Abstract
Background and purpose
Lipoxin A4 (LXA4) has been reported to reduce inflammation in several neurological injury models. We studied the effects of LXA4 on neuroinflammation after subarachnoid hemorrhage (SAH) in a rat model.
Methods
Two hundred and thirty eight Sprague Dawley male rats, weight 280–320 g were used. Exogenous LXA4 (0.3 and 1.0 nmol) were injected intracerebroventricularly at 1.5 hours after SAH. Neurological scores, brain water content and blood-brain barrier were evaluated at 24 hours after SAH; Morris water maze and T-maze tests were examined at 21 days after SAH. The expression of endogenous LXA4 and its receptor formyl peptide receptor 2 (FPR2), as well as p38, IL-1β and IL-6 were studied either by ELISA or western blots. Neutrophil infiltration was observed by myeloperoxidase (MPO) staining. FPR2 siRNA was used to knock down LXA4 receptor.
Results
The expression of endogenous LXA4 decreased and the expression of FPR2 increased after SAH. Exogenous LXA4 decreased brain water content, reduced Evans blue extravasation, and improved neurological functions and improved the learning and memory ability after SAH. LXA4 reduced neutrophil infiltration and phosphorylation of p38, IL-1β and IL-6. These effects of LXA4 were abolished by FPR2 siRNA.
Conclusion
Exogenous LXA4 inhibited inflammation by activating FPR2 and inhibiting p38 after SAH. LXA4 may serve as an alternative treatment to relieve early brain injury after SAH.
Keywords: Subarachnoid hemorrhage, lipoxin A4, formyl peptide receptor 2, inflammation
Introduction
Subarachnoid hemorrhage (SAH) represents a subtype of stroke that carries a high mortality and disability.1 Early brain injury has been reported as the primary cause of mortality in SAH patients and has been considered as a primary target for treatment.2 Recent studies have shown that anti-inflammation may attenuate early brain injury after experimental SAH.3, 4
Lipoxin A4 (LXA4) is one of the important arachidonic acid metabolites and has potent anti-inflammatory properties mediated by its receptor formyl peptide receptor 2 (FPR2),5 including inhibiting pro-inflammatory cytokine production, suppressing the activities of metalloproteinases and enhancing the clearance ability of macrophage.6–8 LXA4 exerted these biological functions through down-regulating the activities of p38 mitogen-activated protein kinase (MAPK), which was mediated by FPR2.7, 9, 10 Several studies have focused on the neuroprotective effects of LXA4 after stroke. Administration of LXA4 methyl ester was reported to reduce pro-inflammatory cytokines TNF-α and IL-1β and up-regulate anti-inflammatory cytokines IL-10 and TGF-β1 in the ischemic brain.11, 12 However, the effects of LXA4 in early brain injury after SAH have not been investigated.
In the present work, we examined the role of LXA4 in a rat model of SAH. The time course of expression of LXA4, its receptor FPR2, and inflammation markers as well as p38 were measured, in the presence of exogenous LXA4.
Materials and Methods
All experiments were approved by the Institutional Animal Care and Use Committee of Loma Linda University.
SAH Animal Model and Experimental Protocol
Two hundred and forty male Sprague-Dawley rats weighing 280 to 320 g (Indianapolis, USA) were used. The endovascular perforation model of SAH in rats was performed as reported previously.13 Briefly, rats were intubated transorally and mechanically ventilated throughout the operation period with 3% isoflurane anesthesia. A sharpened 4-0 monofilament nylon suture was inserted rostrally into the right internal carotid artery from the external carotid artery stump and perforated the bifurcation of the anterior and middle cerebral arteries. Sham-operated rats underwent the same procedures except the suture was withdrawn without puncture.
Two dosages12, 14 (0.3 nmol and 1.0 nmol) of exogenous LXA4 (Cayman Chemical Company, USA) was injected intracerebroventricularly at 1.5 hours after SAH. SAH grades, neurological scores and brain water content were measured at 24 hours.15 Water maze and T-maze were tested from 21 days to 26 days and 27 days respectively.16, 17 LXA4 receptor FPR2 were knocked down by FPR2 siRNA (sc-40123, Santa Cruz Biotechnology, USA) to determine the signaling pathway. All siRNAs were mixed with the same volume of transfection reagent (sc-29528, Santa Cruz Biotechnology, US). The expression and time course of LXA4 and FPR2 were examined by ELISA or western blots both in cortex and hippocampus at 24 hours after SAH. The expression of LXA4 was also examined by ELISA after giving exogenous LXA4. The levels of ALX, p-p38MAPK, IL-1β and IL-6 were measured by western blots at 24 hours after SAH.
Intracerebroventricular Drug Administration
Intracerebroventricular drug administration was performed as previously described.18 Rats were placed in a stereotaxic apparatus under 2.5% isoflurane anesthesia. The needle of a 10-μl Hamilton syringe (Microliter 701, Hamilton Company, USA) was inserted through a burr hole into the right lateral ventricles at the following coordinates relative to bregma: 1.5 mm posterior, 1.0 mm lateral, and 3.2 mm below the horizontal plane of the skull. LXA4 (0.3 nmol and 1.0 nmol) were injected at 1.5 hours after SAH by a pump at a rate of 0.5 μl/min respectively. FPR2 siRNA (500 pmol/3 μl, Santa Cruz Biotechnology, USA) or scrambled siRNA (500 pmol/3 μl, Santa Cruz Biotechnology, USA) dissolved in transfection reagent (Santa Cruz Biotechnology, USA) were injected at 24 hours before SAH induction by a pump at a rate of 0.5 μl/min. We have previously showed that siRNAs were successfully taken up by endothelial cells, ependymal cells, glial cells and neurons in the brain after intracerebroventricular injection.19 To further confirm that siRNA can get to the brain region at the base of skull after intraventricular injection, a fluorescence conjugated siRNA-A (sc-36869, Santa Cruz Biotechnology, US) was injected 24 hours before SAH (n=2).
