The levamisole-sensitive nicotinic acetylcholine receptor present at nematode neuromuscular junctions is composed of multiple different subunits, with the exact composition varying between species. We tested the ability of two well-conserved nicotinic receptor subunits, UNC-38 and UNC-29, from Haemonchus contortus and Ascaris suum to rescue the levamisole-resistance and locomotion defects of Caenorhabditis elegans strains with null deletion mutations in the unc-38 and unc-29 genes. The parasite cDNAs were cloned downstream of the relevant C. elegans promoters and introduced into the mutant strains via biolistic transformation. The UNC-38 subunit of H. contortus was able to completely rescue both the locomotion defects and levamisole resistance of the null deletion mutant VC2937 (ok2896), but no C. elegans expressing the A. suum UNC-38 could be detected. The H. contortus UNC-29.1 subunit partially rescued the levamisole resistance of a C. elegans null mutation in unc-29 VC1944 (ok2450), but did cause increased motility in a thrashing assay. In contrast, only a single line of worms containing the A. suum UNC-29 subunit showed a partial rescue of levamisole resistance, with no effect on thrashing.
Keywords: Caenorhabditis elegans, Haemonchus contortus, Ascaris suum, Nicotinic acetylcholine receptor, Levamisole
Graphical abstract
1. Introduction
The free-living nematode, Caenorhabditis elegans, makes an attractive expression system for genes from parasitic species as it is cheap and easy to maintain in the laboratory, with no need for experimentally infected animals to maintain the life-cycle, and there are a plethora of powerful genetic tools available for its manipulation. In recent years, a number of groups, including us, have reported the successful expression of proteins from parasitic nematodes in C. elegans [1–8]. However, most, if not all, of these proteins are active as either monomers or homomeric multimers. Many nicotinic acetylcholine receptors (nAChRs) and related pentameric ligand-gated ion channels are composed of multiple subunits which have to assemble to form an active protein. Nematode nAChR, especially those found at the neuromuscular junction (nmj), are important anthelmintic drug targets and the sites of action of compounds such as levamisole, pyrantel, tribendimidine, monepantel and derquantel [9, 10].
The levamisole-sensitive nAChR present at the C. elegans neuromuscular junction are made up of five subunits, LEV-1, LEV-8, UNC-29, UNC-38 and UNC-63 and, at least when expressed in vitro in the Xenopus oocyte, the loss of any one of these makes the receptor non-functional [11]. In addition, there is a levamisole-insensitive nAChR that consists of a homo-pentamer of ACR-16 subunits [12]. The analogous receptors of parasitic nematodes are likely to be similar; the levamisole-sensitive receptors of Haemonchus contortus and Oesophagostonum dentatum can be reconstituted in vitro from ACR-8, UNC-29, UNC-38 and UNC-63 subunits, though this is complicated by the presence of multiple paralogs of the unc-29 gene in these organisms [13, 14]. The predicted protein product of the Hco-lev-1 gene (nomenclature as described by Beech et al [15]) is predicted to lack a signal peptide and does not seem to be incorporated into the receptor in vitro [13]. Omission of the Hco-ACR-8 subunit resulted in the expression of a pharmacologically distinct form of the receptor in vitro [13]. Functional nAChR can be formed in vitro by the expression of only two subunits from Ascaris suum, Asu-UNC-29 and Asu-UNC-38, and the properties of the receptor vary with the subunit composition [16]. However, the genome sequence shows that orthologs of the unc-63 and acr-8 genes are present in this organism [17]. This complexity mirrors that revealed by in vivo measurements on the muscle cells of A. suum and O. dentatum where three or four distinct sub-types of receptor can be resolved at the single-channel level [14, 18, 19].
Successful expression of individual nAChR subunits from parasitic nematodes in C. elegans therefore requires that they are able to co-assemble with endogenous host subunits to form a functional receptor, unlike the glutamate-gated chloride channels we studied previously[1, 2] many of which form functional homomeric receptors when expressed in vitro [20–23]. The aim of the experiments reported here was to test whether or not subunits of nAChR from parasitic nematodes could assemble with endogenous C. elegans subunits to form a functional levamisole-sensitive nicotinic receptor. We chose to use the UNC-29 and UNC-38 subunits of H. contortus and A. suum in these experiments because these subunits are conserved in all nematode genomes for which we have sequence information and they are constituents of all the recombinant nematode levamisole-sensitive nAChR that have been reconstituted in the Xenopus oocyte system to date. In order to prevent endogenous C. elegans UNC-29 and UNC-38 subunits, whether functional or not, interfering with the assembly of receptors incorporating the parasite subunits we used unc-29 and unc-38 deletion mutants of C. elegans as the hosts for these experiments. H. contortus is fairly closely related to C. elegans on nematode phylogenetic trees, being placed in the same clade (clade V) as C. elegans by Blaxter et al. [24] and in clade 9B by van Megen et al., who place C. elegans in clade 9A [25]. A. suum is more distantly related to C. elegans: Blaxter et al. place it in clade III and van Megen et al. in clade 8B [24, 25]. We were interested in comparing the two to see if phylogenetic distance may have any effect on the ability of the parasite subunits to functionally express in the C. elegans background.
2 Methods and Materials
2.1 Materials
All the C. elegans strains used in this project were supplied by the Caenorhabditis Genetics Center, St. Paul, MN, USA. The plasmids pPD95.75 and pPD138.11 (Addgene plasmids # 1494 and # 1721) were a gift from Andrew Fire. Unless otherwise stated, all chemicals were from Sigma (St. Louis, MO, USA).
