Abstract
Purpose
Data from two noninferiority trials of a dexamethasone-sparing regimen were assessed for the impact of acute nausea and vomiting on delayed outcome in patients undergoing moderately emetogenic chemotherapy (MEC) or anthracycline plus cyclophosphamide (AC).
Methods
Chemo-naive patients were randomized to receive palonosetron (0.25 mg IV) plus dexamethasone (8 mg IV) on day 1 of chemotherapy, or the same regimen followed by oral dexamethasone on days 2 and 3 in the MEC (n = 237) and AC (n = 380) cohorts. Patients were divided into two groups according to whether or not they experienced vomiting and/or moderate-to-severe nausea during the acute phase (high- and low-risk groups, respectively). Primary efficacy endpoint was the complete protection (CP) against delayed vomiting and moderate-to-severe nausea. Patient’s satisfaction (0–100 mm visual analog scale) was also analyzed.
Results
Among the 209 low-risk patients undergoing MEC, delayed CP occurred in 82.9 % of those who received single-dose dexamethasone and 89.8 % of those who received 3-day dexamethasone (P = 0.165). Of the 271 low-risk patients undergoing AC, CP was achieved in 71.7 % of those treated with single-dose dexamethasone and 84.2 % treated with 3-day dexamethasone (P = 0.019). In spite of these observations, the patient satisfaction data was not influenced by dexamethasone regimen. In both cohorts, occurrence of acute vomiting or moderate-to-severe nausea was the key independent-predictor for delayed vomiting or nausea, respectively.
Conclusions
The dexamethasone-sparing regimen provides adequate delayed protection in patients undergoing MEC who are at low risk for delayed symptoms, and can still be discussed for low-risk AC patients as the daily difference in control is modest. Additional dexamethasone doses can be customized on the basis of occurrence or absence of acute symptoms in the first cycle of MEC and even AC.
Keywords: Palonosetron, Dexamethasone, Delayed CINV, MEC, AC
Introduction
Optimal prevention for chemotherapy-induced nausea and vomiting (CINV) depends on recognition of the intrinsic emetogenicity of a chemotherapeutic agent as well as an understanding of its potential to induce acute symptoms (within the first 24 h) or delayed symptoms (typically between days 2 and 5 after single-day chemotherapy) [1]. Delayed CINV may occur in patients receiving moderately emetogenic chemotherapy (MEC) containing cyclophosphamide, anthracyclines, carboplatin, oxaliplatin, or irinotecan [1]. In addition, combination chemotherapy regimens often increase the potential to induce CINV compared to most individual agents. Accordingly, women who receive the combination of an anthracycline and cyclophosphamide (AC) are at a particularly high risk of acute and delayed CINV [2, 3]. In light of this, some of the major anti-emetic guidelines have reclassified this combination as highly emetogenic [3, 4], while those of the MASCC organization have categorized MEC as AC or non-AC MEC [2].
Two recent phase III trials comparing a 1-day dexamethasone regimen to a 3-day dexamethasone regimen, both combined with palonosetron on day 1, in patients undergoing either a broad range of MEC regimens or AC showed similar outcomes with the two regimens in both settings [5, 6]. In addition, a meta-analysis of individual patient data from these two studies provided evidence that, irrespective of age, the 1-day regimen is not associated with a loss in overall anti-emetic protection in women undergoing AC [7]. In spite of routine use of prophylactic dexamethasone in oncology practice [8], concerns remain about tolerability of this agent, especially when administered as part of multi-day anti-emetic regimens [9]. It is well known that the occurrence of delayed nausea and vomiting may be strongly influenced by the control of these symptoms within the first 24 h after chemotherapy initiation [10, 11]. This evidence prompted us to reanalyze data from the cohorts of patients undergoing either MEC or AC who were included in the two clinical trials of the dexamethasone-sparing regimen to provide separate estimates of effectiveness against delayed CINV depending on whether or not vomiting and significant (i.e., moderate-to-severe) nausea were controlled in the acute phase. In the current analysis, efficacy findings from the high-risk cohort of AC can provide a framework for interpretation of the results from the cohort of MEC. Overall, the estimates may permit to evaluate anti-emetic effectiveness in the delayed phase without the confounding effect due to the occurrence of acute symptoms. They also should help clinicians in customized decision making about whether or not to administer additional dexamethasone doses in prevention of delayed CINV.
Patients and methods
Patients and treatment
The present reanalysis is based on two cohorts of chemo-naive patients who were included in two randomized clinical studies investigating the dexamethasone-sparing regimen [5, 6]. Patients who received either MEC for a solid tumor or AC-containing chemotherapy for breast cancer, took the study medication, and completed the follow-up period (days 1–5 following chemotherapy initiation) were evaluable according to a modified intention-to-treat analysis.
