Abstract
Intrinsic immune responses to acute leukemia are inhibited by a variety of mechanisms, such as aberrant antigen expression by leukemia cells, secretion of immunosuppressive cytokines and expression of inhibitory enzymes in the tumor microenvironment, expansion of immunoregulatory cells, and activation of immune checkpoint pathways, all leading to T cell dysfunction and/or exhaustion. Leukemic cells, similar to other tumor cells, hijack these inhibitory pathways to evade immune recognition and destruction by cytotoxic T lymphocytes. Thus, blockade of immune checkpoints has emerged as a highly promising approach to augment innate anti-tumor immunity in order to treat malignancies. Most evidence for the clinical efficacy of this immunotherapeutic strategy has been seen in patients with metastatic melanoma, where anti-CTLA-4 and anti-PD-1 antibodies have recently revolutionized treatment of this lethal disease with otherwise limited treatment options. To meet the high demand for new treatment strategies in acute leukemia, clinical testing of these promising therapies is commencing. Herein, we review the biology of multiple inhibitory checkpoints (including CTLA-4, PD-1, TIM-3, LAG-3, BTLA, and CD200R) and their contribution to immune evasion by acute leukemias. In addition, we discuss the current state of preclinical and clinical studies of immune checkpoint inhibition in acute leukemia, which seek to harness the body’s own immune system to fight leukemic cells.
Keywords: Acute lymphoblastic leukemia, acute myeloid leukemia, co-inhibitory receptor, immune checkpoint pathway, immune evasion, immunotherapy, monoclonal antibody, T cells
1. INTRODUCTION
The functionality of T cells during immunity and tolerance is determined by the balance between co-stimulatory and co-inhibitory signals that fine-tune the amplitude, quality, and duration of T cell responses that are initiated upon antigen recognition by T cell receptors (TCRs) [1–3]. While engagement of a co-stimulatory receptor such as CD28 strongly amplifies antigen-specific TCR signaling with a resultant increase in lymphocyte proliferation and activation, co-inhibitory receptors serve as immune checkpoints to prevent and limit uncontrolled T cell responses. In healthy individuals, immune checkpoints are crucial for maintaining self-tolerance, and thus preventing autoimmunity, and for limiting tissue damage when the immune system responds to infection. Tumor cells, however, can hijack these inhibitory pathways to evade immune recognition and consequent destruction by tumor antigen-specific cytotoxic T lymphocytes (CTLs; also known as CD8+ effector T cells).
Under normal physiological conditions, the adaptive immune system is highly capable of recognizing and killing foreign cells, including aberrant tumor cells. However, differential expression of co-stimulatory and/or co-inhibitory molecules, among other immune escape mechanisms coopted by tumor cells, can dominantly diminish tumor-specific T cell responses [4, 5]. Sustained signaling via co-inhibitory molecules results in functional exhaustion of T cells, during which their ability to proliferate, secrete cytokines, and mediate lysis of tumor cells is sequentially lost [6]. Thus, these mechanisms ultimately contribute to immune escape by cancer cells [4, 7–9]. In this review, we will address the role of co-inhibitory molecules in immune evasion in acute leukemias and discuss the impact of blocking co-inhibitory molecule signaling in order to circumvent T cell inhibition.
2. ANTI-LEUKEMIC IMMUNE RESPONSES
The most convincing evidence that an intact immune system is highly potent in eliminating leukemia cells is seen in recipients of allogeneic hematopoietic stem cell transplantation (allo-HSCT) [10]. The anti-leukemic effect of allo-HSCT depends on the ability of immunocompetent donor T cells to recognize major histocompatibility antigens (MHAs) and/or minor histocompatibility antigens (MiHAs) present on residual leukemia cells and eliminate them, mediating the so-called graft-versus-leukemia (GVL) effect [11]. The best demonstration for the T cell-mediated GVL effect has been provided by the efficacy of donor lymphocyte infusion (DLI) in inducing disease remission in patients relapsing after allo-HSCT [12].
Besides this allogeneic anti-leukemic effect, there is also evidence for autologous anti-leukemic reactivity. Leukemia-specific cytolytic activity has been reported in patients after autologous HSCT [13], and T cell lines capable of lysing autologous acute myeloid leukemia (AML) cells have been generated in vitro [14]. Moreover, simple co-culture of monocyte-derived dendritic cells (DCs) with leukemic blasts, both derived from the peripheral blood (PB) of patients with AML, effectively activated leukemia-specific autologous T cells [15]. Nonetheless, leukemia cells exploit a variety of mechanisms to evade T cell-mediated immunity, leading to disease progression or relapse.
3. DYSREGULATION OF THE IMMUNE SYSTEM IN ACUTE LEUKEMIA
With the exception of immune checkpoint pathways, which will be discussed separately below, several innate and adaptive immune system aberrations encountered in patients with acute leukemia are summarized in Table 1. However, in any given leukemia patient, multiple mechanisms likely cooperate to create an environment that supports the immune escape of leukemia cells.
Table 1.
Immunologic Changes in Patients with Acute Leukemia*.
| References | No. of Patients | Major Findings | Condition | Specimen |
|---|---|---|---|---|
| LeDieu et al. [186] | 36 | ↑ absolute number of CD8+ T cells. ↑ numbers of CD3+CD56+ NK-T cells. Aberrant T cell activation pattern. Impaired immune synapse formation between T cells and AML blasts. |
Newly diagnosed AML | PB |
| Kanakry et al. [25] | 20 | Tregs represent an expanded T cell population in early lymphocyte recovery after intensive induction chemotherapy. Tregs are oligoclonally skewed and primarily peripherally derived. |
Newly diagnosed AML | PB |
| Shenghui et al. [26] | 182 | ↑ Tregs compared to healthy control. ↑ Treg frequency in BM > PBMC of the same patient. ↓ Treg frequency in patients who achieved CR compared to patients with persistent AML. Higher Tregs at diagnosis associated with poor response to therapy. |
Newly diagnosed AML | BM and PB |
| Szczepanski et al. [24] | 31 | ↑ Tregs and suppressive activity in AML patients compared to normal controls. ↑ pretreatment Treg frequency associated with poor response to chemotherapy. |
Newly diagnosed AML | PB |
| Wang et al. [23] | 36 | ↑Tregs in PBMC > BM. ↑ apoptotic fraction of Tregs in AML > control Tregs suggesting a rapid turnover of Tregs. |
AML | BM and PB |
| Curti et al. [42, 44] | 76 | AML blasts constitutively express IDO. Co-culture of CD4+CD25− T cells with IDO+AML cells results in conversion into CD4+CD25+ Tregs. |
Newly diagnosed AML | BM and PB |
| Chamuleau et al. [43] | 286 | High IDO gene expression levels in leukemic blasts correlates with significantly shortened overall and relapse-free survival. | AML | BM and PB |
| Aurelius et al. [36] | 26 | Monocytic AML cells produce reactive oxygen species (ROS) that kill T cells and NK cells by triggering PARP-1 dependent apoptosis. | Newly diagnosed AML | PB and BM |
| Luczynski et al. [187] | 20 | ALL blasts express low levels of co-stimulatory molecules. | Pre-B ALL | PB |
| Kebelmann et al. [188] | 10 | BM blasts lack expression of CD80. ↓ expression of the adhesion molecule ICAM-1. ↑ secretion of IL-10 by leukemic cells. |
Pediatric Relapsed Pre-B ALL |
BM |
| Zheng et al. [189] | 48 | Leukemic cells lack expression of CD80. 50% of AML samples express CD86. |
ALL (n=6), AML(n=30), other hem. malignancies (n=12) | PB |
| Costello et al. [37] | 18 | ↓ NK cell cytolytic activity. Leukemic blasts resistant to lysis by NK cells. |
AML | PB |
Abbreviations: NK cells - natural killer cells; PB - peripheral blood, BM - bone marrow; PBMC - peripheral blood mononuclear cells; hem. - hematologic
Although a number of genetic and epigenetic changes within leukemia cells provide antigens that should easily be recognized by T cells [16, 17], leukemic blasts have been reported to downregulate human leukocyte antigen (HLA) molecules and to have altered antigen presentation properties that prevent T cell activation [18, 19]. Recently, AML cell loss of the non-shared HLA haplotype has been demonstrated in patients relapsing after HLA-haploidentical HSCT [20]. Furthermore, many leukemia-associated antigens (LAAs) are widely expressed on other tissues, thus generating only weak T cell responses [16, 21, 22].
Besides antigen presentation, other mechanisms contributing to the dysfunctional T cell responses in AML include expansion of suppressive immune cell populations, such as regulatory T cells (Tregs) (Box 1) [23–27] and myeloid-derived suppressor cells (MDSCs) [28, 29] as well as down-regulation of co-stimulatory receptors on the leukemia cells [30–34]. Other mechanisms of immune escape include production of immunosuppressive cytokines or reactive oxygen species (ROS) by leukemia cells themselves or within their microenvironment [35, 36] and impaired natural killer (NK) cell function [37, 38].
Box 1. Regulatory T cells (Tregs).
Tregs are a diverse population of CD4+ T cells with immunosuppressive, rather than inflammatory properties [190]. A key characteristic of Tregs is the expression of the forkhead/winged-helix transcription factor FoxP3 [191, 192]. Tregs suppress the activation and proliferation of other T cells, and therefore may hamper the activation of anti-tumor effector cells. Mechanisms of suppression include production of inhibitory cytokines such as interleukin (IL)-10, transforming growth factor (TGF)-β, and IL-35 [193]. Tregs express high levels of several immune checkpoint molecules. Inhibitory antibodies targeting the many immune checkpoints are likely to enhance anti-tumor immunity by blocking Treg-mediated immunosuppression.
Furthermore, enzymes that degrade metabolically important amino acids (e.g. tryptophan, arginine) [39, 40] and ectonucleotidases like CD39 [41] may contribute to altered T cell immunity. In particular, expression of indoleamine 2,3 dioxygenase (IDO), an enzyme that catalyzes the rate-limiting step of tryptophan degradation along the kynurenine pathway, has been reported in AML blasts [42] and is associated with shortened relapse-free and overall survival (OS) [43]. Depletion of tryptophan and accumulation of its metabolites results in inhibition of effector T cell proliferation, increased T cell apoptosis, and peripheral induction of Tregs, which all lead to an impairment of the anti-leukemic cellular immune response [39, 44]. Several IDO inhibitors are under active clinical investigation in hematologic malignancies, including myelodysplastic syndrome (MDS) and B cell acute lymphoblastic leukemia (B-ALL) (NCT01822691 and NCT01595048).
A recent study has revealed yet another mechanism of immune escape in AML:deletional T cell tolerance, which is likely mediated by immature antigen-presenting cells (APCs) that present LAAs in a context resulting in T cell deletion or anergy [45]. Lastly, and the subject of this review, upregulation of inhibitory ligands and their receptors (immune checkpoints) has been described on leukemia cells, serving as important mechanisms of immune evasion as well as potential therapeutic targets.
4. EMERGING ROLE OF IMMUNE CHECKPOINTS IN IMMUNE ESCAPE BY ACUTE LEUKEMIA
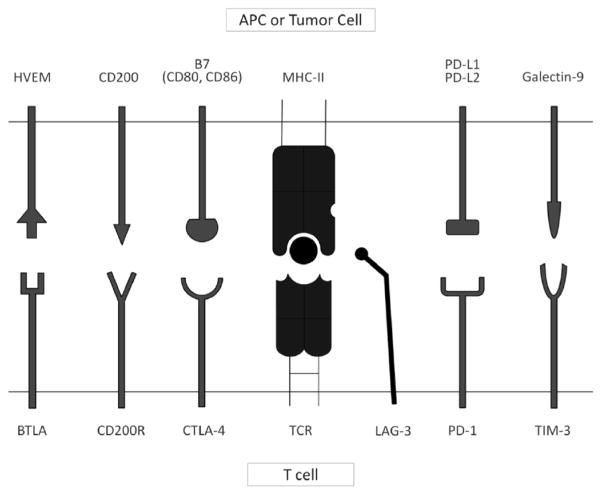
Multiple immune checkpoint molecules, described below, have been implicated in immune escape by tumors, including acute leukemia. A summary of the co-inhibitory receptors and their respective ligands discussed in this review is shown in Fig. (1).
