Abstract
Background and Purpose
Exhaled carbon monoxide (CO) is associated with cardiometabolic traits, subclinical atherosclerosis, and cardiovascular disease, but its specific relations with stroke are unexplored. We related exhaled CO to MRI measures of subclinical cerebrovascular disease cross-sectionally, and to incident stroke/ transient ischemic attack (TIA) prospectively in the Framingham Offspring Study.
Methods
We measured exhaled CO in 3313 participants (age 59±10 years, 53% women), and brain MRI imaging was available in 1982 individuals (age 58±10, 54% women).
Participants were analyzed according to tertiles of exhaled CO concentration.
Results
In age- and sex-adjusted models, the highest tertile of exhaled CO was associated with lower total cerebral brain volumes (TCBV), higher white matter hyperintensity volumes (WMHV), and greater prevalence of silent cerebral infarcts (p <0.05 for all). The results for TCBV and WMHV were consistent after removing smokers from the sample, and the association with WMHV persisted after multivariable adjustment (p=0.04). In prospective analyses (mean follow-up 12.9 years), higher exhaled CO was associated with 67% (second tertile) and 97% (top tertile) increased incidence of stroke/TIA relative to the first tertile that served as referent (p <0.01 for both). These results were consistent in nonsmokers, and were partially attenuated upon adjustment for vascular risk factors.
Conclusions
In this large, community-based sample of individuals without clinical stroke/TIA at baseline, higher exhaled CO was associated with a greater burden of subclinical cerebrovascular disease cross-sectionally, and with increased risk of stroke/TIA prospectively. Further investigation is necessary to explore the biological mechanisms linking elevated CO with stroke.
Keywords: stroke, transient ischemic attack, carbon monoxide, biomarker, heme oxygenase
Introduction
Stroke is the second leading cause of death, and third leading cause of disability worldwide.1, 2 Clinical risk factors such as aging, diabetes, smoking, obesity, poor diet, physical inactivity, and atrial fibrillation have been reported to explain as much as 60-80% of stroke incidence in the community.3-5 Despite efforts to address these risk factors, stroke prevalence and stroke-related disability continue to rise.6, 7 By better understanding the biological mechanisms that modulate the progression from risk factor development to the occurrence of stroke, we may be able to further refine risk prediction methods, and discover novel targets for stroke prevention.
Carbon monoxide (CO), a diatomic gasotransmitter endogenously produced during heme metabolism, serves as an essential regulator of numerous biological processes.8 In animal models, physiologic concentrations of CO in vivo promote vascular and endothelial integrity,9, 10 prevent hypoxia-induced apoptosis,11 and attenuate the development of obesity.12 By contrast, elevated endogenous CO concentrations have been found to antagonize NO-mediated vasodilation,13 promote hyperglycemia,14 foster oxidative stress,15 and mediate the hypertensive response to physiologic stress in several disease models.16, 17 The apparently paradoxical effects of endogenous CO have been further explored in studies of human disease. Since CO can rapidly equilibrate across the alveolar-capillary barrier, endogenous CO levels can be approximated by measuring exhaled CO concentrations.18 Paredi and colleagues demonstrated that higher exhaled CO concentrations were correlated positively with the presence of diabetes, and that CO levels were directly related to blood glucose concentrations.19 In the Framingham Heart Study (FHS), we have previously reported that exhaled CO levels are positively associated with prevalent and incident metabolic syndrome, prevalent subclinical cardiovascular disease (CVD), and incident CVD.20, 21 Furthermore, subclinical CVD was associated with incident CVD in the setting of high but not low CO, suggesting that CO may be a biological modulator of the progression from subclinical to manifest CVD.21 Accordingly, we hypothesized that exhaled CO is associated with the presence of subclinical cerebrovascular disease on magnetic resonance imaging (MRI) cross-sectionally, and with the incidence of stroke/ transient ischemic attack (TIA) prospectively. We tested these hypotheses in the large, community-based Framingham Heart Study (FHS).
Methods
Study Sample
Enrollment and design of the Framingham Offspring Study have been described previously.22 For the present investigation, 3532 participants attending the sixth examination cycle (1995-1998) were considered eligible for analysis and two study samples were constructed. For both samples, participants were excluded for prevalent stroke/TIA (n=87), serum creatinine ≥ 2 mg/dL (n=14), missing CO measurements (n=71), unknown smoking status (n=3), and missing covariates (n=44), yielding a baseline study sample of 3313 individuals. In order to analyze cross-sectional associations of exhaled CO with MRI traits, participants were additionally excluded for refusing MRI or unavailable MRI measures (n=1269, Supplemental Table I), prevalent dementia or other neurologic disease (n=45), and interim stroke/TIA between the sixth examination cycle and MRI (n=17), yielding a second final study sample of 1982 individuals for these analyses. For assessing the prospective associations of exhaled CO and incident stroke/TIA, the baseline sample (n=3313) was used. Medical history, anthropometry, a cardiovascular-targeted physical examination, electrocardiography and phlebotomy were performed for all attendees of the sixth examination cycle at the FHS clinic. All study protocols were approved by the Boston University Medical Center Institutional Review Board, and all participants provided written informed consent.
