Abstract
Rationale
Excessive alcohol (EtOH) drinking is difficult to model in animals despite the extensive human literature demonstrating that stress increases EtOH consumption.
Objective
The current experiments show escalations in voluntary EtOH drinking caused by a history of social defeat stress and intermittent access to EtOH in C57BL/6J mice compared to non-stressed mice given intermittent EtOH or continuous EtOH. To explore a mechanistic link between stress and drinking, we studied the role of corticotropin-releasing factor type-1 receptors (CRF-R1) in the dopamine-rich ventral tegmental area (VTA).
Results
Intra-VTA infusions of a CRF-R1 antagonist, CP376395, infused into the VTA dose-dependently and selectively reduced intermittent EtOH intake in stressed and non-stressed mice, but not in mice given continuous EtOH. In contrast, intra-VTA infusions of the CRF-R2 antagonist astressin2B non-specifically suppressed both EtOH and H2O drinking in the stressed group without effects in the non-stressed mice. Using in vivo microdialysis in the nucleus accumbens shell (NAc), we observed that stressed mice drinking EtOH intermittently had elevated levels of tonic dopamine concentrations compared to non-stressed drinking mice. Also, VTA CP376395 potentiated dopamine output to the NAc only in the stressed group causing further elevations of dopamine post-infusion.
Conclusions
These findings illustrate a role for extrahypothalamic CRF-R1 as especially important for stress-escalated EtOH drinking beyond schedule-escalated EtOH drinking. CRF-R1 may be a mechanism for balancing the dysregulation of stress and reward in alcohol use disorders.
Keywords: Alcohol, intermittent, social defeat stress, ventral tegmental area, CRF-R1, CRF-R2, dopamine, nucleus accumbens, microdialysis
INTRODUCTION
The anxiolytic, stress-relieving effects of EtOH are well established in humans and animal models, as the tension-reduction hypothesis remains one of the oldest theories for why individuals consume EtOH (Conger 1956). There have been many experimental methods to increase EtOH drinking in animals, but not all generate stress-enhanced consumption (Becker et al. 2011). Social defeat and subordination stress can lead to increased EtOH drinking in mice and monkeys compared to non-stressed animals or higher ranking individuals (Sillaber et al. 2002; Peretti and Lewis 1969; but see Van Erp and Miczek 2001). We recently explored specific defeat stress parameters that escalated two-bottle choice EtOH drinking in outbred mice (Norman et al. 2015). This ten-day episodic defeat caused chronic elevations in corticosterone (Norman et al. 2015), indicative of altered hypothalamic-pituitary-adrenal (HPA) stress function. This cascade of endocrine stress responses is initiated by corticotropin-releasing factor (CRF), coordinating behavioral responses in socially defeated animals (Heinrichs et al. 1992).
Not only is CRF integral to social defeat stress, the neuropeptide has also been implicated in heavy EtOH drinking. CRF receptor type-1 (CRF-R1) antagonists have been used as preclinical treatments for EtOH dependence (Phillips et al. 2015) since it was found that CRF increased in the amygdala in rats withdrawing from EtOH liquid diet (Merlo Pich et al. 1995). CRF-R1 antagonists can reduce anxiety-like behavior during EtOH withdrawal, block responding for EtOH reinforcement in dependent animals, and suppress stress-induced EtOH reinstatement (Valdez et al. 2002; Liu and Weiss 2002). Also, CRF-R1 antagonists decreased binge-like drinking and intermittent access to EtOH drinking (Sparta et al. 2008; Simms et al. 2014; Hwa et al. 2013). Importantly, CRF-R1 antagonist treatment was effective in the dependent or heavy-drinking animals and not in non-dependent animals or those that drank moderate EtOH levels. One prevailing hypothesis postulates that chronic drug intake induces a long-lasting dysregulation of extrahypothalamic CRF mechanisms, primarily in the extended amygdala, including the basolateral and central amygdala (CeA) and the bed nucleus of the stria terminalis (BNST; Zorrilla et al. 2014). However, CRF-R1 may be activated in other areas of the brain, such as the ventral tegmental area (VTA).
The VTA is a site for the modulation of dopamine by CRF and its output to forebrain regions like the nucleus accumbens (NAc) and the medial prefrontal cortex (Swanson et al. 1983; Rodaros et al. 2007). CRF-R1s in the VTA have been critical in stress-elevated cocaine self-administration and nicotine dependence (Boyson et al. 2014; Grieder et al. 2014). Neurochemically, acute EtOH administration increases extracellular dopamine concentrations in the NAc (Imperato and Di Chiara 1986). EtOH can affect VTA dopaminergic neurons as indicated by augmented cell firing and dopamine uptake (Brodie et al. 1990; Budygin et al. 2001). Alternatively, stress, aversive stimuli, and CRF also increase dopamine (Tidey and Miczek 1996; Brischoux et al. 2009). Stress activation of CRF systems may facilitate glutamatergic input to dopamine neurons (Ungless et al. 2003; Wang et al. 2005), thereby providing a means whereby stress (and/or withdrawal) could engage appetitive motivational systems in the transition to dependence.
Previous studies have shown that intra-VTA CRF-R1 antagonist CP154526 suppressed consumption in drinking-in-the-dark and intermittent access to EtOH in C57BL/6J mice (Sparta et al. 2008; Hwa et al. 2013). The current experiments investigated the role of VTA CRF-R1 in an additional population, mice that drank excessive levels of EtOH after social defeat stress. It was expected that CRF-R1 antagonist CP376395 intra-VTA would specifically reduce EtOH drinking escalated by schedule of access and social defeat stress, but not in mice drinking moderately given continuous access to EtOH. Although some research has focused on CRF-R1 as a modulator of dopamine neurons in the VTA, CRF-R2 may also play a role in stress-induced drug taking (Wang et al. 2007). In contrast, intra-VTA CRF-R2 antagonism with astressin2B may suppress both EtOH and water drinking only in the stressed mice. Another objective was to measure extracellular NAc dopamine as affected by CRF-R1 antagonism in the stressed versus non-stressed drinking animals using in vivo microdialysis. Tonic dopamine levels may be elevated in the stressed mice based on our previous observations in rats that experienced episodic social defeat (Miczek et al. 2011). Also, CP376395 in the VTA may potentiate dopaminergic output to the NAc in the stressed, EtOH-drinking animals. This increase in accumbal dopamine may be coupled to decreased motivation to drink EtOH. These experiments would confirm a critical role for CRF-R1 influence over the VTA-NAc pathway in stress-escalated EtOH drinking.
MATERIALS AND METHODS
Animals
Experimental animals were eight-week old adult male C57BL/6J mice (n=110; Jackson Laboratories, Bar Harbor, ME). Mice acclimated to a 12 hr reversed light/dark cycle (lights off 0700, lights on 1900) with constant temperature (21 ± 2°C) and humidity (25%). Water (H2O) and standard food (Purina LabDiet 5001, PMI Nutrition International, Brentwood, MO) were available ad libitum. All experimental procedures were approved by the Tufts University Institutional Animal Care and Use Committee.
Social Defeat Stress
C57BL/6J mice were socially defeated as previously reported (Norman et al. 2015). Male Swiss-derived, Carworth Farm Webster mice (Charles River Laboratories International Inc., Wilmington, MA) served as stimulus residents that reliably showed consistent aggression against an intruder. Both before and after the defeat, the intruder was placed into a perforated, protective cage within the home cage of an aggressive resident mouse for 5 min. During the defeat, residents attacked the intruder for 5 min or until 30 bites were counted. Intruders faced a different resident every session. After 10 consecutive days of episodic defeat, intruders rested for 10 days before access to EtOH. Non-defeated mice remained singly housed for 20 days before EtOH drinking.
