Abstract
Rationale
Recent reports on the abuse of novel synthetic cathinone derivatives call attention to serious public health risks of these substances. In response to this concern, a growing body of preclinical research has characterized the psychopharmacology of these substances, particularly mephedrone (MEPH) or methylenedioxypyrovalerone (MDPV), noting their similarities to MDMA and cocaine. Few studies have utilized drug discrimination methodology to characterize the psychopharmacological properties of these substances.
Objectives
The present study employed a rodent drug discrimination assay to further characterize the stimulus effects of MEPH and MDPV in comparison to MDMA and to a drug mixture comprised of d-amphetamine and MDMA.
Methods
Eight male Sprague-Dawley rats were trained to discriminate 1.5 mg/kg 3, 4-methylenedioxymethamphetamine (MDMA) and eight rats were trained to discriminate a mixture of 1.5 mg/kg MDMA and 0.5 mg/kg d-amphetamine (MDMA+AMPH) from vehicle. Substitution tests were conducted with MDMA, d-amphetamine, MDPV, MEPH, and cocaine.
Results
Dose response curves generated with MDMA and MEPH were comparable between training groups. In contrast, AMPH, MDPV, and cocaine produced only partial substitution in animals trained to discriminate MDMA but produced full substitution in animals trained to discriminate the MDMA+AMPH mixture.
Conclusions
These findings indicate MDPV's effects may be more similar to those of traditional psychostimulants, whereas MEPH exerts stimulus effects more similar to those of MDMA. Additional experiments with selective DA and 5-HT receptor antagonists are required to further elucidate specific receptor mechanisms mediating the discriminative stimulus effects of MDPV and mephedrone.
Keywords: mephedrone, MDPV, cocaine, d-amphetamine, drug discrimination, rats
Designer drugs, including the illicit bath salts or synthetic cathinones, have grown in popularity in the United States and Europe in an attempt to circumvent current drug laws (Gibbons and Zloh 2010; Rosenbaum et al. 2012). Cathinone is the naturally occurring amphetamine-like alkaloid found in Catha edulis (Khat), a plant native to Africa and the Middle East. Although the extracts of Khat leaves have been used for centuries for their psychostimulant properties, medical and law enforcement reports of serious toxicities associated with synthetic cathinone derivatives have appeared only within the last decade in the U.S. (Goodnough and Zezima 2011; Winstock and Ramsey 2010).
The emergence of this public health threat began in the mid to late 2000s, when synthetic cathinones gained popularity among recreational drug users. Presumably as a method of diversion and evasion of FDA regulations, mixtures of synthetic cathinones were falsely marketed under a variety of product descriptions, such as “bath salts”, “plant food”, and “research chemicals”. Toxicities resulting from use of these products have received widespread media attention, including reports of violent and bizarre behavior (e.g. Campbell 2012; “Police: Man on” 2013). In response to a growing public health concern, several chemical constituents of these products and their analogs are now classified as Schedule I controlled substances in the United States (DEA 2011).
The chemical constituents of illicit “bath salts” contain a variety of synthetic cathinone derivatives, presenting a considerable challenge to medical and scientific investigations to determine which of these chemicals pose the greatest health threat. The majority of published preclinical studies on synthetic cathinones have examined either mephedrone or MDPV, although a few have also included other cathinone derivatives (Wright et al. 2012; Baumann et al. 2012; Huang et al. 2012; Lisek et al. 2012; Motbey 2012; Aarde et al. 2013; Varner et al. 2013; Shortall et al. 2013; Fantegrossi et al. 2013; Gatch et al. 2013). It is now well established that the synthetic cathinones dose-dependently increase locomotor activity in rodents. Furthermore, repeated daily dosing with mephedrone for five to seven days (Lisek et al. 2012; Gregg et al. 2013a; Berquist et al. 2015) as well as repeated intermittent dosing (Shortall et al. 2013) produced behavioral sensitization in rodents. Additionally, at least one study demonstrated cross-sensitization to the acute locomotor effects of cocaine (15 mg/kg) 10 days after a five day treatment regimen with mephedrone (15 mg/kg) in rats (Gregg et al. 2013b), although this effect was not bidirectional. Mephedrone (30 mg/kg) has also been shown to produce conditioned place preference (CPP) in both rats and mice (Lisek et al. 2012) and methylone was reported to produce CPP in mice (Miyazawa et al. 2011). Moreover, mephedrone supports intravenous self-administration in rats (Aarde et al. 2013).
