Abstract
Pharmacogenomics and personalized medicine incorporate genetic factors, historical data, and environmental exposures to predict individual variation in response to medications. The study of pharmacology and pharmacogenomics is challenging in obstetrics, and knowledge lags behind other disciplines of medicine. However, some preliminary data suggest that some of the inter-individual variation seen in response to medications given for the prevention (progesterone) and treatment (nifedipine, terbutaline, others) of preterm labor may be due to pharmacogenomic effects. A comprehensive approach, integrating clinical data, environmental factors including concomitant medications, and genotype to optimize prevention and treatment strategies for preterm birth, is urgently needed.
Keywords: pharmacogenomics, preterm birth, prematurity prevention, personalized medicine, progesterone, tocolysis
Personalized Medicine and Pharmacogenomics – Introduction
Pharmacogenomics is the application of genomic and molecular data in order to better target the delivery of healthcare, facilitate the discovery and clinical testing of new products, and/or help determine an individual’s predisposition to a particular disease or condition.(1) Importantly, it helps to correlate genetic data with a drug’s efficacy or toxicity. Broadly defined, personalized medicine includes pharmacogenomics, and is a rapidly expanding area of medicine. Personalized medicine is multifaceted, and includes genetic factors, historical factors (e.g., family history, personal medical, obstetric history), and environmental factors (e.g. concurrent medication use, exposure to tobacco smoke), and synthesizes this information with the overall goal of individualizing medical care and treatment to optimize outcomes. The goal of personalized medicine is to provide the ‘right dose of the right drug for the right patient at the right time.’(1) The number of identified associations between genetic factors and disease has recently increased at an exponential level due to improvements in genetic technology, helping to accelerate the development of genetic factors as biomarkers.
It is important to recognize that personalized medicine and pharmacogenomics are not just about finding the ‘right medication’ to treat a condition. Adverse drug reactions are a leading cause of morbidity and mortality in developed countries among both children and adults. It has been estimated that between 0.6% and 17.7% of hospitalized patients experience a serious adverse drug reaction.(2, 3) This places a substantial burden on healthcare resources, estimated at nearly 20% additional increase in cost of care and an increase in average length of hospital stay of 8.3%.(4) Application of pharmacogenomics knowledge has the potential to individualize medications and doses, and may minimize adverse effects.
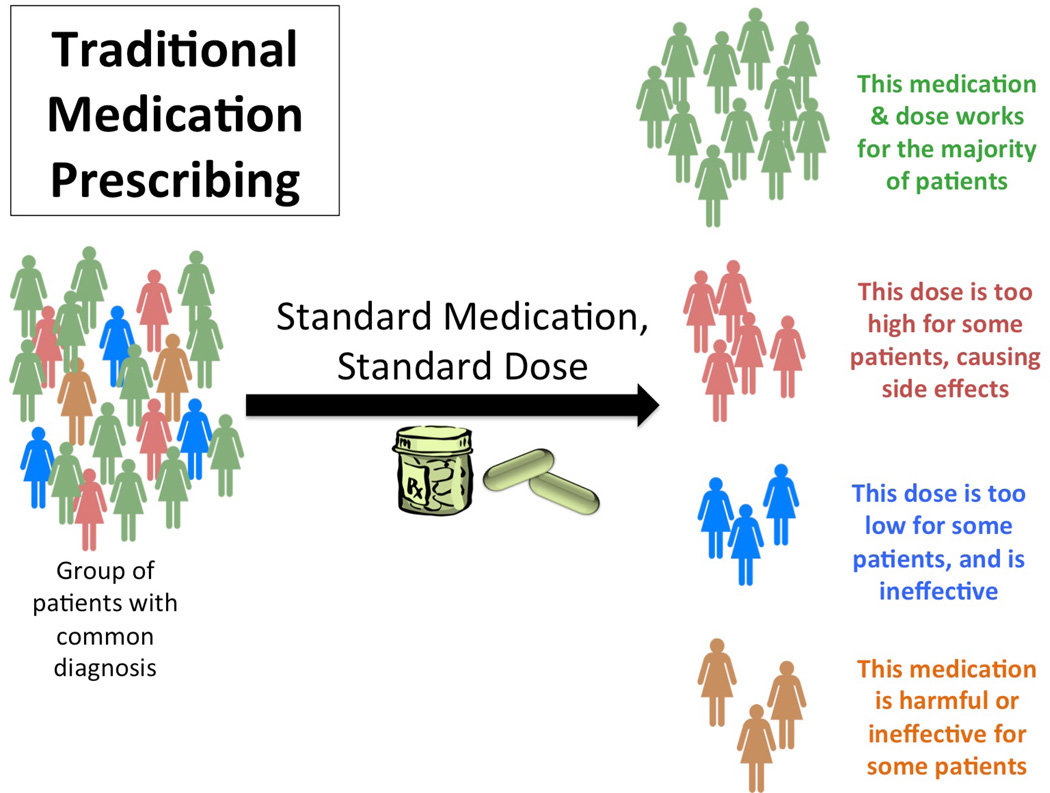
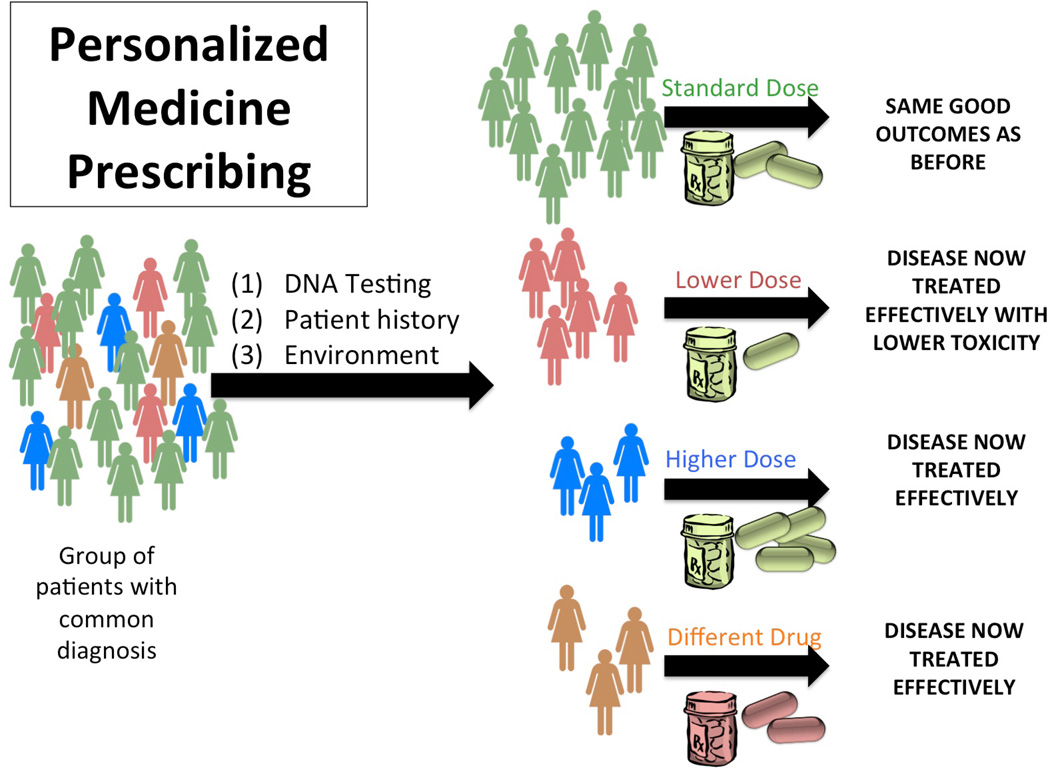