Assessments
Patients made daily entries in their diary for 5 days after starting chemotherapy. Patients included in the analysis assessed the severity of nausea by either a visual analog scale (VAS) [6] or a verbal category scale (no nausea, mild = did not interfere with normal daily life; moderate = interfered with normal daily life; or severe = required the patient to be bedridden) [5]. For the purpose of this post hoc analysis, patients were divided into two groups according to the effectiveness of prophylaxis against nausea and vomiting during the first 24 h (acute phase): a low-risk group, which included patients who experienced neither vomiting nor moderate-to-severe nausea within the acute phase, and a high-risk group, which included patients who experienced one or both of these symptoms. The primary efficacy end point was the proportion of patients with protection against both vomiting and moderate-to-severe nausea on days 2 through 5 (delayed phase) after the first cycle of chemotherapy. The secondary end points were protection against delayed vomiting, protection against delayed moderate-to-severe nausea, duration of delayed symptoms, and maximum severity of delayed nausea. Duration was defined according to the number of days during the delayed phase (4-day period) when patients experienced either vomiting or nausea: ≥2 out of the 4 days was considered severe, and 1 out of the 4 days was considered less severe. The patient’s satisfaction with anti-emetic coverage was assessed by a satisfaction VAS that was a 100-mm line marked “not at all satisfied” at the left-hand end and “totally satisfied” at the right-hand end. Patients completed the satisfaction VAS on day 6 during their first cycle of chemotherapy.
Statistical analysis
For patients who rated the severity of their nausea on a VAS, the daily scores were converted into verbal categories according to the following schema: no nausea defined as a VAS score of less than 5 mm; mild nausea defined as a VAS score of 5 to 24 mm; moderate nausea defined as a VAS score of 25 to 74 mm; and severe nausea defined as a VAS score of 75 to 100 mm. The cutoff selected for severe nausea was defined according to the results of a prospective study evaluating the concordance between a four-point verbal category scale and a VAS in assessing nausea severity in patients undergoing chemotherapy [12]. Fisher’s exact test was used to compare categorical variables in the low-risk and high-risk groups.
Multivariable logistic-regression models with occurrence (yes vs. no) of either delayed vomiting or nausea (moderate-to-severe or mild) as the dependent variable were generated to test for differences between anti-emetic regimens, while controlling for standard covariates (i.e., age, gender, and alcohol consumption as appropriate) and the occurrence of acute vomiting and moderate-to-severe nausea. To determine the occurrence of first-order interactions between anti-emetic regimen and standard covariates, they were included one by one into the statistical model. If the interaction term was not statistically significant, it was removed from the model. Results were reported as odds ratios (ORs) with associated 95 % confidence intervals (CIs) and two-tailed P values. All the secondary analyses were evaluated in an explorative or descriptive manner, and therefore, no adjustment for multiplicity was applied. The reported P values are intended for interpretation of trends, rather than for claims of significance. All P values were two-tailed, and a P < 0.05 was considered statistically significant. All statistical analyses were performed using SAS software (version 9.1; SAS Institute, Cary, NC).
Results
Of the 624 evaluable patients who were enrolled in the two clinical trials, 237 received a MEC regimen for a solid tumor and 380 received AC for breast cancer. Seven patients undergoing AC for malignancy other than breast cancer (four in the 1-day regimen, and three in the 3-day regimen) were excluded from the analysis. Table 1 lists the characteristics of the patients.
Table 1.
Baseline characteristics of the patients by chemotherapy cohort
| Characteristic by cohort | Palo plus 1-day Dex | Palo plus 3-day Dex | P valuea | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| MEC, evaluable patients | 125 | 100 | 112 | 100 | |
| Age (years) | |||||
| Median (min–max) | 59 (31–77) | 60 (35–80) | |||
| <50 years | 27 | 21.6 | 24 | 21.4 | 1.0 |
| Gender | 0.515 | ||||
| Male | 63 | 50.4 | 51 | 45.5 | |
| Female | 62 | 49.6 | 61 | 54.5 | |
| Early stage disease | 61 | 48.8 | 65 | 58.0 | 0.192 |
| Primary tumor | 0.792 | ||||
| Breast | 28 | 22.4 | 31 | 27.7 | |
| Colorectal | 66 | 52.8 | 53 | 47.3 | |
| Lung | 15 | 12.0 | 13 | 11.6 | |
| Other | 16 | 12.8 | 15 | 13.4 | |
| Chemotherapy regimen | 0.840 | ||||
| Oxaliplatin-based | 63 | 50.4 | 52 | 46.4 | |
| Carboplatin-based | 20 | 16.0 | 16 | 14.3 | |
| Irinotecan-based | 14 | 11.2 | 15 | 13.4 | |
| Otherb | 28 | 22.4 | 29 | 25.9 | |
| Alcohol consumption | 0.604 | ||||
| Never | 67 | 53.6 | 56 | 50.0 | |
| Everyday | 58 | 46.4 | 56 | 50.0 | |
| Risk group | 0.841 | ||||
| Lowc | 111 | 88.8 | 98 | 87.5 | |
| Highd | 14 | 11.2 | 14 | 12.5 | |
| AC, evaluable patients | 186 | 100 | 194 | 100 | |
| Age (years) | |||||
| Median (min–max) | 51 (28–77) | 50 (26–78) | |||
| <50 years | 81 | 43.5 | 93 | 47.9 | 0.411 |
| Early-stage breast cancer | 186 | 100 | 194 | 100 | |
| Alcohol consumption | 0.559 | ||||
| Never/Occasionallye | 174 | 93.5 | 178 | 91.7 | |
| Everyday | 12 | 6.5 | 16 | 8.3 | |
| Risk group | 0.257 | ||||
| Lowc | 138 | 74.2 | 133 | 68.6 | |
| Highd | 48 | 25.8 | 61 | 31.4 | |
Palo palonosetron, Dex dexamethasone, MEC moderately emetogenic chemotherapy, AC anthracycline (doxorubicin or epirubicin) plus cyclophosphamide
aFisher’s exact test or chi-square test (two-tailed)
bPatients undergoing anthracycline or cyclophosphamide-containing chemotherapy
cLow-risk patients were free from both vomiting and moderate-to-severe nausea in the acute phase
dHigh-risk patients experienced vomiting or moderate-to-severe nausea in the acute phase
eIncluding 53 patients with missing data for alcohol consumption
Protection against delayed vomiting and nausea
Among the low-risk patients undergoing MEC, there were no statistically significant between-treatment differences according to each of the three measures of protection: no occurrence of both vomiting and moderate-to-severe nausea during days 2 through 5 (primary end point 82.9 vs. 89.8 %, respectively, in the 1- and 3-day regimens; risk difference [RD] = −6.9 %; 95 % CI, −16.3 to 2.5 %; P = 0.165); no occurrence of vomiting alone (89.2 vs. 93.9 %; RD = −4.7 %; 95 % CI, −12.3 to 2.9 %; P = 0.324); and no occurrence of moderate-to-severe nausea alone (94.6 vs. 92.9 %; RD = 3.3 %; 95 % CI, −4.8 to 8.3 %; P = 0.776). Among the 28 patients at high-risk, there were also no statistically significant differences between the two treatments according to any of the three measures of delayed protection (data not shown).