Drug discrimination methodology is commonly employed to characterize the behavioral stimulus properties of novel psychoactive substances in comparison to known drugs of abuse. Drugs sharing similar discriminative functions in nonhumans generally tend to have common psychoactive effects (i.e. intoxicating effects) in humans (Young 2009). Moreover, drugs that are determined to have similar discriminative stimulus properties can be predicted to share some pharmacological mechanisms of action as well as similar abuse liabilities (Nicholson and Balster 2001). To date, four published studies have examined one or more of the synthetic cathinones using drug discrimination methodology with rodents. In the earliest of these studies, methylone was found to substitute fully in rats trained to discriminate d-amphetamine (AMPH) or 3,4-methylenedioxymethamphetamine (MDMA) from saline, but not in rats trained to discriminate DOM from saline (Dal Cason et al. 1997). More recently, Gatch et al. (2013) reported that several cathinone derivatives (MDPV, mephedrone, flephedrone, naphyrone, methylone, and butylone) all produced dose-dependent increases in drug-lever responding and fully substituted in male Sprague-Dawley rats trained to discriminate either cocaine (10 mg/kg) or methamphetamine (1 mg/kg). Varner et al. (2013) were the first to report that male Long Evans hooded rats could be successfully trained to discriminate 3.2 mg/kg mephedrone. MDMA produced complete substitution for mephedrone, while 18 mg/kg cocaine (76%) and 1 mg/kg methamphetamine (73%) produced only partial substitution in these rats. Fantegrossi et al. (2013) demonstrated full substitution with MDMA and methamphetamine in male NIH Swiss mice trained to discriminate 0.3 mg/kg MDPV from saline. The main conclusion gleaned from these initial studies is that the synthetic cathinones share similar discriminative stimulus functions with psychomotor stimulants (cocaine, d-amphetamine, methamphetamine) as well as the serotonergic “entactogen”, MDMA, although in some instances only partial substitution was observed.
Although both MDPV and mephedrone share similar discriminative stimulus functions with MDMA (Fantegrossi et al. 2013; Varner et al. 2013), it is noteworthy that mephedrone's actions on monoamine transporters are comparable to the actions of MDMA, whereas MDPV's actions on dopamine transporters are more similar to cocaine's actions (Bauman et al. 2012; Cameron et al. 2013). In rodent drug discrimination studies, stimulus generalization between cocaine and MDMA has been reported to be asymmetrical (Khorana et al., 2004) or partial (Kueh and Baker 2007). Furthermore, MDMA produces a complex drug cue with both serotonergic and dopaminergic components that can be dissociated dependent on the discrimination training methods (Goodwin and Baker 2000; Goodwin et al. 2003). It is likely that discriminative stimulus effects of mephedrone and MDPV can also be dissociated and the extent of their similarity may be dependent on the discrimination training methods. None of the published studies to date have assessed MDPV or mephedrone in animals trained to discriminate MDMA. Thus, the primary aim of the current study was to do so. Considering the prevalence of concurrent abuse of bath salts with MDMA or psychostimulants, a secondary aim was to assess the effects of MDPV and mephedrone in animals trained to discriminate a drug mixture. Recognizing that most psychoactive drugs have complex stimulus functions involving multiple pharmacological mechanisms of action, some researchers have utilized drug discrimination methods to evaluate the effects of drug mixtures in comparison to novel substances as a way to assess distinct components of a drug's complex stimulus functions (Stolerman 2011). Therefore, in an attempt to dissociate the discriminative stimulus effects of MDPV and mephedrone, the current study assessed these substances for stimulus generalization in rats trained to discriminate either MDMA or a drug mixture consisting of d-amphetamine and MDMA.
Methods
Subjects
Sixteen adult male Sprague-Dawley rats were housed individually in polycarbonate cages lined with corn cob bedding (Harlan Teklad, Conrad, Iowa) in animal facilities maintained at constant temperature (20±2°C) and humidity (50±5%) under a 12:12 light/dark cycle, (lights on from 0900 to 2100). Water was provided ad libitum in the home cages. Commercial rodent diet (Purina® 5001, Richmond, Indiana) was restricted to daily feeding to maintain animals at 80-90% of free-feeding weights. All procedures were reviewed and approved by the Western Michigan University Institutional Animal Care and Use Committee and were in accordance with the guidelines of the Guide for the Care and Use of Laboratory Animals (National Academy of Sciences, 2011) and EU Directive 2010/63/EU.
Apparatus
Training and testing were conducted in eight sound-attenuated operant conditioning chambers (ENV-001; MED Associates Inc., Georgia, VT, USA) equipped with three retractable levers and a food pellet dispenser located on the front panel, a 28-V house light, and fan. Reinforcers for lever pressing consisted of 45 mg Dustless Precision Pellets® (Product# F0021, BioServ, Flemington, NJ). Experimental events were programmed and controlled using Med-PC software (version IV; MED Associates Inc., St. Albans, VT, USA).