Traditionally, medication prescribing has been at a diagnosis level. A group of patients with a common diagnosis are administered a standard medication at a standard dose. This medication and dose will work for the majority of patients; however, the dose may be too high for some, causing adverse side effects, too low for others, who may not achieve the desired efficacy, and may be harmful or ineffective all together (regardless of dose) for a smaller subset of individuals. In contrast, in an era of personalized medicine prescribing, the same group of individuals with a common diagnosis would first undergo DNA or other testing prior to medication prescribing; this information, together with history and environmental data, would aid the prescribing provider in determining the optimal dose for that individual. Additionally, the group of patients unlikely to respond to that medication could be identified and could be given a different medication, minimizing side effects and toxicity, while improving outcomes (Figures 1a and 1b). This concept, sometimes also referred to as ‘stratified medicine,’ has the potential to improve not only individual outcomes, but have also have positive societal economic impacts.(5, 6)
Figure 1.
a. Schematic of traditional medication prescribing
b. Schematic of medication prescribing in an era of personalized medicine.
Current Real World Applications of Pharmacogenomics in Medicine
Pharmacogenomic information is currently provided by the FDA for over 130 drugs; this list is continuously updated online.(7) Many of the medications with pharmacogenomic labeling are commonly prescribed; it has been estimated that 25% of all outpatient prescriptions includes one of these medications.(8) Currently, more than 50 genes have been implicated as genomic biomarkers; most commonly, these biomarkers pertain to polymorphisms in cytochrome P450 (CYP) enzyme metabolism. Polymorphisms in CYP enzymes are relatively common. For example, CYP2D6 is estimated to metabolize approximately 25% of drugs, and is known to be very polymorphic. Over 70 alleles have been identified in this gene, and as a result, CYP2D6 activity ranges widely, even within populations.(9, 10) Up to 8% of European Americans will be identified as ‘poor metabolizers.’ Given that this is only one hepatic enzyme involved with metabolism, this bespeaks the importance of developing companion diagnostic tests to accompany pharmaceuticals.
Although arguably the translation of pharmacogenetic research findings into clinical practice has been slow, the best pharmacogenetic evidence exists in disease areas outside of womens health, including asthma, anti-coagulation, antibiotics, and oncology. Examples of drugs with pharmacogenomics information include warfarin, cyclosporine, and clopidogrel. Another example is the drug ivacaftor, which was approved by the FDA as a ‘targeted’ therapy for individuals with cystic fibrosis due to a specific genetic mutation, and is somewhat revolutionary in the field because it targets the underlying cause of cystic fibrosis rather than the symptoms of the disease. In the field of obstetrics, this would be the equivalent of targeting the underlying etiology of short cervix with a medication, rather than waiting for the cervix to shorten and then treating it with progesterone. Arguably, this would be more challenging in the situation of short cervix (or other underlying etiologies of preterm birth), which is likely to be a polygenic condition.