EtOH drinking
C57BL/6J mice were given intermittent access to two-bottle choice of 20% EtOH (w/v) and H2O according to Hwa and colleagues (2011) for four weeks. Separate groups of mice were given continuous access to 20% EtOH and H2O for four weeks. Mice drank EtOH for four weeks stably, after stress or non-stress, before surgical preparation for either antagonist treatment or microdialysis. During drug testing, 2 hr, 4 hr, and 24 hr fluid intakes were measured by assessing bottle weights before and after drug manipulations. Fluid intake of 20% EtOH and H2O were measured in mL to calculate EtOH grams per kilogram (g/kg) and EtOH preference (%). To control for evaporation or spillage, ‘drip’ measurements (ca. 0.3 ml/24 hr) were taken from bottles on an empty cage and subtracted from individual intakes. Submandibular blood was collected after 2 hr access to two-bottle choice to measure blood EtOH concentrations (BECs). Samples were centrifuged at 3000 rpm for 10 min, and plasma was analyzed for BEC using the Analox AM1 Alcohol Analyzer and reagent (Analox Instruments Inc, Lunenberg, MA). Groups of mice were assessed for handling-induced convulsions (HICs) as a physical measure of EtOH dependence according to a 0–4 ordinal scale (Goldstein 1972) every two hr for 0–10 hr after the EtOH bottle was removed.
VTA microinjection of CRF receptor antagonists
Mice were implanted with a dual cannulae system (Plastics One, Roanoke, VA) to bilaterally target the mouse posterior VTA (AP-3.2, ML±0.75, DV-4.5mm from bregma and dura). Dummy cannulae and dust caps fitted the length of the cannula while VTA dual injectors protruded 0.1mm past the cannula. Other groups of mice were implanted with a unilateral VTA cannula and an ipsilateral microdialysis guide cannula (CMA7, Harvard Apparatus, Holliston, MA) targeting the NAc shell (AP+1.7, ML-0.7, DV-4.0mm from bregma and dura). On test days, doses of the CRF-R1 antagonist CP376395 (0.3–0.6µg, Tocris, Ellisville, MO) or CRF-R2 antagonist astressin2B (0.25–0.5µg, Tocris, Ellisville, MO) were freshly dissolved in an artificial cerebral spinal fluid (aCSF) vehicle (0.2µl infused at 0.1µl/min). Doses were chosen based on previous microinjection procedures (Hwa et al. 2013, Boyson et al. 2014). EtOH and H2O were given to animals 10 min post-infusion. After testing, mice were intracardiacally perfused with 0.9% saline and 4% paraformaldehyde, followed by brain removal. Coronal sections were Nissl stained to check placement of guide cannulae. Animals with incorrect placements into target sites were excluded from analysis.
Dopamine measurement in the NAc after microinjection
The night before sample collection, the microdialysis probe with a 1-mm active membrane (CMA7, Harvard Apparatus, Holliston, MA) was inserted into the NAc under isoflurane anesthesia (Webster Veterinary, Devens, MA) at an overnight perfusion rate of aCSF at 0.5 µl/min. On the test day, the flow rate was increased to 1.5 µl/min for 1 hr until sample collection every 10 min. There were four baseline samples followed by 0.2 µl microinjections of aCSF and 0.6 µg CP376395. Microinjection of the CRF-R1 antagonist occurred 10 min before 3 hr into the dark cycle, mimicking microinjection procedures when two-bottle choice was given (Hwa et al. 2014). Dopamine was measured by HPLC by electrochemical detection (Miczek et al. 2011). A stabilizing agent of 20 mM phosphate buffer with 25 mM EDTA was added to 15 µl dialysate samples. A cation-exchange column (Capcell Pak SCX, 1.5mm × 250mm, 5 µm I.D, Shiseido, Tokyo, Japan) separated dopamine at 30°C and a flow rate of 0.2 ml/min. The mobile phase consisted of 150 mM ammonium acetate, 50 mM citric acid, 27 µM EDTA, 10% MeOH, and 1% acetonitrile with pH adjusted to 4.6. Dopamine concentrations were determined by using standard curves with known amounts of dopamine in a range of 0.125–0.5 pg. The limit of detection was 2 fg under these conditions with a 10.5% recovery rate.
Statistical Analyses