Measuring Exhaled Carbon Monoxide
Exhaled CO was measured at rest using the Ecolyzer (2000 series) instrument (Energetics Science Inc., Elmsford, NY, USA) during the third through sixth examination cycles as described previously.20 This method has been shown to be reproducible and to accurately reflect blood concentrations of CO.23 At each examination, the average of two measurements was recorded. Exhaled CO levels >50 p.p.m. (n=4) were censored and considered equivalent to 50 p.p.m. in order to reduce excess leverage by extreme outliers. For the present analyses, the available CO measurements from examination cycles three through six were averaged for each participant.23
Brain MRI Outcomes
Details of brain MRI image acquisition, analysis and inter-rater reliability have been previously reported.24 Briefly, brain MRIs were performed (1999-2005) on 2,230 Framingham Offspring participants who attended the seventh examination cycle. Images were acquired on a Siemens 1T or 1.5T scanner, with 3-dimensional T1- and T2-weighted double spin-echo images acquired in 4mm contiguous slices (repetition time was 2,420 milliseconds, echo time 1 = 20 milliseconds/echo time 2 = 90 milliseconds, echo train of 8 milliseconds, field of view 22 cm, matrix 182 × 256 interpolated to 256 × 256 with one excitation). The white-matter hyperintensity volume (WMHV) and total cerebral brain parenchymal volume (TCBV) were measured by automated procedure,25 and each was divided by total cranial volume to control for head size. Prevalent silent cerebral infarcts (SCI) >3mm were identified manually. The images were analyzed by operators blinded to the participants' clinical characteristics, exposures, and outcomes.
Outcome Events
The FHS protocol for stroke surveillance and adjudication have been reported elsewhere.26 Briefly, participants in the FHS are under active surveillance for stroke/ TIA. An acute onset neurologic deficit of presumed or definite vascular pathogenesis is described as stroke if lasting for >24 hours, and TIA if symptoms last ≤24 hours. Clinical features, imaging studies and information from post-mortem examinations are used when available. These events have been adjudicated by a committee of at least 2 neurologists at a consensus review.
Covariates
Blood pressure, body mass index, plasma total and high-density lipoprotein cholesterol, fasting blood glucose, B-type natriuretic peptide (BNP) and C-reactive protein (CRP) were measured at the FHS clinic during the sixth examination cycle. Diabetes was defined as fasting blood glucose ≥126 mg/dL, or receiving treatment for diabetes. Self-reported smoking status, and hypertension treatment status were ascertained by questionnaire. FHS participants are under continuous surveillance for cardiovascular disease (CVD) and atrial fibrillation, which are adjudicated by a committee of three physicians based on review of clinic and hospital records.
Statistical Analysis
Exhaled carbon monoxide was the exposure of interest for all analyses. Each participant was placed into one of three groups (creating whole number approximate tertiles) according to average CO concentration ≤4 p.p.m., >4 and ≤5 p.p.m., and >5 p.p.m., as detailed previously.21 In primary analyses, the CO categories were analyzed separately with the lowest tertile serving as the referent. In secondary analyses, 2 categories were grouped together and CO was analyzed as a dichotomous variable. The analyses were performed in the whole sample, and repeated in nonsmokers only.
To evaluate the associations of exhaled CO with brain MRI traits, we used multivariable linear (for WMHV and TCBV) and logistic (for SCI) regression models. WMHV was natural logarithmically transformed (due to its skewed distribution) and analyzed according to age-specific z-scores. Model 1 was adjusted for sex, age, and age*age, due to a previously demonstrated quadratic relation between age and several of the MRI measures.27 Model 2 was adjusted additionally for systolic blood pressure, hypertension treatment status, diabetes mellitus, total/ high-density lipoprotein (HDL) cholesterol, body mass index, smoking status, prevalent atrial fibrillation, prevalent cardiovascular disease, and the time interval between examination cycle 6 and the MRI measurements.
For evaluation of the prospective associations of exhaled CO and stroke/TIA, we used Cox proportional hazards regression models after confirming that the proportionality of hazards assumption was met. Model 1 was adjusted for age and sex. Model 2 was additionally adjusted for systolic blood pressure, hypertension treatment status, diabetes, total/HDL cholesterol, body mass index, smoking status, prevalent atrial fibrillation, and prevalent cardiovascular disease. Model 3 was adjusted additionally for the cardiovascular biomarkers of BNP and CRP, which were log-transformed. Penalized splines were constructed to examine for non-linearity. In secondary analyses, we evaluated the associations of exhaled CO with stroke only.
In both the cross-sectional and prospective analyses, we examined for evidence of effect modification by systolic blood pressure and antihypertensive treatment status. A two-tailed p-value of <0.05 was used to assess statistical significance. Analyses were performed using SAS Software version 9.3 (Cary, NC).
Results
Characteristics of the two samples are shown in Table 1 and Supplemental Table II. Our total study sample consisted of middle-aged to older individuals and over half were women.
Table 1. Characteristics of the study samples.
| Characteristics | Stroke sample (N=3313) |
MRI sample (N=1982) |
|---|---|---|
| Age (years) | 59.0±9.7 | 58.2±9.5 |
| Women (%) | 53.0 | 53.6 |
| Current smoking (%) | 14.9 | 13.3 |
| Diabetes mellitus (%) | 9.5 | 8.4 |
| Body mass index (kg/m2) | 27.9±5.1 | 27.7±5.0 |
| Systolic blood pressure (mm Hg) | 128±19 | 126±18 |
| Diastolic blood pressure (mm Hg) | 76±10 | 75±9 |
| Hypertension treatment (%) | 27.4 | 23.7 |
| Total cholesterol (mmol/L) | 5.3±1.0 | 5.3±0.9 |
| HDL cholesterol (mmol/L) | 1.3±0.4 | 1.3±0.4 |
| Cardiovascular disease (%) | 9.2 | 7.3 |
| Atrial fibrillation (%) | 3.0 | 1.9 |
| Average CO (ppm) | 7.0±6.3 | 6.7±6.0 |
| Average log CO (log ppm) | 1.7±0.6 | 1.7±0.6 |
| CO category 1 (≤4 ppm), (%) | 38.1 | 39.7 |
| CO category 2 (>4 to ≤5 ppm), (%) | 29.6 | 29.8 |
| CO category 3 (≥5 ppm), (%) | 32.3 | 30.5 |
| BNP (pg/mL), median (Q1, Q3)* | 8.3 (4.0, 18.7) | |
| CRP (mg/L), median (Q1, Q3)† | 2.0 (0.9, 4.6) | |
| MRI measures | ||
| TCBV (%) | 79.5±3.3 | |
| WMHV (%), median (Q1, Q3) | 0.05 (0.03, 0.09) | |
| SCI (%) | 10.9 |
HDL indicates high-density lipoprotein; CO, carbon monoxide; ppm, parts per million; BNP, B-type natriuretic peptide; CRP, C-reactive protein; TCBV, total cerebral brain volume; WMHV, white matter hyperintensity volume; SCI, silent cerebral infarct
Data are mean±SD (continuous variables) or percentages (categorical variables).
