Abstract
Multiple myeloma (MM) remains an essentially incurable hematologic malignancy originating from clonal plasma cells. This study evaluated the usefulness of the radiotracers 11C-methionine (MET) and 18F-2`-deoxy-2`-fluorodeoxyglucose (FDG) for staging and re-staging in MM.
43 patients with MM underwent both MET- and FDG-PET/CT for staging or re-staging within 3±2 days. Scans were compared on a patient and on a lesion basis. Tracer uptake was correlated with the degree of bone marrow (BM) involvement and standard clinical parameters of disease activity. Additionally, BM samples were stained for L-type amino acid transporter 1 (LAT1) expression in 15 patients. MET-PET detected focal lesions (FL) in 39/43 subjects (90.7%), whereas 10 patients were missed in FDG-PET/CT (detection rate, 33/43; 76.7%; p<0.05). MET depicted more FL in 28/43 patients (65.1%; p<0.001), whereas in the remainder (34.9%, n=15) both tracers yielded comparable results. LAT1 was highly expressed on the cell surface of myeloma cells. Both FDG and MET uptake correlated significantly with biopsy-proven BM involvement (p<0.001), with MET demonstrating a stronger correlation (SUVmean, r=0.9 vs r=0.6; SUVmax, r=0.88 vs r=0.58). Abnormal beta-2-microglobulin and free light chain levels correlated with the presence of focal intramedullary lesions detected in MET- or FDG-PET/CT (MET, p=0.006 and p=0.01, respectively; FDG, p=0.02 and p=0.01). MET appears to be superior to FDG for staging and re-staging of both intra- and extramedullary MM lesions. Tracer uptake correlates with BM involvement, β2m and FLC levels and appears to be a more accurate marker of tumor burden and disease activity.
Keywords: PET/CT, 11C-methionine, multiple myeloma, FDG
Introduction
Multiple myeloma (MM) accounts for approximately 1% of all cancers and around 10% of hematological malignancies 1, 2. Although MM essentially remains incurable, overall survival has improved over the last decade due to availability of various novel drugs and treatment options. Several studies have demonstrated the utility of molecular imaging using positron emission tomography (PET) and the radiolabeled glucose analog 18F-2`-deoxy-2`-fluorodeoxyglucose (FDG) for diagnosis, staging and estimation of prognosis 3-7. However, limitations of FDG include lack of sensitivity and specificity, e.g. in cases with diffuse bone marrow infiltration (false negative) or with inflammatory lesions (false positive) 8. We previously reported a significantly higher retention of the radiolabelled amino acid L-methyl-[11C]-methionine (MET) in well characterized myeloma cell lines and patient-derived CD138+-plasma cells and the feasibility of monitoring very early treatment response with MET in vitro and in vivo. Furthermore, MET-uptake could be linked to underlying tumor biology by demonstrating an association with intracellular Ig light chain levels and treatment-induced changes in CD138 cell surface expression 9, 10. First human studies also suggested a potential for MET in MM diagnosis 11, 12. This study aimed at further validation of MET as superior radiotracer for functional imaging of MM in comparison to FDG.
Materials and Methods
This prospective study was approved by the local ethics committee of the University of Würzburg. All patients gave written informed consent to FDG- and MET- PET/computed tomography (CT) imaging. MET was administered under the conditions of the pharmaceutical law (German Medicinal Products Act, AMG §13 2b) according to the German law and in accordance with the responsible regulator bodies (Regierung von Oberfranken).
Subjects
43 patients (24 males, 19 females, age 39-82 y, mean 61±9 y) with newly diagnosed (n=11) or a history of (n=32) MM were prospectively enrolled. All patients with longer-standing disease had been pre-treated with a number of various chemotherapeutic drug regimens including novel agents such as bortezomib, lenalidomide and others. 25/32 pre-treated subjects had undergone autologous stem cell transplantation (SCT). At the time point of PET/CT scanning, serum free immunoglobulin light chains (FLC; (all patients) and the M gradient (40/43) were recorded. Serum chemistry including lactate dehydrogenase (LDH), albumin, creatinine (all patients) and β2-microglobulin (40/43 patients) were obtained. Information on degree of myeloma bone marrow (BM) infiltration based on iliac crest biopsy was present in 31/43 patients. Additionally, interphase molecular cytogenetics based on fluorescence in situ hybridization (FISH) were available in 33/43 patients. Presence of del(17p), t(4;14), t(14;16), t(14;20) and chromosome 1 abnormalities were considered as high-risk, whereas all other karyotypes were classified as standard risk.