Drugs
Mephedrone-hydrochloride, methylenedioxypyrovalerone-hydrochloride, cocaine-hydrochloride, and 3,4-methylenedioxymethamphetamine-hydrochloride were generously provided by the National Institute on Drug Abuse drug control supply program (Bethesda, MD). d-Amphetamine-hemisulfate was purchased from Sigma Chemical Company (St Louis, MO). All drugs were dissolved in bacteriostatic 0.9% sodium chloride and administered by intraperitoneal (i.p.) injection. For the training drug mixture of d-amphetamine and MDMA, these substances were dissolved together in a single solution. Doses were calculated based on the weights of the salts.
Preliminary Training
Subjects were acclimated to the operant chambers for two 60 minute sessions, one per day for two consecutive days. During these two sessions, no levers were extended and food pellets were delivered under a fixed-time 60 sec (FT60″) schedule to familiarize the animals with the location and sound of the pellet dispenser. Subsequent training sessions lasted 20 min per day and were conducted five to six days per week. Animals were initially trained to lever press with only the center lever extended and reinforcement was delivered under a fixed-ratio (FR) schedule that was gradually incremented from FR 1 to FR 20 over the course of seven training sessions. Once subjects were reliably lever pressing on the FR 20 schedule, errorless training sessions commenced with either the left lever or right lever extended. During this phase, subjects received i.p. injections of either the training drug (see below) or saline 10 min prior to the beginning of each session. Half the animals in each training group were reinforced for responses on the right lever following drug injections (D) and for responses on the left lever following saline vehicle injections (V). Conditions were reversed for the remaining animals in each group. A total of 12 errorless training sessions were conducted in the following order: V, V, D, D, V, D, V, V, D, D, V, D. Once subjects were responding reliably on an FR20 schedule on both the drug-paired and vehicle-paired levers, discrimination training commenced.
Discrimination Training
Both left and right levers were present during discrimination training sessions. These sessions were 20 min in duration and were conducted only once per day, five to six days a week. One group of rats (n=7) was trained to discriminate 1.5 mg/kg MDMA from saline injections and the other group (n=8) was trained to discriminate a mixture of 1.5 mg/kg MDMA + 0.5 mg/kg d-amphetamine (MDMA + AMPH) from saline injections. Similar to the preliminary training sessions, responding was initially reinforced under a FR1 schedule that was progressively incremented to a FR20 schedule under drug and vehicle conditions, independently based on each subject's performance. Once animals were reliably responding under the FR20 schedule under both drug and vehicle conditions, this schedule remained in effect for the remainder of the training sessions. Drug and vehicle training sessions were alternated with sessions under the same stimulus conditions occurring no more than twice consecutively. The performance criteria for stimulus control was a minimum of 8 out of 10 consecutive discrimination trials with an 80% or better correct lever response prior to delivery of the first reinforcer and for the total session.
Stimulus Generalization Tests
When the discrimination criteria were met, stimulus generalization tests commenced and dose-response curves were established with the following test compounds: AMPH (0.25 – 2.0 mg/kg), MDMA (0.19 – 1.5 mg/kg), MDPV (0.13 – 3.0 mg/kg), mephedrone (0.25 – 2.0 mg/kg), and cocaine (1.25 – 10 mg/kg). All compounds were administered via i.p. injection 10 min prior to commencing test sessions. Test sessions were conducted under extinction and ended immediately following the completion of 20 consecutive responses on either lever or until 20 min elapsed, which ever occurred first. The order of the test doses were counterbalanced among animals in each training group. Approximately half of the animals in each group were tested with a particular dose following a drug training session, and the other half was tested following a vehicle training session. Each subject completed a minimum of one drug and one vehicle training session between generalization test sessions and was required to meet the 80% discrimination criteria on the most recent drug and vehicle training sessions prior to each test.
Data Analysis
The mean (±SEM) number of sessions to criterion was calculated for each training group and statistically analyzed with a t-test. Dose-response curves were graphed for each training drug and test compound, with the mean (±SEM) percentage of drug-appropriate lever responses as well as the mean (±SEM) response rate (lever presses per second) plotted as a function of dose. Response rates were statistically analyzed using a mixed model two-way analysis of variance (ANOVA) with training drug as a between-subjects comparison and test dose as a within-subjects comparison. For drugs that produced full substitution (80% or higher drug-lever responding at any dose), a nonlinear regression was conducted on the dose-response curve to estimate ED50 values. Statistical analyses were conducted and graphs were created using GraphPad Prism (version 6.0) software (La Jolla, CA, USA).
