Abstract
P-glycoprotein (P-gp) is encoded by the multidrug resistance (MDR1) gene and is well studied as a multi-drug resistance transporter. Peritoneal adhesion formation following abdominal surgery remains an important clinical problem. Here, we found that P-gp was highly expressed in human adhesion fibroblasts and promoted peritoneal adhesion formation in a rodent model. Knockdown of P-gp expression by intraperitoneal injection of MDR1-targeted siRNA significantly reduced both the peritoneal adhesion development rate and adhesion grades. Additionally, we found that operative injury up-regulated P-gp expression in peritoneal fibroblasts through the TGF-β1/Smad signaling pathway and histone H3 acetylation. The overexpression of P-gp accelerated migration and proliferation of fibroblasts via volume-activated Cl- current and cell volume regulation by enhancing phosphorylation of the chloride channel-3. Therefore, P-gp plays a critical role in postoperative peritoneal adhesion formation and may be a valuable therapeutic target for preventing the formation of peritoneal adhesions.
Keywords: P-glycoprotein, Peritoneal Adhesions, Chloride Channel-3
Introduction
Intra-abdominal peritoneal adhesions continue to be a significant cause of long-term complications related to abdominal surgery. Peritoneal adhesions develop after 93-100% of upper abdominal laparotomies and after 67-93% of lower abdominal laparotomies1. They may lead to serious complications such as intestinal obstructions, infertility, chronic abdominal and pelvic pain, as well as difficult reoperations2. Current prevention is based on careful surgical techniques and the occasional use of physical barriers, but there is no substantial evidence that their use improves fertility, decreases pain, or reduces the incidence of postoperative bowel obstruction3.
Peritoneal adhesions are the consequence of tissue trauma that may result from sharp, mechanical, or thermal injury during abdominal surgery. The development of adhesions involves the migration, proliferation, and/or differentiation of several cell types, including inflammatory, immune, mesothelial, and fibroblast cells4. Several studies have shown that fibroblasts from the injured peritoneum may have a crucial role in the formation of adhesion tissues4-7. Genes encoding proteins involved in cell adhesion, proliferation, migration, and protein/vesicle trafficking are differentially expressed between normal and adhesion fibroblasts8. Taken together, these results suggest that identification of the proteins produced in fibroblasts that are critical to the attachment of injured peritoneum and the development of fibrous tissue may provide novel targets for preventing peritoneal adhesion formation.
P-glycoprotein (P-gp), the product of the multidrug-resistance gene (MDR1), is a membrane-associated active transport protein that pumps cytotoxic drugs out of cells. P-gp is widely expressed in a variety of cells and limits absorption of toxins into tissues. It is well known that expression of P-gp results in increased efflux of drugs, which is the major cause of tumor cell resistance to chemotherapy9. P-gp is also a regulator of volume-activated Cl- channel10. Directed mutations in the nucleotide-binding domains of the protein has shown that the transport and channel functions of P-gp are separate11. The volume-activated Cl- channel has been found to be involved in cell volume regulation, cell proliferation, cell migration, apoptosis, and cell differentiation12. However, the physiological and pathological implications of P-gp regulating the volume-activated Cl- channel are still unclear.
Here we demonstrate a new key role of P-gp in postoperative peritoneal adhesion formation. We show how peritoneal injury induces up-regulation of P-gp expression in peritoneal fibroblasts. We also describe the underlying mechanism by which overexpression of P-gp promotes the formation of peritoneal adhesions.
Results
Up-regulation of P-gp Protein in Human and Rat Fibroblasts from Peritoneum Adhesions
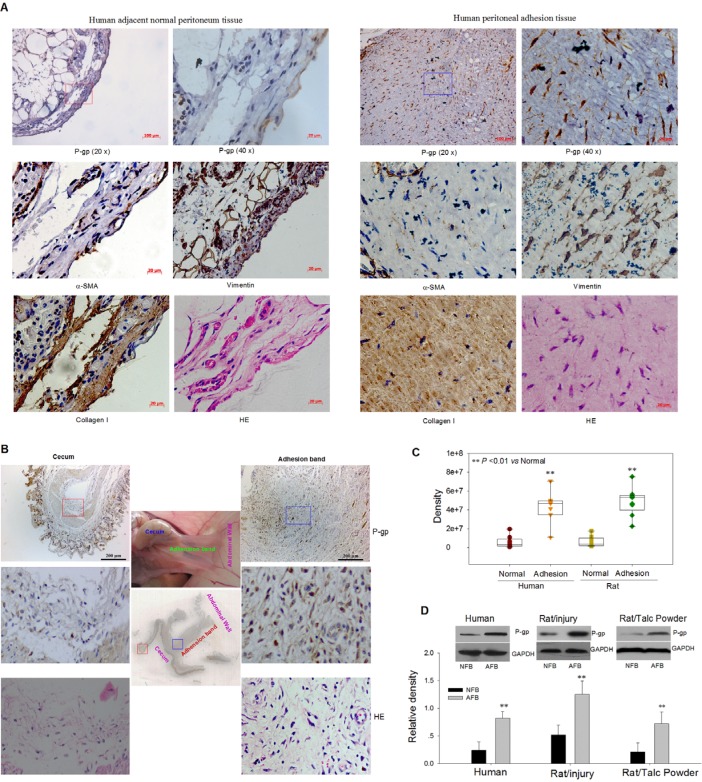
We assessed differences in gene expression levels between human normal and adhesion fibroblasts of several genes investigated in our other projects and related to cell proliferation, migration, and vesicle trafficking13, 14. Of the genes compared between primary cultured adhesion fibroblasts (AFB) and their matched normal fibroblasts (NFB), only the MDR1 gene was significantly upregulated in adhesion fibroblasts (Figure S1). Using immunohistochemistry we found that the expression of P-gp in fibroblasts of human and rat adhesion tissues was obviously higher than in those of adjacent normal tissues (Figure 1A-C). The higher expression of P-gp protein in human and rat cultured adhesion fibroblasts compared to matched normal fibroblasts was verified by Western blot (Figure 1D). Collectively, these data suggest that P-gp may contribute to postoperative peritoneal adhesion formation.
Figure 1.
High Expression of P-gp Protein in Human or Rat Fibroblasts of Adhesion Peritoneum. (A and B) Representative immunohistochemical images for P-gp, a-SMA, vimentin, and collagen I in human (A) and rat (B) peritoneal adhesion tissues and adjacent normal peritoneum tissues. (C) Quantitative analysis of immunohistochemical results indicates that P-gp is highly expressed in the adhesion fibroblasts (AFB). (D) Western blot analysis shows that the P-gp protein expression was significantly upregulated in cultured human and rat AFB. ** P < 0.01. See also Figure S1.
Involvement of P-gp in Rat Models of Postoperative Peritoneal Adhesion
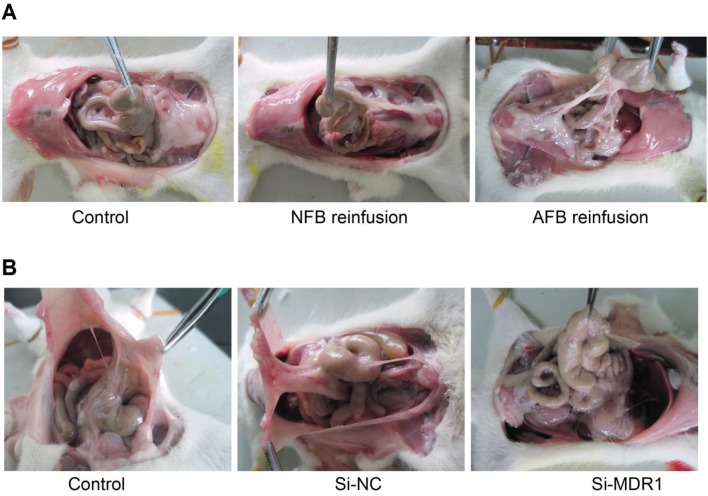
We next observed the effect of up-regulation of P-gp expression on peritoneal adhesion formation by reinfusion of primary cultured adhesion or normal rat fibroblasts (cells were infused into the abdominal cavities of rats with mild peritoneal injuries). Compared to the normal fibroblasts, the reinfusion of adhesion fibroblasts overexpressing P-gp resulted in more serious peritoneal adhesions of higher grade and a higher rate of adhesions (Figure 2A, Table 1). We also found that knockdown of P-gp expression by intraperitoneal injections of MDR1-targeted siRNAs significantly reduced peritoneal adhesion development rates and adhesion grades induced by moderate peritoneal injury (Figure 2B, Table 2 and Figure S2). Together, these data demonstrate that P-gp plays a crucial role in postoperative peritoneal adhesion formation.
Figure 2.
Overexpression or Knockdown of P-gp Promotes or Degrades Peritoneal Adhesion in a Rodent Model, Respectively. (A) Promotion of peritoneal adhesion formation following mild peritoneal injury and reinfusion of rat adhesion fibroblasts overexpressing P-gp into peritoneal cavity (For detailed statistical results, see Table 1). (B) Knockdown of P-gp expression by intraperitoneal injection of MDR1 siRNAs reduced peritoneal adhesion development rate and adhesion grades induced by moderate peritoneal injury (For detailed statistical results, see Table 2). Gene silencing efficiency of siRNA targeting MDR1 is shown in Figure S2.
