Introduction
Stroke is a devastating disease accounting for 5.5 million deaths annually worldwide1. Despite significant preclinical and clinical investigations, with more than 1026 candidate neuroprotective stroke drugs investigated and nearly 200 clinical trials, no effective therapy other than tissue plasminogen activator (TPA) has been approved2. The reasons for these failures are numerous. Recent Stroke Treatment and Academic Roundtable (STAIR) guidelines focusing on the appropriate use of animal models and clinical trial design will undoubtedly improve the odds of identifying effective therapeutics. However improvements will need to be made across all levels of the drug discovery pipeline if new therapies are to be effectively identified and developed. This review will cover current methods used for modelling ischemic stroke in vitro, along with some of the insights that have been gained and the technological developments that may allow for the production of more effective and relevant models for research in stroke.
In vivo vs. in vitro
In vivo models have enabled a great insight into the pathophysiology of human disease and have been critical in our understanding of stroke. Rodents are often the chosen species, due to availability of genetically altered strains. However, around 70 million years of evolution separate humans from rodents and just a 10% difference in genome implies that ~ 3,000 genes differ3. Aside from macro structural discrepancies, a number of cellular and molecular differences exist. For example, the expression levels of transporters and pumps that contribute to the BBB, and the functional diversity and abundance of astrocytes distinguish the human BBB from the rodent4. Duration of excitotoxicity, has also been demonstrated to differ significantly between humans and mice5.
Considering the large inflammatory component of stroke pathology, it is also important to note differences in immune biology between species. A number of important immune signaling molecules (IL-8, CXCL7, CCL18, MCP-4 and CCL24/CCL26) are expressed in humans but not mice, while CCL6, CCL9, and MCP-5 are present in mice but not humans3. As such, the possibility of therapeutic targets being present in humans, but not rodents, or vice versa, should not be overlooked.
Certain non-human primates provide a more anatomically and genetically relevant model species, however even primates’ genes with homology have been shown to have evolved with different biochemistry and function. This is exemplified by the recent clinical trial of TGN1412 that almost resulted in the deaths of six healthy volunteers. Differences in binding characteristics have now been found to account for the severe inflammatory response seen in humans, but not in rhesus monkeys6. Since then in vitro testing of TGN1412 in appropriate human cell in vitro models have replicated the dramatic cytokine release that evaded previous preclinical trials6, 7. Thus, human in vitro systems may provide highly valuable information as to a drug’s safety whilst also allowing for cost effective high-throughput screening assays (HTSAs)8. However, of 100,000 studies on stroke investigated in 2011, only 5 were performed in human in vitro systems8. With the advent of new cellular technologies and genetic and molecular manipulation there is a great opportunity for the development of in vitro systems to model stroke and improve the drug discovery pipeline.
Modeling stroke in vitro: Causing the damage
Chemical and enzymatic methods
Ischemic conditions can either be modeled by removal of oxygen and glucose or via chemical or enzymatic inhibition of metabolism. Kurian et al., have previously compared a number of chemical and enzymatic methods against oxygen glucose deprivation (OGD) with reperfusion (OGDr) in a renal epithelial cell model9, to our knowledge no such comparison has been made for stroke models. In this study they compared chemical methods using rotenone, antimycin and sodium azide to inhibit the electron transport chain. The enzymatic methods used were the GOX/CAT system consisting of glucose oxidase (GOX) and catalase (CAT) and 2-deoxyglucose (an isomer of L-glucose, which cannot be metabolized). These methods were compared to OGD for 1h followed by 3h reperfusion. Antimycin mediated cell injury was found to be the most reproducible, whilst OGD required a much longer time to produce cell injury9.
While chemical and enzymatic methods may be suited to high throughput (HT), due to ease of application and rapidity of response, this is at the expense of relevance (for instance chemical hypoxia results in more free radical generation than anoxia).
Oxygen–glucose deprivation (OGD)
Ischemia-like conditions can be induced by replacing the normal O2/CO2 equilibrated medium with N2/CO2 equilibrated medium and maintaining cells in a hypoxic chamber. While oxygen deprivation is usually used in combination with glucose deprivation, a number of in vitro studies have demonstrated hypoxia alone causes dramatic alterations in endothelial cell (EC) actin cytoskeleton and tight junction protein localization in BBB models10. However, the physiological relevance of anoxia in the absence of glucose depletion is most relevant to conditions where blood flow is maintained, such as carbon monoxide poisoning, thus in the modelling of stroke, OGD is most relevant. In murine primary cortical cultures the rate of neuronal cell loss in anoxia is dependent on the concentration of glucose in the media. Half maximal neuronal loss occurs by 4–8h with 2mM glucose and in>24h with 20mM glucose (a concentration typical of most classical media)11. GD alone is rarely used to model stroke and over 4h does not significantly influence survival of rat cortical neurons in culture12. However, in organotypic mouse hippocampus cultures, GD alone for 2.5h resulted in significant CA1 cellular damage13. Such discrepancies may be explained by the relative sensitivity of CA-1 neurons and highlights the importance of using heterogeneous cell populations.
Combining oxygen and glucose deprivation, causes primary neurons to undergo acute cell body swelling within less than 60min followed by apoptotic14 and excitotoxic necrotic cell death15. In OGDr experiments, neuronal degeneration occurs over several hours, despite return to standard culture conditions11, as is consistent with observations of in vivo I/R injury. OGD in primary mouse cortical neurons is also associated with a large increase in extracellular glutamate concentration11 consistent with excitotoxic effects in vivo.
Excitotoxicity
In vitro models also allow for the study of excitotoxicity in isolation of ischemic damage, through application of glutamate receptor agonists such as N-Methyl-D-aspartate (NMDA) or glutamate16. Use of specific agonists allows investigation of the involvement of glutamate receptor subtypes in excitotoxic events
Cellular platforms used in stroke models
An overview of the cellular platforms used in stroke research is given in table 1.
Table 1.