Results
Discrimination Acquisition
Rats trained to discriminate MDMA+AMPH met the specified criteria for discrimination within 16.9 (±0.4, SEM) training sessions (range 16 – 19) while rats trained to discriminate MDMA met these criteria in an average of 29.1 (±4.4, SEM) training sessions (range 16 – 43). This difference was statistically significant (t(6.1) = 2.781, p < 0.05). Interestingly, there was a bimodal distribution in the sessions to criteria among the MDMA training group, with a range of 16 to 18 sessions among three animals and a range of 35 to 43 sessions among the other four animals.
Stimulus Generalization
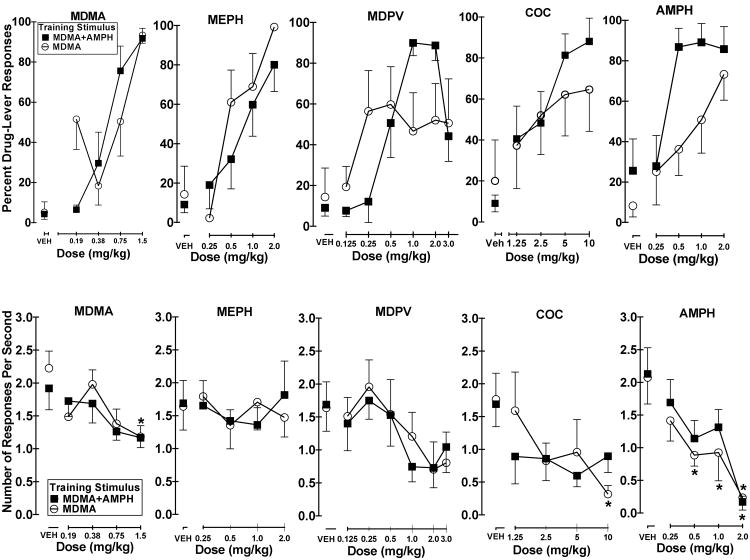
Dose response curves for MDMA, MEPH, MDPV, cocaine, and d-amphetamine are displayed in figure 1. MDMA produced a dose-dependent increase in drug-appropriate responding and substituted fully at 1.5 mg/kg in both MDMA-trained and MDMA+AMPH-training groups. . The ED50 values for MDMA were 0.21 mg/kg (95% CI [0.12 – 0.37 mg/kg]) and 0.35 mg/kg (95% CI [0.15 – 0.82 mg/kg]) in the MDMA+AMPH and MDMA training groups, respectively. A two-factor mixed model ANOVA showed a statistically significant main effect of test dose on response rate (F4, 52 = 5.56, p < 0.05). There was no statistically significant effect of training group nor was there a significant training group by test dose interaction on response rate. Bonferroni multiple comparison tests indicated response rate following 1.5 mg/kg was significantly different from response rate after saline injections (p < 0.05) only in the MDMA-trained animals.
Fig. 1.
Dose response curves determined from stimulus generalization tests with MDMA, mephedrone, MDPV, cocaine, and d-amphetamine in rats trained to discriminate a 1.5 mg/kg MDMA (n = 7) or a mixture of 1.5 mg/kg MDMA + 0.5 mg/kg d-amphetamine (n = 8) from saline. Graphs in the upper panel depict percentage of responses on the drug-appropriate lever. Graphs in the lower panel depict response rate. Individual points represent group means (± S.E. M.). MDMA+AMPH mixture group (■) and MDMA alone group (○). For response rate, significant Bonferroni multiple comparison tests between selected doses and saline are represented by * (p < 0.05).
d-Amphetamine (AMPH) also produced a dose-dependent increase in drug-appropriate responding in both training groups. Full substitution was observed in the MDMA+AMPH training group at 0.5, 1.0 and 2.0 mg/kg, however, only partial substitution was observed in the MDMA training group. The ED50 for AMPH in the MDMA+AMPH training group was calculated at 0.06 mg/kg (95% CI [0.03 – 0.14 mg/kg]). A two-factor mixed model ANOVA found that response rate was significantly affected by test dose (F4, 52 = 15.91, p < 0.05). No significant effects of training group or test dose by training group were found. Bonferroni multiple comparison tests found response rate following the 2.0 mg/kg dose was significantly different from response rate following saline in the MDMA+AMPH training group (p < 0.05), and response rate following all but the 0.25 mg/kg dose was significantly different from response rate following saline in the MDMA training group (0.5 and 1.0 mg/kg, p < 0.05 and 2.0 mg/kg, p < 0.05).