PET/CT
FDG and MET were synthesized in house with a 16 MeV Cyclotron (GE PETtrace 6; GE Healthcare, Milwaukee, USA). PET/CT was performed on a PET/CT scanner (Siemens Biograph mCT 64, Siemens, Knoxville, USA) within a mean interval of 3±2 days between scans.
FDG (299 ± 22 MBq) and MET (755 ± 82 MBq) were injected intravenously. CT scans were acquired after 60 (FDG) or 20 min (MET), respectively, using contrast-enhanced (FDG; depending on kidney function; dose modulation with a quality reference of 210 mAs) or low-dose spiral CT (80 mAs, 120 kV, a 512 × 512 matrix, 5 mm slice thickness, increment of 30 mm/s, rotation time of 0.5 s, and pitch index of 0.8) including the skull to the proximal thighs. Consecutively, PET emission data were acquired in 3D-mode with a 200 × 200 matrix with 2 min emission time per bed position. After decay and scatter correction, PET data were reconstructed iteratively with attenuation correction using a dedicated software (HD. PET, Siemens Esoft, Erlangen, Germany).
Image analysis
For FDG, criteria to define lesions as PET-positive were applied according to Zamagni et al. 13, for MET, lesions were visually determined as focally increased tracer retention as compared to surrounding normal tissue or contralateral structures. Presence and number of intra- and extramedullary (EMD) disease as well as location of lesions were recorded. Analysis both on a patient and a lesion basis was performed.
In order to correlate tracer uptake with BM involvement, a circular region of interest (ROI; diameter 20 mm) was placed over the respective posterior superior iliac spine and maximum (SUVmax) and mean standardized uptake values (SUVmean) were derived. Both SUV were compared to infiltration of malignant myeloma cells, as determined by bone marrow biopsy.
Immunhistochemical quantification of L-type amino acid transporter 1 (LAT1)-expression
In 15/43 patients, imaging results could be compared with histologic LAT1 expression of biopsy samples from the iliac crest. After deparaffinization and rehydration, the slides were placed in a pressure cooker in 0.01M citrate buffer (pH 7.0) and were heated for 7 min. Incubation with anti-CD98 antibody (Santa Cruz) was carried out at room temperature for 1 hour. Detection was performed with DAKO en vision system according to the manufacturer´s protocol. BM involvement was assessed as percentage of positive cells relative to total cell count (per example 40% of nuclear cells) in 10 high power fields (HPFs) per sample. To minimize selection bias due to heterogeneous bone marrow involvement, an average of 10 bone marrow sections per patient were analyzed.
Statistical analysis
Statistical analyses were performed using PASW Statistics software (version 22.0; SPSS, Inc. Chicago, IL). Quantitative values were expressed as mean ± standard deviation or median and range as appropriate. Comparisons of related metric measurements were performed using Wilcoxon-signed rank test. The Chi square- or Fisher exact test was conducted for comparison of frequency data between independent subgroups. For bivariant correlation analyses Spearman or Pearson correlation coefficients were calculated. All statistical tests were performed two-sided and a p-value < 0.05 was considered to indicate statistical significance. No correction for p-values was applied to adjust for multiple tests.
Results
42/43 (97.7%) patients presented with MM; one patient was diagnosed with solitary plasmacytoma. High-risk cytogenetics occurred in 18/43 (41.9%) subjects. Information on the degree of iliac BM infiltration with MM plasma cells was available for 31/43 patients and ranged from 0 to 90% (median, 30). Serum FLC ranged from 9 to 25100 mg/l (median, 337.4) and M gradients from 0 to 58.9 g/dl (median, 3.9) Patients´ characteristics are summarized in table 1 and supplementary table 1.
Table 1.