Fig. (1). Selected Immune Checkpoint Receptors and their Respective Ligands.
Tumors co-opt immune checkpoint pathways as a critical mechanism of immune evasion. Immune checkpoints are initiated by ligand-receptor interactions and inhibit T cell activation and proliferation. Fig. (1) shows selected immune checkpoint receptors including BTLA (B- and T-lymphocyte attenuator) that binds to its ligand HVEM (herpes virus entry mediator), CD200R that engages with CD200, CTLA-4 (cytotoxic T-lymphocyte associated antigen 4) that binds the B7 molecules (CD80 and CD86), LAG-3 (lymphocyte activation gene 3) that binds to the MHC complex, PD-1 (programmed cell death-1) that engages with PD-L1 or PD-L2, and TIM-3 (T cell immunoglobulin and mucin domain-containing protein 3) that binds galectin 9 on the antigen presenting cell or tumor cell.
4.1. CTLA-4
Biology
CTLA-4 (cytotoxic T-lymphocyte associated antigen 4, CD152) is one of the most well studied co-inhibitory receptors and the first one to be targeted in a clinical setting. CTLA-4 was discovered in 1987, when Brunet et al. [46] screened murine cytotoxic T cell-derived cDNA libraries and came across a 223-amino acid protein that clearly belonged to the B7 immunoglobulin superfamily. CTLA-4 is homologous to CD28, and they share identical ligands, CD80 and CD86. CTLA-4, however, binds CD86 and in particular CD80 with much higher avidity and affinity than does CD28 [47–49]. CTLA-4 is expressed predominantly on activated T cells [46] and on Tregs [50–52]. Engagement of CD80 or CD86 with CTLA-4, in contrast with that seen with the activating ligand CD28, results in inhibition of the early stages of T cell activation, thus dampening T cell responses (Box 2) [53]. Besides diminishing effector T cell activation, CTLA-4 signaling in Tregs controls autoreactive T cells and thereby promotes tolerance to self-antigens, further underscoring the diverse role of CTLA-4 in maintaining immune homeostasis. [52, 54] CTLA-4’s major role as a central inhibitory checkpoint was demonstrated through the use of CTLA-4 knockout mice, which had hyperactivated immune systems, consequently resulting in lethal lymphoproliferative disease with massive multi-organ T cell infiltration [55, 56].
Box 2. The Biology of T cell Activation.
Almost 30 years ago Jenkins and Schwartz [194] had shown that engagement of the T cell receptor (TCR) is not sufficient to fully activate T cells. T cell activation is dependent on a two step signaling. Signal 1 involves the TCR recognizing a specific antigen peptide presented on the major histocompatibility complex (MHC complex) expressed on antigen-presenting cells (APCs) and is assisted by the presence of CD4+ or CD8+ on the T cell. Effective activation of naïve T cells, however, requires a second co-stimulatory signal (signal 2) that strongly amplifies TCR signaling to activate T cells. The best characterized co-stimulatory molecules that deliver signal 2 are the B7 molecules (CD80/B7.1 and CD86/B7.2). B7 molecules, expressed on APCs, are encountered by CD28, its receptor on the T cell surface. Ligation of CD28 by B7 molecules is necessary for the optimal clonal expansion of naïve T cells. Importantly, CD28 does not interfere with T cell activation unless the TCR has firstly engaged its antigen through signal 1.
Mechanisms of Action
Multiple cell-intrinsic and cell-extrinsic mechanisms of action have been proposed for CTLA-4-mediated inhibition of T cell responses and are still being elucidated [57, 58]. Reports of cell-extrinsic mechanisms include increased secretion of cytokines such as TGF-β [59] (resulting in reduced antigen presentation, impaired effector T cell function, and expanded Treg activity), sequestration and active removal of CD80 and CD86 from the APCs surface through transendocytosis to prevent their engagement with CD28 [60, 61], activation of the tryptophan-degrading enzyme IDO, and enhancement of Treg function [62–64]. Cell-intrinsic pathways include the activation and recruitment of the inhibitory protein phosphatases PP2A and PTPN11 to the T cell synapse where they inhibit TCR and CD28-induced signaling, transcriptional inhibition of cell-cycle regulators, and competition with CD28 for their shared ligands [65–67]. Recent studies suggest that cell-intrinsic functions are not required for systemic control of effector T cell function as CTLA-4-deficient effector T cells did not demonstrate any apparent abnormality when mixed with wild-type cells in vivo [68, 69]. Further elucidation of CTLA-4’s mechanisms of action is needed to fully understand its effects in clinical application.
CTLA-4 in Solid Tumor Malignancies
The concept of CTLA-4 blockade relies on reactivation of endogenous immune responses against antigens expressed on tumor cells. There was an initial concern that blocking CTLA-4 activity might lead to overt systemic hyperimmunity as seen in CTLA-4 knockout mice. Fortunately, antibody-mediated CTLA-4 blockade resulted in rejection of established immunogenic mouse tumors and provided long-lasting immunity without overt immune toxicities, thus encouraging subsequent clinical translation [70]. Two fully humanized anti-CTLA-4 monoclonal antibodies (mAbs) ipilimumab (MDX-101, Yervoy®; Bristol-Myers Squibb) and tremelimumab (CP-675,206; Pfizer) were brought to clinical testing [71–73]. In a phase III study of advanced melanoma patients comparing treatment with ipilimumab with or without the melanoma-specific vaccine gp100 versus the gp100 vaccine alone, ipilimumab treatment produced a 3.5 month survival benefit, and 18% of patients on the ipilumimab arms were still alive 2 years after treatment [74]. So-called immune-related adverse events (IRAEs) were observed in 10% to 15% of patients treated with ipilimumab. The success of ipilimumab in advanced melanoma resulted in its approval by the US Food and Drug Administration (FDA) in 2010. Not all melanoma patients, however, benefit from ipilumumab therapy, and no consistent predictive biomarkers of response have been validated [75–77]. A number of ongoing clinical trials are investigating the role of ipilimumab in various solid tumors and hematologic malignancies, either alone or in combination with other modalities such as cancer vaccines, other immunomodulatory agents, chemotherapy, or radiation [78].
CTLA-4 in Acute Leukemia
As described above, one immune escape mechanism involves the defective expression of ligands important for T cell co-stimulation by tumor cells. As CTLA-4 and CD28 share CD80 and CD86 as their common ligands, resulting in either T cell inhibition or stimulation, investigators have explored the expression of CD80 and CD86 by leukemic cells. Several groups have shown that leukemia cells (mainly M2, M4, and M5 AML by the French-American-British (FAB) classification) from both PB and bone marrow (BM) have a more consistent but heterogeneous expression of CD86 at baseline and generally lack or have a very low expression of CD80 [30–34]. Expression of CD80 and CD86 together on AML blasts was reported to positively correlate with prolonged remission post induction chemotherapy in one study [79], while other studies suggested that CD86 expression on leukemia blasts is associated with worse outcomes [32, 33]. LaBelle and colleagues directly examined the relative impact of CD80 and CD86 expression on anti-tumor immunity by challenging mice with C1498, a murine myelogenous leukemia cell line, expressing either CD80 or CD86 [80]. They demonstrated that C1498/CD80+ AML tumors grew progressively, whereas expression of CD86 on AML cells resulted in tumor rejection implying effective induction of tumor-specific T cell immunity against wild-type C1498 leukemia. The escape of C1498/CD80+ tumors from T cell-mediated immune elimination was found to be CTLA-4-dependent based on experiments showing that C1498/CD80+ tumors regress after antibody blockade of CTLA-4 or after genetically deleting CTLA-4 from responding T cells [80].
Most data on CTLA-4 expression are limited to T cells. However, an Italian group of investigators examined CTLA-4 expression at the protein and mRNA level in leukemia cell lines and primary leukemia samples (AML, ALL) and found that CTLA-4 is universally expressed in all types of leukemia [81]. Binding of CTLA-4 on leukemia cells with soluble recombinant ligands r-CD80 and r-CD86 led to apoptosis of AML cells [82]. Furthermore, leukemic cells may have the ability to interact with the CD80/CD86 ligands on APCs, potentially modulating immune responses and ultimately promoting the growth and persistence of leukemic cells [81].
The important role of CTLA-4 in modulating anti-leukemia immune responses has been demonstrated by the association between CTLA-4 polymorphisms and outcomes in AML patients [83]. In those patients who achieved a first complete remission (CR) after induction chemotherapy, the CTLA-4 CT60 A/A genotype was associated with a higher rate of leukemic relapse and decreased overall survival (OS) at three years [83]. In children with ALL, certain polymorphisms associated with higher CTLA-4 expression appear to be increased compared to healthy controls and were in fact highest within the high-risk group [84]. However, donor CTLA-4 genotypes were not found to affect the post-transplant outcomes (OS, relapse free survival, and incidence of acute and chronic graft-versus-host disease [GVHD]) among 780 patients with AML and/or MDS following HLA matched or one antigen mismatched unrelated donor HSCT [85]. Besides membrane-bound CTLA-4, a native soluble form of CTLA-4 (sCTLA-4), produced by alternative CTLA-4 mRNA splicing, has been described for a variety of autoimmune diseases and may play a role in regulating immune responses [86]. Significantly elevated levels of circulating sCTLA-4 also have been reported in pediatric patients with B-ALL and were associated with a poor prognosis [87, 88].
Several experimental studies have demonstrated that CTLA-4 can be targeted to enhance a clinical anti-leukemia immune response. CTLA-4 blockade significantly enhanced the capacity of AML-derived DCs to induce T cell responses against autologous AML cells in vitro [89, 90]. This effect was manifested by increased frequency and number of AML-specific T cells, which produced more IFN-γ and were more cytotoxic towards autologous AML cells than those cultured without an anti-CTLA-4 mAb [90]. In the DA1-3b mouse model (tumor dormancy model) of AML, long-term persistent murine leukemia cells (minimal residual disease, MRD) were more resistant to CTL-mediated killing and had increased expression of PD-L1 and CD80 [89]. Upon CTLA-4 blockade (and PD-L1 blockade, described below), CTL-mediated lysis of these persistent AML cells was enhanced as was survival of naïve mice injected with AML cells at MRD levels.
The timing of CTLA-4 blockade may be important for its use in the allo-HSCT setting, as demonstrated in murine models of MHC- and/or MiHA-mismatched allo-HSCT [91, 92]. If administered immediately after transplant, anti-CTLA-4 mAb increased donor T cell expansion and GVHD, as well as host-mediated BM graft rejection due to the dominant effect of the CD28:B7 pathway. However, when administered later after allo-HSCT, CTLA-4 blockade potently augmented GVL immunity without substantially increasing GVHD-related side effects [91, 92].
In the clinical setting, a single infusion of ipilimumab (phase I study, NCT00060372) given to 29 patients with recurrent or progressive hematologic malignancies (including 2 AML patients) after a minimum of 90 days post-allo-HSCT or DLI (median 1 year, range 0.3–6.5 years) did not induce or exacerbate GVHD or promote graft rejection [93]. Organ-specific IRAEs occurred in 14% of patients, but importantly, two patients with Hodgkin lymphoma achieved CR and one patient with mantle cell lymphoma achieved a partial remission (PR) [93]. Analysis of the peripheral blood T lymphocytes before and after single-dose ipilimumab administration revealed that CTLA-4 blockade is associated with increased conventional CD4+ T cell activation and intracellular CTLA-4 expression without resulting in changes in levels of Tregs [94]. Ipilimumab is now being evaluated in patients with relapsed MDS/AML (NCT01757639) and in patients with relapsed hematologic malignancies after allogeneic-HSCT (NCT01822509).