Severity of SAH
The severity of SAH was quantified by use of the previously published grading scale at the time of euthanasia.20 Briefly, animals were euthanized and the brains were removed. The basal cistern was divided into 6 segments. Each segment was allotted a grade from 0 to 3 depending on the amount of subarachnoid blood in the segment. The animals received a total score ranging from 0 to 18 after adding the scores from all 6 segments. Rats with mild SAH (SAH grades ≤ 7 at 24 hours) were excluded from the study.
Neurological Scores
Neurological scores were evaluated in a blinded fashion at 24 hours after SAH by a blinded observer according to the scoring system of Garcia et al with modifications.21
Morris Water Maze
At day 21 to day 26 after SAH, Morris water maze was performed in a blinded setup as previously described.17 Briefly, it consists of three trials (cued, spatial, and probe) and all trials lasted a maximum of 60 sec. The cued trials have a visible platform above the water level where the animals were allowed to remain on the platform for 10 sec after finding it or being guided to it. The spatial trials have the platform submerged in the water. Once released, they were allowed to swim in search of the platform. Here the time taken to find the platform measured spatial learning. In the probe trial, platform is removed completely and rats were allowed to swim again in search of the platform measuring spatial memory.
T-maze Test
T-maze test for spontaneous alternation has been used to examine exploratory behavior and working memory of hippocampus. As studied previously,22 rats were placed in the stem of a T-shaped maze and allowed to freely explore the 2 arms of the maze throughout a 10-trial continuous alternation session. The spontaneous alternation rate was expressed as the ratio of the alternating choices to the total number of the choice.
Brain Water Content
Brain water content was measured as previously described.19 Animals were euthanized and the brains were removed at 24 hours after surgery. The brains were separated into left hemisphere, right hemisphere, cerebellum and brain stem. Each part was weighed immediately after removal (wet weight). Brain specimens were dried in an oven at 105°C for 72 hours and weighed again (dry weight). The percentage of water content was calculated as ([wet weight dry weight] /wet weight)×100%.
Blood brain barrier Disruption
The permeability of blood brain barrier was evaluated on the basis of Evans blue extravasation, as described previously.23 The brain level of Evans blue was determined at 615 nm for spectrophotometric quantification.
ELISA for LXA4
The level of LXA4 was measured as previously described24 by a rat LXA4 ELISA kit (MyBioSource Inc, USA) following the manufacturer’s instructions. The brain samples were collected at 24 hours after SAH and homogenized with 0.01 M Phosphate-buffered saline. The supernatant was incubated together with LXA4-HRP conjugate in precoated plate and then incubated with a substrate for HRP enzyme. Finally, the absorbance was measured spectrophotometrically at 450 nm in a microplate reader (Bio-Rad iMark, USA).
Fluorescent immunostaining
Double-fluorescence staining was performed at 24 hours after SAH as described previously.19 Sections were incubated overnight at 4°C with rabbit anti-FPR2 antibody (Santa Cruz Biotechnology), goat anti-Ionized calcium binding adaptor molecule 1 (Iba1, Abcam, USA), goat anti-glial fibrillary acidic protein (GFAP, Santa Cruz Biotechnology, USA), mouse anti-Neuronal nuclei (NeuN, EMD Millipore, USA) primary antibodies. Neutrophil infiltration was observed by myeloperoxidase (MPO, Santa Cruz Biotechnology, USA) staining. Appropriate fluorescence dye–conjugated secondary antibodies (Jackson Immunoresearch, USA) were applied in the dark for 1 hour at 21°C. For negative controls, the primary antibodies were omitted and the same staining procedures were performed. Five random microscope fields (×20) in the base of the brain coronal section were imaged by Olympus-BX51 (Olympus, Japen). The number of positive cells was calculated as the mean of the numbers obtained from the 5 pictures.
Western Blotting
The brain samples were collected at 24 hours after SAH. Proteins of the ipsilateral cortex and hippocampus were extracted by homogenizing in RIPA buffer (Santa Cruz Biotechnology, USA). Western blotting was performed as described previously19 using FPR2 (Santa Cruz Biotechnology, USA), phospho-p38 (Santa Cruz Biotechnology), p38 (Santa Cruz Biotechnology), IL-1β (Abcam, USA) and IL-6 (Santa Cruz Biotechnology, USA).
Statistical Analysis
Data were expressed as the mean±SEM. Statistical differences among groups were analyzed by using one-way ANOVA followed by Turkey post hoc test. Mortality was evaluated by Fisher’s exact test. P value of <0.05 was considered statistically significant. All statistical analyses were performed using GraphPad Prism for Windows.
Results
SAH decreased the level of LXA4 and increased the expression of its receptor FPR2. FPR2 expressed in microglias and astrocytes, but not in neurons after SAH
After SAH, there were clear blood clots around basal cistern and the SAH grades decreased gradually as time passed (data not shown). There were significant differences of SAH grades between sham and SAH animals up to 72 hours (Supplement Figure I, p<0,05 between sham and SAH group), but no significantly difference between SAH groups (Supplement Figure I, p>0,05 vs. SAH groups).