Another example of current real world use of pharmacogenomics can be seen in the online pharmacogenetic clinical calculator, developed to assist physicians with dosing warfarin (www.warfarindosing.org). Traditionally, warfarin dosing for anticoagulation has been fraught with inter-individual variability, and has resulted in prolonged hospitalizations for some individuals (while awaiting a therapeutic INR) and potential serious adverse effects for others, who become supra-therapeutic on standard dosing regimens. The online warfarin calculator includes input fields for demographic factors (age, sex, ethnicity), environmental factors (smoking, liver disease, concomitant medications), laboratory values (baseline INR, target INR), and genetic data (genotype for several single nucleotide polymorphisms SNPS in drug metabolizing enzymes). The output provides patient specific estimated warfarin dosing information. This tool is freely available and is just one example of how pharmacogenomics information can be directly applied to improve patient care.
Challenges Involved with Pharmacogenomics Applications in Obstetrics
Given the challenges involved with pharmacologic research during pregnancy, pharmacogenetic studies among pregnant women are limited. There are pharmacogenetic data, although many preliminary and on non-pregnant subjects, on several medications commonly used among pregnant women, including nifedipine, omeprazole, and many selective serotonin reuptake inhibitors among others (Table 1).(7) Unfortunately, obstetric providers are often forced to extrapolate knowledge regarding prescribing of many medications from the non-pregnant population when caring for gravidas in need of treatment. The same holds true for the application of pharmacogenomics; again, clinicians and researchers are left to deduce information based on studies among non-pregnant individuals. Although current literature in the study of pharmacogenomics during pregnancy (for a spectrum of medications and indications) is limited, researchers have suggested a role in of maternal and/or fetal genotype and response to medications for epilepsy and depression/anxiety.(11–13) Others have suggested that genotype should also be considered when studying drug-induced teratogenicity.(14, 15)
Table 1.
Pharmacogenetic information in the FDA label of medications commonly prescribed to pregnant women.
| Drug name | Indication for use during pregnancy |
Gene* | Pharmacogenomic Information in FDA Label |
|---|---|---|---|
| Glyburide | Diabetes mellitus | G6PD | Precautions: Hemolytic anemia related to G6PD deficiency |
| Omeprazole, Lansoprazole | Gastroesophageal reflux disease | CYP2C19 | Drug interactions: CYP2C19 poor metabolizers should avoid concomitant use of clopidogrel |
| Metoclopramide | Nausea | G6PD | Precautions: individuals who are NADH cytochrome b5 reductase deficient are at risk of methemoglobinemia |
| Nitrofurantoin | Urinary tract infection | G6PD | Warnings: individuals with G6PD deficiency are at risk of hemolytic anemia |
| Fluoxetine | Depression, anxiety | CYP2D6 | Warnings, Precautions: CYP2D6 poor metabolizers may have higher drug levels; concomitant administration with other drugs metabolized by CYP2D6 may exacerbate this effect |
| Paroxetine | Depression, anxiety | CYP2D6 | Warnings, Precautions: CYP2D6 poor metabolizers may have higher drug levels; concomitant administration with other drugs metabolized by CYP2D6 may exacerbate this effect |
| Metoprolol | Hypertension | CYP2D6 | Warnings, Precautions: drugs that inhibit CYP2D6 such as quinidine, fluoxetine, paroxetine are likely to increase metoprolol concentration and decrease the cardioselectivity of metoprolol; this is exacerbated in subjects with CYP2D6 extensive metabolizer phenotypes |
| Codeine | Analgesia | CYP2D6 | Black box warnings: respiratory depression and death have occurred in children who received codeine who were CYP2D6 ultra-rapid metabolizers. Deaths have also occurred in nursing infants who were exposed to high levels of morphine (metabolite of codeine) in breast milk because their mothers were ultra-rapid metabolizers of codeine |
Standard nomenclature as per the Human Genome Organization symbol
Source: US Food and Drug Administration: Table of Pharmacognomic Biomarkers in Drug Labeling. (Accessed 5/5/2015, at http://www.fda.gov/Drugs/ScienceResearch/ResearchAreas/Pharmacogenetics/ucm083378.htm.)
Variability in patient response to medications is very common in general, but pregnancy further accentuates these inter-individual differences. This can be summarized by the changes in physiology that occur during pregnancy that alter the absorption, distribution, metabolism, and elimination of drugs. When any of these properties are altered, efficacy can also be altered. During pregnancy, there are 2 patients to consider, and the fetus has a different genotype than the mother. This is important to remember, particularly since medications are commonly prescribed to a pregnant woman with the intention of preventing an adverse outcome in her fetus or fetuses. The placental unit needs to be accounted for; some medications easily cross the placenta, whereas others do not. Physiology changes at least every trimester, and pharmacodynamics and pharmacokinetics are also altered, due to changes in volume of distribution and protein binding, among other factors.(16–18) For example, the half-life of labetalol, a commonly used anti-hypertensive during pregnancy, decreases significantly during pregnancy due to altered pharmacokinetics during pregnancy. (19) The exact effects of some of these physiologic changes are hard to quantify on an individual drug basis, as studies are limited, and many are performed in only the third trimester when the fetal risk is thought to be the lowest. A thorough review of the pharmacokinetic and pharmacodynamics changes that occur during pregnancy is beyond the scope of this review, but an overview of major changes is summarized in Table 2.
Table 2.