Table 1.
The effect of AFB or NFB reinfusion on peritoneal adhesions
| Group | n | Grade of adhesion(case) | Average grade | Rate of adhesion [case(%)] | ||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||||
| Control ( with mild peritoneal injury) | 9 | 7 | 2 | 0 | 0 | 0 | 0.22 | 2(22) |
| Reinfusion of NFB | 11 | 4 | 5 | 2 | 0 | 0 | 0.82 | 7(55) |
| Reinfusion of AFB* | 11 | 1 | 4 | 1 | 2 | 3 | 2.36 | 10(90) |
* P < 0.05 as compared to the group of rat NFB reinfusion. Adhesion rate describes the presence of an adhesion in any grade.
Table 2.
The effect of Knockdown of P-gp expression on peritoneal adhesions
| Group | n | Grade of adhesion(case) | Average grade | Rate of adhesion [case(%)] | ||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||||
| Control ( with moderate peritoneal injury) | 5 | 0 | 0 | 0 | 1 | 4 | 3.8 | 5(100) |
| Transfection of Si-NC | 5 | 0 | 0 | 0 | 2 | 3 | 3.6 | 5(100) |
| Transfection of Si-MDR1** | 5 | 3 | 2 | 0 | 0 | 0 | 0.4 | 2(40) |
** P < 0.01 as compared to the Si-NC group.
MDR1 Expression is Upregulated following Peritoneal Injury via the TGF-β1/Smad Signaling Pathway and Histone H3 Acetylation
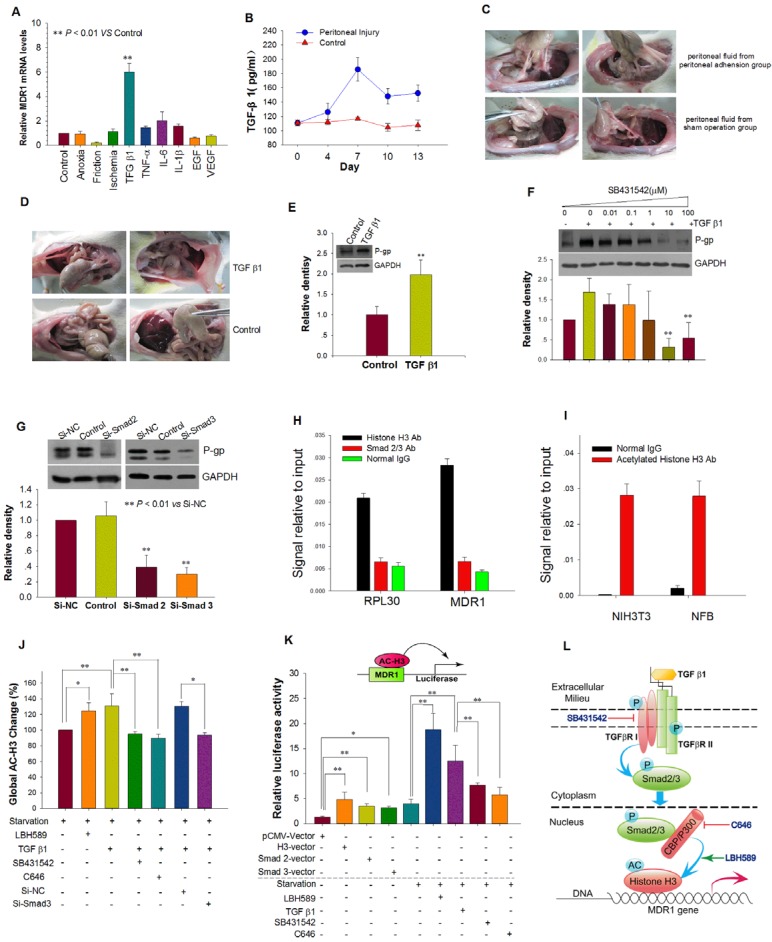
We next tested the effects of several physical and chemical factors associated with peritoneal injury on the expression of MDR1. Only TGF-β1 up-regulated MDR1 mRNA expression and elevated P-gp expression in NFB (Figure 3A and Figure S3A). Both reinfusion of peritoneal fluids with high endogenous TGF-β1 and intraperitoneal injection of exogenous TGF-β1 resulted in more severe peritoneal adhesions and increased P-gp expression in adhesion tissues (Figure 3B-E, Supplemental table 1 and 2). TGF-β type I receptor (TβRI) inhibitor SB431542 and siRNAs targeted to Smad 2 or 3 prevented induction of P-gp expression by TGF-β1 (Figure 3F and G). These results indicate that peritoneal injury up-regulates MDR1 expression via the TGF-β1/Smad signaling pathway.
Figure 3.
TGF-β1 Up-regulated MDR1 Expression by TGF-β1/Smad Signaling Pathway and Histone H3 Acetylation. (A) The effect of physical factors, cytokines and growth factors associated with peritoneal injury on MDR1 mRNA expression in NFB. (B and C) Reinfusion of peritoneal fluid obtained from rats with peritoneal adhesions resulted in more serious peritoneal adhesions. (B) Peritoneal injury increased the concentration of TGF-β1 in peritoneal fluid, which reached the highest level at day 7. (C) Representative photographic images of rat peritoneal adhesions. Peritoneal fluid with high endogenous TGF-β1 was reinfused into peritoneal cavities of rats with mild peritoneal injuries, resulting in more serious peritoneal adhesions of higher grade and a greater rate of adhesions (For detailed statistical results, see Supplemental Table 1). (D and E) TGF-β1 promoted peritoneal adhesion formation and P-gp expression of adhesion tissue. (D) Representative photographic images of rat peritoneal adhesions. Intraperitoneal injection of exogenous TGF-β1 (150 ng/kg) induced more serious peritoneal adhesions of higher grade and a greater rate of adhesions (For detailed statistical results, see Supplemental Table 2). (E) Intraperitoneal injection of TGF-β1 upregulated P-gp expression in adhesion tissues. ** P < 0.01 vs Control. (F and G) Inhibition of the TGF-β1/Smad pathway prevented induction of P-gp expression by TGF-β1. (F) TGF-β type I receptor (TβRI) inhibitor SB431542 inhibited P-gp expression induced by TGF-β1. (G) Silencing of Smad 2 or 3 expression by transfection with Si-Smad 2 or -Smad 3, respectively, prevented induction of P-gp expression by TGF-β1. (H - K) TGF-β1 enhances activity of the MDR1 promoter via the TGF-β1/Smad signaling pathway and promotion of histone H3 acetylation. Chromatin immunoprecipitation (ChIP) analysis of the binding of Smad2/3 and histone H3 to the MDR1 promoter in AFB (H) and acetylated H3 at the MDR1 promoter in rat NFB and NIH3T3 cells treated with TGF-β1 (I). RPL30 was used as a positive control. (J) Detection of global acetylated histone H3 in rat NFB treated with different factors. (K) Induction of MDR1 promoter activity by co-transfection with either histone H3, Smad 2, or Smad 3 expression plasmids plus pMDR1(-1202) reporter plasmid and TGF-β1 or the histone deacetylase (HDAC) inhibitor panobinostat (LBH589). TGF-β type I receptor (TβRI) inhibitor SB431542 and histone acetyltransferase CBP/p300 inhibitor C646 abolished the increased activity induced by TGF-β1. * P < 0.05; ** P < 0.01. (L) Schematic model depicting the proposed mechanism of up-regulation of MDR1 expression induced by TGF-β1 via the TGF-β-Smad signaling pathway and histone H3 acetylation.
We then investigated MDR1 transcriptional activation. ChIP assay results revealed that histone H3 and acetylated histone H3 but not Smad2/3 directly interact with the MDR1 promoter (Figure 3H and I). TGF-β1 promoted acetylation of histone H3, and SB431542 or Si-Smad2 abrogated the increased acetylation of histone H3 and subsequent MDR1 mRNA expression. Moreover, C646, an inhibitor of histone acetyltransferase CBP/p300 that can bind to Smad2/315, 16, also abolished the increased acetylation of histone H3 and MDR1 mRNA expression induced by TGF-β1 (Figure 3J and Figure S3B).
In order to determine whether activation of the TGF-β1/Smad signaling pathway and acetylation of histone H3 modulates the activity of the MDR1 promoter, we used a MDR1 promoter-driven luciferase gene construction (pMDR1 (-1202) plasmid). We found that pMDR1(-1202) activity was greatly increased by TGF-β1 stimulation, histone deacetylase (HDAC) inhibitor panobinostat (LBH589) treatment, co-transfection with histone H3, Smad2, or Smad3 plasmids. SB431542 and C646 blocked the increased activity induced by TGF-β1 (Figure 3K). Together, these results suggest that TGF-β1 enhances the activity of the MDR1 promoter and the transcription of MDR1 via the TGF-β1/Smad signaling pathway and subsequent histone H3 acetylation (Figure 3L).