N=3296
N=3196
Association of Exhaled CO with Subclinical Vascular Brain Injury
Cross-sectional associations of exhaled CO and MRI measures of subclinical cerebrovascular disease are shown in Table 2. Compared with the low CO tertile (≤4 p.p.m.), individuals with the highest CO (>5 p.p.m.) had increased WMHV in both minimally-adjusted and multivariable-adjusted models. These results were consistent in the whole sample, and in nonsmokers. Minimal attenuation of the effect estimates was observed upon multivariable adjustments (∼13% reduction in the β-coefficients).
Table 2. Associations of exhaled carbon monoxide and subclinical cerebrovascular disease.
| Whole sample (N=1982) | ||||||||
|---|---|---|---|---|---|---|---|---|
| MRI Measure | CO ≤4 ppm | p value | CO >4 and ≤5 ppm | p value | CO >5 ppm | p value | p for trend | |
| TCBV, β±SE | ||||||||
| Model 1 | Referent | -- | 0.16±0.15 | 0.3 | -0.36±0.15 | 0.02 | 0.03 | |
| Model 2 | Referent | -- | 0.33±0.15 | 0.03 | -0.17±0.18 | 0.3 | 0.7 | |
| WMHV, β±SE | ||||||||
| Model 1 | Referent | -- | 0.01±0.05 | 0.9 | 0.16±0.05 | 0.0029 | 0.0036 | |
| Model 2 | Referent | -- | 0.00±0.05 | 1.0 | 0.14±0.06 | 0.0297 | 0.05 | |
| SCI, OR (95% CI) | ||||||||
| Model 1 | Referent | -- | 1.42 (0.99-2.02) | 0.05 | 1.46 (1.02-2.09) | 0.04 | 0.03 | |
| Model 2 | Referent | -- | 1.47 (1.03-2.11) | 0.04 | 1.38 (0.89-2.12) | 0.1 | 0.08 | |
| Nonsmokers (N=1718) | ||||||||
| TCBV, β±SE | ||||||||
| Model 1 | Referent | -- | 0.15±0.15 | 0.3 | -0.36±0.18 | 0.05 | 0.1 | |
| Model 2 | Referent | -- | 0.33±0.15 | 0.03 | -0.16±0.18 | 0.4 | 0.8 | |
| WMHV, β±SE | ||||||||
| Model 1 | Referent | -- | 0.02±0.05 | 0.7 | 0.15 ±0.06 | 0.02 | 0.04 | |
| Model 2 | Referent | -- | 0.00±0.05 | 1.0 | 0.13±0.06* | 0.04 | 0.07 | |
| SCI, OR (95% CI) | ||||||||
| Model 1 | Referent | -- | 1.38 (0.97-1.97) | 0.07 | 1.30 (0.85-2.00) | 0.2 | 0.1 | |
| Model 2 | Referent | -- | 1.42 (0.99-2.05) | 0.06 | 1.34 (0.86-2.09) | 0.2 | 0.1 | |
CO indicates carbon monoxide; ppm, parts per million; TCBV, total cerebral brain volume; β, regression coefficient; SE, standard error; WMHV, white matter hyperintensity volume; SCI, silent cerebral infarct; OR, odds ratio; CI, confidence interval.
TCBV and WMHV are expressed as % of intracranial volume. WMHV was log-transformed and analyzed according to age-specific z-score.
Regression coefficient and odds ratios represent changes associated with each CO tertile compared with the lowest CO tertile (referent).
Model 1 was adjusted for sex, age, and age*age
Model 2 was adjusted for sex, age, age*age, systolic blood pressure, hypertension treatment, diabetes, total/HDL cholesterol, body mass index, smoking status, prevalent atrial fibrillation, prevalent cardiovascular disease, interval between exam 6 and MRI.
Prevalence of silent cerebral infarcts was also marginally associated with exhaled CO. The highest CO tertile was associated with a 46% increase in the odds of having a silent cerebral infarct compared with the lowest CO tertile in minimally-adjusted models for the whole sample (p=0.04). Multivariable adjustment attenuated this association. No statistically significant associations were observed in the nonsmokers.
Lower TCBV was observed for the highest CO tertile after adjusting for age and sex, in the whole sample and in nonsmokers. In the intermediate exhaled CO group, however, we observed a statistically significant association in the opposite direction (i.e., an increased total cerebral brain volume) in multivariable-adjusted models.
In secondary analyses, CO was modeled as a dichotomous variable (with categories of CO ≤4 versus CO >4 and CO ≤5 versus CO >5) and similar findings were observed (Supplemental Table III). Effect modification by systolic blood pressure and antihypertensive treatment status were investigated using multiplicative interaction terms that were not statistically significant.
