Abstract
The cerebellum is organized into a map of zones that is manifested in various ways according to gene expression, anatomical connectivity, neuronal firing properties, behavioral specificity, and susceptibility to disease. At the center of every zone is the Purkinje cell, the principal cell type of the cerebellum and sole output of the cerebellar cortex. During development, Purkinje cells are thought to coordinate the zonal patterning of all other cell types. However, the morphogenetic mechanism that mediates the interaction between Purkinje cells and afferent fibers remains unclear. To address this problem in vivo, I took advantage of a rapid fluorescent-based transynaptic tracing approach to determine the nature of mossy fiber to Purkinje cell connectivity during early postnatal development, a period when the afferent map is assembling into clear-cut zonal circuits. By injecting WGA-Alexa 555 into the lower thoracic-upper lumber spinal cord, I found that spinocerebellar mossy fibers transynaptically transfer tracer into zones of Purkinje cells that are directly adjacent to the fibers. The traced Purkinje cell zones formed a zebrin-like pattern that was defined by the expression of neurofilament heavy chain (NFH), a marker of zones in the postnatal developing cerebellum. These results suggest that Purkinje cells generate the zonal circuit map by using molecular cues, neuronal activity, and synaptic contact.
Keywords: spinocerebellar, zones, postnatal, circuitry, neural tracing, transynaptic
INTRODUCTION
Several developmental stages cooperate to generate the mature pattern of cerebellar zones [1]. One of the first stages of forming zones is dependent on the timing of Purkinje cell birth; this occurs between ~ embryonic day 10-13 in mouse. Different clusters of Purkinje cells are born on each day [2,3]. During the next stage, the clusters acquire specific molecular properties that are under the control of at least two families of transcription factors: the atypical helix-loop-helix transcription factor early B-cell factor 2 (EBF2) and the homeodomain transcription factors Engrailed1 and 2 (EN1/2). EBF2 directs the fate of clusters towards a zebrinII positive versus zebrinII negative lineage [4], whereas EN1/2 control the anterior-posterior and medial-lateral positioning of marker expression in each cluster [1,5]. Purkinje cell marker expression is temporally regulated as early markers demarcate embryonic clusters [6,7], late markers define adult zones [8], and constitutively expressed markers link the clusters and zones [1,9,10]. The zones are then refined into precise functional circuit modules by neuronal activity [11].
A critical function of this coordinated program of Purkinje cell patterning is to establish the rest of the cerebellar circuit. This is reflected in the organization of mossy fiber and climbing fiber afferents, which assemble into a zonal plan that is identical to the Purkinje cell map [12,13]. For mossy fibers, we previously showed that the process is both genetically determined [14] and refined by activity [11]. However, the morphogenetic mechanism that guides mossy fiber patterning is still unclear. Part of the hurdle in solving this problem is that mossy fiber development is very dynamic. They initially contact Purkinje cells and then switch postnatally to make synaptic contacts with their adult targets, granule cells [15]. Here, I used a transynaptic tracing approach to better understand the stage when mossy fibers interact with Purkinje cells.
MATERIALS AND METHODS
Animal maintenance
Mouse husbandry and experiments were performed under an approved Institutional Animal Care and Use Committee (IACUC) protocol at Baylor College of Medicine. Male and female Swiss Webster mice were obtained from Taconic (Hudson, NY, USA) and a colony established and maintained in house. Noon on the day a vaginal plug was detected was considered embryonic day (E) 0.5. The day of birth was designated as postnatal day (P) 0.
Neural tracing
Anterograde tracing was performed according to previous protocols [16]. Approximately 250 nl of a 2% solution of WGA (wheat germ agglutinin) conjugated to Alexa Fluor 555 (Cat. #W32464, Invitrogen, Carlsbad, CA) was pressure injected over 5 min into the lower thoracic-upper lumbar spinal cord of P4 mice. After a 24-hour survival period the mice were anesthetized with avertin (2, 2, 2-Tribromoethanol), the blood flushed with 0.1 M phosphate-buffered saline (PBS; pH 7.2), and then perfused with 4% paraformaldehyde (PFA). The WGA-Alexa 555 traced tissue was cut and mounted for imaging using a Zeiss Apotome.2 acquisition system (see below) or counterstained by immunohistochemistry and then imaged.
