The National Institute of Neurological Disorders and Stroke (NINDS) established the NIH StrokeNet to facilitate the rapid initiation and efficient implementation of small and large multi-site exploratory and confirmatory clinical trials focused on promising interventions for stroke prevention, treatment, and recovery, as well as validation studies of biomarkers or outcome measures. The network is open to execute high impact trial ideas that can come from any corner of the wide stroke community. This network, which was initiated in the Fall of 2013, currently involves 288 hospitals across the U.S. and is designed to serve as the infrastructure and pipeline for new potential treatments for patients with stroke and those at risk for stroke. NIH StrokeNet also provides a tremendous educational platform for stroke physicians and other health care professionals, particularly those individuals in training and focused on an academic career. To maximize the impact of NIH StrokeNet, it is important for the larger stroke community to know its structure and the process and timeline by which stroke trials are developed and implemented. For more detailed information regarding the NIH StrokeNet, its ongoing trials, and educational webinars, one can visit the website https://www.nihstrokenet.org/
Organizational Structure
The NIH StrokeNet is a cooperative research program supported by the NINDS. The main components include the National Coordinating Center (NCC) at the University of Cincinnati, the National Data Management Center (NDMC) at the Medical University of South Carolina and 25 academic regional coordinating centers (RCC) across the U.S.
The NCC is responsible for the network infrastructure, initiation of collaborative relationships, facilitation of the design, oversight, and management of network studies. Operationally, the NCC is the home for a Central Institutional Review Board (cIRB) for NIH StrokeNet. Each participating network hospital signs a reliance agreement with the University of Cincinnati that delegates the responsibility of human subject protection review to the cIRB at the University of Cincinnati. The site IRBs remain in close communication with the cIRB to provide knowledge of the local research context. Each hospital also signs a master trial agreement that includes a standardized rate which outlines the participation requirements and financial payment structure for all trials conducted within the network. The NCC works with the protocol PI and his/her team to manage trials within NIH StrokeNet.
The NCC houses the NIH StrokeNet educational core which works closely with the StrokeNet RCCs who have funded programs to advance the training of new clinical stroke investigators, including trainees from many different disciplines. The core provides a detailed educational program for trainees, including webinars focused on stroke, research methodology, and professional development.
The NDMC is the centralized data management structure for NIH StrokeNet. Working with the Protocol PI and statistical investigators for a given trial, the NDMC assists in the development of the protocol, case report forms, and statistical analysis plan, creates the study database, and programs the randomization process within WebDCU™, the clinical trial management software that is used for all NIH StrokeNet Trials. During trial implementation, the NDMC is responsible for data management, site monitoring, interim data analysis and statistical reports generation, tracking of neuroimaging, and interaction with the Data Safety Monitoring Board through a unblinded statistician at the NDMC. The NDMC can also provide the primary blinded statistical effort for a proposed NIH StrokeNet trial, but many proposed trials have a separate primary blinded statistician from another institution. Finally, upon completion of the study, the NDMC provides assistance to the primary statistician with the final analyses and creates and submits to the NINDS public use data sets of the trial, as appropriate.
The RCCs are the backbone of NIH StrokeNet. Each RCC is a hub for multiple clinical sites that include acute care hospitals, rehabilitation facilities and pediatric hospitals. At some of the participating hospitals, the research staff at the RCC provides clinical research support for NIH StrokeNet trials. These hospitals are referred to as performance sites. Some of the participating hospitals within the region of an RCC have their own research staff and infrastructure that handles all of the clinical research support for NIH StrokeNet Trials. These participating hospitals are designated as satellite hospitals, and each of them sign a reliance agreement and master trial agreement with the NCC, just like the RCC. The RCC determines whether to include an interested hospital within their network.
There are multiple key subgroups within NIH StrokeNet that work with protocol PI(s) to provide input on trial design. Physician leaders from the NCC lead the three primary working groups within NIH StrokeNet: Prevention, Acute treatment, and Recovery. The working group composition includes members from the 25 RCCs with interest and expertise specific for a given working group, a statistician from the NDMC, and a member from the Imaging Core. These three groups provide the opportunity to give scientific and clinical input for potential study designs generated within and outside of NIH StrokeNet. Most importantly, they help to assess the feasibility of specific trial proposals (see process below) and become a resource as trials are implemented.
NIH StrokeNet also provides an Imaging Core, which provides scientific input and practical guidance for proposed trials within NIH StrokeNet. Members of this core can participate in the imaging component of a given trial, but imaging analysis expertise for a given trial is decided by the protocol PI(s).
Finally, advisory committees have been developed within the NIH StrokeNet. For example, the Minority Recruitment and Retention Advisory Committee helps to develop strategies that address recruitment of women and minorities. The Interventional Advisory Committee has helped to address the changes in clinical practice and trial design necessitated by the positive findings in the recent endovascular trials.