Exhaled Carbon Monoxide and Incident Stroke/TIA
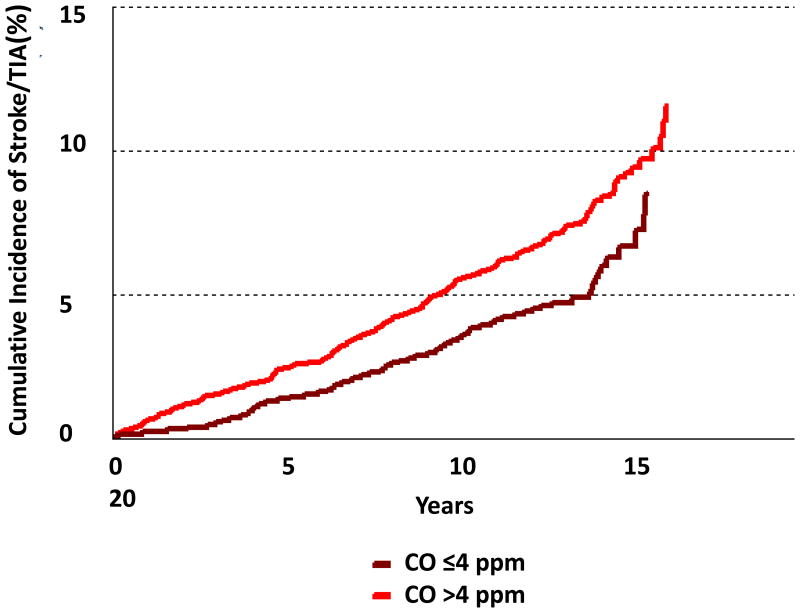
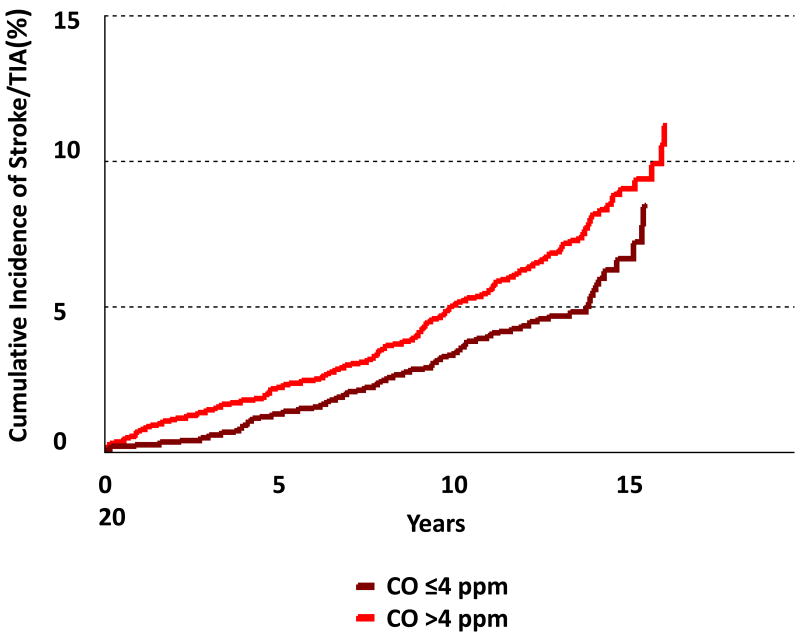
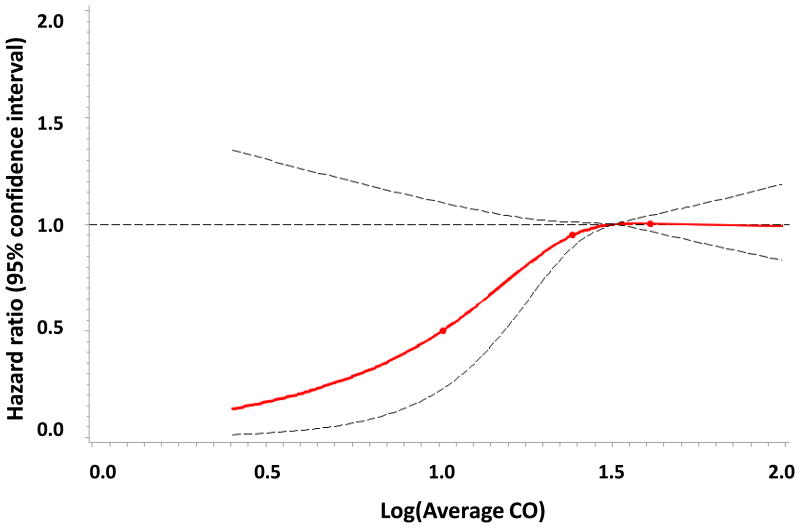
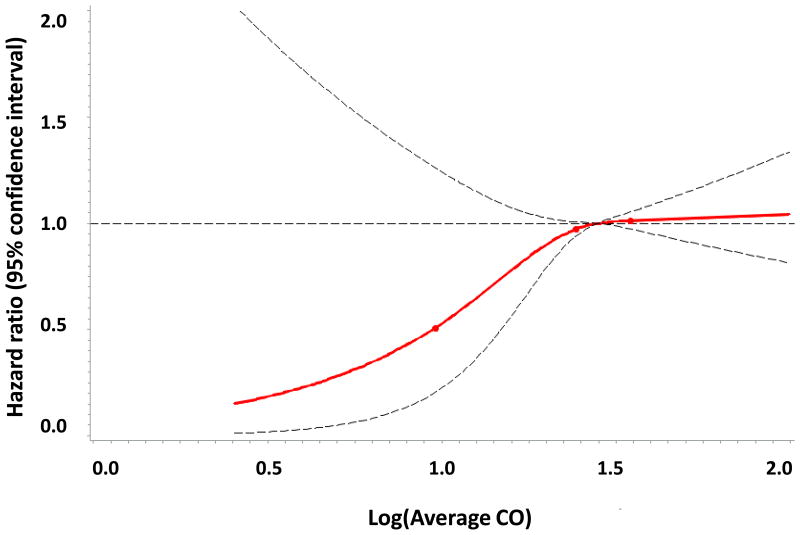
A total of 3313 individuals (53% women) were followed for a mean of 12.9 years. Incident stroke/TIA developed in 231 participants (7.0%; 52% of whom were women). In age- and sex-adjusted models, the intermediate and highest tertiles of exhaled CO had 67% and 97% higher incidence of stroke/TIA when compared to the lowest CO tertile, respectively (p for both <0.01), Table 3 and Figure 1. These associations were also statistically significant in nonsmokers. The hazard ratios demonstrated some attenuation upon multivariable adjustment for known stroke risk factors, but no additional attenuation was observed when additional adjustments were made for the circulating biomarkers BNP and CRP.
