Abstract
Background
Peppermint oil (PO) has shown promise as an IBS therapy, but previous trials have demonstrated variable efficacy and tolerability results.
Aims
To evaluate the efficacy and tolerability of a novel formulation of PO designed for sustained release in the small intestine in patients with IBS-M and IBS-D.
Methods
This is a 4-week, randomized, double-blind, placebo-controlled clinical trial of PO or identical placebo 3 times daily in patients fulfilling Rome III criteria for IBS-M or IBS-D. The primary endpoint was the change from baseline in the Total IBS Symptom Score (TISS) after 4 weeks of treatment.
Results
Seventy-two patients (mean age 40.7 years, 75 % female, 77.8 % white) were randomized to PO (n = 35) or placebo (n = 37). At 4 weeks, PO was associated with a 40 % reduction in the TISS from baseline (mean change −1.16, SD ± 0.807), superior to the 24.3 % decrease (mean change −0.70, SD ± 0.737) observed with placebo (P = 0.0246). The decrease in the TISS of 19.6 % (mean change −0.55, SD ± 0.613) in the PO group at 24 h was also significantly larger than placebo (−10.3 %, mean change −0.27, SD ± 0.342) (P = 0.0092). At trial completion, patients in the PO group experienced greater improvement in multiple individual gastrointestinal symptoms as well as in severe or unbearable symptoms, compared to placebo. PO was well tolerated with few adverse events.
Conclusions
A novel PO formulation designed for sustained release in the small intestine is a safe, effective treatment capable of providing rapid relief of IBS symptoms.
Keywords: Irritable bowel syndrome, Peppermint oil, Abdominal pain, Bloating, Diarrhea, l-menthol
Introduction
Irritable bowel syndrome (IBS) is a chronic functional bowel disorder with an estimated global prevalence of between 10 and 15 % [1–3]. Multiple symptom-based criteria for IBS have been developed, including the Manning criteria and several variations of the Rome criteria [4, 5], and IBS may be considered a syndrome of symptoms rather than a single, unique disease. IBS is characterized by periodic exacerbations of multiple gastrointestinal symptoms including, but not limited to, abdominal pain or discomfort, abdominal bloating, constipation, diarrhea, a sensation of incomplete evacuation, pain at evacuation, passage of gas or mucus, and urgency of bowel movement (BM) [6, 7]. Patients with IBS typically demonstrate one of three recurring bowel habit patterns. According to the Rome III criteria [5], the primary subtypes of IBS include mixed IBS (IBS-M), diarrhea-predominant IBS (IBS-D), and constipation-predominant IBS (IBS-C), and their relative distribution, based on an international survey, has been reported elsewhere [8].
IBS is characterized by variable frequency and intensity of symptoms. Approximately 25 % of patients with IBS describe their symptoms as severe [9]. Patients with severe IBS symptoms experience impaired quality of life, high rates of absenteeism from work or school, and significant health-care resource utilization [10]. Although IBS is not life-threatening, one survey found that its symptoms can be so distressing that some patients would be willing to give up 25 % of their remaining life span (average 15 years) and 14 % would risk a 1/1000 chance of death to receive a treatment that would make them symptom-free [8].
Multiple underlying mechanisms have been implicated in the pathophysiology of IBS, including alterations of gastrointestinal transit, gastrointestinal secretion, and visceral hypersensitivity [11]. Gastrointestinal infections and post-infectious inflammation have been postulated to contribute to the development of IBS symptoms, as have dietary intolerances to complex carbohydrates and proteins [12–14]. Other data suggest that small bowel dysmotility [15] and altered permeability [16] may be associated with IBS symptoms. Disturbances of the small intestinal microbiome may play an important role in the development of IBS symptoms such as bowel habit changes and bloating [17], and the small intestine has been implicated in ineffective gas handling in patients with this prominent symptom [18]. The diverse pathophysiology of IBS has led to the use of a wide variety of therapeutic approaches in clinical practice, including lifestyle and dietary modifications, pharmacotherapy directed toward individual symptoms and potential etiologies, psychological therapies, and complementary and alternative medicine treatments.
Peppermint has been used for centuries as a digestive aid, and PO specifically has been evaluated as a potential IBS therapy for several decades. Peppermint oil and its active ingredient, l-menthol, are known to provide smooth muscle calcium channel antagonism [19], normalization of orocecal transit time [20], carminative effects [21], kappa opioid agonism [22], anti-infective [23] and anti-inflammatory [24] effects, and serotonergic (5HT3) antagonism [25]. All of these proposed mechanisms of action make PO an attractive pharmacotherapy for IBS. A meta-analysis of 121 treatment trials for IBS found PO to be more effective than anti-spasmodics, tricyclic antidepressants, and fiber [26]. This meta-analysis reported that the number needed to treat (NNT) for PO was 2 to 3, a range that was reiterated in the recent American College of Gastroenterology (ACG) Monograph on the Management of IBS [27]. In Europe, PO has been approved in the UK and is often used as frontline IBS pharmacotherapy [28].