Patients' characteristics
| No. | Sex | Age | Myeloma type | Disease duration (months) | M gradient (g/l) | FLC (mg/l) | BM involvement (%) | Previous therapies |
|---|---|---|---|---|---|---|---|---|
| 1 | m | 76 | IgG κ | PD | 3.4 | 4440 (κ) | 40 | none |
| 2 | f | 64 | Plasmocytoma λ | PD | 0 | 9.0 (λ) | 0 | none |
| 3 | f | 65 | IgA κ | PD | 0 | 233 (κ) | 30 | none |
| 4 | f | 65 | IgA κ | 3 | 0 | 498 (κ) | 50 | CTx |
| 5 | m | 64 | IgG κ | 4 | 51.1 | 293 (κ) | 40 | CTx |
| 6 | f | 60 | IgG κ | 120 | 16.2 | 1237 (κ) | n/a | CTx, Auto-Tx |
| 7 | m | 54 | IgA κ | PD | 41.3 | 11.6 (κ) | 70 | none |
| 8 | f | 56 | IgA λ | PD | n/a | 345 (λ) | 25 | none |
| 9 | m | 60 | IgA λ | 9 | 3.5 | 2525 (λ) | 90 | CTx, Auto-Tx |
| 10 | m | 82 | IgA κ | 55 | n/a | 23.0 (κ) | n/a | CTx, |
| 11 | m | 67 | IgG λ | 15 | n/a | 15.7 (κ) | 5 | CTx, Auto-Tx |
| 12 | m | 59 | light chain κ | 7 | 3.4 | n/a | n/a | RTx |
| 13 | m | 48 | IgG κ | PD | 58.9 | 48.0 (κ) | n/a | none |
| 14 | m | 70 | IgG κ | 103 | 23.2 | 8833 (κ) | n/a | CTx, Auto-Tx |
| 15 | f | 69 | IgG κ | 10 | 26.4 | 687 (κ) | n/a | CTx, Auto-Tx |
| 16 | f | 63 | light chain κ | 32 | 0 | 3079 (κ) | n/a | CTx, Auto-Tx |
| 17 | f | 74 | IgG λ | PD | 31.9 | 337 (λ) | n/a | none |
| 18 | f | 64 | IgA κ | 6 | 0 | 27,4 (κ) | 0 | CTx; Auto-Tx |
| 19 | m | 56 | IgA κ | 32 | 44.7 | 0.5 (κ) | 50 | CTx, Auto-Tx |
| 20 | f | 62 | IgG κ | 11 | 24.4 | 254.3 (κ) | 15 | CTx |
| 21 | m | 63 | light chain κ | PD | 1.9 | 14014 (κ) | 70 | none |
| 22 | f | 48 | IgA λ | 63 | 6 | 177.7 (λ) | 3 | CTx, Auto-Tx |
| 23 | m | 51 | IgG κ | 34 | 24.6 | 828.0 (κ) | 60 | CTx, Auto-Tx |
| 24 | m | 47 | light chain κ | PD | 0 | 904.9 (κ) | 90 | none |
| 25 | m | 62 | light chain λ | PD | 1.8 | 444.0 (λ) | 15 | none |
| 26 | m | 59 | IgG κ | 37 | 0 | 5.0 (κ) | 0 | CTx, Auto-Tx |
| 27 | m | 59 | IgG κ | 4 | 26.3 | 551.2 (κ) | 40 | CTx |
| 28 | m | 65 | IgG κ | 89 | 10.4 | 37.8 (κ) | n/a | CTx, Auto-Tx |
| 29 | f | 65 | IgA κ | 12 | 0 | 2387 (κ) | 90 | CTx, Auto-Tx |
| 30 | f | 39 | IgG λ | 58 | 31.0 | 97.5 (λ) | n/a | CTx, Auto-Tx |
| 31 | f | 68 | IgG λ | 31 | 29.8 | 1673 (λ) | 35 | CTx, Auto-Tx |
| 32 | m | 73 | IgG κ | 72 | 4.7 | 25100 (κ) | 70 | CTx, Auto-Tx |
| 33 | m | 62 | IgG κ | 199 | 13.0 | 14015 (κ) | 20 | CTx, Auto-Tx |
| 34 | m | 62 | light chain λ | 47 | 0 | 240.8 (λ) | 5 | CTx, Auto-Tx |
| 35 | m | 72 | IgG λ | PD | 35.4 | 5297 (λ) | 90 | none |
| 36 | f | 53 | light chain κ | 122 | 0 | 99.0 (κ) | 15 | CTx, Auto-Tx |
| 37 | m | 63 | light chain λ | 6 | 0 | 12.4 (λ) | 0 | CTx |
| 38 | f | 63 | IgG κ | 22 | 47.5 | 1907 (κ) | 30 | CTx, Auto-Tx |
| 39 | m | 63 | IgG κ | 86 | 4.1 | 535.0 (κ) | n/a | CTx, Auto-Tx |
| 40 | m | 60 | light chain λ | 22 | 0 | 124.2 (λ) | 20 | CTx, Auto-Tx |
| 41 | f | 63 | light chain λ | 23 | 0 | 1876 (λ) | 10 | CTx, Auto-Tx |
| 42 | f | 49 | IgA λ | 72 | 0 | 30.8 (λ) | 0 | CTx, Auto-Tx |
| 43 | f | 40 | light chain κ | 39 | 0 | 50.0 (κ) | n/a | CTx, Auto-Tx |
m = male. f = female. Disease duration is given in months. PD = primary diagnosis. CTx = chemotherapy including novel agents, RTx = radiotherapy. Auto-Tx = autologous stem cell transplantation. n/a = information not available
Patient-based analysis
In PET/CT examinations with MET, MM lesions were detected in 39/43 subjects (90.7%). In contrast, FDG did not identify any focal lesions in 10 patients (FDG-positive lesions in 33/43 patients, 76.7%; p<0.05; figure 1). The case presenting with solitary plasmacytoma was identified by PET using both tracers.
