Abstract
Background and Aims
Recognition of abnormal glycosylation in virtually every cancer type has raised great interest in exploration of the tumor glycome for biomarker discovery. Identifying glycan markers of circulating tumor cells (CTCs) represents a new development in tumor biomarker discovery. The aim of this study was to establish an experimental approach to enable rapid screening of CTCs for glycan marker identification and characterization.
Methods
We applied carbohydrate microarrays and a high-speed fiber-optic array scanning technology (FAST scan) to explore potential glycan markers of breast CTCs (bCTCs) and targeting antibodies. An anti-tumor monoclonal antibody, HAE3-C1 (C1), was identified as a key immunological probe in this study.
Results
In our carbohydrate microarray analysis, C1 was found to be highly specific for an O-glycan cryptic epitope, gpC1. Using FAST-scan technology, we established a procedure to quantify expression levels of gpC1 in tumor cells. In blood samples from five stage IV metastatic breast cancer patients, the gpC1 positive CTCs were detected in all subjects; ~40% of bCTCs were strongly gpC1 positive. Interestingly, CTCs from a triple-negative breast cancer patient with multiple sites of metastasis were predominantly gpC1 positive (92.5%, 37/40 CTCs).
Conclusions
Together we present here a practical approach to examine rare cell expression of glycan markers. Using this approach, we identified an O-core glyco-determinant gpC1 as a potential immunological target of bCTCs. Given its bCTC-expression profile, this target warrants an extended investigation in a larger cohort of breast cancer patients.
Keywords: Carbohydrate microarrays, O-glycan cryptic antigens, Circulating tumor cells, Metastatic breast cancer, Tumor immunotyping
Introduction
Breast cancer (BCa) is among the most prevalent cancers and accounts for the highest number of cancer-related deaths among women worldwide. Identifying biomarkers of immunological significance is important in developing precision diagnostic and therapeutic strategies to advance current BCa healthcare (1). Recognition of abnormal glycosylation in virtually every cancer type has raised great interest in exploration of the tumor glycome for biomarker discovery (2–5). Potential glycan markers of BCa identified may include, but are not limited to, mucin-1 (CA 15-3) (6), carcinoembryonic antigen (CEA) (7), sialyl Lewis x (sLex/CD15s) (8,9), and glycoforms of a number of serum acute phase proteins such as α1-acid glycoprotein, α1-antichymotrypsin, and haptoglobin β-chain (10). Because carbohydrate moieties are often surface-exposed and easily accessible by antibodies, some targets have been employed for antibody therapeutics (11–14). Exploring glycan markers of breast circulating tumor cells (bCTCs) represents a new development in tumor biomarker discovery. Although bCTCs are rare in blood, they play a key role in tumor metastasis (15,16). Detection of CTCs has been explored as a non-invasive “liquid biopsy” for tumor diagnosis and prognosis (17–19). Glycan markers of bCTCs may have unique value in BCa healthcare, especially in the personalized therapy that targets specific immunotypes of BCa. Thus, our team has worked to identify potential glycan markers of bCTCs (20).
A key immunological probe of this investigation is an anti-tumor glycan monoclonal antibody (mAb), HAE3. This antibody was raised by a murine mammary tumor antigen epiglycanin (EPGN) (21) but was found to cross-react with a number of human epithelial tumors in tissues including the lung, prostate, bladder, esophagus, and ovaries (22–24). In a recent flow-cytometry-based screening for tumor cell surface biomarkers, we found that HAE3 also strongly cross-reacts with human breast tumors (20).
This striking cross-species tumor-binding profile suggests the possibility that HAE3 may recognize a conserved tumor glycan marker that is co-expressed by both mouse- and human-derived epithelial cancers. We therefore explored the potential natural ligands of HAE3 in the repertoire of carbohydrate-based autoantigens. By scanning a large collection of carbohydrate antigens using carbohydrate microarrays, we found that HAE3 is highly specific for a blood group precursor cryptic epitope normally hidden in the cores or internal chains of blood group substances (20). In this study, we further investigated whether this target is applicable for detection and immunotyping analysis of CTCs in patients with metastatic breast cancer. To ensure the observed cross-species antigenic reactivities are not owing to the unexpected presence of oligoclonal populations in the original HAE3 hybridoma cell line, we further subcloned HAE3 and produced antibody from a single clone, HAE3-C1 (C1), for this study. As summarized below, antibody C1 was verified by carbohydrate microarrays and a glycan-specific enzyme-linked immunosorbent assay (ELISA) to be highly specific for a conserved O-glycan cryptic glyco-epitope gpC1 in human blood group precursors. With this key reagent, we further established a FAST-scan-based method for monitoring gpC1 expression in bCTCs.
