Abstract
Controversy remains whether the leukocyte genomic response to trauma or sepsis is dependent upon the initiating stimulus. Previous work illustrated poor correlations between historical models of murine trauma and sepsis (i.e., trauma-hemorrhage and lipopolysaccharide injection, respectively). The aim of this study is to examine the early genomic response in improved murine models of sepsis [cecal ligation and puncture (CLP)] and trauma [polytrauma (PT)] with and without pneumonia (PT+Pp). Groups of naïve, CLP, PT, and PT+Pp mice were killed at 2 h, 1 or 3 days. Total leukocytes were isolated for genome-wide expression analysis, and genes that were found to differ from control (false discovery rate adjusted P < 0.001) were assessed for fold-change differences. Spearman correlations were also performed. For all time points combined (CLP, PT, PT+Pp), there were 10,426 total genes that were found to significantly differ from naïve controls. At 2 h, the transcriptomic changes between CLP and PT showed a positive correlation (rs) of 0.446 (P < 0.0001) but were less positive thereafter. Correlations were significantly improved when we limited the analysis to common genes whose expression differed by a 1.5 fold-change. Both pathway and upstream analyses revealed the activation of genes known to be associated with pathogen-associated and damage-associated molecular pattern signaling, and early activation patterns of expression were very similar between polytrauma and sepsis at the earliest time points. This study demonstrates that the early leukocyte genomic response to sepsis and trauma are very similar in mice.
Keywords: mouse, transcriptomics, correlations, polytrauma, cecal ligation and puncture
despite improvements and advances in clinical management, sepsis and trauma continue to remain substantial burdens to both hospitals and society (24, 32, 42). Sepsis, as a whole, is the leading cause of death within noncardiac intensive care units and mortality rates remain ∼20–30% (23). Mortality rates for septic shock can be as high as 50% (30). Trauma also remains a significant problem, and to date, pharmacotherapeutic agents targeting host immunity have had little effect in regards to modifying outcome (37).
Since Matzinger (29) first published the “danger hypothesis,” much research has focused on identifying pathways that lead to a host inflammatory response. Specific pattern recognition receptor (PRR) pathways rely on both damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs) to signal the presence of tissue damage or microbial infection, respectively. However, both endogenous and exogenous “alarmins” appear to signal through many of the same PRRs, including toll-like receptors (TLRs) (19, 31). Due to these apparent similarities, significant effort has been dedicated to understanding these common pathways that lead to an activation of early inflammatory and innate immune responses.
Recently, murine models of sepsis and trauma have received scrutiny for their failure to fully recapitulate comparable human responses at the level of the leukocyte transcriptome (10, 38). Interestingly, the genomic responses to these various stressors were very similar in humans, but not in mice (38). One explanation is that the current murine models are poor representations of the human condition (9, 10). In support of that conclusion, we have reported that increasing injury severity in a murine trauma model produced a modest but significant improvement in correlation between patterns of human and murine gene expression (16).
An alternative explanation is that the timing of the host response varies significantly between humans and mice, and among different injury models in mice. In this report, we sought to compare the murine blood leukocyte gene expression responses to various inflammatory states including polytrauma, sepsis, and polytrauma followed by pneumonia. We specifically asked whether each form of inflammation would lead to a unique leukocyte genomic response or whether there existed a core genomic response common to different inflammatory challenges produced by either microbial invasion or tissue injury and hypovolemic shock. In addition, we sought to examine whether microbial invasion after trauma induced a response more similar the initial genomic expression pattern after severe sepsis or severe injury.
MATERIALS AND METHODS
Mice.
All experiments were approved by the Institutional Animal Care and Use Committee at the University of Florida. Specific pathogen-free male C57BL/6 (B6) mice were purchased from The Jackson Laboratory (Bar Harbor, ME) at 6–7 wk and allowed to acclimatize for 1 wk before being used for experimental procedures. Mice were maintained on standard rodent food and water ad libitum. For each experiment, four or five mice were used per group for each time point.
Cecal ligation and puncture.
For induction of polymicrobial sepsis, cecal ligation and puncture (CLP) was performed under isoflurane anesthesia, as described previously (6, 8). In brief, the cecum was exposed after a midline laparotomy, ligated with 2-0 silk suture 1 cm from the tip, and punctured through and through with a 25-gauge needle. The cecum was returned to the abdomen, and the incision was closed with surgical clips. After the procedure, the mice were administered 0.05–0.20 mg/kg buprenorphine every 12 h for 48 h and returned to their respective cages. This murine model induces an LD10–30 within the first 7 days.
Polytrauma.
Groups of mice were anesthetized with inhalational isoflurane and restrained in the supine position. Mice underwent 90 min of hemorrhagic shock followed by long bone fracture and cecectomy as previously described (15). The combined level of injury produces an equivalent injury severity score in humans of 18. After injury, mice were housed in groups, and all mice were administered buprenorphine (0.2 mg/kg body wt) prior to arousal from anesthesia and every 12 h afterward until death. Of note, this model is nonlethal, and the animals were able to maintain the ability to ambulate, groom, feed, and drink.
Polytrauma followed by pseudomonal pneumonia.
Pseudomonal pneumonia (Pp) following polytrauma was induced using Pseudomonas aeruginosa (PAK) as described previously (8) 1 day after PT. PAK was grown overnight, transferred to fresh medium, and grown to midlog phase. The bacterial density was measured at optical density 600λ (DU 640 Spectrophotometer, Beckman Coulter, CA) and washed with saline. Under isoflurane anesthesia, these mice received intranasal instillation of 1 × 107 bacteria, delivered in 50 μl. This murine infection model has an LD10–20 over 7 days after polytrauma; it is a nonlethal model when administered to healthy animals (34).
Transcriptomics.
Blood was collected by intracardiac puncture by 1 ml syringes containing 100 μl 169 mM EDTA at 2 h or 1 or 3 days after CLP or polytrauma, and 1 day after Pp in polytrauma mice. Red blood cells were lysed with Buffer EL (Qiagen, Valencia, CA). The supernatant was decanted after centrifugation, and the cell pellet was homogenized in RLT buffer (Qiagen) supplemented with 2-mercaptoethanol and passed through the homogenizer (Qiagen). Subsequently, total RNA was isolated using RNeasy kit (Qiagen, Valencia, CA), and the quality and quantity were assessed using an Agilent Bioanalyzer 2000. Nucleic acids were labeled using the 3′ IVT Express Kit, and 15 μg labeled cRNA was hybridized to mouse genome 430 2.0 arrays (Affymetrix, Santa Clara, CA). Arrays were hybridized for 16 h at 45°C. Following hybridization, arrays were stained and washed using a FS450 Affymetrix Fluidics Station and Affymetrix FlexFS 450-0004 protocol. Arrays were then scanned in an Affymetrix GeneChip scanner 7G Plus. Genome-wide expression was performed on total blood (circulating) leukocytes (9, 33). All array data were submitted to the Gene Expression Omnibus (GEO) genomics data repository; GEO accession number GSE69245.