2.2 Transformation of C. elegans
We constructed plasmids for transfection into C. elegans by sub-cloning the full-length parasite cDNAs (Accession Numbers: Hco-unc-29.1 = EU006786.1; Asu-unc-29 = EU006073.1; Hco-unc-38 = GU060984.1; Asu-unc-38 = EU053155.1) into a pGEM3-derived plasmid, downstream of the putative C. elegans unc-29 or unc-38 promoters. The promoter fragments used corresponded to 1.4 kbp (unc-29) or 1.1 kbp (unc-38) of genomic sequence upstream of the start codons, as these regions have been demonstrated previously to drive body-wall muscle expression of the subunits [26, 27].
Biolistic transformation of C. elegans wild-type and mutant strains was carried out essentially as described by Glendinning et al [2], except that a myo-2::GFP construct (pPD138.11) was used as a co-transformant and candidate transformed lines picked on the basis of pharyngeal GFP expression. This was to avoid the muscle cell expression of GFP under the myo-3 promoter causing any problems in the interpretation of the results. All plasmids were linearized with Apa-1 (New England Biolabs, Ipswich, MA, USA) at 25C for 3 hours prior to transformation.
2.3 Identification of transgenic lines
To select the GFP-expressing transformants, the nematodes were viewed under a dissecting microscope (Olympus SZX7) with a Lumen Dynamics X-cite series 120Q light source and a GFP filter. Individuals expressing GFP in the pharynx were transferred to individual 60mm×15mm NGM plates with an OP50 lawn. The presence of the test DNA was verified for each line using single worm PCR with primers specific for the parasite cDNA as described [2].
2.4 C. elegans Synchronization and Motility Scoring
Wild-type and mutant worms were plated on NGM plates seeded with OP50 E. coli and allowed to mature to adulthood and lay eggs (4–6 days). Plates were washed with M9 to remove adults and gently scraped with a Pasteur pipette to dislodge eggs. M9 containing worms and eggs was centrifuged in a 15 ml conical tube for 2 minutes at 1500 RPM to pellet worms. The pellet was washed with fresh M9 to remove residual OP50 and centrifuged once more. Following the second wash, the supernatant was removed and 5 ml of bleach solution (0.5 ml 5 M NaOH + 1.5 ml 6% bleach +3.5 ml deionized water) was added. Tubes were shaken, vortexed, and rotated to dissolve worms for 5 minutes, or until only eggs were visible. Tubes were then centrifuged at 1500 RPM for 2 minutes to pellet eggs, and the supernatant was immediately removed and replaced by 3 ml fresh M9. The solution was poured over an unseeded NGM plate (60 mm) and placed on a bench-top rocker (KSA 250 Basic) set to 150 RPM overnight. Once hatched, L1 worms were collected and placed on seeded plates and allowed to grow to early adulthood (~56 hours, pre-egg laying) at 19° C for use in motility assays. Assays were setup in 96 well plates with approximately 100 third-stage larvae (L3) or 50 adults per well. All experiments were run in triplicate and their motility measured on the ‘Worminator’ as described [28]. Videos were recorded for approximately 30 seconds per well.
2.5 Levamisole sensitivity assays
The drug sensitivity of the transgenic lines, as well as the wild type and mutant strains, was tested by incubation in different concentrations of levamisole. Worms were washed off a week old 60mmx 15mm NGM plate with 1 ml of M9 buffer; this population contained a mixture of developmental stages. This was then diluted with M9 buffer to a concentration of approximately 50 worms per 20µl. A stock solution of 1mM levamisole was made by dissolving 0.024g of levamisole in 100ml of distilled water. This was then divided into 10ml aliquots and immediately frozen until required. From these 10ml 1mM stocks 1ml was used in 6× 1:10 serial dilutions to generate the following drug concentrations for the assay: 1mM, 0.1mM, 0.01mM, 1µM, 0.1µM, 0.01µM and 1nM. The assay was set up in a 48-well plate with each well containing 1ml of drug or M9 buffer as a control and then 20µl of worms in M9 Buffer (approximately 50 worms) was added to each well. The plate was left to incubate at 19°C for 40 minutes before the worms were scored using an inverted microscope. Worms were scored as ‘moving’ or ‘paralyzed’. Most worms fell clearly into one of these two groups, however some were observed as ‘twitching’, showing occasional movement along part of the body length. These were scored as ‘moving’. Each assay was completed at least three times for each worm line. In order to test which lines showed significantly (p< 0.05) different levamisole responses to the parent strain of C. elegans a one-way ANOVA and the post hoc Dunnett’s test was used, this analysis was carried out using Graph Pad Prism 5 software.
