Abstract
Traditionally, women with node-positive operable breast cancer have received complete axillary lymph node dissection (ALND), which is associated with significant morbidity, but recently less invasive alternatives have been explored. We conducted a systematic review of randomised controlled trials assessing alternative approaches to axillary surgery in patients with pathologically-confirmed sentinel node-positive operable breast cancer. We searched on 16/3/15 the Specialized Register of the Cochrane Breast Cancer group; CENTRAL; MEDLINE; PreMEDLINE; EMBASE; WHO International Clinical Trials Registry Portal; ClinicalTrials.gov; conference proceedings from ASCO and the San Antonio Breast Cancer meetings; checked reference lists and contacted authors to identify relevant studies. Double, independent study sifting, extraction, appraisal and summarising were undertaken using standard Cochrane Collaboration methodology. We included three studies (2020 patients) comparing ALND with sentinel lymph node dissection (SLND) to SLND alone, and two studies (1899 patients) comparing ALND to axillary radiotherapy (aRT). No differences in survival or recurrence were observed between ALND and SLND or aRT, but morbidity may have been increased in ALND, and all the results were subject to different biases, such as recruitment bias, performance bias, and outcome-reporting bias. Whilst it is encouraging that there appears to be no adverse effect on recurrence or survival, it will be appropriate to confirm these findings and provide additional data confirming quality of life effects and long term outcomes.
Electronic supplementary material
The online version of this article (doi:10.1186/s40064-016-1712-9) contains supplementary material, which is available to authorized users.
Keywords: Breast cancer, Axillary surgery, Radiotherapy, Operable, Node positive, Sentinel lymph node dissection
Background
Current NICE Guidance for patients treated in the United Kingdom National Health Service makes the following recommendations:
- Offer further axillary treatment to patients with early invasive breast cancer who:
- have macrometastases or micrometastases shown in a sentinel lymph node.
- have a preoperative ultrasound-guided needle biopsy with histologically proven metastatic cancer.
Do not offer further axillary treatment to patients found to have only isolated tumour cells in their sentinel lymph nodes. These patients should be regarded as lymph node-negative (NICE 2009).
This guidance was last updated in 2009 and is currently under review. Since then a number of studies have evaluated whether all patients identified as having metastatic breast cancer in the axillary sentinel nodes require completion axillary lymph node dissection (ALND) and whether radiotherapy might be an effective alternative to ALND in patients where further treatment is recommended following the identification of a positive axillary node. Traditionally complete or partial excision of axillary lymph nodes was common practice in the surgical treatment of patients with early breast cancer regardless of the presence or absence of metastatic disease. This provided information on likely prognosis and guidance on the selection of appropriate adjuvant therapies, including chemotherapy and radiotherapy, following mastectomy. Concerns relating to the morbidity associated with ALND, particularly arm swelling (lymphoedema), shoulder stiffness and neuropathic pain, resulted in the development of targeted procedures (sentinel lymph node biopsy [SNLB], axillary node sampling) designed to stage the axilla, with ALND only recommended for those patients where positive evidence of metastatic disease was identified. These techniques were shown to be associated with less morbidity in the group undergoing SLNB alone without any clear adverse impact on overall survival or disease free survival (Bromham et al.: Axillary staging for operable primary breast cancer (Cochrane Review), submitted). However, for those patients with a positive SLNB a second procedure (ALND) was required in most cases although preoperative molecular assessment or other techniques such as imprint cytology have been utilised in some centres to facilitate completion axillary node clearance as a single procedure in those found to have metastatic spread to the sentinel node/s.
The increased use of molecular markers (e.g. HER2, Oestrogen and Progesterone receptor status) has resulted in a reduced reliance on numerical axillary node status for adjuvant therapy decision-making and resulted in the proposal that ALND may not be indicated in patients with (limited) axillary node disease and, similarly, the proposal that radiotherapy might be associated with fewer side effects and similar outcomes to ALND.
Objectives
To assess in a systematic review conducted and reported according to the PRISMA guidelines (Moher et al. 2009) the benefits and harms of alternative approaches to axillary surgery (including omitting such surgery altogether) in terms of overall survival; disease-free survival; local, regional and distant recurrences; short-term adverse events; and long-term complications in patients with pathologically-confirmed sentinel node-positive operable breast cancer.
Methods
We included randomised controlled trials in women with clinically-defined operable primary breast cancer with a positive sentinel lymph node, comparing the following interventions as part of the initial surgical treatment of early breast cancer: ALND versus no axillary surgery; and ALND versus axillary radiotherapy without ALND; and reporting the following outcomes: Overall survival; disease-free survival; disease control in the axilla; breast cancer recurrence; adverse events; long-term complications; and quality-of-life. For all studies involving full axillary surgery or axillary sampling, the number of nodes removed and method of node analysis was recorded where available, to indicate whether an adequate sampling or clearance procedure was performed.