Statistical tests were run using SigmaPlot 11.0 (Systat Software Inc., San Jose, CA). Two-bottle choice drinking parameters, like daily EtOH (g/kg, ml, preference) and H2O (ml) intake were evaluated with two-way repeated measures analyses of variance (ANOVA). Blood EtOH concentrations were compared with a one-way ANOVA. Convulsions during withdrawal were analyzed using the Kruskall-Wallis ANOVA on ranks. One-way repeated measures ANOVAs were run for 2, 4, and 24 hr drinking data after microinjections. A two-way repeated measures ANOVA tested the effect of CP376395 on NAc dopamine over time. Post-hoc analyses were Holm-Sidak t-tests when a main effect of drug was found (p<0.05).
RESULTS
Stress and access schedule escalated EtOH drinking
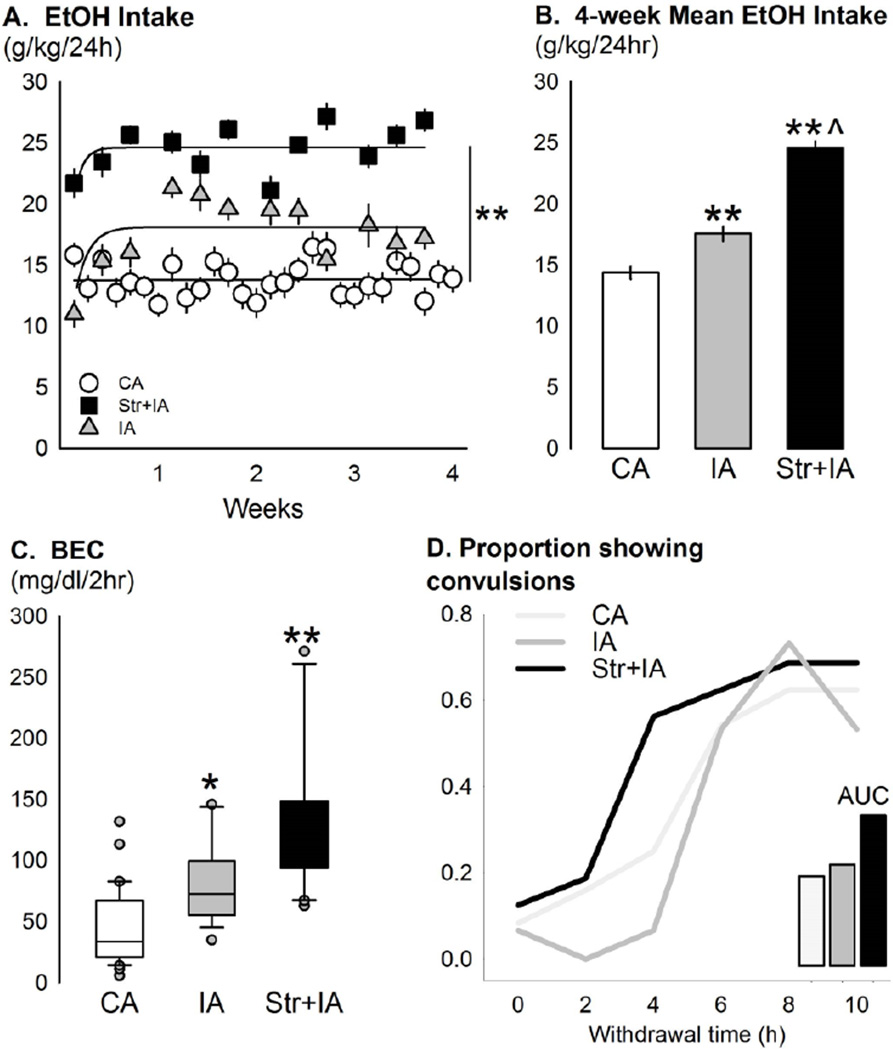
There were clear differences between non-stressed continuous EtOH drinkers, non-stressed intermittent EtOH drinkers, and stressed intermittent EtOH drinkers in daily EtOH drinking (g/kg) [Fig 1a]. There was a main effect for stress condition [F(2,846)=92.08, p<0.001], a main effect for time [F(11,846)=4.06, p<0.001], and an interaction between the two factors [F(22,846)=5.02, p<0.001]. Non-stressed mice given continuous access to 20% EtOH drank significantly less EtOH (g/kg/24hr) than the non-stressed mice given intermittent access to EtOH [p<0.001]. Mice with a history of social defeat stress given intermittent access to EtOH had the largest voluntary daily EtOH drinking, more than both the non-stressed intermittent EtOH and continuous EtOH groups [both p<0.001]. Average EtOH intake per group is summarized in Fig. 1b. For EtOH preference, a there was a main effect for stress condition [F(2,844)=43.99, p<0.001], a main effect for time [F(11,844)=4.17, p<0.001], and an interaction [F(22,844)=3.17, p<0.001]. Mice with a history of social defeat stress given intermittent access to EtOH had the largest preference for 20% EtOH, 0.65±0.02, greater than both the non-stressed intermittent EtOH and continuous EtOH groups [both p<0.001]. Groups of mice significantly differed in blood EtOH concentrations (BECs) after 2 hr two-bottle choice [Fig. 1c; F(2,64)=23.92, p<0.001]. Intermittent EtOH access generated higher BECs than continuous EtOH access [p<0.05]. Stressed intermittent EtOH drinkers showed the highest BECs, greater than both the non-stressed drinking groups [vs. intermittent EtOH p<0.05, vs. continuous EtOH p<0.001]. There were no group differences for raw convulsion scores [H(2)=0.70, p>0.05]. When the data were analyzed according to proportion of animals showing tonic-clonic convulsions, more mice in the stressed condition showed severe convulsions earlier in withdrawal than the non-stressed groups [Fig. 1d]. The area under the curve was highest for the stressed group.
Fig 1.
(a) Mean ± SEM daily ethanol intake (g/kg/24hr) across four weeks in C57BL/6J male mice given intermittent EtOH after a history of social defeat (black squares, Str+IA, n=27), non-defeated mice given intermittent EtOH (grey triangles, IA, n=23), and non-defeated mice given continuous EtOH (white circles, CA, n=30). **p<0.001 main effect of group. (b) The mean ± SEM average 4-week EtOH intake (g/kg/24hr) for the groups, (CA, white bar; IA, grey bar; Str+IA, black bar) is displayed. **p<0.001 vs. CA, ^p<0.001 vs. IA. (c) Box plots with median (black line) and interquartile ranges of blood ethanol concentrations (BEC, mg/dl) after 2 hr access to continuous EtOH (CA, n=30), intermittent EtOH (IA, n=15), or intermittent EtOH with a history of social defeat stress (Str+IA, n=20). Grey dots represent outliers. *p<0.05 vs. CA, **p<0.001 vs. CA. (d) Proportion of mice showing tonic-clonic convulsions, scores of at least “2”, during acute withdrawal from six weeks of EtOH drinking on continuous access (CA, light grey, n=24), intermittent access (IA, grey, n=15), or stress and intermittent access (Str+IA, black, n=16). Inset bars represent area under the curve (AUC) for the three groups.