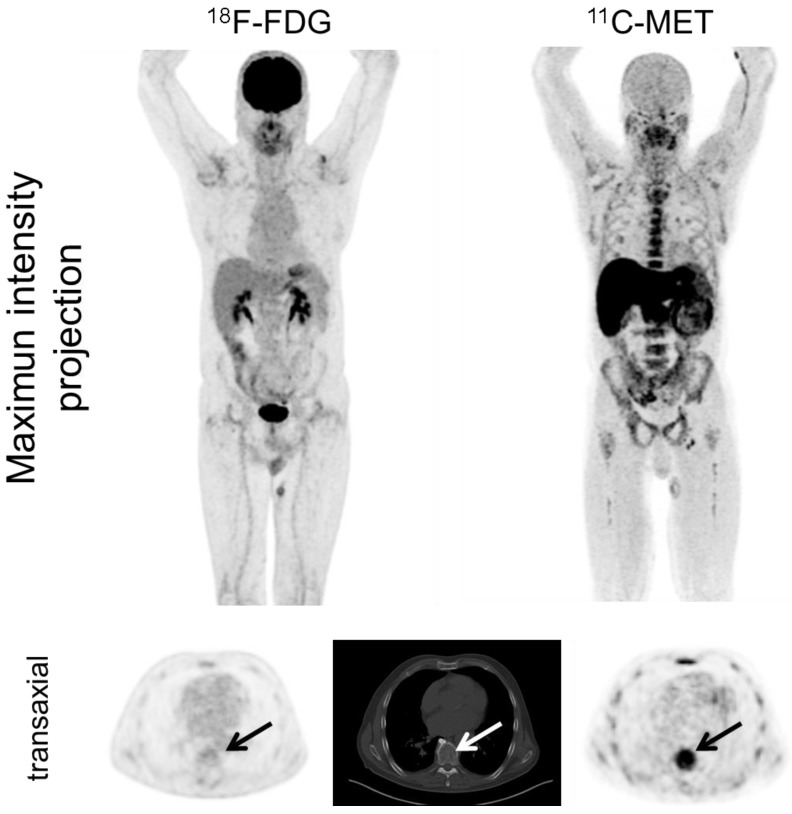
Figure 1.
Display of a patient (patient #11) with a history of Ig G λ MM after autologous stem cell transplant who was referred due to still low but rising serum free light chains. Whereas PET/CT with FDG did not depict hypermetabolic intra- or extramedullary foci suspicious for active MM, MET demonstrated inhomogenous, focally increased tracer uptake of the axial (transaxial slice of thoracic vertebra Th 8, arrows) as well as appendicular skeleton (maximum intensity projection). Bone marrow biopsy confirmed low tumor cell burden of 5%.
Additionally, MET identified extramedullary lesions in 12/43 patients (27.9%), whereas FDG recorded 10/43 patients with EMD (23.3%). Lymph node involvement was most commonly seen (7/43 [MET] vs. 6/43 [FDG]), followed by manifestations in soft tissue (6/43 [MET] vs. 4/43 [FDG]), and lungs (2/43 [MET] vs. 2/43 [FDG]). Regarding intramedullary MM, MET revealed involvement of the appendicular skeleton in 37/43 patients (86.0%), whereas 9 cases were missed with FDG (FDG-positive lesions in 28/43 patients, 65.1%) (supplementary table 3).
Lesion-based analysis
Imaging with MET demonstrated more focal lesions than FDG in 28/43 patients (65.1%, p<0.001). In the remaining patients, an equal number of MM manifestations were detected with both tracers. Most of the lesions exclusively identified by MET located within the bone marrow compartment (28/28; 100%) and in the appendicular skeleton (24/28; 85.7%; figure 2).