The results of ELISA showed the level of LXA4 both in cortex and hippocampus decreased from 6 hours after SAH and reached minimum at 24 hours (Figure 1A and 1B, p<0.05 vs. Sham). Administration of LXA4 significantly increased the level of brain LXA4 both in sham and SAH animals at 24 hours after intracerebroventricular injection (Supplement Figure II, p<0,05 vs. vehicle groups). However, the protein expression of FPR2 increased after SAH and peaked at 24 hours (Figure 1C and 1D, p<0.05 vs. Sham). Double immunostaining of FPR2 with Iba1 (marker for microglia), GFAP (marker for astrocyte), and NeuN (marker for neuron) showed that FPR2 is intensely expressed and highly co-localized with Iba1 and astrocytes at 24 hours after SAH (Figure 2A and 2B, p<0.05 vs. Sham). There was no colocalization of FPR2 with NeuN after SAH (Figure 2A and 2B, p>0.05 vs. Sham).
Figure 1.
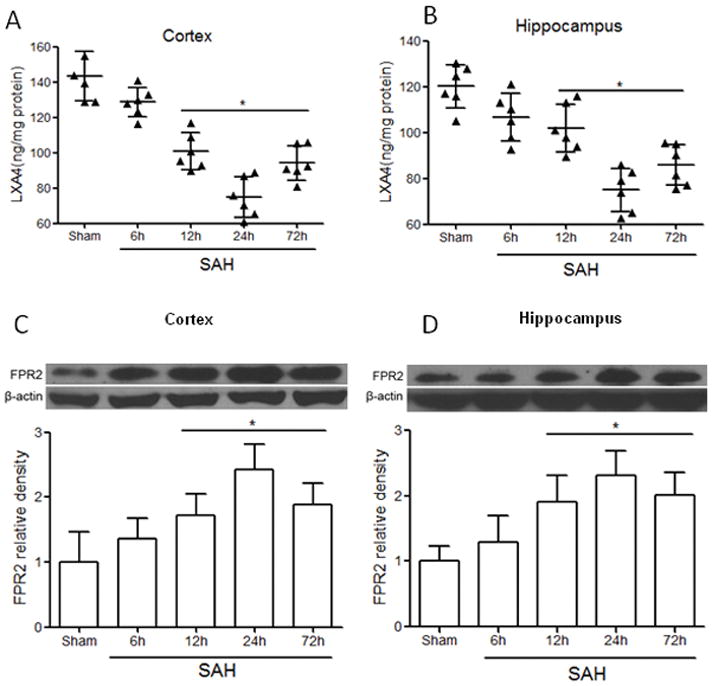
Time course of expression of LXA4 and FPR2 after SAH. (A) and (B) The temporal expression of LXA4 in cortex and hippocampus after SAH by ELISA. (C) and (D) Expression of FPR2 in cortex and hippocampus after SAH by western blots. n=6 for each group. *p<0.05 vs. Sham.
Figure 2.
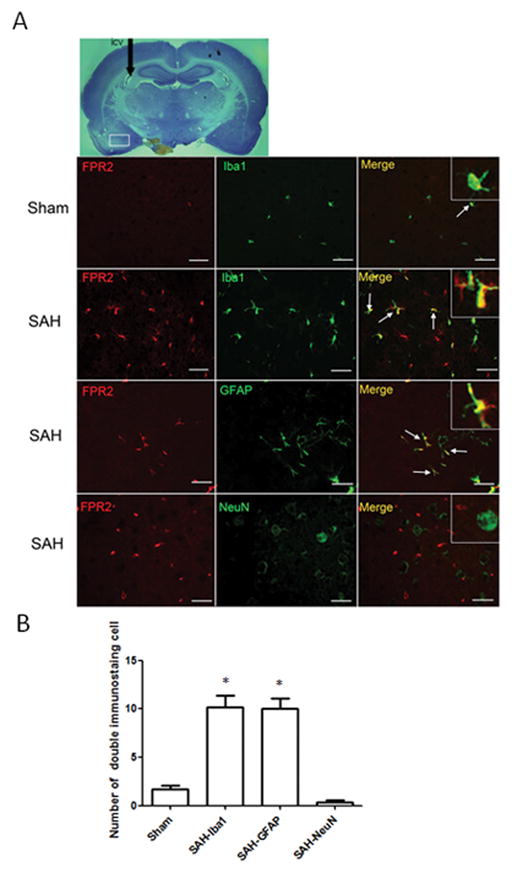
Immunostaining of FPR2 at 24 hours in Sham and SAH animals. (A) Double staining of FPR2 with Iba1, GFAP and NeuN. (B) Statistical analysis of the double staining cells. The expression of FPR2 is low in brain in sham animals, and SAH increased its expression in microglias and astrocytes, but not in neurons at 24 hours after SAH. n=3 for each group. *p<0.05 vs. Sham. Scale bar=30 μm.
Administration of LXA4 decreased brain edema and blood brain barrier permeability, improved neurological functions, and reduced mortality at 24 hours after SAH
Two dosages of LXA4 (0.3 nmol and 1.0 nmol) were administrated intracerebroventricularly 1.5 hours after SAH. Brain water content, Evans blue extravasation, neurological scores and mortality were measured at 24 hours after SAH. The data showed that LXA4 at 1.0 nmol decreased the brain water content (Figure 3A, p<0.05 vs. SAH+vehicle) and BBB permeability (Figure 3B, p<0.05 vs. SAH+vehicle), improved the neurological deficits (Figure 3C, p<0.05 vs. SAH+vehicle) and decreased the mortality (Figure 3D, p<0.05 vs. SAH+vehicle) at 24 hours after SAH. The results indicated that high dosage of LXA4 is an effective treatment for EBI, so we used this dosage to further determine the long-term effects and investigate the mechanism.