The Irritable Bowel Syndrome Reduction Evaluation and Safety Trial (IBSREST) was conducted to compare a novel formulation of triple-coated microspheres of solid-state, highly purified PO (IBgard, IM HealthScience, Boca Raton, FL, USA) with placebo in patients with moderate to severe IBS-M and IBS-D. This PO formulation was designed to provide quick, reliable, and sustained release in the small intestine. The aim of the IBSREST was to evaluate the effectiveness, safety, and tolerability of this novel formulation of PO for the management of global and individual gastrointestinal symptoms in patients with non-constipated IBS.
Methods
Study Subjects
To be eligible for the trial, subjects had to meet Rome III criteria for IBS-M or IBS-D with an average daily IBS-related abdominal pain rating of ≥4 on a 0–10 scale and a Total IBS Symptom Score (TISS) of ≥2 on a 0–4 scale. Subjects had to be between 18 and 60 years of age, and had to confirm that they were not planning to change their usual diet and lifestyle during the study.
Exclusion criteria included a diagnosis of IBS-C or IBS-U as defined by the Rome III criteria or a history of inflammatory or immune-mediated gastrointestinal disorders, including celiac disease. Also excluded were subjects with a history of organic gastrointestinal disorders including intestinal obstruction, stricture, toxic megacolon, perforation, fecal impaction, adhesions, ischemic colitis or impaired intestinal circulation, cholecystitis, or major gastrointestinal surgery, including cholecystectomy. Additional exclusion criteria included a history of cardiovascular events, uncontrolled hypertension, unstable renal, hepatic, metabolic, or hematologic conditions, human immunodeficiency virus (HIV) infection, or a history of alcohol abuse or binge drinking. Subjects who refused to discontinue one or more prohibited medications for at least 7 days before beginning the baseline diary and throughout the remainder of the study were excluded. The protocol did not allow concomitant or rescue medications during the trial.
Experimental Design
The trial was conducted at four geographically diverse study sites in the USA, in accordance with good clinical practice (GCP) and applicable regulatory requirements and ethical principles. The protocol was approved by the Chesapeake Institutional Review Board and the Palm Beach Clinical Research Organization (West Palm Beach, FL, USA) was responsible for conduct of the study.
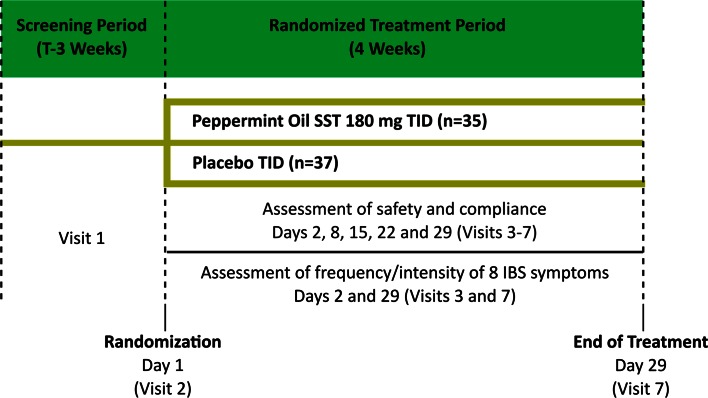
Subjects were enrolled by gastroenterologists, family practitioners, internists, and general medicine practitioners who were qualified as investigators by the clinical research organization overseeing the protocol. Recruitment was via print, radio, and televised advertisements. After a 3-week period for exclusion of organic disease and prohibited medication washout, subjects were randomly allocated to receive double-blind PO 180 mg or identical placebo 3 times daily for 4 weeks (Fig. 1). The PO and placebo capsules contained beads of the same size (≈1.2 mm in diameter) and density. Active beads in the PO capsule contained 60 % fiber while placebo contained 100 % fiber. All beads were triple coated in the same fashion in order to ensure similar gastrointestinal transit and prevent the ability to distinguish active therapy from placebo through smelling PO. Prohibited medications included antibiotics (with the exception of topical antibiotics or a 1-day course of an antibiotic), anticholinergic agents, antidepressants and anxiolytics, antidiarrheal agents, aspirin or medications that contain aspirin (≥325 mg/day) or other salicylates, colchicine, non-steroidal anti-inflammatory drugs (NSAIDs), opioids (including tramadol), probiotics, soluble and insoluble fiber, laxatives (osmotic, stimulant, or secretagogues), stool softeners, and anti-spasmodic agents.
Fig. 1.
IBSREST trial design
Subjects completed a daily diary to capture their assessment of BMs and IBS symptoms 2 weeks before the randomization visit in order to confirm eligibility and ability to comply with study procedures as well as to permit establishment of baseline symptom scores. After successful completion of the screening and washout phase, each subject was assigned a randomization number based on his or her IBS subtype. The randomization scheme was computer generated, using the PLAN procedure with SAS® software version 9.2 (SAS Institute Inc., Cary, North Carolina, USA) and concealed allocation of assignment was utilized via a central randomization center. Randomization numbers consisted of the 2-digit site number, followed by an identifier for the subject’s IBS subtype (D for IBS-D and M for IBS-M), followed by a 4-digit kit number. Kit numbers were assigned consecutively to each subject as he or she was randomized. Patients were instructed to take 2 capsules of the study drug (PO or identical placebo) between 30 and 90 min before breakfast, lunch, and dinner. The frequency and intensity of abdominal pain or discomfort, bloating or distension, pain at evacuation, urgency of BM, constipation, diarrhea, passage of mucus or gas, and sense of incomplete evacuation were assessed at 24 h and 4 weeks after the start of treatment. Compliance was assessed through pill counts at each weekly visit. Safety and tolerability of PO treatment also were assessed at each weekly visit.
