Abstract
The control of deep cerebellar nuclear (DCN) neuronal firing is central to cerebellar function, but is not well understood. The large majority of synapses onto DCN neurons derive from Purkinje cells (PCs), suggesting that PC activity is an important determinant of DCN firing; however, PCs fire both simple and complex spikes (CSs), and little is known about how the latter's action affects DCN activity. Thus, here we explored the effects of CSs on DCN activity. CSs were recorded from PC arrays along with individual DCN neurons. Presumed synaptically connected PC-DCN cell pairs were identified using CS-triggered correlograms of DCN activity, which also showed that CS activity was associated with a predominantly inhibitory effect on DCN activity. The strength of the CS effect varied as a function of synchrony, such that isolated CSs produced only weak inhibition of DCN activity, whereas highly synchronous CSs caused a larger drop in firing levels. Although the present findings were obtained in anesthetized animals, similar CS synchrony levels exist in awake animals, and changes in synchrony level have been observed in association with movements in awake animals. Thus, the present data suggest that synchronous CS activity may be a mechanism for shaping DCN output related to motor commands.
The DCN are central to cerebellar function, as their activity represents the majority of cerebellar output. Yet, relatively little is known about how the spiking patterns of individual DCN neurons are determined by their synaptic input. For example, the major source of synapses (70% – 80%) onto DCN neurons are PCs [1, 2]. Yet, surprisingly, given the inhibitory nature of PCs, DCN activity and PC simple spike levels often co-vary during behavior, at least on a population level (e.g., the majority of PCs and DCN neurons showing locomotion-related activity increase their firing rates during the swing phase of walking, as can be seen by comparing figure 6's in [3, 4]. Such co-variation could be taken to imply that the massive PC input to the DCN only serves to tamp down the response of DCN cells to the excitatory drive from collaterals of mossy and/or climbing fibers, as opposed to being the prime determinant of behaviorally-related modulation of DCN activity. However, this conclusion rests on the assumption that average firing rate is the critical functional parameter of PC and DCN activity.
Recent evidence, in fact, suggests that the pattern of PC spiking may be a critical parameter for determining DCN firing [5]. Consistent with this possibility, and excepting for the nucleo-olivary projection cells [6], PC-evoked IPSCs in DCN neurons are very brief (τdecay = 2.4 ms) [5]. The shortness of the IPSC duration suggests that effective synaptic integration in most DCN neurons requires highly synchronized input. Intriguingly, such synchronization of both simple spikes [7–10] and CSs [11–13] have been shown to occur. The patterns of synchronous CSs suggest that they, in particular, would provide highly convergent synchronous inhibition to individual DCN neurons. Specifically, CS activity has been shown to be synchronized among PCs within extended strips of cerebellar cortex that are relatively restricted (typically 250–500 µm wide) along the longitudinal folial axis, but that can extend for millimeters in the transverse folial direction [12–14]. Furthermore, such strips of PCs tend to align with the zebrin compartments [15], and PCs within a single zebrin compartment project to the same DCN region [16, 17]. Therefore, DCN neurons are likely to receive convergent input from PCs whose CS activity is synchronized, and thus we investigated the importance of synchrony for the inhibitory effect of CS activity on DCN firing.
Methods
Experiments were performed in accordance with the NIH’s Guide for the Care and Use of Laboratory Animals. Experimental protocols were approved by the Institutional Animal Care and Use Committee of New York University School of Medicine.
PC and DCN Recordings
All data were obtained from a series of recordings that were previously reported, and the details of the experimental methods can be found in that report [18]. In brief, all experiments were performed on female Sprague-Dawley rats (225–300 g) under ketamine/ xylazine anesthesia delivered as an initial dose (~100 mg/kg, 8 mg/kg ip) followed by a continuous infusion via a femoral vein catheter. Extracellular recordings of CS activity were made using a multielectrode array implanted on crus IIa. Electrodes were typically implanted to a depth of 100–150 µm, where CS activity could be isolated in the absence of simple spikes and stably recorded for the duration of the experiment. Once the multielectrode array was completed, a single microelectrode was used to locate DCN neurons. The typical recording session, in which CS activity from the PC array and an individual DCN neuron were recorded, lasted 20 min. At the end of the recording sessions the animal was perfused under deep anesthesia to allow histological reconstruction of the electrode track and verification of the location of the DCN recordings.