Materials and Methods
Patient Samples
CTCs analyzed were from patients undergoing treatment for metastatic breast cancer at the City of Hope Cancer Center. Blood samples were collected and used under protocols approved by the Institutional Review Boards of the City of Hope Cancer Center, Palo Alto Research Center, and SRI International. All patients gave their written, voluntary, informed consent (www.clinicaltrials.gov: NCT01048918 and NCT00295893). Patient demographics and clinical characteristics are described in the results section.
Carbohydrate Antigens, Anti-glycan Antibodies, and Tumor Cell Lines
Carbohydrate antigens for carbohydrate microarray analysis are listed in Supplementary Table 1. Antibody C1 (IgM) was produced in this study by cell line HAE3-C1, which is a subclone of the parent murine hybridoma, HAE3 (22). Tumor cell lines used include lung (A549)- and breast (T47D and SKBR3)-derived epithelial tumor cell lines. Both T47D and SKBR3 were derived from metastatic sites in breast cancer patients. All tumor cell lines were acquired from the American Type Culture Collection (ATCC), Manassas, VA.
Carbohydrate Microarrays and ELISA
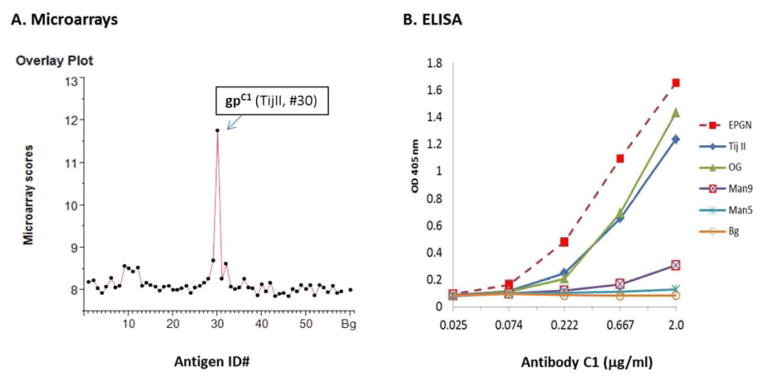
Carbohydrate antigens of various structural compositions were dissolved in phosphate-buffered saline (PBS) (glycoprotein conjugates) or saline (polysaccharides) and spotted onto SuperEpoxy 2 Protein slides (ArrayIt Corporation, Sunnyvale, CA) by a high-precision robot designed to produce cDNA microarrays (Cartesian Technologies PIXSYS 5500C). Immediately before use, the printed microarray slides were washed in 1X PBS at room temperature for 5 min and blocked with 1% bovine serum albumin (BSA)-PBS at room temperature for 30 min. They were then incubated at room temperature with C1 (IgM) antibody at 5.0 μg/mL in 1% (wt/vol) BSA in PBS containing 0.05% (wt/vol) NaN3 and 0.05% (vol/vol) Tween 20. An R-phycoerythrin (R-PE)-conjugated affinity-purified F(ab′) fragment of goat anti-mouse IgM secondary antibody preparation (Rockland Immunochemicals, Inc., Limerick, PA) was applied at 2.0 μg/mL to reveal the C1-specific staining signal. The stained slides were rinsed five times with PBS with 0.05% (vol/vol) Tween 20, air-dried at room temperature, and then scanned for fluorescent signal using a ScanArray5000A Microarray Scanner (PerkinElmer Life Science). The SAS Institute JMP-Genomics software package (http://www.jmp.com/) was used for further microarray data standardization and statistical analysis. Results of the microarray assay are shown as microarray scores, i.e., means of the log2-transformed fluorescent intensities (MFIs) of multiple detections of a given antigen preparation (Figure 1A and Supplementary Table 1). Glycan-specific ELISA was performed as described in Figure 1 legend following our standard protocol (20).
Figure 1.
Carbohydrate microarrays and glycan-specific ELISA verified C1-binding of blood group precursor O-cores autoantigens. A. Carbohydrate microarrays: The customized microarrays of 58 features were applied to capture glycan-binding profiles of C1. Results were shown as an overlay plot of microarray scores of one representative assay. Each score is the Log2-transformed mean fluorescent intensities (MFIs) of triplicate detections of a given antigen preparation. Microarray dataset of two microarray assays were summarized and presented in Supplementary Table 1. B. Glycan-specific ELISA: ELISA plates were coated with carbohydrate antigens at 10.0 μg/mL and incubated with C1 at specified concentrations. The bound C1 antibody was revealed by an alkaline phosphatase- (AP-) conjugated goat anti-mouse IgM (Sigma Chemical Co., St. Louis, MO). ELISA results were plotted as C1 binding curves with corresponding antigens, including the immunogen EPGN (Egenix, Millbrook, NY), blood group precursors, TijII and OG, and oligomannoses, Man9 and Man5. ELISA data shown here are representative results of multiple assays.