Up-regulation of P-gp Expression Elevated Volume-activated Cl- Current (ICl,vol) and Regulatory Volume Decrease (RVD) of Peritoneal Fibroblasts
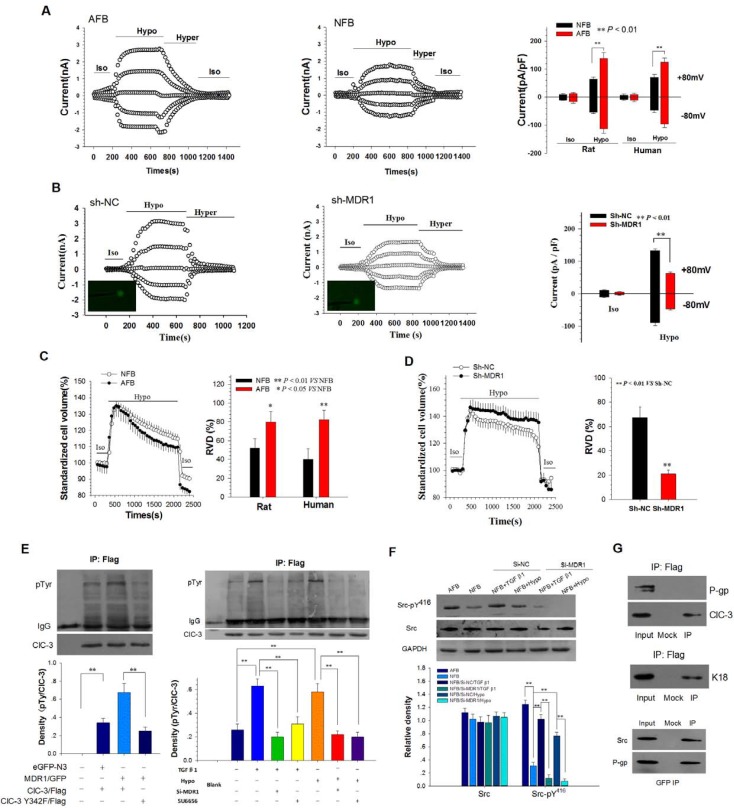
Whole-cell chloride currents were recorded to assess the activity of the chloride channels in normal and adhesion rat fibroblasts. The currents were activated by extracellular treatment with a 47% hypotonic bath solution. The hypotonicity-activated Cl- currents were eliminated by the cell shrinkage induced by the hypertonic and isotonic bath solutions, indicating that the Cl- currents were volume-sensitive (Figure 4A and B). The reversal potential of the I-V curve was similar to the calculated ECl (-0.8 mV), with a mean value of 2.23 ± 1.8 mV (Figure S4A). The sequence of anion permeability was I- > Br- > Cl- > gluconate (Figure S4B) and gluconate shifted the reversal potential at -22.85 ± 3.5 mV (Figure S4C). Silencing of ClC-3 by transfection with Si-ClC3 impaired both hypotonicity-induced Cl- currents and regulatory volume decrease (RVD, Figure S4D and E). Further analysis showed that current densities were different between the NFB and AFB cells. The ICl,vol in the AFB was larger than in the NFB. The current densities of ICl,vol at +80 mV were 138.9 ± 19.9 pA/pF in AFB and 64.5 ± 7.7 pA/pF in NFB (Figure 4A). Furthermore, knockdown of P-gp expression by transfection with MDR1-specific shRNAs significantly inhibited the Cl- currents induced by the hypotonic solution (Figure 4B and Figure S5).
Figure 4.
Up-regulation of P-gp Expression Elevated ICl,vol and RVD of Peritoneal Fibroblast by Phosphorylating Chloride Channel-3 (ClC-3) tyrosine 342 via Src Tyrosine Kinase. (A) Comparison of volume-activated chloride currents in AFB and NFB. Iso, isotonic bath solution; Hypo, 47% hypotonic bath solution; Hyper, 47% hypertonic bath solution. (B) Suppression of MDR1 expression by sh-MDR1 transfection on ICl,vol in AFB. Cells successfully transfected with sh-MDR1 or sh-NC (green) were selected for whole-cell current recordings under the fluorescence microscope. (C and D) Differential capability of RVD in AFB and NFB and inhibition of RVD by silencing of MDR1 expression in AFB. (C) Changes in AFB and NFB cell volumes in isotonic (Iso) and 47% hypotonic (Hypo) bath solutions and RVD capacity in the hypotonic bath solution. (D) RVD capacity of AFB after treatment with Sh-NC or Sh-MDR1 (n = 20). (E) P-gp promoted phosphorylation of human ClC-3 tyrosine 342. (left panels) NIH-3T3 cells were co-transfected with human MDR1-GFP vectors and ClC-3-Flag or ClC-3-Y432F. Immunoblots of IP with anti-phosphotyrosine and anti-ClC-3 antibodies from extracts of NIH-3T3 cells were probed with an antibody against Flag. (right panels) Effects of down-regulation of P-gp expression or inhibition of Src kinase by SU6656 on phosphorylation of human ClC-3 tyrosine induced by TGF β1 and hypotension. pTyr: phosphotyrosine. **: P < 0.01. (F) P-gp-mediated Src phosphorylation induced by hypotonicity and TGF β1. Representative western blot (upper) and densitometric analysis (lower) showed that phospho-Src (pY416) induced by hypotonicity and TGF β1 was abolished by down-regulation of P-gp expression. **: P < 0.01. (G) P-gp binds to Src kinase. (left two panels) P-gp does not bind ClC-3. Immunoblots of co-IP with anti-Flag antibody from extracts of NIH-3T3 cells co-transfected with MDR1/GFP and ClC-3/Flag, probed with antibodies against P-gp or ClC-3 (upper panel). Control experiment with anti-Flag antibody from extracts of NIH-3T3 cells co-transfected with K18/GFP and ClC-3/Flag probed with anti-K18 (centre panel). (Lower panel) Co-IP with anti-GFP antibody from NIH-3T3 cells extract was probed with anti-Src and anti-P-gp. Cells were transfected with human MDR1-GFP vectors.
We next compared the RVD capability between normal and adhesion rat fibroblasts. Cells swelled immediately following the hypotonic challenge, and then cell volume decreased gradually to normal levels. The extent of volume recovery was clearly different between NFB and AFB (Figure 4C). The capability of RVD in human and rat adhesion fibroblasts was significantly higher than in corresponding normal fibroblasts. Moreover, the transfection of cells with MDR1-specific shRNAs significantly inhibited the RVD induced by hypotonic stress in adhesion fibroblasts (Figure 4D). Collectively, the results indicate that P-gp may promote shape changes in adhesion fibroblasts through cell volume regulation by increasing the activity of volume-activated chloride channels.
P-gp Regulates ICl,vol by Promoting Phosphorylation of ClC-3 Tyrosine 342 via Src Tyrosine Kinase
Then we investigated how overexpression of P-gp enhanced ICl,vol. The results showed that P-gp co-localized with ClC-3, a member of the ClC voltage-gated Cl- channel superfamily that may function as a key component of the volume-activated Cl- channel, at the leading edge of lamellipodia and at the rear of migrating cells (Figure S6). TGF β1 stimulation induced trafficking of ClC-3 to the plasma membrane (Figure S7). Silencing of P-gp expression did not effect ClC-3 expression (Figure S8). P-gp did not directly bind to ClC-3 (Figure 4G) but promoted phosphorylation of human ClC-3 tyrosine 342 (Figure 4E), which has been found to be an important molecular mechanism for volume-activated Cl- channel activation17. Moreover, we found that both hypotonicity and TGF β1 stimulation induced ClC-3 tyrosine 342 phosphorylation. Silencing of P-gp expression by transfection with Si-MDR1 or treatment with Src kinase inhibitor SU6656 abolished the increased phosphorylation of ClC-3 tyrosine induced by either hypotonicity or TGF β1 (Figure 4E). Phosphorylation of the Src activation residue Tyr416 was elevated in adhesion fibroblasts when compared to normal fibroblasts. Both hypotonicity and TGF β1 treatment increased phosphorylation of Src Tyr416 but did not effect Src kinase expression. Silencing of P-gp expression blocked the increased activity induced by either hypotonicity or TGF-β1 (Figure 4F). A co-immunoprecipitation (co-IP) assay revealed that P-gp interacts with Src kinase in NIH3T3 cells (Figure 4G). Together, these results suggest that overexpression of P-gp activates Src kinase by binding to the kinase and inducing phosphorylation of Src Tyr416.
