Abstract
Background and purpose
Three-dimensional pseudo-continuous arterial spin labeling (3D pCASL) with multiple post-labeling delays (PLDs) has been used to assess cerebral blood flow (CBF). We used this modality to estimate antegrade and collateral flow in patients with unilateral middle cerebral artery (MCA) stenosis.
Methods
Consecutive patients with unilateral MCA 50% to 99% stenosis at two centers underwent pCASL with a PLD of 1.5s and 2.5s. Mean CBF of bilateral MCA territory at the PLD 1.5s and 2.5s were measured. Early-arriving flow proportion was defined as [CBF 1.5s at lesion side/CBF 2.5s at normal side] × 100%. Late-arriving retrograde flow proportion was defined as [(CBF 2.5s minus CBF 1.5s) at lesion side minus (CBF 2.5s minus CBF 1.5s) at normal side]/CBF 2.5s at normal side × 100%. Antegrade and collateral scales were evaluated in patients with conventional angiography. Spearman correlation coefficients were calculated between early-arriving flow and late-arriving retrograde flow proportions on ASL and antegrade and collateral scales on conventional angiography, respectively.
Results
Forty-one patients (46.0±12.0 years) were enrolled. The mean early-arriving flow proportion was 78.3%±14.9%. The mean late-arriving retrograde flow proportion was 16.1%±10.2%. In 21 patients with conventional angiography, Spearman correlation coefficient was 0.53 (95%CI 0.11–0.79) between antegrade grade and early-arriving flow proportion (p=0.01) and 0.81 (95%CI 0.56–0.92) between collateral grade and late-arriving retrograde flow proportion (p<0.0001).
Conclusion
Three-dimensional pCASL with two-PLD may provide an empirical approach for estimating antegrade and collateral flow in patients with unilateral MCA stenosis.
Keywords: ASL, MRI, Collateral circulation, Stroke, Atherosclerosis
Introduction
In patients with hemodynamic impairment due to middle cerebral artery (MCA) atherosclerotic stenosis, the blood perfusion distal to the lesion site incorporates antegrade flow through the stenotic MCA and the retrograde flow through pial arteries form anterior cerebral artery and/or posterior cerebral artery.1, 2 Antegrade and collateral perfusion combined account for target downstream territory perfusion in ischemic stroke and have a strong correlation with clinical outcome.3–7 Past studies have attempted to estimate or quantify collateral blood flow using computed tomography (CT) angiography or conventional angiography with graded scales.8, 9 It remains difficult to quantify the proportion of cerebral blood flow via antegrade flow through the stenotic vessel versus retrograde flow via collateral vessels with current imaging modalities.10
Arterial spin labeling (ASL) perfusion is a novel method to evaluate cerebral hemodynamic impairment. It is sensitive to both temporal (arterial bolus arrival) and perfusion information with varying post-labeling delays (PLDs), a technique termed dynamic perfusion imaging.11 Three dimensional pseudo-continuous arterial spin labeling (3D pCASL) is an improved ASL technology with high signal-to-noise ratio and spatial resolution for quantitative cerebral blood flow (CBF) measurement.12–14
To the target downstream territory supplied by the stenotic vessel, antegrade flow traverses a relatively short vessel segment, while collateral flow passes through relatively longer pathways.1 This difference in flow pathways may cause a critical distinction in that collateral perfusion is delivered with a substantially delayed transit time compared to that of antegrade flow.15, 16 ASL is generally applied to measure CBF at a single PLD. Due to its sensitivity to arterial transit time, delayed arterial transit artifact has been utilized to identify and grade collateral flow in Moyamoya disease.17 In the present study, we presented an empirical approach to estimate antegrade and collateral flow using a two-PLD 3D pCASL protocol in a cohort of patients with unilateral MCA atherosclerotic stenosis.
Methods
Patients
The study was approved by the institutional ethnic committees of two centers. Written informed consent was obtained from all enrolled patients or his or her legally authorized representative.