The primary endpoint was change from baseline in the TISS 28 days after the start of therapy. The TISS is calculated by adding the means of the intensity and frequency scores for each assessed IBS symptom and dividing by 8. Symptom intensity and frequency were both reported by the patients on a scale from 0 to 4, where 0 equaled absence of symptom and 4 equalled unbearable (i.e., very severe) for intensity or ≥3 times per week for frequency. The modified intent-to-treat (mITT) population consisted of all randomized subjects who received at least 1 dose of therapy and had at least 1 post-baseline diary entry. Last observation carried forward was used for any patient withdrawals. The per-protocol population included all subjects in the mITT population who completed the 4-week treatment period with the exception of major protocol violators including: violation of eligibility criteria, use of prohibited medications, randomization errors, and/or poor treatment compliance (<80 %).
Secondary outcomes included the TISS score at 24 h after start of therapy, reduction from baseline in the frequency and intensity of the 8 individual symptoms included in the TISS (abdominal pain or discomfort, bloating or distension, pain at evacuation, urgency of BM, constipation, diarrhea, passage of mucus or gas, and sense of incomplete evacuation), reduction in severe or unbearable symptom intensity and frequency, and treatment-emergent adverse events (TEAEs). Since the TISS is designed to assess symptoms during the previous week, a modified version, limited to the 24 h after first therapy administration, was used to assess response at 24 h.
Statistical Analysis
Continuous variables were summarized using descriptive statistics. Categorical variables were summarized descriptively using counts and percentages. All percentages were rounded to 1 decimal place. Unless otherwise specified, summaries were presented by treatment group and visit. Statistical comparisons were made with two-sided, 95 % confidence intervals and/or P values rounded to 4 decimal places. The Wilcoxon rank-sum test was used to compare results from the PO and placebo groups. Paired t tests were utilized to compare follow-up score to baseline within each treatment group. Results from five previous clinical trials [6, 29–32] of PO for IBS were used for the sample size determination. With 64 randomized subjects, the study was planned to have >95 % power to show 1-point differences in changes from baseline symptoms between the active and placebo groups. This calculation assumes two-sided tests at the 0.05 alpha level and common standard deviations of 1.0. A sample size of 64 total subjects was considered adequate for a continuous primary endpoint or a dichotomous endpoint. Assuming up to a 10 % premature discontinuation of enrolled subjects, a sample size of 72 subjects was selected.
Results
Patients
Seventy-two patients satisfied the inclusion criteria and were randomized to PO (n = 35) or placebo (n = 37) between June 2013 and June 2014. The mean age was 40.7 years (standard deviation ± 11.23 years; range 18–60 years), 75 % were female, and 77.8 % were Caucasian (Table 1). In the PO group, 16 patients had IBS-M and 19 patients had IBS-D. The distribution in the placebo group was similar: 18 patients had IBS-M and 19 patients had IBS-D (P ≥ 0.35 for all comparisons). Baseline TISS scores are also shown in Table 1 and were not significantly different between subjects randomized to PO or placebo. Baseline individual symptom scores are shown in Table 2 and were not significantly different between subjects randomized to PO or placebo. One patient from each treatment group withdrew from the study before completion of the 4-week treatment period. One patient in the PO group was withdrawn for non-adherence to protocol requirements after 1 week and thus only had evaluable baseline and 24-h data.
Table 1.
Subject characteristics
| PO n (%) |
Placebo n (%) |
P value | |
|---|---|---|---|
| n | 35 | 37 | ns |
| Age (years) | |||
| Mean (SD) | 40.2 (11.15) | 41.1 (11.45) | ns |
| Median | 40.0 | 41.0 | ns |
| Range | 20, 60 | 18, 59 | ns |
| IBS subtype | |||
| IBS-M | 16 (45.7) | 18 (48.6) | ns |
| IBS-D | 19 (54.3) | 19 (51.4) | ns |
| Gender | |||
| Female | 28 (80.0) | 26 (70.3) | ns |
| Male | 7 (20.0) | 11 (29.7) | ns |
| Race | |||
| Caucasian | 29 (82.9) | 27 (73.0) | ns |
| African–American | 6 (17.1) | 8 (21.6) | ns |
| Asian | 0 | 1 (2.7) | ns |
| Other | 0 | 1 (2.7) | ns |
| TISS at baseline | |||
| Mean (SD) | 2.93 (0.394) | 2.76 (0.411) | ns |
| Median | 2.94 | 2.75 | ns |
| Range | 2.2, 4.0 | 2.0–4.0 | ns |
| Subject completion | |||
| Completed | 34 (97.1) | 36 (97.3) | ns |
| Withdrawn | 1 (2.9) | 1 (2.7) | ns |
ns not significant (P ≥ 0.05), SD standard deviation, TISS Total IBS Symptom Score
Table 2.