Similar to AMPH, cocaine also produced only partial substitution in animals trained to discriminate MDMA, but fully substituted in those trained to discriminate the MDMA+AMPH mixture. The ED50 value for animals trained to discriminate MDMA+AMPH was 4.65 mg/kg (95% CI [1.31 – 16.57 mg/kg]). A two-factor mixed model ANOVA found a significant main effect of test dose on response rates (F4, 40 = 3.70, p < 0.05). No significant effects of training group or training group by test dose interaction were found. Bonferroni multiple comparison tests found response rate at the 10.0 mg/kg dose to be significantly lower than that after saline injections in the MDMA-trained group (p < 0.05).
Mephedrone produced a dose-dependent increase in drug-appropriate responding and full substitution at the 2.0 mg/kg dose in both training groups. The ED50 values were 0.56 mg/kg (95% CI [0.25 – 1.23 mg/kg]) in the MDMA+AMPH training group and 0.22 mg/kg (95% CI [0.10 – 0.49 mg/kg]) in the MDMA training group. A two-factor mixed model ANOVA revealed no statistically significant effects of MEPH on response-rate.
Dose response curves for MDPV were distinctly different in the two training groups, similar to the distinction evident with cocaine and AMPH. As such, full substitution with MDPV was attained only in the MDMA+AMPH training group. The ED50 value for MDPV in the MDMA+AMPH training group was 0.30 mg/kg (95% CI [0.11 – 0.82 mg/kg]). A two-factor mixed model ANOVA also showed a significant main effect of MDPV dose on response rate (F5, 65 = 3.460, p < 0.05) but no statistically significant effect of training group or dose by training group interaction. Bonferroni multiple comparison tests on response rate did not reveal any individual doses to be significantly different from saline in either training group.
Discussion
Illicit designer drugs continue to increase in popularity, due in part to ubiquitous sources and lower costs compared to older, controlled psychostimulants, and the potential adverse psychological effects of these drugs are a growing public health concern. At the forefront of these new drugs are multiple new variations of synthetic cathinones, with mephedrone and MDPV among the most widely abused constituents, often found together in “bath salt” mixtures.
The primary finding of the present study is the differential substitution produced by mephedrone and MDPV in rats trained to discriminate MDMA or a drug mixture consisting of MDMA and d-amphetamine. Specifically, mephedrone produced similar dose-dependent increases in drug-lever responses and reached full substitution in both training groups at the 2.0 mg/kg dose. In contrast, MDPV produced full substitution only in the MDMA+AMPH training group, whereas a flat dose response curve and only partial substitution was obtained in the MDMA group. While it appears that MDPV reached a maximal effect with partial substitution in the MDMA group, it is possible that higher MDPV doses might substitute for MDMA. However, rate suppressant effects precluded testing higher doses. It is also possible that a plateau was reached because higher MDPV doses produce neurochemical effects unlike those produced by MDMA. Of particular interest, 3.0 mg/kg actually produced less substitution than 2.0 mg/kg MDPV in the MDMA+AMPH-trained animals, due to the fact that only two animals in this training group displayed complete stimulus generalization to 3.0 mg/kg MDPV while the 2.0 mg/kg dose substituted in nearly all the animals in this group. The reason for this is unclear, though the possibility that MDPV exerts distinctly different neurochemical actions at low and high doses might explain the U-shaped dose response function observed with MDPV in the MDMA+AMPH group.
While only a handful of studies have been published to date on the discriminative stimulus effects of mephedrone and MDPV, a comparison of the present study results with previous reports is worth discussing. The current findings, in concert with a previous report that MDMA substitutes in rats trained to discriminate mephedrone (Varner et al. 2013) provide convincing evidence for symmetrical substitution between mephedrone and MDMA. The current results are also in agreement with a report that methamphetamine substituted in mice trained to discriminate MDPV (Fantegrossi et al., 2013), but inconsistent with the observation in the same study that MDMA fully substituted for MDPV. This discrepancy could be attributed to species differences, but may also indicate that stimulus generalization between MDMA and MDPV is asymmetrical. Indeed, asymmetrical substitution has been noted between MDMA and other psychostimulants (Khorana et al., 2004).
The full substitution of mephedrone observed in both training groups in the current study suggests that this substance produces similar interoceptive stimuli (i.e., subjective effects) to those produced by MDMA (the component common to both training groups). This hypothesis is consistent with self-reports by human subjects equating the subjective effects of mephedrone to those of MDMA (Carhart-Harris et al. 2011). Moreover, recent reports indicate the pharmacological mechanisms of action of mephedrone closely resemble those of MDMA and are distinct from those of MDPV (Cameron et al. 2013; Bauman et al. 2012; Kehr et al. 2011). For example, unlike MDPV, mephedrone produces significant increases in serotonin (5-HT) release in rat nucleus accumbens in vivo (Kehr et al. 2011; Baumann et al. 2012), whereas MDPV reported blocks dopamine reuptake, similar to cocaine (Cameron et al. 2013). The current finding that mephedrone was slightly more potent in the MDMA group compared to the MDMA+AMPH group implicates 5-HT release as a more salient feature of mephedrone's stimulus effects. Additional studies in animals trained to discriminate mephedrone are required to directly assess this hypothesis.