4.2. PD-1
Biology
Programmed death-1 (PD-1, CD279) is another inhibitory receptor that belongs to the B7/CD28 family. Almost two decades ago, PD-1 was identified on T cells undergoing apoptosis [95]. It was only later that its major role in inhibiting T cell activation and effector function in an inflammatory environment and controlling autoimmunity in peripheral tissues was elucidated [96, 97]. The importance of PD-1 as a peripheral inhibitory regulator was confirmed in PD-1 knockout mice that developed organ- and strain-specific autoimmune complications including cardiomyopathy, lupus-like arthritis, and glomerulonephritis [98, 99]. In contrast to CTLA-4 deficient mice, PD-1 deficient mice have milder phenotypes [100]. PD-1 functions during the effector phase of T cell activation and is expressed on a broad variety of cells including activated T cells, B cells, monocytes, dendritic cells, and NK cells [101]. Notably, naïve lymphocytes do not express PD-1 prior to activation [102].
PD-1 has two ligands, PD-L1 (B7-H1, CD274) and PD-L2 (B7-DC, CD273), that bind PD-1 with different affinity [96, 103–105]. Although PD-L1 is expressed on different cell types, including hematopoietic and epithelial cells, its expression is generally low on normal human tissues [106]. The inflammatory milieu, including that encountered in the tumor microenvironment, promotes PD-L1 upregulation on a variety of different tumor cells in response to proinflammatory cytokines such as IFN-γ [106]. Thus, PD-L1 plays a key role in mediating tumor cell immune resistance to endogenous immune responses (adaptive immune resistance) within the tumor microenvironment. Upregulation of PD-L1 by activation of oncogenic pathways may contribute to innate immune resistance [107]. The increased expression of PD-L1 on tumor cells has been associated with poor prognosis in certain solid tumors [108–112]. PD-L2, the second known ligand for PD-1, has both stimulatory and inhibitory functions on T cell activation. While it is mostly expressed on APCs, PD-L2 also is upregulated on certain tumors such as B cell lymphoma [104, 113, 114]. Moreover, CD86+ HL-60 myeloid leukemia cells were shown to provoke T-helper cell responses and become immunosuppressive through the upregulation of both PD-L1 and PD-L2 [115].
Mechanisms of Action
Upon engagement by one of its ligands, PD-1 becomes phosphorylated at its cytoplasmic tail signaling motif, which then recruits protein phosphatases like SHP2 and results in dephosphorylation of the proximal TCR signaling molecules [29]. These changes also suppress CD28/TCR-mediated activation of phosphoinositide 3-kinase (PI3K) and Akt signaling, leading to inhibition of T cell activation [67]. In contrast to PD-1 signaling, CTLA-4 signaling inhibits Akt independently of PI3K [67]. Recent studies reported an interaction between PD-L1 and the CD80 co-stimulatory molecules, resulting in T cell inhibition [116, 117]. This finding has added an increased layer of complexity to the role of CD80 as a control nexus for mediating stimulatory and inhibitory signals from CD28, CTLA-4, and PD-1. Further investigation is needed to understand this additional dimension to the complex binding interactions of checkpoint proteins and their immunoregulatory functions. Furthermore, epigenetic regulation of the PD-1 gene after TCR stimulation regulates its expression and controls T cells responses. Demethylation of the PD-1 gene is observed upon activation of CD8+ T cells, but becomes remethylated in functional memory T cells [118]. However, in chronic viral infections, exhausted effector memory T cells have upregulated PD-1 and maintained demethylation of the PD-1 locus [118, 119]. In addition, as PD-1 is expressed not only on effector T cells, but also on Tregs, NK cells, and B cells, inhibition of PD-1 may augment anti-tumor immunity through a variety of mechanisms including activation of T effector function, suppression of Treg inhibition, promotion of NK cell activity, and production of antibodies.
PD-1 in Solid Tumor Malignancies
A critical milestone in the clinical development of PD1/PD-L1/-L2 pathway inhibitors was achieved in 2014 with the FDA approval of pembrolizumab (Keytruda®, Merck), and shortly after, nivolumab (Opdivo®, Bristol-Myers Squibb), two anti-PD-1 antibodies, for the treatment of patients with metastatic melanoma who have failed prior therapies with ipilumumab and, if BRAF V600 mutation positive, a BRAF inhibitor. Pembrolizumab produced a 26% response rate in this patient population, and, at most recent data analysis, 88% (36/41) of patients with objective responses had ongoing responses at follow-up of 1.4 to 8.5+ months [120]. Nivolumab produced responses in 32% of patients compared to 11% response rate in chemotherapy arm, with 87% (33/38) of patients having ongoing responses at follow up of 2.6 to 10+ months [121].
A number of agents targeting PD-1 (nivolumab, pembrolizumab, and CT-011) or PD-L1 (MPDL3280A, BMS936559, and MEDI4736) are under clinical investigation. This fast-paced development of agents targeting PD-1 and PD-L1 was prompted by a favorable safety and efficacy profile observed in an initial Phase I study of repeat-dose nivolumab in patients with advanced solid tumors [122]. Nivolumab produced objective and durable responses in 28% of patients with melanoma, 18% of patients with non small cell lung cancer, and 27% of patients with renal cell carcinoma [122]. Notably, the expression of PD-L1 on tumor cells seemed to correlate with response. Furthermore, the frequency of IRAEs appeared less than with anti-CTLA-4 mAb treatment [122]. Updated survival data for advanced (but non-ipilimumab-treated) melanoma patients treated with nivolumab show 1-, 2-, and 3-year survival rates of 62%, 48%, and 41%, respectively [123]. Inhibiting both CTLA-4 and PD-1 in melanoma patients appeared to be more effective when given concurrently (objective response (OR) 53%) than when given sequentially (OR 20%) [124]. At the most promising dose level of ipilimumab 3 mg/kg and nivolumab 1 mg/kg, 2-year survival was 79% [124, 125]. While toxicities were increased with concurrent therapy, the side effects were qualitatively similar to that seen with monotherapy and dose-limiting in 21% of patients. Notably, IRAEs were generally manageable and reversible with treatment interruption, treatment discontinuation, or the administration of glucocorticoids [124]. Enthusiasm over the use of PD-1 inhibitors in hematologic malignancies comes from promising preclinical data and the success of early clinical trials in patients with Hodgkin lymphoma (HL). Nivolumab and pembrolizumab produced objective responses in 87% and 53% of relapsed or refractory HL patients, respectively, and 86% of patients treated with nivolumab maintained responses at 6 months [126–128]. Based on these results, the FDA granted nivolumab breakthrough status in relapsed HL.
PD-1 and PD-L1 in Acute Leukemia
Signaling through the PD-1/PD-L1 axis has been shown to impair anti-leukemic immunity. As described earlier in this review, PD-L1 and CD80 are upregulated on long-lived murine myeloid leukemia cells (MRD) that exhibit resistance to CTL-mediated killing [89]. Upon PD-L1 checkpoint blockade, CTL-mediated lysis of leukemic cells was enhanced in vitro, which was accompanied by increased levels of IFN-γ and TNF-α and decreased IL-10 production, and the survival of naïve mice injected MRD-derived leukemia cells was improved [89]. Zhang et al. [129] evaluated the role of PD-1/PD-L interactions in a murine model of AML and found that murine leukemia C1498 cells had low PD-L1 expression in vitro, but PD-L1 became upregulated in vivo. One could speculate that the leukemic microenvironment may stimulate upregulation of PD-L1, thus contributing to immune evasion. These investigators also demonstrated that leukemia-specific T cell immunity and survival upon AML challenge was increased in PD-1 knockout mice or in wild-type mice upon PD-L1 blockade using a mAb [129]. In another murine model of advanced AML, tumor progression was associated with upregulation of PD-1 expression on CD8+ CTLs, but also correlated with an increased frequency of Tregs [130]. PD-1 knockout mice were less susceptible to C1498 challenge, despite having similar Treg percentages compared to wild-type mice. Interestingly, AML-associated Tregs impaired the function of adoptively transferred CTLs in vivo, and Treg depletion followed by blockade of PD-L1 resulted in superior anti-leukemia responses than PD-L1 blockade alone [130].
Similar to what is seen in other malignancies, PD-L1 expression has been inconsistently found on human AML cells. A Japanese group reported low or absent PD-L1 expression on de novo AML cells, whereas PD-L2 was more frequently expressed [131]. High PD-L2 expression (>25% of leukemia cells) was associated with significantly shortened survival [131]. In contrast, several groups reported that PD-L1 is expressed on acute leukemia cells (AML and ALL) in PB and/or BM, but the frequency of PD-L1-positive leukemia varied across different studies from 18% to more than 50% [132–134]. A few studies also have reported that PD-L1 expression increased at the time of relapse [133, 134]. PD-L1 appeared to be expressed more frequently on acute monocytic leukemia (M5) and predicted for poor prognosis [133]. These different observations on PD-L1 expression on leukemic cells are likely due to multiple factors, including diverse patient populations studied (particularly given that AML is a very heterogenous disease), different assays and techniques used to detect PD-L1, different cell source (PB or BM) examined, and the use of different treatment regimens. Most recently, PD-L1 expression was measured on freshly isolated leukemic blasts from the PB and BM of 154 AML patients with or without ex vivo stimulation with IFN-γ [135]. IFN-γ exposure strongly increased PD-L1 expression on leukemia blasts, particularly in patients who had completed induction chemotherapy, suggesting that its upregulation is driven by an inflammatory tumor microenvironment [135]. Treatment with a hypomethylating agent (decitabine) appears to up-regulate PD-1, PD-L1, PD-L2, and CTLA-4 gene expression in PB mononuclear cells of patients with myeloid malignancies (MDS, AML, and chronic myelomonocytic leukemia) [136].
The impact of the PD-1/PD-L1 inhibitory pathway on acute leukemias in the post-transplant setting has been evaluated in both mice and humans. In different murine models, heightened PD-1 expression on donor T cells post allo-HSCT was found to be associated with a lack of GVL reactivity [137, 138]. Functional exhaustion of donor T cells was fostered by the expression of allo-antigens on the recipient’s non-hematopoietic cells [137, 138]. Similarly, adoptive transfer of allogeneic T cells, engineered to express TCRs against LAAs, provided a potent GVL effect without causing GVHD, but only if administered early post-transplant [139]. If administered later post-transplant, however, adoptive transfer is effective only with concurrent PD-L1 blockade due to PD-1 upregulation on T cells in leukemia-bearing mice [139]. In these three studies, donor T cell dysfunction could partially be restored by blockade of PD-L1; delayed blockade of PD-L1 after allo-HSCT improved the beneficial GVL effect without inducing GVHD [137–139]. In two patients relapsing after allo-HSCT, PD-L1 was found to be highly expressed on leukemic progenitor cells, whereas co-stimulatory molecules CD80 and CD86 were generally absent [34]. Furthermore, high PD-1 expression was noted on alloreactive MiHA-specific effector memory CD8+ T cells. Ex vivo treatment with mAbs against PD-1 or PD-L1 restored proliferation and IFN-γ production of MiHA-specific effector memory T cells, thus enhancing alloreactive T cell responses, while stimulation with MiHA-loaded DCs alone was insufficient to activate these exhausted cells [34]. In addition, PD-1 blockade was more potent in activating MiHA-specific T cells from relapsed patients than those in remission [34]. Another study evaluated ex vivo vaccination with MiHA-loaded DCs that had undergone short interfering RNA (siRNA) silencing of PD-L1 and PD-L2 [140]. DC vaccination augmented expansion and cytokine production of MiHA-specific effector memory CD8+ T cells from leukemia patients early after DLI or later at relapse [140]. Furthermore, these expanded MiHA-specific CD8+ T cells retained the capacity to degranulate and exert cytotoxic activity upon recognition of MiHA+ target cells [140]. A phase I clinical study evaluated a single infusion of anti-PD-1 mAb CT-011 (CureTech Ltd, Yavne, Israel) in 17 patients with advanced hematologic malignancies, including 8 patients with AML [141]. Treatment with anti-PD-1 was well tolerated, and 6 patients benefited from treatment, including one patient with non-Hodgkin lymphoma (NHL) who achieved a CR. While one AML patient demonstrated disease stabilization and reduction in circulating blasts, another AML patient who received anti-PD-1 mAb 8 weeks post allo-HSCT, developed worsening of mild GVHD that was present at treatment, culminating in grade 4 acute GVHD [141]. As with CTLA-4 blockade, these studies underscore the importance of appropriate timing for the administration of checkpoint inhibitors in the post-transplant setting.