Figure 3.
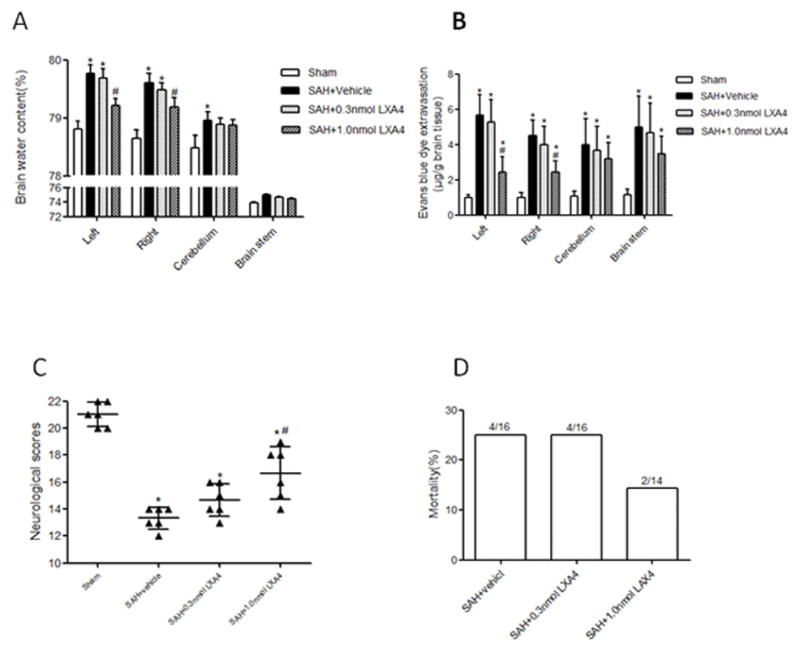
Administration of LXA4 decreased brain water content (A) and blood brain barrier permeability (B), improved neurological functions (C) and reduced mortality (D) at 24 hours after SAH. n=6 for each group. *p<0.05 vs. Sham, #p<0.05 vs. SAH+vehicle.
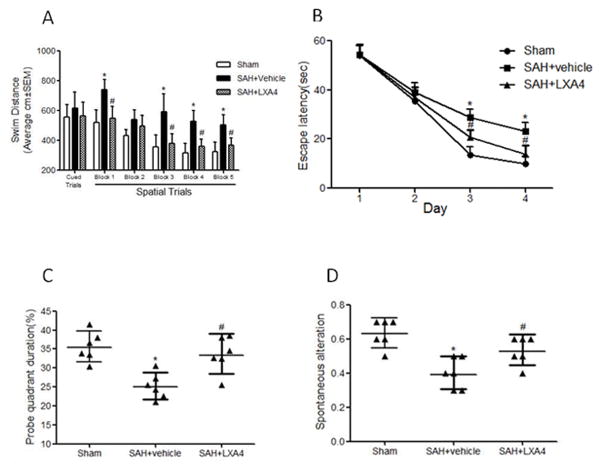
LXA4 improved spatial learning and memory abilities 21 days after SAH
Morris water maze was executed at 21–26 days after SAH. The results showed the animals in vehicle group had a significantly greater distance moved from the target (Figure 4A, p<0.05 vs. Sham), need more time to reach the platform (Figure 4B, p<0.05 vs. Sham), spent less time in the probe quadrants (Figure 4C, p<0.05 vs. Sham) when compared with sham group; Treatment with LXA4 reduced the distance moved (Figure 4A, p<0.05 vs. SAH+vehicle), decreased the latency for platform (Figure 4B, p<0.05 vs. SAH+vehicle) and increased the time duration in the probe quadrants (Figure 4C, p<0.05 vs. SAH+vehicle). T-maze testing for spontaneous alternation demonstrated a significantly worse performance in the SAH+vehicle group compared with the sham (Figure 4D, p<0.05 vs. Sham) and high dosage LXA4 improved the performance (Figure 4D, p<0.05 vs. SAH+vehicle).
Figure 4.
High dosage of exogenous LXA4 improved spatial learning and memory abilities 21 days after SAH. In Morris water maze, treatment with LAX4 reduced the distance moved (Figure 4A), decreased the latency for platform (Figure 4B) and increased the time duration in the probe quadrants (Figure 4C). LAX4 also increased the spontaneous alteration in T-maze (Figure 4D). n=6 for each group. *p<0.05 vs. Sham, #p<0.05 vs. SAH+vehicle.