The search strategy consisted of the following searches (see Additional file 1 for full search strategies):
The Specialized Register of the Cochrane Breast Cancer group on 16 March 2015. Details of the sources and search strategies used to populate this register are described in the Group’s module in The Cochrane Library (http://onlinelibrary.wiley.com/o/cochrane/clabout/articles/BREASTCA/frame.html) Studies coded as “AXILLARY NODE(S)”, “EARLY BREAST CANCER”, “LOCALLY ADVANCED BREAST CANCER”, “PSYCHOSOCIAL”, or “SURGERY” on the specialised register has been extracted for consideration;
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, issue 2 on 16 March 2015);
MEDLINE via OvidSP (2007–12 March 2015) PreMEDLINE via OvidSP (12 March 2015) and EMBASE via OvidSP (2002–12 March 2015). We used a validated filter to identify reports of randomised controlled trials in the initial search of MEDLINE (Lefebvre and Clarke 2001) and for the updated searches used the revised filter (Lefebvre et al. 2011); we used the Scottish Intercollegiate Guidelines Network RCT filter for Embase (http://www.sign.ac.uk/methodology/filters.html);
The World Health Organisation International Clinical Trials Registry Portal (WHO ICTRP) and ClinicalTrials.gov for prospectively registered and ongoing trials, both on 16 March 2015;
The conference proceedings from the American Society of Clinical Oncology (ASCO) 41st–50th Annual Meetings (2005–2014) via the Journal of Clinical Oncology (http://jco.ascopubs.org/site/meetings) and the conference proceedings from the San Antonio Breast Cancer (SABCS) 29th-37th Annual Symposium Meetings (2006–2014) via Cancer Research web site (http://cancerres.aacrjournals.org/), both on 12 March 2015;
The authors of included or ongoing trials were contacted by e-mail and asked if they knew of any relevant studies, but no further studies were identified. The reference lists of the included studies as well as published reviews were also checked for relevant studies.
Two authors independently screened the titles and abstracts of the records identified in the electronic searches, excluding all obviously not relevant studies, and examined the full text of potentially eligible trials. If required, and possible, additional information was sought from the principal investigator of any trial of uncertain eligibility. Any discrepancies in eligibility judgements were resolved by discussion between the authors.
Study data from each trial were extracted independently by two authors with any disagreements in data extraction resolved by discussion between the authors. The authors of included studies were contacted by e-mail and asked to share unpublished data from their trial and to clarify any details about their trial that were missing or unclear in the published reports.
We assessed the risk of bias in the included studies using the standard Cochrane Collaboration methods for randomised trials (Higgins et al. 2011). Selection bias (random sequence generation, allocation concealment) and reporting bias (selective reporting) were assessed at study level, whereas detection bias (blinding of outcome assessment) and attrition bias (incomplete outcome data) were assessed at outcome level. We did not assess detection bias for the outcome of survival because this in an objective outcome, and we also did not assess performance bias because blinding of either healthcare personnel or patients is not possible with the interventions under consideration in this review.
The study data were meta-analysed where possible. The meta-analysis of time-to-event outcomes in Review Manager 5.3 (Nordic Cochrane Centre 2014) uses ‘O-E’ and ‘V’ statistics or hazard ratios (HR) for each trial. If these were not reported in a given trial we calculated them from the available statistics, if possible, using the methods described in Tierney (Tierney et al. 2007). Heterogeneity in meta-analyses was assessed using the I2 statistic. If the I2 value was >50 % we did not pool the effect estimates but used the range of effects from the individual studies instead. Time-to-event outcomes, entered as ‘O–E and Variance’ outcomes, were statistically synthesised using a fixed-effect model and arranged so that HRs > 1 favoured the ALND group and HRs < 1 favoured the comparison group. Dichotomous outcomes were summarised as risk ratios (RR) and analysed using a fixed-effects model according to the Mantel–Haenszel method and arranged so that RRs < 1 favoured the ALND group and RRs > 1 favoured the comparison group. All analyses were conducted in Review Manager 5.3 (Nordic Cochrane Centre 2014). We included only the data available in trial reports or through contact with the trial authors. No data imputation was attempted.
Results
The search identified 7436 unique records, of which 7273 were excluded based on the title and abstract while the full publications of 163 potentially relevant studies were examined. Of these, 5 trials reported in 13 publications met the inclusion criteria, two studies were still ongoing (comparing ALND to SLNB [NCT01796444 (Wang 2013), and ALND or axillary radiotherapy [aRT] + adjuvant treatment versus adjuvant treatment alone [POSNOC (Goyal 2014a, b)], respectively) while the remaining 147 records were excluded because they were: not a randomised trial (n = 20), ineligible population (n = 101), unclear intervention (n = 2) and ineligible intervention (n = 24); See also Additional file 2). The five included studies compared ALND with sentinel lymph node dissection (SLND) to SLND alone [ACOSOG Z0011 (Lucci et al. 2007; Olsen and McCall 2008; Giuliano et al. 2010, 2011); ATTRM-048-13-2000 (Sola et al. 2013); IBCSG-23-01 (Galimberti et al. 2011, 2012, 2013)], and ALND to aRT [AMAROS (Straver et al. 2010a, b; Donker et al. 2014); OTOASOR (Savolt et al. 2013a, b)]. See Tables 1 and 2 for summary study details and risk-of-bias levels, respectively, and Additional file 3 for full study details and risk-of-bias assessments.
Table 1.