4.3. Other Immune Checkpoints
A number of other immune checkpoints are emerging as interesting targets for anti-cancer immunotherapy [4]. In vitro studies identified many of these receptors as important contributors to T cell dysfunction and immune evasion in a variety of hematologic malignancies [7]. Data on acute leukemias are limited but suggest that the key to enhancing anti-tumor immunity may lie in combinatorial approaches of checkpoint inhibition. Studies evaluating immune checkpoint blockade for the treatment of acute leukemia in mice or humans are summarized in Table 2.
Table 2.
Immune Checkpoint Blockade for Treating Acute Myeloid Leukemia in Mice or Humans*.
| Checkpoint Molecule | PRECLINICAL STUDIES | CLINICAL STUDIES | |
|---|---|---|---|
| Non Transplant | Transplant | ||
| CTLA-4 |
LaBelle et al., 2002 [80]; mouse, in vivo
|
Blazar et al., 1999 [91]; mouse, in vivo
|
NCT00060372, Bashey et al., 2009 [93]
|
Saudemont & Quesnel, 2004 [89]; mouse, in vitro
|
Fevery et al., 2007 [92]; mouse, in vivo
|
NCT01757639
|
|
Zhong et al., 2006 [90]; human, in vitro
|
NCT01822509
|
||
| PD-1 |
Saudemont & Quesnel, 2004 [89]; mouse, in vitro
|
Flutter et al., 2010 [138]; mouse, in vivo Koestner et al., 2011 [139]; mouse, in vivo
|
Berger et al., 2008 [141]
|
Zhang et al., 2009 [129]; mouse, in vivo
|
Hobo et al., 2010 [140]; human, in vitro
|
NCT01096602
|
|
Zhou et al., 2010 [130]; mouse, in vivo
|
Norde et al., 2011 [34]; human, in vitro
|
NCT02275533
|
|
| TIM-3 |
Zhou et al., 2011 [146]; mouse,in vivo
|
||
| BTLA |
Hobo et al., 2012 [170]; human, in vitro
|
||
| CD200R |
Coles et al., 2011 [174]; human, in vitro
|
Gorczynski et al., 2001 [173]; mouse, in vivo
|
|
Memarian et al., 2013 [179] human, in vitro
| |||
Abbreviations: allo-HSCT - allogeneic hematopoietic stem cell transplantation; BMT - bone marrow transplant; CTL - cytotoxic T lymphocyte; GVHD - graft versus host disease; GVL - graft versus leukemia; HL - Hodgkin’s Lymphoma; MCL - Mantle Cell Lymphoma; MLR - mixed lymphocyte reaction; NHL - Non Hodgkin Lymphoma; Tregs - regulatory T cells
TIM-3 (T cell immunoglobulin and mucin domain-containing protein 3), an inducible inhibitory receptor expressed on IFN-γ producing T helper 1 cells (TH1), was recently shown to contribute to T cell co-inhibition in acute leukemias. TIM-3 inhibits TH1 responses through interaction with its ligand galectin-9, which is expressed in a variety of tissues in mice and humans, including on tumor cells [142]. Increased frequency of TIM-3 expressing dysfunctional (exhausted) T cells was observed in the tumor microenvironment in murine models as well as in patients with melanoma or chronic viral infections and TIM-3 antibody blockade enhanced anti-tumor immunity [143–145]. Both mice and human AML cells express galectin-9, while co-expression of TIM-3 and PD-1 was observed on a subset of exhausted CD8+ T cells in a systemic murine AML model: TIM-3 and PD-1 co-expression increased with AML progression [146]. Overexpression of TIM-3 on T cells also was shown to suppress immune responses through upregulation of MDSCs at the tumor site [147]. Higher expression of TIM-3 was observed on PB CD4+ and CD8+ T cells of newly diagnosed AML patients compared to healthy controls and appeared to correlate with the disease risk group and FLT3-ITD mutation status [148]. Furthermore, increased expression of TIM-3 on endothelial cells in human lymphoma contributes to impaired CD4+ T cell responses and promotes lymphomagenesis [149]. In the murine AML model, only combined blockade of the TIM-3/galectin-9 and PD-1/PD-L1 pathways, using a TIM-3 fusion protein and a mAb against PD-L1, decreased tumor burden and lethality, whereas blockade of either pathway alone was insufficient to rescue mice from death from AML [146]. Besides its expression on T cells, TIM-3 has emerged as a specific marker for CD34+CD38− myeloid leukemia stem cells (LSC) and seems to be a promising candidate for LSC-targeted therapies [150–152].
LAG-3 (lymphocyte activation gene 3; CD223), another co-inhibitory receptor, is a transmembrane protein that is structurally similar to CD4 and binds the MHC II complex with high affinity [153]. LAG-3 is expressed on activated T cells, regulatory T cells, and NK cells [154, 155]. LAG-3 expression on effector T cells limits effective viral- or tumor-specific T cell responses [156]. Furthermore, LAG-3 is frequently co-expressed with PD-1 on exhausted T cells, and dual blockade of LAG-3 and PD-1 during T cell priming augmented proliferation and cytokine production by tumor-antigen-specific T cells [157]. However, activation of T cells under tolerizing conditions yielded distinct CD8+ T cell populations expressing either or both of PD-1 and LAG3; these different populations had distinct functional characteristics, suggesting that, while both are inhibitory, PD-1 and LAG-3 do not have redundant effects on T cell function [158]. The synergistic potential of PD-1 and LAG-3 inhibition in activating T cells is further underscored by evidence that dual PD-1 and LAG-3 knockout mice reject even poorly immunogenic tumors and develop lethal autoimmunity albeit at a slower pace than seen in CTLA-4-deficient mice [159, 160]. In patients with Hodgkin lymphoma, the presence of both CD4+LAG3+ and CD4+FOXP3+ cells in the tumor environment coincided with defective tumor-specific (LMP1/2) T cell responses [161]. FoxP3 and LAG-3 were expressed on different regulatory T cell subsets, and deletion of CD4+LAG3+ T cells augmented tumor-specific responses in vitro [161]. Data on LAG-3 expression in acute leukemia are limited; one report found that only T cells isolated from CD30L- but not CD30L+ AML patients expressed LAG-3 [162].
A soluble form of the LAG-3 receptor (IMP321) has been in clinical testing in patients with solid tumor malignancies and most recently an anti-LAG-3 mAb (BMS-986016) is being tested either alone or in combination with anti-PD1 in solid tumor malignancies as well as patients with lymphoma and multiple myeloma.
BTLA (B- and T-lymphocyte attenuator; CD272) is another inhibitory receptor belonging to the B7 family and has structural and functional similarities to CTLA-4 and PD-1 [163]. BTLA is mainly expressed on T cells, B cells, and DCs [164]. Unlike the other B7 family members, BTLA binds to HVEM (herpes virus entry mediator), a member of the TNF receptor superfamily [165]. HVEM itself has a complex signaling network with multiple other binding partners including CD160, LIGHT (homologous to lymphotoxin; TNFSF14), lymphotoxin-α, and herpes simplex glycoprotein D [166]. Furthermore, the signaling can be bi-directional, depending on the type and combination of interactions [164]. While engagement of HVEM with BTLA or CD160 leads to T cell inhibition, its ligation with LIGHT results in T cell co-stimulation [164]. Naïve T cells express both HVEM and BTLA, which form a heterodimeric complex and consequently make HVEM inaccessible to external ligands, thus inhibiting co-stimulation [167]. This mechanism appears to be important in self-tolerance as BTLA4-deficient mice developed autoimmune diseases [163, 168]. In melanoma patients, dysfunction of tumor antigen (NY-ESO-1)-specific effector CD8+ T cells correlated with increased expression of BTLA together with co-expression of PD-1 and TIM-3 [169]. Importantly, T cell dysfunction could be reversed by triple blockade of BTLA/PD-1/TIM-3 [169].
The role of BTLA in suppressing T cell immunity against MiHA post allo-HSCT was evaluated in patients with hematologic malignancies, including AML. HVEM was found to be highly expressed on primary leukemic cells, whereas BTLA was moderately and more heterogeneously expressed [170]. The expression of LIGHT and CD160 was generally low or absent. MiHA specific CD8+ effector memory T cells were found to express high levels of BTLA and PD-1. Ex vivo blockade of the BTLA-HVEM pathway using an anti-BTLA mAb and/or inhibition of PD-1 by an anti-PD1 mAb restored proliferation of highly functional MiHA-specific CD8+ T cells and augmented alloreactive T cell responses. In several patients, the effect of BTLA blockade was more pronounced compared to PD-1 blockade, underscoring its importance in allogeneic tumor-specific T cell immunity [170].
CD200R is an inhibitory receptor expressed by cells of either the myeloid or lymphoid lineage, including NK and T cells [171, 172]. CD200R exclusively binds CD200 (OX2), a type I membrane glycoprotein. CD200 is expressed by a variety of cells and tissues and has been shown to be expressed by AML blasts in both mice and humans [173–175]. In mice, CD200 expression by leukemia cells was associated with enhanced tumor growth that could be inhibited by CD200 Fc administration [173]. In humans, an association between CD200 expression by AML blasts (43% of AMLs; most commonly seen on core binding factor AMLs) and poor prognosis was reported [176]. Signaling through the CD200/CD200R was shown to promote immune evasion in AML patients by reducing the function and frequency of activated NK cells and memory T cells, as well as by promoting the expansion of FoxP3+ Tregs [174, 177–179]. Notably, in vitro mAb blockade of the CD200R/CD200 ligation restored the cytolytic activity of NK cells [174].
Novel Checkpoint Receptors
A number of other inhibitory receptors are emerging as promising new targets for augmenting anti-tumor immunity and have been reviewed elsewhere [4, 7, 180]. Among them are A2aR, an adenosine receptor that promotes peripheral tolerance by inducing T cell anergy and generation of Tregs, specifically LAG-3+ Tregs [181], and PD-1H/VISTA, a PD-1 homolog expressed on hematopoietic cells and T cells that when inhibited prevented acute GVHD in a murine transplant model [182, 183].
Although inhibitory receptors associated with NK cell functionality are technically not immune checkpoints, significant defects in NK cell function have been shown to contribute to immune escape by acute leukemias [38]. The two common AML fusion proteins PML-RARA and AML1-ETO were recently shown to specifically downregulate CD48, the ligand of the NK cell receptor 2B4 (CD244), thus leading to impaired cytolytic activity of NK cells [184]. Therefore, receptors on NK cells, such as 2B4 (CD244) and the broad category of killer cell immune globulin-like receptors (KIR), may also be attractive targets to enhance anti-tumor immunity of acute leukemias [38, 185].
5. INTEGRATION OF CHECKPOINT BLOCKADE INTO CLINICAL MANAGEMENT OF ACUTE LEUKEMIAS - FUTURE PROSPECTS
Harnessing the body’s own immune system to fight cancer through immune checkpoint inhibition represents a breakthrough in cancer treatment. The recent FDA approval of the three checkpoint inhibitor mAbs targeting CTLA-4 or PD-1 for the treatment of metastatic melanoma has paved the way for the evaluation of this highly promising strategy in other malignancies, including acute leukemias. However, the clinical translation of checkpoint inhibitors faces some critical challenges. Firstly, only a limited number of patients respond to this therapeutic intervention and predictive bio-markers of response have not thus far been identified. Such reliable biomarkers are urgently needed both for evaluating a tumor’s immunogenicity and for assessing the likelihood of achieving a response to treatment. Second of all, not all tumors are equally immunogenic, and these tumors frequently employ a variety of different mechanisms to promote immune escape and survival. For example, tumor cells including leukemia blasts may aberrantly express antigens or fail to express co-stimulatory molecules, which are necessary for T cell activation. Also, certain tumors have been shown to poorly express MHC molecules or recruit Tregs or MDSCs to suppress other T cell subsets including CTLs. Another layer of complexity in AML stems from the different immune milieu of the BM and PB wherein dissimilar immune escape mechanisms may be operational. Thorough evaluation of a tumor’s potential to be immunologically targeted seems to be crucial for a successful implementation of such novel therapeutic strategies. Acute leukemias, due to ease of obtaining samples before and after treatment, may provide an ideal setting to address such questions.