Fiberoptic Array Scanning Technology (FAST Scan)
An established procedure for multiple marker measurement using the FAST scan (17,25,26) was followed with minor modifications to cover glycan marker detection. In brief, 8 mL samples of blood from patients with stage IV breast cancer were drawn into Cell Free DNA tubes (Streck Inc., Omaha, NE) shipped overnight at room temperature and processed within 24 hr. Following erythrocyte lysis, the cell pellet was washed, resuspended in PBS, and plated on custom-designed (12.7 × 7.6 cm slide with an active area of 63.68 cm2) adhesive glass substrates (Paul Marienfeld GmbH & Co., KG, Bad Mergentheim, Germany) at a density of up to 25 million cells per slide. The slides were incubated for 40 min at 37°C and 95% humidity to allow cell attachment and were then processed with a cocktail of antibody staining reagents for the desired cell markers. Primary antibodies used in this study include: a) C1 (IgM) for detection of the O-core crptic epitope gpC1; b) a mouse anti-human CD45 IgG1 (MCA87, AbD Serotec, Raleigh, NC) directly conjugated with Qdot 800 (Invitrogen custom conjugation, www.lifetechnologies.com) for labeling of white blood cells; and c) a preparation of mouse anti-cytokeratin (CK) IgG1 antibodies (C2562, Sigma; RCK108, DAKO) for identification of epithelial-derived cells in circulation. A biotinylated goat anti-mouse IgG1 (A10519, Invitrogen) and a Streptavidin-Alexa555 conjugate (S-32355, Invitrogen) were applied in combination to reveal CK+ CTCs. The R-PE-tagged goat anti-mouse IgM preparation described in microarray assay was applied to reveal C1-specific glycan marker staining. Nuclear counterstaining was done using 4′,6-diamidino-2-phenylindole (DAPI) (D-21490, Invitrogen) at 0.5 μg/mL. CTCs were identified by their morphology and immuno-phenotype (CK+, CD45−, and DAPI+), expression of the cryptic O-glycan epitopes was scored using the C1 staining, and the FAST-scan-located CTCs were imaged by an automated digital microscopy.
Results
Antibody C1 recognizes a conserved O-core cryptic epitope in murine and human tumor glycomes. We performed a carbohydrate microarray analysis to determine the antigen-binding specificity and potential cross-reactivities of antibody C1. Figure 1A illustrates its glycan-binding profiles as overlay plots of microarray scores. The corresponding microarray datasets are listed in Supplementary Table 1. As expected, C1 is highly specific for a blood group precursor antigen, Tij II 20%fr. 2nd 10% (TijII, ID#30) (27–29), which is one of the natural ligands recognized by its parent antibody HAE3 (20). Nevertheless, C1 also illustrates weak but significant binding to Man9GlcNAc2Asn-BSA (Man9) (#12) and αGal-BSA (#32), which were spotted in the same microarrays.
Using glycan-specific ELISA, we compared the C1-binding curves for five selected antigens. These include the murine tumor immunogen, EPGN, two human blood group precursor substances, TijII and OG 10% 2X (OG) (30,31), and two synthetic oligomannose antigens, Man9 and Man5GlcNAc2Asn-BSA (Man5) (32). Each antigen was coated on ELISA plates at a constant concentration to react with antibody C1 in a series of 1:3 dilutions with an initial concentration of 2.0 μg/mL. Such an assay design is practical for measuring the relative antibody binding affinities against different antigens.
In Figure 1B, C1 binding curves were produced by plotting ELISA values (y axis) against antibody concentrations (x axis). As illustrated, C1 is highly reactive with EPGN, TijII, and OG; the three binding curves are nearly linear in the range of antibody concentrations from 0.2–2.0 μg/mL. Thus, the gpC1-epitope is well preserved in the two protein-free human blood group precursor substances in addition to the native murine glycoprotein immunogen. The weak C1-binding of Man9 observed in microarrays was also reproduced in the ELISA. However, C1 is negative with Man5, which differs from Man9 in that it lacks the terminal Manα1,2M.