Table 3. Associations of exhaled carbon monoxide and incident stroke/TIA.
| Whole sample (N=3313) | |||||||
|---|---|---|---|---|---|---|---|
| CO ≤4 ppm | p value | CO >4 and ≤5 ppm | p value | CO >5 ppm | p value | p for trend | |
| Model 1, HR (95% CI) | Referent | -- | 1.67 (1.20-2.32) | 0.0022 | 1.97 (1.41-2.76) | <0.0001 | <0.0001 |
| Model 2, HR (95% CI) | Referent | -- | 1.60 (1.15-2.23) | 0.0053 | 1.43 (0.96-2.14) | 0.08 | 0.04 |
| Model 3, HR (95% CI) | Referent | -- | 1.57 (1.12-2.20) | 0.0083 | 1.45 (0.97-2.17) | 0.07 | 0.04 |
| Nonsmokers (N=2819) | |||||||
| Model 1, HR (95% CI) | Referent | -- | 1.69 (1.21-2.35) | 0.0019 | 1.63 (1.09-2.44) | 0.02 | 0.0056 |
| Model 2, HR (95% CI) | Referent | -- | 1.68 (1.20-2.35) | 0.0025 | 1.55 (1.03-2.33) | 0.04 | 0.01 |
| Model 3, HR (95% CI) | Referent | -- | 1.66 (1.18-2.34) | 0.0036 | 1.57 (1.04-2.38) | 0.03 | 0.01 |
TIA indicates transient ischemic attack; CO, carbon monoxide; ppm, parts per million; HR, hazard ratio; CI, confidence interval.
HR represents the relative hazard for each CO category compared with the referent.
Model 1 was adjusted for age and sex.
Model 2 was additionally adjusted for systolic blood pressure, hypertension treatment status, diabetes, total/HDL cholesterol, body mass index, smoking, prevalent cardiovascular disease, prevalent atrial fibrillation.
Model 3 was additionally adjusted for log-BNP and log-CRP. N=3189 for the whole sample and N=2715 for nonsmokers.
Figure 1.
Figure 1A. Cumulative incidence of stroke/TIA by dichotomous exhaled CO categories in the whole sample.
Figure 1B. Cumulative incidence of stroke/TIA by dichotomous exhaled CO categories in nonsmokers.
Figure 1C. Age- and sex- adjusted associations of exhaled CO and stroke/TIA in the whole sample.
Figure 1D. Age- and sex- adjusted associations of exhaled CO and stroke/TIA in nonsmokers.
In secondary analyses, CO was modeled as a dichotomous variable (CO ≤4 versus CO >4) and similar associations were observed (Figure 1 and Supplemental Table IV). Effect modification by systolic blood pressure and antihypertensive status was not observed (data not shown). We also evaluated the associations of exhaled CO tertiles with stroke only (after removing TIA events from the outcome) and the findings were similar (data not shown).
Discussion
In the present investigation of mostly middle-aged individuals in the community, exhaled CO concentrations were associated with MRI measures of subclinical cerebrovascular disease cross-sectionally, and with the incidence of stroke/TIA prospectively. In minimally-adjusted models, persons in the highest exhaled CO tertile had lower TCBV, greater burden of WMHV, and were more likely to have prevalent SCI when compared to persons in the lowest CO tertile. These associations were partially attenuated upon adjustment for known stroke risk factors. In prospective analyses, higher exhaled CO was associated with an increased risk of stroke during approximately 13 years of follow-up. The effect estimate was attenuated after adjustment for traditional stroke risk factors, but remained robust after adjusting for the circulating biomarkers BNP and CRP. These findings are consistent with those of Cheng and colleagues, which established that exhaled CO is associated with cardiometabolic traits, subclinical CVD, and the incidence of CVD, a composite outcome comprising different types of vascular outcomes.20, 21 Our findings support a similar role for exhaled CO when stroke/TIA was analyzed as a distinct outcome. Furthermore, the partial attenuation of the effect estimates after adjusting for stroke risk factors supports the hypothesis that CO may be physiologically related to vascular risk factors. The lack of attenuation upon adjustment for BNP and CRP, in contrast, suggests that CO acts independently of myocardial stress (BNP) and inflammation (CRP) to affect stroke risk.