Full substitution of MDPV in the MDMA+AMPH training group but not the MDMA training group indicates MDPV produces discriminable stimuli that are dissimilar to those produced by MDMA alone and more similar to the d-amphetamine component of the MDMA+AMPH mixture. This is supported by the current results that both AMPH and cocaine also produced full substitution in the MDMA+AMPH training group, suggesting AMPH was the dominant component of the drug mixture stimulus. In consideration of previous findings indicating MDPV's higher potency compared to other psychostimulants (e.g. Aarde et al. 2013b; Baumann et al. 2013), the slightly higher potency of AMPH substitution compared to MDPV in the current study was somewhat surprising. However, this is likely due to the selection of a low AMPH training dose. Extensive research on the role of training dose in drug discrimination indicates that low training doses yield greater sensitivity to lower test doses (Stolerman et al. 2011).
Due to the apparent lack of overshadowing by either drug component in the MDMA+AMPH mixture (i.e. both components of the mixture fully substituted individually at their respective training doses), it can be concluded that the MDMA+AMPH mixture does not produce a novel or qualitatively distinct stimulus. Rather, the two components likely have an additive effect. These findings may be compared to those of Shoaib et al. (1997) who found that a mixture of fenfluramine (FEN) and phentermine (PHEN) (agents with similar neurochemical effects to MDMA and d-amphetamine, respectively) produced an additive cue in animals trained to discriminate a FEN+PHEN mixture from saline. As previously noted, the discriminable effects of MDMA involve both serotonergic and dopaminergic activities and the extent to which either 5-HT or DA plays a dominant role in these effects is dependent on the training methods (Goodwin and Baker 2000; Goodwin et al. 2003). Adding d-amphetamine to MDMA in the current study may have strengthened the dopaminergic component of the complex drug stimulus, thus accounting for full substitution with cocaine for the MDMA+AMPH mixture. Following this line of reasoning, dopaminergic activities may be a dominant component of MDPV discrimination and less important to mephedrone discrimination. Further studies, such as tests with receptor-selective antagonists in animals trained to discriminate MDPV or mephedrone, are required to fully evaluate this hypothesis.
In summary, insofar as drug discrimination offers a model of subjective drug effects, the current results are relevant to distinguishing the subjective effects of mephedrone and MDPV and the pharmacological actions contributing to these effects. Utilizing drug mixtures as complex stimuli offers a novel approach to examine the pharmacological mechanisms that may distinguish the subjective effects of mephedrone and MDPV. This study represents the first attempt to do so. In consideration of the common practice of polysubstance use, further investigations on the stimulus functions of drug mixtures may help elucidate the unique subjective effects of commonly co-abused drugs.
References
- Aarde SM, Angrish D, Barlow DJ, Wright MJ, Jr, Vandewater SA, Creehan, Taffe MA, et al. Mephedrone (4-methylmethcathinone) supports intravenous self-administration in Sprague-Dawley and Wistar rats. Addict Biol. 2013a;18(5):786–799. doi: 10.1111/adb.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarde SM, Huang PK, Creehan KM, Dickerson TJ, Taffe MA. The novel recreational drug 3,4-methylenedioxypyrovalerone (MDPV) is a potent psychomotor stimulant: Self-administration and locomotor activity in rats. Neuropharmacol. 2013b;71:130–140. doi: 10.1016/j.neuropharm.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Jr, Partilla JS, Sink JR, Shulgin AT, Daley PF, Cozzi NV, et al. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacol. 2012;37(5):1192–1203. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, Schindler CW, et al. Powerful Cocaine-Like Actions of 3,4-Methylenedioxypyrovalerone (MDPV), a Principal Constituent of Psychoactive ‘Bath Salts’ Products. Neuropsychopharmacology. 2013;38:552–562. doi: 10.1038/npp.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berquist MD, II, Peet MM, Baker LE. Behavioral sensitization following concurrent exposure to mephedrone and d-amphetamine in female mice. Behav Pharmacol. 2015;26:180–183. doi: 10.1097/FBP.0000000000000121. [DOI] [PubMed] [Google Scholar]
- Cameron K, Kolanos R, Verkariya R, Felice L, Glennon R. Mephedrone and methylenedioxypyrovalerone (MDPV), major constituents of “bath salts,” produce opposite effects at the human dopamine transporter. Psychopharmacology. 2013;227(3):493–499. doi: 10.1007/s00213-013-2967-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A. Carl jacquneaux bit a chunk of face off victim todd credeur in louisiana, cops say. The Huffington Post. 2012 Retrieved from http://www.huffingtonpost.com/2012/06/06/carl-jacquneaux-bit-a-chunk-of-victims-face_n_1574316.html.