Among the low-risk women undergoing AC, the 1-day regimen was significantly less effective than the 3-day regimen according to two of the three measures of protection: no occurrence of both symptoms during days 2 through 5 (primary end point 71.7 vs. 84.2 %, respectively; RD = −12.5 %; 95 % CI, −22.4 to −2.6 %; P = 0.019); no occurrence of vomiting alone (87.7 vs. 94 %; RD = −6.3 %; 95 % CI, −13.2 to 0.6 %; P = 0.093); and no occurrence of moderate-to-severe nausea alone (77.5 vs. 88.7 %; RD = −11.2 %; 95 % CI, −20.1 to −2.2 %; P = 0.016). Among the high-risk patients, the 1-day regimen resulted in much less control of delayed symptoms than the 3-day regimen but the differences were statistically significant for only two of the three measures of protection: no occurrence of both symptoms during days 2 through 5 (14.6 vs. 42.6 %, respectively; RD = −28 %; 95 % CI, −45.4 to −10.7 %; P = 0.002); no occurrence of delayed vomiting alone (54.2 vs. 67.2 %; RD = −13.1 %; 95 % CI, −31.4 to 5.4 %; P = 0.173); and no occurrence of moderate-to-severe nausea alone (25 vs. 49.2 %; RD = −24.2 %; 95 % CI, −42.6 to −5.8 %; P = 0.011).
Daily protection against delayed symptoms
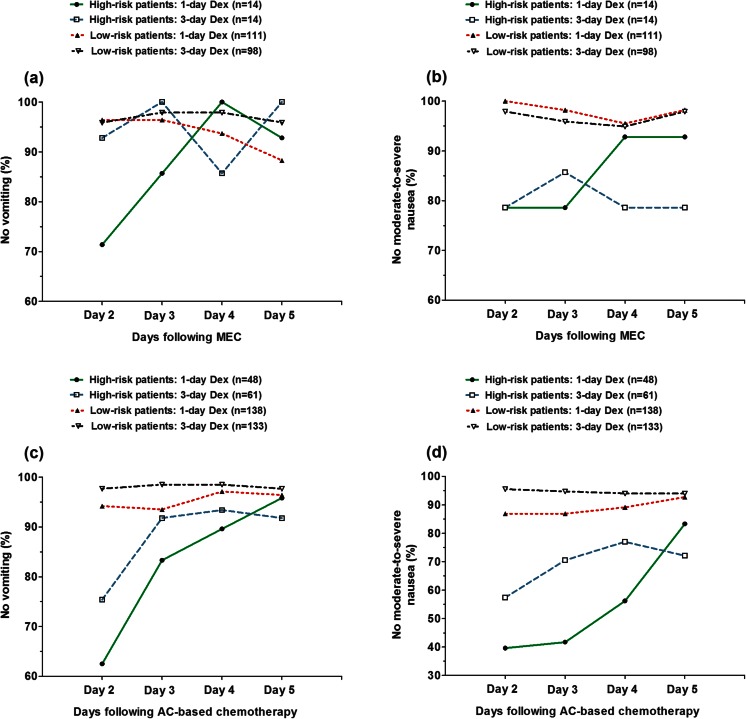
Among the low-risk patients undergoing MEC, no statistically significant incremental improvement (i.e., the between-treatment difference) was observed with additional dexamethasone doses for control of either vomiting or moderate-to-severe nausea during each day of the delayed phase (Fig. 1). Among the patients at high risk, there were no significant between-treatment differences for control of delayed vomiting or moderate-to-severe nausea at each time point.
Fig. 1.