3. Results
3.1 The unc-38 promoter drives GFP expression in muscle cells
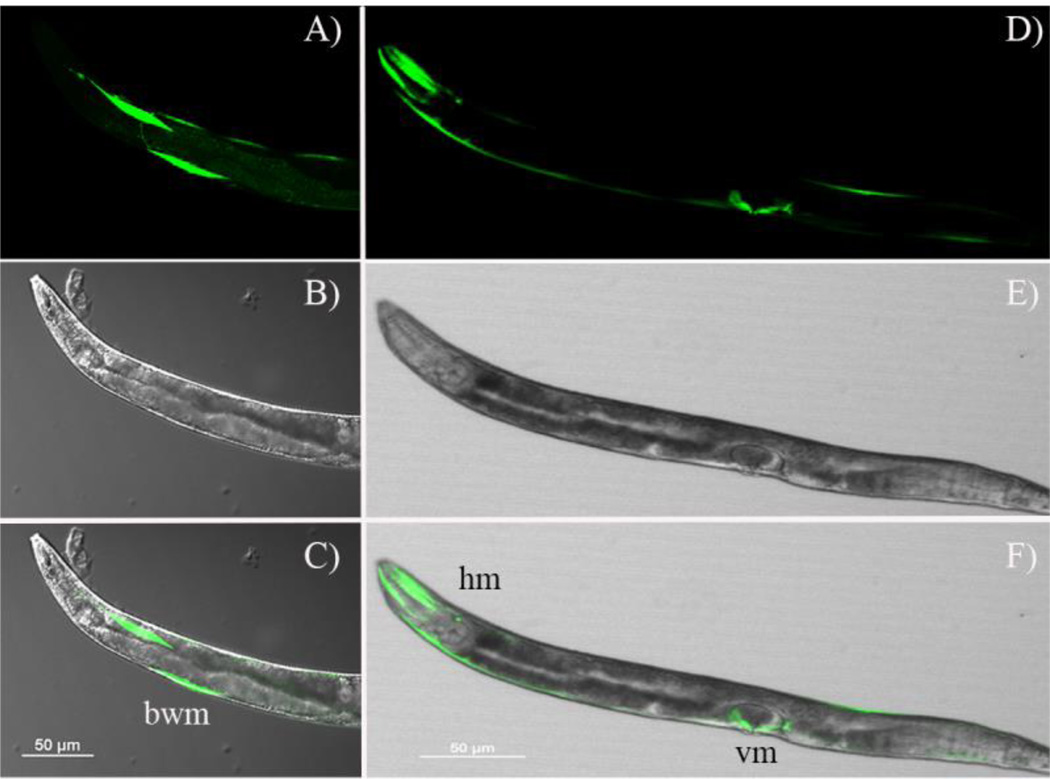
We sub-cloned the unc-29 and unc-38 upstream regions into pPD95.75, upstream of the GFP coding sequence, and used biolistics to transform these constructs into N2 C. elegans. Following transformation, fluorescence was observed in body-wall, head and vulval muscle cells. Figure 1 shows two images of individual worms containing the punc-38::GFP constructs. These animals were not from cloned lines expressing the GFP, but it is clear from both sets of images that there is mosaicism in the expression patterns observed.
Figure 1.
Confocal images of two individual C. elegans expressing GFP under the control of the unc-38 promoter sequence used in these studies. Panels A and D are the GFP images, B and E are the transmitted light images and C and F are the merged images. bwm = body wall muscle cells, hm = head muscle cells and vm = vulval muscle cells.
3.2 Expression of Hco-UNC-38 rescues the behavioral and pharmacological defects in an unc-38 knockout mutant of C. elegans
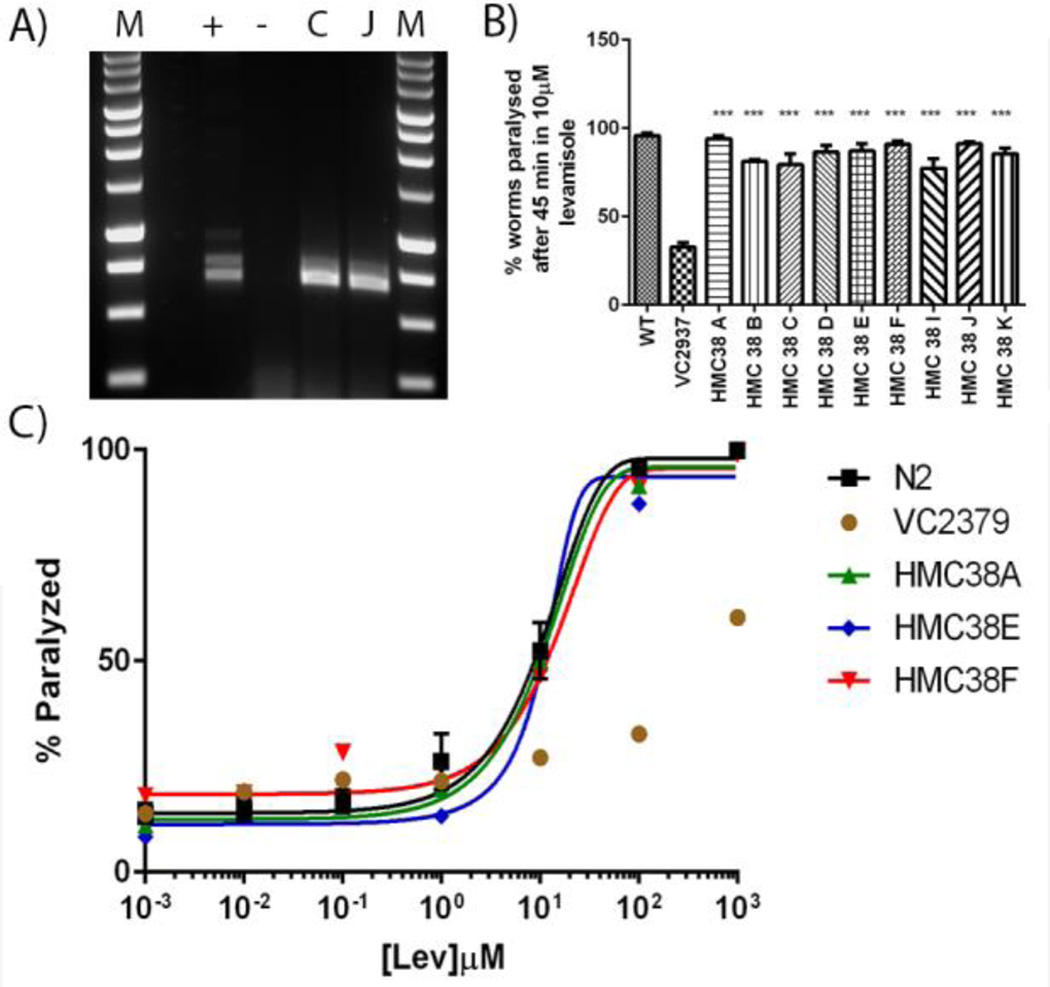
We transformed the unc-38 knock-out strain of C. elegans, VC2937, with plasmids containing either the Hco-unc-38 or Asu-unc-38 cDNA behind the C. elegans unc-38 upstream sequence, together with myo- 2::GFP. After the transformation, worms showing GFP expression in the pharyngeal muscle cells were picked. Multiple such worms were observed following transformation with Hco-unc-38, and these worms also showed considerably greater motility than the parent VC2937 animals (Supplementary Movies 1 & 2). This made them very easy to identify, as VC2937 worms are very sluggish and slow-growing. We selected independent lines of VC2937 transformed with Hco-unc-38 cDNA and demonstrated, by PCR, that they contained the transgene. Positive PCR amplifications for two lines, HNC38/C and HMC38/J, are shown in Figure 2A and identical results were obtained from the other lines (data not shown). We then tested the effect of a single concentration of levamisole, 100µM, to paralyze these strains. As shown in Figure 2B, nearly all (95.9 ± 5.7 %; n=17) of the wild-type C. elegans were paralyzed after 45 minutes incubation in the drug, as opposed to 32.7 ± 4.3% (n=3) of the VC2937 animals. A much higher proportion (77.3 – 94.0%, depending on the strain; n=3 for each strain) of the individual worms in all of the transgenic strains tested were also paralyzed by this incubation, suggesting that the parasite UNC-38 subunit had restored drug sensitivity to the mutant strain. In order to confirm this, we examined the dose-response relationship between levamisole and paralysis for the parent and transformed strains and found that in all cases the response of the transformed lines was almost identical to that of N2, and completely different from that of VC2937 (p = <0.0001). Dose-response curves for three lines are shown in Figure 2C; the results for all the other PCR-positive lines tested were almost identical, but have been omitted in order to reduce the complexity of the graph.