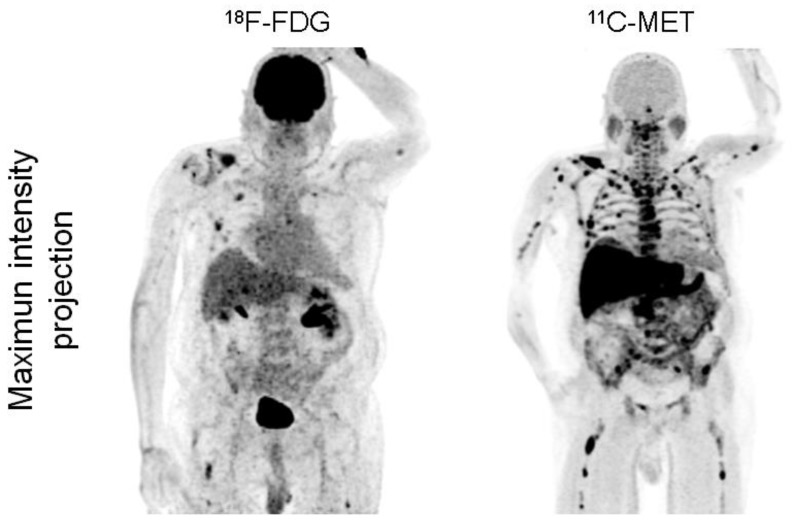
Figure 2.
Display of a patient (patient #1) with newly diagnosed MM Ig G κ. FDG depicts faint to moderate uptake in the skeleton in contrast to highly intense lesions in MET, e.g. in the right clavicle. Multiple additional intramedullary lesions are clearly detected by MET.
MET-PET/CT detected more than 20 FL in 35/39 patients (89.7%): 3/35 (8.6%) had >20 focal lesions detected, 7/35 patients (20.0%) >50 and 25/35 patients (71.4%) >100 FL. The remaining 4/39 (10.3%) subjects had less than 20 FL. With FDG, 16/33 (48.5%) subjects had less than 20 FL, 3/33 (9.1%) >20, 4/33 (12.1%) >50 and 10/33 (30.3%) >100 FL.
Interestingly, in the 12 patients with EMD, MET detected lesions which were missed by FDG (6/12 subjects; 50%; figure 3). In numbers, FDG detected a total of 28 EMD foci (lymph nodes, n=17; soft tissue, n=9; lungs, n=2), whereas MET depicted 42 lesions in lymph nodes (n=23), soft tissue (n=14), and lungs (n=5). The lesions exclusively visualized by MET were lymphonodal (n=6), soft tissue (n=5) and pulmonary (n=3) manifestations of origin (supplementary table 3).
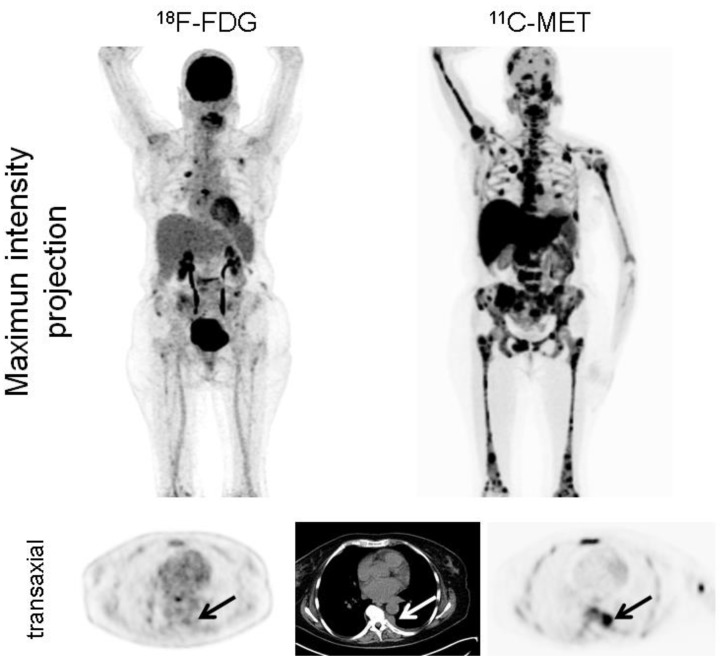
Figure 3.
Display of a patient (patient #6) with MM Ig G κ. FDG depicts moderate uptake in the skeleton in contrast to highly intense lesions in MET (maximum intensity projection, upper row). Additionally, pleural extramedullary disease was exclusively detected by MET (arrows, transaxial slices, lower row). The patient deceased 5 months later.
Immunohistochemical work-up for LAT1
In 15 patients (8 subjects with newly diagnosed and 7 with heavily pre-treated MM), imaging results could be compared to immunohistological staining for LAT1. 13/15 samples displayed homogeneous strong cytoplasmic and membraneous expression of the transporter by all myeloma cells. No differences in LAT1 expression or intensity on the cell level could be observed between the individual specimens (figure 4).