Comparison of suitability of cellular platforms for in vitro neurological research
| Cellular platform |
HT ready |
Availability of human cells |
Physiological relevance |
Structural complexity modelled |
Possible application to stroke research |
Comments |
|---|---|---|---|---|---|---|
| Brain Slice | No | Extremely limited |
High | To a high degree |
OGD, chemical ischemia, excitotoxicity |
Tissue damage during preparation may induce abnormal functioning |
|
Organotypic cell culture |
No | Limited | High | To some degree |
OGD, chemical ischemia, excitotoxicity |
Tissue damage during preparation may induce abnormal functioning |
|
Primary cells |
No | Limited | High | Artificial arrangement required |
OGD, chemical ischemia, excitotoxicity BBB models, thrombosis and leukocyte responses |
Limited proliferative capacity |
| Cell lines | Yes | Unlimited | Low | Artificial arrangement required |
OGD, chemical ischemia, excitotoxicity BBB models |
Oncogenes present and high passage may limit physiological relevance |
|
Embryonic stem cells |
Partial | Extremely limited |
Potentially High |
Artificial arrangement required |
OGD, chemical ischemia, excitotoxicity, BBB models |
Limited use due to ethical issues |
| iPSCs | Yes | Potentially unlimited |
Potentially High |
Artificial arrangement required |
OGD, chemical ischemia, excitotoxicity, BBB models |
Single donor models of the relevant ‘at risk’ populations are possible |
Brain slices
The brain slice method utilizes a thin slice of brain tissue (usually ~400µm), allowing for in-depth probing of neuronal circuitry. The slice is perfused with artificial CSF (aCSF), allowing rodent slices to be maintained for up to 12h. Removing glucose and replacing oxygen with nitrogen in this solution provides global OGD, causing neuronal and astrocyte depolarization within 10min in rat cortical slices17. The benefit of preserving architecture is that different vulnerabilities of neuronal cell types may be assessed17. Additionally, neuronal effects of ischemia can be separated from cerebrovascular influences17. Richard et al., have built upon this model to provide focal OGD achieved by perfusing OGD aCSF over a brain slice in a focal stream, while the remainder of the slice is perfused with normal aCFS. Using this model in rodent brain slices, Richard et al., have demonstrated rapid neuronal depolarization in the anoxic core, followed by progressive depolarization in the “penumbra” region18.
A key benefit of in vitro systems is the opportunity to work with human cells, as such Werth et al., utilized the brain slice method in human cortical slices to provide the first direct evidence of glutamate receptor involvement in ischemic injury in the human brain19. However, such investigations are low throughput and availability of human samples is extremely limited. These samples are often obtained from neurosurgery of young epileptic patients, which may not represent the chemical and molecular physiology of the typical stroke patient.
While it has been recognized that human brain slices might provide a crucial intermediate assay to forecast translational success, it should be recognized that preparation of the slice is associated with tissue trauma and ischemia and as such, basal level readings obtained represent a post post-traumatic state.
Organotypic cell culture
Existing in the realm between brain slice and primary cell culture, these ex vivo cultures are obtained from different anatomical regions of the brain, usually taken from neonatal animals, and allowed to mature in vitro. This method maintains structural organization however many cultures experience synaptic rearrangement due to lack of extrinsic afferent and efferent signals in vitro20. These cultures have increasingly been used to investigate neuronal cell death, myelination, synapse plasticity and potential stroke therapies in OGD models, however they use neonatal rodent tissue, which may be of questionable relevance to the adult-aging human.
Cell culture
Cell cultures, while lacking the physiological architecture and networks that exist in vivo, are compatible with HT and allow for dissection of the contributions of individual cell types to pathological processes.
Primary cells
The particular benefit of primary cells is evident in brain endothelial cell (BEC) primary cultures, where trans-endothelial electrical resistance (TEER) is comparatively high compared to cell lines21. Primary glial cell cultures are some of the most commonly used in vitro model for neurobiological studies and the molecular properties and differentiation of these cells in culture reflect their in vivo phenotype well22. Primary cell isolation and purification is time-consuming, yields can be limited and possible contamination with other cell types raises questions of reproducibility.
Primary cells may also lose phenotypic identity with increased passage number23, thus application to HT is limited. Furthermore neurological research is particularly limited, due to the fact that once terminally differentiated into mature neurons, these primary cells can no longer replicate. On the other hand, human blood samples are readily available as such isolated primary leukocytes and platelets may be utilized within a stroke model, to investigate relevant thrombotic and inflammatory responses.
Cell lines: Immortal cancers with mistaken identities or useful tools?
The main advantage of immortalised cell lines is the ready supply of human cells suitable for use in HTSAs. Immortalized human microglia cells have been produced e.g. HMO6, established from primary human embryonic microglia and display a wide variety of inflammatory responses typical of the primary cell. The human teratoma-derived NT2 cell line is also a promising investigational tool from which neuronal cells, astrocytes, and oligodendrocytes can be derived (reviewed by Haile et al.,)24. However it should be noted that these cells contain oncogenes that distinguish them from endogenous cells by increased proliferation, cell adhesion, and variance of morphologies. The origin of the cell line should also be concidered, for instance SH-SY5Y is often used with OGD to model stroke, however these adrenergic and dopaminergic cells are derived from cells of a bone marrow biopsy taken from a four-year-old female with neuroblastoma. Differences between primary and immortalized cells are also notable in BEC cell lines where BBB specific adhesion molecules, enzymes and transporters are expressed at much lower levels25.
Stem cells
Embryonic stem cells in principle allow for unlimited quantities of every cell type in vitro, and numerous techniques have been established for the generation of lineage-restricted neural progenitor cells followed by their specific differentiation into neurons, astrocytes or oligodendrocytes (reviewed by Selvaraj et al.,)26. Ethical issues limit the use of human embryonic stem cells however reprogramming of somatic cells into induced pluripotent stem cells (iPSCs) provides an attractive alternative.
Since the first reprogramming of somatic cells to iPSCs by Yamanaka et al. new strategies have been developed to induce iPSCs omitting oncogenic factors, using non-viral methods and removing transgenic sequences using recombination methods, such as Cre-loxP or PiggyBac transposition27. Furthermore protocols for iPSC cell differentiation to neurons are improving to give near 100% purity of neurons28. iPSCs have been utilized to model a number of diseases for drug screening, and since 2008 have also been used in modeling neuronal disease29. The particular benefit of these cells is in producing patient specific cells that can aid in modeling neurological disorders with significant genetic contributions, such as Multiple Sclerosis (MS). While stroke is a multifactorial disease, mendelian stroke syndromes do exist, such as Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leucoencephalopathy (CADASIL)30. The use of iPSCs from such patients may aid a further understanding of stroke pathophysiology.