Knockdown FPR2 by FPR siRNA increased brain edema, decreased neurological scores and aggravated neutrophil infiltration in LXA4 treated animals
To further investigate the potential mechanisms of LXA4, we administrated FPR2 siRNA or Scramble siRNA with LXA4 treatment in SAH rats. We first showed siRNAs was transfected into neural cells in the brain region at the base of skull (Supplement figure III). FPR2 siRNA significantly increased brain water content (Figure 5A, p<0.05 vs. SAH+LXA4+Scramble siRNA), and decreased the neurological scores (Figure 5B, p<0.05 vs. SAH+LXA4+Scramble siRNA) when compared with SAH+LXA4+Scramble siRNA group at 24 hours after SAH. We also investigated the effects of LXA4 on neutrophil infiltration, and measured the level of myeloperoxidase in brain cortex by immunostaining at 24 hours following SAH. Results from immunostaining indicated that LXA4 treatment significantly reduced the number of myeloperoxidase positive cells in the cortex compared to vehicle group following SAH (Figure 5C and 5D, p<0.05 vs. SAH+vehicle), and knockdown FPR2 reversed the effects of LXA4 (Figure 5C and 5D, p<0.05 vs. SAH+LXA4+Scramble siRNA).
Figure 5.
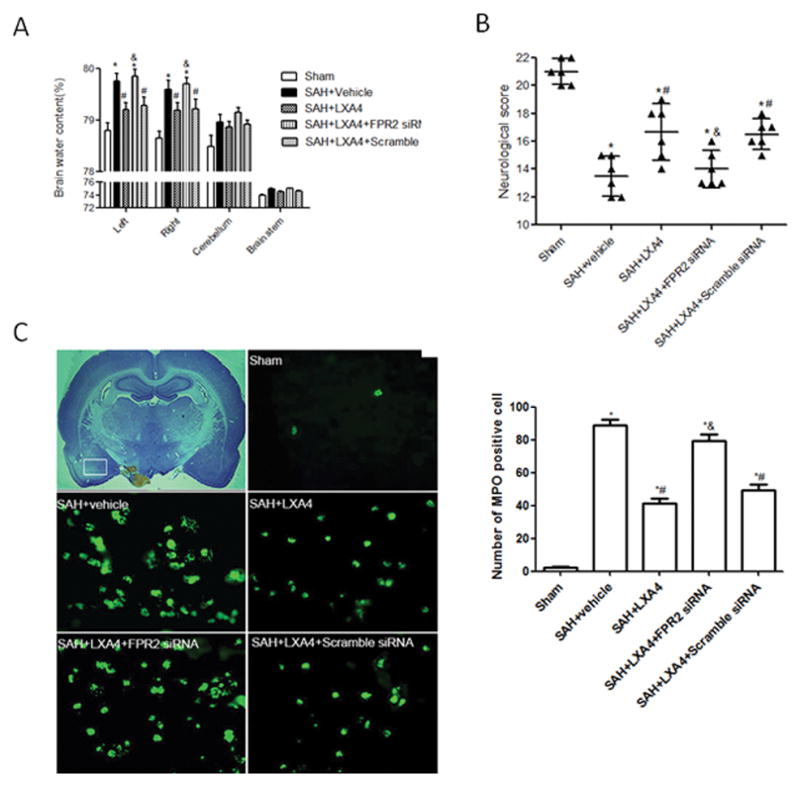
The protective effects of LXA4 depended on FPR2. FPR2 siRNA increased brain edema (A), deteriorated neurobehavioral deficits (B), and aggravated neutrophil infiltration (C and D) in LXA4 treated animals. n=6 for each group in (A) and (B), n=3 for each group in (C) and (D). *p<0.05 vs. Sham, #p<0.05 vs. SAH+vehicle, &p<0.05 vs. SAH+LXA4.
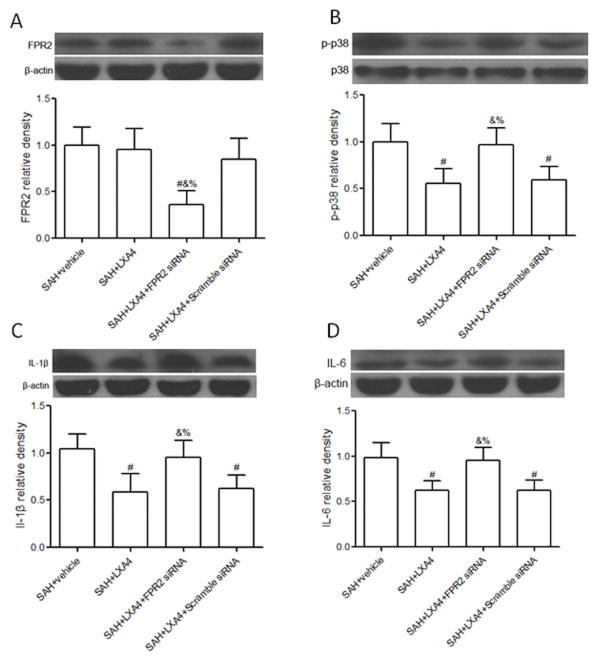
LXA4 inhibited the expression of IL-1β and IL-6 dependent on FPR2/p38 pathway
Administration of LXA4 had no significant effects on the protein level of its receptor FPR2 at 24 hours after SAH (Figure 6A and Supplemental figure IV A, p<0.05 vs. SAH+vehicle), however, administration of FPR2 siRNA knocked down the receptor efficiently (Figure 6A and Supplemental figure IV A, p<0.05 vs. SAH+LXA4+Scramble siRNA). LXA4 treatment inhibited the phosphorylation of p38, and the expression of IL-1β and IL-6 both in brain cortex (Figures 6B–D, p<0.05 vs. SAH+vehicle) and hippocampus (Supplemental Figure IIIB-D, p<0.05 vs. SAH+vehicle) compared with vehicle group. Knockdown FPR2 by FPR2 siRNA remarkably increased the expression of p38, IL-1β and IL-6 in LXA4 treated animals (Figure 6B–D, p<0.05 vs. SAH+LXA4+Scramble siRNA and Supplemental Figure IV B-D, p<0.05 vs. SAH+LXA4+Scramble siRNA).