Proportions of patients in the MEC and AC cohorts who experience no vomiting or moderate-to-severe nausea in the delayed phase, by risk and treatment groups. Low-risk patients were free from vomiting and moderate-to-severe nausea during the acute phase; high-risk patients experienced vomiting or moderate-to-severe nausea during the acute phase. Patients received palonosetron plus dexamethasone on day 1 either with or without dexamethasone on days 2 and 3. MEC moderately emetogenic chemotherapy, AC anthracycline plus cyclophosphamide, Dex dexamethasone
Among the low-risk patients undergoing AC, the incremental improvement observed with additional dexamethasone doses for vomiting control was of only minimal magnitude (≤5 percentage points) during each day of the delayed phase (Fig. 1). The incremental improvement for control of moderate-to-severe nausea was significant but of modest magnitude on day 2 (9 percentage points; P = 0.018) and on day 3 (8 percentage points; P = 0.035) post-chemotherapy. As expected, the incremental improvement observed with additional dexamethasone doses was of greater magnitude among patients in the high-risk group (Fig. 1). There were no significant between-treatment differences in the proportion of patients protected against delayed vomiting on days 2 through 5 post-chemotherapy. Additional dexamethasone doses improved to a remarkable extent the protection rates against moderate-to-severe nausea on day 2 (P = 0.083), on day 3 (P = 0.003), and on day 4 (P = 0.024) post-chemotherapy.
Duration and severity of delayed symptoms
Irrespective of anti-emetic subgroup, there were no significant differences in duration of either delayed vomiting or delayed nausea in patients undergoing MEC who were at high or low risk (Table 2). Severe nausea was seldom observed in the low-risk group: one of 38 patients in the 1-day dexamethasone subgroup (2.6 %) and one of 30 in the 3-day dexamethasone subgroup (3.3 %) recorded severe nausea on at least 1 day in the delayed phase. Likewise, the patients at high risk seldom reported severe nausea: one of nine patients in the 1-day dexamethasone subgroup and none of ten in the 3-day dexamethasone subgroup recorded severe nausea on at least 1 day in the delayed phase.
Table 2.
Duration and severity of delayed CINV in patients undergoing MEC by risk group
| End point | Risk groupa | Palo plus 1-day Dex, n/N (%) | Palo plus 3-day Dex, n/N (%) | P valueb |
|---|---|---|---|---|
| Duration of delayed vomitingc | High | 1.0 | ||
| 1 day | 3/5 (60) | 1/2 (50) | ||
| ≥2 days | 2/5 (40) | 1/2 (50) | ||
| Duration of delayed nauseac | High | 0.303 | ||
| 1 day | 3/9 (33.3) | 1/10 (10) | ||
| ≥2 days | 6/9 (66.7) | 9/10 (90) | ||
| Severity of delayed nausea | High | 1.0 | ||
| Mild | 5/9 (55.6) | 6/10 (60) | ||
| Moderate-to-severe | 4/9 (44.4) | 4/10 (40) | ||
| Duration of delayed vomitingc | Low | 0.615 | ||
| 1 day | 8/12 (66.7) | 5/6 (83.3) | ||
| ≥2 days | 4/12 (33.3) | 1/6 (16.7) | ||
| Duration of delayed nauseac | Low | 0.328 | ||
| 1 day | 15/38 (39.5) | 16/30 (53.3) | ||
| ≥2 days | 23/38 (60.5) | 14/30 (46.7) | ||
| Severity of delayed nausea | Low | 0.539 | ||
| Mild | 32/38 (84.2) | 23/30 (76.7) | ||
| Moderate-to-severe | 6/38 (15.8) | 7/30 (23.3) |
MEC moderately emetogenic chemotherapy, Palo palonosetron, Dex dexamethasone, N total number of patients experiencing symptom
aHigh-risk patients experienced vomiting or moderate-to-severe nausea during the acute phase; low-risk patients were free from vomiting and moderate-to-severe nausea during the acute phase
bFisher’s exact test (two-tailed)
cAssessment over 4-day period
Irrespective of anti-emetic subgroup, there were no significant differences in duration of either delayed vomiting or delayed nausea in patients undergoing AC who were at high or low risk (Table 3). Among the nauseated women at low risk, the severity of delayed nausea significantly differed between the two treatments (P = 0.039). Severe nausea was seldom observed in the low-risk group: 6 of 70 patients in the 1-day dexamethasone subgroup (8.6 %) and 2 of 55 in the 3-day dexamethasone subgroup (3.6 %) recorded severe nausea on at least 1 day in the delayed phase. The high-risk patients were more likely to report severe nausea: 16 of 45 patients in the 1-day dexamethasone subgroup (35.6 %) and 10 of 48 in the 3-day dexamethasone subgroup (20.8 %) recorded severe nausea on at least 1 day in the delayed phase.
Table 3.