Pathophysiologic Mechanisms Linking Elevated Endogenous CO Concentrations with Stroke/TIA
CO is essential for healthy development and, in physiologic concentrations, serves a number of protective functions.28-30 Conversely, elevated CO concentrations may contribute to vascular disease and stroke via several mechanisms.31 At high concentrations, endogenous CO inhibits the production of endothelial NO, thereby reducing the anti-thrombogenic activity of the endothelial layer, and increasing recruitment of inflammatory cells.13 In fact, CO may directly enhance the migration of inflammatory cells via stimulation of cyclic guanosine monophosphate (GMP).32 Furthermore, endogenous CO may lead to increased blood glucose by stimulating glucagon release.14 CO appears to be an important modulator of cardiometabolic disease, as evidenced by a rat model of the metabolic syndrome, in which elevated CO was found to promote hypertension and endothelial dysfunction independent of its effects on metabolic variables.17 Lastly, CO is known to result in oxidative stress and free radical production, which contribute to cardiometabolic disease pathogenesis.15
In addition to its individual associations with cardiometabolic and vascular pathways, CO concentrations reflect activity of heme oxygenase, which produces CO during heme metabolism.8 Heme oxygenase-1 (HO-1) is an increasingly recognized modulator of cardiovascular risk and its expression is inducible by physiologic stressors such as circulating phospholipids, vascular injury, hypertension, hyperglycemia, smoking, and laminar shear stress.8, 33-36 In autopsy samples of human coronary arteries, HO-1 activity was shown to be overexpressed in atherosclerotic plaques and to correlate with the degree of stenosis.37 HO-1 has also been demonstrated to be an essential driver of insulin resistance.38 The degree to which elevated endogenous CO concentrations lead to adverse cardiometabolic adaptations independently, or primarily via its relations with the activity of HO-1 remains unknown.
Exhaled Carbon Monoxide and Stroke Incidence
To our knowledge, this is the first study establishing an association of exhaled CO with stroke incidence. These findings are consistent with previous work by our group demonstrating associations of exhaled CO with cardiometabolic traits, subclinical CVD and incident CVD. Therefore, our findings might be explained by shared risk factors and pathophysiology of both stroke and coronary heart disease. Although animal models and clinical studies have previously demonstrated associations of elevated endogenous CO with various molecular processes that might mediate the increased stroke risk, the precise mechanisms responsible for the observed associations have not been determined. Furthermore, it remains unknown whether endogenous CO may serve as an essential biological modulator of stroke risk versus a biomarker of physiologic stress and, perhaps, HO-1 activity.
Limitations and Strengths
Several limitations of this study warrant mention. Exhaled CO was measured as an approximation of endogenous CO production. Although gas chromatography remains the reference standard for measuring blood CO concentrations, exhaled CO has been previously determined to be accurate and reproducible for measuring circulating CO.23 However, CO in the blood is a reflection of both endogenous CO production, and exogenous CO exposure from several sources including cigarette smoke and ambient air pollution. Current smoking was ascertained by questionnaire, but misclassification due to misreporting of current smoking, previous smoking history, or inability to control for secondhand smoke exposure, is possible. Ambient air pollution is also known to contain CO and to contribute to atherosclerotic vascular disease.39 Environmental CO exposure may be affected by a number of factors including automobile type,40 distance from major roadways,41 and home heating apparatuses such as kerosene stoves.42 Secular trends showing decreasing CO concentrations in ambient air in recent decades further complicate this issue.43 Lastly, we have not evaluated other environmental pollutants such as nitric oxides or sulfur oxides in this present investigation. Thus, comprehensive CO exposure histories have not been assessed in our analyses. Environmental or personal sensors might be used to more completely control for exogenous CO exposure and the role of other air pollutants in future epidemiologic studies evaluating stroke risk. In addition, CO measurements and brain MRI scans were non-contemporaneous, with an average interval of 3.7 years between them (even though we adjusted for this interval in multivariable analyses). This time lag between assessment of exposure and outcome could potentially introduce a regression dilution bias, which would likely lead to underestimation of the true effect, or bias us towards the null hypothesis of no association between CO and the outcomes evaluated. Finally, the study sample was composed of mostly middle-aged white individuals of European descent; thus, the generalizability of our findings to other populations remains unknown. As certain ethnic and geographic groups are disproportionally affected by stroke,44 examining the associations of CO and stroke risk in other populations would be of great interest.
Notwithstanding these limitations, our study has several strengths including use of a rigorously implemented, standardized measure of CO exposure in a large, community-based population of adults free of clinical stroke at baseline. In addition, the opportunity to analyze time-averaged measures of CO allowed for precise estimates of the exposure variable for each individual. Furthermore, the observations that exhaled CO is related both to incident stroke and subclinical cerebrovascular injury on brain MRI in the whole sample and in nonsmokers alone suggests a true biological effect in our study.
Conclusions
In our large, community-based sample of individuals free of clinical stroke at baseline, higher levels of exhaled CO were associated with MRI measures of subclinical cerebrovascular disease cross-sectionally, and with incident stroke/TIA prospectively. These findings are consistent with experimental data linking higher endogenous CO and activation of the heme oxygenase pathway with vascular dysfunction and cardiometabolic risk. Future investigations are warranted to evaluate whether exhaled CO might be a useful clinical biomarker of future stroke risk in select individuals, and if modulation of the CO or heme-oxygenase pathways might serve to reduce stroke risk.
Supplementary Material
Acknowledgments
None
Sources of Funding: This work was supported by the Framingham Heart Study contract from the National Heart, Blood, and Lung Institute: N01-HC-25195 (PI: RSV) and by NIH grant R01-NS-17950 which funded the stroke surveillance in the FHS. In addition, MN was supported by NIH grant T32-HL007604 and training grant U10HL110337 from the National Heart, Lung, and Blood Institute. SC was supported by NIH grant R00-HL-107842, the Ellison Foundation and the Lerner Award. CD was supported by NIH grants P-30-AG10129 and R01-AG-08122.