Characteristics of the included studies
| ATTRM-048-13-2000 | IBCSG-23-01 | ACOSOG Z0011 | AMAROS | OTOASOR | |
|---|---|---|---|---|---|
| Study years | 2001-2008 | 2001-2010 | 1999-2004 | 2001-2010 | 2002-2009 |
| Comparison | SLND + ALND v SLND | SLND + ALND v SLND | SLND + ALND v SLND | ALND v aRT | ALND v aRT |
| ALND intervention | Breast conservation therapy or mastectomy + SLND + ”complete ALND” but not otherwise specified | Surgical resection of primary tumour + SLND + ALND not otherwise specified | Breast conserving surgery + SLND + ALND consisting of removal of all level I and II nodes on affected side with at least 10 identified nodes per surgical specimen | Breast-conserving treatment (including whole-breast radiotherapy or mastectomy with/without radiotherapy to the chest wall) + ALND (level I and II; at least 10 nodes) | Breast-conserving surgery or mastectomy + ALND (level I and II; at least 6 nodes) |
| Comparison intervention | Breast conservation therapy or mastectomy + SLND | Surgical resection of primary tumour + SLND | Breast conserving surgery + SLND: After the blue or hot nodes were removed any remaining axillary nodes were palpated and removed as SLNs if suggestive of disease | Breast-conserving treatment (including whole-breast radiotherapy or mastectomy with/without radiotherapy to the chest wall) + aRT, including the contents of all three levels of the axilla and the medial part of the supraclavicular fossa; 25 fractions of 2 Gy | Breast-conserving surgery or mastectomy + aRT including the contents of all three levels of the axilla and the supraclavicular fossa; 25 fractions of 2 Gy |
| Country | Spain | Europe, South America, Australia | USA | Europe | Hungary |
| Number ALND | 112 | 464 | 436 | 744 | 244 |
| Number Comparison | 121 | 467 | 420 | 681 | 230 |
| Age ALDN (years) | Mean = 55.3 (range 29–75) | Median = 53 (range 28–81) | Median = 56 (range 24–92) | Median = 56 (IQR 48–64) | Mean = 54.7 (range 26–74) |
| Age comparison (years) | Mean = 53.2 (range 33–75) | Median = 54 (range 26–81) | Median = 54 (range 25–90) | Median = 55 (IQR 48–63) | Mean = 55.2 (range 27–74) |
| Follow up | Median = 5.17 (range 2–9.17) years | Median = 5 (IQR 3.6–7.3) years | Median = 6.3 (IQR 5.2–7.7) years | Median = 6.1 (IQR 4.1–8) years | Mean = 41.9–42.3 months |
| Radiotherapy | Total breast (not axillary) both arms | Intraoperative with/without conventional post-operative RT: SLND alone: N = 410; ALND: N = 413 | Whole breast RT. Some patients also received RT to the supraclavicular area (total N = 89). | Adjuvant aRT after ALND when ≥ 4 positive nodes were found. Adjuvant RT received to breast/chest wall/internal mammary chain: aRT: N = 546/51/65; ALND: N = 597/34/72. | ALND: Postoperative RT to the regional nodes when ≥ 4 positive nodes (pN2a-3a) or 1-3 positive nodes (pN1a) with other high-risk characteristics. 232 patients received RT to the breast/chest wall, 76 patients received RT to the axillary/supraclavicular nodes. aRT: 208 patients received RT to the breast/chest wall, 230 patients received RT to the axillary/supraclavicular nodes. |
| Chemo-therapy | 41 ALND; 42 SLND alone | 33 SLND alone; 42 ALND | 243 ALND; 253 SLND alone | Yes according to local GLs | 190 ALND; 159 aRT |
| Hormone therapy | 10 ALND; 7 SLND alone | 315 Surgery alone; 292 ALND | 195 ALND; 203 SLND alone | Yes according to local GLs | 213 ALND; 204 aRT |
| Both chemo- and hormone therapy | 51 ALND; 65 SLND alone | 103 Surgery alone; 107 ALND | Not reported | Not reported | 159 ALND; 133 aRT |
| Baseline differences | Detection by palpation more in ALND | Appear comparable | Appear comparable | Appear comparable | More pT2-3 tumours in ALND |
| Intention-to-treat analyses | 2 SLND alone and 4 ALND patients lost to follow up and not included in analyses | Yes for survival and disease-free survival. For the long term adverse events data were analysed per protocol | Yes for overall survival and recurrence | Yes | Unclear |
| Notes | 14/247 randomised patients dropped out | Non-inferiority trial; closed early | Closed early | Non-inferiority trial | Equivalence trial |
SLND sentinel lymph node dissection, ALND axillary lymph node dissection, aRT axillary radiotherapy, RT radiotherapy, IQR inter-quartile range
Table 2.
Risk of bias in the included studies
| ATTRM-048-13-2000 | IBCSG-23-01 | ACOSOG Z0011 | AMAROS | OTOASOR | |
|---|---|---|---|---|---|
| Random sequence generation (selection bias) | Unclear | Low | Low | Low | Unclear |
| Allocation concealment (selection bias) | Unclear | Low | Low | Low | Unclear |
| Blinding of outcome assessment (detection bias): Disease control in the axilla | Unclear | High—no blinding | Unclear | High—no blinding | Unclear |
| Blinding of outcome assessment (detection bias): Breast cancer recurrence | Unclear | High—no blinding | Unclear | High—no blinding | Unclear |
| Blinding of outcome assessment (detection bias): Short term adverse events | Outcome not reported | High—no blinding | Unclear | Outcome not reported | Unclear |
| Blinding of outcome assessment (detection bias): Long term adverse events | Outcome not reported | High—no blinding | Unclear | High—no blinding | Unclear |
| Incomplete outcome data (attrition bias): Survival | Low | Low | Low | Low | Low |
| Incomplete outcome data (attrition bias): Disease control in the axilla | Low | Low | Low | Low | Unclear—data not reported in sufficient detail to be able to ascertain whether all patients are included |
| Incomplete outcome data (attrition bias): Breast cancer recurrence | Low | Low | Low | Low | Low |
| Incomplete outcome data (attrition bias): Short term adverse events | Outcome not reported | Unclear—denominator not reported | Unclear—data reported at 30 days for 371/411 ALND and 373/399 SLND + ALND | Outcome not reported | Outcome not reported |
| Incomplete outcome data (attrition bias): Long term complications | Outcome not reported | Unclear—14 patients allocated to surgery alone received ALND and 17 patients allocated to ALND did not receive ALND; all excluded from the analyses | High—data missing from progressively larger proportions of patients as follow up progressed; possibly more pronounced in the SLND group. Outcome data reported at 1 year for 242/411 ALND and 226/399 SLND +ALND | High—data available from 655/744 ALND and 586/681 aRT patients at baseline; progressively higher rates of missing data at 1, 3 and 5 years for lympoedema. Unclear how much data were available for shoulder mobility | Outcome not reported |
| Selective reporting (reporting bias) | High—adverse events not reported | Low | Low | High—only lymphedema and should mobility reported as morbidity outcomes | High—morbidity not reported |
SLND sentinel lymph node dissection, ALND axillary lymph node dissection, aRT axillary radiotherapy
ALND with SLND versus SLND
Figure 1 shows that neither overall survival (summary HR = 0.82, 95 % CI 0.58–1.15; p = 0.25; I2 = 0 %) nor disease-free survival (summary HR = 0.81, 95 % CI 0.63–1.04; p = 0.1; I2 = 0 %) differed between the SLND + ALND and SLND treatment groups overall or in any of the trials.