Statistics.
Blood leukocyte genome-wide expression patterns were compared between healthy mice and mice experiencing either CLP sepsis, polytrauma, or polytrauma and pneumonia, using a false discovery adjusted F test (P < 0.001) with BRB Tools. The datasets were analyzed for individual gene expression differences (magnitude of fold change of the significant genes), as well as for individual pathways (Gene Ontology and Biocarta) using the distance from reference (DFR), (P < 0.05) (5, 43), and functional pathway differences (Z-score, <−2, >2) using Ingenuity Pathway Analysis (IPA). A Z-score of <−2 or >2 represents a significant change at a 95% confidence interval (3). The DFR calculation derives a single metric representing the overall differences in gene expression and is calculated as the natural log of the sum of the differences in gene expression (between healthy and experimental animals) for each probe set divided by the pooled variance for that individual probe set. This allows each specimen's overall genomic response to be represented by a single natural log-transformed value. DFR is useful to determine only the magnitude of genomic expression change from baseline and does not describe its direction.
Spearman correlations were calculated to assess the correlation between changes in gene expression in the various forms of injury and time points. All correlations were carried out on individual genes, not individual probe sets (16).
RESULTS
Polytrauma and sepsis induce similar lymphocyte dominant responses.
Complete blood count analyses at 1 day revealed no significant difference in the differential leukocyte count between polytrauma with and without pneumonia (Fig. 1A). At 1 day after CLP, the observed leukocyte count was composed predominantly of lymphocytes, similar to that seen after polytrauma, but to a significantly lesser extent (Fig. 1B). As expected though, the percentage of neutrophils was significantly increased in all injury models at 1 day after severe injury or infection.
Fig. 1.
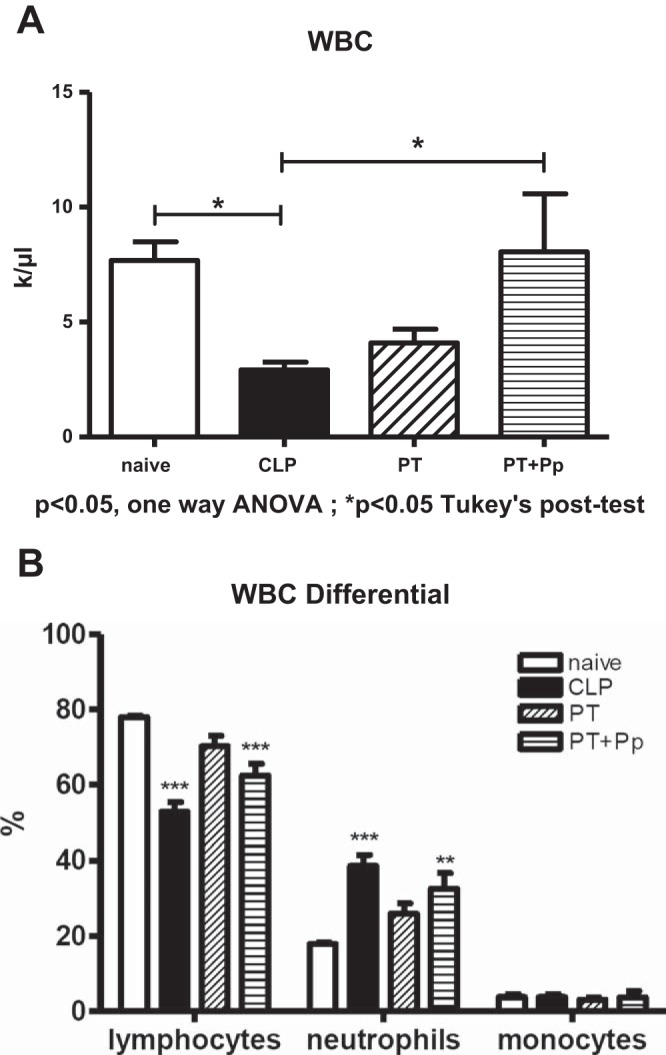
Complete blood count (CBC) analysis 1 day postsepsis [cecal ligation and puncture (CLP)], polytrauma (PT), and polytrauma plus pneumonia (PT+Pp). A: complete white blood count (WBC) shows decreased WBC in CLP compared with naïve mice. PT and PT+Pp do not show a significant difference compared with naïve mice. B: WBC differential. All models show an increase in neutrophils at 1 day. *P < 0.05 Tukey's posttest, **P < 0.01, ***P < 0.001 vs. naïve per 2-way ANOVA, Bonferroni posttests.
Total leukocyte microarray genomic analysis reveals similar genomic responses in the early time points after polytrauma and CLP sepsis.
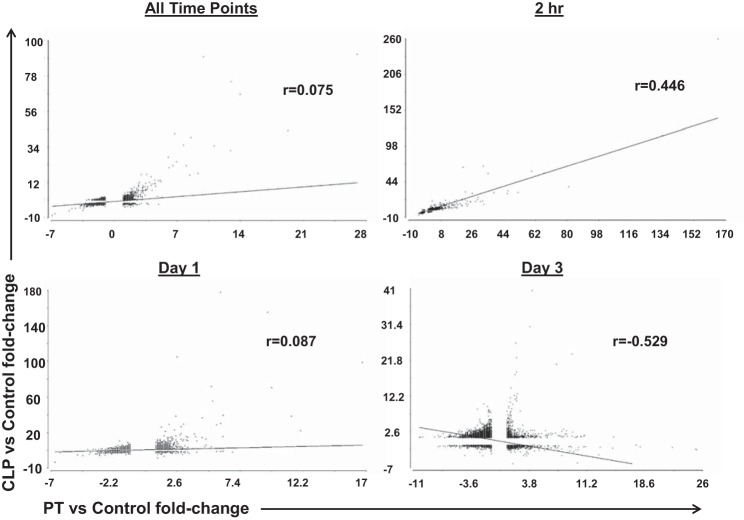
The expression of 16,728 probe sets representing 10,426 unique genes significantly differed between healthy controls (at P < 0.001) (Fig. 2) and any of the injury/sepsis models at any of the time periods. The overall Spearman rank correlation (rs) for the entire leukocyte genome that changed after CLP sepsis vs. polytrauma (10,426 genes) regardless of timing was only 0.075 (P insignificant). When changes in gene expression after polytrauma and CLP sepsis were compared at individual time points, a positive correlation was seen at 2 h (rs = 0.446) (P < 0.0001), while no correlation was seen at 1 day (rs = 0.087) and a highly significant inverse correlation was seen at 3 days (rs = −0.529) (P < 0.0001) (Fig. 3). However, when we compared genome-wide expression at the time of maximal genomic upregulation for both CLP sepsis (1 day) and polytrauma (2 h), the overall rs correlation was 0.755 (P < 0.0001). Similarly, when genome-wide expression was compared between CLP and polytrauma with pneumonia at 1 day, rs was 0.423 (P < 0.0001).