Figure 2.
A) Agarose gel (1%) demonstrating the amplification of Hco-unc-38 cDNA from individual worms of transgenic lines. M = 1 kbp markers (Promega), ‘+’ = Positive control (plasmid containing Hco-unc-38 cDNA), ‘-’ = Negative Control (VC2937), ‘C’ and ‘J” are single worms from lines HMC38/C and HMC38/J respectively. The ~700bp band is indicative of the presence of the H. contortus unc-38 sequences in the strain. B) Paralysis of transgenic VC2937 C. elegans lines expressing Hco-UNC-38 by 45 minutes exposure to 10 µM levamisole. In all cases p < 0.0001 compared to VC2937. C) Dose-response curve for levamisole-induced paralysis of transgenic VC2937 lines. For panels B) and C) n ≥3 and the errors bars indicate ± SEM.
In contrast to the results obtained with Hco-unc-38, we were unable to obtain any worms that exhibited more robust locomotion following transformation with Asu-unc-38, as we were unable to identify any progeny expressing or containing this cDNA.
3.3 Expression of Hco-unc-29 and Asu-unc-29 only partially rescues levamisole resistance
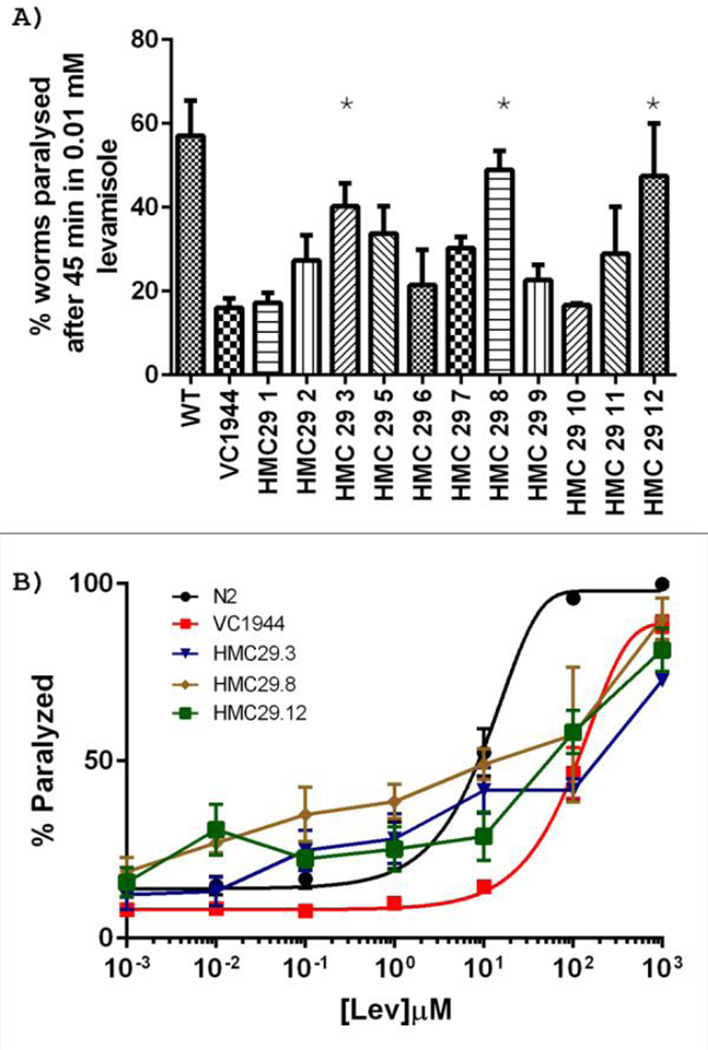
We carried out similar experiments to introduce the Hco-unc-29.1 and Asu-unc-29 cDNAs into the unc-29 knock-out mutant VC1944, along with myo-2::GFP as a marker for transformation. Identification of transformants was not as straightforward as with VC2937, since the motility of VC1944 animals is similar to that of wild-type. We were able to isolate multiple lines expressing GFP in the pharynx following these transformations and several of these contained the parasite cDNA, as assessed by PCR on single adult worms (Figure 3). When the cDNA-containing lines were tested for their ability to continue to move after a 45-minute exposure to 10µM levamisole only 3 out of 11 lines containing the Hco-unc-29.1 cDNA that we tested, HMC29/3 (41.7±12.8%, n=3), HMC29/8 (49.0±7.7%, n=3) and HMC29/12 (28.7±15.3%, n=3), showed statistically significantly increased susceptibility to levamisole compared to VC1944, of which 14.6 ± 8.0% (n = 17) were paralyzed (p = 0.0027) (Figure 4A). This was partially confirmed in dose-response experiments (Figure 4B), though none of the lines showed complete rescue to wild-type levamisole sensitivity.