Footnotes
Disclosures: The authors report no conflicts.
References
- 1.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (dalys) for 291 diseases and injuries in 21 regions, 1990-2010: A systematic analysis for the global burden of disease study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 2.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the global burden of disease study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whisnant JP. Modeling of risk factors for ischemic stroke. The willis lecture. Stroke. 1997;28:1840–1844. doi: 10.1161/01.str.28.9.1840. [DOI] [PubMed] [Google Scholar]
- 4.O'Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the interstroke study): A case-control study. Lancet. 2010;376:112–123. doi: 10.1016/S0140-6736(10)60834-3. [DOI] [PubMed] [Google Scholar]
- 5.Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371:1612–1623. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- 6.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics-2015 update: A report from the american heart association. Circulation. 2015;131:e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 7.Bennett DA, Krishnamurthi RV, Barker-Collo S, Forouzanfar MH, Naghavi M, Connor M, et al. The global burden of ischemic stroke: Findings of the gbd 2010 study. Global heart. 2014;9:107–112. doi: 10.1016/j.gheart.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Fredenburgh LE, Merz AA, Cheng S. Haeme oxygenase signalling pathway: Implications for cardiovascular disease. Eur Heart J. 2015;36:1512–1518. doi: 10.1093/eurheartj/ehv114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wegiel B, Gallo DJ, Raman KG, Karlsson JM, Ozanich B, Chin BY, et al. Nitric oxide-dependent bone marrow progenitor mobilization by carbon monoxide enhances endothelial repair after vascular injury. Circulation. 2010;121:537–548. doi: 10.1161/CIRCULATIONAHA.109.887695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Otterbein LE, Zuckerbraun BS, Haga M, Liu F, Song R, Usheva A, et al. Carbon monoxide suppresses arteriosclerotic lesions associated with chronic graft rejection and with balloon injury. Nature medicine. 2003;9:183–190. doi: 10.1038/nm817. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Wang Y, Kim HP, Nakahira K, Ryter SW, Choi AM. Carbon monoxide protects against hyperoxia-induced endothelial cell apoptosis by inhibiting reactive oxygen species formation. The Journal of biological chemistry. 2007;282:1718–1726. doi: 10.1074/jbc.M607610200. [DOI] [PubMed] [Google Scholar]
- 12.Hosick PA, AlAmodi AA, Storm MV, Gousset MU, Pruett BE, Gray W, et al. Chronic carbon monoxide treatment attenuates development of obesity and remodels adipocytes in mice fed a high-fat diet. Int J Obes (Lond) 2014;38:132–139. doi: 10.1038/ijo.2013.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thorup C, Jones CL, Gross SS, Moore LC, Goligorsky MS. Carbon monoxide induces vasodilation and nitric oxide release but suppresses endothelial nos. Am J Physiol. 1999;277:F882–889. doi: 10.1152/ajprenal.1999.277.6.F882. [DOI] [PubMed] [Google Scholar]
- 14.Henningsson R, Alm P, Ekström P, Lundquist I. Heme oxygenase and carbon monoxide: Regulatory roles in islet hormone release: A biochemical, immunohistochemical, and confocal microscopic study. Diabetes. 1999;48:66–76. doi: 10.2337/diabetes.48.1.66. [DOI] [PubMed] [Google Scholar]
- 15.Thom SR, Xu YA, Ischiropoulos H. Vascular endothelial cells generate peroxynitrite in response to carbon monoxide exposure. Chem Res Toxicol. 1997;10:1023–1031. doi: 10.1021/tx970041h. [DOI] [PubMed] [Google Scholar]
- 16.Motterlini R, Gonzales A, Foresti R, Clark JE, Green CJ, Winslow RM. Heme oxygenase-1 derived carbon monoxide contributes to the suppression of acute hypertensive responses in vivo. Circulation Research. 1998;83:568–577. doi: 10.1161/01.res.83.5.568. [DOI] [PubMed] [Google Scholar]
- 17.Johnson FK, Johnson RA, Durante W, Jackson KE, Stevenson BK, Peyton KJ. Metabolic syndrome increases endogenous carbon monoxide production to promote hypertension and endothelial dysfunction in obese zucker rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R601–608. doi: 10.1152/ajpregu.00308.2005. [DOI] [PubMed] [Google Scholar]
- 18.Jones RH, Ellicott MF, Cadigan JB, Gaensler EA. The relationship between alveolar and blood carbon monoxide concentrations during breathholding; simple estimation of cohb saturation. The Journal of laboratory and clinical medicine. 1958;51:553–564. [PubMed] [Google Scholar]
- 19.Paredi P, Biernacki W, Invernizzi G, Kharitonov SA, Barnes PJ. Exhaled carbon monoxide levels elevated in diabetes and correlated with glucose concentration in blood: A new test for monitoring the disease? Chest. 1999;116:1007–1011. doi: 10.1378/chest.116.4.1007. [DOI] [PubMed] [Google Scholar]