Given that acute leukemias, in particular AML, are a very heterogeneous disease, one has to address the question whether all - or if not all - which of the many distinct subgroups are susceptible to checkpoint blockade. For instance, expression of CD200 and PD-L1 has only been found to be of prognostic significance for core binding factor leukemias and monocytic leukemia (FAB M5), respectively [133, 176]. Secondly, clinical responses to checkpoint blockade in hematologic malignancies may be difficult to measure. It is unlikely that checkpoint blockade alone will be sufficient to eliminate the bulk of leukemic blasts present at initial diagnosis. If agents that inhibit cellular checkpoints must be integrated with cytotoxic chemotherapeutic agents, the optimal timing of each therapy must be explored. Additionally, the expression of certain inhibitory receptors such as PD-L1 on leukemia cells may be promoted by the inflammatory milieu created by chemotherapy. Furthermore, one must not only consider therapeutic efficacy but also tolerance in such studies since IRAEs may not be easily tolerable in patients undergoing intensive chemotherapeutic treatment. Thus, administration of checkpoint inhibitors may be appealing at the time of consolidation chemotherapy when it is also likely to be better tolerated. Beyond its potential in the upfront setting, checkpoint inhibition is an attractive strategy for preventing disease relapse once patients have achieved complete remission or at the very least in the presence of only minimal residual disease. Even in this setting, however, timing is critical as evidenced by tumor-specific changes and differing potential for GVL versus GVHD seen in several murine and human studies that have evaluated CTLA-4 and PD-1 blockade in the post-transplant setting [91, 93, 138, 141].
As preclinical and recent clinical data suggest, the key to success may lie in combinatorial approaches to checkpoint inhibition [120, 124, 125]. Despite these promising results we are still left with the question of which and how many of the multiple checkpoint receptors to target. Which combinations make sense and which do not? Is dual, triple, or even several receptor blockades associated with a higher risk of toxicities or should we aim to target common downstream targets like SHP2 or Akt? Given that some checkpoint receptors signal bi-directionally or have both inhibitory and stimulatory ligands, it is clear that antibodies targeting these checkpoint receptors have to be carefully designed.
Based on the promising preclinical and clinical data discussed in this review, two checkpoint agents have made the long way from murine leukemic models to their evaluation in phase I clinical studies of acute leukemias. Anti-CTLA-4 (ipilimumab) is currently being tested in a US multicenter study enrolling patients with relapsed or refractory high-risk myelodysplastic syndrome (MDS) or acute myeloid leukemia (NCT01757639). Two other trials are evaluating ipilimumab in patients with relapsed hematologic malignancies (including acute leukemias) after allo-HSCT (NCT01822509 and NCT00060372). Blockade of PD-1 (nivolumab) is being tested in two phase I trials of AML patients in complete remission post induction chemotherapy (NCT02275533 and NCT01096602 (anti PD-1 together with a dendritic cell vaccine). Taken together, data from a number of preclinical and phase I trials show clearly that manipulation of inhibitory networks has emerged as an attractive strategy to increase anti-tumor immunity in patients with acute leukemia and that dual blockade of immune checkpoints can produce additive and perhaps even synergistic anti-leukemic responses. Ideally, these emerging therapies will meet the high demand for new treatment strategies to prevent relapses in the post-remission stage of acute leukemia, particularly for patients who are ineligible for allo-HSCT.
Acknowledgments
We gratefully acknowledge Raul Montiel Esparza, M.D. for his input and Benedikt Heidinger, M.D. for generating the figure. The authors’ work is supported in part by Leukemia & Lymphoma Society (LLS) award 6449-13 (LL) and NCI Cooperative Agreement UM1 CA186691 (IG).
ABBREVIATIONS
- ALL
Acute lymphoblastic leukemia
- allo-HSCT
Allogeneic hematopoietic stem cell transplantation
- AML
Acute myeloid leukemia
- APC
Antigen presenting cell
- BM
Bone marrow
- BTLA
B- and T-lymphocyte attenuator, CD272
- CD
Cluster of differentiation
- CR
Complete remission
- CTL
Cytotoxic T lymphocyte (also known as CD8+ effector T cell)
- CTLA-4
Cytotoxic T-lymphocyte associated antigen 4, CD152
- DC
Dendritic cell
- DLI
Donor lymphocyte infusion
- FAB
French-American-British classification of acute leukemias
- FDA
United States Food and Drug Administration
- GVHD
Graft-versus-host disease
- GVL
Graft-versus-leukemia
- HL
Hodgkin’s Lymphoma
- HLA
Human leukocyte antigen
- HVEM
Herpes virus entry mediator
- IDO
Indoleamine 2,3 dioxygenase
- IFN
Interferon
- IL
Interleukin
- IRAE
Immune-related adverse event
- KIR
Killer inhibitory receptor
- LAA
Leukemia associated antigen
- LAG-3
Lymphocyte activation gene 3, CD233
- mAb
Monoclonal antibody
- MCL
Mantle cell lymphoma
- MDS
Myelodysplastic syndrome
- MDSC
Myeloid-derived suppressor cell
- MHA
Major histocompability antigen
- MiHA
Minor histocompability antigen
- MLR
Mixed lymphocyte reaction
- MRD
Minimal residual disease
- NHL
Non-Hodgkin lymphoma
- NK cell
Natural killer cell
- OR
Overall response
- OS
Overall survival
- PB
Peripheral blood
- PBMC
Peripheral blood mononuclear cell
- PD-1
Programmed death-1; CD279
- PI3K
Phosphoinositide 3-kinase
- PR
Partial remission
- ROS
Reactive oxygen species
- siRNA
Short interfering RNA
- TCR
T cell receptor
- TH1
T helper 1 cells
- TIM-3
T cell immunoglobulin and mucin domain-containing protein 3
- TNF
Tumor necrosis factor
- Treg
Regulatory T cell
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
References
- 1.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2(2):116–26. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 2.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–48. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 3.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13(4):227–42. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yao S, Zhu Y, Chen L. Advances in targeting cell surface signalling molecules for immune modulation. Nat Rev Drug Discov. 2013;12(2):130–46. doi: 10.1038/nrd3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12(6):492–9. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 7.Norde WJ, Hobo W, van der Voort R, et al. Coinhibitory molecules in hematologic malignancies: targets for therapeutic intervention. Blood. 2012;120(4):728–36. doi: 10.1182/blood-2012-02-412510. [DOI] [PubMed] [Google Scholar]
- 8.Nirschl CJ, Drake CG. Molecular pathways: coexpression of immune checkpoint molecules: signaling pathways and implications for cancer immunotherapy. Clin Cancer Res. 2013;19(18):4917–24. doi: 10.1158/1078-0432.CCR-12-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greaves P, Gribben JG. The role of B7 family molecules in hematologic malignancy. Blood. 2013;121(5):734–44. doi: 10.1182/blood-2012-10-385591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horowitz MM, Gale RP, Sondel PM, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75(3):555–62. [PubMed] [Google Scholar]
- 11.Warren EH, Deeg HJ. Dissecting graft-versus-leukemia from graft-versus-host-disease using novel strategies. Tissue Antigens. 2013;81(4):183–93. doi: 10.1111/tan.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolb HJ, Schattenberg A, Goldman JM, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995;86(5):2041–50. [PubMed] [Google Scholar]
- 13.Lowdell MW, Ray N, Craston R, et al. The in vitro detection of anti-leukaemia-specific cytotoxicity after autologous bone marrow transplantation for acute leukaemia. Bone Marrow Transplant. 1997;19(9):891–7. doi: 10.1038/sj.bmt.1700756. [DOI] [PubMed] [Google Scholar]
- 14.Zhong RK, Lane TA, Ball ED. Generation of T-cell lines to autologous acute myeloid leukemia cells by competitive limiting dilution culture of acute myeloid leukemia mononuclear cells. Exp Hematol. 2008;36(4):486–94. doi: 10.1016/j.exphem.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 15.Draube A, Beyer M, Wolf J. Activation of autologous leukemia-specific T cells in acute myeloid leukemia: monocyte-derived dendritic cells cocultured with leukemic blasts compared with leukemia-derived dendritic cells. Eur J Haematol. 2008;81(4):281–8. doi: 10.1111/j.1600-0609.2008.01110.x. [DOI] [PubMed] [Google Scholar]
- 16.Greiner J, Dohner H, Schmitt M. Cancer vaccines for patients with acute myeloid leukemia--definition of leukemia-associated antigens and current clinical protocols targeting these antigens. Haematologica. 2006;91(12):1653–61. [PubMed] [Google Scholar]
- 17.Goswami M, Hensel N, Smith BD, et al. Expression of putative targets of immunotherapy in acute myeloid leukemia and healthy tissues. Leukemia. 2014;28(5):1167–70. doi: 10.1038/leu.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Luijn MM, Chamuleau ME, Ossenkoppele GJ, et al. Tumor immune escape in acute myeloid leukemia: Class II-associated invariant chain peptide expression as result of deficient antigen presentation. Oncoimmunology. 2012;1(2):211–3. doi: 10.4161/onci.1.2.18100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stolzel F, Hackmann K, Kuithan F, et al. Clonal evolution including partial loss of human leukocyte antigen genes favoring extramedullary acute myeloid leukemia relapse after matched related allogeneic hematopoietic stem cell transplantation. Transplantation. 2012;93(7):744–9. doi: 10.1097/TP.0b013e3182481113. [DOI] [PubMed] [Google Scholar]
- 20.Vago L, Perna SK, Zanussi M, et al. Loss of mismatched HLA in leukemia after stem-cell transplantation. N Engl J Med. 2009;361(5):478–88. doi: 10.1056/NEJMoa0811036. [DOI] [PubMed] [Google Scholar]
- 21.Anguille S, Van Tendeloo VF, Berneman ZN. Leukemia-associated antigens and their relevance to the immunotherapy of acute myeloid leukemia. Leukemia. 2012;26(10):2186–96. doi: 10.1038/leu.2012.145. [DOI] [PubMed] [Google Scholar]
- 22.Teague RM, Kline J. Immune evasion in acute myeloid leukemia: current concepts and future directions. J Immunother Cancer. 2013;1(13) doi: 10.1186/2051-1426-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Zheng J, Liu J, et al. Increased population of CD4(+)CD25(high), regulatory T cells with their higher apoptotic and proliferating status in peripheral blood of acute myeloid leukemia patients. Eur J Haematol. 2005;75(6):468–76. doi: 10.1111/j.1600-0609.2005.00537.x. [DOI] [PubMed] [Google Scholar]
- 24.Szczepanski MJ, Szajnik M, Czystowska M, et al. Increased frequency and suppression by regulatory T cells in patients with acute myelogenous leukemia. Clin Cancer Res. 2009;15(10):3325–32. doi: 10.1158/1078-0432.CCR-08-3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanakry CG, Hess AD, Gocke CD, et al. Early lymphocyte recovery after intensive timed sequential chemotherapy for acute myelogenous leukemia: peripheral oligoclonal expansion of regulatory T cells. Blood. 2011;117(2):608–17. doi: 10.1182/blood-2010-04-277939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shenghui Z, Yixiang H, Jianbo W, et al. Elevated frequencies of CD4(+) CD25(+) CD127lo regulatory T cells is associated to poor prognosis in patients with acute myeloid leukemia. Int J Cancer. 2011;129(6):1373–81. doi: 10.1002/ijc.25791. [DOI] [PubMed] [Google Scholar]
- 27.Ustun C, Miller JS, Munn DH, et al. Regulatory T cells in acute myelogenous leukemia: is it time for immunomodulation? Blood. 2011;118(19):5084–95. doi: 10.1182/blood-2011-07-365817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mougiakakos D, Jitschin R, von Bahr L, et al. Immunosuppressive CD14+HLA-DRlow/neg IDO+ myeloid cells in patients following allogeneic hematopoietic stem cell transplantation. Leukemia. 2013;27(2):377–88. doi: 10.1038/leu.2012.215. [DOI] [PubMed] [Google Scholar]