Figure 3.
Identification of VC1944-derived transgenic lines containing Hco-unc-29.1 or Asu-unc-29 cDNA by single worm PCR. A) Identification of lines containing Hco-unc-29.1. Lane 1 is a negative control (N2), lanes 3–13 are amplifications on single worms showing pharyngeal expression of GFP. M= 1kbp markers. The amplification product of ~1,500 bp indicates the presence of the H. contortus unc-29 DNA. B) Identification of lines containing Asu-unc-29. Lane 1 is a positive control (Asu-unc-29 cDNA), lane 2 is a negative control (N2), lanes 3–7 are amplifications from individual worms exhibiting pharyngeal GFP expression. The amplification product of ~1,500 bp in lanes 3, 5 and 7 indicate presence of the A. suum unc-29 DNA.
Figure 4.
A) Effects of incubation in 10µM levamisole for 45 minutes on VC1944-derived lines containing the Hco-unc-29.1 cDNA. * = p <0.05 compared to VC1944. B) Dose-response curves for some of the lines. N ≥ 3 in all cases.
When we assessed the sensitivity of worms from the four lines containing the Asu-unc-29 cDNA to 10µM levamisole, none of them showed any statistically significant difference in response to VC1944, (Figure 5A). However, a higher proportion (79.1 ± 1.57%) of the worms from one of the lines, ASC29/3, was paralyzed at 100µM drug, compared to those of VC1944 (46.65 ± 7.18%; p = 0.0116), again suggestion a partial rescue of the drug resistance phenotype by the parasite sub-unit (Figure 5B).
Figure 5.
A) Effects of incubation in 10µM levamisole for 45 minutes on VC1944-derived lines containing the Asu-unc-29 cDNA. None of the lines showed any statistically significant differences from VC1944. B) Dose-response curves for some of these lines. * indicates the single point at which ASC29/3 gave a significantly different response from VC1944 (p=0.0116). N ≥3 in all cases.
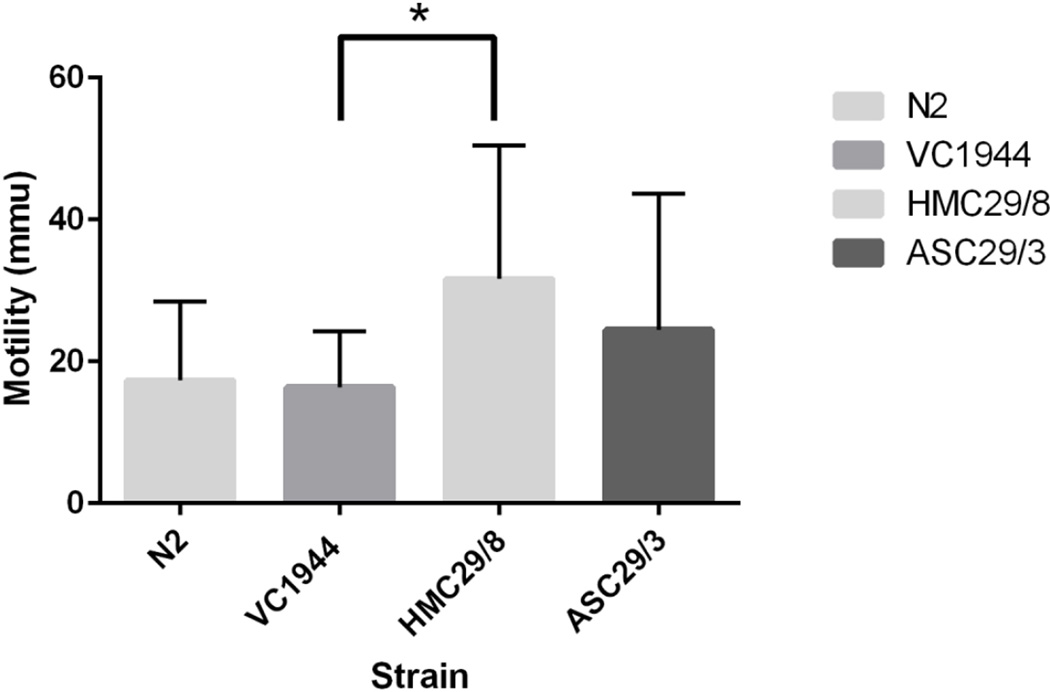
Since the effects of expressing the parasite UNC-29 subunits on the drug sensitivity of VC1944 was limited, we examined the movement of two lines, HMC29/8 and ASC29/3 in order to see if this provided further evidence for the expression of the two proteins in the transgenic strains. Analysis of the motility of some of unc-29 transformants using the ‘Worminator’ [28], essentially a ‘thrashing’ assay, showed that adult HMC29/8 were significantly more mobile than the host strain, VC1944 (p=0.028), whereas those of ASU29/3 were not (p=0.22) (Figure 6). These data provided additional evidence that the HCO-UNC-29.1 subunit was expressed in HMC29/8 and had an effect on the neuromuscular function of this strain.
Figure 6.
Motility of C. elegans lines determined using the ‘Worminator’ (n=5 independent experiments for each strain shown). All recordings were for 30 seconds at room temperature. Mmu = mean motility units calculated by the WormAssay program as previously described [28].