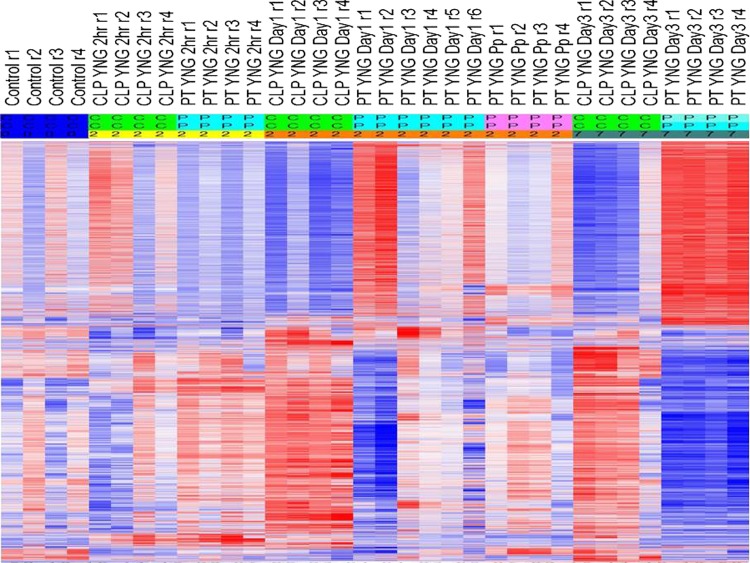
Fig. 2.
Heat map illustrating a supervised analysis of gene expression in murine models of sepsis (CLP), PT, and PT+Pp at various time points after insult (2 h, 1 day, and 3 days). The heat map reveals that the closest correlations are at the early time points. Red, upregulation; blue, downregulation. Heat map represents 10,426 unique genes.
Fig. 3.
Spearman rank correlations for all 10,426 genes significantly differ from control at specific points postinsult. Comparisons of all time points combined (A), 2 h post-PT vs. 2 h post-CLP (B), 1 day post-PT vs. 1 day post-CLP (C), and 3 days post-PT vs. 3 days post-CLP (D) are displayed. Although the correlations are poor when all 10,426 genes are compared at all time points (A), the strongest correlations are seen at the earliest time points. By 3 days, there is a negative correlation due to the downregulation of the previously upregulated genes in PT.
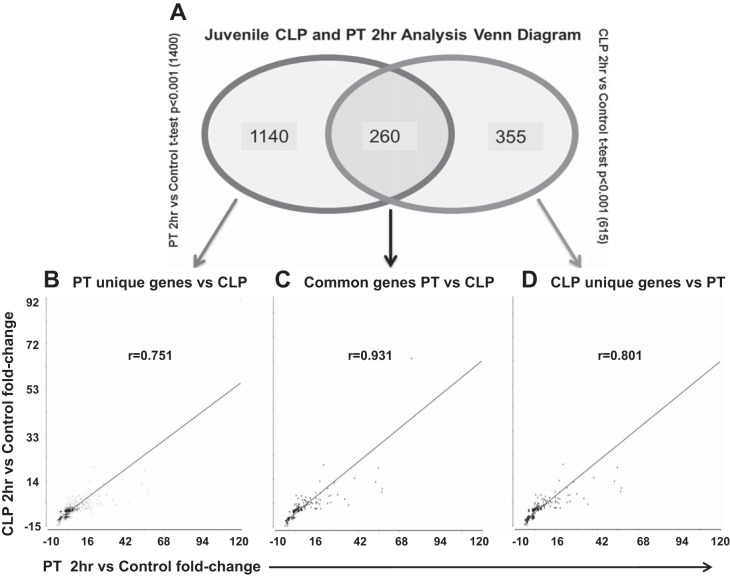
We then filtered the genes whose expression was found to be 1.5-fold different from healthy, control mice (P < 0.001). At 2 h, there were a total of 1,400 genes after polytrauma and 615 genes after CLP that had significant gene expression changes that were also altered ≥1.5-fold from baseline expression (P < 0.001). Of these significant genes, there were 260 commonly altered genes common between the two models (Fig. 4A). Not surprisingly, the rs for these 260 genes was 0.931 (P < 0.001) (Fig. 4B). When we examined the overall 1,400 genes that changed in polytrauma by at least 1.5-fold and compared the change in expression to the same genes in CLP, whether significant or not, we observed an rs of 0.751 (P < 0.001) (Fig. 4B). Subsequently, we analyzed the 615 genes that were changed in CLP and compared them to the same genes in polytrauma, and the rs was 0.801 (P < 0.001) (Fig. 4B).
Fig. 4.
Comparison of genes with significantly differentiated gene expression at 2 h postinsult with a fold change ≥ 1.5. A: Venn diagram illustrating all genes found to be significantly different from controls. B: Spearman rank correlation graph for all genes found to be significantly different from control 2 h post-PT vs. the corresponding genes 2 h post-CLP. C: Spearman rank correlation graph for all genes found to be significantly different from control with at least a 1.5-fold change that were shared between PT and CLP at 2 h post-insult. D: Spearman rank correlations for all genes found to be significantly different from control with at least a 1.5-fold change for CLP at vs. corresponding genes in PT at 2 h postinsult (whether they met the aforementioned criteria or not).
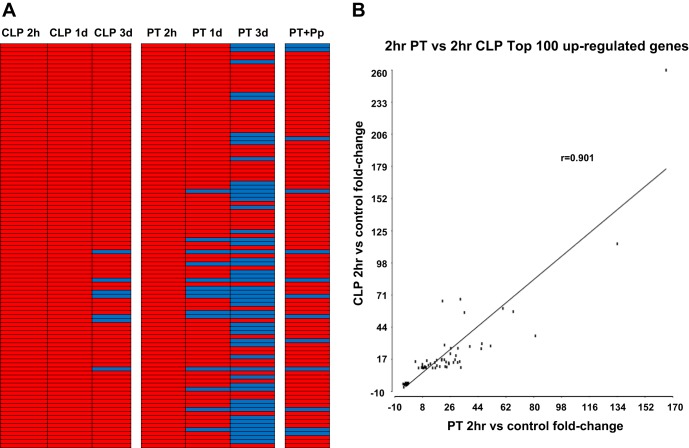
We then evaluated the top 100 responsive genes, whether upregulated or downregulated from baseline controls, from both CLP sepsis and polytrauma for similarities at 2 h after injury or infection. The changes from baseline control mouse expression ranged from two- to 280-fold, and we identified that a large proportion of these genes were involved in the early inflammatory response. Interestingly, the top 100 upregulated genes 2 h after CLP sepsis were similarly upregulated 2 h after polytrauma. The rs for these 100 genes at 2 h was 0.901 (P < 0.001) (Fig. 5, A and B). The same 100 genes continued to be upregulated by CLP at 1 day, but after polytrauma, several of the genes had either returned to baseline or their expression reduced (Fig. 5A). We also examined the top 50 upregulated and top 50 downregulated genes after polytrauma and compared these to the genomic expression patterns of the same genes 2 h after CLP sepsis; their correlation (rs) was 0.865 (P < 0.001).