Data Analysis
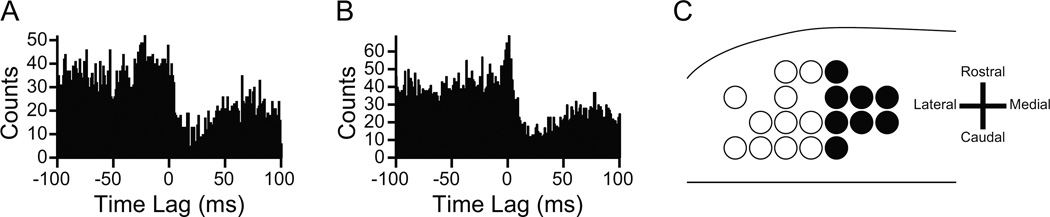
Offline analysis of CS-DCN cross-correlograms was performed to identify presumed synaptically-connected PC-DCN cell pairs. In brief, identification rested on the correlogram showing the presence of inhibition of DCN activity starting within 1–5 ms of the onset of a CS and having a relatively flat baseline in the 50 ms period preceding the CS (Fig. 1A; for further details, see [18]). The inhibition could be preceded by a transient excitation that likely reflected the effect of climbing fiber collaterals on the DCN cell (Fig. 1B; for further details, see [18]). Only experiments in which at least four such PC-DCN cell pairs were present were analyzed for the present paper.
Figure 1.
(A, B) CS triggered correlograms of DCN activity that were used to identify a monosynaptic connection between the PC and DCN neuron being recorded. In (A) the correlogram shows a sharp drop in activity within a few milliseconds of the CS (t = 0). In (B) the inhibition is preceded by a brief increase in activity, presumably due to excitation of the DCN neuron by collaterals of the olivocerebellar axon. (A) and (B) are based on figure 4A, B in Blenkinsop TA and Lang EJ (2011) J Neuroscience 31: 14708–14720 with permission granted under the copyright policy of J Neuroscience. (C) Schematic of the electrode array from one experiment showing the clustering of PCs that projected to the DCN neuron being recorded. Filled circles indicate PCs that projected to the DCN neuron, open circles represent the remaining PCs in the array. The spacing between PCs was 250 µm.
To define synchrony amongst members of a group of PCs that projected to the same DCN cell, the time of a CS was taken as its onset. CSs in the other PCs of the group were considered synchronous with a CS in the reference PC if they occurred within +/− 5 ms of its onset. Each CS was thus assigned a synchrony level according to how many PCs in the group fired synchronously, which could run from 1 (a spike in only the reference cell) to the total number of PCs in the group.
Results
The results presented here are based on an analysis of a subset of experiments reported in [18] in which CS activity was recorded from arrays of PCs simultaneously with the activity of a DCN neuron. Three such experiments were chosen for analysis because they had at least four PCs (n = 4, 7, and 7 PCs) in the array that had correlograms that fit the criteria described in the Methods for being synaptically-connected to the DCN cell being recorded. These groups of PCs were spatially organized into narrow bands on crus IIa (Fig. 1C), and spontaneous CS activity showed a high level of synchrony among the PCs comprising each group.
Analysis of CS-DCN correlograms indicated that the predominant effect of CS activity was to inhibit DCN spiking, as previously reported [18]. This inhibition could last for more than 100 ms, but was strongest during the period from 1 – 50 ms following the CS, and thus we used this initial period of inhibition to quantify how the strength of the inhibition varied with synchrony level. The average height of the 50 1-ms bins of the correlogram during this period was compared to the average bin height for the baseline activity during the 50 bins preceding the time of the CS. When all CSs of a PC were used to construct the CS-DCN correlogram the average percent change in activity from baseline was −36.0 ± 12.8% (n = 18 PCs).
To investigate the effect of synchrony on the strength of the inhibition, each PC's CSs were divided into sets according to their synchrony level. CS-DCN correlograms were then constructed for each set, and the percent inhibition calculated for each. The inhibitory effect of isolated CSs (−20.6 ± 9.8%; one out of four PCs for one experiment or one out of seven PCs for the other two experiments) was significantly weaker than the average inhibitory effect obtained from all CSs (paired t-test, p = 1.0 × 10−5). In contrast, as the synchrony level increased, the inhibitory effect increased until plateauing at the highest levels (−64.5 ± 14.3 %; comparison of inhibition caused by isolated CSs versus highly synchronous CSs, either 4 out of 4 PCs or 6–7 out of 7 PCs; paired t-test, p = 1.9 × 10−7; n = 18 PCs).