4. Discussion
There have been many reports of the successful expression of genes or cDNAs derived from parasitic nematodes in the model organism, C. elegans. However, the majority of these have involved gene products that are active as monomers, or can form functional homo-multimers. This includes our previous study on glutamate-gated chloride channels from Haemonchus contortus, where the ability of Hco-GLC-3, Hco- GLC-5 and Hco-GLC-6 to rescue ivermectin resistance [2] parallels their ability to form functional homomeric channels in vitro [20, 29]. In contrast, the levamisole-sensitive nicotinic acetylcholine receptors studied to date are all composed of multiple subunits, which must assemble with each other and often interact with several chaperones in order to form a functional channel [11, 13, 14, 16]. This poses a far greater challenge to the successful expression of individual subunits from parasitic species, which may be an essential step on the road to a reconstitution of a full parasite nAChR in C. elegans. Given the importance of nAChR as anthelmintic targets, both of ‘old’ and ‘new’ drugs [10], we examined the ability of two subunits, UNC-29 – a non-α subunit – and UNC-38 – an α subunit, derived from two parasitic species, A. suum (clade 3) and H. contortus (clade 5) to express and co-assemble with the other components of the C. elegans levamisole-sensitive receptor.
We confirmed that the promoter sequences we used were capable of driving expression of GFP in body-wall, head and vulval muscle cells (Figure 1), but the individual worms we examined (which were not derived from a clonal line) showed mosaic expression patterns. If this result was repeated in the transformed lines, some of the muscle cells would express the parasite receptor and that this might influence their ability to rescue the drug resistance phenotype. This would be a greater problem with the unc-29 experiments than with those using unc-38, as the severe paralysis of the VC2937 strain made it easy to identify individuals in which the phenotype had been rescued. The UNC-38 of H. contortus is able to completely rescue the locomotion and drug-resistance phenotypes we tested, indicating that it is capable of assembling with the other C. elegans subunits to form a fully functional receptor, which is also capable of interacting with the chaperones and other factors required for post-synaptic expression [11, 13, 30–32]. In contrast, we could not produce any rescue of locomotion following introduction of the Asu-unc-38 cDNA into the same VC2937 strain. If any ASU-UNC-38 protein was produced following this introduction, which we believe is likely given that exactly the same methods were used as for the successful experiments with the H. contortus cDNA, it was presumably incapable of associating with the C. elegans receptor proteins and/or chaperones. The two UNC-29 proteins showed at best a partial rescue of drug sensitivity. This is despite the evidence that both the subunits contribute to functional receptors in the Xenopus oocyte [16], which confirms that the cDNAs do encode functional subunits. The evidence suggests that both the H. contortus and A. suum subunits might co-assemble with the endogenous C. elegans subunits and chaperones to form functional receptors, based on a slightly increased thrashing activity of HMC29/8 compared to N2 or the knock-out mutant strain, VC1944, and a slightly increased levamisole sensitivity of three out of eleven tested lines that contained the Hco-unc-29.1 cDNA and one out of four that contained Asu-unc-29, but that these receptors either did not contain a high-affinity levamisole binding site, or were not present at neuromuscular junctions at very high levels. It is also possible that the expression levels of the parasite UNC-29 subunits was not sufficient to allow them to assemble with the C. elegans proteins. It is worth noting that H. contortus possesses four copies of unc-29 [13, 33, 34] and it possible that copies other than the one used here (Hco-unc-29.1) would co-assemble better with the C. elegans components. The unc-29 mutant strain, VC1944, exhibits almost normal locomotion, in contrast to the unc-38 deletion, which is possibly a little surprising since the ok2450 allele is a large deletion that would be expected to cause a functionally null mutation. The absence of either UNC-29 or UNC-38 prevents the formation of a functional receptor in vitro [11], but it is possible that in vivo another non-α subunit, such as ACR-2 or ACR-3, may be able to substitute for UNC-29. ACR-2 and ACR-3 have both been reported to form functional receptors when co-expressed with UNC-38 [35, 36]. If this does occur, then it would obviously complicate the analysis of experiments such as those reported here.
Overall, our data show that receptor subunit cDNAs from the more closely related parasite, H. contortus, may be better able to rescue C. elegans mutations than those of the more distantly-related A. suum. Though possibly not surprising, this does indicate that it might be possible to fully express a nicotinic receptor from a clade V nematode, which includes many important veterinary and human parasites, in C. elegans. Recent developments in CRSIPR/Cas-9 technology, including its application to C. elegans [37–39], are likely to make such studies much easier in the future.
Supplementary Material
Highlights.
The UNC-29 and UNC-38 of A. suum and H. contortus were expressed in C. elegans
Hco-UNC-38 was able to rescue the lev and unc phenotypes of VC2397
Asu-UNC-38 was not able to rescue these phenotypes
Both UNC-29 proteins partially, but not fully, increased drug susceptibility
Acknowledgments
This work was supported by award R21 AI092022 from NIAID. C. elegans strains were provided by the Caenorhabditis Genetics Center, St. Paul, MN, which is supported by the National Institutes of Health - Office of Research Infrastructure Programs (P40 OD010440). We should like to thank Veronica Benedit for help with some of the experiments reported.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Information
Sup Fig 1) Movies of a) VC2397 and b) HMC38/J.
Sup Table 1) Primers used.