Individual IBS Symptom Scores at baseline (mITT population)
| Individual symptoms (average of frequency and intensity)** | PO | Placebo | P value* |
|---|---|---|---|
| n | 35 | 37 | ns |
| Abdominal pain or discomfort | |||
| Mean (SD) | 3.54 (0.427) | 3.28 (0.547) | ns |
| Median | 3.50 | 3.50 | NA |
| Range | 2.5, 4.0 | 2.0, 4.0 | NA |
| Abdominal bloating or distension | |||
| Mean (SD) | 3.23 (0.780) | 3.08 (0.651) | ns |
| Median | 3.50 | 3.00 | NA |
| Range | 1.0, 4.0 | 0.0, 4.0 | NA |
| Constipation (<3 stools/week) | |||
| Mean (SD) | 1.54 (1.432) | 1.45 (1.252) | ns |
| Median | 1.50 | 1.50 | NA |
| Range | 0.0, 4.0 | 0.0, 4.0 | NA |
| Diarrhea (>3 defecations/day) | |||
| Mean (SD) | 3.10 (0.784) | 3.16 (0.782) | ns |
| Median | 3.50 | 3.50 | NA |
| Range | 1.5, 4.0 | 1.0, 4.0 | NA |
| Pain at evacuation | |||
| Mean (SD) | 2.41 (1.197) | 2.09 (1.178) | ns |
| Median | 2.50 | 2.50 | NA |
| Range | 0.0, 4.0 | 0.0, 4.0 | NA |
| Passage of gas or mucus | |||
| Mean (SD) | 3.14 (0.862) | 2.93 (0.647) | ns |
| Median | 3.50 | 3.00 | NA |
| Range | 0.0, 4.0 | 2.0, 4.0 | NA |
| Sense of incomplete evacuation | |||
| Mean (SD) | 3.23 (0.634) | 2.85 (0.964) | ns |
| Median | 3.50 | 3.00 | NA |
| Range | 1.0, 4.0 | 0.0, 4.0 | NA |
| Urgency of bowel movement | |||
| Mean (SD) | 3.27 (0.657) | 3.22 (0.662) | ns |
| Median | 3.50 | 3.00 | NA |
| Range | 2.0, 4.0 | 1.5, 4.0 | NA |
TISS Total IBS Symptom Score, IBS irritable bowel syndrome, mITT modified intent-to-treat, NA not applicable, ns not significant, PO peppermint oil, SD standard deviation
* Wilcoxon rank-sum test (P < 0.05 considered statistically significant)
** Intensity and frequency were both measured on a scale of 0–4
Response to Treatment
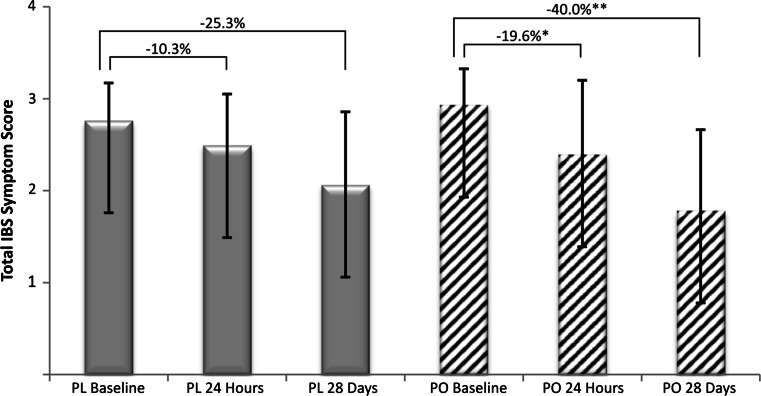
The TISS was calculated at baseline and at 24 h and 28 days after randomization and first dose (Fig. 2; Tables 1, 3). The primary endpoint, the decrease (improvement) in the TISS at 28 days compared to baseline, was 40.0 % (95 % CI −49.5, −30.6 %; mean change −1.16, SD ± 0.807) in subjects randomized to PO compared to 24.3 % (95 % CI −34.9, −15.7 %; mean change −0.70, SD ± 0.737) in subjects randomized to placebo (P = 0.0246). Similarly, at 24 h the decrease in TISS from baseline of 19.6 % (95 % CI −27.6 %, −11.6 %; mean change −0.55, SD ± 0.613) in the PO group was significantly greater than placebo (−10.3 %, 95 % CI −14.5 %, −6.0 %; mean change −0.27, SD ± 0.342) (P = 0.0092) (Table 3).
Fig. 2.
Total IBS Symptom Score (TISS) at baseline and after 24 h and 4 weeks of treatment with peppermint oil or placebo. TISS = mean intensity and frequency score for each of the 8 IBS symptoms (abdominal pain or discomfort, bloating or distension, pain at evacuation, urgency of BM, constipation, diarrhea, mucus or gas, sense of incomplete evacuation) summed and divided by 8 (*P = 0.0092, **P = 0.0246). P values are from generalized linear models with the baseline score as a covariate. Percent reduction from baseline is shown above brackets for 24-h and 4-week time points
Table 3.