- 30.Hirano N, Takahashi T, Takahashi T, et al. Expression of costimulatory molecules in human leukemias. Leukemia. 1996;10(7):1168–76. [PubMed] [Google Scholar]
- 31.Costello RT, Mallet F, Sainty D, et al. Regulation of CD80/B7-1 and CD86/B7-2 molecule expression in human primary acute myeloid leukemia and their role in allogenic immune recognition. Eur J Immunol. 1998;28(1):90–103. doi: 10.1002/(SICI)1521-4141(199801)28:01<90::AID-IMMU90>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 32.Maeda A, Yamamoto K, Yamashita K, et al. The expression of co-stimulatory molecules and their relationship to the prognosis of human acute myeloid leukaemia: poor prognosis of B7-2-positive leukaemia. Br J Haematol. 1998;102(5):1257–62. doi: 10.1046/j.1365-2141.1998.00901.x. [DOI] [PubMed] [Google Scholar]
- 33.Graf M, Reif S, Hecht K, et al. High expression of costimulatory molecules correlates with low relapse-free survival probability in acute myeloid leukemia (AML) Ann Hematol. 2005;84(5):287–97. doi: 10.1007/s00277-004-0978-0. [DOI] [PubMed] [Google Scholar]
- 34.Norde WJ, Maas F, Hobo W, et al. PD-1/PD-L1 interactions contribute to functional T-cell impairment in patients who relapse with cancer after allogeneic stem cell transplantation. Cancer Res. 2011;71(15):5111–22. doi: 10.1158/0008-5472.CAN-11-0108. [DOI] [PubMed] [Google Scholar]
- 35.Sun YX, Kong HL, Liu CF, et al. The imbalanced profile and clinical significance of T helper associated cytokines in bone marrow microenvironment of the patients with acute myeloid leukemia. Hum Immunol. 2014;75(2):113–8. doi: 10.1016/j.humimm.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 36.Aurelius J, Thoren FB, Akhiani AA, et al. Monocytic AML cells inactivate antileukemic lymphocytes: role of NADPH oxidase/gp91(phox) expression and the PARP-1/PAR pathway of apoptosis. Blood. 2012;119(24):5832–7. doi: 10.1182/blood-2011-11-391722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Costello RT, Sivori S, Marcenaro E, et al. Defective expression and function of natural killer cell-triggering receptors in patients with acute myeloid leukemia. Blood. 2002;99(10):3661–7. doi: 10.1182/blood.v99.10.3661. [DOI] [PubMed] [Google Scholar]
- 38.Lion E, Willemen Y, Berneman ZN, et al. Natural killer cell immune escape in acute myeloid leukemia. Leukemia. 2012;26(9):2019–26. doi: 10.1038/leu.2012.87. [DOI] [PubMed] [Google Scholar]
- 39.Curti A, Trabanelli S, Salvestrini V, et al. The role of indoleamine 2,3-dioxygenase in the induction of immune tolerance: focus on hematology. Blood. 2009;113(11):2394–401. doi: 10.1182/blood-2008-07-144485. [DOI] [PubMed] [Google Scholar]
- 40.Mussai F, De Santo C, Abu-Dayyeh I, et al. Acute myeloid leukemia creates an arginase-dependent immunosuppressive microenvironment. Blood. 2013;122(5):749–58. doi: 10.1182/blood-2013-01-480129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dulphy N, Henry G, Hemon P, et al. Contribution of CD39 to the immunosuppressive microenvironment of acute myeloid leukaemia at diagnosis. Br J Haematol. 2014;165(5):722–5. doi: 10.1111/bjh.12774. [DOI] [PubMed] [Google Scholar]
- 42.Curti A, Aluigi M, Pandolfi S, et al. Acute myeloid leukemia cells constitutively express the immunoregulatory enzyme indoleamine 2,3-dioxygenase. Leukemia. 2007;21(2):353–5. doi: 10.1038/sj.leu.2404485. [DOI] [PubMed] [Google Scholar]
- 43.Chamuleau ME, van de Loosdrecht AA, Hess CJ, et al. High INDO (indoleamine 2,3-dioxygenase) mRNA level in blasts of acute myeloid leukemic patients predicts poor clinical outcome. Haematologica. 2008;93(12):1894–8. doi: 10.3324/haematol.13113. [DOI] [PubMed] [Google Scholar]
- 44.Curti A, Pandolfi S, Valzasina B, et al. Modulation of tryptophan catabolism by human leukemic cells results in the conversion of CD25− into CD25+ T regulatory cells. Blood. 2007;109(7):2871–7. doi: 10.1182/blood-2006-07-036863. [DOI] [PubMed] [Google Scholar]
- 45.Zhang L, Chen X, Liu X, et al. CD40 ligation reverses T cell tolerance in acute myeloid leukemia. J Clin Investig. 2013;123(5):1999–2010. doi: 10.1172/JCI63980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brunet JF, Denizot F, Luciani MF, et al. A new member of the immunoglobulin superfamily--CTLA-4. Nature. 1987;328(6127):267–70. doi: 10.1038/328267a0. [DOI] [PubMed] [Google Scholar]
- 47.Azuma M, Ito D, Yagita H, et al. B70 antigen is a second ligand for CTLA-4 and CD28. Nature. 1993;366(6450):76–9. doi: 10.1038/366076a0. [DOI] [PubMed] [Google Scholar]
- 48.Linsley PS, Greene JL, Brady W, et al. Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity. 1994;1(9):793–801. doi: 10.1016/s1074-7613(94)80021-9. [DOI] [PubMed] [Google Scholar]
- 49.Peach RJ, Bajorath J, Brady W, et al. Complementarity determining region 1 (CDR1)- and CDR3-analogous regions in CTLA-4 and CD28 determine the binding to B7-1. J Exp Med. 1994;180(6):2049–58. doi: 10.1084/jem.180.6.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salomon B, Lenschow DJ, Rhee L, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12(4):431–40. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 51.Takahashi T, Tagami T, Yamazaki S, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192(2):303–10. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sharpe AH. Mechanisms of costimulation. Immunol Rev. 2009;229(1):5–11. doi: 10.1111/j.1600-065X.2009.00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Egen JG, Allison JP. Cytotoxic T lymphocyte antigen-4 accumulation in the immunological synapse is regulated by TCR signal strength. Immunity. 2002;16(1):23–35. doi: 10.1016/s1074-7613(01)00259-x. [DOI] [PubMed] [Google Scholar]
- 54.Wing K, Onishi Y, Prieto-Martin P, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322(5899):271–5. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 55.Tivol EA, Borriello F, Schweitzer AN, et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3(5):541–7. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 56.Waterhouse P, Penninger JM, Timms E, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270(5238):985–8. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 57.Walker LS, Sansom DM. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat Rev Immunol. 2011;11(12):852–63. doi: 10.1038/nri3108. [DOI] [PubMed] [Google Scholar]
- 58.Grosso JF, Jure-Kunkel MN. CTLA-4 blockade in tumor models: an overview of preclinical and translational research. Cancer Immun. 2013;13:5. [PMC free article] [PubMed] [Google Scholar]
- 59.Chen W, Jin W, Wahl SM. Engagement of cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) induces transforming growth factor beta (TGF-beta) production by murine CD4(+) T cells. J Exp Med. 1998;188(10):1849–57. doi: 10.1084/jem.188.10.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Onishi Y, Fehervari Z, Yamaguchi T, et al. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc Natl Acad Sci USA. 2008;105(29):10113–8. doi: 10.1073/pnas.0711106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qureshi OS, Zheng Y, Nakamura K, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332(6029):600–3. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grohmann U, Orabona C, Fallarino F, et al. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3(11):1097–101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 63.Fallarino F, Grohmann U, Hwang KW, et al. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4(12):1206–12. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 64.Mellor AL, Chandler P, Baban B, et al. Specific subsets of murine dendritic cells acquire potent T cell regulatory functions following CTLA4-mediated induction of indoleamine 2,3 dioxygenase. Int Immunol. 2004;16(10):1391–401. doi: 10.1093/intimm/dxh140. [DOI] [PubMed] [Google Scholar]
- 65.Marengere LE, Waterhouse P, Duncan GS, et al. Regulation of T cell receptor signaling by tyrosine phosphatase SYP association with CTLA-4. Science. 1996;272(5265):1170–3. doi: 10.1126/science.272.5265.1170. [DOI] [PubMed] [Google Scholar]
- 66.Chuang E, Fisher TS, Morgan RW, et al. The CD28 and CTLA-4 receptors associate with the serine/threonine phosphatase PP2A. Immunity. 2000;13(3):313–22. doi: 10.1016/s1074-7613(00)00031-5. [DOI] [PubMed] [Google Scholar]
- 67.Parry RV, Chemnitz JM, Frauwirth KA, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25(21):9543–53. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Corse E, Allison JP. Cutting edge: CTLA-4 on effector T cells inhibits in trans. J Immunol. 2012;189(3):1123–7. doi: 10.4049/jimmunol.1200695. [DOI] [PubMed] [Google Scholar]
- 69.Wang CJ, Kenefeck R, Wardzinski L, et al. Cutting edge: cell-extrinsic immune regulation by CTLA-4 expressed on conventional T cells. J Immunol. 2012;189(3):1118–22. doi: 10.4049/jimmunol.1200972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271(5256):1734–6. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 71.Hodi FS, Mihm MC, Soiffer RJ, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci U S A. 2003;100(8):4712–7. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100(14):8372–7. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ribas A, Camacho LH, Lopez-Berestein G, et al. Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675,206. J Clin Oncol. 2005;23(35):8968–77. doi: 10.1200/JCO.2005.01.109. [DOI] [PubMed] [Google Scholar]
- 74.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liakou CI, Kamat A, Tang DN, et al. CTLA-4 blockade increases IFNgamma-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc Natl Acad Sci U S A. 2008;105(39):14987–92. doi: 10.1073/pnas.0806075105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yuan J, Adamow M, Ginsberg BA, et al. Integrated NY-ESO-1 antibody and CD8+ T-cell responses correlate with clinical benefit in advanced melanoma patients treated with ipilimumab. Proc Natl Acad Sci U S A. 2011;108(40):16723–8. doi: 10.1073/pnas.1110814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ji RR, Chasalow SD, Wang L, et al. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother. 2012;61(7):1019–31. doi: 10.1007/s00262-011-1172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Blank CU, Enk A. Therapeutic use of anti-CTLA-4 antibodies. Int Immunol. 2015;27(1):3–10. doi: 10.1093/intimm/dxu076. [DOI] [PubMed] [Google Scholar]
- 79.Whiteway A, Corbett T, Anderson R, et al. Expression of co-stimulatory molecules on acute myeloid leukaemia blasts may effect duration of first remission. Br J Haematol. 2003;120(3):442–51. doi: 10.1046/j.1365-2141.2003.04085.x. [DOI] [PubMed] [Google Scholar]
- 80.LaBelle JL, Hanke CA, Blazar BR, et al. Negative effect of CTLA-4 on induction of T-cell immunity in vivo to B7-1+, but not B7-2+, murine myelogenous leukemia. Blood. 2002;99(6):2146–53. doi: 10.1182/blood.v99.6.2146. [DOI] [PubMed] [Google Scholar]
- 81.Pistillo MP, Tazzari PL, Palmisano GL, et al. CTLA-4 is not restricted to the lymphoid cell lineage and can function as a target molecule for apoptosis induction of leukemic cells. Blood. 2003;101(1):202–9. doi: 10.1182/blood-2002-06-1668. [DOI] [PubMed] [Google Scholar]
- 82.Laurent S, Palmisano GL, Martelli AM, et al. CTLA-4 expressed by chemoresistant, as well as untreated, myeloid leukaemia cells can be targeted with ligands to induce apoptosis. Br J Haematol. 2007;136(4):597–608. doi: 10.1111/j.1365-2141.2006.06472.x. [DOI] [PubMed] [Google Scholar]
- 83.Perez-Garcia A, Brunet S, Berlanga JJ, et al. CTLA-4 genotype and relapse incidence in patients with acute myeloid leukemia in first complete remission after induction chemotherapy. Leukemia. 2009;23(3):486–91. doi: 10.1038/leu.2008.339. [DOI] [PubMed] [Google Scholar]
- 84.Hui L, Lei Z, Peng Z, et al. Polymorphism analysis of CTLA-4 in childhood acute lymphoblastic leukemia. Pak J Pharm Sci. 2014;27(4 Suppl):1005–13. [PubMed] [Google Scholar]