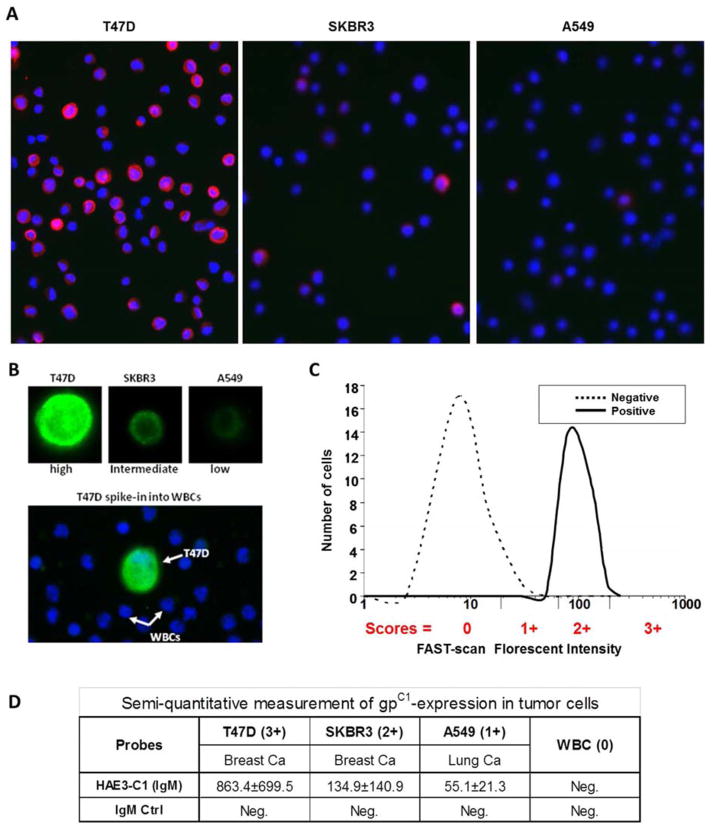
Quantifying the Levels of gpC1 Expression in Tumor Cells
We applied a semiquantitative scoring method to measure the relative expression levels of a glycan marker at the single-cell level. Conceptually, the marker expression level of a targeted cell is scored relative to a moderate expressing cell line during antibody staining of target cells, such as bCTCs in blood circulation. To identify a suitable standard control cell line, we scanned a panel of tumor cell lines to identify those that express different levels of gpC1 glycan marker. As shown in Figures 2A and 2B, T47D (breast), SKBR3 (breast), and A549 (lung) were found to be gpC1 positive (strong, intermediate, and weak, respectively). Figure 2C is a schematic of this scoring method. In essence, the cells with an expression level within the 34th quantile of the median of the standard control cell line are scored a 2. In this case, SKBR3 was identified as the intermediate to serve as a control for scoring the levels of gpC1 expression. Cells expressing higher levels than SKBR3 are scored a 3, and cells with expression levels lower than SKBR3 but higher than background are scored a 1. Figure 2D shows the intensity values of three tumor cell lines captured by FAST scan and the assigned gpC1 scores based on the intensities of gpC1 expression.
Figure 2.
Monitoring differential expression of gpC1 markers in human tumor cells by FAST-scan technology. (A) Three tumor cell lines, including two breast cancer lines (T47D and SKBR3) and a lung cancer line (A549), were stained with antibody C1 (IgM) at 2.0 μg/mL. The gpC1-specific signal was revealed by an R-PE-conjugated goat anti-mouse IgM antibody and the images captured by the FAST-scan technology (gpC1 in red, DAPI in blue, 200X). (B) The tumor cell line was spiked into white blood cells (WBCs) to examine whether C1 specifically detects the gpC1-positive tumor cells in the background of human WBC. An Alexa555-conjugated goat anti-mouse IgM secondary antibody was used to quantify the cell surface-captured IgM-C1 antibody. The stained slides were mounted with ProLong Gold antifade containing DAPI for cell nuclear visualization. T47D, SKBR3, and A549 were identified as gpC1-high, -intermediate, and -low, respectively. (C) A schematic of the scoring method based on the florescent intensities of C1-stained cells. (D) FAST-scan values of gpC1-expression in three tumor cell lines and the assigned scores.
Detection of gpC1-Positive bCTCs in Stage IV Breast Cancer Patients
We further examined bCTC expression of gpC1 in breast cancer patients using the FAST-scan technology. In this pilot clinical case study, we characterized blood samples from five stage IV breast cancer patients. Figure 3A illustrates how CTCs captured from the stage IV breast cancer patients were scored as 3+, 2+, 1+, and 0, left to right. Four representative bCTCs are shown in which the epithelial-derived cells were labeled by anti-CK antibodies in red, and the gpC1 positive cells were stained in green in the background of the DAPI-blue labeling of white blood cells. Figures 3B and 3C show that all subjects characterized had gpC1-positive CTCs. Approximately 40% of CTCs captured in these patients expressed higher levels (2+ and 3+) of the gpC1 markers; gpC1-positive and -negative CTCs were found to co-exist in four subjects. Notably, a triple-negative patient (ID# 189370) produced predominantly gpC1-positive CTCs (37 of 40 CTCs) with 50% scored gpC1 2+/3+. In this patient, metastatic tumors were seen in multiple sites including bone, liver, and skin.
Figure 3.