Total IBS symptom score at 24 h and 28 days
| Placebo 24 h | PO 24 h | Placebo 28 days | PO 28 days | |
|---|---|---|---|---|
| n | 37 | 35 | 37 | 34 |
| Observed data | ||||
| Mean (SD) | 2.49 (0.560) | 2.39 (0.810) | 2.06 (0.796) | 1.78 (0.884) |
| Median | 2.50 | 2.69 | 2.19 | 1.75 |
| Range | 1.4, 4.0 | 0.3, 4.0 | 0.3, 3.4 | 0.2, 3.9 |
| Difference from placebo | NA | −0.10 | NA | −0.28 |
| 95 % CI* | NA | (−0.42, 0.23) | NA | (−0.68, 0.12) |
| P value* | NA | 0.5463 | NA | 0.1650 |
| Change from baseline | ||||
| Mean (SD) | −0.27 (0.342) | −0.55 (0.613) | −0.70 (0.737) | −1.16 (0.807) |
| Median | −0.31 | −0.38 | −0.63 | −0.97 |
| Range | −1.1, 0.3 | −2.3, 0.1 | −2.3, 0.7 | −2.7, 0.3 |
| Difference from placebo | NA | −0.27 | NA | −0.46 |
| 95 % CI** | NA | (−0.55, −0.08) | NA | (−0.81, −0.06) |
| P value** | NA | 0.0092 | NA | 0.0246 |
NA not applicable, PO peppermint oil, SD standard deviation
* P values and two-sided CIs are from t tests comparing treatments
** P values and two-sided CIs are from generalized linear models with the baseline score as a covariate
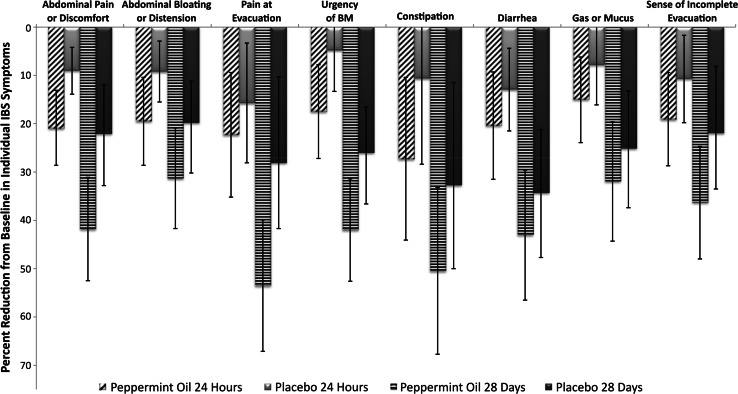
The changes from baseline in the mean intensity and frequency of each of the 8 individual IBS symptom scores comprising the TISS for PO and placebo at 24 h and 28 days are shown in Fig. 3 and Table 5. At 24 h, patients in the PO group experienced a statistically significant reduction from baseline, compared to placebo, in 2 of the 8 individual IBS symptoms evaluated. Subjects randomized to PO experienced a 21.0 % reduction (95 % CI −28.6, −13.1 %; mean change −0.74, SD ± 0.817) from baseline in abdominal pain or discomfort versus 9.0 % with placebo (95 % CI −13.9, −4.2 %; mean change −0.30, SD ± 0.478) (P = 0.0138). Patients in the PO group had a 25.2 % reduction from baseline (mean change −0.59, SD ± 0.919) compared with 5.7 % (mean change −0.22, SD ± 0.703) in the placebo group in mean intensity of BM urgency at 24 h (P = 0.0374). Changes in all other individual symptom scores trended in favor of PO at 24 h, but were not statistically different compared to changes observed with placebo (Fig. 3; Table 4).
Fig. 3.
Percent reduction from baseline in individual IBS symptoms (average of frequency and intensity) after 24 h of treatment with peppermint oil or placebo and 4 weeks of treatment with peppermint oil or placebo (*P < 0.05). P values are from generalized linear models with the baseline score as a covariate
Table 5.
Treatment-emergent adverse events
| PO (n = 35) n (%) |
Placebo (n = 37) n (%) |
All subjects (n = 72) n (%) |
|
|---|---|---|---|
| Total TEAEs | 2 (5.7) | 4 (10.8) | 6 (8.3) |
| Dyspepsia | 1 (2.9) | 0 | 1 (1.4) |
| Flatulence | 0 | 1 (2.7) | 1 (1.4) |
| Gastroesophageal reflux disease | 0 | 1 (2.7) | 1 (1.4) |
| Gastroenteritis (viral) | 0 | 1 (2.7) | 1 (1.4) |
| Upper respiratory tract infection | 1 (2.9) | 0 | 1 (1.4) |
| Back pain | 0 | 1 (2.7) | 1 (1.4) |
| TEAEs > grade 1 | 0 | 1 (2.7) | 1 (1.4) |
| Serious TEAEs and deaths | 0 | 0 | 0 |
| TEAEs that led to discontinuation | 0 | 0 | 0 |
TEAE treatment-emergent adverse events
Grade 1 = mild; asymptomatic or mild symptoms; clinical or diagnostic observations only; intervention not indicated
Table 4.