Duration and severity of delayed CINV in women undergoing AC by risk group
| End point | Risk groupa | Palo plus 1-day Dex, n/N (%) | Palo plus 3-day Dex,n/N (%) | P valueb |
|---|---|---|---|---|
| Duration of delayed vomitingc | High | 1.0 | ||
| 1 day | 14/22 (63.6) | 12/20 (60) | ||
| ≥2 days | 8/22 (36.4) | 8/20 (40) | ||
| Duration of delayed nauseac | High | 0.798 | ||
| 1 day | 10/45 (22.2) | 9/48 (18.8) | ||
| ≥2 days | 35/45 (77.8) | 39/48 (81.2) | ||
| Severity of delayed nausea | High | 0.111 | ||
| Mild | 9/45 (20) | 17/48 (35.4) | ||
| Moderate-to-severe | 36/45 (80) | 31/48 (64.6) | ||
| Duration of delayed vomitingc | Low | 1.0 | ||
| 1 day | 11/17 (64.7) | 6/8 (75) | ||
| ≥2 days | 6/17 (35.3) | 2/8 (25) | ||
| Duration of delayed nauseac | Low | 0.058 | ||
| 1 day | 28/70 (40) | 13/55 (23.6) | ||
| ≥2 days | 42/70 (60) | 42/55 (76.4) | ||
| Severity of delayed nausea | Low | 0.039 | ||
| Mild | 39/70 (55.7) | 41/55 (74.5) | ||
| Moderate-to-severe | 31/70 (44.3) | 14/55 (25.5) |
AC anthracycline plus cyclophosphamide, Palo palonosetron, Dex dexamethasone, N total number of patients experiencing symptom
aHigh-risk patients experienced vomiting or moderate-to-severe nausea during the acute phase; low-risk patients were free from vomiting and moderate-to-severe nausea during the acute phase
bFisher’s exact test (two-tailed)
cAssessment over 4-day period
Prediction of delayed CINV
In the MEC cohort, the first-order interactions of delayed vomiting or nausea between anti-emetic regimen and each standard covariate were not statistically significant. In the adjusted models, only acute vomiting was an independent predictor for delayed vomiting (P = 0.045), while only acute moderate-to-severe nausea independently predicted for delayed nausea (P = 0.0007; Table 4).
Table 4.
Results of multivariable logistic-regression analysis of delayed vomiting and nausea among 237 patients undergoing MEC
| Variable | Odds ratioa (95 % CI) | P value |
|---|---|---|
| Delayed vomiting | ||
| Anti-emetic prophylaxis (1-day vs. 3-day regimen)b | 1.89 (0.76–4.71) | 0.172 |
| Age (<50 vs. ≥50 years) | 0.62 (0.18–2.14) | 0.452 |
| Gender (female vs. male) | 0.69 (0.27–1.77) | 0.435 |
| Alcohol consumption (never vs. regularly) | 1.27 (0.49–3.27) | 0.626 |
| Acute vomiting (yes vs. no) | 5.40 (1.04–28.1) | 0.045 |
| Acute moderate-to-severe nausea (yes vs. no) | 2.04 (0.56–7.39) | 0.277 |
| Delayed nausea (moderate-to-severe or mild) | ||
| Anti-emetic prophylaxis (1-day vs. 3-day regimen)b | 1.19 (0.68–2.07) | 0.546 |
| Age (<50 vs. ≥50 years) | 1.12 (0.55–2.25) | 0.756 |
| Gender (female vs. male) | 1.46 (0.81–2.64) | 0.204 |
| Alcohol consumption (never vs. regularly) | 0.93 (0.52–1.67) | 0.804 |
| Acute vomiting (yes vs. no) | 0.76 (0.17–3.45) | 0.727 |
| Acute moderate-to-severe nausea (yes vs. no) | 5.98 (2.11–16.9) | 0.0007 |
MEC moderately emetogenic chemotherapy, CI confidence interval
aAn odds ratio larger than 1 indicates an increased likelihood of experiencing delayed vomiting or nausea
bPatients received palonosetron plus dexamethasone on day 1 either with or without dexamethasone on days 2 and 3
In the AC cohort, the first-order interaction of delayed vomiting between anti-emetic regimen and age was statistically significant (P = 0.020). In the model including the interaction term, the 1-day dexamethasone regimen, acute vomiting, and acute moderate-to-severe nausea were all associated with a significantly higher odds of developing delayed vomiting (P = 0.005, P = 0.001, and P = 0.0009 for the three comparisons, respectively; Table 5). However, vomiting or moderate-to-severe nausea occurring in the acute phase were strong predictors for delayed vomiting. Only acute moderate-to-severe nausea was an independent predictor for delayed nausea (P < 0.0001; Table 5).
Table 5.
Results of multivariable logistic-regression analysis of delayed vomiting and nausea among 380 women undergoing AC
| Variable | Odds ratioa (95 % CI) | P value |
|---|---|---|
| Delayed vomiting | ||
| Anti-emetic prophylaxis (1-day vs. 3-day regimen)b | 3.28 (1.43–7.52) | 0.005 |
| Age (<50 vs. ≥50 years) | 1.23 (0.50–3.03) | 0.653 |
| Interaction (anti-emetic prophylaxis) (age) | 0.32 (0.09–1.06) | 0.062 |
| Alcohol consumption (never vs. regularly) | 0.42 (0.15–1.13) | 0.085 |
| Acute vomiting (yes vs. no) | 3.48 (1.65–7.33) | 0.001 |
| Acute moderate-to-severe nausea (yes vs. no) | 3.41 (1.66–7.00) | 0.0009 |
| Delayed nausea (moderate-to-severe or mild) | ||
| Anti-emetic prophylaxis (1-day vs. 3-day regimen)b | 1.56 (1.00–2.43) | 0.050 |
| Age (<50 vs. ≥50 years) | 1.29 (0.82–2.02) | 0.271 |
| Alcohol consumption (never vs. regularly) | 0.76 (0.33–1.75) | 0.521 |
| Acute vomiting (yes vs. no) | 1.07 (0.50–2.33) | 0.853 |
| Acute moderate-to-severe nausea (yes vs. no) | 11.6 (4.94–27.2) | <0.0001 |
AC anthracycline plus cyclophosphamide, CI confidence interval
aAn odds ratio larger than 1 indicates an increased likelihood of experiencing delayed vomiting or nausea
bPatients received palonosetron plus dexamethasone on day 1 either with or without dexamethasone on days 2 and 3
Patient’s satisfaction with anti-emetic regimen
A total of 227 VAS satisfaction ratings were available for analysis in the MEC cohort. Mean satisfaction with anti-emetic therapy for the 118 patients in the 1-day regimen subgroup did not differ from that for the 109 patients in the 3-day regimen subgroup (8.62; 95 % CI, 8.27 to 8.96; and 8.42; 95 % CI, 8.02 to 8.82, respectively; P = 0.892; Mann-Whitney U test). There was no significant between-treatment difference in the mean VAS scoring in the low-risk group (1-day regimen: mean 8.67; 95 % CI, 8.31 to 9.03; 3-day regimen: mean 8.72; 95 % CI, 8.37 to 9.08; P = 0.540). There was also no significant between-treatment difference in the mean VAS scoring in the high-risk group (1-day regimen: mean 8.18; 95 % CI, 6.80 to 9.57; 3-day regimen: mean 6.36; 95 % CI, 4.53 to 8.18; P = 0.089). However, irrespective of anti-emetic subgroup, the mean VAS scoring for treatment satisfaction was significantly different between the low-risk and high-risk groups (mean difference, 1.46; 95 % CI, 0.67 to 2.25; P = 0.003).