Inhibition of ICl,vol and RVD by Cl- Channel Blockers Prevents Formation of Peritoneal Adhesions and Reduces Migration and Proliferation of Adhesion Fibroblast
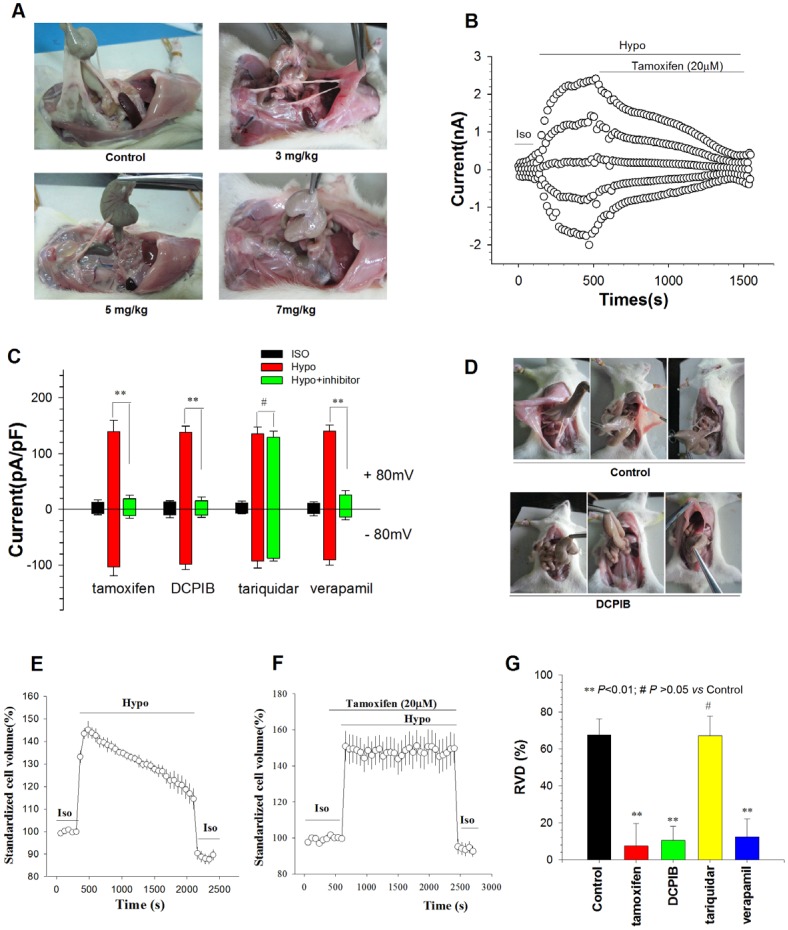
To verify that enhanced ICl,vol and RVD are involved in postoperative peritoneal adhesion formation, we determined the effects of Cl- Channel blockers on peritoneal adhesion formation, and ICl,vol and RVD of adhesion fibroblasts. Tamoxifen, a well-known inhibitor of volume-activated Cl- channels18, mitigated the formation of rat peritoneal adhesions in a dose-dependent manner, with an IC50 of 3.61 mg/kg (Figure 5A,Table 3). Tamoxifen (20 µM) almost completely inhibited ICl,vol and RVD induced by hypotension in rat AFB (Figure 5B, C, E-G). DCPIB, another ICl,vol, blocker19, also significantly mitigated postoperative peritoneal adhesion formation in rats and inhibited ICl,vol and RVD of AFB (Figure 5C-G, Table 4). Moreover, the inhibition of the transport function of P-gp by tariquidar, a third generation inhibitor of P-gp transport20, had no effect on postoperative peritoneal adhesion formation, ICl,vol, or RVD (Figure S9A, Figure 5D and G, Supplemental table 3). However, another P-gp transport function inhibitor verapamil reduced peritoneal adhesion formation and inhibited ICl,vol and RVD (Figure S9B, Figure 5D and G, Supplemental table 4). In addition, tamoxifen, DCPIB, tariquidar, and verapamil had similar effects on migration and proliferation of adhesion fibroblasts (Figure 6D and G). Together, these results provide additional evidence for the roles of ICl,vol and RVD in the mediation of P-gp in postoperative peritoneal adhesion formation.
Figure 5.
Chloride Channel Blockers Mitigated the Formation of Peritoneal Adhesion by Inhibiting ICl,vol and RVD of Peritoneal Fibroblasts. (A) Intraperitoneal injection of chloride channel blocker tamoxifen significantly reduced peritoneal adhesion development rate and adhesion grades induced by moderate peritoneal injury in a dose-dependent manner, with an IC50 of 3.61 mg/kg (For detailed statistical results, see Table 3). (B and C) Chloride channel blockers almost completely inhibited ICl,vol of rat AFB. (B) Time course of the Cl- current activated by a hypotonic solution (Hypo, 160 mosmol/l) in AFB and the effect of 20 µM tamoxifen. Iso, isotonic bath solution; Hyper, 47% hypertonic bath solution. (C) Mean current densities in cells treated with tamoxifen (20 µM), DCPIB (20 µM), tariquidar (0.1 µM), or verapamil (100 µM). ** P < 0.01. (D) Representative photographic images of peritoneal adhesion in rats. DCPIB (4 mg/kg) significantly inhibited peritoneal adhesion formation. (For detailed statistical results, see Table 4). (E - G) Chloride channel blockers decreased the RVD capacity of rat AFB. Time course of RVD activated by the hypotonic solution (Hypo) in control (E) and 20 µM tamoxifen-treated cells (F). Iso, cells in isotonic condition. (G) The effects of tamoxifen (20 µM), DCPIB (20 µM), tariquidar (0.1 µM), or verapamil (100 µM) on the RVD capacity of AFB. Data are mean ± SEM, n=3 with >10 cells.
Table 3.
The effect of chloride channel blocker tamoxifen on peritoneal adhesions
| Group | n | Grade of adhesion(case) | Average grade | Rate of adhesion [case(%)] |
||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||||
| Control | 9 | 0 | 0 | 4 | 5 | 0 | 2.56 | 9(100) |
| 3mg/kg | 9 | 4 | 0 | 3 | 2 | 0 | 1.33 | 5(56) |
| 5mg/kg | 9 | 3 | 2 | 3 | 1 | 0 | 1.22 | 6(67) |
| 7mg/kg | 9 | 6 | 1 | 2 | 0 | 0 | 0.56 | 3(33) |
Crosstabs, Chi-square test P < 0.01; Spearman's correlation analysis, r =-0.55, P < 0.01.
Table 4.
The effect of chloride channel blocker DCPIB on peritoneal adhesions
| Group | n | Grade of adhesion(case) | Average grade | Rate of adhesion [case(%)] |
||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||||
| Control | 9 | 0 | 0 | 1 | 3 | 5 | 3.4 | 9(100) |
| DCPIB** | 9 | 3 | 2 | 4 | 0 | 0 | 1.1 | 6(67) |
**: P < 0.01 vs control (Mann-Whitney U test).
Figure 6.
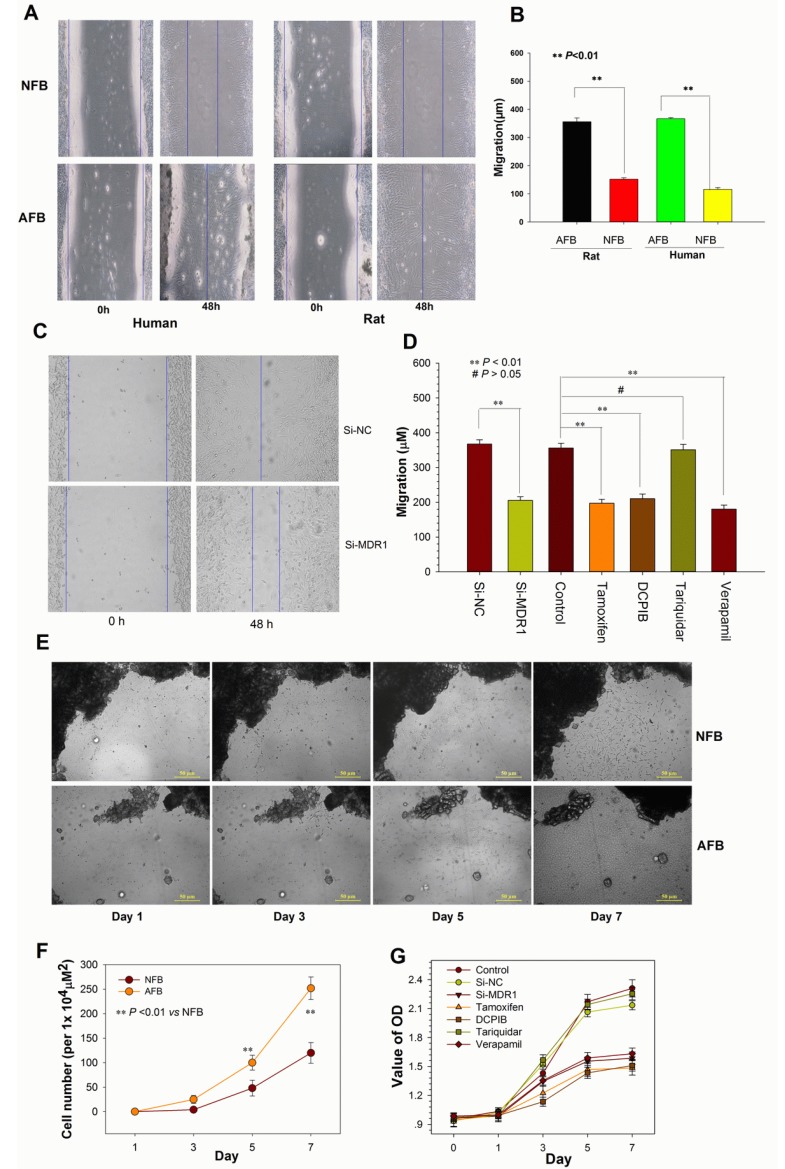
Altering P-gp Expression or Blocking ICl,vol affects Cell Migration and Proliferation. (A and B) Migratory ability of human and rat AFB overexpressing P-gp and NFB. Confluent monolayers were scratched and then cultured in medium containing 0.5% FCS for 48 h (A). Data shown in (B) are mean ± SEM. (C) Representative photographs of wound-healing motility assays for rat AFB cells transiently transfected with siRNA against MDR1. (D) Average migratory width of AFB cells treated with MDR1 siRNA or inhibitors. Data are mean ± SEM. (E and F) Comparison of proliferative capability between rat AFB and NFB cells (E) and quantification of multiple visual fields (n = 4, F). Bright-field photographs of primary cultured live cells were taken at different times. Dark areas indicate peritoneal adhesion tissue or adjacent normal peritoneum tissue. (G) Growth curve for rat AFB cells transfected with si-MDR1 or treated with different inhibitors. Cells were cultured in 96-well plates in medium with 10% serum. Relative cell numbers were determined by CCK-8 colorimetric assay. Data shown are the results of three independent experiments (mean ± SEM). si-MDR1 transfection and all inhibitors except for tariquidar significantly inhibited the proliferation of AFB cells at day 5 and 7 (P < 0.01 vs si-NC).