Analysis of traced tissue
The parasagittal pattern of WGA labeled mossy fibers is remarkably consistent from animal to animal [11,16]. I restricted the injection locus to within 1-2 vertebral segments and delivered the same volume of tracer into each animal; these measures together ensure that a consistent pool of spinal neurons is marked. The 250 nl injections performed in this study are considered relatively large, and therefore the tracer is taken up by neurons across the medial-lateral extent of the injected spinal segment. This strategy ensures that the major spinal sources to the cerebellum, for example Clarke's column, are properly labeled. Note that changing the volume and timing allowed for tracing changes the robustness of axon and terminal labeling within each zone, but these modifications do not affect the number and pattern of zones that are revealed [16]. I examined the spinocerebellar mossy fibers only in the vermis because their terminals are heavily restricted to the medial cerebellum in both developing and adult mice [16].
Immunohistochemistry
Immunohistochemistry was carried out as described previously [11,14,16]. We used an anti-SMI-32 primary antibody (1:1500; Covance, Princeton, NJ,) to reveal Purkinje cell zones. See White and Sillitoe [17] for details about the NFH staining pattern.
Imaging and data analysis
Photomicrographs of tissue sections were captured with a Zeiss AxioCam MRc5 camera mounted on a Zeiss Axio Imager.M2 microscope. Images of tissue sections were acquired with the Zeiss Apotome.2 system and analyzed using Zeiss ZEN software (2012 edition). After imaging, the raw data was imported into Adobe Photoshop CS5 and corrected for brightness and contrast levels. Schematics were drawn in Adobe Illustrator CS5.
RESULTS
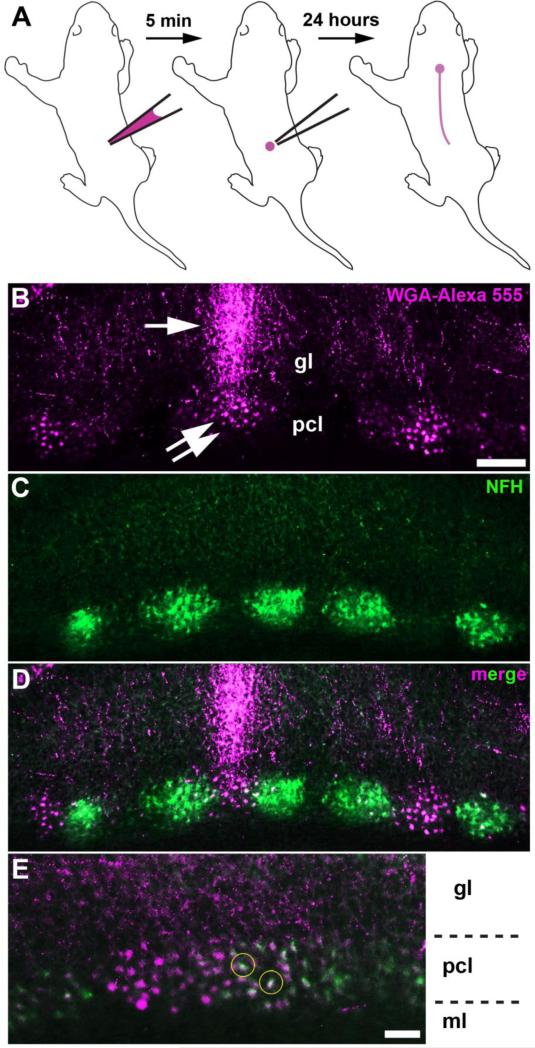
The spinocerebellar map first shows an obvious pattern of parasagittal zones between ~P4 and P5 in mouse [14]. Therefore, to better understand the nature of the mossy fiber to Purkinje cell interaction, I injected WGA-Alexa 555 into the lower-thoracic upper-lumbar spinal cord of P4 mice and then examined the cerebellum one day later at P5 (n > 15 mice; Fig. 1a). I followed the labeled afferents into the cerebellum where they terminated into zones. The pattern of labeled zones was consistent across all animals examined. Remarkably, the afferent zones were associated with clusters of WGA-Alexa 555 labeled neuronal somata (Fig. 1b). This result suggested that the tracer traveled transynaptically, but only into neurons that were in close proximity to the labeled afferents (Fig. 1b). To test if the labeled neurons reflected the known zonal organization of the cerebellum, and to determine whether they were Purkinje cells, I co-labeled the tissue with neurofilament heavy chain (NFH), a marker of postnatal Purkinje cell zones [17]. NFH expression revealed a pattern of zones that was complementary to a subset of traced Purkinje cells (Fig. 1c, d). Some traced neurons were immunoreactive for NFH (Fig. 1e). The zonal pattern of NFH within clusters of Purkinje cells [17] and the spinocerebellar mossy fiber domains [16] are both refined during postnatal development–at later stages, the projections that overlap with NFH are likely partially pruned away when zonal boundaries sharpen. Therefore, the transynaptic transfer of WGA-Alexa 555 not only reveals how the postnatal circuitry is initially organized, but it also suggests a coordinated sculpting of pre- and post-synaptic components during cerebellar wiring.