Modeling the BBB in stroke
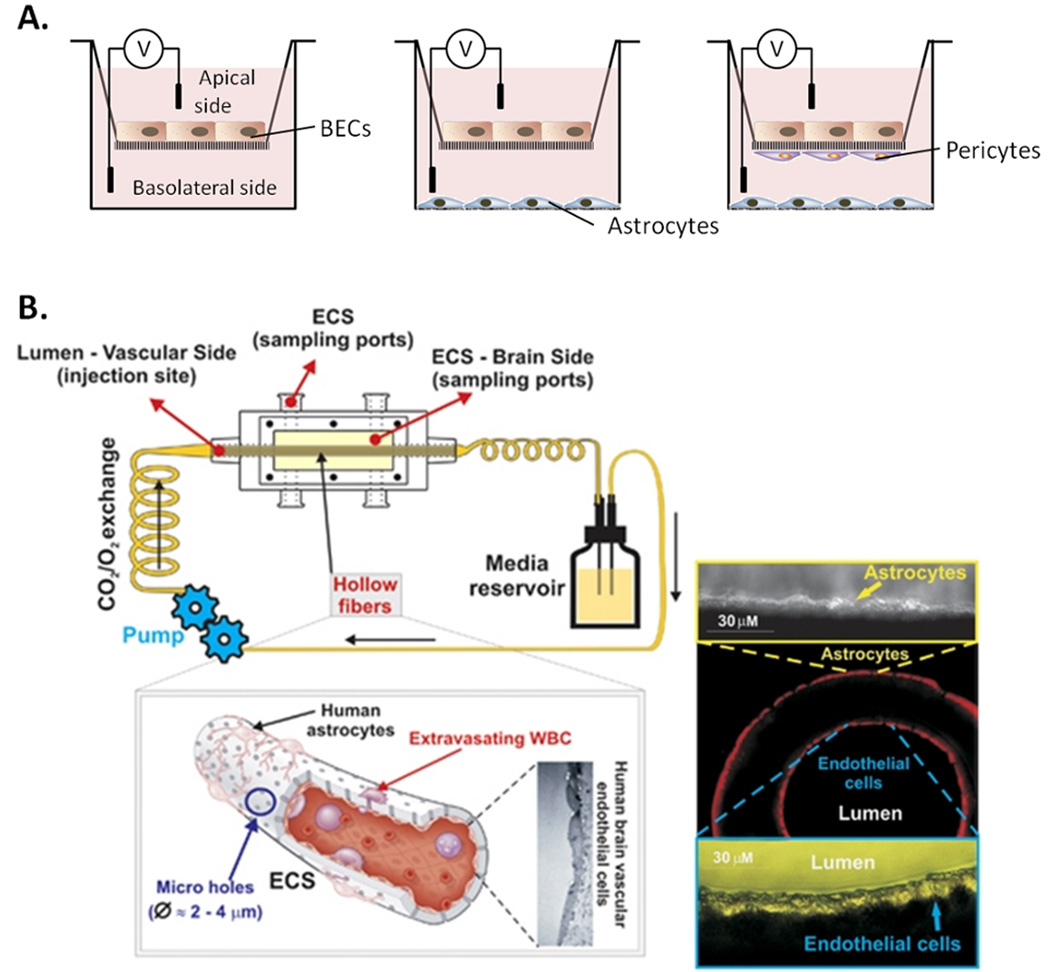
BBB compromise is an important pathological process in stroke causing further disruption of brain homeostasis. A wide variety of in vitro BBB models have been described, to assess both the effects of ischemic damage and potential therapeutic interventions to combat BBB breakdown, a thorough review is given by Gumbleton et al.,23. Such models generally consist of a two-layer or transwell cell culture system where permeability to molecules such as sucrose or TEER can be measured across the monolayer (Figure 1A). Commercially available 3D models allowing co-culture have also more recently been developed31 (Figure 1B).
Figure 1. Currently used blood brain barrier (BBB) models.
A) Diagrams demonstrating the varying complexities of transwell BBB models showing direct and indirect co-culture methods; BECs may be cultured alone on a porous membrane transwell across which electrical resistance may be measured to assess BBB integrity. Astrocytes may be co-cultured in the basolateral side of the well to enhance BBB properties, whist in more complex models pericytes or microglia may be included and cultured on the membrane. B) The commercially available Flocel inc. DIV-BBB3D system uses a series of hollow fibers to allow for 3D co-culture of BECs and astrocytes whilst also allowing luminal flow to investigate leukocyte transmigration (Reprinted by permission from Macmillan Publishers Ltd; Journal of Cerebral Blood Flow & Metabolism31 Copyright 2011). ECS, extraluminal space.
Building on BBB models: The neurovascular unit (NVU)
BBB models using BECs alone, suffer from low TEER and improper transporter and junctional protein localization compared to those found in vivo32. However it is now recognised that the BBB is supported by multiple heterotypic cell interactions as part of a NVU, involving astrocytes, pericytes, microglia, neurons and also extracellular matrix components.
Astrocytes
Astrocytes, the most abundant glial cells in the brain, whose endfeet cover ≥99% of the vascular surface and contribute to the unique properties of BECs and thus BBB integrity25. In agreement with this hypothesis, co-culturing astrocytes with BECs significantly increases TEER and enhances the expression of transporters, BBB enzymes and tight junctions25. Astrocytes also actively participate in stroke pathology, and exposing BECs to media from primary astrocytes which have experienced hypoxia, stimulates inflammatory genes in BECs33. Astrocytes have also been shown to influence immune cell recruitment to BECs34. Such co-culture methods may be used to investigate astrocyte influence on stroke induced BBB breakdown.
Pericytes
Pericyte numbers in the brain correlate with BBB tightness, and their loss contributes to BBB breakdown in vivo25, 35. In vitro pericytes have also been shown to promote BBB characteristics in BECs25. Thus, in vitro models that include pericytes may provide an insight as to whether these cells might present as a potential therapeutic target for the treatment of stroke and other neurovascular diseases.
Microglia
The role of microglia in regulating BBB remains controversial. Microglial activation promotes the restoration of a disrupted BBB, yet microglia-released TNF-α promotes BBB compromise25. Such apparently contradictory results are likely due to the complex microglial phenotype and activation state. Kaushal and Schlicher recently used a novel in vitro model of penumbral damage to help delineate the microglial involvement in stroke36. In this model, neuron and astrocyte co-cultures undergo OGD to simulate the stroke core. Soluble factors from the stressed co-cultures are then used to activate the microglia. These microglia are then incubated with naive neuron/astrocyte co-cultures to reveal how microglia may damage healthy tissue36. Such studies allow for insights into the complex role of microglia in BBB regulation.
Neurons
Considering that salvaging neuronal function is the aim of many prospective stroke treatments, incorporation of neurons in models of stroke is desirable. While simplified models of ischemic damage or excitotoxicity regularly use neuronal cell cultures, their inclusion in models of the BBB is rare. Neurons have however been shown to promote BBB properties in BEC cultures25 suggesting that inclusion of neuronal cultures in BBB models may provide useful mechanistic insights into ischemia-induced BBB breakdown.