Up-regulation of P-gp Expression Promotes Migration and Proliferation of Peritoneal Fibroblasts
We next determined whether altering P-gp expression affected fibroblast migration and proliferation. As shown in Fig. 6A, B, E and F, compared to normal fibroblasts, the adhesion fibroblasts with high P-gp expression had higher potential for migration and proliferation. In contrast, down-regulation of P-gp expression by transfection of si-MDR1 significantly inhibited the migration and proliferation of adhesion fibroblasts (Figure 6C, D and G). Taken together, these data indicate that the mechanisms through which P-gp contributes to postoperative peritoneal adhesion formation are closely related to the acceleration of fibroblast migration and proliferation.
Discussion
Several genes and proteins have been reported to be differentially expressed between fibroblasts of normal and adhesion peritoneum4, 8. However, it remains unknown how these genes or proteins contribute to peritoneal adhesion formation. This study demonstrates that the MDR1 gene encoding P-gp plays a critical role in postoperative peritoneal adhesion formation. Four lines of evidence support this notion. First, expression of P-gp protein was significantly upregulated in fibroblasts of human and rat adhesion tissues when compared to adjacent normal tissues. Second, fibroblasts derived from adhesions, as opposed to nearby peritoneum, were characterized by increased basal expression of MDR1 mRNA and P-gp protein. Third, reinfusion of adhesion fibroblasts overexpressing P-gp resulted in more serious peritoneal adhesions. Finally, knockdown of P-gp expression by intraperitoneal transfection of MDR1-targeted siRNA significantly reduced the peritoneal adhesion development rate and adhesion grades. Our results also indicate that P-gp may be a valuable therapeutic target to prevent postoperative peritoneal adhesion formation.
A crucial process during peritoneal adhesion formation is the infiltration of the fibrinous coagulum by proliferating fibroblasts followed by vascularization and cellular growth21. The high capability of peritoneal fibroblasts to proliferate and migrate would accelerate adhesion formation or increase the grade of adhesions. P-gp promotes transendothelial migration of antigen-presenting dendritic cells and T lymphocytes during an immune response22. In addition, P-gp overexpression has been shown to enhance brain endothelial cell (EC) migration23. P-gp knockdown by MDR1-targeted siRNA decreased the rate of migration in breast cancer MCF-7 cells24. Although some reports have found relationship or a negative correlation between P-gp expression and proliferation in peripheral T-cells25 and some cancer cells26, 27, other studies have demonstrated that P-gp enhances cell proliferation in normal28 and cancer cells29. Our results revealed that adhesive fibroblasts overexpressing P-gp had higher potential for migration and proliferation and knockdown of P-gp inhibited cell migration and proliferation. Taken together, these results suggest that P-gp mediates postoperative peritoneal adhesion formation by promoting the migration and proliferation of peritoneal fibroblasts.
Our results also revealed that, among several physical and chemical factors associated with peritoneal injury30, TGF-β1 was the inducing factor for up-regulating expression of P-gp. TGF-β1 is a major secretory product of macrophages. Macrophages are normal cellular components of reproductive tract tissues and peritoneal fluid. Excessive TGF-β1 secretion by macrophages following operative injury plays an important role in intraperitoneal adhesion formation via the Smad 2/3 signaling pathway, stimulating increased expression of plasminogen activator inhibitor (PAI)-1 and decreased expression of tissue-type plasminogen activator (t-PA)31-33. Consistent with previous studies, we also found that the concentration of TGF-β1 in peritoneal fluid was significantly higher following peritoneal injury. Both reinfusion of peritoneal fluid with high endogenous TGF-β1 and intraperitoneal injection of exogenous TGF-β1 resulted in more severe peritoneal adhesions. Interference in TGF-β1/Smad signaling by inhibitors or siRNAs mitigated the up-regulation of P-gp expression. This suggests that peritoneal injury induces elevation of P-gp expression via the TGF-β1/Smad signaling pathway.
Moreover, our results showed that Smad2/3 did not directly regulate transcription of MDR1. Instead, its activity was directly modulated by acetylated histone H3 bound to the MDR1 promoter. Histone acetylation has been found to be involved in TGF-β1-mediated induction of PAI-1 and p21 expression32. Our results revealed that TGF-β1 promoted acetylation of histone H3 and the activity of the MDR1 promoter. Inhibitors or siRNAs targeting the TGF-β1/Smad signaling pathway abrogated the increased acetylation of histone H3, the activity of the MDR1 promoter, and MDR1 mRNA expression. Smad2/3 can recruit and activate histone acetyltransferase CBP/p300, which enhances histone acetylation16, 34. We found that inhibition of CBP/p300 by C646 prevented the acetylation of histone H3 and induction of MDR1 mRNA expression by TGF-β1. These results indicate that histone H3 acetylation induced by CBP/p300-mediated transcription of MDR1 is downstream of the TGF-β1/Smad signaling pathway. Therefore, targeting the activity of CBP/p300 or acetylation of histone H3 may be a feasible strategy for preventing P-gp-mediated postoperative peritoneal adhesion formation.
P-gp is a bifunctional protein with a transport function that pumps cytotoxic drugs out of cells and a channel-related function that regulates volume-activated Cl- channel activity11. The transport and channel-related functions of P-gp are regulated by different phosphorylation sites35. Based on our results, only the channel-related function of P-gp is involved in peritoneal adhesion formation. Although it is well known that P-gp regulates volume-activated Cl- channels36, the underlying mechanism remains unknown. Based on our results, P-gp enhanced phosphorylation of human ClC-3 tyrosine 342 (or tyrosine 284 in the rat ClC-3 channel). The later has been reported to be an important molecular mechanism for volume-activated Cl- channel activation17. We found that P-gp did not bind to ClC-3 and it appeared that P-gp did not directly phosphorylate ClC-3. Activation of Src kinase activity leads to inhibition of P-gp transport function37. Src kinase is rapidly activated and phosphorylates ClC-3 tyrosine 342 during hypotonic stimulation17. Using immunoprecipitation and immunofluorescence staining techniques, Li et al. demonstrated that P-gp interacts with Src kinase in cancer cells (AACR Annual Meeting presentation abstract, Apr 21, 2015). Our results revealed that P-gp activates Src kinase by binding to the kinase and inducing phosphorylation of Src Tyr416. Therefore, in adhesion fibroblasts increased levels of P-gp, induced by TGF-β1, results in activated Src kinase, which then inhibits its transport activity. Activated Src kinase then phosphorylates ClC-3 tyrosine 342, which results in the opening of volume-activated Cl- channels.
Volume-activated Cl- current (ICl,vol) plays a role in cell migration in several types of cells by controlling changes in cell shape during migration38-40. The opening of the ClC-3 channel facilitates the retraction of the rear of migrating cells by inducing cell shrinkage41. Our data show that P-gp co-localized with ClC-3 at the rear of migrating cell and indicate that P-gp may modulate retraction in migrating cells via ICl,vol and RVD by phosphorylating ClC-3. Of interest, we also found that P-gp co-localized with ClC-3 at the leading edge of lamellipodia. Although ClC-3 has found to be localized at the leading edge of lamellipodia in human glioma cells, the functional implications remain unclear42. We speculate that in coordination with other ion channels and transporters, P-gp may be involved in the increase in local volume before protrusion of lamellipodium and/or the decrease following protrusion through regulation of ICl,vol 43. This may also contribute to the promoting effects of P-gp on migration of dendritic cells and cancer cells22, 24. ICl,vol has also been shown to contribute to cell proliferation via modulation of cell volume during cell cycle progression12, 44. In particular, ICl,vol is important for the G1/S phase transition and cell division in the M-phase45, 46. Thus, P-gp may promote cell migration and proliferation of fibroblasts via ICl,vol and RVD through ClC-3 phosphorylation.