There were no significant group differences in body weight between experimental groups. Throughout the stress period before EtOH access, stressed mice weighed 24.6±0.2 g and non-stressed mice weighed 23.5±0.1 g. During drug testing under vehicle conditions, stressed, intermittent EtOH mice weighed 25.4±0.4 g, non-stressed, intermittent EtOH mice weighed 26.1±0.4 g, and non-stressed, continuous EtOH mice weighed 26.2±0.4 g.
VTA CRF-R1 antagonist reduced stress-enhanced drinking
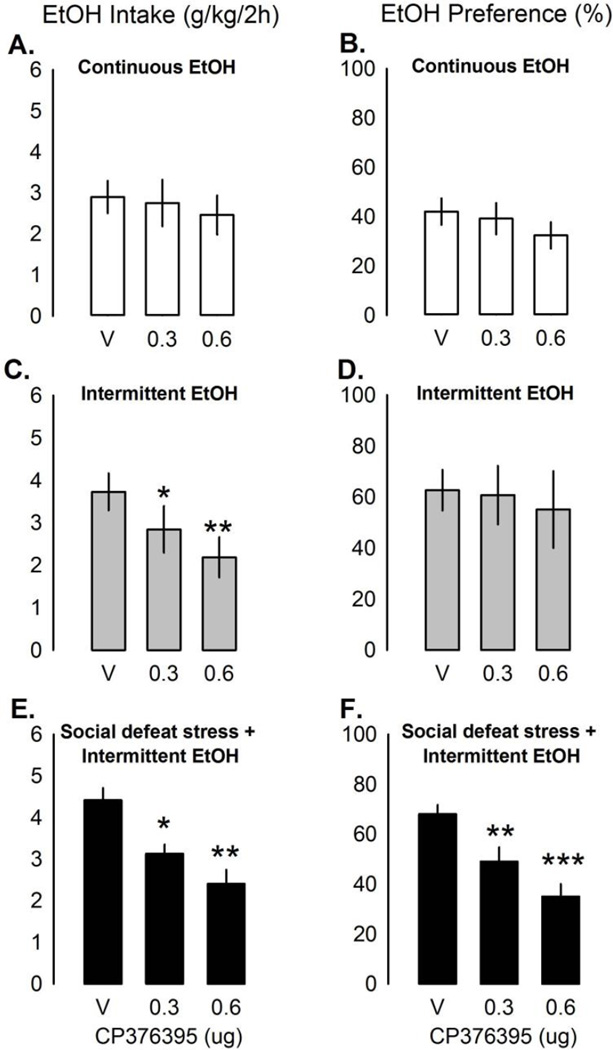
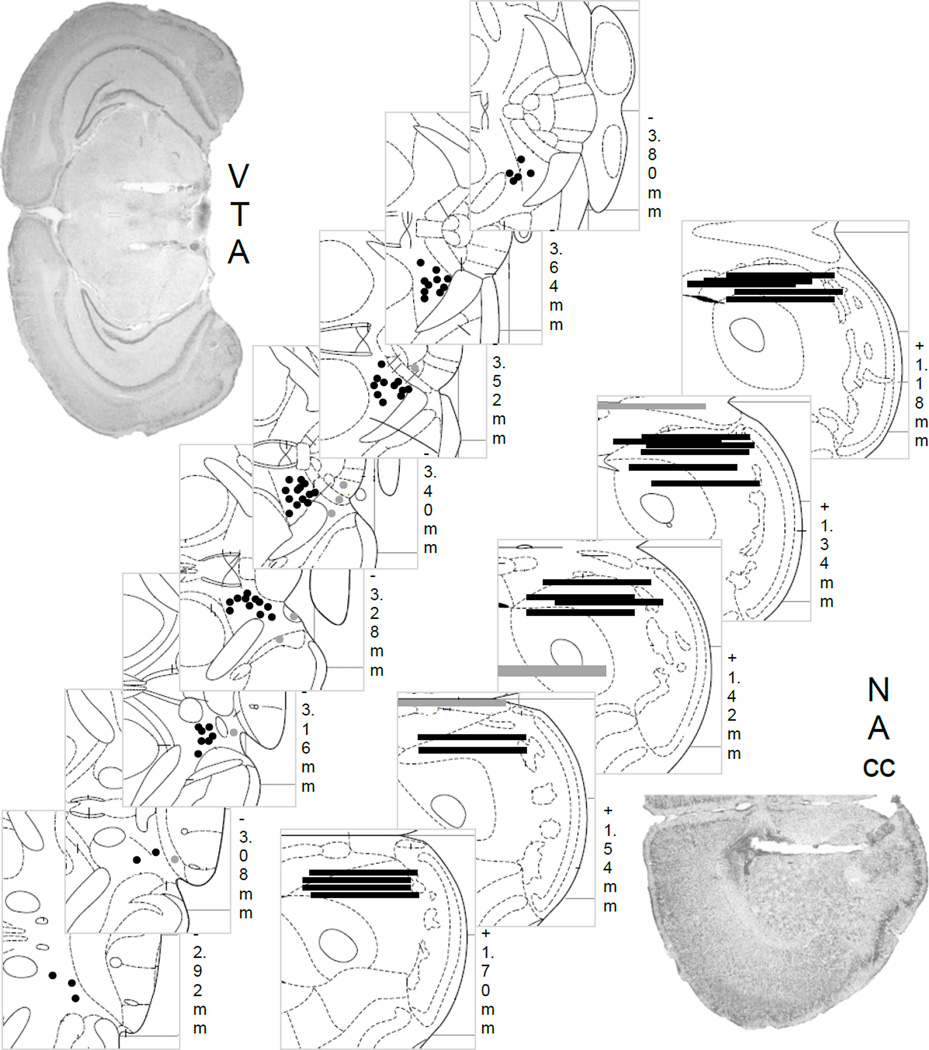
In mice given continuous access, CP376395 in the VTA did not affect EtOH intake [Fig. 2a], EtOH preference [Fig. 2b], or H2O drinking (n=12) at any time point. Treatment with both doses of CP376395 in mice given intermittent access to EtOH (n=10) decreased EtOH (g/kg/2hr) intake [Fig. 2c; F(2,17)=11.77, p<0.001; aCSF vs. 0.3 µg CP376395, p<0.05; aCSF vs. 0.6 µg CP376395, p<0.001] lasting for 24 hr [Table 1; 4 hr, F(2,17)=4.00, p<0.05; 24 hr, F(2,18)=4.43, p<0.05] caused by the highest dose [both p<0.05]. EtOH preference [Fig. 2d] and H2O intake were not significantly affected by CP376395 in the intermittent EtOH mice. Socially defeated mice given intermittent access to EtOH (n=10) also drank less EtOH (g/kg) after intra-VTA CP376395 [Fig. 2e; F(2,18)=9.80, p<0.01; aCSF vs. 0.3 µg CP376395, p<0.05; aCSF vs. 0.6 µg CP376395, p<0.001]. CP376395 in the VTA suppressed EtOH (g/kg) drinking for up to 24 hr [Table 1; 4 hr: F(2,18)=12.01, p<0.001; 24 hr: F(2,18)=7.99, p<0.01]. At 4 hr, both doses were effective [aCSF vs. 0.3 µg, p<0.05; aCSF vs. 0.6 µg, p<0.001], while at 24 hr, only the high dose was effective [aCSF vs. 0.6 µg, p<0.01]. Intra-VTA CP376395 reduced EtOH preference acutely, as well [F(2,18)=14.53, p<0.001; Fig. 2f]. H2O intake (ml/2hr) increased after CP376395 in the VTA in the socially defeated intermittent EtOH mice [F(2,18)=8.38, p<0.01; aCSF vs. 0.6 µg, p<0.01]. Microinjection guide cannulae aimed at the VTA are shown in Fig. 4. Animals were removed from analysis due to missed placement outside the VTA (n=7), overdose of anesthesia during surgery (n=2), and death during the 10-day social defeat (n=1).
Fig 2.
Mean ± SEM ethanol intake (g/kg/2hr) and ethanol preference (%/2hr) in mice given continuous EtOH access (a, intake; b, preference; white bars, n=12), intermittent EtOH access (c, intake; d, preference; grey bars, n=10), and intermittent EtOH access after 10 days of social defeat stress (e, intake; f, preference; black bars, n=10) affected by intra-VTA microinjections of CRF-R1 antagonist CP376395 (0–0.6µg). *p<0.05 vs. vehicle, **p<0.001 vs. vehicle.
Table 1. EtOH Intake (g/kg) affected by CRF-R1 and CRF-R2 antagonists.
Ethanol consumption (g/kg) over time after intra-VTA microinjection of CRF-R1 antagonist CP376395 or CRF-R2 antagonist Astressin2B after 4 weeks of continuous EtOH, intermittent EtOH, or intermittent EtOH and social defeat stress.
| Group | Drug | Dose | 2 hr | 4 hr | 24 hr |
|---|---|---|---|---|---|
| Continuous EtOH | CP376395 | ACSF | 2.90 ± 0.39 | 5.60 ± 0.50 | 16.47 ± 1.22 |
| 0.3 µg | 2.75 ± 0.57 | 5.32 ± 0.61 | 17.67 ± 1.46 | ||
| 0.6 µg | 2.46 ± 0.48 | 4.53 ± 0.55 | 15.41 ± 1.20 | ||
| Astressin2B | ACSF | 3.38 ± 0.39 | 5.86 ± 0.35 | 16.04 ± 1.38 | |
| 0.5 µg | 2.60 ± 0.59 | 5.53 ± 0.96 | 15.53 ± 1.70 | ||
| Intermittent EtOH | CP376395 | ACSF | 3.73 ± 0.44 | 6.36 ± 0.42 | 22.28 ± 2.16 |
| 0.3 µg | 2.85 ± 0.55* | 5.52 ± 0.64 | 20.34 ± 2.59 | ||
| 0.6 µg | 2.19 ± 0.48** | 4.80 ± 0.55* | 18.89 ± 1.72* | ||
| Astressin2B | ACSF | 2.69 ± 0.40 | 5.60 ± 0.38 | 22.10 ± 1.29 | |
| 0.25 µg | 2.36 ± 0.19 | 5.33 ± 0.38 | 19.01 ± 1.42 | ||
| 0.5 µg | 2.19 ± 0.25 | 4.51 ± 0.41 | 19.72 ± 2.14 | ||
| Stress + Intermittent EtOH | CP376395 | ACSF | 4.42 ± 0.29 | 7.35 ± 0.35 | 26.54 ± 1.48 |
| 0.3 µg | 3.13 ± 0.22* | 6.30 ± 0.32* | 25.28 ± 1.61 | ||
| 0.6 µg | 2.41 ± 0.34** | 5.33 ± 0.33** | 20.58 ± 1.40* | ||
| Astressin2B | ACSF | 3.92 ± 0.38 | 6.80 ± 0.52 | 29.11 ± 2.20 | |
| 0.25 µg | 3.05 ± 0.41 | 5.43 ± 0.71 | 26.79 ± 1.18 | ||
| 0.5 µg | 2.27 ± 0.35* | 6.24 ± 0.49 | 26.15 ± 2.57 | ||
p<0.05 vs. ACSF,
p<0.001 vs. ACSF.