Figure 6.
Effects of LXA4 and ALX siRNA on the expression of FPR2, p38, IL-1β and IL-6 in cortex after SAH. LXA4 has no effects on protein level of FPR2 (A), but decreased the phosphorylation of p38 (B), and expression of IL-1β (C) and IL-6 (D). Silencing FPR2 by siRNA reduced the expression of FPR2 (A), abolished the effect of LXA4 on p38 (B), IL-1β (C) and IL-6 (D). n=6 for each group. #p<0.05 vs. SAH+vehicle, &p<0.05 vs. SAH+LXA4, %p<0.05 vs. SAH+LXA4+Scramble siRNA.
Discussion
In this study, we investigated the role of LXA4 in anti-inflammation during early brain injury after SAH. Our data showed that high dosage of exogenous LXA4, reduced brain edema, preserved blood brain barrier integrity, improved neurological scores as well as spatial learning and memory abilities in SAH rats; silence of its receptor FPR2 by FPR2 siRNA abolished the beneficial effects of LXA4; LXA4 ameliorated the outcome by suppressing neutrophil infiltration, inhibiting the expression of phosphorylated p38, IL-1β and IL-6 both in the cortex and hippocampus. Our study confirmed that LXA4 provided neuroprotection in early brain injury through suppressing inflammation via FPR2/p38 signaling pathway. LXA4 might be a promising alternative treatment for SAH patients.
Previous studies have showed that LXA4 had anti-inflammatory actions in vivo when administered to the site of inflammation or systemically by intravenous injection,25 intraperitoneal injection,25 or oral route.26 However, blood brain barrier limits the access of therapeutic agents to the brain and presents a major challenge to the delivery of drugs. The molecular weight of LXA4 is 352.5 Dalton. It is little known whether LXA4 could cross the blood brain barrier. Delivery of LXA4 through intracerebroventricular injection has been reported to protect brain and reduce inflammation in focal cerebral ischemia reperfusion.12, 14 So in the present study, we administrated LXA4 by intracerebroventricular injection.
LXA4 has been shown to exert its activity principally via the G protein-coupled lipoxin receptor FPR2.27 In immune system, it has been reported that LXA4 can decrease IgM and IgG production on activated human B cells through FPR2-dependent signaling, which down regulated NF-κB p65 nuclear translocation.28 In addition, LXA4 suppressed the development of endometriosis through a mechanism that mediated by FPR2.7 LXA4 significantly decreased airway inflammation and regulated the catabasis of eosinophilic inflammation by increasing NK cell-mediated eosinophil apoptosis and decreased IL-13 release by type 2 innate lymphoid cells, which also mediated by FPR2.29 We found the protection of high dosage exogenous LXA4 could be blocked by FPR2 siRNA, which lead to increased brain edema and blood brain barrier damage, and also deteriorated neurological impairments. These results suggested the neuroprotective effects of LXA4 in SAH rats were mediated through its receptor FPR2, which is consistent with the previous reports.12, 14, 30
Inflammation is a key pathologic manifestation of early brain injury and is a crucial factor for poor outcome after SAH. Inflammation can cause blood brain barrier leakage, brain edema and cell death, which result in neurological deficits and mortality. Microglia are the most active cells involved in regulating inflammatory processes in the brain and astrocytes are also known to be directly involved in regulating inflammation after SAH.31 This study demonstrated FPR2 co-localized with microglia and astrocytes after SAH, which suggested that microglia and astrocytes might be the targets of LXA4 in the brain. We further observed that the expression of FPR2 both in cortex and hippocampus increased after SAH, suggesting SAH induces the activation of FPR2, and the activation of FPR2 was not enhanced by LXA4 treatment. Lastly, we observed that FPR2 siRNA suppressed neutrophil infiltration and pro-inflammatory IL-1β and IL-6.
Recent studies have linked p38 MAPK to cytokines activation in response to inflammation after SAH.32, 33 Our previous published data showed significant increased phosphorylation of p38 MAPK was observed in brain after SAH.18 The activities of p38 MAPK could be down-regulated by FPR2 receptor antagonist on endometriosis.7 It has been determined that p38 was activated in the arterial wall after SAH, leading to the development of vasospasm, through the upregulation of inflammatory cytokines, which can be inhibited by p38 selective inhibitor.34 The IL-6 synthesis was inhibited by specific inhibitors of p38 MAPK in human astroglioma cells and primary rat astrocytes.35 In this study, p38 MAPK was phosphorylated and the expression of IL-1β and IL-6 were increased after SAH; high dosage of LXA4 reduced the phosphorylation of p38 MAPK, and inhibited the expression of IL-1β and IL-6. Based on these evidences, we suggest that LXA4 may inhibit the phosphorylation of p38 MAPK via FPR2, and resulted in decreased inflammatory response during early brain injury after SAH.
In conclusion, our observations indicated for the first time that LXA4 treatment reduced inflammation through FPR2/p38 MAPK signaling pathway in SAH rats. This study provided new information on inflammation following SAH and LXA4 may be a future option for the treatment of SAH patients.
Supplementary Material
Acknowledgments
Sources of Funding
This study is supported partially by a grant from National Institutes of Health NS081740 and NS082184 to Dr. Zhang and a grant from National Scientific Foundation of China NSFC81100865 to Zongduo Guo.