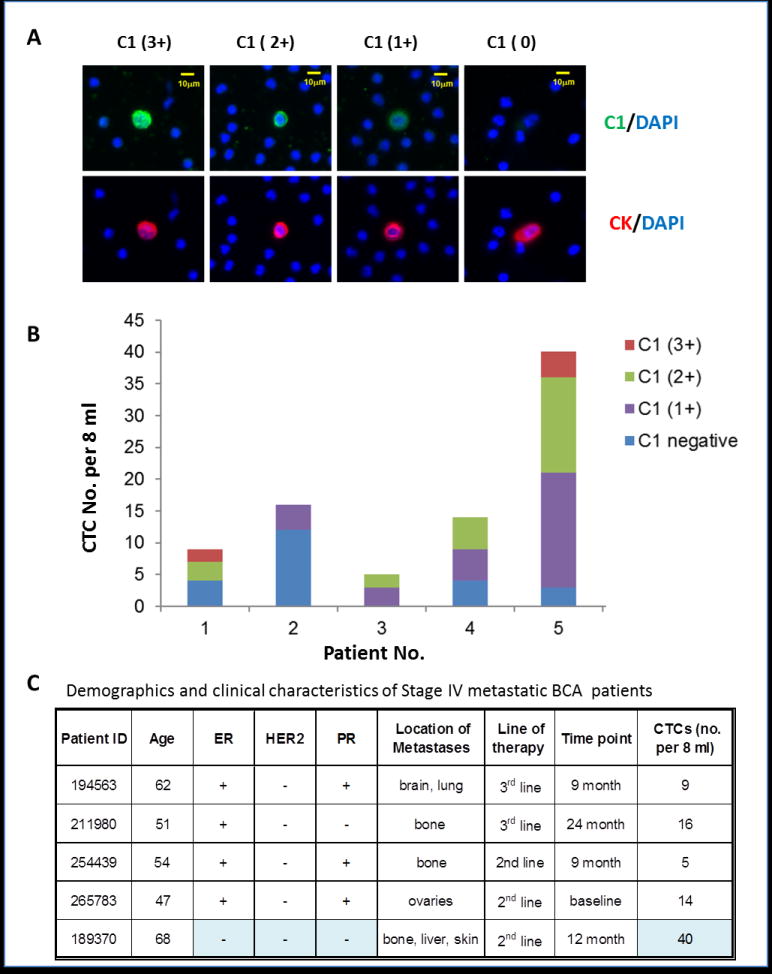
Glycan marker gpC1 is expressed in significant numbers of CTCs in stage IV breast cancer patients. (A) FAST-scan images of bCTCs. Upper Panels: Co-staining of C1 (green) and DAPI (blue); Bottom panels: co-staining of anti-CK (red) and DAPI (blue). (B) Distribution of gpC1-positive and -negative bCTCs in five subjects. C1-staining of bCTCs was semiquantitatively measured by the FAST scan as antibody negative (blue), 1+ (purple), 2+ (green), and 3+ (red) as described. A patient with triple-negative BCa (ID# 189370) was measured gpC1-positive in 37 of 40 CTCs with 50% scored as strong positive (2+ and 3+). (ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor 2). (C) A summary of patients’ demographics and clinical characteristics.
Discussion
Although tumor-associated abnormal glycosylation has been recognized for years (2,3), identifying glycan markers of CTCs is technically challenging. We describe here a practical approach to overcome this difficulty. In essence, we integrated the use of carbohydrate microarrays and FAST-scan technologies to explore potential glycan markers of CTCs and targeting antibodies. Conceptually, we take advantage of the fact that the immune systems of many animal species are able to recognize subtle changes in sugar moieties displayed by cells or soluble antigens and produce specific antibodies for abnormally expressed tumor glycan markers. Experimentally, we first screened anti-tumor mAbs using carbohydrate microarrays to identify those that are specific tumor glycan markers. Subsequently, we determined whether the selected mAbs are specific for the cell-surface glycan markers using flow cytometry and FAST-scan technology. Finally, we used the new probe to monitor CTC-expression of corresponding glycan markers in advanced breast cancer patients. This approach is likely to be useful for exploring potential glycan markers of other cancers.