This retrospective study enrolled consecutive patients from August 2013 to November 2014 who underwent MRI by the inclusion criteria as follows: 1) Symptomatic unilateral moderate to severe MCA stenosis (50% to 99%) as confirmed by magnetic resonance angiography or conventional angiography with ischemic stroke or transit ischemic attack (TIA) within 90 days; 2) Age of patients greater than 18 years old; 3) Patient presenting two or more atherosclerotic risk factors including hypertension, hyperlipidemia, diabetes mellitus, obesity, cigarette smoking;18 and 4) Patients who did not receive endovascular therapy. Patients with multiple moderate to severe stenoses (>50%) in intracranial arteries and extracranial arteries or those with less than two atherosclerotic risk factors were excluded. Extracranial arterial stenosis was evaluated by routine carotid ultrasound. Other clinically and/or radiologically suspected vascular diseases such as vasculitis or dissection were excluded. Patients suffering from stroke with a large infarction area (greater than 1/3 area of MCA territory) were excluded.
Image acquisition
All MRI studies were performed at 3.0T (DISCOVERY MR 750, GE Healthcare). Three dimensional MRA, 3D inversion recovery (IR)-prepared fast spoiled gradient recalled echo (FSPGR) and 3D pCASL were acquired for each patient.
MRA was performed using the following parameters: repetition time (TR) = 34 ms, echo time (TE) = 3.1 ms; field of view (FOV) = 24 cm, matrix = 512×128, slice thickness = 1 mm, and overlap = 0.5 mm. High-resolution volumetric FSPGR was acquired with whole brain coverage and the following parameters: FOV = 24 cm, slice thickness = 1 mm, number of slices = 156, TR = 8.2 msec, TE = 3.2 msec, bandwidth = 31.2 kHz, inversion time = 450 msec, matrix = 256×256, and number of excitations (NEX) = 1.
Three dimensional pCASL was acquired using 3D spiral fast spin echo (FSE) with the following parameters: TR = 4590ms (PLD = 1.5 s), 5285ms (PLD = 2.5 s), labeling duration = 1500 ms, TE = 10.5 ms, FOV = 24 cm, 512 sampling points on eight spirals, spatial resolution = 3.64 mm, slice thickness = 4.0 mm, number of slices = 36, NEX = 2, background suppressed. Three dimensional pCASL was acquired twice with the PLD of 1.5 s and 2.5 s. Total acquisition time was 20 minutes.
Conventional angiography was acquired from arterial phase through late venous phase.
Image post-processing and CBF estimation
The CBF map of 3D pCASL was postprocessed and generated by Function Tool (AW 4.5 Workstation, GE Healthcare). FSPGR was normalized to MNI space (Montreal Neurological Institute template) using SPM8 (Statistical Parametric Mapping, University College of London, available at www.fil.ion.ucl.ac.uk/spm/software/Spm8) on the Matlab platform (R2013b, MathWorks, Natick, MA). CBF map was coregistered to the normalized T1-weighted FSPGR and spatially smoothed. A volumetric MCA territory based on grey matter mask according to previous work was extracted from the AAL template (Automated Anatomical Labeling).19–21 Volume of interest (VOI) covered all the MCA territory including leptomeningeal and insular area but not basal ganglion (Figure I in the online-only Data Supplement). Mean CBF value of each side was obtained by applying the VOI to normalized CBF map.
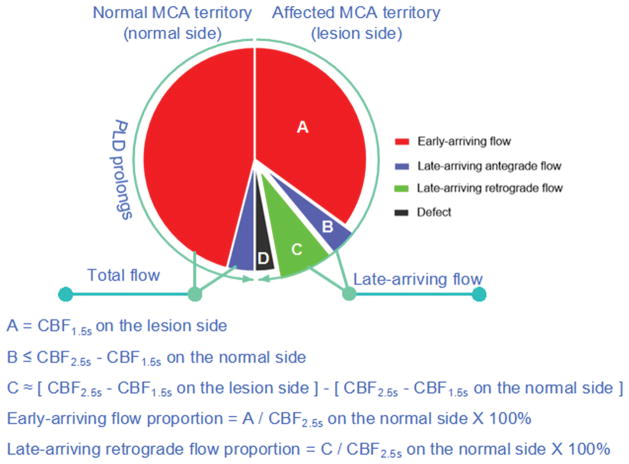
On the lesion side, perfusion on the CBF map of PLD 1.5s was assumed as early-arriving flow, and perfusion on the CBF map of PLD 2.5s was assumed as combination of early-arriving flow, late-arriving antegrade flow and late-arriving retrograde flow (Figure 1). Early-arriving flow was defined as the mean CBF of PLD 1.5s on the lesion side. Late-arriving retrograde flow was defined as [(CBF 2.5s minus CBF 1.5s) on the lesion side minus (CBF 2.5s minus CBF 1.5s) on the normal side]. Early-arriving flow proportion was calculated as [CBF 1.5s on the lesion side/CBF 2.5s on the contralateral side] × 100%, late-arriving retrograde flow proportion was calculated as [the late-arriving retrograde flow/CBF 2.5s on the normal side] × 100%.