Summary of physiologic and metabolic changes potentially affecting pharmacotherapy during pregnancy.
| Change | Impact on Pharmacotherapy |
|---|---|
| Slower gastric emptying, reduced intestinal motility | - May impact drug absorption |
| Increase in gastric pH | - May impact drug absorption |
| Increased glomerular filtration rate (40–80%) | - Enhanced renal drug elimination - Decreased steady-state concentrations of many drugs |
| Increase in total volume of distribution | - Altered drug distribution - Decreased peak serum concentration of many drugs |
| Hypoalbuminemia | - Decreased protein binding, and increased free drug fraction |
| Change in metabolic enzyme activity | - Altered drug metabolism:
|
| Presence of placenta | - Additional metabolism and transport of some drugs |
Lastly, many disease processes that are studied during pregnancy should be regarded as ‘final common pathways.’ Because many pregnancy complications may result from different initial etiologic processes, a ‘one size fits all’ treatment approach that is commonly employed for conditions including pre-eclampsia, preterm birth (PTB), etc. may be ineffective for most individuals. Furthermore, neonatal outcomes are often used as a surrogate for long term outcomes, providing additional challenges in the study of therapeutics during pregnancy.(20)
Current Pharmacogenomics Knowledge Related to Preterm Birth Prevention and Treatment
Preterm birth (PTB) remains the leading cause of neonatal morbidity and mortality among non-anomalous infants in the developed world. A prior PTB is the greatest risk factor for recurrence, and thus, women with a history of a PTB have been the focus of many intervention trials during pregnancy.(21, 22) In 2003, Meis and colleagues published results from a double blind randomized controlled trial demonstrating that prophylaxis with 250mg of weekly intramuscular 17-alpha hydroxyprogesterone caproate (17-OHPC) beginning at 16–20 weeks gestation reduces the recurrence risk of PTB among high risk women. The original Meis study demonstrated a significant reduction in recurrent PTB with 17-OHPC, with a RR of 0.66 (95% CI 0.54–0.81).(23) Despite this significant reduction, 17-OHPC does not help everyone. Additional studies have shown that 17-OHPC (regardless of dose or dosing regimen) is ineffective for other groups of women at high risk for PTB, including those carrying twins or triplets and women with an asymptomatic short cervix in the mid-trimester.(24–26) The reasons for this variation in response are unknown. It is possible that there is a critical 17-OHPC concentration (which may vary among individuals) required to achieve ‘response.’ This theory is supported by a pharmacokinetics study done by Caritis and colleagues. They examined 17-OHPC plasma concentrations from 315 women at 25–28 weeks gestation, and grouped results into quartiles within their patient population. Women with 17-OHPC concentrations in the lowest quartile were significantly more likely to have a recurrent PTB than those who had a 17-OHPC plasma concentration in the upper 3 quartiles (46% vs. 29%, p=0.03); they also demonstrated that those women in the second to fourth quartiles had a 50% reduction in the hazard of delivering preterm (Hazard ratio, 0.48, 95%CI 0.31–0.75, p=0.001) compared to those with plasma 17-OHPC concentrations in the first quartile.(27) Additionally, the lowest PTB rates were seen when median 17-OHPC concentrations exceeded 6.4 ng/mL.
Two specific pharmacogenomics studies have been performed to examine the relationship between genotype and response to 17-OHPC. The first was a candidate gene study, examining 20 single nucleotide polymorphisms (SNPs) in the progesterone receptor gene. Individuals were defined as ‘responding’ to 17-OHPC if they delivered at term with 17-OHPC. The majority of findings in this study were consistent with the expectation that decreased rates of SPTB would be found across genotypes among individuals who received 17-OHPC, consistent with findings from the initial Meis study. For example, self-identified African-Americans with the AA genotype at rs471767 who received placebo had an OR of PTB of 1.0; those who received 17-OHPC had a reduced odds of PTB (0.20); in contrast, those with either the AG or GG genotype for this SNP had similar rates of PTB with 17-OHPC. However, some surprising findings were noted; women with certain genotypes might have an increased risk of PTB when treated with 17-OHPC compared to their ‘background’ rate of prematurity. An example of this is Caucasians and Hispanics at rs503362; those with the GG genotype had an increased odds of PTB <32 weeks gestation with 17-OHPC, while those with the CG or CC genotype at this SNP who received 17-OHPC had a significant reduction in PTB compared to those receiving placebo.(28)
This study was a reasonable initial investigation, and suggested a possible interaction between maternal genotype, 17-OHPC, and response to therapy, but had several limitations. First, the study was limited by the examination of only the progesterone receptor gene. A single gene etiology is very unlikely for this complex phenotype incorporating pharmacogenomics. A broader approach, in an era of improved genetic technology, is to employ next generation sequencing. Sequencing provides all genetic information and is not subject to investigator bias or preconceived ideas regarding causative variants. Second, the study was limited by the definition of progesterone ‘responder.’ Traditionally, responders to progesterone are those individuals who are administered 17-OHPC and deliver at term. This may misclassify many women as non-responders who gain significant gestational age with 17-OHPC but do not reach 37 weeks gestation. Even small gains in gestational age may have large impacts on neonatal and childhood morbidity and mortality. PTB tends to recur at similar gestational ages; e.g. a woman with a prior PTB at 24 weeks who experiences a recurrent PTB is likely to experience this recurrence around 24 weeks gestation. Thus, a woman with a prior 24 week delivery who receives 17-OHPC and reaches 34 weeks gestation would not be considered a 17-OHPC responder using the traditional definition of responder, since she did not reach term, but the outcomes for her 34 week baby are expected to be significantly improved compared to her first, earlier delivery. Given this, a ‘new’ definition of 17-OHPC responder was created. Those who delivered at least 3 weeks later than the gestational age of the earliest prior PTB without 17-OHPC were considered ‘responders’ and those who delivered earlier with 17-OHPC than without it or <3 weeks later with 17-OHPC were considered non-responders.