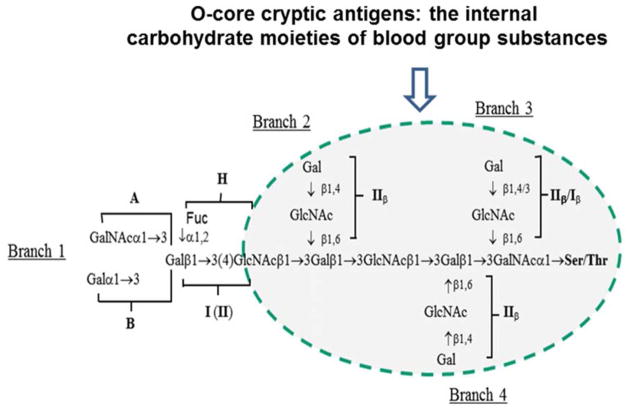
Given that our recent study has identified blood-group precursor substances as the natural ligands of HAE3 (20), we verified whether the HAE3-C1 subclone is specific for the blood-group precursor epitope. As shown in Figure 1, C1 is highly reactive with two blood group precursor reference reagents, TijII (27–29) and OG (30,31). These reference antigens were purified from ovarian cyst fluids after multiple steps of treatments that resulted in elimination or reduction of the A, B, H, and Lea/b-terminal sugar moieties. Both antigens were prepared by pepsin digestion, ethanol precipitation, and solubilization in 90% phenol followed by fractional ethanol precipitation from phenol. After these treatments, they are devoid of A, B, H, or Lea/b activities but preserve their O-glycan core structures, leaving a number of cryptic O-core epitopes exposed for antibody recognition. Figure 4 is a schematic of the conserved cryptic O-cores of blood group precursor substances, such as TijII and OG. In essence, these are polylactosamine chains with branches of Galβ1,4/3GlcNAcβ1,6(IIβ/Iβ)-moieties without the additional fucosylation essential for forming blood group A, B, H, or Le antigens. Antibody C1 was weakly but significantly cross-reactive with oligomannose antigen Man9 in both carbohydrate microarrays (Supplementary Table 1) and the glycan-specific ELISA (Figure 1). This weak cross-reactivity may be attributed to its recognition of terminal Manα1,2Man-moiety of Man9, which is not present in the C1-negative Man5. The latter displays Manα1,3Man and Manα1,6Man moieties at its terminals. Maisonrouge-Mcauliffe and Kabat (28) also observed that a mannose-specific lectin Concanavalin A was specifically precipitated by the gpC1-positive blood-group precursor TijII in quantitative precipitin assays. Nevertheless, TijII is free of mannose residue. Thus, it is not impossible that the conventional antibody binding sites of C1 are able to accommodate both O-core gpC1 and Manα1,2Man-moiety of Man9 with different binding affinities.
Figure 4.
A schematic of the conserved O-core cryptic antigens in blood group substances. As illustrated, blood group precursors are characteristically composed of polylactosamine chains with branches of IIβ (Galβ1,4GlcNAcβ1,6) or Iβ(Galβ1,3GlcNAcβ1,6)-moieties without fucosylation. The latter is essential for forming blood group A, B, H, or Le antigens.
In our carbohydrate microarray analysis (Supplementary Table 1), C1 was also found to be weakly reactive with αGal disaccharide (Galα1,3Galβ1)-BSA, which displays a terminal Gal-residue just as O-core branches (Galβ1,4/3GlcNAcβ1,6-) do. However, the adjacent carbohydrate moiety of αGal differs from the conserved branches of blood group precursors (Figure 4). Thus, antibody C1 may recognize a more complex glyco-epitope that requires both terminal Gal residue and adjacent sugar moieties for higher-affinity binding by C1. The exact molecular composition of the gpC1 epitope displayed by bCTCs and metastatic breast tumor cell lines remains to be further characterized. Identifying antibody C1 and the breast tumor cell lines that express high levels of gpC1 will pave the way for this line of study.
We conducted a pilot study to evaluate the feasibility of translating this finding for clinical application. Using FAST-scan technology, we characterized five cases of stage IV metastatic breast cancer using antibody C1. We found that: a) ~40% of the bCTCs detected in five stage IV breast cancer patients were C1 strongly positive; b) gpC1-positive and -negative CTCs were found to co-exist in four of the five subjects; and c) in a triple-negative (ER−/HER2−/PR−) breast cancer patient with multiple sites of metastasis, CTCs were predominantly gpC1-positive (92.5%, 37/40 CTCs). In our recent flow cytometry analysis, we also determined cell-surface expression of gpC1 by two of four analyzed triple-negative BCa lines, BT-549 and MDA-MB-468 (20). Taken together, we consider that the gpC1 tumor glycan marker warrants an extended investigation in a larger cohort of breast cancer patients.
It is noteworthy that more than one million global cases of BCa are diagnosed each year and ~15% are triple negative. Owing to the lack of an effective therapeutic target, a younger age at onset, and early metastatic spread, these patients often have poor prognoses and clinical outcomes (33,34). If gpC1 were confirmed to be significantly associated with the triple-negative BCa in a larger cohort validation study, this O-core cryptic glycan marker could be used for immunotype-enhanced precision diagnosis and prognosis of BCa and targeted immunotherapy against metastatic cancer. Our laboratory is currently engaged in producing and characterizing humanized anti-gpC1 antibodies.
Supplementary Material
Acknowledgments
The authors thank Zeqi (Joe) Zhou and John L. Daiss for valuable discussion; Barbara Sato, Janey Ly, and Jing Tang for technical assistance; and Lai-Xi Wang and the Kabat Collection of Carbohydrate Antigens at SRI International for a number of carbohydrate antigens that were applied in this study.
This work was supported in part by NIH grants U01CA128416 (DW), R56AI108388 (DW), and CA111359 (GS and RB), and by SRI International IR&D funds (DW, XL, and LS).