A total of 375 VAS satisfaction ratings were available for analysis in the AC cohort. Mean satisfaction with anti-emetic therapy for the 185 patients in the 1-day regimen subgroup did not differ from that for the 190 patients in the 3-day regimen subgroup (6.98; 95 % CI, 6.47 to 7.49, and 7.39; 95 % CI, 6.92 to 7.85, respectively; P = 0.330). There was no significant between-treatment difference in the mean VAS scoring in the low-risk group (1-day regimen: mean 7.82; 95 % CI, 7.31 to 8.33; 3-day regimen: mean 8.24; 95 % CI, 7.77 to 8.71; P = 0.345). There was also no significant between-treatment difference in the mean VAS scoring in the high-risk group (1-day regimen: mean 4.57; 95 % CI, 3.48 to 5.66; 3-day regimen: mean 5.54; 95 % CI, 4.61 to 6.47; P = 0.205). However, irrespective of anti-emetic subgroup, the mean VAS scoring for treatment satisfaction was significantly different between the low-risk and high-risk groups (mean difference, 2.91; 95 % CI, 2.21 to 3.62; P < 0.0001).
Discussion
There may be a specific interest in minimizing the total dose of dexamethasone for the prevention of CINV in all patients, and specifically those who experience dexamethasone-related side effects or in patients with pre-existing conditions like diabetes that could be exacerbated by corticosteroid use [13]. However, any clinical benefit conferred by reducing the overall exposure to dexamethasone should not compromise the ability to effectively control CINV. Hence, we investigated the impact of vomiting or significant nausea occurring in the first 24 h after chemotherapy initiation, the most important risk factor for delayed CINV, on delayed outcome of palonosetron plus single-dose dexamethasone in patients included in two randomized trials [5, 6]. The post hoc analysis described here yielded several key findings in patients at either low- or high-risk for delayed symptoms who received the same prophylaxis for acute CINV:
The vast majority of low-risk patients undergoing commonly used MEC regimens achieve delayed protection against vomiting and moderate-to-severe nausea, regardless of dexamethasone regimen.
In the AC cohort, the dexamethasone-sparing regimen is significantly less effective in delayed protection against either symptom also in patients at low risk; however, the 1-day regimen is significantly less effective against significant nausea alone but not vomiting alone in the delayed phase.
In the MEC cohort, the only independent predictor for delayed CINV is the occurrence of acute symptoms, regardless of anti-emetic regimen, age, gender, and alcohol consumption.
In the AC cohort, a key predictor for delayed vomiting is the occurrence of acute symptoms, while significant nausea occurring in the acute phase is the only predictor for delayed nausea.
Irrespective of chemotherapy cohort, the dexamethasone-sparing regimen has no apparent impact on the overall patient’s satisfaction with anti-emetic treatment in both the low-risk and high-risk groups.
From a clinical point of view, the main findings of the current analysis are that very few patients in the MEC cohort experienced acute CINV and the dexamethasone-sparing regimen achieved an excellent control of delayed symptoms in patients with no acute CINV. In addition, there were no between-treatment differences in the duration of delayed symptoms as well as the severity of delayed nausea occurring in patients at low risk. Since both duration and severity of delayed symptoms may impact on health-related quality of life [14], the finding can be considered as further proof of the effectiveness of the dexamethasone-sparing regimen in the setting of MEC. More recently, a phase III, noninferiority trial involving 305 patients undergoing mainly oxaliplatin-, irinotecan-, or carboplatin-based MEC (73, 13, and 12 % of the enrolled patients, respectively) also demonstrated that palonosetron plus single-dose dexamethasone provide protection against CINV which was noninferior to that of the same regimen with dexamethasone for 3 days [15].