Fig 4.
Histological placements of guide cannula into the VTA for microinjection (black circles, n=62) or outside the VTA not included in analysis (grey circles, n=8). 1-mm microdialysis probes are shown into the NAc (black lines, n=21) or outside the NAc (grey lines, n=4). Representative photomicrographs for each brain site are shown.
Astressin2B in the VTA did not affect EtOH or H2O drinking at any time point in mice given continuous access to EtOH (n=12) or intermittent access to EtOH (n=9) [Table 1]. In the stressed mice given intermittent EtOH, astressin2B decreased EtOH drinking (g/kg/2hr) [Table 1; F(2,16)=4.75, p<0.05], specifically from the highest dose compared to vehicle [aCSF vs. 0.5 µg, p<0.05]. The highest dose of astressin2B did not change EtOH preference during this time period [not shown], from 61.86±0.07% to 59.74±0.08%. As for H2O intake, there was a trend that astressin2B decreased 2 hr H2O drinking [F(2,14)=2.84, p=0.09] from 0.31±0.08 ml to 0.16±0.05 ml, causing a 56.60±14.07% reduction with the highest dose. Microinjection guide cannulae aimed at the VTA are shown in Fig. 4. Animals were removed from analysis due to missed placement outside the VTA (n=4).
Accumbal dopamine potentiated by CRF-R1 antagonist in stressed mice
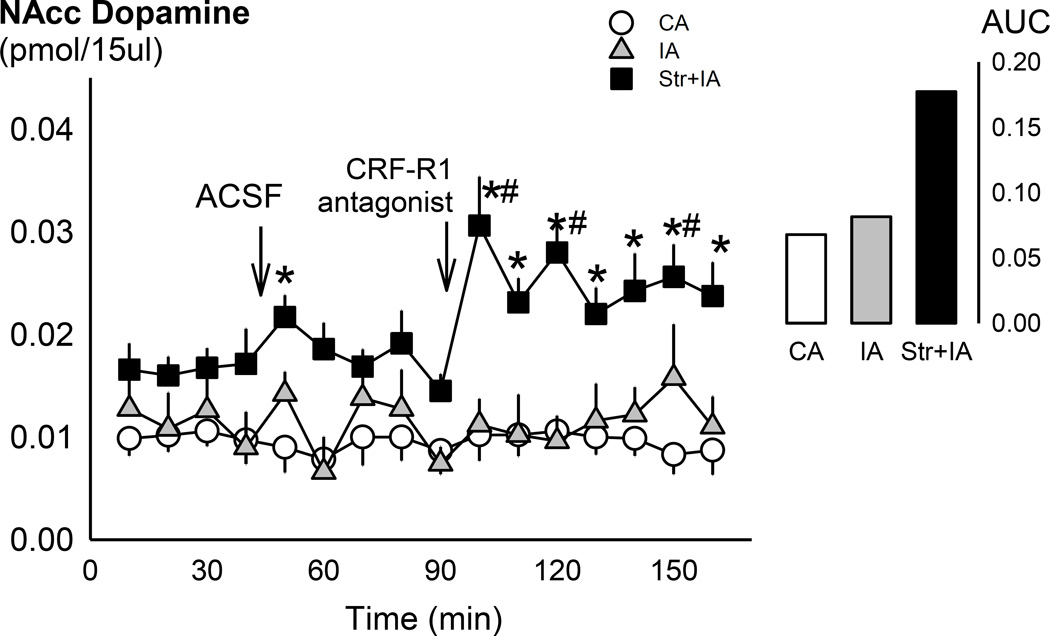
Probes measured extracellular dopamine in the NAc with 10.39% recovery. Social defeat stress enhanced baseline dopamine levels (pmol/15µl), as assessed 2 months after the last episode of social defeat stress [F(2,73)=9.30, p<0.001; Fig. 3]. Stressed intermittent EtOH mice had significantly higher dopamine tone than both the non-stressed groups [vs. intermittent EtOH, p<0.01; vs. continuous EtOH, p<0.001]. There were significant main effects of drinking group [F(2,238)=14.40, p<0.001] and time [F(15,238)=2.17, p<0.01], and a significant interaction [F(30,238)=1.74, p<0.05] on NAc dopamine levels. After the aCSF microinjection, stressed mice differed from the continuous EtOH mice [p<0.01], but not the intermittent EtOH mice. However, this was not a statistically significant deviation from baseline levels in this group. Within stressed mice, dopamine 10 min [p<0.001], 30 min [p<0.01], and 60 min [p<0.05] after CP376395 microinjection was greater than baseline dopamine. Stressed mice had higher dopamine than the two other groups [vs. intermittent EtOH: all p<0.05; vs. continuous EtOH: all p<0.05] after the CP376395 microinjection. Area under the curve after the microinjection confirmed that stressed, intermittent EtOH mice had greater dopamine than the non-stressed groups [Fig. 3]. Microinjection guide cannulae aimed at the VTA and microdialysis probes in the NAc are shown in Fig. 4. Animals were removed from analysis due to missed placement outside the VTA/NAc (n=4), overdose of anesthesia during the surgery (n=1), or the use of a different probe manufacturer (n=1). Other mice were not included because dopamine measurements were analyzed on a different analytical column (n=6). However, changes in dopamine within experimental groups were similar across columns.
Fig 3.
Mean ± SEM dopamine in the nucleus accumbens (pmol/15µl) over time. White circles are continuous EtOH drinkers (CA; n=7), grey triangles are intermittent EtOH drinkers (IA; n=7), and black squares are socially defeated intermittent EtOH drinkers (Str+IA; n=7). Arrows denote intra-VTA microinjections of aCSF and 0.6 µg CP376395. *p<0.05 vs. CA. #p<0.05 vs. baseline. Inset panel shows area under the curve (AUC) after the CP376395 microinjection.
DISCUSSION
The current research pointed to escalations in intermittent, voluntary EtOH drinking caused by a history of social defeat stress in C57BL/6J mice. This excessive drinking approached 25 g/kg/24hr accompanied by high BECs during the initial 2 hr of access. To explore a mechanistic link between stress and drinking, the present data showed that a water-soluble CRF-R1 antagonist infused into the VTA dose-dependently and selectively reduced intermittent EtOH intake and preference in stressed and non-stressed mice, but not in mice given continuous EtOH. By contrast, VTA infusions of the CRF-R2 antagonist astressin2B less specifically suppressed both EtOH and H2O drinking in the stressed group without effects in the non-stressed groups. Stressed mice drinking intermittent EtOH had elevated levels of tonic dopamine compared to non-stressed drinking mice. Additionally, application of CP376395 into the VTA potentiated dopamine output to the accumbens caused further elevations of dopamine, only in the stressed group. These findings illustrate a role for extrahypothalamic CRF-R1 as especially important for stress-escalated EtOH drinking beyond access schedule-escalated EtOH drinking.