Next, using both a broader genetic approach, and the refined definition of progesterone responder, another study was performed on a different cohort of women. This study recruited women from a prematurity prevention clinic in Salt Lake City, UT. Maternal samples underwent whole exome sequencing. Data were analyzed at the gene level using the Variant Annotation, Analysis and Search Tool (VAAST, Salt Lake City, UT).(29, 30) VAAST prioritized those genes with different allele frequencies between responders and non-responders; the top genes were then examined in a pathway analysis. Statistically significant over-representation of several biologic processes and molecular functions was found. For example, genes in the nitric oxide signal transduction pathway were found to be over-represented among non-responders compared to responders.(31) Nitric oxide is known to be a potent uterine smooth muscle relaxant, and may work synergistically with progesterone to inhibit uterine contractility.(32, 33) Small randomized controlled trials of unselected women examining the role of nitroglycerin (typically administered in patch form, converted to nitric oxide) for preterm birth tocolysis have produced mixed results, and a recent metaanalysis concluded there is no apparent benefit for this indication.(34) Whether nitroglycerin may be an appropriate adjunct to 17-OHPC in some high risk women remains unknown and is an area for future research.
Pharmacogenomics of Preterm Birth Treatment
Nifedipine
Nifedipine is a calcium channel blocker commonly used as a tocolytic. It has a favorable efficacy and safety profile overall. It is metabolized by the polymorphic CYP3A family of metabolic enzymes in the liver. One small study of 14 women receiving nifedipine tocolysis genotyped participants for CYP3A5, and analyzed nifedipine concentration over time. These investigators found that CYP3A5 genotype correlated with clearance of nifedipine; those individuals who were CYP3A5 high expressers metabolized nifedipine more quickly and had lower serum levels of nifedipine across the study period.(35) In another pilot study of 20 pregnant women, Haas and colleagues examined 20 pregnant women receiving nifedipine for preterm labor. CYP3A5 genotype was found to correlate with nifedipine levels. Those women who had the high expresser genotype were again found to have lower nifedipine levels. Importantly, this study also showed that these individuals who were CYP3A5 high expressers had less improvement in contraction frequency at several time points – after the loading dose, at the steady state, and in the first hour after the study dose.(36)
Beta-2 adrenergic receptor agonists
Beta-2 adrenergic receptor agonists are commonly used tocolytics in Europe and Canada. They work through binding the beta-2 adrenergic receptor and causing smooth muscle relaxation. There is known genetic variability within the beta-2 adrenergic receptor. An arginine to glycine substitution at codon 16 (Arg16Gly) has been shown to increase receptor desensitization in response to agonist exposure. A substitution of glutamate for glutamine at codon 27 (Gln27Glu) decreased down regulation of the receptor. There have been several candidate gene studies in different populations which have found a relationship between polymorphisms in the beta-2 adrenergic receptor and PTB. For example, Gibson and colleagues reported that a variant of the beta-2 adrenergic receptor gene (ADRB2 Q27E) is more common among a cohort of 147 children born preterm who did not eventually develop cerebral palsy.(37) Other studies have also supported the possible relationship between beta-2 adrenergic receptor polymorphisms and PTB.(38–40)
Adding in the element of pharmacogenomics to response to beta-2 adrenergic agonists thus seemed a logical ‘next step,’ but such studies remain small and limited. In 2005, Landau and colleagues examined 60 women with preterm labor, and genotyped them for the beta-2 adrenergic receptor. Women with the AA genotype at codon 16 were noted to have a significant prolongation in gestation, and the authors reported that tocolysis was 100% successful in delaying delivery for 48 hours (n=10, p=0.069). Among the remaining 50 women with either the AG or GG genotype at codon 16, only 37 had delivery delayed by more than 48 hours with beta-2 agonist tocolysis. Gestation was noted to be significantly prolonged among those homozygote for the minor allele, 69 days vs. 58 days, p=0.04); neonatal outcomes were also improved. The authors concluded that women with an AA genotype may have improved response to beta-2 agnoist tocolysis.(41) An additional pharmacogenetic study of ritrodine therapy demonstrated that individuals homozygous for the minor allele in rs1042713 or who carried at least one copy of the minor allele for rs1042719 had a significantly shorter admission to delivery interval compared to those with the more common wild-type genotypes.(42)
Indomethacin and Magnesium Sulfate
Indomethacin is another commonly used tocolytic. It is metabolized by the polymorphic CYP2C9 and CYP2C19 enzymes in the liver.(43) Magnesium sulfate is also commonly used for tocolysis and among mothers at risk of PTB for the purposes of fetal neuroprotection.(44) Magnesium sulfate is renally excreted, and levels are affected by volume of distribution and glomerular filtration rate.(45) It is possible that genotype may affect indomethacin and/or magnesium sulfate concentration and efficacy, but this is speculation only; no pharmacogenomics data are currently available.