Extracellular matrix
The importance of extacellular matrix is exemplified by Tilling et al., using porcine BECs. These experiments with various combinations of matrix coatings demonstrate significant effects on BEC permeability37.
Beyond the brain: Recruitment of circulating cells
While most in vitro stroke models focus on brain parenchymal cells and BBB, it is important to recognize the significant contribution of circulating immune cells. There are a number of systems in which leukocyte recruitment can be assessed: from static transmigration assays through membranes, to static or flow based leukocyte-EC interaction assays. However, few have utilized these assays to investigate stroke-specific leukocyte recruitment.
Studies by Inglis et al., have shown that neutrophil migration across BECs in response to a chemotactic gradient decreases TEER38. This is consistent with neutrophil migration in vivo causing vasogenic cerebral edema and BBB breakdown following stroke. OGD has also been shown to up-regulate BEC adhesion molecules, chemoattractive factors, and neutrophil binding under static conditions33. Currently we are establishing leukocyte-EC recruitment flow assays with OGD to dissect the contribution of leukocyte subsets to the pathophysiology of stroke.
Improving current models
Culture conditions: Re-thinking the status quo
While there have been a few attempts to mimic the complex architecture of the brain, such complex systems are not always required, however mimicking physiological environment in terms of the chemical microenvironment and relevant culture conditions is crucially important.
Is ‘Hypoxia’ in vitro, normoxia in vivo?
Oxygen is critical to cell function, too little and cells will die whilst too much enhances free radical production. Standard incubator conditions often utilize 5% CO2 to maintain pH, however oxygen is rarely controlled for. Circulating arterial blood typically contains 10.5–13% (80–100mm Hg) oxygen and most organs typically function at 2–8% O2 (19–70mm Hg)39. However, atmospheric oxygen levels are often referred to as “normoxic” despite being substantially higher than those in vivo. While many ODG studies use relevant hypoxia levels (<2%), relatively few return to normoxia (2–5%), instead returning them to atmospheric levels. Thus the oxygen level changes in OGD are more extreme than found in vivo. A number of reports have demonstrated exposure to physiological oxygen conditions (2–5%) to increase survival, proliferation and dopaminergic differentiation of neurons in culture40. In addition to altering basal functioning, high oxygen levels also alter cellular response to challenge, with neurons cultured at atmospheric oxygen being resistant to HIV induced cell death39. It is thus plausible that these cells are resistant to the oxidative stress produced in stroke, considering they have grown in an oxidative environment prior to challenge. As such, the influence of oxygen levels should be taken into account when interpreting data from in vitro experiments.
Glucose: Are cell cultures diabetic?
In vivo, plasma glucose can range from 5.5 to 7.8mM, while brain glucose levels have been reported ranging from 0.82 to 2.4mM41, 42. However, glucose levels found in cell cultures (designed for rapid cell growth) far exceed physiological levels, often containing >20mM glucose. In severe hyperglycemia plasma levels may reach 15.2mM, and in hypoglycemia, may drop to 2.8mM, while brain levels only reach 4.5mM, or fall as low as 0.16mM41. As such, neurons in culture are consistently exposed to >10 times the levels found in vivo. High levels of glucose in standard media have been shown to negatively affect long term neuronal viability and influence AMPK signaling41. Levels of other energy sources including lactate, pyruvate, fatty acids, and ketone bodies have been demonstrated to influence neuronal metabolism both in vivo and in vitro41 and should be considered when investigating neuronal energy crisis. Regardless of the disease state or system being modeled, if the aim of an in vitro study is to predict or investigate the physiological in vivo environment then it is logical that physiologically relevant conditions are maintained. The most widely used media formula were designed a number of decades ago and it may be that it is time for a re-think of the conditions under which cells maintained.
A glimpse into the future
Taking the above considerations into account to improve the physiological relevance, new technologies and understandings may allow for the development of newer models with increased predictive power and providing more faithful representations of pathophysiological microenvironment.
Moving into the third dimension
In Edwin Abbott Abbott’s 1884 “novella flatland”, the 3rd dimension is described from a world in which only 2 dimensions exist. In this imaginary world, a sphere would appear as a series of changing size circles. By ignoring the 3rd dimension in vitro we may equally only be getting a slice of the bigger picture of cells’ behavior.
Most 3D platforms can be broadly categorised into three groups: 1) Hydrogel-forming (such as alginate and collagen), 2) Synthetic microporous scaffolds (such as polystyrene scaffolds) and 3) Scaffold-free formats (such as hanging drop preparations)43. Most headway with regards to 3D BBB, NVU and stroke models has been made with the use of Hydrogels, because cross linking can be adjusted to provide physiological densities and hydrogels can also be functionalised to provide biochemical cues43. Cells grown in 3D microenvironments exhibit distinct phenotypes, often with greater physiological relevance, Peretz et al., have shown that hippocampal neurons and astrocytes grown in 3D have significantly lower mortality rates and a higher neuron:astrocyte ratio than is achievable in 2D cultures44. These 3D cell cultures were also more resistant to nutrient deprivation, and astrocytes may be more easily maintained in a resting state, allowing for rapid activation in response to stimulation45. While this additional layer of complexity may not be suitable for all stroke models, the 3D microenvironment undoubtedly influences cell behavior and accordingly a number of cell culture systems are moving in this direction.
Human cells for a human disease: Conditionally immortalized cells and all human models
Conditional immortalization, with for example a temperature-sensitive, or drug-induced gene expression technologies such as c-mycERTAM, allow the use of cells which maintain their original phenotype, but with the convenience and reproducibility of a cell line. Amemori et al., have used this technology to produce the SPC-01 cell line from human neural stem cells which are currently undergoing testing in a clinical trial for stroke46. Such cells have not yet been utilised for stroke modelling but may provide a valuable tool in future research. Recent advances in iPSC technology have enabled study of not only monogenetic diseases but also sporadic and multifactorial diseases and may provide in vitro researchers with much of the experimental power that transgenic animals have granted in vivo research8. Importantly, such technological advances are also allowing for more widespread use of all human systems. Indeed, iPSCs may pave the way for in vitro stroke models that are not only of human origin but also from a single donor. The benefit of all single donor systems is evident from the work of Reed et al., who reveal that assays used to detect cytokine storms often fail due to mismatch between ECs used (often human umbilical vein cells) and the isolated leukocytes (from healthy adults)47. Their use of an autologous bioassay was able to respond to biologics, such as TGN1412, in a way that accurately reflects the clinical situation, thus providing great predicative power47. In light of the above evidence, and considering a number of stroke models mix cells from different species, ‘all human systems’ should be promoted for the modelling of human disease.