Individual IBS symptom scores—change from baseline of frequencies and intensities at 24 h and 28 days
| Average of frequency and intensity | Placebo 24 h | PO 24 h | Placebo 28 days | PO 28 days |
|---|---|---|---|---|
| n | 37 | 35 | 37 | 34 |
| Abdominal bloating or distension | ||||
| Change from baseline | ||||
| Mean (SD) | −0.28 (0.560) | −0.59 (0.732) | −0.59 (0.780) | −1.10 (1.036) |
| Difference from placebo | NA | −0.30 | NA | −0.51 |
| P value* | NA | 0.0586 | NA | 0.0474 |
| Abdominal pain or discomfort | ||||
| Change from baseline | ||||
| Mean (SD) | −0.30 (0.478) | −0.74 (0.817) | −0.74 (0.983) | −1.50 (1.155) |
| Difference from placebo | NA | −0.45 | NA | −0.76 |
| P value* | NA | 0.0138 | NA | 0.0183 |
| Constipation (<3 stools/week) | ||||
| Change from baseline | ||||
| Mean (SD) | −0.23 (0.723) | −0.29 (0.700) | −0.38 (0.924) | −0.66 (1.283) |
| Difference from placebo | NA | −0.06 | NA | −0.28 |
| P value* | NA | 0.8082 | NA | 0.3150 |
| Diarrhea (>3 defecations/day) | ||||
| Change from baseline | ||||
| Mean (SD) | −0.38 (0.721) | −0.67 (0.970) | −1.14 (1.310) | −1.37 (1.275) |
| Difference from placebo | NA | −0.29 | NA | −0.23 |
| P value* | NA | 0.1328 | NA | 0.3426 |
| Pain at evacuation | ||||
| Change from baseline | ||||
| Mean (SD) | −0.27 (0.804) | −0.47 (0.757) | −0.53 (1.213) | −1.16 (0.959) |
| Difference from placebo | NA | −0.20 | NA | −0.63 |
| P value* | NA | 0.4657 | NA | 0.0328 |
| Passage of gas or mucus | ||||
| Change from baseline | ||||
| Mean (SD) | −0.22 (0.584) | −0.47 (0.822) | −0.70 (0.953) | −1.01 (1.190) |
| Difference from placebo | NA | −0.26 | NA | −0.31 |
| P value* | NA | 0.1659 | NA | 0.3475 |
| Sense of incomplete evacuation | ||||
| Change from baseline | ||||
| Mean (SD) | −0.28 (0.596) | −0.56 (0.829) | −0.68 (1.062) | −1.15 (1.077) |
| Difference from placebo | NA | −0.27 | NA | −0.47 |
| P value* | NA | 0.1723 | NA | 0.1970 |
| Urgency of bowel movement | ||||
| Change from baseline | ||||
| Mean (SD) | −0.22 (0.703) | −0.59 (0.919) | −0.84 (0.921) | −1.35 (1.048) |
| Difference from placebo | NA | −0.37 | NA | −0.52 |
| P value* | NA | 0.0649 | NA | 0.0336 |
NA not applicable, PO peppermint oil, SD standard deviation
* P values derived from generalized linear models with the baseline score as a covariate
After 28 days of treatment (Fig. 3; Table 4), patients in the PO group experienced a statistically significant reduction from baseline compared with patients receiving placebo, of 41.8 % (95 % CI −52.5, −31.1 %) versus 22.1 % (95 % CI −32.2, −12.0 %), respectively, P = 0.0495) in mean symptom scores for abdominal pain or discomfort; 31.3 % (95 % CI −41.7, −20.9 %) versus 19.8 % (95 % CI −28.5, −11.2 %) (P = 0.0474) for abdominal bloating or distension; 53.5 % (95 % CI −67.1, −39.9 %) versus. 28.1 % (95 % CI −45.9, −10.3 %) (P = 0.0328) for pain at evacuation; and 42.0 % (95 % CI −52.6, −31.4 %) versus 26.0 % (95 % CI −35.4, −16.5 %) (P = 0.0336) for urgency of BM. The remainder of the changes in individual IBS symptoms were not statistically significant with PO compared to placebo.
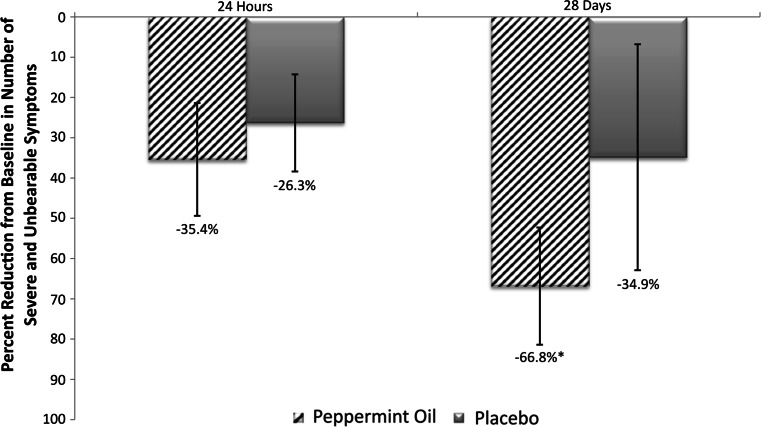
The reduction from baseline in the number of severe and unbearable symptoms was calculated as the number of symptoms for which the average of the frequency and intensity was ≥3 for each of the 8 IBS symptoms assessed (Fig. 4). Subjects receiving PO experienced a significant decrease in the number of severe and unbearable symptoms at 28 days compared to those receiving placebo (66.8 vs. 34.9 %, respectively, P = 0.0282). The reduction from baseline in the number of severe and unbearable symptoms was also more pronounced for the PO group compared with placebo at 24 h, but did not reach statistical significance (35.4 vs. 26.3 %, respectively, P = 0.0910).