Fig 1.
a Schematic illustrating the injection of WGA-Alexa 555 into the spinal cord of a pup. b WGA-Alexa 555 tracing of spinocerebellar mossy fibers (arrow) and transynaptic tracing of neuronal somata (double arrow) in the anterior cerebellum. c, d NFH staining revealed a pattern of zones that was complementary to a subset of traced Purkinje cells. e, Some of the WGA-Alexa 555 traced neuronal somata were co-labeled with NFH, a marker for Purkinje cells (yellow circles). The layers of the cerebellar cortex are indicated by ml (molecular layer), pcl (Purkinje cell layer), and gl (granular layer). The scale bar = 100 μm in B (applies to C, D) and 50 μm in E.
DISCUSSION
WGA based neuronal tracers can travel transynaptically. Of specific interest is WGA-Alexa 488, which was recently reported to undergo retrograde transynaptic transport [18]. Here, I demonstrate that a closely related tracer, WGA-Alexa 555, exhibits anterograde transynaptic transport. The main finding of this study may be integrated into a model for how cerebellar mossy fiber topography might develop. Initially, afferents invade the cerebellum using a process that is guided by molecular cues. Such cues may also mediate a matching mechanism that links afferents with a crude map of Purkinje cell zones [12]. Genetic experiments show that blocking transcription factor function alters Purkinje cell and afferent maps in a predictable manner [14]. During this period, the mossy fibers make transient physical contacts with Purkinje cells [15], and here I show that these contacts are restricted to zones (Fig. 1). Importantly, impulses can be transmitted across these transient direct mossy fiber contacts to elicit simple spikes in the connected Purkinje cells [19]. This is critical because cerebellar cortical activity is required for fine-tuning the afferent map into precise zones [20], and we recently showed that Purkinje cell GABAergic neurotransmission is particularly crucial for the process zone of refinement [11]. Loss of Purkinje cell neurotransmission and its effects on zonal wiring and circuit firing resulted in ataxia and disequilibrium [11]. I therefore raise the intriguing possibility that transient zonal connections might have a long-term impact on the function and dysfunction of the cerebellum.
Acknowledgements
This work was supported by funds from Baylor College of Medicine and Texas Children's Hospital. R.V.S. received support from The Bachmann-Strauss Dystonia and Parkinson Foundation, Inc., The Caroline Wiess Law Fund for Research in Molecular Medicine, BCM IDDRC 1U54HD083092, National Center For Research Resources C06RR029965, The Mrs. Clifford Elder White Graham Endowed Research Fund, and the National Institutes of Neurological Disorders and Stroke 1R01NS089664. The BCM IDDRC Neuropathology Core performed a portion of the immunohistochemistry and histology experiments. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health (NIH).
Footnotes
Conflicts of Interest: I have nothing to disclose.