Fig. 5.
The correlation between the top 100 upregulated genes in CLP compared with the same genes in PT. Correlations between the 2 models are very strong when one considers genes that represent early responders to the inflammatory response. A: illustration showing the top 100 upregulated genes observed for 2 h after CLP and their corresponding changes in various settings (CLP vs. PT vs. PT + Pp) and time points (2 h, 1 day, 3 days). Red, upregulation; blue, downregulation. B: Spearman correlation graph showing the same top 100 upregulated genes in CLP at 2 h vs. the same genes in PT at 2 h.
The DFR calculation provides an overall metric of the deviation in gene expression (5, 43). It is a natural log value whose variance normalizes the contribution of highly expressed genes to overall gene expression, resulting in a more equitable averaging of the contribution of each gene to the total metric. Overall DFRs from the 10,426 genes that differed revealed that the magnitude of leukocyte gene expression change for CLP peaked at 1 day (12.05) and was greater than the change in gene expression after polytrauma, which occurred at both 2 h and 1 day. However, polytrauma shows a rebound effect at 3 days that actually reveals the highest degree of gene expression change by overall DFR but represents an overall downregulation of early activation genes. As a reference, the DFR in healthy controls was 8.95 (Fig. 6).
Fig. 6.
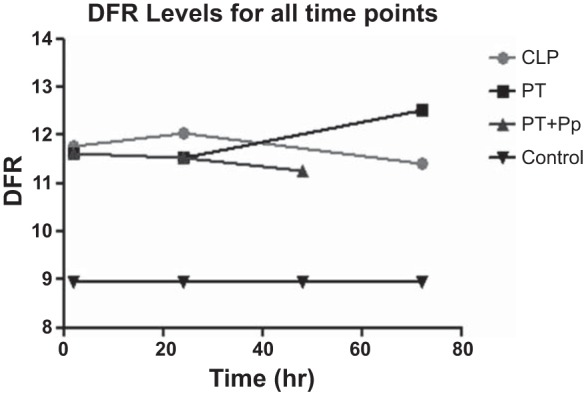
Distance from reference (DFR) analysis for all genes in various injury models and time points. DFR is a simplified method of looking at overall level of change in genomic expression independent of direction, and it can be seen in this graph that all injury models create similar levels of change in expression.
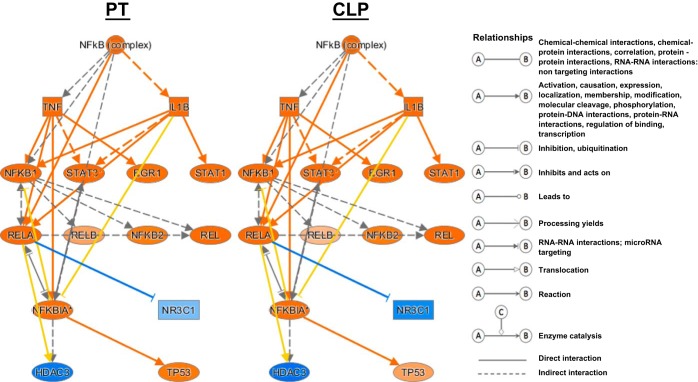
IPA confirmed that the regulation of the genomic responses after CLP sepsis and polytrauma were most similar at the early time points. According to upstream regulation analysis for the total leukocyte population, the requirement for nuclear factor kappa B (NF-κB) activation showed nearly an identical response after sepsis and severe hemorrhagic shock with trauma (Fig. 7). Other early response pathways that were similarly activated in both polytrauma and CLP sepsis at 2 h were pathways dependent on extracellular regulated kinase, myeloid differentiation primary response gene 88 (MYD88), CCAAT/enhancer-binding protein alpha (CEBPA), TLRs, and epidermal growth factor (EGF) receptor expression.
Fig. 7.
Ingenuity Pathway Analysis (IPA) illustration showing upstream regulation of the pathway for NF-κB. The response to injury in both models at 2 h creates a very similar response. Orange, upregulation; blue, downregulation.
Surprisingly, we identified through upstream analysis that microRNA (miR)-223 was predicted to be activated in both polytrauma and CLP sepsis at the early time points (Z score 4.43 and 3.76, respectively). miR-223 is highly expressed in myeloid cells and has been shown to negatively regulate progenitor proliferation (21, 35). It has also been demonstrated to suppress differentiation of myeloid-derived suppressor cells (MDSCs) from bone marrow (26). This is highly relevant since expansion of MDSCs is a common response to trauma, sepsis, and burns (7).
We were able to identify molecules, predicted by the transcriptomic patterns of the mice after the insult, that were uniquely recognized to be activated or inhibited in either CLP or polytrauma at 2 h, an early time point during the innate immune response. Using IPA Causal Analysis we determined that Cullin 4B (CUL4B) was predicted to be inhibited at 2 h after polytrauma, but its expression was not significantly affected in the early CLP sepsis response (Z score −2.093 and 0.246, respectively). Interestingly, CUL4B is a negative regulator of MDSC function (36) and has also been identified as a promoter of proliferation and inhibitor of apoptosis in cancer cell (4). In addition, we found that miR-483-3p, also an inhibitor of proliferation with proapoptotic activity (2), was predicted to be inhibited early in polytrauma, but its expression was not significantly affected in CLP sepsis (Z score −2.449 and 0.707 at the 2 h time points, respectively). Furthermore, regulator of G protein signaling-14 (RGS14) was predicted to be activated early in CLP sepsis, but its expression was not significantly affected in polytrauma (Z score 2.234 and −0.364, respectively). RGS14 is involved in αMβ2-integrin activation, which is important for bidirectional signaling involved in phagocytosis (25); thus, its activation in CLP sepsis and not in trauma would seem appropriate. Lastly, bone marrow stromal cell antigen 2 (BST2) was predicted to be activated early in CLP sepsis but inhibited in polytrauma (Z score 3.883 and −4.982, respectively). The protein encoded by this gene has been demonstrated to mediate antigen delivery to plasmacytoid dendritic cells, which subsequently induces initial T-cell priming (27). Thus, downregulation of BST2 expression in polytrauma might be protective by limiting presentation of self-antigens expressed in tissue injury.
Genes known to be involved with PRRs display similar responses.
Individual fold changes for genes known to be involved with PAMP and DAMP recognition also demonstrated similar degrees of expression changes. These genes include heat shock proteins and TLR, which are almost universally upregulated at 2 h after both sepsis and polytrauma (Table 1). Similar changes were also identified 1 day after pneumonia in polytrauma animals. However, PRR gene expression began returning to baseline levels within 3 days after polytrauma, which was dissimilar to sepsis (Table 1).
Table 1.