Incorporating Physiological flow into complex models
Exposure of ECs to physiological shear rates has been shown to increase longevity and inhibit cell cycle, influencing cell genotype and phenotype48. Indeed, the importance of shear stress on cellular responses is particularly evident when there is a temporary loss of shear stress to ECs, as might occur during ischemic stroke, which causes cytokine release and BBB permeability. The presence of leukocytes was also found to compound these effects49. It should be noted that these experiments simply used cessation of flow, with constant glucose and oxygen. Utilizing models that not only deprive oxygen and glucose, but also flow, may provide the most relevant pathological stimulus to study mechanisms of stroke pathology.
Microfluidic systems
Photolithographic etching techniques developed to fabricate computer microchips have since been adopted by biologists to develop micro-patterned devices for cell culture. Such techniques provide new opportunities to mimic tissue architecture at the micro and nano scale.
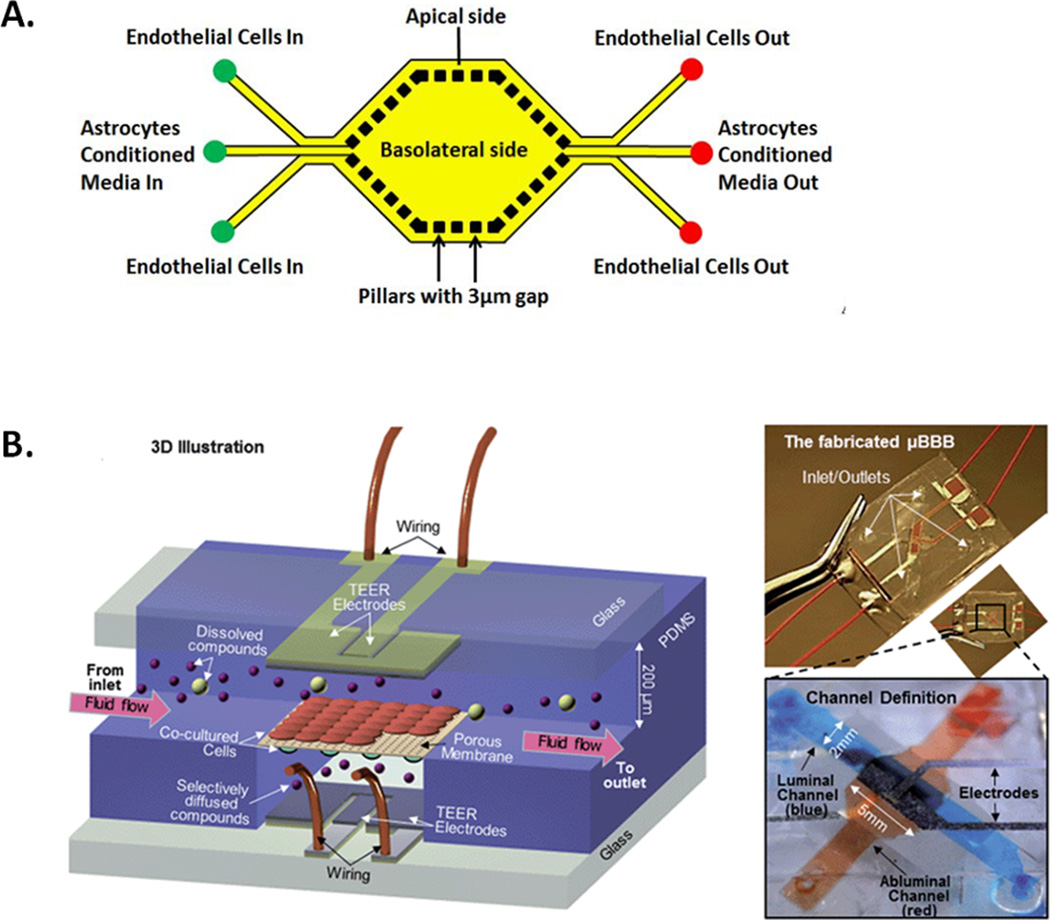
The microfluidic BBB (µBBB) model is one of the first microfluidic systems to be applied to neurological research. Essentially a two-layer (luminal and abluminal) flow chamber the model contains built-in Ag/AgCl electrodes for TEER measurement50. This model allows the establishment of BBB properties in a short timeframe and uses fewer reagents than traditional models. Other models such as the SyM-BBB have also been developed using microfabricated pillars rather than porous membranes51 (Figure 2).
Figure 2. Microfluidic BBB models.
Examples of experimental microfluidic devices designed to model the BBB A) Diagram showing a top view of the SyM-BBB microfluidic BBB model. Micro-pillars are used to separate a basolateral central reservoir to which astocytes or astrocyte-conditioned media is added. BECs are seeded in the peripheral channels whilst the micropillars allow basolateral interaction.51 B) The µBBB model, uses a porous membrane to separate basolateral cells from BECs, inbuilt electrodes allow for TEER measurement across the BEC monolayer50. (Reproduced in part from Ref 51 and Ref 50 with permission of The Royal Society of Chemistry)
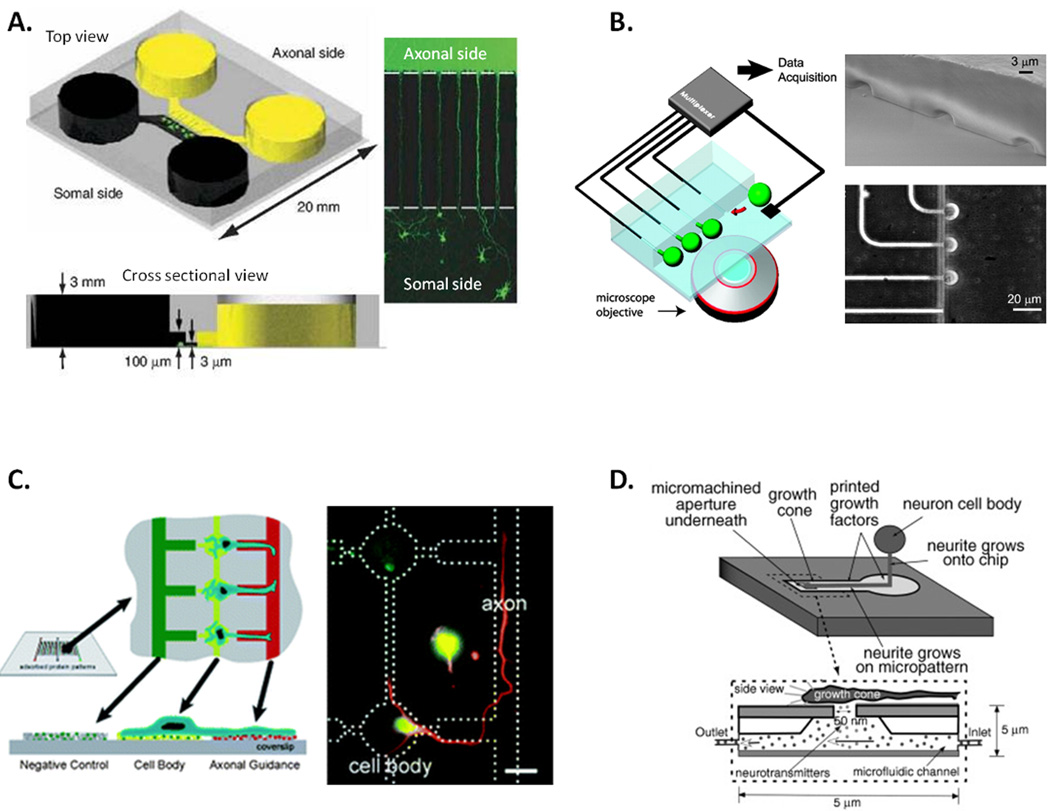
Microfluidic fabrication techniques have also been used to model neuronal architecture (Figure 3), from guiding axons via channels to establish axonal polarization52 and improved ‘capillary free’ electrophysiological devices53, to the complex patterning of adhesion substrates to create artificial neuronal networks54. Furthermore, Peterman et al. have recently created an artificial synapse, in which cultured neurons may be stimulated by the pico liter release of neurotransmitters55. Such devices have exciting implications for the study of excitotoxicity and neuronal interaction within networks. Microfluidic devices are also currently being used to investigate thrombosis and microvascular occlusion in hematologic disease. Tsai et al., have utilized a vascular tree of “endothelialized” microfluidic channels to investigate hemodynamic effects on thrombosis in blood samples from patients with Sickle Cell Disease56. Investigations of thrombosis in microfluidic devices resembling the NVU might in future be utilized to simultaneously investigate thrombolytic and neurological effects of test. A number of microfluidic devices are commercially available and while currently low throughput, such devices are also amenable to automation and as such may allow for complex HTSAs which is currently at odds with physiological relevance25.
Figure 3. Systems for modeling neuronal architecture in vitro.
A) A microfluidic-based culture platform to direct axonal growth of CNS neurons; two chambers (yellow and black) are connected via sub-cellular diameter channels which allow axon growth between chambers and polarization of axonal and somal sides (Reprinted by permission from Macmillan Publishers Ltd; Nature Methods 52 Copyright 2011). B) A microfluidic chip enabling for multiplexed electrophysiological experiments on cultured neurons: microfluidic junctions between the main cell culture chamber and lateral recording capillaries allows for patch clamp recordings of cell (green) activities (Reprinted from Ref 53 with permission from PNAS Copyright 2005 National Academy of Sciences, U.S.A). C) The use of vacuum soft lithography to pattern biomolecules and direct neuronal adhesion and polarization: adhesion molecules are patterned on a glass coverslip using a microfluidic template to allow for directed cell growth (Reproduced in part from Ref 54 with permission of The Royal Society of Chemistry). D) the artificial synapse; a micro-aperture allows for delivery of pico liter volumes of neurotransmitter to neuronal dendrites enabling detailed investigation of synaptic activity (Reprinted from Ref 55 with permission from John Wiley and Sons Inc. Artificial Organs. Copyright 2003).