Figure 1.
Diagram and formulas of blood flow in MCA territory with PLD increasing at normal and lesion side of patients with unilateral MCA atherosclerotic stenosis.
Conventional angiography antegrade scale and collateral grade assessment
Two experienced interventional neuroradiologists (N.M, J.L) who were blinded to clinical findings assessed the status of antegrade and collateral flow in patients with available conventional angiography using the modified Thrombolysis in Cerebral Infarction (mTICI) scale,22 and the American Society of Interventional and Therapeutic Neuroradiology (ASITN/SIR) collateral grading system based on consensus.9
Statistical analysis
Categorical variables were expressed as n (%), and continuous variables were expressed as mean±SD. Comparisons of mean CBF at the PLD 1.5 s and 2.5 s between bilateral MCA territories were analyzed using one-way ANOVA. Spearman correlation coefficient was used to assess the correlation of perfusion proportion with angiographic grading scales in cases with available conventional angiography. A P value < 0.05 (two-sided) was considered to indicate statistically significant difference. All statistical analyses were performed using SPSS 17.0.
Results
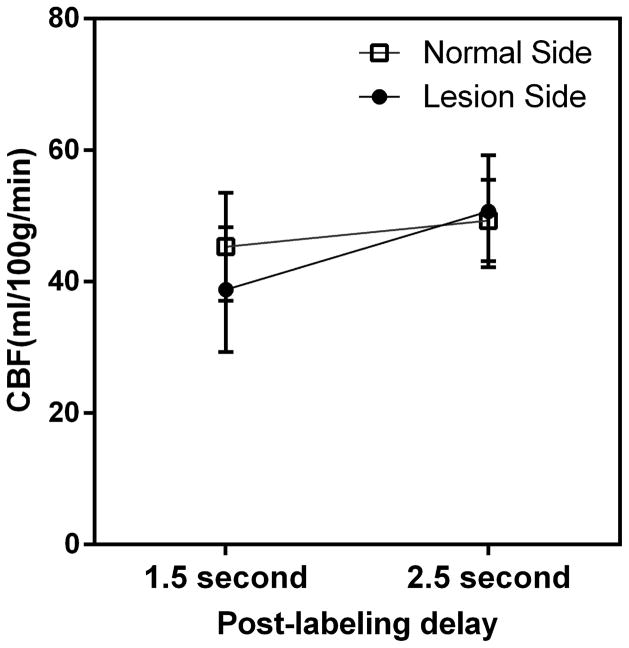
Forty-one patients (mean age, 46.0±12.0 years, 29 men) were included in our study. Thirty-nine stenosis lesions were located in the M1 segment of MCA and 2 lesions were located in the M2 segment. The demographic and clinical information at baseline of all patients were listed in Table 1 (see Table I for more details in the online-only Data Supplement). The mean CBF values of the lesion side at the PLD of 1.5s and 2.5s were 38.8±9.5 ml/100g/min and 50.7±8.5 ml/100g/min, and those of the normal side were 45.3±8.2 ml/100g/min and 49.3±6.2 ml/100g/min respectively. The ANOVA revealed significant differences between mean CBF of normal and lesion side at the PLD of 1.5s (p<0.05) but not for the PLD of 2.5s (p = 0.46). The ANOVA also revealed significant difference between mean CBF of the same side at the two-PLD (p < 0.05). Figure 2 shows that the mean CBF increases from 1.5 s PLD to 2.5 s PLD in bilateral MCA territory with different trends. The mean early-arriving flow proportion was 78.3%±14.9%. The mean late-arriving retrograde flow was 7.9±5.3 ml/100g/min. The mean late-arriving retrograde flow proportion was 16.1%±10.2% (see Table II for more details in the online-only Data Supplement).