Betamethasone
In a study of 109 women and their 117 neonates delivering at a mean 32.2 weeks gestation, 72% due to preterm premature rupture of membranes or PTL, Haas and colleagues examined 73 maternal and fetal SNPs in glucocorticoid pathways. After controlling for confounders, they found that maternal and fetal genetic variants in several SNPs, including CYP3A5 and CYP3A7*1E to be associated with variation in neonatal respiratory outcomes, including respiratory distress syndrome, need for surfactant, and ventilator support.(46) Thus, these authors concluded that genetic variation in key betamethasone pathway genes may be associated with the severity of respiratory morbidity.
Conclusions and Future Directions
Preliminary data suggest that genotype may influence response to commonly used therapeutics administered for recurrent PTB prevention and treatment. Studies to date are small and limited, but evolving. Unfortunately, pharmacogenomics is not yet ready for clinical implementation in obstetrics. Future studies should focus on confirming the results of the preliminary studies presented here, expand to examine the influence of fetal genotype, and also incorporate functional data and protein expression information. A comprehensive approach to personalized medicine, integrating clinical data, environmental factors including concomitant medications, and genotype to optimize prevention and treatment strategies for PTB, is urgently needed. Only then will we be able to screen low-risk women and impact primary PTB prevention.
Acknowledgements
Funding
This work was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development 5K23HD067224
Footnotes
Disclosure of Interests
I serve on the scientific advisory board for Sera Prognostics, a private company that was established to create a commercial test to predict preterm birth and other obstetric complications (the ICMJE disclosure form is available as online supporting information).
Contribution to Authorship
I am the sole author, and contributed to 100% of this manuscript.
Details of Ethics Approval
Not applicable for this review article.
References
- 1.Shastry BS. Pharmacogenetics and the concept of individualized medicine. Pharmacogenomics J. 2006;6(1):16–21. doi: 10.1038/sj.tpj.6500338. [DOI] [PubMed] [Google Scholar]
- 2.Smyth RM, Gargon E, Kirkham J, Cresswell L, Golder S, Smyth R, et al. Adverse drug reactions in children--a systematic review. PLoS One. 2012;7(3):e24061. doi: 10.1371/journal.pone.0024061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thiesen S, Conroy EJ, Bellis JR, Bracken LE, Mannix HL, Bird KA, et al. Incidence, characteristics and risk factors of adverse drug reactions in hospitalized children - a prospective observational cohort study of 6,601 admissions. BMC medicine. 2013;11:237. doi: 10.1186/1741-7015-11-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan LM. Comparative epidemiology of hospital-acquired adverse drug reactions in adults and children and their impact on cost and hospital stay--a systematic review. Eur J Clin Pharmacol. 2013;69(12):1985–1996. doi: 10.1007/s00228-013-1563-z. [DOI] [PubMed] [Google Scholar]
- 5.Trusheim MR, Berndt ER, Douglas FL. Stratified medicine: strategic and economic implications of combining drugs and clinical biomarkers. Nat Rev Drug Discov. 2007;6(4):287–293. doi: 10.1038/nrd2251. [DOI] [PubMed] [Google Scholar]
- 6.Trusheim MR, Burgess B, Hu SX, Long T, Averbuch SD, Flynn AA, et al. Quantifying factors for the success of stratified medicine. Nat Rev Drug Discov. 2011;10(11):817–833. doi: 10.1038/nrd3557. [DOI] [PubMed] [Google Scholar]
- 7.US Food and Drug Administration: Table of Pharmacognomic Biomarkers in Drug Labeling [5/5/2015] Available from: http://www.fda.gov/Drugs/ScienceResearch/ResearchAreas/Pharmacogenetics/ucm083378.htm.