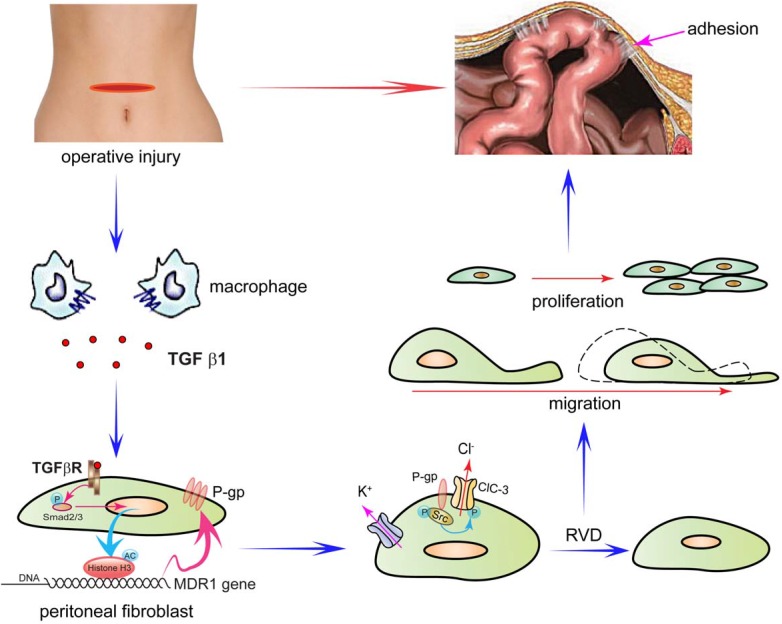
Based on our results, we proposed a four-step model by which P-glycoprotein mediates postoperative peritoneal adhesion formation (Figure 7). First, operative injury induces excessive TGF-β1 secretion by macrophages33, 47. Second, TGF-β1 up-regulates P-gp expression by enhancing the activity of the MDR1 promoter via the TGF-β1/Smad signaling pathway and histone H3 acetylation. Third, P-gp accelerates activation of volume-activated Cl- channels and cell RVD capability by promoting phosphorylation of ClC-3 via Src tyrosine kinase. Fourth, increased ICl,vol and cell volume regulation capability accelerates cell migration and proliferation of fibroblasts, which finally leads to peritoneal adhesion formation.
Figure 7.
Schematic Diagram Depicting the Proposed Role of P-gp in the Formation of Peritoneal Adhesions following an Abdominal Operation. In the proposed model, P-glycoprotein mediates postoperative peritoneal adhesion formation in four steps. First, operative injury induces excessive TGF-β1 secretion by macrophages. Second, TGF-β1 up-regulates P-gp expression by enhancing the activity of the MDR1 promoter via the TGF-β1/Smad signaling pathway and histone H3 acetylation. Third, P-gp accelerates activation of volume-activated Cl- channels and cell RVD capability by promoting phosphorylation of ClC-3 via Src tyrosine kinase. Fourth, increased ICl,vol and cell volume regulation capability accelerates migration and proliferation of fibroblasts, which finally leads to peritoneal adhesion formation.
Materials and Methods
Human Peritoneal Tissue Collection and Peritoneal Fibroblast Culture
Tissues were collected during abdominal surgery from patients who had undergone previous abdominal surgery. All samples were collected in accordance with the guidelines of the Guangdong Pharmaceutical University Institutional Review Board and informed consent was obtained from all patients. Peritoneal adhesion tissues were obtained from the adhesive band. Normal parietal peritoneal tissues near to the site of adhesive tissue collection were also collected from the same patient.
Primary culture of peritoneal fibroblasts was performed as previously described4. Briefly, the tissue samples were cut into smaller pieces (1-2 mm2) in a sterile culture dish and transferred into a fresh T-25 flask for primary explant culture in a humidified chamber at 37°C and 5% CO2. Cells were grown in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal calf serum (FCS), 100 U/ml penicillin, and 100 µg/ml streptomycin. Outgrowth of fibroblasts generally took 2 weeks. When confluence was reached, the cells were subcultured by trypsinization to a maximum of 12 passages. Cells were verified as being fibroblasts by immunofluorescence with a monoclonal antibody against vimentin (1:100, Boster, Beijing, China).
Induction of Adhesions via Peritoneal Injury
All animal studies were conducted with institutional Animal Care and Use Committee approval. Animals received no food for at least 12 hours before surgery. The rats were prepared for surgery with an injection of ketamine (80 mg/kg i.m.) anesthesia. After hair removal, the abdomen was cleansed with 1% antiseptic povidone-iodine solution and a 3 cm midline laparotomy was performed. The cecum was exposed and kept moist. Moderate injury of peritoneum on two parts of the cecum, the left and right sides of the abdominal incision, by clamping 4 times with Hemostatic forceps at a locking tension of three. For mild peritoneal injury, Hemostatic forceps at a locking tension of two were used to clamp the aforementioned cecum peritoneum three times and the abdominal peritoneum one time. Blockers were injected intraperitoneally every day for 2 weeks after the injury. Induction of adhesions by talc powder was performed as described previously48.
At two weeks after the surgery, the animals were sacrificed and the abdominal cavity was opened. Adhesions were evaluated according to the Mazuji classification49. Adhesions were graded from 0 (absent) to 4 (severe). In general, Grades 0 and 1 have no clinical significance, whereas Grades 3 and 4 can cause intestinal obstruction. Adhesion rate described the presence of an adhesion of any grade.
Immunohistochemistry and Immunofluorescence
Immunostaining was performed on tissues using the MaxVision™ two-step system (KIT-5010; Maixin Biotechnology Co., Ltd., Fuzhou, China) following the manufacturer's protocol. The mean optical intensity of the specific immunohistochemical staining reaction was evaluated by IPP6.0 image analysis software. Immunofluorescence was performed as described previously50. Samples were incubated overnight at 4°C with primary antibodies against P-gp (1:100, Boster) and ClC- 3 (1:50, Abcam). A Nikon Eclipse C1 confocal laser scanning microscope (Nikon Corporation, Tokyo, Japan) was used to take a series of images in the z-axis and reconstruct the three-dimensional profile.
Real-time PCR Analysis
Total RNA was extracted from cells using Trizol (LifeTechnologies, Grand Island, NY) and subjected to reverse transcription with the ReverTra Ace-a-kit (Toyobo, Osaka, Japan) according to the manufacturer's instructions. Real-time PCR was performed on an Opticon 2 Real Time Cycler (BioRad, Hercules, CA) with SYBR Green PCR Master Mix kit (Toyobo, Osaka, Japan). PCR conditions consisted of a 30 second hot start at 95°C, followed by 40 cycles of 5 seconds at 95°C and 30 seconds at 60°C. The primer sequences for human MDR1 (ABCB1) mRNA were as follows: 5′-GGAAAAGAAACCAACUGUC-3′ (forward) and 5′-GACAGUUGGUUUCUUUUCC-3′ (reverse). The primer sequences for rat MDR1 (MDR1a and MDR1b) mRNA were as follows: 5′-CCCAAGATCCTTTTGTTGGA-3′ (forward) and 5′- CAAGCGGTGAGCTATCACAA -3′ (reverse).
Immunoprecipitation, Co-Immunoprecipitation, and Western blotting
Whole-cell lysates were prepared using M-PER mammalian protein extraction reagent (Pierce, Rockford, IL). cOmplete Protease Inhibitor and PhosSTOP Phosphatase Inhibitor cocktail (Roche, Indianapolis, IN) were added fresh to the lysis buffer. For immunoprecipitation, Sepharose® Bead Conjugated Flag antibody (5750, Cell Signaling Technology, Beverly, MA) was added to the cell lysates. Precipitation was performed by overnight shaking at 4°C. Then complexes were carefully washed, and proteins were electrophoretically separated by SDS-PAGE followed by Western blotting detection using mouse monoclonal anti-phosphotyrosine antibodies (PY20; Biolegend). For co-immunoprecipation, the Pierce Co-Immunoprecipitation Kit (26149, Pierce; Rockford, Illinois, USA) was used according to the manufacturer's instructions. Cell Signaling Flag antibody (8146) and GeneTex P-gp antibody (C219, specific reactivity to human, mouse and rat, GTX23364) or Biolegend cytokeratin 18 (Poly6172, 617202) were used for immunoprecipitation and immunoblotting, respectively. A total of 50 µg of the primary antibody was immobilized on the affinity columns. Lysates pre-cleared with the control resin were loaded onto columns containing immobilized antibodies covalently linked to an amine-active resin and incubated under constant agitation for 12 h at 4°C. The co-immunoprecipitate was then eluted and analyzed by SDS-PAGE and Western blotting along with the input controls. Western blotting was performed as previously described14. Blots were digitally photographed, and blot density was determined using a Bio-Rad Imaging Densitometer Quantity One 4.1.0 (Bio-Rad, Hercules, California).