Table 1.
Demographics of patients
| Patients (n = 41) | |
|---|---|
| Age, years, mean (SD) | 46.0 (12.0) |
| Female, n (%) | 12 (29) |
| Hypertension, n (%) | 27 (66) |
| Diabetes, n (%) | 9 (22) |
| Coronary artery disease, n (%) | 2 (5) |
| Smoking history, n (%) | 16 (39) |
| Lipid disorder, n (%) | 36 (88) |
| Obesity, n (%) | 0(0) |
| Stenosis of middle cerebral artery, n (%) | |
| Moderate stenosis (50%–70%) | 4 (10) |
| Severe stenosis (70%–99%) | 37 (90) |
| Stroke as a qualifying event, n (%) | 21 (51) |
| Time from qualifying event to MRI scan, d, mean (SD) | 34.1 (21.3) |
| NIH Stroke Scale score at admission, median (IQR) | 0 (0–2.5) |
Figure 2.
Mean CBF of bilateral MCA territory increases from PLD 1.5 s to PLD 2.5s
Modified TICI Scale and Early-arriving Flow Proportion
Twenty-two patients (mean age, 47.2±9.6 years, 17 men) underwent conventional angiography. One patient was excluded because of the lack of late venous phase for assessment. Modified TICI scale and ASITN/SIR collateral grade of 21 patients (mean age, 47.7±9.5 years, 17 men) were assessed.
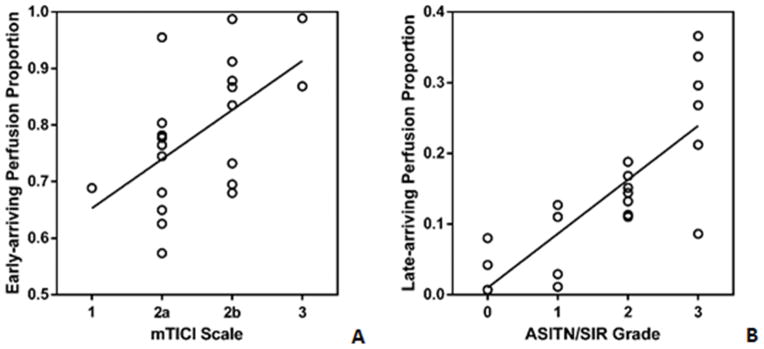
Modified TICI scale were assessed as 0 patient for mTICI 0, 1 patient for mTICI 1, 10 patients for mTICI 2a, 8 patients for mTICI 2b and 2 patients for mTICI 3. The relationship of early-arriving perfusion proportions and mTICI scales in the 21 patients is shown in Figure 3A. Spearman correlation coefficient was 0.53 (95%CI 0.11–0.79) between antegrade mTICI scale 1 to 3 and early-arriving flow proportion (p = 0.01). Early-arriving flow proportion was moderately correlated with mTICI scale 1 to 3.
Figure 3.