Discussion
Why PCs generate two types of spikes is among the most intriguing and central unresolved questions of cerebellar physiology. A common answer to this question is that the output signal of the PC is carried by simple spike activity, while CSs are assumed to play only a role in modulating the synaptic plasticity underlying motor learning. However, CSs do cause axonically-propagated spikes [19–21] that inhibit DCN neurons [18, 22], which raises the possibility that CSs make a direct contribution to the ongoing motor commands being generated by the DCN.
The much greater levels of simple spike activity immediately raise the question of mechanism. There are several potential answers to this question, however, anatomical and physiological evidence point to CS synchrony as a likely, and perhaps predominant part, of the solution. In particular, the results presented here show that PCs having synchronous CS activity can converge onto the same DCN neuron and as a result cause greater inhibition of its activity. This finding is consistent with our previous work showing high levels of CS synchrony among PCs located within the same zebrin band [15], because such PCs project to the same DCN region [16, 17].
It is important to note that the dominant effect of synchronous CS activity we observed was inhibitory, and so is consistent with the CS itself, and not the subsequent pause in simple spikes, being the main determinant of CS-associated changes in DCN activity. It should also be noted that in many instances (e.g., Fig. 1B) a transient initial increase in DCN firing is associated with CS activity; however, the timing of this transient is more consistent with its being caused by excitation of the DCN neuron via an olivocerebellar axonal collateral than with its being the result of the post-CS pause in simple spike activity [18].
Interestingly, there is evidence that timing (asynchronous versus synchronous) may also be an important determinant of simple spike action on the DCN [5], raising the possibility that synchrony is an important parameter for both CSs and simple spikes. Dynamic clamp simulations of simple spike triggered IPSCs showed that more synchronous patterns allowed greater levels of DCN firing [5], the opposite of what we found for synchronization of CS activity. However, in the experiments of [5] it is not fully clear whether the presence of synchronous IPSCs or the opening of large gaps of time in which no IPSCs were present was the main underlying factor of the resulting changes in DCN firing. Furthermore, synchronization of simple spike activity occurs over a much more restricted area than does CS synchrony, and so the absolute number of simultaneous IPSCs comprising synchronous simple spike events likely will be much less than the number resulting from synchronous CS events, although how much less will depend on the exact spatial distribution of PCs that converge to a single DCN neuron, something that is not known.
In sum, the results provide evidence for synchrony being a mechanism by which CS activity can directly modulate DCN firing patterns, and thus directly contribute to ongoing motor commands. Although these results were obtained in anesthetized animals, the levels of CS synchrony recorded here are similar to those in awake animals [13], and changes in CS synchrony levels have been shown to occur with movement [23–25], indicating that synchronous CSs would likely have a similar effect on DCN activity during behavior.
Acknowledgments
Funding
This work was supported by grants to EJL from the National Science Foundation (IOS-1051858) and the National Institute of Neurological Disorders (NS-37028, NS-95089).
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
References
- 1.De Zeeuw CI, Berrebi AS. Postsynaptic targets of Purkinje cell terminals in the cerebellar and vestibular nuclei of the rat. Eur J Neurosci. 1995;7:2322–2333. doi: 10.1111/j.1460-9568.1995.tb00653.x. [DOI] [PubMed] [Google Scholar]
- 2.Palkovits M, Mezey E, Hamori J, Szentagothai J. Quantitative histological analysis of the cerebellar nuclei in the cat. I. Numerical data on cells and on synapses. Exp Brain Res. 1977;28:189–209. doi: 10.1007/BF00237096. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong DM, Edgley SA. Discharges of nucleus interpositus neurones during locomotion in the cat. J Physiol (Lond) 1984a;351:411–432. doi: 10.1113/jphysiol.1984.sp015253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong DM, Edgley SA. Discharges of Purkinje cells in the paravermal part of the cerebellar anterior lobe during locomotion in the cat. J Physiol (Lond) 1984b;352:403–424. doi: 10.1113/jphysiol.1984.sp015300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Person AL, Raman IM. Purkinje neuron synchrony elicits time-locked spiking in the cerebellar nuclei. Nature. 