References
- 1.Cook A, Aptel N, Portillo V, Siney E, Sihota R, Holden-Dye L, et al. Caenorhabditis elegans ivermectin receptors regulate locomotor behaviour and are functional orthologues of Haemonchus contortus receptors. Mol Biochem Parasitol. 2006;147:118–125. doi: 10.1016/j.molbiopara.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Glendinning SK, Buckingham SD, Sattelle DB, Wonnacott S, Wolstenholme AJ. Glutamate-gated chloride channels of Haemonchus contortus restore drug sensitivity to ivermectin resistant Caenorhabditis elegans. Plos One. 2011;6:e22390. doi: 10.1371/journal.pone.0022390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Britton C, Murray L. Using Caenorabditis elegans for functional analysis of genes of parasitic nematodes. Int J Parasit. 2006;36:651–659. doi: 10.1016/j.ijpara.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Couthier A, Smith J, McGarr P, Craig B, Gilleard JS. Ectopic expression of a Haemonchus contortus GATA transcription factor in Caenorhabditis elegans reveals conserved function in spite of extensive sequence divergence. Mol Biochem Parasitol. 2004;133:241–253. doi: 10.1016/j.molbiopara.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 5.Crook M, Grant K, Grant WN. Failure of Parastrongyloides trichosuri daf-7 to complement a Caenorhabditis elegans daf-7 (e1372) mutant: implications for the evolution of parasitism. Int J Parasit. 2010;40:1675–1683. doi: 10.1016/j.ijpara.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Massey HC, Castelletto ML, Bhopale VM, Schad GA, Lok JB. Sst-tgh-1 from Strongyloides stercoralis encodes a proposed ortholog of daf-7 in Caenorhabditis elegans. Mol Biochem Parasitol. 2005;142:116–120. doi: 10.1016/j.molbiopara.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Welz C, Kruger N, Schniederjans M, Miltsch SM, Krucken J, Guest M, et al. SLO-1-channels of parasitic nematodes reconstitute locomotor behaviour and emodepside sensitivity in Caenorhabditis elegans slo-1 loss of function mutants. PLoS Pathogens. 2011;7:e1001330. doi: 10.1371/journal.ppat.1001330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li FC, Lok JB, Gasser RB, Korhonen PK, Sandeman MR, Shi DS, et al. Hc-daf-2 encodes an insulin-like receptor kinase in the barber's pole worm Haemonchus contortus, and restores partial dauer regulation. Int J Parasit. 2014;44:485–496. doi: 10.1016/j.ijpara.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolstenholme AJ. Ion channels and receptors as targets for the control of parasitic nematodes. International Journal for Parasitology - Drugs and Drug Resistance. 2011;1:2–13. doi: 10.1016/j.ijpddr.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin RJ, Robertson AP, Buxton SK, Beech RN, Charvet CL, Neveu C. Levamisole receptors: a second awakening. Trends Parasitol. 2012;28:289–296. doi: 10.1016/j.pt.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boulin T, Gielen M, Richmond JE, Williams DC, Paoletti P, Bessereau J-L. Eight genes are required for functional reconstitution of the Caenorhabditis elegans levamisole-sensitive acetylcholine receptor. Proc Natl Acad Sci U S A. 2008;105:18590–18595. doi: 10.1073/pnas.0806933105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Touroutine D, Fox RM, Von Stetina SE, Burdina A, Miller DM, Richmond JE. acr-16 encodes an essential subunit of the levamisole-resistant nicotinic receptor at the Caenorhabditis elegans neuromuscular junction. Journal of Biological Chemistry. 2005;280:27013–27021. doi: 10.1074/jbc.M502818200. [DOI] [PubMed] [Google Scholar]
- 13.Boulin T, Fauvin A, Charvet CL, Cortet J, Cabaret J, Bessereau JL, et al. Functional reconstitution of Haemonchus contortus acetylcholine receptors in Xenopus oocytes provides mechanistic insights into levamisole resistance. British Journal of Pharmacology. 2011;164:1421–1432. doi: 10.1111/j.1476-5381.2011.01420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buxton SK, Charvet CL, Neveu C, Cabaret J, Cortet J, Peineau N, et al. Investigation of acetylcholine receptor diversity in a nematode parasite leads to characterization of tribendimidine-and derquantel-sensitive nAChRs. Plos Pathogens. 2014;10:e1003870. doi: 10.1371/journal.ppat.1003870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beech RN, Wolstenholme AJ, Neveu C, Dent JA. Nematode parasite genes: what's in a name? Trends Parasitol. 2010;26:334–340. doi: 10.1016/j.pt.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Williamson SM, Robertson AP, Brown L, Williams T, Woods DJ, Martin RJ, et al. The nicotinic acetylcholine receptors of the parasitic nematode Ascaris suum: formation of two distinct drug targets by varying the relative expression levels of two subunits. PLoS Pathogens. 2009;5:e1000517. doi: 10.1371/journal.ppat.1000517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jex AR, Liu SP, Li B, Young ND, Hall RS, Li YR, et al. Ascaris suum draft genome. Nature. 2011;479:529-U257. doi: 10.1038/nature10553. [DOI] [PubMed] [Google Scholar]
- 18.Martin RJ, Robertson AP, Bjorn H, Sangster NC. Heterogeneous levamisole receptors: A singlechannel study of nicotinic acetylcholine receptors from Oesophagostomum dentatum. Eur J Pharmacol. 1997;322:249–257. doi: 10.1016/s0014-2999(96)00996-x. [DOI] [PubMed] [Google Scholar]
- 19.Qian H, Martin RJ, Robertson AP. Pharmacology of N-, L-, and B-subtypes of nematode nAChR resolved at the single-channel level in Ascaris suum. FASEB J. 2006;20:E2108–E2116. doi: 10.1096/fj.06-6264fje. [DOI] [PubMed] [Google Scholar]