Footnotes
Conflict of interest
There are no conflicts of interest to declare for any of the authors involved in this work.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chatterjee SK, Zetter BR. Cancer biomarkers: knowing the present and predicting the future. Future Oncol. 2005;1:37–50. doi: 10.1517/14796694.1.1.37. [DOI] [PubMed] [Google Scholar]
- 2.Hakomori S. Aberrant glycosylation in tumors and tumor-associated carbohydrate antigens. Adv Cancer Res. 1989;52:257–331. doi: 10.1016/s0065-230x(08)60215-8. [DOI] [PubMed] [Google Scholar]
- 3.Hakomori S. Tumor-associated carbohydrate antigens defining tumor malignancy: basis for development of anti-cancer vaccines. Adv Exp Med Biol. 2001;491:369–402. doi: 10.1007/978-1-4615-1267-7_24. [DOI] [PubMed] [Google Scholar]
- 4.Fukuda M. Possible roles of tumor-associated carbohydrate antigens. Cancer Res. 1996;56:2237–2244. [PubMed] [Google Scholar]
- 5.Dube DH, Bertozzi CR. Glycans in cancer and inflammation--potential for therapeutics and diagnostics. Nat Rev Drug Discov. 2005;4:477–488. doi: 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]
- 6.Persson J, Backstrom M, Johansson H, et al. Molecular evolution of specific human antibody against MUC1 mucin results in improved recognition of the antigen on tumor cells. Tumour Biol. 2009;30:221–231. doi: 10.1159/000240634. [DOI] [PubMed] [Google Scholar]
- 7.Haidopoulos D, Konstadoulakis MM, Antonakis PT, et al. Circulating anti-CEA antibodies in the sera of patients with breast cancer. Eur J Surg Oncol. 2000;26:742–746. doi: 10.1053/ejso.2000.0996. [DOI] [PubMed] [Google Scholar]
- 8.Renkonen J, Paavonen T, Renkonen R. Endothelial and epithelial expression of sialyl Lewis(x) and sialyl Lewis(a) in lesions of breast carcinoma. Int J Cancer. 1997;74:296–300. doi: 10.1002/(sici)1097-0215(19970620)74:3<296::aid-ijc11>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 9.Nakagoe T, Fukushima K, Itoyanagi N, et al. Expression of ABH/Lewis-related antigens as prognostic factors in patients with breast cancer. J Cancer Res Clin Oncol. 2002;128:257–264. doi: 10.1007/s00432-002-0334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abd Hamid UM, Royle L, Saldova R, et al. A strategy to reveal potential glycan markers from serum glycoproteins associated with breast cancer progression. Glycobiology. 2008;18:1105–1118. doi: 10.1093/glycob/cwn095. [DOI] [PubMed] [Google Scholar]
- 11.Vassilaros S, Tsibanis A, Tsikkinis A, et al. Up to 15-year clinical follow-up of a pilot Phase III immunotherapy study in stage II breast cancer patients using oxidized mannan-MUC1. Immunotherapy. 2013;5:1177–1182. doi: 10.2217/imt.13.126. [DOI] [PubMed] [Google Scholar]
- 12.Apostolopoulos V, Pietersz GA, Tsibanis A, et al. Pilot phase III immunotherapy study in early-stage breast cancer patients using oxidized mannan-MUC1 [ISRCTN71711835] Breast Cancer Res. 2006;8:R27. doi: 10.1186/bcr1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shibata S, Raubitschek A, Leong L, et al. A phase I study of a combination of yttrium-90-labeled anti-carcinoembryonic antigen (CEA) antibody and gemcitabine in patients with CEA-producing advanced malignancies. Clin Cancer Res. 2009;15:2935–2941. doi: 10.1158/1078-0432.CCR-08-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomlinson IP, Whyman A, Barrett JA, et al. Tumour marker CA15-3: possible uses in the routine management of breast cancer. Eur J Cancer. 1995;31A:899–902. doi: 10.1016/0959-8049(94)00447-1. [DOI] [PubMed] [Google Scholar]
- 15.Jacob K, Sollier C, Jabado N. Circulating tumor cells: detection, molecular profiling and future prospects. Expert Rev Proteomics. 2007;4:741–756. doi: 10.1586/14789450.4.6.741. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi N, Yamauchi H. Role of circulating tumor cells and disseminated tumor cells in primary breast cancer. Breast Cancer. 2012;19:110–117. doi: 10.1007/s12282-011-0282-5. [DOI] [PubMed] [Google Scholar]