- 20.Cheng S, Lyass A, Massaro JM, O'Connor GT, Keaney JF, Jr, Vasan RS. Exhaled carbon monoxide and risk of metabolic syndrome and cardiovascular disease in the community. Circulation. 2010;122:1470–1477. doi: 10.1161/CIRCULATIONAHA.110.941013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng S, Enserro D, Xanthakis V, Sullivan LM, Murabito JM, Benjamin EJ, et al. Association of exhaled carbon monoxide with subclinical cardiovascular disease and their conjoint impact on the incidence of cardiovascular outcomes. Eur Heart J. 2014;35:2980–2987. doi: 10.1093/eurheartj/ehu052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The framingham offspring study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 23.Hedblad B, Ogren M, Engstrom G, Wollmer P, Janzon L. Heterogeneity of cardiovascular risk among smokers is related to degree of carbon monoxide exposure. Atherosclerosis. 2005;179:177–183. doi: 10.1016/j.atherosclerosis.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 24.DeCarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, et al. Measures of brain morphology and infarction in the framingham heart study: Establishing what is normal. Neurobiol Aging. 2005;26:491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Jeerakathil T, Wolf PA, Beiser A, Massaro J, Seshadri S, D'Agostino RB, et al. Stroke risk profile predicts white matter hyperintensity volume: The framingham study. Stroke. 2004;35:1857–1861. doi: 10.1161/01.STR.0000135226.53499.85. [DOI] [PubMed] [Google Scholar]
- 26.Wolf PA, D'Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: A risk profile from the framingham study. Stroke. 1991;22:312–318. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 27.Seshadri S, DeStefano AL, Au R, Massaro JM, Beiser AS, Kelly-Hayes M, et al. Genetic correlates of brain aging on mri and cognitive test measures: A genome-wide association and linkage analysis in the framingham study. BMC Med Genet. 2007;8(Suppl 1):S15. doi: 10.1186/1471-2350-8-S1-S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yachie A, Niida Y, Wada T, Igarashi N, Kaneda H, Toma T, et al. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Invest. 1999;103:129–135. doi: 10.1172/JCI4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poss KD, Tonegawa S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc Natl Acad Sci U S A. 1997;94:10925–10930. doi: 10.1073/pnas.94.20.10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Motterlini R, Otterbein LE. The therapeutic potential of carbon monoxide. Nature reviews. Drug discovery. 2010;9:728–743. doi: 10.1038/nrd3228. [DOI] [PubMed] [Google Scholar]
- 31.Owens EO. Endogenous carbon monoxide production in disease. Clin Biochem. 2010;43:1183–1188. doi: 10.1016/j.clinbiochem.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 32.VanUffelen BE, de Koster BM, VanSteveninck J, Elferink JG. Carbon monoxide enhances human neutrophil migration in a cyclic gmp-dependent way. Biochem Biophys Res Commun. 1996;226:21–26. doi: 10.1006/bbrc.1996.1305. [DOI] [PubMed] [Google Scholar]
- 33.Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: From basic science to therapeutic applications. Physiological reviews. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 34.Jonas JC, Guiot Y, Rahier J, Henquin JC. Haeme-oxygenase 1 expression in rat pancreatic beta cells is stimulated by supraphysiological glucose concentrations and by cyclic amp. Diabetologia. 2003;46:1234–1244. doi: 10.1007/s00125-003-1174-9. [DOI] [PubMed] [Google Scholar]
- 35.Favatier F, Polla BS. Tobacco-smoke-inducible human haem oxygenase-1 gene expression: Role of distinct transcription factors and reactive oxygen intermediates. The Biochemical journal. 2001;353:475–482. doi: 10.1042/0264-6021:3530475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ishizaka N, de Leon H, Laursen JB, Fukui T, Wilcox JN, De Keulenaer G, et al. Angiotensin ii-induced hypertension increases heme oxygenase-1 expression in rat aorta. Circulation. 1997;96:1923–1929. doi: 10.1161/01.cir.96.6.1923. [DOI] [PubMed] [Google Scholar]
- 37.Song J, Sumiyoshi S, Nakashima Y, Doi Y, Iida M, Kiyohara Y, et al. Overexpression of heme oxygenase-1 in coronary atherosclerosis of japanese autopsies with diabetes mellitus: Hisayama study. Atherosclerosis. 2009;202:573–581. doi: 10.1016/j.atherosclerosis.2008.05.057. [DOI] [PubMed] [Google Scholar]
- 38.Jais A, Einwallner E, Sharif O, Gossens K, Lu TT, Soyal SM, et al. Heme oxygenase-1 drives metaflammation and insulin resistance in mouse and man. Cell. 2014;158:25–40. doi: 10.1016/j.cell.2014.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Puett RC, Schwartz J, Hart JE, Yanosky JD, Speizer FE, Suh H, et al. Chronic particulate exposure, mortality, and coronary heart disease in the nurses' health study. Am J Epidemiol. 2008;168:1161–1168. doi: 10.1093/aje/kwn232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Potchter O, Oz M, Brenner S, Yaakov Y, Schnell I. Exposure of motorcycle, car and bus commuters to carbon monoxide on a main road in the tel aviv metropolitan area, israel. Environmental monitoring and assessment. 2014;186:8413–8424. doi: 10.1007/s10661-014-4013-1. [DOI] [PubMed] [Google Scholar]
- 41.Hagler GS, Thoma ED, Baldauf RW. High-resolution mobile monitoring of carbon monoxide and ultrafine particle concentrations in a near-road environment. J Air Waste Manag Assoc. 2010;60:328–336. doi: 10.3155/1047-3289.60.3.328. [DOI] [PubMed] [Google Scholar]
- 42.Lam NL, Smith KR, Gauthier A, Bates MN. Kerosene: A review of household uses and their hazards in low- and middle-income countries. J Toxicol Environ Health B Crit Rev. 2012;15:396–432. doi: 10.1080/10937404.2012.710134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cabon Monoxide: National Trends in CO Levels. [Accessed July 13, 2015];Environmental Protection Agency web site. http://www.epa.gov/airtrends/carbon.html.
- 44.Lanska DJ, Kuller LH. The geography of stroke mortality in the united states and the concept of a stroke belt. Stroke. 1995;26:1145–1149. doi: 10.1161/01.str.26.7.1145. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.