2012;481:502–505. doi: 10.1038/nature10732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Najac M, Raman IM. Integration of purkinje cell inhibition by cerebellar nucleo-olivary neurons. J Neurosci. 2015;35:544–549. doi: 10.1523/JNEUROSCI.3583-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heck DH, Thach WT, Keating JG. On-beam synchrony in the cerebellum as the mechanism for the timing and coordination of movement. Proc Natl Acad Sci USA. 2007;104:7658–7663. doi: 10.1073/pnas.0609966104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bell CC, Grimm RJ. Discharge properties of Purkinje cells recorded on single and double microelectrodes. J Neurophysiol. 1969;32:1044–1055. doi: 10.1152/jn.1969.32.6.1044. [DOI] [PubMed] [Google Scholar]
- 9.de Solages C, Szapiro G, Brunel N, Hakim V, Isope P, Buisseret P, et al. High-frequency organization and synchrony of activity in the purkinje cell layer of the cerebellum. Neuron. 2008;58:775–788. doi: 10.1016/j.neuron.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Wise AK, Cerminara NL, Marple-Horvat DE, Apps R. Mechanisms of synchronous activity in cerebellar Purkinje cells. J Physiol (Lond) 2010;588:2373–2390. doi: 10.1113/jphysiol.2010.189704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bell CC, Kawasaki T. Relations among climbing fiber responses of nearby Purkinje cells. J Neurophysiol. 1972;35:155–169. doi: 10.1152/jn.1972.35.2.155. [DOI] [PubMed] [Google Scholar]
- 12.Sasaki K, Bower JM, Llinas R. Multiple Purkinje cell recording in rodent cerebellar cortex. Eur J Neurosci. 1989;1:572–586. doi: 10.1111/j.1460-9568.1989.tb00364.x. [DOI] [PubMed] [Google Scholar]
- 13.Lang EJ, Sugihara I, Welsh JP, Llinás R. Patterns of spontaneous Purkinje cell complex spike activity in the awake rat. J Neurosci. 1999;19:2728–2739. doi: 10.1523/JNEUROSCI.19-07-02728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sugihara I, Lang EJ, Llinás R. Uniform olivocerebellar conduction time underlies Purkinje cell complex spike synchronicity in the rat cerebellum. J Physiol (Lond) 1993;470:243–271. doi: 10.1113/jphysiol.1993.sp019857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sugihara I, Marshall SP, Lang EJ. Relationship of complex spike synchrony to the lobular and longitudinal aldolase C compartments in crus IIA of the cerebellar cortex. J Comp Neurol. 2007;501:13–29. doi: 10.1002/cne.21223. [DOI] [PubMed] [Google Scholar]
- 16.Chung SH, Marzban H, Hawkes R. Compartmentation of the cerebellar nuclei of the mouse. Neuroscience. 2009;161:123–138. doi: 10.1016/j.neuroscience.2009.03.037. [DOI] [PubMed] [Google Scholar]
- 17.Sugihara I, Fujita H, Na J, Quy PN, Li BY, Ikeda D. Projection of reconstructed single Purkinje cell axons in relation to the cortical and nuclear aldolase C compartments of the rat cerebellum. J Comp Neurol. 2009;512:282–304. doi: 10.1002/cne.21889. [DOI] [PubMed] [Google Scholar]
- 18.Blenkinsop TA, Lang EJ. Synaptic action of the olivocerebellar system on cerebellar nuclear spike activity. J Neurosci. 2011;31:14708–14720. doi: 10.1523/JNEUROSCI.3323-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ito M, Simpson JI. Discharges in Purkinje cell axons during climbing fiber activation. Brain Res. 1971;31:215–219. doi: 10.1016/0006-8993(71)90648-2. [DOI] [PubMed] [Google Scholar]
- 20.Monsivais P, Clark BA, Roth A, Häusser M. Determinants of action potential propagation in cerebellar Purkinje cell axons. J Neurosci. 2005;25:464–472. doi: 10.1523/JNEUROSCI.3871-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khaliq ZM, Raman IM. Axonal propagation of simple and complex spikes in cerebellar Purkinje neurons. J Neurosci. 2005;25:454–463. doi: 10.1523/JNEUROSCI.3045-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lang EJ, Blenkinsop TA. Control of cerebellar nuclear cells: a direct role for complex spikes? Cerebellum. 2011;10:694–701. doi: 10.1007/s12311-011-0261-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Welsh JP, Lang EJ, Sugihara I, Llinás R. Dynamic organization of motor control within the olivocerebellar system. Nature. 1995;374:453–457. doi: 10.1038/374453a0. [DOI] [PubMed] [Google Scholar]
- 24.Ozden I, Sullivan MR, Lee HM, Wang SS. Reliable coding emerges from coactivation of climbing fibers in microbands of cerebellar Purkinje neurons. J Neurosci. 2009;29:10463–10473. doi: 10.1523/JNEUROSCI.0967-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mukamel EA, Nimmerjahn A, Schnitzer MJ. Automated analysis of cellular signals from large-scale calcium imaging data. Neuron. 2009;63:747–760. doi: 10.1016/j.neuron.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]