- Carhart-Harris RI, King LA, Nutt DJ. A web-based survey on mephedrone. Drug and Alcohol Depend. 2011;118(1):19–22. doi: 10.1016/j.drugalcdep.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Dal Cason T, Young R, Glennon R. Cathinone: An investigation of several N-alkyl and methylenedioxy-substituted analogs. Pharmacol Biochem and Behav. 1997;58(4):1109–1116. doi: 10.1016/S0091-3057(97)00323-7. [DOI] [PubMed] [Google Scholar]
- Drug Enforcement Administration (DEA) Federal Register. Vol. 76. Washington, DC: U.S. Government Printing Office; 2011. Schedules of controlled substances: Temporary placement of three synthetic cathinones into schedule I; p. 204. [PubMed] [Google Scholar]
- Fantegrossi W, Gannon B, Zimmerman S, Rice K. In vivo effects of abused ‘bath salt’ constituent 3,4-methylenedioxypyrovalerone (MDPV) in mice: Drug discrimination, thermoregulation, and locomotor activity. Neuropsychopharmacol. 2013;38(4):563–573. doi: 10.1038/npp.2012.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch M, Taylor C, Forster M. Locomotor stimulant and discriminative stimulus effects of ‘bath salt’ cathinones. Behav Pharmacol. 2013;24(5-6):437–447. doi: 10.1097/FBP.0b013e328364166d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons S, Zloh M. An analysis of the ‘legal high’ mephedrone. Bioorganic & Medicinal Chemistry Letters. 2010;20(14):4135–4139. doi: 10.1016/j.bmcl.2010.05.065. [DOI] [PubMed] [Google Scholar]
- Goodnough A, Zezima K. The New York Times; 2011. Jul 16, [Accessed 7 Nov 2014]. An alarming new stimulant, legal in many states. http://www.nytimes.com/2011/07/17/us/17salts.html. [Google Scholar]
- Goodwin A, Baker L. A three-choice discrimination procedure dissociates the discriminative stimulus effects of d-amphetamine and (±)-MDMA in rats. Exp and Clin Psychopharmacol. 2000;8(3):415–423. doi: 10.1037/1064-1297.8.3.415. [DOI] [PubMed] [Google Scholar]
- Goodwin A, Pynnonen D, Baker L. Serotonergic–dopaminergic mediation of MDMA's discriminative stimulus effects in a three-choice discrimination. Pharmacol Biochem and Behav. 2003;74(4):987–995. doi: 10.1016/S0091-3057(03)00029-7. [DOI] [PubMed] [Google Scholar]
- Gregg R, Tallarida C, Reitz A, Mccurdy C, Rawls S. Mephedrone (4-methylmethcathinone), a principal constituent of psychoactive bath salts, produces behavioral sensitization in rats. Drug and Alcohol Depend. 2013;133(2):746–750. doi: 10.1016/j.drugalcdep.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg R, Tallarida C, Reitz A, Rawls S. Mephedrone interactions with cocaine: Prior exposure to the ‘bath salt’ constituent enhances cocaine-induced locomotor activation in rats. Behav Pharmacol. 2013;24(8):684–688. doi: 10.1097/FBP.0000000000000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Aarde S, Angrish D, Houseknecht K, Dickerson T, Taffe M. Contrasting effects of d-methamphetamine, 3,4-methylenedioxymethamphetamine, 3,4-methylenedioxypyrovalerone, and 4-methylmethcathinone on wheel activity in rats. Drug and Alcohol Depend. 2012;126(1-2):168–175. doi: 10.1016/j.drugalcdep.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehr J, Ichinose F, Yoshitake S, Goiny M, Sievertsson T, Nyberg F, Yoshitake T. Mephedrone, compared with MDMA (ecstasy) and amphetamine, rapidly increases both dopamine and 5-HT levels in nucleus accumbens of awake rats. Br J of Pharmacol. 2011;164(8):1949–1958. doi: 10.1111/j.1476-5381.2011.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorana N, Pullagurla M, Young R, Glennon R. Comparison of the discriminative stimulus effects of 3,4-methylenedioxymethamphetamine (MDMA) and cocaine: Asymmetric generalization. Drug and Alcohol Depend. 2004;74(3):281–287. doi: 10.1016/j.drugalcdep.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Kueh D, Baker L. Reinforcement schedule effects in rats trained to discriminate 3,4-methylenedioxymethamphetamine (MDMA) or cocaine. Psychopharmacology. 2006;189(4):447–457. doi: 10.1007/s00213-006-0523-z. [DOI] [PubMed] [Google Scholar]