Conclusions
While current in vitro systems have a limited capacity to replicate the complex situation that occurs in stroke, when appropriately utilized for a specific research question, in vitro systems are a highly useful research tool to help understand basic biochemical and cellular mechanisms. With the increased availability of human cells, the development of new technologies and more physiologically relevant models, in vitro systems may provide not only a HT low cost base for the drug discovery pyramid, but also composite all human assays at the top, supplementing in vivo research to improve clinical translation.
Supplementary Material
Acknowledgments
Sources of Funding
The NIH/NHLBI (HL125572-01A1).
Footnotes
Disclosures
None.
References
- 1.Mukherjee D, Patil CG. Epidemiology and the global burden of stroke. World Neurosurg. 2011;76:S85–S90. doi: 10.1016/j.wneu.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 2.Minnerup J, Sutherland BA, Buchan AM, Kleinschnitz C. Neuroprotection for stroke: Current status and future perspectives. International journal of molecular sciences. 2012;13:11753–11772. doi: 10.3390/ijms130911753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mestas J, Hughes CCW. Of mice and not men: Differences between mouse and human immunology. The Journal of Immunology. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 4.Syvanen S, Lindhe O, Palner M, Kornum BR, Rahman O, Langstrom B, et al. Species differences in blood-brain barrier transport of three positron emission tomography radioligands with emphasis on p-glycoprotein transport. Drug Metab. Dispos. 2009;37:635–643. doi: 10.1124/dmd.108.024745. [DOI] [PubMed] [Google Scholar]
- 5.Dávalos A, Castillo J, Serena J, Noya M. Duration of glutamate release after acute ischemic stroke. Stroke. 1997;28:708–710. doi: 10.1161/01.str.28.4.708. [DOI] [PubMed] [Google Scholar]
- 6.Hanke T. Lessons from tgn1412. The Lancet. 2006;368:1569–1570. doi: 10.1016/S0140-6736(06)69651-7. [DOI] [PubMed] [Google Scholar]
- 7.Romer PS, Berr S, Avota E, Na SY, Battaglia M, ten Berge I, et al. Preculture of pbmcs at high cell density increases sensitivity of t-cell responses, revealing cytokine release by cd28 superagonist tgn1412. Blood. 2011;118:6772–6782. doi: 10.1182/blood-2010-12-319780. [DOI] [PubMed] [Google Scholar]
- 8.Antonic A, Sena E, Donnan G, Howells D. Human in vitro models of ischaemic stroke: A test bed for translation. Transl. Stroke Res. 2012;3:306–309. doi: 10.1007/s12975-012-0201-x. [DOI] [PubMed] [Google Scholar]
- 9.Kurian G, Pemaih B. Standardization of in vitro cell-based model for renal ischemia and reperfusion injury. Indian J. Pharm. Sci. 2014;76:348–353. [PMC free article] [PubMed] [Google Scholar]
- 10.Brown RC, Davis TP. Hypoxia/aglycemia alters expression of occludin and actin in brain endothelial cells. Biochem. Biophys. Res. Commun. 2005;327:1114–1123. doi: 10.1016/j.bbrc.2004.12.123. [DOI] [PubMed] [Google Scholar]
- 11.Goldberg MP, Choi DW. Combined oxygen and glucose deprivation in cortical cell culture: Calcium-dependent and calcium-independent mechanisms of neuronal injury. J. Neurosci. 1993;13:3510–3524. doi: 10.1523/JNEUROSCI.13-08-03510.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsumoto K, Yamada K, Kohmura E, Kinoshita A, Hayakawa T. Role of pyruvate in ischaemia-like conditions on cultured neurons. Neurol. Res. 1994;16:460–464. doi: 10.1080/01616412.1994.11740274. [DOI] [PubMed] [Google Scholar]
- 13.Newell DW, Barth A, Papermaster V, Malouf AT. Glutamate and non-glutamate receptor mediated toxicity caused by oxygen and glucose deprivation in organotypic hippocampal cultures. J. Neurosci. 1995;15:7702–7711. doi: 10.1523/JNEUROSCI.15-11-07702.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalda A, Eriste E, Vassiljev V, Zharkovsky A. Medium transitory oxygen-glucose deprivation induced both apoptosis and necrosis in cerebellar granule cells. Neurosci. Lett. 1998;240:21–24. doi: 10.1016/s0304-3940(97)00914-2. [DOI] [PubMed] [Google Scholar]
- 15.Gwag BJ, Lobner D, Koh JY, Wie MB, Choi DW. Blockade of glutamate receptors unmasks neuronal apoptosis after oxygen-glucose deprivation in vitro. Neuroscience. 1995;68:615–619. doi: 10.1016/0306-4522(95)00232-8. [DOI] [PubMed] [Google Scholar]
- 16.von Engelhardt J, Coserea I, Pawlak V, Fuchs EC, Kohr G, Seeburg PH, et al. Excitotoxicity in vitro by nr2a- and nr2b-containing nmda receptors. Neuropharmacology. 2007;53:10–17. doi: 10.1016/j.neuropharm.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 17.Dong WQ, Schurr A, Reid KH, Shields CB, West CA. The rat hippocampal slice preparation as an in vitro model of ischemia. Stroke. 1988;19:498–502. doi: 10.1161/01.str.19.4.498. [DOI] [PubMed] [Google Scholar]