Footnotes
Disclosure
All authors claimed no conflict of interest.
References
- 1.Chen S, Feng H, Sherchan P, Klebe D, Zhao G, Sun X, et al. Controversies and evolving new mechanisms in subarachnoid hemorrhage. Prog Neurobiol. 2014;115:64–91. doi: 10.1016/j.pneurobio.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cahill J, Zhang JH. Subarachnoid hemorrhage: Is it time for a new direction? Stroke. 2009;40:S86–87. doi: 10.1161/STROKEAHA.108.533315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang T, Su J, Guo B, Wang K, Li X, Liang G. Apigenin protects blood-brain barrier and ameliorates early brain injury by inhibiting TLR4-mediated inflammatory pathway in subarachnoid hemorrhage rats. Int Immunopharmacol. 2015;28:79–87. doi: 10.1016/j.intimp.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 4.Wang CX, Xie GB, Zhou CH, Zhang XS, Li T, Xu JG, et al. Baincalein alleviates early brain injury after experimental subarachnoid hemorrhage in rats: Possible involvement of TLR4/NF-kB-mediated inflammatory pathway. Brain Res. 2015;1594:245–255. doi: 10.1016/j.brainres.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 5.Chiang N, Takano T, Arita M, Watanabe S, Serhan CN. A novel rat lipoxin A4 receptor that is conserved in structure and function. Br J Pharmacol. 2003;139:89–98. doi: 10.1038/sj.bjp.0705220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Q, Tian Y, Wang ZF, Liu SB, Mi WL, Ma HJ, et al. Involvement of the spinal nalp1 inflammasome in neuropathic pain and aspirin-triggered-15-epi-lipoxin A4 induced analgesia. Neuroscience. 2013;254:230–240. doi: 10.1016/j.neuroscience.2013.09.028. [DOI] [PubMed] [Google Scholar]
- 7.Wu R, Zhou W, Chen S, Shi Y, Su L, Zhu M, et al. Lipoxin A4 suppresses the development of endometriosis in an ALX receptor-dependent manner via the p38 MAPK pathway. Br J Pharmacol. 2014;171:4927–4940. doi: 10.1111/bph.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morris T, Stables M, Colville-Nash P, Newson J, Bellingan G, de Souza PM, et al. Dichotomy in duration and severity of acute inflammatory responses in humans arising from differentially expressed proresolution pathways. Proc Natl Acad Sci U S A. 2010;107:8842–8847. doi: 10.1073/pnas.1000373107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen XQ, Wu SH, Zhou Y, Tang YR. Lipoxin A4-induced heme oxygenase-1 protects cardiomyocytes against hypoxia/reoxygenation injury via p38 MAPK activation and Nrf2/ARE complex. Plos One. 2013;8:e67120. doi: 10.1371/journal.pone.0067120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lv W, Lv C, Yu S, Yang Y, Kong H, Xie J, et al. Lipoxin A4 attenuation of endothelial inflammation response mimicking pancreatitis-induced lung injury. Exp Biol Med (Maywood) 2013;238:1388–1395. doi: 10.1177/1535370213502611. [DOI] [PubMed] [Google Scholar]
- 11.Wu Y, Ye XH, Guo PP, Xu SP, Wang J, Yuan SY, et al. Neuroprotective effect of lipoxin A4 methyl ester in a rat model of permanent focal cerebral ischemia. J Mol Neurosci. 2010;42:226–234. doi: 10.1007/s12031-010-9355-8. [DOI] [PubMed] [Google Scholar]
- 12.Ye XH, Wu Y, Guo PP, Wang J, Yuan SY, Shang Y, et al. Lipoxin A4 analogue protects brain and reduces inflammation in a rat model of focal cerebral ischemia reperfusion. Brain Res. 2010;1323:174–183. doi: 10.1016/j.brainres.2010.01.079. [DOI] [PubMed] [Google Scholar]
- 13.Bederson JB, Germano IM, Guarino L. Cortical blood flow and cerebral perfusion pressure in a new noncraniotomy model of subarachnoid hemorrhage in the rat. Stroke. 1995;26:1086–1091. doi: 10.1161/01.str.26.6.1086. [DOI] [PubMed] [Google Scholar]
- 14.Wu L, Liu ZJ, Miao S, Zou LB, Cai L, Wu P, et al. Lipoxin A4 ameliorates cerebral ischaemia/reperfusion injury through upregulation of nuclear factor erythroid 2-related factor 2. Neurol Res. 2013;35:968–975. doi: 10.1179/1743132813Y.0000000242. [DOI] [PubMed] [Google Scholar]
- 15.Okubo S, Strahle J, Keep RF, Hua Y, Xi G. Subarachnoid hemorrhage-induced hydrocephalus in rats. Stroke. 2013;44:547–550. doi: 10.1161/STROKEAHA.112.662312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen G, Li Q, Feng D, Hu T, Fang Q, Wang Z. Expression of NR2B in different brain regions and effect of NR2B antagonism on learning deficits after experimental subarachnoid hemorrhage. Neuroscience. 2013;231:136–144. doi: 10.1016/j.neuroscience.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 17.Sherchan P, Lekic T, Suzuki H, Hasegawa Y, Rolland W, Duris K, et al. Minocycline improves functional outcomes, memory deficits, and histopathology after endovascular perforation-induced subarachnoid hemorrhage in rats. J Neurotrauma. 2011;28:2503–2512. doi: 10.1089/neu.2011.