Fig. 1.
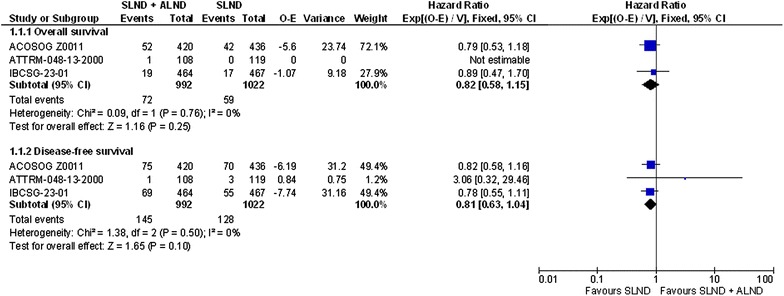
Overall survival and disease-free survival in the studies comparing SLND + ALND to SLND alone
Meta-analysis of breast cancer recurrence as a dichotomous outcome, rather than as a time-to-event outcome, was undertaken as the data were not reported as time-to-event outcomes. However, the length of follow-up for these data was comparable between the trials (see Table 1). These analyses are illustrated in Fig. 2, which shows that axillary (summary RR = 0.46, 95 % CI 0.14–1.49; p = 0.2; I2 = 0 %), local (summary RR = 1.6, 95 % CI 0.86–2.97; p = 0.14; I2 = 0 %), regional (summary RR = 0.34, 95 % CI 0.1–1.15; p = 0.08; I2 = 0 %) and distant breast cancer recurrence (summary RR = 1.31, 95 % CI 0.8–2.15; p = 0.28; I2 = 0 %) did not differ between the treatment groups.
Fig. 2.
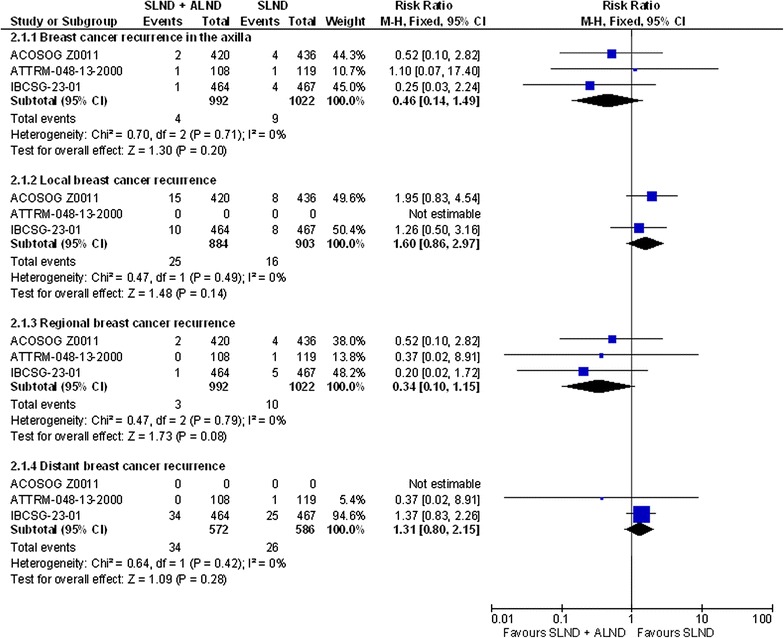
Breast cancer recurrence in the studies comparing SLND + ALND to SLND alone. Please note the following regarding the data included for ACOSOG ZOO11 for regional breast cancer recurrence: Regional recurrence defined as recurrence in the axillary, supraclavicular or internal mammary nodes. The authors only report local recurrence, axillary recurrence and locoregional recurrence. We have subtracted the local recurrence data from locoregional recurrence data to obtain the regional recurrence data, which is equal to the disease recurrence in the axilla data, suggesting that no patients recurred in the supraclavicular or internal mammary nodes, provided all these data only count each patient once. An entry of 0 in the total number of events column signifies that the study did not report this outcome
The ATTRM-048-13-2000 trial did not report on short-term adverse events or long term complications (Table 3) and is therefore at high risk of reporting bias for these outcomes. Inadequate details were reported on the selection of patients and the outcome assessment, which puts the results at risk of both patient selection bias and detection bias (Table 2). Moreover, at baseline the tumours were detected by palpation in more ALND than SLND patients. In IBCSG-23-01 the authors did not report inferential analyses of the short-term adverse events or long-term complications, but the rates of post-operative infection, sensory neuropathy (any, grade 3–4), lymphoedema (any, grade 3–4) and motor neuropathy were all numerically higher in the ALND group (Table 3). No blinding was undertaken of the outcome assessment, which means that the results are at high risk of detection bias. Moreover, it was unclear whether the results were subject to attrition bias for short-term adverse events and long-term complications (Table 2).
Table 3.