We showed that, among low-risk AC patients, there is a statistically significant improvement associated with additional dexamethasone doses in protection against delayed vomiting and significant nausea, and this benefit is due to an improved control of moderate-to-severe nausea. However, clinicians should also keep in mind that the between-treatment difference in daily control of delayed moderate-to-severe nausea is of only modest magnitude (less than 10 % on each single day) among low-risk patients. It is interesting to note that the occurrence of significant nausea in the acute phase was the only independent predictor for delayed nausea in the AC cohort, and there was no apparent impact on the overall patient’s satisfaction with the 3-day dexamethasone regimen. Overall, the results from the current analysis should encourage clinicians to discuss the dexamethasone-sparing regimen for low-risk AC patients. To put the findings in a proper clinical perspective, we must consider that current guidelines recommend adding a neurokinin-1 (NK1) receptor antagonist (RA) to the 5-HT3RA and dexamethasone regimen for the optimal prevention of CINV caused by AC [2–4]. A recent phase III, superiority trial comparing palonosetron, dexamethasone, and aprepitant on day 1 followed by either aprepitant or dexamethasone on days 2 and 3 in this setting failed to detect any dexamethasone-induced improvement in the rate of delayed complete response (primary end point) as well as in any secondary end point including no nausea, no significant nausea, maximum severity of nausea, and duration of nausea in the delayed phase [16]. In addition, significantly more women receiving dexamethasone on days 2 and 3 experienced insomnia or heartburn in the delayed period following the first cycle of AC. Since most of the improvement produced by the three-drug regimen is expected to occur in patients at low risk, these findings support the view that the potential benefit of further dexamethasone in these patients may be overcome when a NK1RA is given on day 1. Consistent with this supposition, in a recent trial of breast cancer patients receiving AC, 67 % had delayed complete protection, 82 % had no delayed vomiting and 77 % experienced no delayed moderate-to-severe nausea among patients given the 1-day three-drug regimen of NEPA (a combination of the novel NK1RA netupitant and palonosetron) and dexamethasone, versus 60, 76, and 71 %, respectively, of patients who received palonosetron plus single-dose dexamethasone [17]. Given the consistency of results and the total number of patients enrolled onto these two trials with NK1RAs, we suggest that in breast cancer patients at low risk treated with the 1-day three-drug regimen the benefit of further dexamethasone may have little relevance for the control of delayed CINV. Interestingly, it has been reported that in breast cancer patients undergoing the first cycle of AC nausea continued to affect more patients than vomiting, even when they received aprepitant plus dexamethasone on days 2 and 3 post-chemotherapy [18]. If a NK1RA is not available, the clinicians should consider that there are currently no data from adequately powered randomized trials that evaluated the efficacy of a dexamethasone-sparing approach with an older 5-HT3RA compared to the same approach with palonosetron in the setting of AC. It also should be noted that the dexamethasone-sparing approach has been developed taking advantage of the superiority of palonosetron, when administered alone or in combination with dexamethasone, compared to a single dose of older antagonists in the control of delayed CINV caused by non-AC and AC MEC [19–21].
One limitation of this study is that it is a post hoc analysis. In spite of this, the overall results we gathered on the effectiveness of the dexamethasone-sparing regimen in the homogeneous cohort of AC, which has a particularly high-risk for CINV as a whole, support and extend the clinical relevance of the findings observed in the MEC cohort at lower risk. Another limitation is that pre-chemotherapy patient’s anxiety and personal history of nausea or vomiting were not recorded, and these data might help the decision-making process [22].
The current analysis strongly supports the position that optimizing the overall risk-benefit ratio for dexamethasone is possible because the choice of using additional steroid doses can be customized on the basis of the occurrence or absence of acute CINV in the first cycle of MEC, and possibly in AC. Accordingly, additional dexamethasone doses could be offered selectively to patients receiving the dexamethasone-sparing regimen who experienced vomiting or significant nausea in the acute or delayed phases following the first cycle of MEC or AC.
Acknowledgments
Conflict of interest
L. Celio and M. Aapro have received consulting fees from Helsinn Healthcare. The authors have had full access to all data in the study and had full responsibility for the design of the study, collection, analysis and interpretation of data, and the writing of the report.