- 17.Somlo G, Lau SK, Frankel P, et al. Multiple biomarker expression on circulating tumor cells in comparison to tumor tissues from primary and metastatic sites in patients with locally advanced/inflammatory, and stage IV breast cancer, using a novel detection technology. Breast Cancer Res Treat. 2011;128:155–163. doi: 10.1007/s10549-011-1508-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Das M, Riess JW, Frankel P, et al. ERCC1 expression in circulating tumor cells (CTCs) using a novel detection platform correlates with progression-free survival (PFS) in patients with metastatic non-small-cell lung cancer (NSCLC) receiving platinum chemotherapy. Lung Cancer. 2012;77:421–426. doi: 10.1016/j.lungcan.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Liu X, Hsieh HB, Campana D, et al. A new method for high speed, sensitive detection of minimal residual disease. Cytometry A. 2012;81:169–175. doi: 10.1002/cyto.a.21124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang D, Tang J, Liu S, et al. Blood group precursor cryptic glyco-epitopes as breast cancer cell surface markers for immune recognition and targeting. J Immunol Res. 2015:Article ID 510810, 9. doi: 10.1155/2015/510810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Codington JF, Sanford BH, Jeanloz RW. Glycoprotein coat of the TA3 cell. Isolation and partial characterization of a sialic acid containing glycoprotein fraction. Biochemistry. 1972;11:2559–2564. doi: 10.1021/bi00764a001. [DOI] [PubMed] [Google Scholar]
- 22.Li R, Yao JL, Bourne PA, et al. Frequent expression of human carcinoma-associated antigen, a mucin-type glycoprotein, in cells of prostatic carcinoma. Arch Pathol Lab Med. 2004;128:1412–1417. doi: 10.5858/2004-128-1412-FEOHCA. [DOI] [PubMed] [Google Scholar]
- 23.Liang S, Yao J, Bourne PA, et al. Overexpression of human carcinoma-associated antigen in esophageal adenocarcinoma and its precursor lesions. Am J Clin Pathol. 2004;122:747–751. doi: 10.1309/02AP-A6AP-GL43-GCTR. [DOI] [PubMed] [Google Scholar]
- 24.Yao JL, Bourne PA, Yang Q, et al. Overexpression of human carcinoma-associated antigen in urothelial carcinoma of the bladder. Arch Pathol Lab Med. 2004;128:785–787. doi: 10.5858/2004-128-785-OOHCAI. [DOI] [PubMed] [Google Scholar]
- 25.Krivacic RT, Ladanyi A, Curry DN, et al. A rare-cell detector for cancer. Proc Natl Acad Sci USA. 2004;101:10501–10504. doi: 10.1073/pnas.0404036101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsieh HB, Marrinucci D, Bethel K, et al. High speed detection of circulating tumor cells. Biosens Bioelectron. 2006;21:1893–1899. doi: 10.1016/j.bios.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 27.Maisonrouge McAuliffe F, Kabat EA. Immunological studies on blood groups. Structures and immunochemical properties of oligosaccharides from two fractions of blood group substance from ovarian cyst fluid differeing in B, I, and i activities and reactivity toward Concanavalin A. Arch Biochem Biophys. 1976;175:90–113. doi: 10.1016/0003-9861(76)90488-4. [DOI] [PubMed] [Google Scholar]
- 28.Maisonrouge-McAuliffe F, Kabat EA. Immunochemical studies on blood groups. Heterogeneity of oligosaccharides liberated by degradation with alkaline borohydride of two human ovarian cyst fractions differing in B, I, and i activities and in reactivity toward concanavalin A. Arch Biochem Biophys. 1976;175:81–89. doi: 10.1016/0003-9861(76)90487-2. [DOI] [PubMed] [Google Scholar]
- 29.Maisonrouge-McAuliffe F, Kabat EA. Immunochemical studies on blood groups. Fractionation, heterogeneity, and chemical and immunochemical properties of a blood group substance with B, I and i activities purified from human ovarian cyst fluid. Arch Biochem Biophys. 1976;175:71–80. doi: 10.1016/0003-9861(76)90486-0. [DOI] [PubMed] [Google Scholar]
- 30.Feizi T, Kabat EA. Immunochemical studies on blood groups. LIV. Classification of anti-I and anti-i sera into groups based on reactivity patterns with various antigens related to the blood group A,B,H, Lea, Leb and precursor substances. J Exp Med. 1972;135:1247–1258. doi: 10.1084/jem.135.6.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feizi T. Blood group antigens. Ii antigens. Proc R Soc Med. 1975;68:799–802. doi: 10.1177/003591577506801229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang D, Dafik L, Nolley R, et al. Anti-oligomannose antibodies as potential serum biomarkers of aggressive prostate cancer. Drug Dev Res. 2013;74:65–80. doi: 10.1002/ddr.21063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anders CK, Carey LA. Biology, metastatic patterns, and treatment of patients with triple-negative breast cancer. Breast Cancer. 2009;9(suppl 2):S73–S81. doi: 10.3816/CBC.2009.s.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brenton JD, Carey LA, Ahmed AA, et al. Molecular classification and molecular forecasting of breast cancer: ready for clinical application? J Clin Oncol. 2005;23:7350–7360. doi: 10.1200/JCO.2005.03.3845. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.