Morbidity outcomes in the included studies
| Comparison | SLND + ALND versus SLND alone | ALND versus aRT | |||
|---|---|---|---|---|---|
| Study | ATTRM-048-13-2000 | IBCSG-23-01 | ACOSOG Z0011 | AMAROS | OTOASOR |
| Short-term adverse events | Not reported |
Post-operative infection: ALND: 1/464 SLND: 0/467 |
Wound infection: ALND: 31/373 SLND: 11/371; Axillary seromas: ALND: 53/373 SLND: 21/371; Axillary paresthesias: ALND: 174/373 SLND: 43/371; Objective lymphodema b: ALND: 23/255 SLND: 17/272 |
Not reported | Not reported |
| Long-term complications | Not reported |
Sensory neuropathy
a: Any: ALND: 82/447 SLND: 55/453 Grade 3–4: ALND: 1/447 SLND: 0/453 Lymphoedema a: Any: ALND: 59/447 SLND: 15/453 Grade 3–4: ALND: 3/447 SLND: 0/453 Motor neuropathy a: Any: ALND: 37/447 SLND: 13/453 Grade 3–4: ALND: 3/447 SLND: 1/453 |
Brachial plexus injury: 6 months: ALND: 5/406; SLND: 3/415; 12 months: ALND: 1/406; SLND: 0/415; Axillary paresthesias: 6 months: ALND: 146/335; SLND: 35/288; 12 months: ALND: 113/287; SLND: 24/268; Objective lymphoedema b: 6 months: ALND: 29/270; SLND: 21/271; 12 months: ALND: 26/242; SLND: 14/226; Subjective lymphodema c: 6 months: ALND: 27/327; SLND: 19/339; 12 months: ALND: 37/288; SLND: 12/268; >12 months: ALND: 52/272; SLND: 14/253 |
Sign of lymphoedema
d: Baseline: ALND: 3/655; aRT: 0/586, p = 0.25; 12 months: ALND: 114/410; aRT: 62/410, p < 0.0001; 3 years: ALND: 84/373; aRT: 47/341, p = 0.003; 5 years: ALND: 76/328; aRT: 31/286, p < 0.0001; Arm circumference increase ≥ 10 % e: Baseline: ALND: 33/655; aRT: 24/586, p = 0.5; 12 months: ALND: 32/410; aRT: 24/410, p = 0.332; 3 years: ALND: 38/373; aRT: 22/341, p = 0.08; 5 years: ALND: 43/328; aRT: 16/286, p = 0.0009; Shoulder mobility f: No differences found in the range of motion in the four excursions at 1 (p = 0.29) or 5 years (p = 0.47). |
Not reported |
| Quality of life | Not reported | Not reported | Not reported | No differences foundg | Not reported |
ALND axillary lymph node dissection, SLND sentinel lymph node dissection, aRT axillary radiotherapy
aThe treating physician assessed and reported long-term surgical events (sensory neuropathy, lymphoedema, and motor neuropathy) at every follow-up visit (every 4 months from the date of randomisation for the first year, and every 6 months for years 2–5) on the basis of the National Cancer Institute Common Toxicity Criteria version 2. No more information reported
bLympheoedema (objective): 2 cm or greater post-operative increase in the ipsilateral arm circumference (assessed by phycisian)
cLympheoedema (subjective): according to patient self-report or physician diagnosis
dAny clinical sign of lymphoedema
eArm circumference was measure 15 cm above the medial epicondyle (upper arms) and 15 cm below the medial epicondyle (lower arms). An increase in arm circumference of at least 10 % in the lower arm or the upper arm, or both, compared with the contralateral arm at the same timepoint was judged to be clinically significant lymphoedema
fThe range of motion in both arms was measured in four excursions: abduction, adduction, anteversion, and retroversion and compared between arms. The four relative excursions were combined in a multivariate composite endpoint at 1 and 5 years
gAssessed using the EORTC quality-of-life questionnaire (EORTC-QLQ-C30; version 3) and breast cancer module (QLQ-BR23) using the pain, body image, and arm symptoms scales. The arm symptoms scale was composed of three items: pain in arm or shoulder, swollen arm or hand, and difficulties moving arm. Questionnaires were completed at baseline and at years 1, 2, 3, 5, and 10. All outcome data at 10 years subject to a future report
Inferential analyses of the rates of short-term adverse events were not presented in the ACOSOG Z0011 trial, but the rates of wound infection, axillary seromas, axillary paresthesias and objective lymphoma are all numerically higher in the group that received ALND. The same pattern of results was also observed for the long-term complications of brachial plexus injury, axillary paresthesias, and objective and subjective lymphoma at 6 and 12 months; and for subjective lymphoma at >12 months (Table 3). In this trial it was unclear whether outcome assessment was blinded, 30-day short-term adverse event data were not reported for all the patients, and the outcome data for long-term complications were missing for progressively larger proportions of patients in both treatment groups, but possibly more so for the SLND group. This in turn means that the results must be interpreted with some caution because they are at risk of detection bias for all outcomes and of attrition bias for the short-term adverse events outcome; and for the long-term complications the results are at high risk of attrition bias (Table 2). Moreover, all three trials randomised patients after the results of SLND were known, which puts these trials at risk of recruitment bias to the extent that patients perceived at higher risk (e.g., multiple micrometastatic foci) were not invited or chose not to take part in the studies. This is because any tendency not to recruit patients perceived to be at higher risk would influence the relative performance of the interventions in the direction that less extensive surgery (SLND) would appear relatively more beneficial because the patients who are more likely to benefit from more extensive surgery (ALND), that is, patients at higher risk, would not be part of the study population. This could mean that the results are only applicable to the patients seen in clinical practice who meet the inclusion criteria of these trials, but are also perceived to be at low risk.
ALND versus aRT
In the AMAROS trial no differences in overall survival, disease-free survival, shoulder mobility or quality of life were observed between the groups that received ALND and aRT (Tables 3, 4). However, the rates of (any clinical sign of) lymphoedema were higher in the ALND group at 1, 3 and 5 years. When lymphoedema was defined as an arm circumference increase ≥10 %, the rates only differed significantly at 5 years (Table 3). The trial was open label and did not report short-term adverse events or long-term complications other than lymphoedema and shoulder mobility for which either progressively larger or unclear proportions of data were missing, respectively. The results are therefore at high risk of both detection bias (all outcomes), attrition bias (lymphoedema and shoulder mobility) and reporting bias (short-term adverse events and long term complications; Table 2).