The damage-associated molecular pattern and pathogen-associated molecular pattern with their pattern-recognition receptor
| Gene | CLP 2 h | CLP 1 d | CLP 3 d | PT 2 h | PT 1 d | PT 3 d | PT+Pp |
|---|---|---|---|---|---|---|---|
| Hsp90aa1 | −1.4 | 3.3 | 1.6 | 1.5 | −1.2 | −1.5 | 1.7 |
| Hsp90ab1 | −1.7 | 2 | 1.2 | 1.4 | −1.5 | −1.1 | 1.5 |
| Hsp90b1 | −1.2 | 4.5 | 1.5 | 1.4 | −1.3 | −1.2 | 1.6 |
| Hspa13 | 1.4 | 3.1 | 1.7 | 1.6 | −1.3 | −2.9 | 1.2 |
| Nod1 | −1.1 | 1.6 | 1.8 | 1.2 | −1.8 | −2.2 | −1.3 |
| NLRP3 | 1.8 | −1.2 | −1.3 | 4.2 | −1.2 | −1.2 | −1 |
| Tlr1 | −1.7 | 1.3 | 1.9 | −1.7 | −2.3 | −3.5 | −1 |
| Tlr12 | −1 | −1.2 | −1.2 | −1.2 | 1.1 | 1.2 | −1 |
| Tlr13 | 2 | 3.7 | 2.7 | 4.7 | 1.2 | −1.7 | 1.7 |
| Tlr2 | 12.4 | 4.6 | 3.7 | 26.7 | 1.6 | 1.1 | 2 |
| Tlr4 | 2.8 | 5.5 | 3.2 | 2.9 | 1.3 | −1.5 | 2.3 |
| Tlr6 | 1.8 | 1.9 | 1.9 | 2.3 | 1.1 | −1 | 1.1 |
| Tlr9 | 1.1 | −1.3 | −1.1 | −1.1 | 1.3 | 1.5 | 1.1 |
| Cxcr2 | 3.7 | 3.4 | 4.1 | 8 | 1.4 | −1.5 | 2 |
| Sap130 | −1.3 | 1.4 | 1.1 | 1.2 | −1.1 | −2.1 | −1.2 |
| CD36 | −1 | −2.3 | −2.1 | −1.4 | 1.7 | 6.3 | 2.9 |
| CD44 | 1 | 2.2 | 1.4 | 1.8 | 1.1 | −3.7 | 1 |
| Il1r2 | 33.2 | 34.4 | 9.7 | 46.1 | 6.7 | 1.8 | 2.8 |
Values represent fold-change vs. healthy control mice. CLP, cecal ligation and puncture; PT, polytrauma; Pp, pseudomonal pneumonia; d, day.
DISCUSSION
The discovery of PRRs has led to a greater understanding of how the host recognizes microbial pathogens and tissue injury and has opened the possibility of novel therapeutic immunomodulation (20). Murine models remain the bedrock for preclinical mechanistic and therapeutic studies. Recent studies examining the host genomic responses to injury and sepsis have increased the scrutiny of a murine model's capacity to recapitulate the human processes (10). Recently, we reported that the leukocyte genomic response to human models of injury showed significant overlap, while comparable murine models demonstrated little to no correlation among themselves (38). This overlap in the human response is thought to be due to the initiation of the systemic inflammatory response syndrome, which is common to both microbial infection and tissue injury.
In contrast, the failure of murine models to recapitulate the human response may well be due to the nature of the injury models and time periods selected (10, 16). Although the response to a 30% burn injury, administration of endotoxin, and hemorrhagic shock with a laparotomy were transcriptomically dissimilar, it can be argued that these murine models are suboptimal representations of human infection and injury (10). For example, endotoxin administration is not equivalent to septic polymicrobial infection (6, 15). In fact, the method of bacterial infection can have huge implications on the murine transcriptomic response (18). Additionally, although a steadfast component of trauma research for decades, the murine model of hemorrhagic shock/laparotomy can certainly be improved to increase its similarity to human severe trauma (6, 15).
This study asked a different but fundamental question regarding how the murine model responds to two different inflammatory challenges of similar severity: polymicrobial sepsis produced by a CLP, and polytrauma with hemorrhagic shock. One model represents the host response to an overwhelming infectious cascade, whereas the other represents significant tissue injury and hypovolemic shock in the absence of microbial infection. Although the CLP sepsis model is associated with 10–20% mortality, whereas the polytrauma is not, polytrauma mice become susceptible to nonlethal challenges with Pp. Based on the overall hypothesis that PRRs can recognize both microbial pathogens and endogenous alarmins resulting from tissue injury, we postulated that the early genomic responses should be more similar than disparate. Furthermore, we proposed that not only should there be common genomic responses, but upstream promoter analyses dependent upon PRR signaling should also reveal significant overlap. We also hypothesized that the genomic responses would grow more dissimilar with time, as the CLP represents an ongoing infectious process, while polytrauma is nonfatal and animals rapidly recover.
Using a CLP model of sepsis and an improved model of trauma, we demonstrated that there truly are dramatic leukocyte transcriptomic changes after severe murine infection or injury, with nearly 40% of the host genome expression significantly changing. This is similar to what has been revealed after human trauma, a true “genomic storm” (29). Looking at the entire leukocyte transcriptome that changed (10,426 genes), one sees that the correlations in gene expression changes remained poor when timing of the response is not considered (rs = 0.075). These findings are consistent with the changes in genome-wide expression seen between burns, hemorrhagic shock, and endotoxemia, as we reported (38). However, the failure to see strong correlations appears to be due primarily to the difference in the timing of the genomic response. Rather, if one focused on the individual time points, the genome-wide associations between severe trauma and sepsis were most similar at 2 h (rs = 0.446) and then declined dramatically over time until there was an inverse correlation at 3 days (rs = −0.529). The overall differences in the kinetics of gene expression are best revealed by the DFR, which peaked in polymicrobial sepsis at 1 day. In polytrauma, changes in gene expression show a significant overall upregulation at 2 h, but by 3 days there is actually a rebound effect in which we see our overall highest level of gene downregulation. However, in CLP, there is actually a prolonged upregulation of inflammatory genes. This likely reflects the ongoing presence of nonviable tissue (ligated cecum) and unresolved infection in this model.
Such numbers can be overanalyzed since in any cell type only ∼50% of the genome is expressed (22). In blood leukocytes in particular, one would purposefully focus on genes involved in activation of protective immunity, inflammation, and metabolic activity in response to polytrauma or polymicrobial sepsis. If the genes are filtered to include only those genes that change after injury or sepsis at least 1.5-fold, the number of genes was dramatically reduced (1,400 in polytrauma and 615 in sepsis); in fact, their commonality improved dramatically! This was the approach taken by Takao and Miyakawa (40) when they re-evaluated our earlier analyses on human and murine gene expression. Although the number of genes whose change in expression exceeded 1.5-fold, the rs for the 1,400 and 615 genes, respectively, was excellent (rs = 0.751 and 0.801, respectively), suggesting that there are subsets of genes whose expression moves in a comparable direction in these two distinct injury and infection models. Further refining the list to the top 100 genes that changed the greatest magnitude after polytrauma or sepsis revealed even greater concordance between the two injuries (rs = 0.901). Not surprisingly, 88% of these genes were directly involved in early inflammatory and innate immune responses. Such findings demonstrate that there is a core genomic response in murine trauma and polymicrobial sepsis that is shared, although the timing of the response may differ.