Chromatin Immunoprecipitation (ChIP) Assays
To determine whether acetylated histone H3 was present at the MDR1 promoter region, we performed ChIP assays according to the manufacturers' protocols (SimpleChIP® Enzymatic Chromatin IP Kit for Histone H3, Cell Signaling Technology; EpiQuik Acetyl-Histone H3 ChIP Kit for acetylated histone H3, Epigentek). For histone H3, cells were incubated in 1% formaldehyde to cross-link the DNA and proteins. Chromatin was sheared by sonication. Samples were incubated with Histone H3 (D2B12) XP®, Smad2/3 Rabbit mAb (Cell Signaling Technology), or with an equivalent amount of normal IgG (anti-Rabbit). A portion of the sonicated DNA was left untreated to serve as the input control. For acetylated histone H3, chromatin in the cells was extracted, sheared, and added into a microwell immobilized with the antibody. The acetyl-histone H3 protein-DNA complex was captured by the antibody, and then the DNA was released and purified through the specifically designed Fast-Spin Column. Eluted DNA was used for real-time PCR analysis. Immunoprecipitated or eluted DNA was subjected to quantitative real-time PCR using primers specific to the MDR1 promoter51 (forward: 5′- TTTGCCACAGGAAGCCTGA-3′, reverse: 5′-AAAGGAAACGAACAGCGGC-3′). Signals were expressed as a percent of the total input chromatin.
Luciferase Assays
For MDR1 promoter-luciferase transcriptional assays, cells were transiently transfected with MDR1-luc plasmid (pMDR1-1202, Addgene plasmid 37627) following treatment with different blockers or co-transfected with pMDR1-1202 and histone H3, Smad 2 or Smad 3 vectors. Then cells were assayed for luciferase and renilla activity using the Dual-Luciferase®Reporter Assays System (E1910, Promega, Milwaukee, WI) and Varioskan Flash (Thermo Fisher Scientific, Waltham, MA, USA). All experiments were performed in triplicate and normalized for renilla activity.
Additional Methods
Detailed methodology is described in the Supplementary Methods.
Supplementary Material
Supplemental Methods, Supplemental Figures and Tables.
Acknowledgments
We are grateful to Prof. David Piwnica-Worms (Washington University School of Medicine) for kindly providing the MDR1-GFP expression plasmids. This work was supported by the National Natural Science Foundation of China (81170339, 31371144, 81101666, and 30800435).
Abbreviations
- P-gp
P-glycoprotein
- ClC-3
chloride channel-3
- MDR1
multidrug-resistance gene 1
- ICl,vol
volume-activated Cl- current
- RVD
regulatory volume decrease
- AFB
adhesion fibroblasts
- NFB
normal fibroblasts
- ChIP
Chromatin immunoprecipitation
- sh-MDR1
pGPU6/GFP-MDR1 shRNA
- sh-NC
pGPU6/GFP sh-NC.
References
- 1.Ouaissi M, Gaujoux S, Veyrie N, Deneve E, Brigand C, Castel B. et al. Post-operative adhesions after digestive surgery: their incidence and prevention: review of the literature. J Visc Surg. 2012;149:e104–14. doi: 10.1016/j.jviscsurg.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Reed KL, Stucchi AF, Leeman SE, Becker JM. Inhibitory effects of a neurokinin-1 receptor antagonist on postoperative peritoneal adhesion formation. Ann N Y Acad Sci. 2008;1144:116–26. doi: 10.1196/annals.1418.010. [DOI] [PubMed] [Google Scholar]
- 3.Pfeifer S LR, Goldberg J, Thomas M, Pisarska M, Widra E, Licht M, Sandlow J, Collins J, Cedars M, Vernon M, Davis O, Dumesic D, Gracia C, Catherino W, Odem R, Thornton K, Rebar R, La Barbera A. Pathogenesis, consequences, and control of peritoneal adhesions in gynecologic surgery: a committee opinion. Fertil Steril. 2013;99:1550–5. doi: 10.1016/j.fertnstert.2013.02.031. [DOI] [PubMed] [Google Scholar]
- 4.Saed GM, Zhang W, Diamond MP. Molecular characterization of fibroblasts isolated from human peritoneum and adhesions. Fertil Steril. 2001;75:763–8. doi: 10.1016/s0015-0282(00)01799-4. [DOI] [PubMed] [Google Scholar]
- 5.Kawanishi K, Yamato M, Sakiyama R, Okano T, Nitta K. Peritoneal cell sheets composed of mesothelial cells and fibroblasts prevent intra-abdominal adhesion formation in a rat model. J Tissue Eng Regen Med. 2013 doi: 10.1002/term.1860. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Ambler DR, Fletcher NM, Diamond MP, Saed GM. Effects of hypoxia on the expression of inflammatory markers IL-6 and TNF-a in human normal peritoneal and adhesion fibroblasts. Syst Biol Reprod Med. 2012;58:324–9. doi: 10.3109/19396368.2012.713439. [DOI] [PubMed] [Google Scholar]
- 7.White JC, Jiang ZL, Diamond MP, Saed GM. Macrophages induce the adhesion phenotype in normal peritoneal fibroblasts. Fertil Steril. 2011;96:758–63. doi: 10.1016/j.fertnstert.2011.06.046. e3. [DOI] [PubMed] [Google Scholar]
- 8.Rout UK, Saed GM, Diamond MP. Expression pattern and regulation of genes differ between fibroblasts of adhesion and normal human peritoneum. Reprod Biol Endocrinol. 2005;3:1–14. doi: 10.1186/1477-7827-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luqmani YA. Mechanisms of drug resistance in cancer chemotherapy. Med Princ Pract. 2005;14(Suppl 1):35–48. doi: 10.1159/000086183. [DOI] [PubMed] [Google Scholar]
- 10.Higgins CF. P-glycoprotein and cell volume-activated chloride channels. J Bioenerg Biomembr. 1995;27:63–70. doi: 10.1007/BF02110332. [DOI] [PubMed] [Google Scholar]
- 11.Gill DR, Hyde SC, Higgins CF, Valverde MA, Mintenig GM, Sepulveda FV. Separation of drug transport and chloride channel functions of the human multidrug resistance P-glycoprotein. Cell. 1992;71:23–32. doi: 10.1016/0092-8674(92)90263-c. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann EK, Holm NB, Lambert IH. Functions of volume-sensitive and calcium-activated chloride channels. IUBMB Life. 2014;66:257–67. doi: 10.1002/iub.1266. [DOI] [PubMed] [Google Scholar]
- 13.Xu B, Jin X, Min L, Li Q, Deng L, Wu H. et al. Chloride channel-3 promotes tumor metastasis by regulating membrane ruffling and is associated with poor survival. Oncotarget. 2015;6:2434–2450. doi: 10.18632/oncotarget.2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang LW, Chen LX, Jacob T. ClC-3 expression in the cell cycle of nasopharyngeal carcinoma cells. Sheng Li Xue Bao. 2004;56:230–6. [PubMed] [Google Scholar]
- 15.Wotton D, Lo RS, Lee S, Massague J. A Smad transcriptional corepressor. Cell. 1999;97:29–39. doi: 10.1016/s0092-8674(00)80712-6. [DOI] [PubMed] [Google Scholar]
- 16.Janknecht R, Wells NJ, Hunter T. TGF-beta-stimulated cooperation of smad proteins with the coactivators CBP/p300. Genes Dev. 1998;12:2114–9. doi: 10.1101/gad.12.14.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang XG, Tao J, Ma MM, Tang YB, Zhou JG, Guan YY. Tyrosine 284 phosphorylation is required for ClC-3 chloride channel activation in vascular smooth muscle cells. Cardiovasc Res. 2013;98:469–78. doi: 10.1093/cvr/cvt063. [DOI] [PubMed] [Google Scholar]
- 18.Abdullaev IF, Rudkouskaya A, Schools GP, Kimelberg HK, Mongin AA. Pharmacological comparison of swelling-activated excitatory amino acid release and Cl- currents in cultured rat astrocytes. J Physiol. 2006;572:677–89. doi: 10.1113/jphysiol.2005.103820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Decher N, Lang HJ, Nilius B, Bruggemann A, Busch AE, Steinmeyer K. DCPIB is a novel selective blocker of I(Cl,swell) and prevents swelling-induced shortening of guinea-pig atrial action potential duration. Br J Pharmacol. 2001;134:1467–79. doi: 10.1038/sj.bjp.0704413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wagner CC, Bauer M, Karch R, Feurstein T, Kopp S, Chiba P. et al. A pilot study to assess the efficacy of tariquidar to inhibit P-glycoprotein at the human blood-brain barrier with (R)-11C-verapamil and PET. J Nucl Med. 2009;50:1954–61. doi: 10.2967/jnumed.109.063289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diamond MP, Decherney AH. Pathogenesis of adhesion formation/reformation: application to reproductive pelvic surgery. Microsurgery. 1987;8:103–7. doi: 10.1002/micr.1920080215. [DOI] [PubMed] [Google Scholar]