Table 4.
Overall survival, disease-free survival and breast cancer recurrence in the axilla in the studies comparing ALND and aRT
| Overall survival | Disease-free survival | Breast cancer recurrence in the axilla | ||
|---|---|---|---|---|
| AMAROS | ALND: 71/744 aRT: 76/681; 5-year: ALND: 93.3 % (95 % CI 91–95); aRT: 92.5 % (95 % CI 90–94.4); HR = 1.17 (95 % CI 0.85–1.62), p = 0.34 |
ALND: 124/744 aRT: 134/681; 5-year: ALND: 86.9 % (95 % CI 84.1–89.3); aRT: 82.7 % (95 % CI 79.3–85.5); HR = 1.18 (95 % CI 0.93–1.51), p = 0.18 |
ALND: 4/744 aRT: 7/681; 5-year: ALND: 0.43 % (95 % CI 0–0.92); aRT: 1.19 % (95 % CI 0.31–2.08) |
None of the studies reported local, regional, locoregional or distant breast cancer recurrence |
| OTOASOR | No significant difference, although no overall rates reported | ALND: 94.3 % aRT: 97 %; non-significant |
ALND: 0.82 % aRT: 1.3 %; non-significant |
The relative effects of treatments on time-to-event outcomes were reported so that HRs less than 1.0 favour the aRT arm and HRs greater than 1.0 favour the ALND arm
ALND axillary lymph node dissection, aRT axillary radiotherapy
The OTOASOR trial did also not find any significant differences between the treatment groups in overall survival, disease-free survival or axillary recurrence rates (Table 4), however, the OTOASOR trial did not report any morbidity outcomes, which puts the trial at risk of reporting bias. Moreover, very little information was reported about patient selection and allocation as well as about potential blinding of outcome assessment, which exposes the results to risk of both selection bias (all outcomes) and detection bias (all outcomes) to the extent that these were compromised (Table 2). At baseline, however, more ALND than aRT patients had pT2-3 tumours. On the other hand, both trials randomised patients before sentinel lymph node biopsy, which suggests that the study populations are representative of the risk spectrum of those patients seen in clinical practice that meet the inclusion criteria of these trials.
Discussion
The evidence for ALND compared to other less invasive strategies for axillary treatment consisted of 5 studies including 3919 patients and reporting on 2 different comparisons: ALND versus aRT and SLND + ALND versus SLND. None of the included trials found a difference between the ALND groups and their respective comparison group in overall survival, disease-free survival or breast cancer recurrence. Two of the studies (IBCSG-23-01, ACOSOG Z0011) reported short-term adverse events and found that the rates were numerically higher in the ALND groups than in their respective comparison groups (neither study reported inferential analyses of these rates). Three of the studies (IBCSG-23-01, ACOSOG Z0011, AMAROS) reported long-term complications and found that lymphoedema tended to be higher in the ALND arms, either statistically significantly (AMAROS) or numerically (IBCSG-23-01, ACOSOG Z0011). Moreover, the rates of sensory neuropathy (IBCSG-23-01), motor neuropathy (IBCSG-23-01), brachial plexus injury (ACOSOG Z0011), and axillary paresthesias (ACOSOG Z0011) were also numerically higher in the ALND groups, although these results were also not analysed inferentially. Shoulder mobility and quality of life were not found to differ significantly between the treatment groups in the only study reporting these outcomes (AMAROS). These results were, however, subject to varying risks of a number of biases, not least detection bias, attrition bias, reporting bias and recruitment bias.
Although blinding of the patients and personnel is conceivably not feasible in the types of trials included in this review, blinding of outcome assessment may be undertaken in an effort to minimise the risk of detection bias. This bias is more likely to be at play for outcomes that are more subjective in evaluation rather than objective (e.g., survival). We therefore only considered this bias for breast cancer recurrence and morbidity. Detection bias in this context may lead to an overestimation of short-term adverse events and long-term complications in patients who received ALND. Similarly patients receiving less extensive axillary treatment may have been checked more carefully for breast cancer recurrence, because in both cases, the expected results would be of more morbidity in ALND and more recurrences in the less extensively treated axilla. The risk of attrition bias was particularly associated with the morbidity outcomes. This tended to be because these outcomes were assessed in a subset of the trial population. This subgroup of patients assessed for adverse events could be systematically different from the trial population as a whole, especially in the case of assessment for long-term complications when patients may have died or been too sick to participate. The morbidity outcomes were also at risk of reporting bias in the two studies that did not report morbidity at all (ATTRM, OTOASOR), while a third study only reported lymphoedema and shoulder mobility. Given the finding that none of the studies found any differences in overall or disease-free survival or in breast cancer recurrence, the assessment of treatment-associated morbidity arguably becomes less important when considering which treatment strategy to choose because it may be safe to assume that treatment-related morbidity will be less in less extensive treatments compared to ALND. However, it would still be preferable to be able to confirm the veracity of this assumption by being able to test it though appropriate analyses. Finally, we were unable to evaluate the risk of patient selection in two studies (ATTRM, OTOASOS) because not enough information was reported, which is of some concern because patient selection bias is a powerful bias that can affect the results markedly. Taken together, the risk of the different biases discussed above serves to compromise the validity of the results to the extent that they are at play and this must be borne in mind when considering the results.