Scattered plots shows the relationship of early-arriving flow perfusion proportion and mTICI scale (A), late-arriving retrograde flow perfusion proportion and ASITN/SIR collateral grade (B) in patients with an available conventional angiography. The line represents linear regression
Collateral Circulation Grade and Late-arriving Retrograde Flow Perfusion Proportion
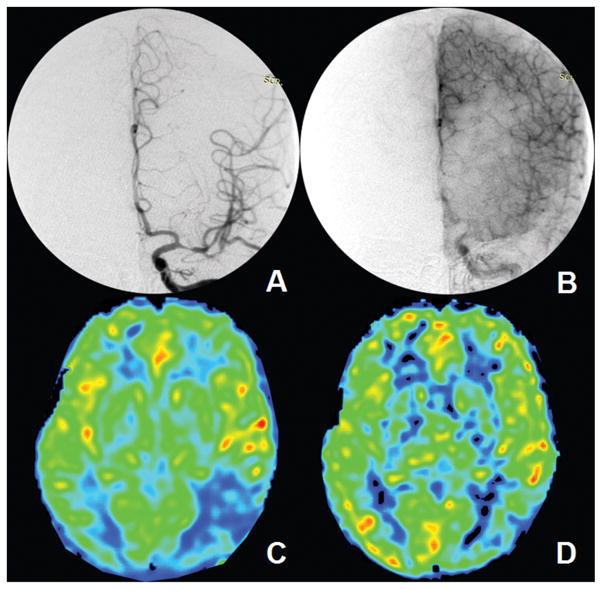
Collateral ASITN/SIR grades were assessed as 3 patients for grade 0, 4 patients for grade 1, 8 patients for grade 2, 6 patients for grade 3 and 0 for grade 4. The relationship of late-arriving retrograde flow proportions of collateral grades in the 21 patients are shown in Figure 3B. Spearman correlation coefficient was 0.81 (95%CI 0.56–0.92) between collateral ASITN/SIR grade 0 to 3 and late-arriving retrograde flow proportion (p < 0.0001). Late-arriving retrograde flow proportion was strongly correlated with ASITN/SIR collateral grade 0 to 3. Figure 4 illustrates a representative patient with conventional angiographic antegrade and collateral scales as well as the CBF maps at two-PLD (see Figure II in the online-only Data Supplement for one more example).
Figure 4.
A 52-year-old male patient with severe left M1 segment stenosis is scored mTICI 2b and ASITN/SIR grade 2 (A and B). Early-arriving flow perfusion proportion is measured as 87.8% and late-arriving retrograde flow perfusion proportion is 13.2%. C and D shows the CBF maps of PLD 1.5 s and 2.5 s
Discussion
There remain no existing imaging modalities to quantify antegrade flow through a stenotic vessel versus the retrograde flow via potential collateral blood vessels in patients with intracranial atherosclerotic stenosis.23 Our study showed significant correlations between early-arriving flow and late-arriving flow on two-PLD pCASL with conventional angiographic antegrade and collateral scales, suggesting that the early-arriving flow and late-arriving retrograde flow to the territory supplied by the stenotic MCA may primarily represent antegrade and collateral flow, respectively.
Antegrade flow assessment is important in predicting the clinical outcome of patients with intracranial arterial stenosis. In studies focusing on acute ischemic stroke, patients with better antegrade mTICI scale typically have an improved 90-day outcome.5, 6 However, this scale is categorical, and there are only a handful studies on using it to predict clinical outcome in patients with chronic intracranial arterial stenosis.24 Our study reveals that antegrade flow may be quantified using two-PLD ASL and that it accounts for a larger proportion of perfusion than collateral flow in patients with unilateral MCA stenosis. This indicates that antegrade flow plays an important role in maintaining perfusion of the target downstream territory in this population. The proposed approach may help establish or define a critical threshold of antegrade perfusion to predict a good clinical outcome in future studies.
Quantification of collateral perfusion is likely as important as antegrade flow. Patients with the same amount of antegrade flow exhibited a varied degree of clinical outcomes, which is likely due to the variation of collateral compensation. Previous studies indicated that ASITN/SIR collateral grade is an independent prognostic factor for patients with intracranial arteries stenosis.7, 24 Since conventional angiography is invasive, alternative approaches and imaging modalities have been applied but rarely used in a routine clinical setting. Quantification of collateral perfusion may help to evaluate the capacity of collateral vessels and to stratify patients at high risk of recurrent ischemic events and patients with high risk of hemorrhage due to hyperperfusion after recanalization. Our study showed that the mean fraction of collateral perfusion was 16.1%±10.2% of total cerebral perfusion in patients with unilateral MCA stenosis using 3D pCASL, which may reflect the extent of cerebral blood flow contributed by collateral vessels.
Multi-PLD ASL has been investigated by a number of studies in both health volunteers and stroke patients. 25–28 It often refers to ASL using more than five PLDs to quantitatively estimate arterial transit time (ATT) and CBF. ATT maps generated using multi-PLD ASL may offer a method to define the optimal PLDs of specific vascular territories. The early-arriving flow and late-arriving retrograde flow proportions acquired from optimal PLDs according to ATT map from multi-PLD ASL may be more accurate than that from two empirical PLDs in present study.