Fig. 4.
Percent reduction from baseline in the number of severe and unbearable symptoms. Calculated as the number of symptoms for which the average of the frequency and intensity is ≥3 for each of the 8 IBS symptoms (abdominal pain or discomfort, bloating or distension, pain at evacuation, urgency of BM, constipation, diarrhea, mucus or gas, sense of incomplete evacuation) (*P = 0.0282). P values are from generalized linear models with the baseline score as a covariate
Safety and Tolerability
Peppermint oil was safe and well tolerated. TEAEs were similar in both treatment groups and are listed in Table 5. Six subjects reported a total of 6 TEAEs (PO group: 2; placebo group: 4). No TEAE was reported more than once. Treatment-related adverse events were reported by 3 subjects (PO group: 1; placebo group: 2) and consisted of flatulence, dyspepsia, and gastroesophageal reflux, respectively. All adverse events were mild in intensity with the exception of moderate gastroesophageal reflux reported by 1 patient in the placebo group. No patients reported smelling menthol on expired breath, flatus, or after BMs. There were no discontinuations due to adverse events. All TEAEs resolved prior to study completion with the exception of 1 subject in the PO group with mild dyspepsia. No subjects withdrew after experiencing a TEAE and there were no serious adverse events or deaths during the study.
Discussion
Peppermint oil is extracted from the mentha plant and is a complex mixture of terpenes, which can vary with growing conditions, time of harvest, and method of distillation. l-menthol is the principal component of PO, accounting for 35–50 % of the compound with more than 90 other minor components making up the remainder. The specifications of the PO included in the formulation used in the current trial were established to ensure a high level (47.5 ± 2.5 %) of free l-menthol. The specifications for the active formulation included the level of PO (90 mg) and free l-menthol (41.5 mg) per capsule. A standard dose of two capsules contains approximately 83 mg of l-menthol, designed to release over 4 h after exiting the stomach. A pharmacokinetic (PK) study of a single immediate-release, 100-mg dose of l-menthol in healthy adults detected only menthol glucuronide in plasma or urine, while no free menthol was detected [33].
In this randomized clinical trial, subjects with moderate to severe non-constipated IBS who received a novel formulation of PO 3 times a day for 28 days experienced a statistically significant decrease from baseline in mean TISS, a global IBS symptom score, compared with placebo 28 days after the start of treatment. Additionally, subjects in the PO group experienced a statistically significant reduction from baseline in abdominal pain and discomfort compared with the placebo group as well as a statistically significant reduction from baseline in the mean intensity of urgency of BM at 24 h. This rapid amelioration of symptoms suggests that PO may have potential as an on-demand pharmacotherapy for non-constipated IBS.
After 4 weeks of treatment, subjects in the PO group experienced a statistically significant reduction from baseline, compared with placebo, in the mean individual symptom score in 4 of the 8 IBS symptoms assessed. The 4 individual IBS symptoms that were more responsive to PO (abdominal pain or discomfort, abdominal bloating or distension, pain at evacuation, and urgency of BM) were clustered around viscerosensory perception, compared to motility related symptoms such as constipation, diarrhea, or passage of gas or mucus. As suggested by others, viscerosensory symptoms may be dissociated from bowel-related symptoms in patients with IBS and these observations suggest that PO may selectively modify important viscerosensory symptoms that IBS patients endorse [34]. In addition, PO treatment was associated with a statistically significant improvement in the number of severe and unbearable gastrointestinal symptoms after 4 weeks of treatment.
Other PO products are available as single-unit, liquid-filled, enteric-coated capsules originally developed in the 1970s. Treatment-related adverse events reported with these formulations of PO typically reflect vagaries in their delivery systems. Single-unit, liquid-filled, enteric-coated PO capsules can rupture in the stomach and have been associated with heartburn and nausea [35]. Additionally, delayed release of l-menthol has been associated with anal burning [36]. Such single-unit, non-disintegrating dosage forms can be subject to an unpredictable risk of dose-dumping [37].