- 18.Richard MJP, Saleh TM, El Bahh B, Zidichouski JA. A novel method for inducing focal ischemia in vitro. J. Neurosci. Methods. 2010;190:20–27. doi: 10.1016/j.jneumeth.2010.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Werth JL, Park TS, Silbergeld DL, Rothman SM. Excitotoxic swelling occurs in oxygen and glucose deprived human cortical slices. Brain Res. 1998;782:248–254. doi: 10.1016/s0006-8993(97)01286-9. [DOI] [PubMed] [Google Scholar]
- 20.Vornov JJ, Tasker RC, Coyle JT. Delayed protection by mk-801 and tetrodotoxin in a rat organotypic hippocampal culture model of ischemia. Stroke. 1994;25:457–464. doi: 10.1161/01.str.25.2.457. discussion 464-455. [DOI] [PubMed] [Google Scholar]
- 21.Eigenmann D, Xue G, Kim K, Moses A, Hamburger M, Oufir M. Comparative study of four immortalized human brain capillary endothelial cell lines, hcmec/d3, hbmec, ty10, and bb19, and optimization of culture conditions, for an in vitro blood-brain barrier model for drug permeability studies. Fluids and Barriers of the CNS. 2013;10:33. doi: 10.1186/2045-8118-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abney ER, Bartlett PP, Raff MC. Astrocytes, ependymal cells, and oligodendrocytes develop on schedule in dissociated cell cultures of embryonic rat brain. Dev. Biol. 1981;83:301–310. doi: 10.1016/0012-1606(81)90476-0. [DOI] [PubMed] [Google Scholar]
- 23.Gumbleton M, Audus KL. Progress and limitations in the use of in vitro cell cultures to serve as a permeability screen for the blood-brain barrier. J. Pharm. Sci. 2001;90:1681–1698. doi: 10.1002/jps.1119. [DOI] [PubMed] [Google Scholar]
- 24.Haile Y, Fu W, Shi B, Westaway D, Baker G, Jhamandas J, et al. Characterization of the nt2-derived neuronal and astrocytic cell lines as alternative in vitro models for primary human neurons and astrocytes. J. Neurosci. Res. 2014;92:1187–1198. doi: 10.1002/jnr.23399. [DOI] [PubMed] [Google Scholar]
- 25.He Y, Yao Y, Tsirka SE, Cao Y. Cell-culture models of the blood–brain barrier. Stroke. 2014;45:2514–2526. doi: 10.1161/STROKEAHA.114.005427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Selvaraj V, Jiang P, Chechneva O, Lo UG, Deng W. Differentiating human stem cells into neurons and glial cells for neural repair. Frontiers in bioscience (Landmark edition) 2012;17:65–89. doi: 10.2741/3916. [DOI] [PubMed] [Google Scholar]
- 27.Durnaoglu S, Genc S, Genc K. Patient-specific pluripotent stem cells in neurological diseases. Stem Cells International. 2011;2011:1–17. doi: 10.4061/2011/212487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imaizumi Y, Okano H. Modeling human neurological disorders with induced pluripotent stem cells. J. Neurochem. 2014;129:388–399. doi: 10.1111/jnc.12625. [DOI] [PubMed] [Google Scholar]
- 29.Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, et al. Induced pluripotent stem cells generated from patients with als can be differentiated into motor neurons. Science. 2008;321:1218–1221. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- 30.Joutel A, Vahedi K, Corpechot C, Troesch A, Chabriat H, Vayssiere C, et al. Strong clustering and stereotyped nature of notch3 mutations in cadasil patients. Lancet. 1997;350:1511–1515. doi: 10.1016/S0140-6736(97)08083-5. [DOI] [PubMed] [Google Scholar]
- 31.Cucullo L, Marchi N, Hossain M, Janigro D. A dynamic in vitro bbb model for the study of immune cell trafficking into the central nervous system. J. Cereb. Blood Flow Metab. 2011;31:767–777. doi: 10.1038/jcbfm.2010.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al Ahmad A, Taboada CB, Gassmann M, Ogunshola OO. Astrocytes and pericytes differentially modulate blood-brain barrier characteristics during development and hypoxic insult. J. Cereb. Blood Flow Metab. 2011;31:693–705. doi: 10.1038/jcbfm.2010.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang W, Smith C, Howlett C, Stanimirovic D. Inflammatory activation of human brain endothelial cells by hypoxic astrocytes in vitro is mediated by il-1beta. J. Cereb. Blood Flow Metab. 2000;20:967–978. doi: 10.1097/00004647-200006000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Hudson LC, Bragg DC, Tompkins MB, Meeker RB. Astrocytes and microglia differentially regulate trafficking of lymphocyte subsets across brain endothelial cells. Brain Res. 2005;1058:148–160. doi: 10.1016/j.brainres.2005.07.071. [DOI] [PubMed] [Google Scholar]
- 35.Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaushal V, Schlichter LC. Mechanisms of microglia-mediated neurotoxicity in a new model of the stroke penumbra. J. Neurosci. 2008;28:2221–2230. doi: 10.1523/JNEUROSCI.5643-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tilling T, Korte D, Hoheisel D, Galla H-J. Basement membrane proteins influence brain capillary endothelial barrier function in vitro. J. Neurochem. 1998;71:1151–1157. doi: 10.1046/j.1471-4159.1998.71031151.x. [DOI] [PubMed] [Google Scholar]