REFERENCES
- 1.White JJ, Sillitoe RV. Development of the cerebellum: from gene expression patterns to circuit maps. Wiley Interdiscip. Rev. Dev. Biol. 2013;2:149–64. doi: 10.1002/wdev.65. [DOI] [PubMed] [Google Scholar]
- 2.Hashimoto M, Mikoshiba K. Mediolateral Compartmentalization of the Cerebellum Is Determined on the “ Birth Date ” of Purkinje Cells. 2003;23:11342–51. doi: 10.1523/JNEUROSCI.23-36-11342.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sudarov A, Turnbull RK, Kim EJ, Lebel-Potter M, Guillemot F, Joyner AL. Ascl1 genetics reveals insights into cerebellum local circuit assembly. J. Neurosci. 2011;31:11055–69. doi: 10.1523/JNEUROSCI.0479-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Croci L, Chung S-H, Masserdotti G, Gianola S, Bizzoca A, Gennarini G, et al. A key role for the HLH transcription factor EBF2COE2,O/E-3 in Purkinje neuron migration and cerebellar cortical topography. Development. 2006;133:2719–29. doi: 10.1242/dev.02437. [DOI] [PubMed] [Google Scholar]
- 5.Millen K, Hui C, Joyner A. A role for En-2 and other murine homologues of Drosophila segment polarity genes in regulating positional information in the developing cerebellum. Development. 1995;121:3935–45. doi: 10.1242/dev.121.12.3935. [DOI] [PubMed] [Google Scholar]
- 6.Larouche M, Hawkes R. From clusters to stripes: the developmental origins of adult cerebellar compartmentation. Cerebellum. 2006;5:77–88. doi: 10.1080/14734220600804668. [DOI] [PubMed] [Google Scholar]
- 7.Wassef M, Zanetta JP, Brehier A, Sotelo C. Transient biochemical compartmentalization of Purkinje cells during early cerebellar development. Dev. Biol. 1985;111:129–37. doi: 10.1016/0012-1606(85)90441-5. [DOI] [PubMed] [Google Scholar]
- 8.Brochu G, Maler L, Hawkes R. Zebrin II: a polypeptide antigen expressed selectively by Purkinje cells reveals compartments in rat and fish cerebellum. J. Comp. Neurol. 1990;291:538–52. doi: 10.1002/cne.902910405. [DOI] [PubMed] [Google Scholar]
- 9.Marzban H, Chung S, Watanabe M, Hawkes R. Phospholipase Cbeta4 expression reveals the continuity of cerebellar topography through development. J. Comp. Neurol. 2007;502:857–71. doi: 10.1002/cne.21352. [DOI] [PubMed] [Google Scholar]
- 10.Fujita H, Morita N, Furuichi T, Sugihara I. Clustered fine compartmentalization of the mouse embryonic cerebellar cortex and its rearrangement into the postnatal striped configuration. J. Neurosci. 2012;32:15688–703. doi: 10.1523/JNEUROSCI.1710-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White JJ, Arancillo M, Stay TL, George-Jones NA, Levy SL, Heck DH, et al. Cerebellar zonal patterning relies on Purkinje cell neurotransmission. J. Neurosci. 2014;34:8231–45. doi: 10.1523/JNEUROSCI.0122-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sotelo C. Cellular and genetic regulation of the development of the cerebellar system. Prog. Neurobiol. 2004;72:295–339. doi: 10.1016/j.pneurobio.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Apps R, Hawkes R. Cerebellar cortical organization: a one-map hypothesis. Nat. Rev. Neurosci. 2009;10:670–81. doi: 10.1038/nrn2698. [DOI] [PubMed] [Google Scholar]
- 14.Sillitoe RV, Vogel MW, Joyner AL. Engrailed homeobox genes regulate establishment of the cerebellar afferent circuit map. J. Neurosci. 2010;30:10015–24. doi: 10.1523/JNEUROSCI.0653-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalinovsky A, Boukhtouche F, Blazeski R, Bornmann C, Suzuki N, Mason CA, et al. Development of axon-target specificity of ponto-cerebellar afferents. PLoS Biol. 2011;9:e1001013. doi: 10.1371/journal.pbio.1001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reeber SL, Gebre SA, Sillitoe RV. Fluorescence mapping of afferent topography in three dimensions. Brain Struct. Funct. 2011;216:159–69. doi: 10.1007/s00429-011-0304-2. [DOI] [PubMed] [Google Scholar]
- 17.White JJ, Sillitoe RV. Postnatal development of cerebellar zones revealed by neurofilament heavy chain protein expression. Front. Neuroanat. 2013;7:9. doi: 10.3389/fnana.2013.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goshgarian HG, Buttry JL. The pattern and extent of retrograde transsynaptic transport of WGA-Alexa 488 in the phrenic motor system is dependent upon the site of application. J. Neurosci. Methods. 2014;222:156–64. doi: 10.1016/j.jneumeth.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takeda T, Maekawa K. Transient direct connection of vestibular mossy fibers to the vestibulocerebellar Purkinje cells in early postnatal development of kittens. Neuroscience. 1989;32:99–111. doi: 10.1016/0306-4522(89)90110-3. [DOI] [PubMed] [Google Scholar]
- 20.Tolbert DL, Pittman T, Alisky JM, Clark BR. Chronic NMDA receptor blockade or muscimol inhibition of cerebellar cortical neuronal activity alters the development of spinocerebellar afferent topography. Dev. Brain Res. 1994;80:268–74. doi: 10.1016/0165-3806(94)90112-0. [DOI] [PubMed] [Google Scholar]