Mice that experienced brief episodes of defeat stress for 10 days consumed more EtOH, preferred EtOH more, and had higher BECs than non-stressed mice given intermittent EtOH beginning on the first day of EtOH access. It has been challenging to develop a social stress procedure that consistently and reliably escalates EtOH consumption in rodents given the variable nature of defeat protocols. For example, social defeat stress in rats reduced EtOH drinking and self-administration compared to non-stressed rats (Van Erp and Miczek 2001; Funk et al. 2005). Previous social defeat experiments in mice have found little or no change in EtOH drinking during the stressor phase of the study, yet some observed greater EtOH intake two-three weeks after the stress (Croft et al. 2005; Sillaber et al. 2002). It appears that durations ranging from 7–19 days of defeat can increase drinking in C57BL/6J mice compared to non-stressed controls (Kudryavtseva et al. 1991; Bahi 2013), supporting the current ten-day protocol originating in outbred, Swiss-derived mice (Norman et al. 2015). This 30-bite condition across ten consecutive days prompted high daily EtOH drinking behavior, evident on the first day of access and persistent for at least four weeks. To our knowledge, the history of social defeat stress and intermittent access to EtOH schedule has led to the highest reported voluntary drinking in adult, male mice reported to date.
The present studies showed that the CRF-R1 antagonist decreased EtOH drinking in mice that drank in excess as a result of intermittent EtOH or defeat-stress but not in those that drank continuously in moderation and were not stressed by defeat. The present work supports the role of CRF-R1 in EtOH drinking and withdrawal, which has been known for some time (Merlo Pich et al. 1995). Chronic EtOH and withdrawal activate otherwise quiescent extrahypothalamic CRF-R1 stress systems, according to the opponent process theory (Koob and Le Moal 2005). Previous work confirmed the involvement of CRF-R1 specifically in intermittent EtOH drinkers (Hwa et al. 2013, 2014; Simms et al. 2014). Under the present conditions, infusions of a CRF-R1 antagonist into the VTA reduced EtOH intake, confirming similar effects in a binge drinking protocol (Sparta et al. 2008). The long-lasting suppression of drinking after CP376395 implies that the drinking behavior did not recover despite the drug metabolizing locally. Other experiments have also observed latent CRF-R1 antagonist effects on behavior, where rats pre-treated with intra-VTA CP154526 before episodes of social defeat self-administer significantly less cocaine in a 24-hr binge protocol compared to aCSF treatment (Boyson et al. 2011). It is also possible that Urocortin I may play a role in stress-enhanced EtOH drinking, as Urocortin I remains another possible ligand for CRF receptors. Urocortin immunoreactivity is located in the cell bodies of dopaminergic neurons in the VTA (Yamamoto et al. 1998). However, urocortin in this region is insufficiently characterized regarding alcohol drinking and should be explored. Drinking without a history of social stress was not affected by astressin2B in the VTA, suggesting that schedule-escalated drinking may depend on a CRF-R1-specific mechanism in the VTA.
Here, intra-VTA astressin2B decreased defeat stress-enhanced drinking, but the effects were less specific to EtOH, possibly as a result of CRF-R2 activation and decreases in ingestive behavior (Spina et al. 1996). Though the effects of astressin2B on H2O intake were not statistically significant, EtOH preference was not affected by astressin2B. These effects on drinking behavior were also short-lasting compared to the enduring effects on both EtOH drinking and preference with CP376395. Other studies have also found non-specific effects of CP376395 on H2O drinking with only access to H2O (Giardino and Ryabinin 2013), so drug effects may be exclusive to protocols differing in inherent stress intensities. It appears that the roles of CRF-R1 and CRF-R2 may be dissociated in stressed, heavy EtOH-drinking mice for their different effects on drinking behavior. Altogether, CRF acting on CRF-R1 may become particularly relevant under drinking conditions of excess, induced by stressful experiences. In addition to increases in EtOH drinking, social defeat stress could have also affected intake of other appetitive tastants like sucrose or saccharin, as a potential measure of anhedonia-like behavior (Krishnan et al. 2007). Though there may be baseline differences in sucrose consumption across stress groups, it would be expected that CRF-R1 antagonism may not affect sucrose intake, as previously found with sucrose drinking and sucrose pellet self-administration (Sparta et al. 2008; Blacktop et al. 2011).The current experiments use H2O intake as a control fluid, but studying sucrose consumption in the stressed mice would be an interesting future avenue.
The current experiments explored how CRF-R1 antagonism affected VTA dopamine output to the NAc in the stressed, EtOH-drinking mice. Alterations in extracellular dopamine can reflect either heightened low-frequency tonic firing or high-bursting phasic firing of dopamine (Grace 2000). The presently observed elevated NAc tonic dopamine in the stressed group corroborates work showing that rats subjected to intermittent, brief episodes of defeat stress have significantly higher NAc dopamine than non-defeated controls (Miczek et al. 2011). In contrast, several weeks of continuous subordination stress blunts tonic NAc dopamine (Miczek et al. 2011) suggesting that the intermittent nature of the defeat may be critical for increasing dopamine tone. In the mouse episodic defeat protocol, Han et al. (2015) measured NAc dopamine 6–10 days post-stress, and revealed no differences in basal levels of dopamine. It seems that social defeat alone was not sufficient to elevate tonic dopamine in mice. To further tease apart the contribution of stress and drinking on dopamine output, another control group given continuous EtOH after a history of stress could have been studied. However, we wanted to test dopamine in the highest drinking conditions, which were the intermittent EtOH stress and non-stressed mice. The current experiments show that four weeks of intermittent EtOH drinking alone did not affect dopamine tone. Heightened tonic dopamine levels may be the result of an interaction between the social stress and the long-term intake.
In the stressed, EtOH-drinking mice, CP376395 administered into the VTA promoted significant elevations in NAc dopamine. Augmented NAc dopamine after the CRF-R1 antagonist may reflect changes in the brain 3 hr into the dark cycle, the time of EtOH access. The results may suggest that accumbal dopamine may be more involved with cues that predict the availability of EtOH, instead of post-ingestion pharmacological effects (Schultz et al. 1997). Enhanced dopamine after the antagonist may be the tempering signal to reduce drinking since there is less of a need to compensate for low NAc dopamine during withdrawal. Again, intra-VTA microinjection of CP376395 may have elevated dopamine levels that occur with withdrawal from intermittent access to EtOH drinking, suppressing EtOH intake, as has been recently shown with reinstatement to cocaine seeking (Twining et al. 2015). It is possible that intra-VTA astressin2B, in the same manner as CP376395, may have also increased NAc dopamine in the stressed, EtOH-drinking mice given the similar behavioral reductions in EtOH intake. Alternatively, others have demonstrated that a CRF-R2 antagonist, but not a CRF-R1 antagonist, blocks stress-induced increases in VTA dopamine in vitro (Wang et al. 2007). However, since intra-VTA astressin2B did not decrease EtOH preference in the stressed group in the current preparation, microdialysis experiments with the CRF-R2 antagonist may not have been as behaviorally relevant.