- 20.Horoszok L, Raymond V, Sattelle DB, Wolstenholme AJ. GLC-3: a novel fipronil and BIDN-sensitive, but picrotoxinin-insensitive, L-glutamate-gated chloride channel subunit from Caenorhabditis elegans. British Journal of Pharmacology. 2001;132:1247–1254. doi: 10.1038/sj.bjp.0703937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCavera S, Rogers AT, Yates DM, Woods DJ, Wolstenholme AJ. An ivermectin-sensitive glutamate-gated chloride channel from the parasitic nematode Haemonchus contortus. Mol Pharmacol. 2009;75:1347–1355. doi: 10.1124/mol.108.053363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cully DF, Vassilatis DK, Liu KK, Paress P, Van der Ploeg LHT, Schaeffer JM, Arena JP. Cloning of an avermectin-sensitive glutamate-gated chloride channel from Caenorhabditis elegans. Nature. 1994;371:707–711. doi: 10.1038/371707a0. [DOI] [PubMed] [Google Scholar]
- 23.Vassilatis DK, Arena JP, Plasterk RHA, Wilkinson H, Schaeffer JM, Cully DF, et al. Genetic and biochemical evidence for a novel avermectin sensitive chloride channel in C. elegans isolation and characterisation. Journal of Biological Chemistry. 1997;272:33167–33174. doi: 10.1074/jbc.272.52.33167. [DOI] [PubMed] [Google Scholar]
- 24.Blaxter ML, De ley P, Garey JR, Liu LX, Scheldeman P, Vierstrate A, Vanfleteren JR, Mackey LY, Dorris M, Frisse LM, Vida JT, Thomas WK. A molecular evolution framework for the phylum Nematoda. Nature. 1998;392:71–75. doi: 10.1038/32160. [DOI] [PubMed] [Google Scholar]
- 25.van Megen H, van den Elsen S, Holterman M, Karssen G, Mooyman P, Bongers T, et al. A phylogenetic tree of nematodes based on about 1200 full-length small subunit ribosomal DNA sequences Nematology. 2009;11:927–954. [Google Scholar]
- 26.Fleming JT, Squire MD, Barnes TM, Tornoe CT, Matsuda K, Sulston JE, et al. Caenorhabditis elegans levamisole resistance genes lev-1, unc-29 and unc-38 encode functional nicotinic acetylcholine receptor subunits. Journal of Neuroscience. 1997;17:5843–5857. doi: 10.1523/JNEUROSCI.17-15-05843.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu YS, LeBeouf B, Guo XY, Correa PA, Gualberto DG, Lints R, et al. A cholinergic-regulated circuit coordinates the maintenance and bi-stable states of a sensory-motor behavior during Caenorhabditis elegans male copulation. Plos Genetics. 2011;7:e1001326. doi: 10.1371/journal.pgen.1001326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Storey B, Marcellino C, Miller M, Maclean M, Mostafa E, Howell S, et al. Utilization of computer processed high definition video imaging for measuring motility of microscopic nematode stages on a quantitative scale: "The Worminator". International Journal for Parasitology-Drugs and Drug Resistance. 2014;4:233–243. doi: 10.1016/j.ijpddr.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forrester SG, Prichard RK, Dent JA, Beech RN. Haemonchus contortus: HcGluCla expressed in Xenopus oocytes forms a glutamate-gated ion channel that is activated by ibotenate and the antiparasitic drug ivermectin. Mol Biochem Parasitol. 2003;129:115–121. doi: 10.1016/s0166-6851(03)00102-6. [DOI] [PubMed] [Google Scholar]
- 30.Halevi S, McKay J, Palfreyman M, Yassin L, Eshel M, Jorgensen E, et al. The C-elegans ric-3 gene is required for maturation of nicotinic acetylcholine receptors. EMBO J. 2002;21:1012–1020. doi: 10.1093/emboj/21.5.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boulin T, Rapti G, Briseno-Roa L, Stigloher C, Richmond JE, Paoletti P, et al. Positive modulation of a Cys-loop acetylcholine receptor by an auxiliary transmembrane subunit. Nat Neurosci. 2012;15:1374–1381. doi: 10.1038/nn.3197. [DOI] [PubMed] [Google Scholar]
- 32.Richard M, Boulin T, Robert VJP, Richmond JE, Bessereau JL. Biosynthesis of ionotropic acetylcholine receptors requires the evolutionarily conserved ER membrane complex. Proc Natl Acad Sci U S A. 2013;110:E1055–E1063. doi: 10.1073/pnas.1216154110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laing R, Kikuchi T, Martinelli A, Tsai IJ, Beech RN, Redman E, et al. The genome and transcriptome of Haemonchus contortus, a key model parasite for drug and vaccine discovery. Genome Biology. 2013:14. doi: 10.1186/gb-2013-14-8-r88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwarz EM, Korhonen PK, Campbell BE, Young ND, Jex AR, Jabbar A, et al. The genome and developmental transcriptome of the strongylid nematode Haemonchus contortus. Genome Biology. 2013:14. doi: 10.1186/gb-2013-14-8-r89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Squire MD, Tornoe C, Baylis HA, Fleming JT, Barnard EA, Sattelle DB. Molecular cloning and functional coexpression of a Caenorhabditis elegans nicotinic acetylcholine-receptor subunit (acr-2) Recept Channels. 1995;3:107–115. [PubMed] [Google Scholar]
- 36.Baylis HA, Matsuda K, Squire MD, Fleming JT, Harvey RJ, Darlison MG, et al. ACR-3, a Caenorhabditis elegans nicotinic acetylcholine receptor subunit - Molecular cloning and functional expression. Recept Channels. 1997;5:149–158. [PubMed] [Google Scholar]
- 37.Dickinson DJ, Ward JD, Reiner DJ, Goldstein B. Engineering the Caenorhabditis elegans genome using Cas9-triggered homologous recombination. Nature Methods. 2013;10:1028–1034. doi: 10.1038/nmeth.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Friedland AE, Tzur YB, Esvelt KM, Colaiacovo MP, Church GM, Calarco JA. Heritable genome editing in C. elegans via a CRISPR-Cas9 system. Nature Methods. 2013;10:741–743. doi: 10.1038/nmeth.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lo TW, Pickle CS, Lin S, Ralston EJ, Gurling M, Schartner CM, et al. Precise and heritable Genome editing in evolutionarily diverse nematodes using TALENs and CRISPR/Cas9 to engineer insertions and deletions. Genetics. 2013;195:331–348. doi: 10.1534/genetics.113.155382. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.