Analysis of predicted upstream regulatory control suggested that the two inflammatory models, whether infectious or traumatic, induce a similar set of response elements. The common patterns of gene expression reflect common activation of NF-κB and several other early response pathways including MYD88, TLRs, and EGF. There was also early activation in both models of CEBPA, which leads to downstream inhibition of peroxisome proliferator-activated receptors (PPAR). PPAR is known to have significant effects on substrate metabolism essential for the cell's oxidative response (28).
Interestingly, when we compared pneumonia in polytrauma to CLP sepsis at 1 day, there was still a significant correlation at 0.423, albeit less than the correlation between polytrauma and CLP sepsis at 1 day. This may be due to the model itself in that the infection occurs 24 h following polytrauma. Trauma clearly altered the baseline changes in expression as well as immune suppression (45), and infection in this setting is likely to induce genomic changes markedly different than would have occurred in a healthy, naïve animal. This may lead to less activation of the immune response following a second stimulus potentially via MDSCs (7). MDSCs, initially described in the cancer literature, consist of a heterogeneous subset of immature myeloid cells whose numbers increase dramatically after inflammation and are potently immunosuppressive while simultaneously contributing to low-grade, chronic inflammation (13).
MicroRNAs (miRNAs), small segments of noncoding RNA, are involved in both pre- and posttranscriptional gene regulation (1). Several studies performed in the past have demonstrated that various expression levels of these miRNAs can affect myeloid differentiation (12, 21). miR-223 is a widely studied miRNA that has been shown to be highly expressed in myeloid cells (35). Interestingly, in tumors, it has been shown to decrease differentiation of tumor-induced MDSCs (21). It is possible that these MDSCs, elevated posttrauma and sepsis, are key players in both the chronic inflammation and immunosuppression displayed after severe sepsis and trauma (7).
We have previously demonstrated that, in response to injury, the elderly do not have an exaggerated inflammatory response nor greater suppression of adaptive immunity genes but, rather, a delayed return to baseline (33, 34, 41). Thus, return to homeostasis is a key objective for future immunomodulation therapy of elderly trauma and sepsis patients (33, 34, 41). However, it is clear that juveniles do not return to baseline genomic expression patterns after severe trauma within 3 days in this study. Without further insults, such as a bacterial pneumonia, there appears to be a complete “inversion” of the transcriptomic expression pattern, completely different from the response to CLP sepsis (Fig. 2). Whether this represents an evolutionary benefit to the host, by trying to reduce the likelihood of an autoimmune response to released damaged cell products or organ injury, or a period of increased host susceptibility after the initial emergency myelopoiesis response, has yet to be determined. Unfortunately, animal welfare issues prevent us from determining how long it truly takes the juvenile and elderly mouse to return to baseline genomic expression patterns, but preliminary data in elderly humans may indicate that the process may not be rapid (11, 14).
There are a number of significant limitations to the present study that warrant caution. First, this study makes no attempt to compare murine to human responses but focuses entirely on the similarities and differences in leukocyte gene expression to different inflammatory stimuli in mice. Second, we are focusing on a single tissue compartment, and that compartment is heterogeneous and its composition varies with both the polytrauma and polymicrobial sepsis (Fig. 1). Whether these findings can be extrapolated to other tissues and models of injury is unknown. Third, the findings are conducted in juvenile mice, and mortality from these conditions, especially sepsis, is most commonly associated with human patients of increased age (17, 33). We have recently demonstrated that aged animals have a relatively attenuated early inflammatory response to both trauma and sepsis and fail to re-establish homeostasis (33, 34). Furthermore, this study uses C57BL/6 mice, a Th1 dominant strain (44). It is well known that different inbred mouse strains can have a different response to injury (39, 44). A Th1 dominant strain has predominant interferon-γ cytokine production leading to immune activation via a macrophage dominant response (44), thus favoring the innate immune response. However, as with all of our prior experiments, we use this strain as it has proven to provide high reproducibility. In addition, C57BL/6 mice constitute the majority of knockout strains and transgenics, thus providing a platform for genomic manipulation if pursued in future studies.
With these limitations in mind, however, the findings are quite striking in demonstrating that although the overall genome-wide response varies over time between polytrauma and sepsis, there is a core of 1,000–2,000 genes whose expression changes and changes in a common pattern, regardless of the initiating inflammatory challenge. The commonality of this response is greatest early after the inflammatory challenge, and the gene expression changes are primarily related to the early inflammatory and innate immunity response. Examination of upstream regulatory elements also reveals a commonality regardless of whether the stimulus was polytrauma or polymicrobial sepsis in many of the early regulatory activators of the immune response.
GRANTS
B. E. Szpila, B. J. Mathias, J. C. Mira, and L. F. Gentile were supported by National Institutes of Health (NIH) training grant in burn and trauma research T32 GM-08721. This work was also supported by NIH Grants R01 GM-40586-24 and R01 GM-081923-06. A. M. Mohr was supported by NIH Grant R01 GM-105893-01A1. In addition, P. A. Efron was supported by NIH Grants P30 AG-028740 and R01 GM-113945-01. Finally, P. A. Efron, F. A. Moore, H. V. Baker, S. Brakenridge and L. L. Moldawer were supported by NIH Grant P50 GM-111152-01.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.C.M., B.E.S., D.C.N., F.A.M., S.B., A.M.M., L.L.M., and P.A.E. conception and design of research; J.C.M., B.E.S., D.C.N., R.U., and P.A.E. performed experiments; J.C.M., B.E.S., D.C.N., M.-C.L., L.F.G., B.J.M., E.L.V., R.U., D.H., M.R., J.R., P.T.V., S.D.L., F.A.M., H.V.B., and P.A.E. analyzed data; J.C.M., B.E.S., D.C.N., M.-C.L., L.F.G., B.J.M., E.L.V., D.H., M.R., J.R., P.T.V., S.D.L., F.A.M., S.B., H.V.B., L.L.M., and P.A.E. interpreted results of experiments; J.C.M., B.E.S., and D.C.N. prepared figures; J.C.M., B.E.S., L.L.M., and P.A.E. drafted manuscript; J.C.M., B.E.S., D.C.N., S.B., A.M.M., L.L.M., and P.A.E. edited and revised manuscript; J.C.M., B.E.S., D.C.N., M.-C.L., L.F.G., B.J.M., E.L.V., R.U., D.H., M.R., J.R., P.T.V., S.D.L., F.A.M., S.B., A.M.M., H.V.B., L.L.M., and P.A.E. approved final version of manuscript.