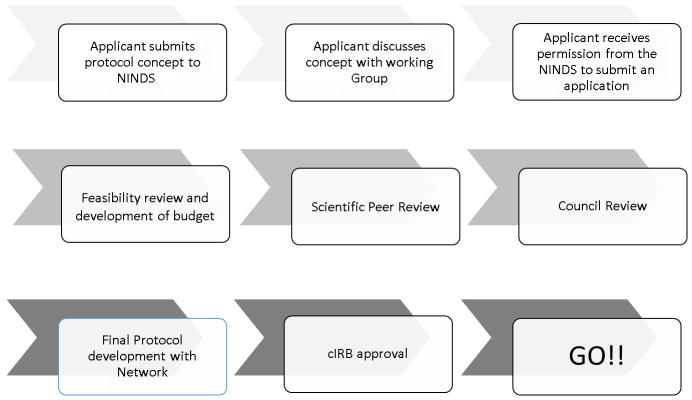
The Introduction and Implementation of Stroke Trials within NIH StrokeNet (Figure 1)
Figure.
The Introduction and Implementation of Stroke Trials within NIH StrokeNet
Working with StrokeNet is a cooperative venture between NINDS, the StrokeNet network, and the applicant. The NIH StrokeNet is an open network; trial concepts can be initiated by investigators outside of NIH StrokeNet as well as by NIH StrokeNet investigators. Studies to be conducted through the network are funded through an NINDS grant application process summarized below. As for all clinical trials, an applicant with a reasonably developed concept for a trial should first contact NINDS program staff for preliminary determination of fit with NINDS mission and priorities. If a project fits within the NINDS mission and appropriate for the Stroke network, the applicant has the option to obtain feedback from the respective working group within NIH StrokeNet, as well as general guidance from the NCC and NDMC about developing a budget. As per any clinical trial proposal, the protocol PI(s) must seek formal permission from NINDS to submit a grant application. Included with this request is a completed trial concept form with an estimated total budget (direct costs) for the project. Projects receiving permission to proceed by the NINDS are then referred to the NIH StrokeNet Executive Committee to assess network feasibility for the project. Once NINDS permission is received, it takes approximately 3 months to complete the feasibility assessment, prepare a detailed budget, receive input from the various network advisory groups and cores, and submit the full grant for NIH peer review as detailed below.
The feasibility assessment is an essential step in the process that is overseen by the specific network working group, the protocol PIs, and the NCC epidemiologic assessment. It includes two key parts; a survey which is distributed by the NDMC to all potential sites and an epidemiologic assessment done by the NCC. The survey focuses on key feasibility questions about availability of subjects, technology, expertise, and enthusiasm of investigators to participate in such a trial at each of the participating hospitals within NIH StrokeNet. More than half of RCCs and satellites who are part of the Get with the Guidelines Stroke Network can use this database to estimate available subjects, particularly for acute treatment trials. The epidemiologic assessment focuses on the specific eligibility criteria for a trial by applying them to a population-based database of all strokes and TIAs from the Greater Cincinnati/Northern Kentucky Stroke Study (R01 NS030678) to determine the proportion of subjects who would qualify for a trial. Based upon the results of the survey and the population assessment, the trial’s feasibility and the number of needed clinical sites are determined. This information is critical for the science of the proposal and finalization of the budget. Final development of the grant application is the responsibility of the protocol PI, who submits according to NIH policy and receipt dates.
All StrokeNet grant applications receive scientific peer review from the NINDS clinical trials study section. Following secondary review by the NINDS National Advisory Council, final funding decisions are made by the NINDS Director. Applications selected for funding are referred to StrokeNet for implementation, which includes finalization of the protocol by the Protocol PI (s) working with NCC, NDMC, and working group leadership, approval of a final protocol by the cIRB, development of case report forms, FDA approval of any changes for IND or IDE studies, and approval by the NIH StrokeNet DSMB. NINDS and the NIH StrokeNet Executive Committee will closely monitor all trials competing for similar participants. If a newly approved study is determined to overlap with currently enrolling network trials, the implementation of the study may be delayed until the implications for enrollment of the overlapping trials are resolved.
Industry and small businesses are especially encouraged to apply to NIH to conduct trials within the NIH StrokeNet. NIH has established unique mechanisms for consideration of Industry supported trials. Details can be found at http://grants.nih.gov/grants/guide/pa-files/PAR-14-253.html. The NINDS NeuroNext phase 2 trial network has executed a number of clinical trials of novel therapies coming from industry.
Summary
We are excited by the opportunities that StrokeNet provides for advancing stroke prevention, treatment, and recovery and look forward to working with the larger stroke community. Hospitals in the U.S. interested in participating within NIH StrokeNet may approach an RCC to be added as a potential recruitment site, although the RCC ultimately has the decision-making authority whether to add a site based upon their own capacity to manage additional centers. Because some StrokeNet trials will require more recruitment sites than are currently available within StrokeNet, centers may participate in such StrokeNet trials, even if they are not part of an RCC, if they can demonstrate the ability and expertise to recruit study subjects. Finally, over the past year, we have initiated discussions with other national stroke networks worldwide to coordinate efforts in decreasing the burden of stroke.
Acknowledgments
Funding: Dr. Broderick and Dr. Palesch’s efforts within NIH StrokeNet are supported by NIH grants U01NS086872 and NS087748.
Footnotes
Disclosures: Joseph Broderick: research monies to Department of Neurology from Genentech for role on PRISMS Trial, monies paid to Department of Neurology by Pfizer as consultant. Yuko Palesch: research monies to her department for her role as DSMB member for the Biogen and Brainsgate trials. Scott Janis: none.