- 8.Frueh FW, Amur S, Mummaneni P, Epstein RS, Aubert RE, DeLuca TM, et al. Pharmacogenomic biomarker information in drug labels approved by the United States food and drug administration: prevalence of related drug use. Pharmacotherapy. 2008;28(8):992–998. doi: 10.1592/phco.28.8.992. [DOI] [PubMed] [Google Scholar]
- 9.Zhou SF. Polymorphism of human cytochrome P450 2D6 and its clinical significance: part II. Clin Pharmacokinet. 2009;48(12):761–804. doi: 10.2165/11318070-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 10.Zhou SF. Polymorphism of human cytochrome P450 2D6 and its clinical significance: Part I. Clin Pharmacokinet. 2009;48(11):689–723. doi: 10.2165/11318030-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 11.Atkinson DE, Brice-Bennett S, D'Souza SW. Antiepileptic medication during pregnancy: does fetal genotype affect outcome? Pediatr Res. 2007;62(2):120–127. doi: 10.1203/PDR.0b013e3180a02e50. [DOI] [PubMed] [Google Scholar]
- 12.Weikum WM, Brain U, Chau CM, Grunau RE, Boyce WT, Diamond A, et al. Prenatal serotonin reuptake inhibitor (SRI) antidepressant exposure and serotonin transporter promoter genotype (SLC6A4) influence executive functions at 6 years of age. Frontiers in cellular neuroscience. 2013;7:180. doi: 10.3389/fncel.2013.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oberlander TF, Bonaguro RJ, Misri S, Papsdorf M, Ross CJ, Simpson EM. Infant serotonin transporter (SLC6A4) promoter genotype is associated with adverse neonatal outcomes after prenatal exposure to serotonin reuptake inhibitor medications. Mol Psychiatry. 2008;13(1):65–73. doi: 10.1038/sj.mp.4002007. [DOI] [PubMed] [Google Scholar]
- 14.Daud AN, Bergman JE, Bakker MK, Wang H, de Walle HE, Plosch T, et al. Pharmacogenetics of drug-induced birth defects: the role of polymorphisms of placental transporter proteins. Pharmacogenomics. 2014;15(7):1029–1041. doi: 10.2217/pgs.14.62. [DOI] [PubMed] [Google Scholar]
- 15.Wilffert B, Altena J, Tijink L, van Gelder MM, de Jong-van den Berg LT. Pharmacogenetics of drug-induced birth defects: what is known so far? Pharmacogenomics. 2011;12(4):547–558. doi: 10.2217/pgs.10.201. [DOI] [PubMed] [Google Scholar]
- 16.Fischer JH, Sarto GE, Hardman J, Endres L, Jenkins TM, Kilpatrick SJ, et al. Influence of gestational age and body weight on the pharmacokinetics of labetalol in pregnancy. Clin Pharmacokinet. 2014;53(4):373–383. doi: 10.1007/s40262-013-0123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bologa M, Tang B, Klein J, Tesoro A, Koren G. Pregnancy-induced changes in drug metabolism in epileptic women. J Pharmacol Exp Ther. 1991;257(2):735–740. [PubMed] [Google Scholar]
- 18.Anderson GD. Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin Pharmacokinet. 2005;44(10):989–1008. doi: 10.2165/00003088-200544100-00001. [DOI] [PubMed] [Google Scholar]
- 19.Rogers RC, Sibai BM, Whybrew WD. Labetalol pharmacokinetics in pregnancy-induced hypertension. Am J Obstet Gynecol. 1990;162(2):362–366. doi: 10.1016/0002-9378(90)90386-l. [DOI] [PubMed] [Google Scholar]
- 20.Manuck TA, Sheng X, Yoder BA, Varner MW. Correlation between initial neonatal and early childhood outcomes following preterm birth. Am J Obstet Gynecol. 2014;210(5):426, e1–e9. doi: 10.1016/j.ajog.2014.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manuck TA, Henry E, Gibson J, Varner MW, Porter TF, Jackson GM, et al. Pregnancy outcomes in a recurrent preterm birth prevention clinic. Am J Obstet Gynecol. 2011;204(4):320, e1–e6. doi: 10.1016/j.ajog.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 22.Mazaki-Tovi S, Romero R, Kusanovic JP, Erez O, Pineles BL, Gotsch F, et al. Recurrent preterm birth. Semin Perinatol. 2007;31(3):142–158. doi: 10.1053/j.semperi.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meis PJ, Klebanoff M, Thom E, Dombrowski MP, Sibai B, Moawad AH, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348(24):2379–2385. doi: 10.1056/NEJMoa035140. [DOI] [PubMed] [Google Scholar]
- 24.Grobman WA, Thom EA, Spong CY, Iams JD, Saade GR, Mercer BM, et al. 17 alpha-hydroxyprogesterone caproate to prevent prematurity in nulliparas with cervical length less than 30 mm. American journal of obstetrics and gynecology. 2012;207(5):390, e1–e8. doi: 10.1016/j.ajog.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rouse DJ, Caritis SN, Peaceman AM, Sciscione A, Thom EA, Spong CY, et al. A trial of 17 alpha-hydroxyprogesterone caproate to prevent prematurity in twins. N Engl J Med. 2007;357(5):454–461. doi: 10.1056/NEJMoa070641. [DOI] [PubMed] [Google Scholar]