REFERENCES
- 1.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 136: 215–233, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertero T, Bourget-Ponzio I, Puissant A, Loubat A, Mari B, Meneguzzi G, Auberger P, Barbry P, Ponzio G, Rezzonico R. Tumor suppressor function of miR-483-3p on squamous cell carcinomas due to its pro-apoptotic properties. Cell Cycle 12: 2183–2193, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheadle C, Cho-Chung YS, Becker KG, Vawter MP. Application of z-score transformation to Affymetrix data. Appl Bioinformat 2: 209–217, 2003. [PubMed] [Google Scholar]
- 4.Chen Z, Shen BL, Fu QG, Wang F, Tang YX, Hou CL, Chen L. CUL4B promotes proliferation and inhibits apoptosis of human osteosarcoma cells. Oncol Rep 32: 2047–2053, 2014. [DOI] [PubMed] [Google Scholar]
- 5.Cobb JP, Mindrinos MN, Miller-Graziano C, Calvano SE, Baker HV, Xiao W, Laudanski K, Brownstein BH, Elson CM, Hayden DL, Herndon DN, Lowry SF, Maier RV, Schoenfeld DA, Moldawer LL, Davis RW, Tompkins RG, Baker HV, Bankey P, Billiar T, Brownstein BH, Calvano SE, Camp D, Chaudry I, Cobb JP, Davis RW, Elson CM, Freeman B, Gamelli R, Gibran N, Harbrecht B, Hayden DL, Heagy W, Heimbach D, Herndon DN, Horton J, Hunt J, Laudanski K, Lederer J, Lowry SF, Maier RV, Mannick J, McKinley B, Miller-Graziano C, Mindrinos MN, Minei J, Moldawer LL, Moore E, Moore F, Munford R, Nathens A, O'Keefe G, Purdue G, Rahme L, Remick D, Sailors M, Schoenfeld DA, Shapiro M, Silver G, Smith R, Stephanopoulos G, Stormo G, Tompkins RG, Toner M, Warren S, West M, Wolfe S, Xiao W, Young V, Inflammation Host Response to Injury Large-Scale Collaborative Research P . Application of genome-wide expression analysis to human health and disease. Proc Natl Acad Sci USA 102: 4801–4806, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cuenca AG, Delano MJ, Kelly-Scumpia KM, Moldawer LL, Efron PA. Cecal ligation and puncture. Curr Protoc Immunol 19: Unit 19 13, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuenca AG, Delano MJ, Kelly-Scumpia KM, Moreno C, Scumpia PO, Laface DM, Heyworth PG, Efron PA, Moldawer LL. A paradoxical role for myeloid derived suppressor cells in sepsis and trauma. Mol Med 17: 281–292, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delano MJ, Thayer T, Gabrilovich S, Kelly-Scumpia KM, Winfield RD, Scumpia PO, Cuenca AG, Warner E, Wallet SM, Wallet MA, O'Malley KA, Ramphal R, Clare-Salzer M, Efron PA, Mathews CE, Moldawer LL. Sepsis induces early alterations in innate immunity that impact mortality to secondary infection. J Immunol 186: 195–202, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dyson A, Singer M. Animal models of sepsis: why does preclinical efficacy fail to translate to the clinical setting? Crit Care Med 37: S30–S37, 2009. [DOI] [PubMed] [Google Scholar]
- 10.Efron PA, Mohr AM, Moore FA, Moldawer LL. The future of murine sepsis and trauma research models. J Leukoc Biol 98: 945–952, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finnerty CC, Jeschke MG, Qian WJ, Kaushal A, Xiao W, Liu T, Gritsenko MA, Moore RJ, Camp DG 2nd, Moldawer LL, Elson C, Schoenfeld D, Gamelli R, Gibran N, Klein M, Arnoldo B, Remick D, Smith RD, Davis R, Tompkins RG, Herndon DN. Determination of burn patient outcome by large-scale quantitative discovery proteomics. Crit Care Med 41: 1421–1434, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fontana L, Pelosi E, Greco P, Racanicchi S, Testa U, Liuzzi F, Croce CM, Brunetti E, Grignani F, Peschle C. MicroRNAs 17-5p-20a-106a control monocytopoiesis through AML1 targeting and M-CSF receptor upregulation. Nat Cell Biol 9: 775–787, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 9: 162–174, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gentile LF, Cuenca AG, Efron PA, Ang D, Bihorac A, McKinley BA, Moldawer LL, Moore FA. Persistent inflammation and immunosuppression: a common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg 72: 1491–1501, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gentile LF, Nacionales DC, Cuenca AG, Armbruster M, Ungaro RF, Abouhamze AS, Lopez C, Baker HV, Moore FA, Ang DN, Efron PA. Identification and description of a novel murine model for polytrauma and shock. Crit Care Med 41: 1075–1085, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gentile LF, Nacionales DC, Lopez MC, Vanzant E, Cuenca A, Cuenca AG, Ungaro R, Baslanti TO, McKinley BA, Bihorac A, Cuschieri J, Maier RV, Moore FA, Leeuwenburgh C, Baker HV, Moldawer LL, Efron PA. A better understanding of why murine models of trauma do not recapitulate the human syndrome. Crit Care Med 42: 1406–1413, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gentile LF, Nacionales DC, Lopez MC, Vanzant E, Cuenca A, Cuenca AG, Ungaro R, Szpila BE, Larson S, Joseph A, Moore FA, Leeuwenburgh C, Baker HV, Moldawer LL, Efron PA. Protective immunity and defects in the neonatal and elderly immune response to sepsis. J Immunol 192: 3156–3165, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gentile LF, Nacionales DC, Lopez MC, Vanzant E, Cuenca A, Szpila BE, Cuenca AG, Joseph A, Moore FA, Leeuwenburgh C, Baker HV, Moldawer LL, Efron PA. Host responses to sepsis vary in different low-lethality murine models. PLoS One 9: e94404, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gosemann JH, van Griensven M, Barkhausen T, Kobbe P, Thobe BM, Haasper C, Pape HC, Krettek C, Hildebrand F, Frink M. TLR4 influences the humoral and cellular immune response during polymicrobial sepsis. Injury 41: 1060–1067, 2010. [DOI] [PubMed] [Google Scholar]
- 20.Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol 54: 1–13, 1989. [DOI] [PubMed] [Google Scholar]
- 21.Johnnidis JB, Harris MH, Wheeler RT, Stehling-Sun S, Lam MH, Kirak O, Brummelkamp TR, Fleming MD, Camargo FD. Regulation of progenitor cell proliferation and granulocyte function by microRNA-223. Nature 451: 1125–1129, 2008. [DOI] [PubMed] [Google Scholar]