- 85.Sengsayadeth S, Wang T, Lee SJ, et al. Cytotoxic T-lymphocyte antigen-4 single nucleotide polymorphisms are not associated with outcomes after unrelated donor transplantation: a center for international blood and marrow transplant research analysis. Biol Blood Marrow Transplant. 2014;20(6):900–3. doi: 10.1016/j.bbmt.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Oaks MK, Hallett KM. Cutting edge: a soluble form of CTLA-4 in patients with autoimmune thyroid disease. J Immunol. 2000;164(10):5015–8. doi: 10.4049/jimmunol.164.10.5015. [DOI] [PubMed] [Google Scholar]
- 87.Simone R, Tenca C, Fais F, et al. A soluble form of CTLA-4 is present in paediatric patients with acute lymphoblastic leukaemia and correlates with CD1d+ expression. PLoS One. 2012;7(9):e44654. doi: 10.1371/journal.pone.0044654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mansour A, Elkhodary T, Darwish A, et al. Increased expression of costimulatory molecules CD86 and sCTLA-4 in patients with acute lymphoblastic leukemia. Leuk Lymphoma. 2014;55(9):2120–4. doi: 10.3109/10428194.2013.869328. [DOI] [PubMed] [Google Scholar]
- 89.Saudemont A, Quesnel B. In a model of tumor dormancy, long-term persistent leukemic cells have increased B7-H1 and B7. 1 expression and resist CTL-mediated lysis. Blood. 2004;104(7):2124–33. doi: 10.1182/blood-2004-01-0064. [DOI] [PubMed] [Google Scholar]
- 90.Zhong RK, Loken M, Lane TA, et al. CTLA-4 blockade by a human MAb enhances the capacity of AML-derived DC to induce T-cell responses against AML cells in an autologous culture system. Cytotherapy. 2006;8(1):3–12. doi: 10.1080/14653240500499507. [DOI] [PubMed] [Google Scholar]
- 91.Blazar BR, Taylor PA, Panoskaltsis-Mortari A, et al. Opposing roles of CD28: B7 and CTLA-4: B7 pathways in regulating in vivo alloresponses in murine recipients of MHC disparate T cells. J Immunol. 1999;162(11):6368–77. [PubMed] [Google Scholar]
- 92.Fevery S, Billiau AD, Sprangers B, et al. CTLA-4 blockade in murine bone marrow chimeras induces a host-derived antileukemic effect without graft-versus-host disease. Leukemia. 2007;21(7):1451–9. doi: 10.1038/sj.leu.2404720. [DOI] [PubMed] [Google Scholar]
- 93.Bashey A, Medina B, Corringham S, et al. CTLA4 blockade with ipilimumab to treat relapse of malignancy after allogeneic hematopoietic cell transplantation. Blood. 2009;113(7):1581–8. doi: 10.1182/blood-2008-07-168468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhou J, Bashey A, Zhong R, et al. CTLA-4 blockade following relapse of malignancy after allogeneic stem cell transplantation is associated with T cell activation but not with increased levels of T regulatory cells. Biol Blood Marrow Transplant. 2011;17(5):682–92. doi: 10.1016/j.bbmt.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ishida Y, Agata Y, Shibahara K, et al. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11(11):3887–95. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–34. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Keir ME, Liang SC, Guleria I, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203(4):883–95. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nishimura H, Nose M, Hiai H, et al. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11(2):141–51. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 99.Nishimura H, Okazaki T, Tanaka Y, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291(5502):319–22. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 100.Okazaki T, Chikuma S, Iwai Y, et al. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat Immunol. 2013;14(12):1212–8. doi: 10.1038/ni.2762. [DOI] [PubMed] [Google Scholar]
- 101.Sharpe AH, Wherry EJ, Ahmed R, et al. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8(3):239–45. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 102.Agata Y, Kawasaki A, Nishimura H, et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8(5):765–72. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 103.Dong H, Zhu G, Tamada K, et al. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5(12):1365–9. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 104.Latchman Y, Wood CR, Chernova T, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2(3):261–8. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 105.Youngnak P, Kozono Y, Kozono H, et al. Differential binding properties of B7-H1 and B7-DC to programmed death-1. Biochem Biophys Res Commun. 2003;307(3):672–7. doi: 10.1016/s0006-291x(03)01257-9. [DOI] [PubMed] [Google Scholar]
- 106.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 107.Parsa AT, Waldron JS, Panner A, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13(1):84–8. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 108.Thompson RH, Gillett MD, Cheville JC, et al. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci USA. 2004;101(49):17174–9. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hamanishi J, Mandai M, Iwasaki M, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci USA. 2007;104(9):3360–5. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nomi T, Sho M, Akahori T, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13(7):2151–7. doi: 10.1158/1078-0432.CCR-06-2746. [DOI] [PubMed] [Google Scholar]
- 111.Gao Q, Wang XY, Qiu SJ, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15(3):971–9. doi: 10.1158/1078-0432.CCR-08-1608. [DOI] [PubMed] [Google Scholar]
- 112.Hino R, Kabashima K, Kato Y, et al. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer. 2010;116(7):1757–66. doi: 10.1002/cncr.24899. [DOI] [PubMed] [Google Scholar]
- 113.Tseng SY, Otsuji M, Gorski K, et al. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med. 2001;193(7):839–46. doi: 10.1084/jem.193.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shi M, Roemer MG, Chapuy B, et al. Expression of programmed cell death 1 ligand 2 (PD-L2) is a distinguishing feature of primary mediastinal (thymic) large B-cell lymphoma and associated with PDCD1LG2 copy gain. Am J Surg Pathol. 2014;38(12):1715–23. doi: 10.1097/PAS.0000000000000297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dolen Y, Esendagli G. Myeloid leukemia cells with a B7-2(+) subpopulation provoke Th-cell responses and become immunosuppressive through the modulation of B7 ligands. Eur J Immunol. 2013;43(3):747–57. doi: 10.1002/eji.201242814. [DOI] [PubMed] [Google Scholar]
- 116.Butte MJ, Keir ME, Phamduy TB, et al. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27(1):111–22. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Park JJ, Omiya R, Matsumura Y, et al. B7-H1/CD80 interaction is required for the induction and maintenance of peripheral T-cell tolerance. Blood. 2010;116(8):1291–8. doi: 10.1182/blood-2010-01-265975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Youngblood B, Hale JS, Ahmed R. T-cell memory differentiation: insights from transcriptional signatures and epigenetics. Immunology. 2013;139(3):277–84. doi: 10.1111/imm.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Youngblood B, Oestreich KJ, Ha SJ, et al. Chronic virus infection enforces demethylation of the locus that encodes PD-1 in antigen-specific CD8(+) T cells. Immunity. 2011;35(3):400–12. doi: 10.1016/j.immuni.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384(9948):1109–17. doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 121.Weber JS, Minor DR, D’Angelo SP, et al. A phase 3 randomized, open-label study of nivolumab (anti-PD-1, BMS-936558; ONO-4538) versus investigator’s choice chemotherapy (ICC) in patients with advanced melanoma after prior anti-CTLA-4 therapy. Ann Oncol. 2014;25:1–14. [Google Scholar]
- 122.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hodi FS, Sznol M, Kluger HM, et al. Long-term survival of ipilimumab-naive patients (pts) with advanced melanoma (MEL) treated with nivolumab (anti-PD-1, BMS-936558, ONO-4538) in a phase I trial. 2014 ASCO Annual Meeting: J Clin Oncol. 2014;32:5s. suppl; abstr 9002. [Google Scholar]
- 124.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sznol M, Harriet M, Kluger H, et al. Survival, response duration, and activity by BRAF mutation (MT) status of nivolumab (NIVO, anti-PD-1, BMS-936558, ONO-4538) and ipilimumab (IPI) concurrent therapy in advanced melanoma (MEL) 2014 ASCO Annual Meeting Clin Oncol. 2014;32:5s. suppl; abstr LBA9003^. [Google Scholar]
- 126.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311–9. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lesokhin AM, Ansell SM, Armand P, et al. Preliminary results of a phase i study of nivolumab (BMS-936558) in patients with relapsed or refractory lymphoid malignancies. Blood. 2014;124(21) [Google Scholar]
- 128.Moskowitz CH, Ribrag V, Michot JM, et al. PD-1 Blockade with the monoclonal antibody pembrolizumab (MK-3475) in patients with classical hodgkin lymphoma after brentuximab vedotin failure: preliminary results from a phase 1b study (KEYNOTE-013) Blood. 2014;124(21) [Google Scholar]
- 129.Zhang L, Gajewski TF, Kline J. PD-1/PD-L1 interactions inhibit antitumor immune responses in a murine acute myeloid leukemia model. Blood. 2009;114(8):1545–52. doi: 10.1182/blood-2009-03-206672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhou Q, Munger ME, Highfill SL, et al. Program death-1 signaling and regulatory T cells collaborate to resist the function of adoptively transferred cytotoxic T lymphocytes in advanced acute myeloid leukemia. Blood. 2010;116(14):2484–93. doi: 10.1182/blood-2010-03-275446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tamura H, Dan K, Tamada K, et al. Expression of functional B7-H2 and B7. 2 costimulatory molecules and their prognostic implications in de novo acute myeloid leukemia. Clin Cancer Res. 2005;11(16):5708–17. doi: 10.1158/1078-0432.CCR-04-2672. [DOI] [PubMed] [Google Scholar]
- 132.Salih HR, Wintterle S, Krusch M, et al. The role of leukemia-derived B7-H1 (PD-L1) in tumor-T-cell interactions in humans. Exp Hematol. 2006;34(7):888–94. doi: 10.1016/j.exphem.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 133.Chen X, Liu S, Wang L, et al. Clinical significance of B7-H1 (PD-L1) expression in human acute leukemia. Cancer Biol Ther. 2008;7(5):622–7. doi: 10.4161/cbt.7.5.5689. [DOI] [PubMed] [Google Scholar]
- 134.Berthon C, Driss V, Liu J, et al. In acute myeloid leukemia, B7-H1 (PD-L1) protection of blasts from cytotoxic T cells is induced by TLR ligands and interferon-gamma and can be reversed using MEK inhibitors. Cancer Immunol Immunother. 2010;59(12):1839–49. doi: 10.1007/s00262-010-0909-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kronig H, Kremmler L, Haller B, et al. Interferon-induced programmed death-ligand 1 (PD-L1/B7-H1) expression increases on human acute myeloid leukemia blast cells during treatment. Eur J Haematol. 2014;92(3):195–203. doi: 10.1111/ejh.12228. [DOI] [PubMed] [Google Scholar]
- 136.Yang H, Bueso-Ramos C, DiNardo C, et al. Expression of PD-L1, PD-L2, PD-1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia. 2014;28(6):1280–8. doi: 10.1038/leu.2013.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Asakura S, Hashimoto D, Takashima S, et al. Alloantigen expression on non-hematopoietic cells reduces graft-versus-leukemia effects in mice. J Clin Investig. 2010;120(7):2370–8. doi: 10.1172/JCI39165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Flutter B, Edwards N, Fallah-Arani F, et al. Nonhematopoietic antigen blocks memory programming of alloreactive CD8+ T cells and drives their eventual exhaustion in mouse models of bone marrow transplantation. J Clin Investig. 2010;120(11):3855–68. doi: 10.1172/JCI41446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Koestner W, Hapke M, Herbst J, et al. PD-L1 blockade effectively restores strong graft-versus-leukemia effects without graft-versus-host disease after delayed adoptive transfer of T-cell receptor gene-engineered allogeneic CD8+ T cells. Blood. 2011;117(3):1030–41. doi: 10.1182/blood-2010-04-283119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hobo W, Maas F, Adisty N, et al. siRNA silencing of PD-L1 and PD-L2 on dendritic cells augments expansion and function of minor histocompatibility antigen-specific CD8+ T cells. Blood. 2010;116(22):4501–11. doi: 10.1182/blood-2010-04-278739. [DOI] [PubMed] [Google Scholar]