There may be several mechanisms for how the CRF-R1 antagonist led to a peak in dopamine. Lodge and Grace (2005) have shown that an acute CRF-R1 antagonist significantly increased spontaneously active dopamine neuronal firing in the NAc. The CRF-R1 antagonist may have enhanced dopamine through disinhibitory action on GABA interneurons or alternatively via D2 autoreceptors (Karkhanis et al. 2015). Specifically, CRF-R1s located on VTA GABA interneurons may inhibit GABAergic medium spiny neurons to the NAc, leading to increased phasic dopamine. These current results appear to contrast with past studies demonstrating CRF increasing dopamine neuron firing and release (Kalivas et al. 1987; Wanat et al. 2008) while CRF receptor antagonists in the VTA reduce glutamate and dopamine release in the VTA (Wang et al. 2005). We found that NAc dopamine was increased, instead of decreased, in response to a CRF-R1 antagonist. The present mice had a history of social defeat and EtOH drinking, which may have induced neuroadaptations in the CRF receptors. Moreover, the antagonist was microinjected into the behaving animal, in contrast to the earlier in vitro work (Sparta et al. 2013). Wanat and colleagues (2013) found increased NAc dopamine transients after intra-VTA CRF, which corresponded to stress-depressed lever pressing for food reinforcement. Here, we observed an enhancement of motivational behavior after stress, to contrast with more typical behavioral inhibition after stress or CRF microinfusion. Stress-disinhibited versus stress-inhibited drinking may certainly evoke disparate dopaminergic mechanisms.
We have demonstrated that a history of episodic social defeat can escalate EtOH drinking using C57BL/6J mice. Perturbations in the CRF-R1 system, but not CRF-R2, were important in the VTA, containing populations of cells linked to either stress or the rewarding effects of EtOH. Dopamine signals from the VTA to the NAc in the mesolimbic pathway were altered only in the stressed, EtOH-drinking mice. An alternative neurochemical marker distinguishing intermittent and continuous EtOH drinking mice could be instead dorsal raphe serotonin, as we have seen measuring extracellular mPFC serotonin after administration of CP154526 in non-stressed intermittent drinkers (Hwa et al. 2013, 2014). Individual differences in the response of the CRF system to social stress have been observed in a number of rodent models (Wood et al. 2010). There was little variability among inbred C57BL/6J mice for daily intake, indicating a profound stress effect compared to non-stressed EtOH drinking mice. In conclusion, the intermittency of both the EtOH availability and the stressor may be major factors that influence the brain stress systems. Understanding how stress- and reward-specific mechanisms in the VTA interact should be a next goal in the effort to understand EtOH use disorders.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the technical efforts of Ashley Siegel, Madeline Kuppe, and Tom Sopko, and the guidance of Dr. Lucas Albrechet-Souza. Also, the authors thank Drs. Kathleen Grant and Lisa Shin for their valuable feedback on this research.
FUNDING
This research was supported by NIH grants AA013983 (KAM) and AA021622 (LSH), and the American Psychological Association’s Dissertation Award (LSH).
Footnotes
DISCLOSURE
There were no competing financial interests in relation to the work described.
REFERENCES
- Bahi A. Increased anxiety, voluntary alcohol consumption and ethanol-induced place preference in mice following chronic psychosocial stress. Stress. 2013;16:441–451. doi: 10.3109/10253890.2012.754419. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF, Doremus-Fitzwater TL. Effects of stress on alcohol drinking: a review of animal studies. Psychopharmacology (Berl) 2011;218:131–156. doi: 10.1007/s00213-011-2443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacktop JM, Seubert C, Baker DA, Ferda N, Lee G, Graf E, Mantsch JR. Augmented cocaine seeking in response to stress or CRF delivered into the ventral tegmental area following long-access self-administration is mediated by CRF receptor type 1 but not CRF receptor type 2. J Neurosci. 2011;31:11396–11403. doi: 10.1523/JNEUROSCI.1393-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyson CO, Holly EN, Shimamoto A, Albrechet-Souza L, Weiner LA, DeBold JF, et al. Social stress and CRF-dopamine interactions in the VTA: role in long-term escalation of cocaine self-administration. J Neurosci. 2014;34:6659–6667. doi: 10.1523/JNEUROSCI.3942-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyson CO, Miguel T, Quadros IM, DeBold JF, Miczek KA. Prevention of social stress-escalated cocaine self-administration by CRF-R1 antagonist in the rat VTA. Psychopharmacology (Berl) 2011;218:257–269. doi: 10.1007/s00213-011-2266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci USA. 2009;106:4894–4899. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie MS, Shefner SA, Dunwiddie TV. Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res. 1990;508:65–69. doi: 10.1016/0006-8993(90)91118-z. [DOI] [PubMed] [Google Scholar]
- Budygin EA, Phillips PE, Wightman RM, Jones SR. Terminal effects of ethanol on dopamine dynamics in rat nucleus accumbens: an in vitro voltammetric study. Synapse. 2001;42:77–79. doi: 10.1002/syn.1101. [DOI] [PubMed] [Google Scholar]
- Conger JJ. Alcoholism: Theory, problem and challenge. II. Reinforcement theory and the dynamics of alcoholism. Q J Stud Alcohol. 1956;17:296–305. [PubMed] [Google Scholar]
- Croft AP, Brooks SP, Cole J, Little HJ. Social defeat increases alcohol preference of C57BL/10 strain mice; effect prevented by a CCKB antagonist. Psychopharmacology (Berl) 2005;183:163–170. doi: 10.1007/s00213-005-0165-6. [DOI] [PubMed] [Google Scholar]
- Funk D, Harding S, Juzytsch W, Le AD. Effects of unconditioned and conditioned social defeat on alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 2005;183:341–349. doi: 10.1007/s00213-005-0194-1. [DOI] [PubMed] [Google Scholar]
- Giardino WJ, Ryabinin AE. CRF1 Receptor Signaling Regulates Food and Fluid Intake in the Drinking-in-the-Dark Model of Binge Alcohol Consumption. Alcohol Clin Exp Res. 2013;37:1161–1170. doi: 10.1111/acer.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DB. Relationship of alcohol dose to intensity of withdrawal signs in mice. J Pharmacol Exp Ther. 1972;180:203–215. [PubMed] [Google Scholar]
- Grace AA. The tonic/phasic model of dopamine system regulation and its implications for understanding alcohol and psychostimulant craving. Addiction. 2000;95:S119–S128. doi: 10.1080/09652140050111690. [DOI] [PubMed] [Google Scholar]
- Grieder TE, Herman MA, Contet C, Tan LA, Vargas-Perez H, Cohen A, et al. VTA CRF neurons mediate the aversive effects of nicotine withdrawal and promote intake escalation. Nature neuroscience. 2014;17:1751–1758. doi: 10.1038/nn.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Albrechet-Souza L, Doyle MR, Shimamoto A, DeBold JF, Miczek KA. Social stress and escalated drug self-administration in mice II. Cocaine and dopamine in the nucleus accumbens. Psychopharmacology (Berl) 2015;232:1003–1010. doi: 10.1007/s00213-014-3734-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs SC, Pich EM, Miczek KA, Britton KT, Koob GF. Corticotropin-releasing factor antagonist reduces emotionality in socially defeated rats via direct neurotropic action. Brain Res. 1992;581:190–197. doi: 10.1016/0006-8993(92)90708-h. [DOI] [PubMed] [Google Scholar]