Limitations of our study include a relatively small sample size. Second, because of the complexity of cerebral hemodynamics in patients with artery stenosis, antegrade flow may be slower than normal flow in contralateral side and the transit time of collateral flow may be longer than 2.5s. The ASL signal acquired at the PLD of 1.5s may also contain a small portion from collateral supply. It is therefore challenging to absolutely quantify antegrade flow and separate slow antegrade flow from late-arriving flow completely. Such differentiation may have to utilize vessel-selective ASL. However, considering the moderate to high correlations between the proposed ASL quantification at the two-PLD and DSA mTICI and ASITN/SIR scores in our patient cohort, the formulas proposed in our study may provide an empirical estimation of antegrade and retrograde flow. Third, the temporal parameters including PLD and delayed filling time through the stenotic lesion were based on an empirical definition, as no standards exist. To estimate antegrade perfusion and collateral perfusion in a given individual, time to reach the peak flow of the affected hemisphere and contralateral normal side is essential. Our analyses demonstrated that the PLDs of 1.5s and 2.5s may be rational, considering that it may not be feasible to obtain a reliable CBF map if PLD is longer than 3s with current technology.25 Furthermore, arterial transit artifacts caused by slow blood flow within the vessels may also influence the measurement. Fourth, this quantification method could not provide information about the sources of collateral perfusion. Vessel-selective or vessel-encoded ASL may be used to quantify collateral perfusion in those patients with intracranial stenosis and depict the sources of collateral vessels in future studies.29,30 Finally, we hypothesized that the “ideal” perfusion of affected territory would equal to that of the contralateral normal MCA territory. The hemodynamic status of the normal side may also influence the results, as well (Figure III in the online-only Data Supplement).
Conclusion
Three-dimensional pCASL with two-PLD may provide a useful tool to quantify early-arriving and late-arriving flow in patients with unilateral MCA stenosis and may provide an empirical index of antegrade and collateral flow. The clinical utility of the proposed approach for identifying patients with high risk of recurrent stroke requires evaluation in further studies.
Supplementary Material
Acknowledgments
We thank Dr. Linfeng Zhang, MD, PhD at Department of Epidemiology, the Cardiovascular Institute, Fu Wai Hospital of the Chinese Academy of Medical Sciences and Peking Union Medical College, and the National Center for Cardiovascular Disease Control and Research, Beijing, China, 100037, for his help in statistical analysis.
Sources of Funding
This work was supported by the National Natural Science Foundation of China (Contract grant number: 81471390 and 81371290), Beijing High-level Personnel Funds (Contract grant number: 2013-2-19) and the US National Institutes of Health (Contract grant number: K24-NS072272 and R01-NS081077).
Footnotes
Clinical Trial Registration-URL: http://www.clinicaltrials.gov.
Unique identifier: NCT02479243.
Disclosures
None.