- 38.Inglis VI, Jones MPJ, Tse ADY, Easton AS. Neutrophils both reduce and increase permeability in a cell culture model of the blood–brain barrier. Brain Res. 2004;998:218–229. doi: 10.1016/j.brainres.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 39.Tiede LM, Cook EA, Morsey B, Fox HS. Oxygen matters: Tissue culture oxygen levels affect mitochondrial function and structure as well as responses to hiv viroproteins. Cell Death Dis. 2011;2:e246. doi: 10.1038/cddis.2011.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Studer L, Csete M, Lee SH, Kabbani N, Walikonis J, Wold B, et al. Enhanced proliferation, survival, and dopaminergic differentiation of cns precursors in lowered oxygen. J. Neurosci. 2000;20:7377–7383. doi: 10.1523/JNEUROSCI.20-19-07377.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kleman AM, Yuan JY, Aja S, Ronnett GV, Landree LE. Physiological glucose is critical for optimized neuronal viability and ampk responsiveness in vitro. J. Neurosci. Methods. 2008;167:292–301. doi: 10.1016/j.jneumeth.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abi-Saab WM, Maggs DG, Jones T, Jacob R, Srihari V, Thompson J, et al. Striking differences in glucose and lactate levels between brain extracellular fluid and plasma in conscious human subjects: Effects of hyperglycemia and hypoglycemia. J. Cereb. Blood Flow Metab. 2002;22:271–279. doi: 10.1097/00004647-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 43.Asthana A, Kisaalita WS. Biophysical microenvironment and 3d culture physiological relevance. Drug discovery today. 2013;18:533–540. doi: 10.1016/j.drudis.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 44.Peretz H, Talpalar AE, Vago R, Baranes D. Superior survival and durability of neurons and astrocytes on 3-dimensional aragonite biomatrices. Tissue Eng. 2007;13:461–472. doi: 10.1089/ten.2005.0522. [DOI] [PubMed] [Google Scholar]
- 45.East E, Golding JP, Phillips JB. A versatile 3d culture model facilitates monitoring of astrocytes undergoing reactive gliosis. J. Tissue Eng. Regen. Med. 2009;3:634–646. doi: 10.1002/term.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amemori T, Romanyuk N, Jendelova P, Herynek V, Turnovcova K, Prochazka P, et al. Human conditionally immortalized neural stem cells improve locomotor function after spinal cord injury in the rat. Stem Cell. Res. Ther. 2013;4:68. doi: 10.1186/scrt219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reed DM, Paschalaki KE, Starke RD, Mohamed NA, Sharp G, Fox B, et al. An autologous endothelial cell:Peripheral blood mononuclear cell assay that detects cytokine storm responses to biologics. The FASEB Journal. 2015;29:2595–2602. doi: 10.1096/fj.14-268144. [DOI] [PubMed] [Google Scholar]
- 48.Desai SY, Marroni M, Cucullo L, Krizanac-Bengez L, Mayberg MR, Hossain MT, et al. Mechanisms of endothelial survival under shear stress. Endothelium. 2002;9:89–102. doi: 10.1080/10623320212004. [DOI] [PubMed] [Google Scholar]
- 49.Krizanac-Bengez L, Mayberg MR, Cunningham E, Hossain M, Ponnampalam S, Parkinson FE, et al. Loss of shear stress induces leukocyte-mediated cytokine release and blood-brain barrier failure in dynamic in vitro blood-brain barrier model. J. Cell. Physiol. 2006;206:68–77. doi: 10.1002/jcp.20429. [DOI] [PubMed] [Google Scholar]
- 50.Booth R, Kim H. Characterization of a microfluidic in vitro model of the blood-brain barrier (mubbb) Lab Chip. 2012;12:1784–1792. doi: 10.1039/c2lc40094d. [DOI] [PubMed] [Google Scholar]
- 51.Prabhakarpandian B, Shen MC, Nichols JB, Mills IR, Sidoryk-Wegrzynowicz M, Aschner M, et al. Sym-bbb: A microfluidic blood brain barrier model. Lab Chip. 2013;13:1093–1101. doi: 10.1039/c2lc41208j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taylor AM, Blurton-Jones M, Rhee SW, Cribbs DH, Cotman CW, Jeon NL. A microfluidic culture platform for cns axonal injury, regeneration and transport. Nature methods. 2005;2:599–605. doi: 10.1038/nmeth777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ionescu-Zanetti C, Shaw RM, Seo J, Jan YN, Jan LY, Lee LP. Mammalian electrophysiology on a microfluidic platform. Proc. Natl. Acad. Sci. U.S.A. 2005;102:9112–9117. doi: 10.1073/pnas.0503418102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nevill JT, Mo A, Cord BJ, Palmer TD, Poo M-m, Lee LP, et al. Vacuum soft lithography to direct neuronal polarization. Soft Matter. 2011;7:343–347. [Google Scholar]
- 55.Peterman MC, Mehenti NZ, Bilbao KV, Lee CJ, Leng T, Noolandi J, et al. The artificial synapse chip: A flexible retinal interface based on directed retinal cell growth and neurotransmitter stimulation. Artif. Organs. 2003;27:975–985. doi: 10.1046/j.1525-1594.2003.07307.x. [DOI] [PubMed] [Google Scholar]
- 56.Tsai M, Kita A, Leach J, Rounsevell R, Huang JN, Moake J, et al. In vitro modeling of the microvascular occlusion and thrombosis that occur in hematologic diseases using microfluidic technology. The Journal of Clinical Investigation. 2012;122:408–418. doi: 10.1172/JCI58753. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.