References
- 1.Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med. 2008;358:2482–2494. doi: 10.1056/NEJMra0706547. [DOI] [PubMed] [Google Scholar]
- 2.Roila F, Herrstedt J, Aapro M, et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol. 2010;21(suppl 5):v232–v243. doi: 10.1093/annonc/mdq194. [DOI] [PubMed] [Google Scholar]
- 3.Basch E, Prestrud AA, Hesketh PJ, et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2011;29:4189–4198. doi: 10.1200/JCO.2010.34.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NCCN . Clinical Practice Guidelines. Antiemesis v.2. 2014. [Google Scholar]
- 5.Celio L, Frustaci S, Denaro A, et al. Palonosetron in combination with 1-day versus 3-day dexamethasone for prevention of nausea and vomiting following moderately emetogenic chemotherapy: a randomized, multicenter, phase III trial. Support Care Cancer. 2011;19:1217–1225. doi: 10.1007/s00520-010-0941-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aapro M, Fabi A, Nolè F, Medici M, Steger G, Bachmann C, Roncoroni S, Roila F. Double-blind, randomised, controlled study of the efficacy and tolerability of palonosetron plus dexamethasone for 1 day with or without dexamethasone on days 2 and 3 in the prevention of nausea and vomiting induced by moderately emetogenic chemotherapy. Ann Oncol. 2010;21:1083–1088. doi: 10.1093/annonc/mdp584. [DOI] [PubMed] [Google Scholar]
- 7.Celio L, Bonizzoni E, Bajetta E, Sebastiani S, Perrone T, Aapro MS. Palonosetron plus single-dose dexamethasone for the prevention of nausea and vomiting in women receiving anthracycline/cyclophosphamide-containing chemotherapy: meta-analysis of individual patient data examining the effect of age on outcome in two phase III trials. Support Care Cancer. 2013;21:565–573. doi: 10.1007/s00520-012-1558-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grunberg SM. Antiemetic activity of corticosteroids in patients receiving cancer chemotherapy : dosing, efficacy, and tolerability analysis. Ann Oncol. 2007;18:233–240. doi: 10.1093/annonc/mdl347. [DOI] [PubMed] [Google Scholar]
- 9.Vardy J, Chiew KS, Galica J, Pond GR, Tannock IF. Side effects associated with the use of dexamethasone for prophylaxis of delayed emesis after moderately emetogenic chemotherapy. Br J Cancer. 2006;94:1011–1015. doi: 10.1038/sj.bjc.6603048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Italian Group for Antiemetic Research Delayed emesis induced by moderately emetogenic chemotherapy: do we need to treat all patients? Ann Oncol. 1997;8:561–567. doi: 10.1023/A:1008229721099. [DOI] [PubMed] [Google Scholar]
- 11.The Italian Group for Antiemetic Research Dexamethasone alone or in combination with ondansetron for the prevention of delayed nausea and vomiting induced by chemotherapy. N Engl J Med. 2000;342:1554–1559. doi: 10.1056/NEJM200005253422102. [DOI] [PubMed] [Google Scholar]
- 12.Borjeson S, Hursti TJ, Peterson C, Fredikson M, Furst CJ, Avall-Lundqvist E, Steineck G. Similarities and differences in assessing nausea on a verbal category scale and a visual analogue scale. Cancer Nurs. 1997;20:260–266. doi: 10.1097/00002820-199708000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Olver I, Molassiotis A, Aapro M, Herrstedt J, Grunberg S, Morrow G. Antiemetic research: future directions. Support Care Cancer. 2011;19(suppl 1):s49–s55. doi: 10.1007/s00520-010-1036-1. [DOI] [PubMed] [Google Scholar]
- 14.Ballatori E, Roila F, Ruggeri B, Betti M, Sarti S, Soru G, Cruciani G, Di Maio M, Andrea B, Deuson RR. The impact of chemotherapy-induced nausea and vomiting on health-related quality of life. Support Care Cancer. 2007;15:179–185. doi: 10.1007/s00520-006-0109-7. [DOI] [PubMed] [Google Scholar]
- 15.Komatsu Y, Okita K, Yuki S et al (2015) Open-label, randomized, comparative, phase III study on effects of reducing steroid use in combination with palonosetron. Cancer Sci 106:891--895. doi:10.1111/cas.12675 [DOI] [PMC free article] [PubMed]
- 16.Roila F, Ruggeri B, Ballatori E, Del Favero A, Tonato M. Aprepitant versus dexamethasone for preventing chemotherapy-induced delayed emesis in patients with breast cancer: a randomized double-blind study. J Clin Oncol. 2014;32:101–106. doi: 10.1200/JCO.2013.51.4547. [DOI] [PubMed] [Google Scholar]
- 17.Aapro M, Rugo H, Rossi G, et al. A randomized phase III study evaluating the efficacy and safety of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy. Ann Oncol. 2014;25:1328–1333. doi: 10.1093/annonc/mdu101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hesketh PJ, Sanz-Altamira P. Aprepitant, dexamethasone, and palonosetron in the prevention of doxorubicin/cyclophosphamide-induced nausea and vomiting. Support Care Cancer. 2012;20:653–656. doi: 10.1007/s00520-011-1312-8. [DOI] [PubMed] [Google Scholar]
- 19.Gralla R, Lichinitser M, Van der Vegt S, et al. Palonosetron improves prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy: results of a double-blind randomized phase III trial comparing single doses of palonosetron with ondansetron. Ann Oncol. 2003;14:1570–1577. doi: 10.1093/annonc/mdg417. [DOI] [PubMed] [Google Scholar]
- 20.Eisenberg P, Figueroa-Vadillo J, Zamora R, Charu V, Hajdenberg J, Cartmell A, Macciocchi A, Grunberg S. Improved prevention of moderately emetogenic chemotherapy-induced nausea and vomiting with palonosetron, a pharmacologically novel 5-HT3 receptor antagonist. Cancer. 2003;98:2473–2482. doi: 10.1002/cncr.11817. [DOI] [PubMed] [Google Scholar]
- 21.Saito M, Aogi K, Sekine I, et al. Palonosetron plus dexamethasone versus granisetron plus dexamethasone for prevention of nausea and vomiting during chemotherapy: a double-blind, double-dummy, randomized, comparative phase III trial. Lancet Oncol. 2009;10:115–124. doi: 10.1016/S1470-2045(08)70313-9. [DOI] [PubMed] [Google Scholar]
- 22.Molassiotis A, Aapro M, Dicato M, Gascon P, Novoa SA, Isambert N, Burke TA, Gu A, Roila F. Evaluation of risk factors predicting chemotherapy-related nausea and vomiting: results from a European prospective observational study. J Pain Symptom Manage. 2014;47:839–848. doi: 10.1016/j.jpainsymman.2013.06.012. [DOI] [PubMed] [Google Scholar]