Ram and colleagues (Ram et al. 2014) conducted a systematic review on SNLD alone versus SLND + ALND and included the same three RCTs included in the current review for that comparison, and unsurprisingly their conclusions are similar to ours for this comparison. Systematic reviews by Glechner et al. (2013) and Li et al. (2015), which included both RCTs and retrospective studies, also found no differences in overall survival, disease-free survival and recurrence between SLND alone compared to SLND + ALND, and higher rates of some adverse events associated with SLND + ALND, although Glechner et al. (2013) noted that a number of these results are subject to low event rates and/or the potential influence of different confounding variables and must be interpreted with caution. Moreover Li et al. (2015) treated ACOSOG ZOO11 as three RCTs, rather than one, which is potentially confusing and certainly at high risk of giving an inflated impression of the amount of evidence available. These reviews did not consider ALND versus aRT in patients with node-positive operable breast cancer, and we have found no other systematic reviews on that comparison either.
Implications for practice
The studies described above have resulted in changes in guidelines and practice in some countries with implementation of the findings of the ACOSOG Z0011 study in patients who specifically meet the entrance criteria in many centres in the USA. This may be partly due to the inclusion of a significant number of patients with micrometastases in ACOSOG Z011 which are not now regarded as an indication for ALND or radiotherapy. Similarly there is increased use of axillary radiotherapy as an alternative to ALND following positive SLND in several European centres. However for the reasons identified above and in the following section these studies have not resulted in universal changes in practice and further data are required to confirm these results. Whilst it is encouraging that there appears to be no adverse effect on local, regional or distant recurrence or overall survival further evidence, particularly in those settings where evidence is lacking (e.g. mastectomy) will be appropriate to confirm these findings. At present many clinicians and multidisciplinary teams are interpreting and implementing these findings on a case by case basis in patients that strictly comply with the inclusion criteria of the relevant studies and whilst it is hoped this review will provide useful confirmation of the appropriateness of this practice, the provision of further data will be valuable not least in providing additional data confirming quality of life effects and long term outcomes.
Implications for research
In this review, we only found 3 and 2 studies, respectively, evaluating each of the two target comparisons. Moreover, not all of these studies reported all the target outcomes. Quality-of-life was for example only reported by one of the included trials. We did however identify two ongoing trials that with time will contribute further data to this area (NCT01796444, POSNOC). These trials notwithstanding, the evidence base cannot be considered complete at this stage, and it would be preferable to see further well-designed and adequately powered studies conducted confirming the current results. Furthermore the increased stratification of the treatment of the axilla is being explored in further studies in both ‘low risk’ patients (e.g. no axillary staging) and ‘high risk’ patients (e.g. ALND followed by radiotherapy). It appears that research in the area of axillary node management in breast cancer will continue to drive the evolution from a ‘one size fits all’ approach to a more personalised evidence-based approach in future.
Authors’ contributions
MSH and NB conceived the idea for the review, designed the study, screened the literature searches, and extracted, appraised and interpreted the data. MSH drafted the first version of the article. EH designed and carried out the literature searches. MR interpreted the results, wrote the Introduction and the Implications for Practice and Research sections. All authors read and approved the final manuscript.
Acknowledgements
We would like to thank Dr Laszlo Igali, Consultant Histopathologist, Norfolk and Norwich University Hospital, United Kingdom, for help with the translation of a trial published in Hungarian.
Competing interests
MR is a co-investigator of POSNOC. Otherwise, the authors declare they have no competing interest.
Ethical standards
The research reported in this manuscript comply with the current laws of the UK, but were not subject to review by an ethics committee as only data from already published studies were used.
Funding
The work reported in this article was not subject to any funding.
Additional files
10.1186/s40064-016-1712-9 Full search strategies.
10.1186/s40064-016-1712-9 PRISMA diagram detailing the search results.
10.1186/s40064-016-1712-9 Full study characteristics of the included and ongoing studies.
Contributor Information
Mia Schmidt-Hansen, Phone: +44 2920 402910, Email: Mia.Schmidt-Hansen@wales.nhs.uk.
Nathan Bromham, Email: Nathan.Bromham@wales.nhs.uk.
Elise Hasler, Email: Elise.Hasler@wales.nhs.uk.
Malcolm W. Reed, Email: m.reed@bsms.ac.uk
References
- Donker M, Tienhoven G, Straver ME, Meijnen P, Velde CJH, Mansel RE, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014;15:1303–1310. doi: 10.1016/S1470-2045(14)70460-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galimberti V, Chifu C, Rodriguez Perez S, Veronisi P, Intra M, Botteri E, Mastropasqua M, Colleoni M, Luini A, Veronisi U. Positive axillary sentinel lymph node: is axillary dissection always necessary? Breast. 2011;20(Supplement 3):S96–S98. doi: 10.1016/S0960-9776(11)70303-4. [DOI] [PubMed] [Google Scholar]
- Galimberti V, Cole BF, Zurrida S, Viale G, Luini A, Veronesi P, Baratella P, Chifu C, Sargenti M, Intra M, Gentilini O, Massarut S, Garbay JR, Zgajnar J, Galatius H, Recalcati A, Littlejohn D, Bamert M, Price KN, Goldhirsch A, Gelber RD, Veronesi U. S3-1: Update of International Breast Cancer Study Group Trial 23-01 to compare axillary dissection versus no axillary dissection in patients with clinically node negative breast cancer and micrometastases in the sentinel node. Cancer Res. 2012;71(224, Supplement):S3–1. [Google Scholar]