References
- 1.Liebeskind DS. Collateral circulation. Stroke. 2003;34:2279–2284. doi: 10.1161/01.STR.0000086465.41263.06. [DOI] [PubMed] [Google Scholar]
- 2.Higashida RT, Furlan AJ, Roberts H, Tomsick T, Connors B, Barr J, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003;34:e109–137. doi: 10.1161/01.STR.0000082721.62796.09. [DOI] [PubMed] [Google Scholar]
- 3.Bang OY, Saver JL, Kim SJ, Kim GM, Chung CS, Ovbiagele B, et al. Collateral flow averts hemorrhagic transformation after endovascular therapy for acute ischemic stroke. Stroke. 2011;42:2235–2239. doi: 10.1161/STROKEAHA.110.604603. [DOI] [PubMed] [Google Scholar]
- 4.Bang OY, Saver JL, Kim SJ, Kim GM, Chung CS, Ovbiagele B, et al. Collateral flow predicts response to endovascular therapy for acute ischemic stroke. Stroke. 2011;42:693–699. doi: 10.1161/STROKEAHA.110.595256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaidat OO, Suarez JI, Sunshine JL, Tarr RW, Alexander MJ, Smith TP, et al. Thrombolytic therapy of acute ischemic stroke: Correlation of angiographic recanalization with clinical outcome. AJNR Am J Neuroradiol. 2005;26:880–884. [PMC free article] [PubMed] [Google Scholar]
- 6.Fields JD, Lutsep HL, Smith WS, Investigators MMM. Higher degrees of recanalization after mechanical thrombectomy for acute stroke are associated with improved outcome and decreased mortality: Pooled analysis of the merci and multi merci trials. AJNR Am J Neuroradiol. 2011;32:2170–2174. doi: 10.3174/ajnr.A2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liebeskind DS, Cotsonis GA, Saver JL, Lynn MJ, Turan TN, Cloft HJ, et al. Collaterals dramatically alter stroke risk in intracranial atherosclerosis. Ann Neurol. 2011;69:963–974. doi: 10.1002/ana.22354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miteff F, Levi CR, Bateman GA, Spratt N, McElduff P, Parsons MW. The independent predictive utility of computed tomography angiographic collateral status in acute ischaemic stroke. Brain. 2009;132:2231–2238. doi: 10.1093/brain/awp155. [DOI] [PubMed] [Google Scholar]
- 9.Zaidat OO, Yoo AJ, Khatri P, Tomsick TA, von Kummer R, Saver JL, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: A consensus statement. Stroke. 2013;44:2650–2663. doi: 10.1161/STROKEAHA.113.001972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shuaib A, Butcher K, Mohammad AA, Saqqur M, Liebeskind DS. Collateral blood vessels in acute ischaemic stroke: A potential therapeutic target. The Lancet Neurology. 2011;10:909–921. doi: 10.1016/S1474-4422(11)70195-8. [DOI] [PubMed] [Google Scholar]
- 11.Bokkers RP, van Laar PJ, van de Ven KC, Kapelle LJ, Klijn CJ, Hendrikse J. Arterial spin-labeling MR imaging measurements of timing parameters in patients with a carotid artery occlusion. AJNR Am J Neuroradiol. 2008;29:1698–1703. doi: 10.3174/ajnr.A1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu G, Rowley HA, Wu G, Alsop DC, Shankaranarayanan A, Dowling M, et al. Reliability and precision of pseudo-continuous arterial spin labeling perfusion MRI on 3.0 t and comparison with 15o-water pet in elderly subjects at risk for alzheimer’s disease. Nmr Biomed. 2010;23:286–293. doi: 10.1002/nbm.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gevers S, van Osch MJ, Bokkers RP, Kies DA, Teeuwisse WM, Majoie CB, et al. Intra- and multicenter reproducibility of pulsed, continuous and pseudo-continuous arterial spin labeling methods for measuring cerebral perfusion. J Cereb Blood Flow Metab. 2011;31:1706–1715. doi: 10.1038/jcbfm.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang D, Wu B, Shi K, Ma L, Cai Y, Lou X. Reliability of three-dimensional pseudo-continuous arterial spin labeling MR imaging for measuring visual cortex perfusion on two 3t scanners. PLoS One. 2013;8:e79471. doi: 10.1371/journal.pone.0079471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng XQ, Tian JM, Zuo CJ, Liu J, Zhang Q, Lu GM. Quantitative perfusion computed tomography measurements of cerebral hemodynamics: Correlation with digital subtraction angiography identified primary and secondary cerebral collaterals in internal carotid artery occlusive disease. Eur J Radiol. 2012;81:1224–1230. doi: 10.1016/j.ejrad.2011.02.046. [DOI] [PubMed] [Google Scholar]