- 22.Randolph GJ, Beaulieu S, Pope M, Sugawara I, Hoffman L, Steinman RM. et al. A physiologic function for p-glycoprotein (MDR-1) during the migration of dendritic cells from skin via afferent lymphatic vessels. Proc Natl Acad Sci U S A. 1998;95:6924–9. doi: 10.1073/pnas.95.12.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barakat S, Turcotte S, Demeule M, Lachambre MP, Regina A, Baggetto LG. et al. Regulation of brain endothelial cells migration and angiogenesis by P-glycoprotein/caveolin-1 interaction. Biochem Biophys Res Commun. 2008;372:440–6. doi: 10.1016/j.bbrc.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 24.Miletti-Gonzalez KE, Chen S, Muthukumaran N, Saglimbeni GN, Wu X, Yang J. et al. The CD44 receptor interacts with P-glycoprotein to promote cell migration and invasion in cancer. Cancer Res. 2005;65:6660–7. doi: 10.1158/0008-5472.CAN-04-3478. [DOI] [PubMed] [Google Scholar]
- 25.Eisenbraun MD, Miller RA. mdr1a-encoded P-glycoprotein is not required for peripheral T cell proliferation, cytokine release, or cytotoxic effector function in mice. J Immunol. 1999;163:2621–7. [PubMed] [Google Scholar]
- 26.Volm M, Mattern J, Samsel B. Relationship of inherent resistance to doxorubicin, proliferative activity and expression of P-glycoprotein 170, and glutathione S-transferase-pi in human lung tumors. Cancer. 1992;70:764–9. doi: 10.1002/1097-0142(19920815)70:4<764::aid-cncr2820700408>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 27.Takanishi K, Miyazaki M, Ohtsuka M, Nakajima N. Inverse relationship between P-glycoprotein expression and its proliferative activity in hepatocellular carcinoma. Oncology. 1997;54:231–7. doi: 10.1159/000227694. [DOI] [PubMed] [Google Scholar]
- 28.Batetta B, Pani A, Putzolu M, Sanna F, Bonatesta R, Piras S. et al. Correlation between cholesterol esterification, MDR1 gene expression and rate of cell proliferation in CEM and MOLT4 cell lines. Cell Prolif. 1999;32:49–61. doi: 10.1046/j.1365-2184.1999.3210049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katoh SY, Ueno M, Takakura N. Involvement of MDR1 function in proliferation of tumour cells. J Biochem. 2008;143:517–24. doi: 10.1093/jb/mvm242. [DOI] [PubMed] [Google Scholar]
- 30.Gorvy DA, Herrick SE, Shah M, Ferguson MW. Experimental manipulation of transforming growth factor-beta isoforms significantly affects adhesion formation in a murine surgical model. Am J Pathol. 2005;167:1005–19. doi: 10.1016/s0002-9440(10)61190-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dou Q, Williams RS, Chegini N. Inhibition of transforming growth factor-beta 1 alters the growth, anchor-dependent cell aggregation and integrin mRNA expression in human promonocytes: implications for endometriosis and peritoneal adhesion formation. Mol Hum Reprod. 1997;3:383–91. doi: 10.1093/molehr/3.5.383. [DOI] [PubMed] [Google Scholar]
- 32.Yuan H, Reddy MA, Sun G, Lanting L, Wang M, Kato M. et al. Involvement of p300/CBP and epigenetic histone acetylation in TGF-beta1-mediated gene transcription in mesangial cells. Am J Physiol Renal Physiol. 2013;304:F601–13. doi: 10.1152/ajprenal.00523.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang G, Wu K, Li W, Zhao E, Shi L, Wang J. et al. Role of IL-17 and TGF-beta in peritoneal adhesion formation after surgical trauma. Wound Repair Regen. 2014;22:631–9. doi: 10.1111/wrr.12203. [DOI] [PubMed] [Google Scholar]
- 34.Furumatsu T, Tsuda M, Taniguchi N, Tajima Y, Asahara H. Smad3 induces chondrogenesis through the activation of SOX9 via CREB-binding protein/p300 recruitment. J Biol Chem. 2005;280:8343–50. doi: 10.1074/jbc.M413913200. [DOI] [PubMed] [Google Scholar]
- 35.Idriss HT, Hannun YA, Boulpaep E, Basavappa S. Regulation of volume-activated chloride channels by P-glycoprotein: phosphorylation has the final say! The Journal of physiology. 2000;524(Pt 3):629–36. doi: 10.1111/j.1469-7793.2000.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sardini A, Amey JS, Weylandt KH, Nobles M, Valverde MA, Higgins CF. Cell volume regulation and swelling-activated chloride channels. Biochim Biophys Acta. 2003;1618:153–62. doi: 10.1016/j.bbamem.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 37.Hawkins BT, Sykes DB, Miller DS. Rapid, reversible modulation of blood-brain barrier P-glycoprotein transport activity by vascular endothelial growth factor. J Neurosci. 2010;30:1417–25. doi: 10.1523/JNEUROSCI.5103-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mao J, Wang L, Fan A, Wang J, Xu B, Jacob TJ. et al. Blockage of volume-activated chloride channels inhibits migration of nasopharyngeal carcinoma cells. Cell Physiol Biochem. 2007;19:249–58. doi: 10.1159/000100644. [DOI] [PubMed] [Google Scholar]
- 39.Ransom CB, O'Neal JT, Sontheimer H. Volume-activated chloride currents contribute to the resting conductance and invasive migration of human glioma cells. J Neurosci. 2001;21:7674–83. doi: 10.1523/JNEUROSCI.21-19-07674.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cuddapah VA, Sontheimer H. Ion channels and transporters [corrected] in cancer. 2. Ion channels and the control of cancer cell migration. Am J Physiol Cell Physiol. 2011;301:C541–9. doi: 10.1152/ajpcell.00102.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwab A, Fabian A, Hanley PJ, Stock C. Role of ion channels and transporters in cell migration. Physiol Rev. 2012;92:1865–913. doi: 10.1152/physrev.00018.2011. [DOI] [PubMed] [Google Scholar]
- 42.Olsen ML, Schade S, Lyons SA, Amaral MD, Sontheimer H. Expression of voltage-gated chloride channels in human glioma cells. J Neurosci. 2003;23:5572–82. doi: 10.1523/JNEUROSCI.23-13-05572.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cuddapah VA, Robel S, Watkins S, Sontheimer H. A neurocentric perspective on glioma invasion. Nat Rev Neurosci. 2014;15:455–65. doi: 10.1038/nrn3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen LX, Zhu LY, Jacob TJ, Wang LW. Roles of volume-activated Cl- currents and regulatory volume decrease in the cell cycle and proliferation in nasopharyngeal carcinoma cells. Cell Prolif. 2007;40:253–67. doi: 10.1111/j.1365-2184.2007.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang B, Hattori N, Liu B, Nakayama Y, Kitagawa K, Inagaki C. Suppression of cell proliferation with induction of p21 by Cl(-) channel blockers in human leukemic cells. Eur J Pharmacol. 2004;488:27–34. doi: 10.1016/j.ejphar.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 46.Habela CW, Olsen ML, Sontheimer H. ClC3 is a critical regulator of the cell cycle in normal and malignant glial cells. J Neurosci. 2008;28:9205–17. doi: 10.1523/JNEUROSCI.1897-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holmdahl L, Kotseos K, Bergstrom M, Falk P, Ivarsson ML, Chegini N. Overproduction of transforming growth factor-beta1 (TGF-beta1) is associated with adhesion formation and peritoneal fibrinolytic impairment. Surgery. 2001;129:626–32. doi: 10.1067/msy.2001.113039. [DOI] [PubMed] [Google Scholar]
- 48.Tander B, Bicakci U, Kilicoglu-Aydin B, Ariturk E, Rizalar R, Bernay F. Antiadhesive effects of mitomycin C and streptopeptidase A in rats with intraperitoneal adhesions. Pediatric surgery international. 2007;23:785–8. doi: 10.1007/s00383-007-1886-x. [DOI] [PubMed] [Google Scholar]
- 49.Mazuji MK, Kalambaheti K, Pawar B. Prevention of Adhesions with Polyvinylpyrrolidone. Preliminary Report. Arch Surg. 1964;89:1011–5. doi: 10.1001/archsurg.1964.01320060079015. [DOI] [PubMed] [Google Scholar]
- 50.Mao J, Li X, Chen W, Xu B, Zhang H, Li H. et al. Cell cycle-dependent subcellular distribution of ClC-3 in HeLa cells. Histochem Cell Biol. 2012;137:763–76. doi: 10.1007/s00418-012-0937-0. [DOI] [PubMed] [Google Scholar]
- 51.Tabe Y, Konopleva M, Contractor R, Munsell M, Schober WD, Jin L. et al. Up-regulation of MDR1 and induction of doxorubicin resistance by histone deacetylase inhibitor depsipeptide (FK228) and ATRA in acute promyelocytic leukemia cells. Blood. 2006;107:1546–54. doi: 10.1182/blood-2004-10-4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Methods, Supplemental Figures and Tables.