- Lisek R, Xu W, Yuvasheva E, Chiu Y, Reitz A, Liu-Chen L, Rawls S. Mephedrone (‘bath salt’) elicits conditioned place preference and dopamine-sensitive motor activation. Drug and Alcohol Depend. 2012;126(1-2):257–262. doi: 10.1016/j.drugalcdep.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa M, Kojima T, Nakaji S. Behavioral and rewarding effects of methylone, an analog of MDMA in mice. Hirosaki Med J. 2011;62(1):56–71. [Google Scholar]
- Motbey CP, Hunt GE, Bowen MT, Artiss S, McGregor IS. Mephedrone (4-methylmethcathinone, ‘meow’): acute behavioural effects and distribution of Fos expression in adolescent rats. Addict Biol. 2012;17:409–422. doi: 10.1111/j.1369-1600.2011.00384.x. [DOI] [PubMed] [Google Scholar]
- National Research Council of the National Academies. Guide for the care and use of laboratory animals. Washington, D C.: The National Academies Press; 2011. [Google Scholar]
- Nicholson KL, Balster RL. GHB: a new and novel drug of abuse. Drug and Alcohol Depend. 2001;63(1):1–22. doi: 10.1016/S0376-8716(00)00191-5. [DOI] [PubMed] [Google Scholar]
- Police: Man on bath salts runs naked down street. Altoona Mirror. [Accessed 19 Mar 2013];2013 http://www.altoonamirror.com/page/content.detail/id/569536/Police--Man-on-bath-salts-runs-naked-down-street.html?nav=742.
- Rosenbaum C, Carreiro S, Babu K. Here today, gone tomorrow…and back again? A review of herbal marijuana alternatives (K2, spice), synthetic cathinones (bath salts), kratom, salvia divinorum, methoxetamine, and piperazines. J of Med Toxicol. 2012;8(1):15–32. doi: 10.1007/s13181-011-0202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoaib M, Baumann MH, Rothman RB, Goldberg SR, Schindler CW. Behavioural and neurochemical characteristics of phentermine and fenfluramine administered separately and as a mixture in rats. Psychopharmacology. 1997;131(3):296–306. doi: 10.1007/s002130050296. [DOI] [PubMed] [Google Scholar]
- Shortall S, Macerola A, Swaby R, Jayson R, Korsah C, Pillidge K, King M, et al. Behavioural and neurochemical comparison of chronic intermittent cathinone, mephedrone and MDMA administration to the rat. Eur Neuropsychopharmacol. 2013;23(9):1085–1095. doi: 10.1016/j.euroneuro.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Stolerman I. The discrimination of drug mixtures. In: Glennon R, Young R, editors. Drug discrimination: applications to medicinal chemistry and drug studies, 1st edn. Wiley; Hoboken: 2011. pp. 323–359. [Google Scholar]
- Stolerman I, Childs E, Ford M, Grant K. Role of training dose in drug discrimination. Behav Pharmacol. 2011;22(5-6):415–429. doi: 10.1097/FBP.0b013e328349ab37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varner K, Daigle K, Weed P, Lewis P, Mahne S, Sankaranarayanan A, Winsauer P. Comparison of the behavioral and cardiovascular effects of mephedrone with other drugs of abuse in rats. Psychopharmacology. 2013;225(3):675–685. doi: 10.1007/s00213-012-2855-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstock AR, Ramsey JD. Legal highs and the challenges for policy makers. Addict. 2010;105(10):1685–1687. doi: 10.1111/j.1360-0443.2010.03163.x. [DOI] [PubMed] [Google Scholar]
- Wright MJ, Jr, Angrish D, Aarde SM, Barlow DJ, Buczynski MW, Creehan KM, Taffe MA, et al. Effect of ambient temperature on the thermoregulatory and locomotor stimulant effects of 4-methylmethcathinone in Wistar and Sprague-Dawley rats. PLoS ONE. 2012;7(8):e44652. doi: 10.1371/journal.pone.0044652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. Drug discrimination. Buccafusco JJ, editor. Methods of behavior analysis in neuroscience, 2nd edn. 2009 Retrieved from http://www.ncbi.nlm.nih.gov/books/NBK5225/ [PubMed]