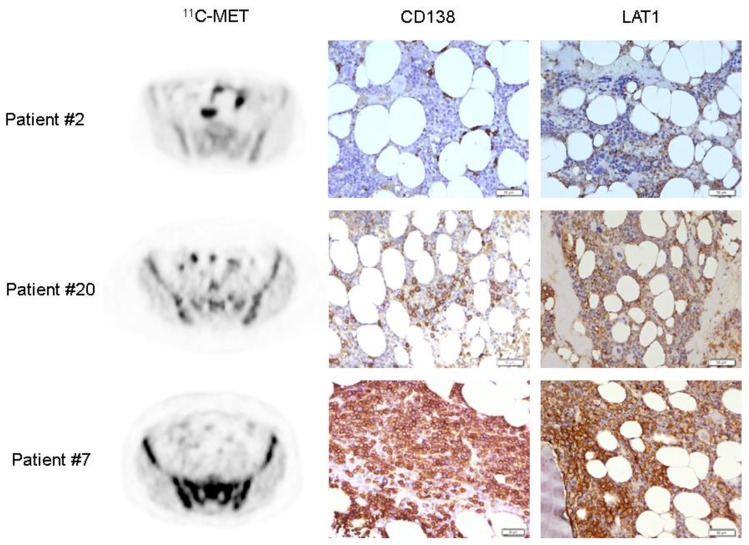
Figure 4.
Histological assessment of LAT1 expression on MM bone marrow biopsies. LAT1 expression was detected in 13/15 samples using an anti-CD98 antibody. Sections were counterstained with H&E. Three exemplary cases are shown with corresponding anti-CD138 staining for comparison with the degree of MM-cell bone marrow infiltration (patient #2, 0%; patient #20, 15%; patient #7, 70%). Magnification 200x.
Correlation of imaging parameters with laboratory findings and cytogenetics
MET- as well as FDG-positivity was independent of FLC levels and/or M gradients. The presence of focal intramedullary lesions in MET or FDG-PET was indicative for abnormal β2m and FLC levels (MET, p=0.006 and p=0.01, respectively; FDG, p=0.02 and p=0.01). A slight trend towards higher intramedullary SUVmax in patients with high-risk cytogenetics (p=0.12) was also observed for MET-PET. Semiquantiative parameters (SUVmean; SUVmax) as well as presence or number of extramedullary lesions did not correlate with cytogenetics or pathologic changes laboratory values including albumin (<3.5 g/dl), creatinine (>1.2 mg/dl), β2m (>3.5 mg/l) or levels of free light chains (>100).
Correlation of tracer uptake with bone marrow involvement
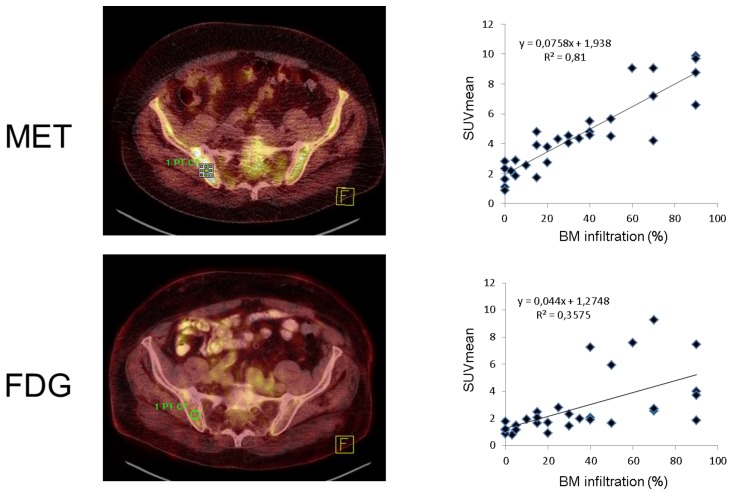
In the 31 patients in whom BM infiltration was histologically assessed, semiquantative FDG and MET uptake values were highly correlated (SUVmean, r=0.66; SUVmax, r=0.64). Both SUVmean as well as SUVmax significantly correlated with the degree of malignant plasma cell infiltration for FDG- as well as MET-PET (p<0.001). SUVmean (r=0.90) and SUVmax (r=0.88) of MET-PET demonstrated stronger correlations with tumor cell burden than FDG-PET derived parameters (r=0.60 and r=0.58, respectively) (figure 5). The individual numbers for all biopsies available are given in supplementary table 2.
Figure 5.
Correlation of radiotracer uptake with bone marrow infiltration. Assessment of iliac crest bone marrow involvement by FDG and MET-PET for a single patient (patient #31, transaxial fused PET/CT slices, left). Dot plots for SUVmean for all individual patients (n=31) with a strong correlation for FDG (r=0.6) and a very strong correlation for MET (r=0.9).