- Hwa LS, Chu A, Levinson SA, Kayyali TM, DeBold JF, Miczek KA. Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% ethanol. Alcohol Clin Exp Res. 2011;35:1938–1947. doi: 10.1111/j.1530-0277.2011.01545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa LS, DeBold JF, Miczek KA. Alcohol in excess: CRF(1) receptors in the rat and mouse VTA and DRN. Psychopharmacology (Berl) 2013;225:313–327. doi: 10.1007/s00213-012-2820-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa LS, Shimamoto A, Kayyali T, Norman KJ, Valentino RJ, DeBold JF, et al. Dissociation of µ-opioid receptor and CRF-R1 antagonist effects on escalated ethanol consumption and mPFC serotonin in C57BL/6J mice. Addict Biol. 2014 doi: 10.1111/adb.12189. Online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperato A, Di Chiara G. Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. J Pharmacol Expl Ther. 1986;239:219–228. [PubMed] [Google Scholar]
- Kalivas PW, Duffy P, Latimer LG. Neurochemical and behavioral effects of corticotropin-releasing factor in the ventral tegmental area of the rat. J Pharmacol Exp Ther. 1987;242:757–763. [PubMed] [Google Scholar]
- Karkhanis AN, Rose JH, Huggins KN, Konstantopoulos JK, Jones SR. Chronic intermittent ethanol exposure reduces presynaptic dopamine neurotransmission in the mouse nucleus accumbens. Drug Alcohol Depend. 2015;150:24–30. doi: 10.1016/j.drugalcdep.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the 'dark side' of drug addiction. Nature Neurosci. 2005;8:1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Kudryavtseva NN, Bakshtanovskaya IV, Koryakina LA. Social model of depression in mice of C57BL/6J strain. Pharmacol Biochem Behav. 1991;38:315–320. doi: 10.1016/0091-3057(91)90284-9. [DOI] [PubMed] [Google Scholar]
- Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci. 2002;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Acute and chronic corticotropin-releasing factor 1 receptor blockade inhibits cocaine-induced dopamine release: correlation with dopamine neuron activity. J Pharmacol ExpTher. 2005;314:201–206. doi: 10.1124/jpet.105.084913. [DOI] [PubMed] [Google Scholar]
- Merlo Pich E, Lorang M, Yeganeh M, Rodriguez De Fonseca F, Raber J, Koob GF, et al. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Nikulina EM, Shimamoto A, Covington HE., III Escalated or suppressed cocaine reward, tegmental BDNF and accumbal dopamine due to episodic vs. continuous social stress in rats. J Neurosci. 2011;31:9848–9857. doi: 10.1523/JNEUROSCI.0637-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman KJ, Seiden JA, Klickstein JA, Han X, Hwa LS, DeBold JF, et al. Social stress and escalated drug self-administration in mice I. Alcohol and corticosterone. Psychopharmacology (Berl) 2015;232:991–1001. doi: 10.1007/s00213-014-3733-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretti PO, Lewis BR. Affects of alcoholic consumption on the activity patterns of individual rhesus monkeys and their behavior in a social group. Primates. 1969;10:181–188. [Google Scholar]
- Phillips TJ, Reed C, Pastor R. Preclinical Evidence Implicating Corticotropin Releasing Factor Signaling in Ethanol Consumption and Neuroadaptation. Genes, Brain and Behavior. 2015;14:98–135. doi: 10.1111/gbb.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodaros D, Caruana DA, Amir S, Stewart J. Corticotropin-releasing factor projections from limbic forebrain and paraventricular nucleus of the hypothalamus to the region of the ventral tegmental area. Neuroscience. 2007;150:8–13. doi: 10.1016/j.neuroscience.2007.09.043. [DOI] [PubMed] [Google Scholar]
- Sillaber I, Rammes G, Zimmermann S, Mahal B, Zieglgansberger W, Wurst W, et al. Enhanced and delayed stress-induced alcohol drinking in mice lacking functional CRH1 receptors. Science. 2002;296:931–933. doi: 10.1126/science.1069836. [DOI] [PubMed] [Google Scholar]
- Simms JA, Nielsen CK, Li R, Bartlett SE. Intermittent access ethanol consumption dysregulates CRF function in the hypothalamus and is attenuated by the CRF-R1 antagonist, CP-376395. Addict Biol. 2014;19:606–611. doi: 10.1111/adb.12024. [DOI] [PubMed] [Google Scholar]
- Sparta DR, Hopf FW, Gibb SL, Cho SL, Stuber GD, Messing RO, Ron D, Bonci A. Binge Ethanol-Drinking Potentiates Corticotropin Releasing Factor R1 Receptor Activity in the Ventral Tegmental Area (2013) Alcohol Clin Exp Res. 2013;37:1680–1687. doi: 10.1111/acer.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparta DR, Sparrow AM, Lowery EG, Fee JR, Knapp DJ, Thiele TE. Blockade of the corticotropin releasing factor type 1 receptor attenuates elevated ethanol drinking associated with drinking in the dark procedures. Alcohol Clin Exp Res. 2008;32:259–265. doi: 10.1111/j.1530-0277.2007.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spina M, Merlo Pich E, Chan RKW, Basso AM, Rivier J, Vale W, et al. Appetite-surpressing effects of urocortin a CRF-related neuropeptide. Science. 1996;273:1561–1564. doi: 10.1126/science.273.5281.1561. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA. Social defeat stress selectively alters mesocorticolimbic dopamine release: An in vivo microdialysis study. Brain Res. 1996;721:140–149. doi: 10.1016/0006-8993(96)00159-x. [DOI] [PubMed] [Google Scholar]
- Twining RC, Wheeler DS, Ebben AL, Jacobsen AJ, Robble MA, Mantsch JR, Wheeler RA. Aversive Stimuli Drive Drug Seeking in a State of Low Dopamine Tone. Biol Psych. 2015;77:895–902. doi: 10.1016/j.biopsych.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A. Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron. 2003;39:401–407. doi: 10.1016/s0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, et al. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Van Erp AMM, Miczek KA. Persistent suppression of ethanol self-administration by brief social stress in rats and increased startle response as index of withdrawal. Physiology & Behavior. 2001;73:301–311. doi: 10.1016/s0031-9384(01)00458-9. [DOI] [PubMed] [Google Scholar]
- Wanat MJ, Bonci A, Phillips PE. CRF acts in the midbrain to attenuate accumbens dopamine release to rewards but not their predictors. Nat Neurosci. 2013;16:383–385. doi: 10.1038/nn.3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanat MJ, Hopf FW, Stuber GD, Phillips PE, Bonci A. Corticotropin-releasing factor increases mouse ventral tegmental area dopamine neuron firing through a protein kinase C-dependent enhancement of Ih. J Physiol. 2008;586:2157–2170. doi: 10.1113/jphysiol.2007.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci. 2005;25:5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, You ZB, Rice KC, Wise RA. Stress-induced relapse to cocaine seeking: roles for the CRF2 receptor and CRF-binding protein in the ventral tegmental area of the rat. Psychopharmacology (Berl) 2007;193:283–294. doi: 10.1007/s00213-007-0782-3. [DOI] [PubMed] [Google Scholar]
- Wood SK, Walker HE, Valentino RJ, Bhatnagar S. Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: role of corticotropin-releasing factor. Endocrinology. 2010;151:1795–1805. doi: 10.1210/en.2009-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Meada T, Fujimura M, Fujimiya M. Urocortin-like immunoreactivity in the substantia nigra, ventral tegmental area and Edinger-Westphal nucleus of rat. Neurosci Lett. 1998;243:21–24. doi: 10.1016/s0304-3940(98)00071-8. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Logrip ML, Koob GF. Corticotropin releasing factor: a key role in the neurobiology of addiction. Front Neuroendocrinol. 2014;35:234–244. doi: 10.1016/j.yfrne.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]