- 22.Jojic V, Shay T, Sylvia K, Zuk O, Sun X, Kang J, Regev A, Koller D, Immunological Genome Project C, Best AJ, Knell J, Goldrath A, Joic V, Koller D, Shay T, Regev A, Cohen N, Brennan P, Brenner M, Kim F, Rao TN, Wagers A, Heng T, Ericson J, Rothamel K, Ortiz-Lopez A, Mathis D, Benoist C, Bezman NA, Sun JC, Min-Oo G, Kim CC, Lanier LL, Miller J, Brown B, Merad M, Gautier EL, Jakubzick C, Randolph GJ, Monach P, Blair DA, Dustin ML, Shinton SA, Hardy RR, Laidlaw D, Collins J, Gazit R, Rossi DJ, Malhotra N, Sylvia K, Kang J, Kreslavsky T, Fletcher A, Elpek K, Bellemarte-Pelletier A, Malhotra D, Turley S. Identification of transcriptional regulators in the mouse immune system. Nat Immunol 14: 633–643, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA 311: 1308–1316, 2014. [DOI] [PubMed] [Google Scholar]
- 24.Lee J, Quraishi SA, Bhatnagar S, Zafonte RD, Masiakos PT. The economic cost of firearm-related injuries in the United States from 2006 to 2010. Surgery 155: 894–898, 2014. [DOI] [PubMed] [Google Scholar]
- 25.Lim J, Thompson J, May RC, Hotchin NA, Caron E. Regulator of G-protein signalling-14 (RGS14) regulates the activation of alphaMbeta2 integrin during phagocytosis. PLoS One 8: e69163, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Q, Zhang M, Jiang X, Zhang Z, Dai L, Min S, Wu X, He Q, Liu J, Zhang Y, Zhang Z, Yang R. miR-223 suppresses differentiation of tumor-induced CD11b(+) Gr1(+) myeloid-derived suppressor cells from bone marrow cells. Int J Cancer 129: 2662–2673, 2011. [DOI] [PubMed] [Google Scholar]
- 27.Loschko J, Schlitzer A, Dudziak D, Drexler I, Sandholzer N, Bourquin C, Reindl W, Krug AB. Antigen delivery to plasmacytoid dendritic cells via BST2 induces protective T cell-mediated immunity. J Immunol 186: 6718–6725, 2011. [DOI] [PubMed] [Google Scholar]
- 28.Mansour M. The roles of peroxisome proliferator-activated receptors in the metabolic syndrome. Prog Mol Biol Transl Sci 121: 217–266, 2014. [DOI] [PubMed] [Google Scholar]
- 29.Matzinger P. The danger model: a renewed sense of self. Science 296: 301–305, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Mayr FB, Yende S, Angus DC. Epidemiology of severe sepsis. Virulence 5: 4–11, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGhan LJ, Jaroszewski DE. The role of toll-like receptor-4 in the development of multi-organ failure following traumatic haemorrhagic shock and resuscitation. Injury 43: 129–136, 2012. [DOI] [PubMed] [Google Scholar]
- 32.Moore L, Stelfox HT, Turgeon AF, Nathens A, Bourgeois G, Lapointe J, Gagne M, Lavoie A. Hospital length of stay after admission for traumatic injury in Canada: a multicenter cohort study. Ann Surg 260: 179–187, 2014. [DOI] [PubMed] [Google Scholar]
- 33.Nacionales DC, Gentile LF, Vanzant E, Lopez MC, Cuenca A, Cuenca AG, Ungaro R, Li Y, Baslanti TO, Bihorac A, Moore FA, Baker HV, Leeuwenburgh C, Moldawer LL, Efron PA. Aged mice are unable to mount an effective myeloid response to sepsis. J Immunol 192: 612–622, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nacionales DC, Szpila B, Ungaro R, Lopez MC, Zhang J, Gentile LF, Cuenca AL, Vanzant E, Mathias B, Jeevan J, Westerveld D, Bihorac A, Joseph A, Mohr A, Duckworth LV, Moore FA, Baker HV, Leeuwenburgh C, Moldawer LL, Brakenridge S, Efron PA. A detailed characterization of the dysfunctional immunity and abnormal myelopoiesis induced by severe shock and trauma in the aged. J Immunol 195: 2396–2407, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Connell RM, Zhao JL, Rao DS. MicroRNA function in myeloid biology. Blood 118: 2960–2969, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qian Y, Yuan J, Hu H, Yang Q, Li J, Zhang S, Jiang B, Shao C, Gong Y. The CUL4B/AKT/beta-catenin axis restricts the accumulation of myeloid-derived suppressor cells to prohibit the establishment of a tumor permissive microenvironment. Cancer Res 75: 5070–5083, 2015. [DOI] [PubMed] [Google Scholar]
- 37.Remick KN, Schwab CW, Smith BP, Monshizadeh A, Kim PK, Reilly PM. Defining the optimal time to the operating room may salvage early trauma deaths. J Trauma Acute Care Surg 76: 1251–1258, 2014. [DOI] [PubMed] [Google Scholar]
- 38.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, Lopez CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA 110: 3507–3512, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stewart D, Fulton WB, Wilson C, Monitto CL, Paidas CN, Reeves RH, De Maio A. Genetic contribution to the septic response in a mouse model. Shock 18: 342–347, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Takao K, Miyakawa T. Genomic responses in mouse models greatly mimic human inflammatory diseases. Proc Natl Acad Sci USA 112: 1167–1172, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vanzant EL, Hilton RE, Lopez CM, Zhang J, Ungaro RF, Gentile LF, Szpila BE, Maier RV, Cuschieri J, Bihorac A, Leeuwenburgh C, Moore FA, Baker HV, Moldawer LL, Brakenridge SC, Efron PA,. Inflammation, and host response to injury I. Advanced age is associated with worsened outcomes and a unique genomic response in severely injured patients with hemorrhagic shock. Crit Care 19: 77, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vaughan-Sarrazin MS, Bayman L, Cullen JJ. Costs of postoperative sepsis: the business case for quality improvement to reduce postoperative sepsis in veterans affairs hospitals. Arch Surg 146: 944–951, 2011. [DOI] [PubMed] [Google Scholar]
- 43.Warren HS, Elson CM, Hayden DL, Schoenfeld DA, Cobb JP, Maier RV, Moldawer LL, Moore EE, Harbrecht BG, Pelak K, Cuschieri J, Herndon DN, Jeschke MG, Finnerty CC, Brownstein BH, Hennessy L, Mason PH, Tompkins RG. A genomic score prognostic of outcome in trauma patients. Mol Med 15: 220–227, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watanabe H, Numata K, Ito T, Takagi K, Matsukawa A. Innate immune response in Th1- and Th2-dominant mouse strains. Shock 22: 460–466, 2004. [DOI] [PubMed] [Google Scholar]
- 45.Xiao W, Mindrinos MN, Seok J, Cuschieri J, Cuenca AG, Gao H, Hayden DL, Hennessy L, Moore EE, Minei JP, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Brownstein BH, Mason PH, Baker HV, Finnerty CC, Jeschke MG, Lopez MC, Klein MB, Gamelli RL, Gibran NS, Arnoldo B, Xu W, Zhang Y, Calvano SE, McDonald-Smith GP, Schoenfeld DA, Storey JD, Cobb JP, Warren HS, Moldawer LL, Herndon DN, Lowry SF, Maier RV, Davis RW, Tompkins RG. A genomic storm in critically injured humans. J Exp Med 208: 2581–2590, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]