Discussion
This study aimed at the validation of the radiolabeled amino acid MET as advanced radiotracer for functional imaging of multiple myeloma. In addition, a comparison to the established radiotracer FDG has been performed. In our cohort, MET provided more accurate information on both intra- as well as extramedullary disease and appeared to be superior to FDG in the vast majority of patients. It proved useful in all stages of the disease ranging from untreated to heavily pre-treated patients having undergone SCT. Due to its potential to reliably reflect MM biology by depicting amino acid metabolism, MET served as a superior readout for non-invasive determination of tumor burden.
This finding is in line with previously published reports on the feasibility and potential diagnostic superiority of MET-PET/CT in comparison to FDG 12, 14. However, in clinical studies conducted in Japan, the advantage of 11C-MET was confined to intramedullary lesions. In our cohort, this tracer also depicted more extramedullary lesions in half of patients (6/12) presenting with EMD. Of note, EMD was exclusively depicted by MET in 2 patients.
Interestingly, MET-PET-positivity was independent of presence and extent of FLC and/or M gradient elevation. The fact that MET-PET/CT was positive in EMD as well as in subjects with non-elevated M gradient and/or FLC suggests that tracer uptake might be due to increased tumor anabolism and might therefore reflect true tumor burden 15. This notion is corroborated by the fact that MET uptake in the posterior superior iliac spine highly correlated with the degree of malignant plasma cell infiltration and even outperformed FDG. Of note, L-type amino acid transporter 1 (LAT1) as the major uptake mechanism of 11C-MET was highly expressed by all myeloma cells in almost all samples analyzed. This finding is in contrast to a recent study which reported on an association of LAT1 expression with proliferation and poor prognosis in newly diagnosed MM patients 16. A possible explanation might be given be the different study populations as both newly diagnosed but also heavily pre-treated patients were enrolled in our study. Further larger studies are needed to fully assess the prognostic value of LAT1 overexpression.
Based on the data presented here, MET might prove a more versatile marker of disease burden, especially as it depicts both low- and high-grade myeloma lesions. In contrast, FDG might be limited to more aggressive subclones of MM and therefore prone to underestimation of true disease extent. However, more research is necessary to further investigate this finding. Additionally, the impact of MET positivity in MM has not been clarified yet. Whereas FDG has proven its prognostic value in a number of studies 7, 15, 17, the additional value of the increased sensitivity of MET has to be demonstrated. Furthermore, no study has assessed the performance of this tracer in therapy monitoring so far. A pilot trial investigating the performance of very early treatment response MET-PET in patients with MM would be warranted. Potential drawbacks of the amino acid tracer concerning reduced sensitivity within the liver due to high physiologic uptake can be neglected, given the very rare hepatic involvement in this disease and the broad availability of combined PET/CT imaging. Accumulation of the radiotracer in normal bone marrow could be discriminated from active disease in our series. Even faint BM invasion of 5% was correctly demonstrated. However, a limitation of MET for routine clinical applications might be the requirement for an on-site cyclotron.
This study has several limitations. It only comprised a limited number of patients, thereby limiting the statistical power. Histopathological confirmation of active disease, especially MET-positive, FDG-negative EMD manifestations was not present in all lesions. However, uptake of exclusively MET-avid lesions was typical for MM and projected on pathological substrates in CT imaging. Selection bias affecting the assessment of bone marrow involvement due to heterogeneous involvement cannot be completely excluded (though an average number of10 sections per patient were analysed to minimize bias).
Concluding, our results suggest that MET is superior to FDG for staging and re-staging of MM. It is able to detect both intra- as well as extramedullary MM manifestations. Additionally, tracer uptake correlates with BM involvement and seems to be a more accurate marker of tumor biology. Further research investigating this tracer including its potential in early treatment response prediction is warranted.
Conclusion
In this prospective study, MET was superior to FDG for staging and re-staging of both intra- and extramedullary MM lesions. Tracer uptake correlates with BM involvement and appears to be a more accurate marker of tumor biology.
Supplementary Material
Supplementary Tables.
Acknowledgments
This work was supported by the Wilhelm-Sander-Stiftung (grant no. 2013.906.1).