- 16.Cao W, Campbell B, Dong Q, Davis S, Yan B. Relative filling time delay based on ct perfusion source imaging: A simple method to predict outcome in acute ischemic stroke. AJNR Am J Neuroradiol. 2014;35:1683–1687. doi: 10.3174/ajnr.A3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaharchuk G, Do HM, Marks MP, Rosenberg J, Moseley ME, Steinberg GK. Arterial spin-labeling MRI can identify the presence and intensity of collateral perfusion in patients with moyamoya disease. Stroke. 2011;42:2485–2491. doi: 10.1161/STROKEAHA.111.616466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma N, Jiang WJ, Lou X, Ma L, Du B, Cai JF, et al. Arterial remodeling of advanced basilar atherosclerosis: A 3-tesla MRI study. Neurology. 2010;75:253–258. doi: 10.1212/WNL.0b013e3181e8e714. [DOI] [PubMed] [Google Scholar]
- 19.Nowinski WL, Qian G, Kirgaval Nagaraja BP, Thirunavuukarasuu A, Hu Q, Ivanov N, et al. Analysis of ischemic stroke MR images by means of brain atlases of anatomy and blood supply territories. Acad Radiol. 2006;13:1025–1034. doi: 10.1016/j.acra.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Kim SJ, Kim IJ, Kim YK, Lee TH, Lee JS, Jun S, et al. Probabilistic anatomic mapping of cerebral blood flow distribution of the middle cerebral artery. J Nucl Med. 2008;49:39–43. doi: 10.2967/jnumed.107.045724. [DOI] [PubMed] [Google Scholar]
- 21.Tatu L, Moulin T, Bogousslavsky J, Duvernoy H. Arterial territories of the human brain cerebral hemispheres. Neurology. 1998;50:1699–1708. doi: 10.1212/wnl.50.6.1699. [DOI] [PubMed] [Google Scholar]
- 22.Yoo AJ, Simonsen CZ, Prabhakaran S, Chaudhry ZA, Issa MA, Fugate JE, et al. Refining angiographic biomarkers of revascularization: Improving outcome prediction after intra-arterial therapy. Stroke. 2013;44:2509–2512. doi: 10.1161/STROKEAHA.113.001990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McVerry F, Liebeskind DS, Muir KW. Systematic review of methods for assessing leptomeningeal collateral flow. AJNR Am J Neuroradiol. 2012;33:576–582. doi: 10.3174/ajnr.A2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liebeskind DS, Cotsonis GA, Saver JL, Lynn MJ, Cloft HJ, Chimowitz MI, et al. Collateral circulation in symptomatic intracranial atherosclerosis. J Cereb Blood Flow Metab. 2011;31:1293–1301. doi: 10.1038/jcbfm.2010.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang DJ, Alger JR, Qiao JX, Gunther M, Pope WB, Saver JL, et al. Multi-delay multi-parametric arterial spin-labeled perfusion MRI in acute ischemic stroke - comparison with dynamic susceptibility contrast enhanced perfusion imaging. Neuroimage Clin. 2013;3:1–7. doi: 10.1016/j.nicl.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dai W, Robson PM, Shankaranarayanan A, Alsop DC. Reduced resolution transit delay prescan for quantitative continuous arterial spin labeling perfusion imaging. Magn Reson Med. 2012;67:1252–1265. doi: 10.1002/mrm.23103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mezue M, Segerdahl AR, Okell TW, Chappell MA, Kelly ME, Tracey I. Optimization and reliability of multiple postlabeling delay pseudo-continuous arterial spin labeling during rest and stimulus-induced functional task activation. J Cereb Blood Flow Metab. 2014;34:1919–1927. doi: 10.1038/jcbfm.2014.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin Q, Huang AJ, Hua J, Desmond JE, Stevens RD, van Zijl PC. Three-dimensional whole-brain perfusion quantification using pseudo-continuous arterial spin labeling MRI at multiple post-labeling delays: Accounting for both arterial transit time and impulse response function. NMR Biomed. 2014;27:116–128. doi: 10.1002/nbm.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu B, Wang X, Guo J, Xie S, Wong EC, Zhang J, et al. Collateral circulation imaging: MR perfusion territory arterial spin-labeling at 3t. AJNR Am J Neuroradiol. 2008;29:1855–1860. doi: 10.3174/ajnr.A1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chng SM, Petersen ET, Zimine I, Sitoh YY, Lim CC, Golay X. Territorial arterial spin labeling in the assessment of collateral circulation: Comparison with digital subtraction angiography. Stroke. 2008;39:3248–3254. doi: 10.1161/STROKEAHA.108.520593. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.