- 141.Berger R, Rotem-Yehudar R, Slama G, et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14(10):3044–51. doi: 10.1158/1078-0432.CCR-07-4079. [DOI] [PubMed] [Google Scholar]
- 142.Zhu C, Anderson AC, Schubart A, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6(12):1245–52. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 143.Fourcade J, Sun Z, Benallaoua M, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207(10):2175–86. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sakuishi K, Apetoh L, Sullivan JM, et al. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207(10):2187–94. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gorman JV, Colgan JD. Regulation of T cell responses by the receptor molecule Tim-3. Immunol Res. 2014;59(1–3):56–65. doi: 10.1007/s12026-014-8524-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhou Q, Munger ME, Veenstra RG, et al. Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood. 2011;117(17):4501–10. doi: 10.1182/blood-2010-10-310425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dardalhon V, Anderson AC, Karman J, et al. Tim-3/galectin-9 pathway: regulation of Th1 immunity through promotion of CD11b+Ly-6G+ myeloid cells. J Immunol. 2010;185(3):1383–92. doi: 10.4049/jimmunol.0903275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Li C, Chen X, Yu X, et al. Tim-3 is highly expressed in T cells in acute myeloid leukemia and associated with clinicopathological prognostic stratification. Int J Clin Exp Pathol. 2014;7(10):6880–8. [PMC free article] [PubMed] [Google Scholar]
- 149.Huang X, Bai X, Cao Y, et al. Lymphoma endothelium preferentially expresses Tim-3 and facilitates the progression of lymphoma by mediating immune evasion. J Exp Med. 2010;207(3):505–20. doi: 10.1084/jem.20090397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kikushige Y, Shima T, Takayanagi S, et al. TIM-3 is a promising target to selectively kill acute myeloid leukemia stem cells. Cell Stem Cell. 2010;7(6):708–17. doi: 10.1016/j.stem.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 151.Jan M, Chao MP, Cha AC, et al. Prospective separation of normal and leukemic stem cells based on differential expression of TIM3, a human acute myeloid leukemia stem cell marker. Proc Natl Acad Sci U S A. 2011;108(12):5009–14. doi: 10.1073/pnas.1100551108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gao L, Yu S, Zhang X. Hypothesis: Tim-3/galectin-9, a new pathway for leukemia stem cells survival by promoting expansion of myeloid-derived suppressor cells and differentiating into tumor-associated macrophages. Cell Biochem Biophys. 2014;70(1):273–7. doi: 10.1007/s12013-014-9900-0. [DOI] [PubMed] [Google Scholar]
- 153.Baixeras E, Huard B, Miossec C, et al. Characterization of the lymphocyte activation gene 3-encoded protein. A new ligand for human leukocyte antigen class II antigens. J Exp Med. 1992;176(2):327–37. doi: 10.1084/jem.176.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Huard B, Gaulard P, Faure F, et al. Cellular expression and tissue distribution of the human LAG-3-encoded protein, an MHC class II ligand. Immunogenetics. 1994;39(3):213–7. doi: 10.1007/BF00241263. [DOI] [PubMed] [Google Scholar]
- 155.Huang CT, Workman CJ, Flies D, et al. Role of LAG-3 in regulatory T cells. Immunity. 2004;21(4):503–13. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 156.Grosso JF, Kelleher CC, Harris TJ, et al. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J Clin Investig. 2007;117(11):3383–92. doi: 10.1172/JCI31184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci U S A. 2010;107(17):7875–80. doi: 10.1073/pnas.1003345107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Grosso JF, Goldberg MV, Getnet D, et al. Functionally distinct LAG-3 and PD-1 subsets on activated and chronically stimulated CD8 T cells. J Immunol. 2009;182(11):6659–69. doi: 10.4049/jimmunol.0804211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Okazaki T, Okazaki IM, Wang J, et al. PD-1 and LAG-3 inhibitory co-receptors act synergistically to prevent autoimmunity in mice. J Exp Med. 2011;208(2):395–407. doi: 10.1084/jem.20100466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Woo SR, Turnis ME, Goldberg MV, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72(4):917–27. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gandhi MK, Lambley E, Duraiswamy J, et al. Expression of LAG-3 by tumor-infiltrating lymphocytes is coincident with the suppression of latent membrane antigen-specific CD8+ T-cell function in Hodgkin lymphoma patients. Blood. 2006;108(7):2280–9. doi: 10.1182/blood-2006-04-015164. [DOI] [PubMed] [Google Scholar]
- 162.Rossi FM, Degan M, Mazzocco FT, et al. Co-expression of CD30 ligand and interleukin 4 (IL-4) receptors by acute myeloid leukaemia blasts is associated with the expansion of IL-4-producing CD30+ normal T cells. Br J Haematol. 2002;117(1):59–69. doi: 10.1046/j.1365-2141.2002.03398.x. [DOI] [PubMed] [Google Scholar]
- 163.Watanabe N, Gavrieli M, Sedy JR, et al. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat Immunol. 2003;4(7):670–9. doi: 10.1038/ni944. [DOI] [PubMed] [Google Scholar]
- 164.Murphy TL, Murphy KM. Slow down and survive: Enigmatic immunoregulation by BTLA and HVEM. Annu Rev Immunol. 2010;28:389–411. doi: 10.1146/annurev-immunol-030409-101202. [DOI] [PubMed] [Google Scholar]
- 165.Sedy JR, Gavrieli M, Potter KG, et al. B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat Immunol. 2005;6(1):90–8. doi: 10.1038/ni1144. [DOI] [PubMed] [Google Scholar]
- 166.del Rio ML, Lucas CL, Buhler L, et al. HVEM/LIGHT/BTLA/CD160 cosignaling pathways as targets for immune regulation. J Leukocyte Biol. 2010;87(2):223–35. doi: 10.1189/jlb.0809590. [DOI] [PubMed] [Google Scholar]
- 167.Cheung TC, Oborne LM, Steinberg MW, et al. T cell intrinsic heterodimeric complexes between HVEM and BTLA determine receptivity to the surrounding microenvironment. J Immunol. 2009;183(11):7286–96. doi: 10.4049/jimmunol.0902490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Oya Y, Watanabe N, Owada T, et al. Development of autoimmune hepatitis-like disease and production of autoantibodies to nuclear antigens in mice lacking B and T lymphocyte attenuator. Arthritis Rheum. 2008;58(8):2498–510. doi: 10.1002/art.23674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Fourcade J, Sun Z, Pagliano O, et al. CD8(+) T cells specific for tumor antigens can be rendered dysfunctional by the tumor microenvironment through upregulation of the inhibitory receptors BTLA and PD-1. Cancer Res. 2012;72(4):887–96. doi: 10.1158/0008-5472.CAN-11-2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hobo W, Norde WJ, Schaap N, et al. B and T lymphocyte attenuator mediates inhibition of tumor-reactive CD8+ T cells in patients after allogeneic stem cell transplantation. J Immunol. 2012;189(1):39–49. doi: 10.4049/jimmunol.1102807. [DOI] [PubMed] [Google Scholar]
- 171.Wright GJ, Cherwinski H, Foster-Cuevas M, et al. Characterization of the CD200 receptor family in mice and humans and their interactions with CD200. J Immunol. 2003;171(6):3034–46. doi: 10.4049/jimmunol.171.6.3034. [DOI] [PubMed] [Google Scholar]
- 172.Rijkers ES, de Ruiter T, Baridi A, et al. The inhibitory CD200R is differentially expressed on human and mouse T and B lymphocytes. Mol Immunol. 2008;45(4):1126–35. doi: 10.1016/j.molimm.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 173.Gorczynski RM, Chen Z, Hu J, et al. Evidence of a role for CD200 in regulation of immune rejection of leukaemic tumour cells in C57BL/6 mice. Clin Exp Immunol. 2001;126(2):220–9. doi: 10.1046/j.1365-2249.2001.01689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Coles SJ, Wang EC, Man S, et al. CD200 expression suppresses natural killer cell function and directly inhibits patient anti-tumor response in acute myeloid leukemia. Leukemia. 2011;25(5):792–9. doi: 10.1038/leu.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rygiel TP, Meyaard L. CD200R signaling in tumor tolerance and inflammation: A tricky balance. Curr Opin Immunol. 2012;24(2):233–8. doi: 10.1016/j.coi.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 176.Tonks A, Hills R, White P, et al. CD200 as a prognostic factor in acute myeloid leukaemia. Leukemia. 2007;21(3):566–8. doi: 10.1038/sj.leu.2404559. [DOI] [PubMed] [Google Scholar]
- 177.Coles SJ, Hills RK, Wang EC, et al. Expression of CD200 on AML blasts directly suppresses memory T-cell function. Leukemia. 2012;26(9):2148–51. doi: 10.1038/leu.2012.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Coles SJ, Hills RK, Wang EC, et al. Increased CD200 expression in acute myeloid leukemia is linked with an increased frequency of FoxP3+ regulatory T cells. Leukemia. 2012;26(9):2146–8. doi: 10.1038/leu.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Memarian A, Nourizadeh M, Masoumi F, et al. Upregulation of CD200 is associated with Foxp3+ regulatory T cell expansion and disease progression in acute myeloid leukemia. Tumour Biol. 2013;34(1):531–42. doi: 10.1007/s13277-012-0578-x. [DOI] [PubMed] [Google Scholar]
- 180.Driessens G, Kline J, Gajewski TF. Costimulatory and coinhibitory receptors in anti-tumor immunity. Immunol Rev. 2009;229(1):126–44. doi: 10.1111/j.1600-065X.2009.00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zarek PE, Huang CT, Lutz ER, et al. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood. 2008;111(1):251–9. doi: 10.1182/blood-2007-03-081646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Flies DB, Wang S, Xu H, et al. Cutting edge: A monoclonal antibody specific for the programmed death-1 homolog prevents graft-versus-host disease in mouse models. J Immunol. 2011;187(4):1537–41. doi: 10.4049/jimmunol.1100660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wang L, Rubinstein R, Lines JL, et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J Exp Med. 2011;208(3):577–92. doi: 10.1084/jem.20100619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Elias S, Yamin R, Golomb L, et al. Immune evasion by oncogenic proteins of acute myeloid leukemia. Blood. 2014;123(10):1535–43. doi: 10.1182/blood-2013-09-526590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Watzl C, Claus M. WhatSAP - 2B4 sends mixed messages in the absence of SAP. Eur J Immunol. 2014;44(5):1281–4. doi: 10.1002/eji.201444562. [DOI] [PubMed] [Google Scholar]
- 186.Le Dieu R, Taussig DC, Ramsay AG, et al. Peripheral blood T cells in acute myeloid leukemia (AML) patients at diagnosis have abnormal phenotype and genotype and form defective immune synapses with AML blasts. Blood. 2009;114(18):3909–16. doi: 10.1182/blood-2009-02-206946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Luczynski W, Stasiak-Barmuta A, Ilendo E, et al. Low expression of costimulatory molecules and mRNA for cytokines are important mechanisms of immunosuppression in acute lymphoblastic leukemia in children? Neoplasma. 2006;53(4):301–4. [PubMed] [Google Scholar]
- 188.Kebelmann-Betzing C, Korner G, Badiali L, et al. Characterization of cytokine, growth factor receptor, costimulatory and adhesion molecule expression patterns of bone marrow blasts in relapsed childhood B cell precursor all. Cytokine. 2001;13(1):39–50. doi: 10.1006/cyto.2000.0794. [DOI] [PubMed] [Google Scholar]
- 189.Zheng Z, Takahashi M, Aoki S, et al. Expression patterns of costimulatory molecules on cells derived from human hematological malignancies. J Exp Clin Cancer Res : CR. 1998;17(3):251–8. [PubMed] [Google Scholar]
- 190.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–64. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 192.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 193.Miyara M, Sakaguchi S. Natural regulatory T cells: mechanisms of suppression. Trends Mol Med. 2007;13(3):108–16. doi: 10.1016/j.molmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 194.Jenkins MK, Schwartz RH. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J Exp Med. 1987;165(2):302–19. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]