References
- 1.Phekoo KJ, Schey SA, Richards MA, Bevan DH, Bell S, Gillett D. et al. A population study to define the incidence and survival of multiple myeloma in a National Health Service Region in UK. British journal of haematology. 2004;127:299–304. doi: 10.1111/j.1365-2141.2004.05207.x. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: a cancer journal for clinicians. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Durie BG. The role of anatomic and functional staging in myeloma: description of Durie/Salmon plus staging system. European journal of cancer. 2006;42:1539–43. doi: 10.1016/j.ejca.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 4.Durie BG, Waxman AD, D'Agnolo A, Williams CM. Whole-body (18)F-FDG PET identifies high-risk myeloma. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2002;43:1457–63. [PubMed] [Google Scholar]
- 5.Bartel TB, Haessler J, Brown TL, Shaughnessy JD Jr, van Rhee F, Anaissie E. et al. F18-fluorodeoxyglucose positron emission tomography in the context of other imaging techniques and prognostic factors in multiple myeloma. Blood. 2009;114:2068–76. doi: 10.1182/blood-2009-03-213280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hillner BE, Siegel BA, Shields AF, Liu D, Gareen IF, Hunt E. et al. Relationship between cancer type and impact of PET and PET/CT on intended management: findings of the national oncologic PET registry. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2008;49:1928–35. doi: 10.2967/jnumed.108.056713. [DOI] [PubMed] [Google Scholar]
- 7.Lapa C, Luckerath K, Malzahn U, Samnick S, Einsele H, Buck AK. et al. 18 FDG-PET/CT for prognostic stratification of patients with multiple myeloma relapse after stem cell transplantation. Oncotarget. 2014;5:7381–91. doi: 10.18632/oncotarget.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terpos E, Moulopoulos LA, Dimopoulos MA. Advances in imaging and the management of myeloma bone disease. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2011;29:1907–15. doi: 10.1200/JCO.2010.32.5449. [DOI] [PubMed] [Google Scholar]
- 9.Luckerath K, Lapa C, Spahmann A, Jorg G, Samnick S, Rosenwald A. et al. Targeting paraprotein biosynthesis for non-invasive characterization of myeloma biology. PloS one. 2013;8:e84840. doi: 10.1371/journal.pone.0084840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luckerath K, Lapa C, Albert C, Herrmann K, Jorg G, Samnick S. et al. 11C-Methionine-PET: a novel and sensitive tool for monitoring of early response to treatment in multiple myeloma. Oncotarget. 2015;6:8418–29. doi: 10.18632/oncotarget.3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dankerl A, Liebisch P, Glatting G, Friesen C, Blumstein NM, Kocot D. et al. Multiple Myeloma: Molecular Imaging with 11C-Methionine PET/CT-Initial Experience. Radiology. 2007;242:498–508. doi: 10.1148/radiol.2422051980. [DOI] [PubMed] [Google Scholar]
- 12.Nakamoto Y. Clinical contribution of PET/CT in myeloma: from the perspective of a radiologist. Clinical lymphoma, myeloma & leukemia. 2014;14:10–1. doi: 10.1016/j.clml.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Zamagni E, Patriarca F, Nanni C, Zannetti B, Englaro E, Pezzi A. et al. Prognostic relevance of 18-F FDG PET/CT in newly diagnosed multiple myeloma patients treated with up-front autologous transplantation. Blood. 2011;118:5989–95. doi: 10.1182/blood-2011-06-361386. [DOI] [PubMed] [Google Scholar]
- 14.Okasaki M, Kubota K, Minamimoto R, Miyata Y, Morooka M, Ito K. et al. Comparison of (11)C-4'-thiothymidine, (11)C-methionine, and (18)F-FDG PET/CT for the detection of active lesions of multiple myeloma. Annals of nuclear medicine. 2015;29:224–32. doi: 10.1007/s12149-014-0931-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okubo S, Zhen HN, Kawai N, Nishiyama Y, Haba R, Tamiya T. Correlation of L-methyl-11C-methionine (MET) uptake with L-type amino acid transporter 1 in human gliomas. Journal of neuro-oncology. 2010;99:217–25. doi: 10.1007/s11060-010-0117-9. [DOI] [PubMed] [Google Scholar]
- 16.Isoda A, Kaira K, Iwashina M, Oriuchi N, Tominaga H, Nagamori S. et al. Expression of L-type amino acid transporter 1 (LAT1) as a prognostic and therapeutic indicator in multiple myeloma. Cancer science. 2014;105:1496–502. doi: 10.1111/cas.12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nanni C, Zamagni E, Celli M, Caroli P, Ambrosini V, Tacchetti P. et al. The value of 18F-FDG PET/CT after autologous stem cell transplantation (ASCT) in patients affected by multiple myeloma (MM): experience with 77 patients. Clinical nuclear medicine. 2013;38:e74–9. doi: 10.1097/RLU.0b013e318266cee2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Tables.