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen S, Ma Q, Krafft PR, Chen Y, Tang J, Zhang J, et al. P2X7 receptor antagonism inhibits p38 mitogen-activated protein kinase activation and ameliorates neuronal apoptosis after subarachnoid hemorrhage in rats. Crit Care Med. 2013;41:e466–474. doi: 10.1097/CCM.0b013e31829a8246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu Q, Chen C, Yan J, Yang X, Shi X, Zhao J, et al. Therapeutic application of gene silencing MMP-9 in a middle cerebral artery occlusion-induced focal ischemia rat model. Exp Neurol. 2009;216:35–46. doi: 10.1016/j.expneurol.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Sugawara T, Ayer R, Jadhav V, Zhang JH. A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. J Neurosci Methods. 2008;167:327–334. doi: 10.1016/j.jneumeth.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altay O, Suzuki H, Hasegawa Y, Caner B, Krafft PR, Fujii M, et al. Isoflurane attenuates blood-brain barrier disruption in ipsilateral hemisphere after subarachnoid hemorrhage in mice. Stroke. 2012;43:2513–2516. doi: 10.1161/STROKEAHA.112.661728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drunalini Perera PN, Hu Q, Tang J, Li L, Barnhart M, Doycheva DM, et al. Delayed remote ischemic postconditioning improves long term sensory motor deficits in a neonatal hypoxic ischemic rat model. Plos One. 2014;9:e90258. doi: 10.1371/journal.pone.0090258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Altay O, Suzuki H, Hasegawa Y, Ostrowski RP, Tang J, Zhang JH. Isoflurane on brain inflammation. Neurobiol Dis. 2014;62:365–371. doi: 10.1016/j.nbd.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pamplona FA, Ferreira J, Menezes de Lima O, Jr, Duarte FS, Bento AF, Forner S, et al. Anti-inflammatory lipoxin A4 is an endogenous allosteric enhancer of CB1 cannabinoid receptor. Proc Natl Acad Sci U S A. 2012;109:21134–21139. doi: 10.1073/pnas.1202906109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Svensson CI, Zattoni M, Serhan CN. Lipoxins and aspirin-triggered lipoxin inhibit inflammatory pain processing. J Exp Med. 2007;204:245–252. doi: 10.1084/jem.20061826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serhan CN. Lipoxins and aspirin-triggered 15-epi-lipoxins are the first lipid mediators of endogenous anti-inflammation and resolution. Prostaglandins Leukot Essent Fatty Acids. 2005;73:141–162. doi: 10.1016/j.plefa.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Chiang N, Serhan CN, Dahlen SE, Drazen JM, Hay DW, Rovati GE, et al. The lipoxin receptor ALX: Potent ligand-specific and stereoselective actions in vivo. Pharmacol Rev. 2006;58:463–487. doi: 10.1124/pr.58.3.4. [DOI] [PubMed] [Google Scholar]
- 28.Ramon S, Bancos S, Serhan CN, Phipps RP. Lipoxin A4 modulates adaptive immunity by decreasing memory B-cell responses via an ALX/FPR2-dependent mechanism. Eur J Immunol. 2014;44:357–369. doi: 10.1002/eji.201343316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barnig C, Cernadas M, Dutile S, Liu X, Perrella MA, Kazani S, et al. Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Sci Transl Med. 2013;5:174ra126. doi: 10.1126/scitranslmed.3004812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu L, Miao S, Zou LB, Wu P, Hao H, Tang K, et al. Lipoxin A4 inhibits 5-lipoxygenase translocation and leukotrienes biosynthesis to exert a neuroprotective effect in cerebral ischemia/reperfusion injury. J Mol Neurosci. 2012;48:185–200. doi: 10.1007/s12031-012-9807-4. [DOI] [PubMed] [Google Scholar]
- 31.Pan H, Wang H, Zhu L, Mao L, Qiao L, Su X. Depletion of Nrf2 enhances inflammation induced by oxyhemoglobin in cultured mice astrocytes. Neurochem Res. 2011;36:2434–2441. doi: 10.1007/s11064-011-0571-6. [DOI] [PubMed] [Google Scholar]
- 32.Vikman P, Ansar S, Edvinsson L. Transcriptional regulation of inflammatory and extracellular matrix-regulating genes in cerebral arteries following experimental subarachnoid hemorrhage in rats. Laboratory investigation. J Neurosurg. 2007;107:1015–1022. doi: 10.3171/JNS-07/11/1015. [DOI] [PubMed] [Google Scholar]
- 33.Kusaka G, Ishikawa M, Nanda A, Granger DN, Zhang JH. Signaling pathways for early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2004;24:916–925. doi: 10.1097/01.WCB.0000125886.48838.7E. [DOI] [PubMed] [Google Scholar]
- 34.Sasaki T, Kasuya H, Onda H, Sasahara A, Goto S, Hori T, et al. Role of p38 mitogen-activated protein kinase on cerebral vasospasm after subarachnoid hemorrhage. Stroke. 2004;35:1466–1470. doi: 10.1161/01.STR.0000127425.47266.20. [DOI] [PubMed] [Google Scholar]
- 35.Fiebich BL, Schleicher S, Spleiss O, Czygan M, Hull M. Mechanisms of prostaglandin E2-induced interleukin-6 release in astrocytes: possible involvement of EP4-like receptors, p38 mitogen-activated protein kinase and protein kinase C. J Neurochem. 2001;79:950–958. doi: 10.1046/j.1471-4159.2001.00652.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.