- 26.Caritis SN, Rouse DJ, Peaceman AM, Sciscione A, Momirova V, Spong CY, et al. Prevention of preterm birth in triplets using 17 alpha-hydroxyprogesterone caproate: a randomized controlled trial. Obstet Gynecol. 2009;113(2 Pt 1):285–292. doi: 10.1097/AOG.0b013e318193c677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caritis SN, Venkataramanan R, Thom E, Harper M, Klebanoff MA, Sorokin Y, et al. Relationship between 17-alpha hydroxyprogesterone caproate concentration and spontaneous preterm birth. Am J Obstet Gynecol. 2014;210(2):128, e1–e6. doi: 10.1016/j.ajog.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manuck TA, Lai Y, Meis PJ, Dombrowski MP, Sibai B, Spong CY, et al. Progesterone receptor polymorphisms and clinical response to 17-alpha-hydroxyprogesterone caproate. Am J Obstet Gynecol. 2011;205(2):135, e1–e9. doi: 10.1016/j.ajog.2011.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu H, Huff CD, Moore B, Flygare S, Reese MG, Yandell M. VAAST 2.0: improved variant classification and disease-gene identification using a conservation-controlled amino acid substitution matrix. Genetic epidemiology. 2013;37(6):622–634. doi: 10.1002/gepi.21743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yandell M, Huff C, Hu H, Singleton M, Moore B, Xing J, et al. A probabilistic disease-gene finder for personal genomes. Genome research. 2011;21(9):1529–1542. doi: 10.1101/gr.123158.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manuck TA, Watkins WS, Moore B, Esplin MS, Varner MW, Jackson GM, et al. Pharmacogenomics of 17-alpha hydroxyprogesterone caproate for recurrent preterm birth prevention. Am J Obstet Gynecol. 2014;210(4):321, e1–e21. doi: 10.1016/j.ajog.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yallampalli C, Buhimschi I, Chwalisz K, Garfield RE, Dong YL. Preterm birth in rats produced by the synergistic action of a nitric oxide inhibitor (NG-nitro-L-arginine methyl ester) and an antiprogestin (onapristone) Am J Obstet Gynecol. 1996;175(1):207–212. doi: 10.1016/s0002-9378(96)70276-4. [DOI] [PubMed] [Google Scholar]
- 33.Izumi H, Garfield RE. Relaxant effects of nitric oxide and cyclic GMP on pregnant rat uterine longitudinal smooth muscle. Eur J Obstet Gynecol Reprod Biol. 1995;60(2):171–180. doi: 10.1016/0028-2243(95)02096-b. [DOI] [PubMed] [Google Scholar]
- 34.Conde-Agudelo A, Romero R. Transdermal nitroglycerin for the treatment of preterm labor: a systematic review and metaanalysis. Am J Obstet Gynecol. 2013;209(6):551, e1–e18. doi: 10.1016/j.ajog.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haas DM, Quinney SK, Clay JM, Renbarger JL, Hebert MF, Clark S, et al. Nifedipine pharmacokinetics are influenced by CYP3A5 genotype when used as a preterm labor tocolytic. American journal of perinatology. 2013;30(4):275–281. doi: 10.1055/s-0032-1323590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haas DM, Quinney SK, McCormick CL, Jones DR, Renbarger JL. A pilot study of the impact of genotype on nifedipine pharmacokinetics when used as a tocolytic. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2012;25(4):419–423. doi: 10.3109/14767058.2011.583700. [DOI] [PubMed] [Google Scholar]
- 37.Gibson CS, MacLennan AH, Dekker GA, Goldwater PN, Dambrosia JM, Munroe DJ, et al. Genetic polymorphisms and spontaneous preterm birth. Obstet Gynecol. 2007;109(2 Pt 1):384–391. doi: 10.1097/01.AOG.0000252712.62241.1a. [DOI] [PubMed] [Google Scholar]
- 38.Suh YJ, Park HJ, Lee KA, Lee BE, Ha EH, Kim YJ. Associations between genetic polymorphisms of beta-2 adrenergic receptor and preterm delivery in Korean women. Am J Reprod Immunol. 2013;69(1):85–91. doi: 10.1111/aji.12022. [DOI] [PubMed] [Google Scholar]
- 39.Ozkur M, Dogulu F, Ozkur A, Gokmen B, Inaloz SS, Aynacioglu AS. Association of the Gln27Glu polymorphism of the beta-2-adrenergic receptor with preterm labor. Int J Gynaecol Obstet. 2002;77(3):209–215. doi: 10.1016/s0020-7292(02)00035-8. [DOI] [PubMed] [Google Scholar]
- 40.Doh K, Sziller I, Vardhana S, Kovacs E, Papp Z, Witkin SS. Beta2-adrenergic receptor gene polymorphisms and pregnancy outcome. J Perinat Med. 2004;32(5):413–417. doi: 10.1515/JPM.2004.138. [DOI] [PubMed] [Google Scholar]
- 41.Landau R, Morales MA, Antonarakis SE, Blouin JL, Smiley RM. Arg16 homozygosity of the beta2-adrenergic receptor improves the outcome after beta2-agonist tocolysis for preterm labor. Clin Pharmacol Ther. 2005;78(6):656–663. doi: 10.1016/j.clpt.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 42.Park JY, Lee NR, Lee KE, Park S, Kim YJ, Gwak HS. Effects of beta2-adrenergic receptor gene polymorphisms on ritodrine therapy in pregnant women with preterm labor: prospective follow-up study. International journal of molecular sciences. 2014;15(7):12885–12894. doi: 10.3390/ijms150712885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakajima M, Inoue T, Shimada N, Tokudome S, Yamamoto T, Kuroiwa Y. Cytochrome P450 2C9 catalyzes indomethacin O-demethylation in human liver microsomes. Drug Metab Dispos. 1998;26(3):261–266. [PubMed] [Google Scholar]
- 44.Rouse DJ, Hirtz DG, Thom E, Varner MW, Spong CY, Mercer BM, et al. A randomized, controlled trial of magnesium sulfate for the prevention of cerebral palsy. N Engl J Med. 2008;359(9):895–905. doi: 10.1056/NEJMoa0801187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu JF, Nightingale CH. Magnesium sulfate in eclampsia and pre-eclampsia: pharmacokinetic principles. Clin Pharmacokinet. 2000;38(4):305–314. doi: 10.2165/00003088-200038040-00002. [DOI] [PubMed] [Google Scholar]
- 46.Haas DM, Dantzer J, Lehmann AS, Philips S, Skaar TC, McCormick CL, et al. The impact of glucocorticoid polymorphisms on markers of neonatal respiratory disease after antenatal betamethasone administration. Am J Obstet Gynecol. 2013;208(3):215, e1–e6. doi: 10.1016/j.ajog.2012.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]