- Galimberti V, Cole BF, Zurrida S, Viale G, Luini A, Veronesi P, Baratella P, Chifu C, Sargenti M, Intra M, Gentilini O, Mastropasqua MG, Mazzarol G, Massarut S, Garbay JR, Zgajnar J, Galatius H, Recalcati A, Littlejohn D, Bamert M, Colleoni M, Price KN, Regan MM, Goldhirsch A, Coates AS, Gelber RD, Veronesi U, International Breast Cancer Study Group Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): a phase 3 randomised controlled trial. Lancet Oncol. 2013;14(4):297–305. doi: 10.1016/S1470-2045(13)70035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano AE, McCall L, Beitsch P, Whitworth PW, Blumencranz P, Leitch AM, Saha S, Hunt KK, Morrow M, Ballman K. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg. 2010;252:426–432. doi: 10.1097/SLA.0b013e3181f08f32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano AE, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW, Blumencranz PW, Leitch AM, Saha S, McCall LM, Morrow M. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305:569–575. doi: 10.1001/jama.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glechner A, Wockel A, Gartlehner G, Thaler K, Strobelberger M, Griebler U, Kreienberg R. Sentinel lymph node dissection only versus complete axillary lymph node dissection in early invasive breast cancer: a systematic review and meta-analysis. Eur J Cancer. 2013;49(4):812–825. doi: 10.1016/j.ejca.2012.09.010. [DOI] [PubMed] [Google Scholar]
- Goyal A (2014a) POSNOC—a randomised trial of armpit (axilla) treatment for women with early stage breast cancer (ISRCTN54765244). www.isrctn.com/ISRCTN54765244. Accessed on 14 July 2015
- Goyal A (2014b) POSNOC—POsitive Sentinel NOde: adjuvant therapy alone versus adjuvant therapy plus Clearance or axillary radiotherapy: a randomised controlled trial of axillary treatment in women with early stage breast cancer who have metastases in one or two sentinel nodes (Project record). Health Technology Assessment Database [DOI] [PMC free article] [PubMed]
- Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre C, Clarke M. Identifying randomised trials. In: Egger M, Davey Smith G, Altman DG, editors. Systematic reviews in health care: meta-analysis in context. London: BMJ Publishing Group; 2001. pp. 69–86. [Google Scholar]
- Lefebvre C, Manheimer E, Glanville J. Searching for studies. In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.1.0. Oxford: Cochrane Collaboration; 2011. pp. 95–150. [Google Scholar]
- Li CZ, Zhang P, Li RW, Wu CT, Zhang XP, Zhu HC. Axillary lymph node dissection versus sentinel lymph node biopsy alone for early breast cancer with sentinel node metastasis: a meta-analysis. Eur J Surg Oncol. 2015;41:958–966. doi: 10.1016/j.ejso.2015.05.007. [DOI] [PubMed] [Google Scholar]
- Lucci A, McCall LM, Beitsch PD, Whitworth PW, Reintgen DS, Blumencranz PW, et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol. 2007;25(24):3657–3663. doi: 10.1200/JCO.2006.07.4062. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICE (2009) Early and locally advanced breast cancer: Diagnosis and treatment. https://www.nice.org.uk/guidance/cg80. Accessed on 6/10/2015
- Nordic Cochrane Centre . Review manager (RevMan) Copenhagen: The Cochrane Collaboration; 2014. [Google Scholar]
- Olson JA, Jr, McCall LM. Impact of immediate versus delayed axillary node dissection on surgical outcomes in breast cancer patients with positive sentinel nodes: results from American College of Surgeons Oncology Group trials Z0010 and Z0011. J Clin Oncol. 2008;26(21):3530–3535. doi: 10.1200/JCO.2007.15.5630. [DOI] [PubMed] [Google Scholar]
- Ram R, Singh J, McCaig E. Sentinel node biopsy alone versus completion axillary node dissection in node positive breast cancer: systematic review and meta-analysis. Int J Breast Cancer. 2014 doi: 10.1155/2014/513780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savolt A, Musonda P, Matrai Z, Polgar C, Renyi-Vamos F, Rubovszky G, et al. Optimal treatment of the axilla after positive sentinel lymph node biopsy in early invasive breast cancer. Early results of the OTOASOR trial. Orv Hetil. 2013;154:1934–1942. doi: 10.1556/OH.2013.29765. [DOI] [PubMed] [Google Scholar]
- Savolt A, Polgar C, Musonda P, Matrai Z, Renyi-Vamos F, Toth L, et al. Does the result of completion axillary lymph node dissection influence the recommendation for adjuvant treatment in sentinel lymph node-positive patients? Clin Breast Cancer. 2013;13:364–370. doi: 10.1016/j.clbc.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Sola MS, Alberro JA, Fraile M, Santesteban P, Ramos M, Fabregas R, Moral A, Ballester B, Vidal S. Complete axillary lymph node dissection versus clinical follow-up in breast cancer patients with sentinel node micrometastasis: final results from the multicenter clinical trial AATRM 048/13/2000. Ann Surg Oncol. 2013;20:120–127. doi: 10.1245/s10434-012-2569-y. [DOI] [PubMed] [Google Scholar]
- Straver ME, Meijnen P, van Tienhoven G, van de Velde CJ, Mansel RE, Bogaerts J, Duez N, Cataliotti L, Klinkenbijl JH, Westenberg HA, van der Mijle H, Snoj M, Hurkmans C, Rutgers EJ. Sentinel node identification rate and nodal involvement in the EORTC 10981-22023 AMAROS trial. Ann Surg Oncol. 2010;17:1854–1861. doi: 10.1245/s10434-010-0945-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straver ME, Meijnen P, van Tienhoven G, van de Velde CJ, Mansel RE, Bogaerts J, et al. Role of axillary clearance after a tumor-positive sentinel node in the administration of adjuvant therapy in early breast cancer. J Clin Oncol. 2010;28(5):731–737. doi: 10.1200/JCO.2008.21.7554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8(16):1–16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y (2013) Axillary lymph node dissection versus no dissection in breast cancer with positive sentinel lymph node (Z0011-China). https://clinicaltrials.gov/ct2/show/NCT01796444. Accessed 14 July 2015