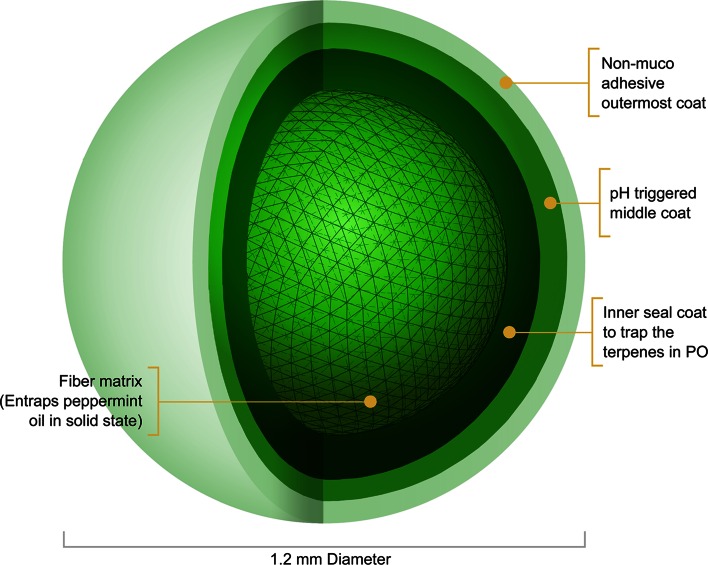
The site-specific targeting (SST) technology used for the PO evaluated in this trial consists of a triple-coated microsphere formulation designed to promote sustained release of PO in the small intestine (Figure 5). This controlled release is designed to overcome unpredictable delivery and tolerability issues with older PO technology and is implemented by converting PO into a solid-state matrix with microcrystalline cellulose as a spherical core, designed to release over 4 h in a simulated intestinal medium. A seal coat surrounds the core to trap the terpenes, the middle coat is an enteric polymer that dissolves at the intestinal pH and is insoluble at the gastric pH, and the external coat contains a non-mucoadhesive polymer, which facilitates transit through the stomach. The microspheres have an average diameter of less than 1.5 mm to allow rapid transit through the pylorus irrespective of the digestive stage of the stomach.
Fig. 5.
The delivery system for PO used in this study consists of a triple-coated microsphere formulation with sustained release of PO in the small intestine. The SST technology is implemented by converting the PO into a solid-state matrix with microcrystalline cellulose as a spherical core. The core is designed for release over 4 h. There is a seal coat surrounding the core to trap the terpenes. The middle coat is an enteric polymer, which dissolves at the intestinal pH and is insoluble at the gastric pH. The external coat contains a non-mucoadhesive polymer that facilitates faster transit through the stomach. The microspheres have an average diameter of less than 1.5 mm to allow flow through the pylorus
In addition to being the first clinical trial to use this novel formulation of PO, this trial has several notable strengths. Only subjects with moderate to severe IBS-M or IBS-D were recruited. Patients had an average daily IBS-related abdominal pain rating ≥4.0 on a 0–10 scale in each of the 2 weeks of the baseline diary, which is more severe than the abdominal pain ratings reported in many previous PO trials. This was done to enrich the trial population with patients who had more severe and unbearable symptoms, because this remains an area of unmet need for patients with non-constipated IBS. The measurement of global and individual symptom scores 24 h after the initial PO dose is unprecedented. Withdrawals were rare, with more than 94 % of randomized subjects completing the trial.
There are several limitations in this trial. The sample size is relatively small; however, our sample size calculations suggest that the population was adequate to demonstrate statistical significance versus placebo for the primary endpoint. Symptom assessment was limited to baseline, 24 h, and 28 days after randomization, so weekly changes or assessment of progressive improvement of symptoms is not possible. However, the observed results suggest that there is cumulative improvement with longer administration of PO. The trial duration of 4 weeks, although not typical for a FDA registration trial [38], is considered appropriate by the EMA based on its recent guideline for short-term IBS treatment protocols [7]. Additionally, most previous trials of PO for IBS have been ≤4 weeks in duration [32, 36]. The current trial did not include patients with IBS-C; however, we preferentially evaluated the effects of PO on subjects with IBS-M and IBS-D because effective pharmacotherapy options for these subgroups are limited. It is conceivable that patients randomized to PO may have been unblinded by noticing a menthol odor on their breath, flatus, or stool, but no patients reported noticing such an odor.
The primary analysis of this trial was the TISS, a global IBS symptom measure. Although use of the TISS as a primary endpoint was previously described in a PO trial by Cappello et al. [6], this scale has never been used to measure 24-h efficacy and has not been previously validated. This scale was chosen based on its previous use in a trial of PO in patients with IBS as well as the fact that there is no specific regulatory guidance regarding endpoints for a randomized controlled trial including patients with more than one IBS subtype. We therefore elected to use a global IBS symptom assessment tool that we felt was neutral with respect to IBS subtype. It was understood during the design of the IBSREST that frequency evaluations after 24 h would be limited by the design of the questionnaire, which included the 6 days before the start of therapy.
In summary, our results demonstrated that a novel formulation of PO, designed to release in the small intestine, was associated with a rapid and sustained symptomatic improvement in patients with non-constipated IBS based on significant reductions in a global IBS symptom score and reduced frequency and/or intensity of individual IBS symptoms. Peppermint oil was also associated with a reduction in the number of severe or unbearable IBS symptoms over 4 weeks of therapy and was well tolerated. This novel formulation of PO is a promising addition to the unmet need for a rapidly acting, safe, and effective pharmacotherapy for patients with non-constipated IBS.
Acknowledgments
The authors thank the principal investigators on the trial: Dennis S Riff, MD, FACG, CPI; Steven C Bowman, MD; Gigi Claire Lefebvre, MD; and Richard Krause, MD; Palm Beach CRO, LLC for help conducting the trial; SDC Biostatistics and Data Management for providing power and statistical analyses; Hubbell Consulting, LLC for preparing the clinical study report; and Premier Healthcare and Whitney Smalley-Freed, Ph.D., for editorial support.
Funding
This study was funded by IM HealthScience, LLC.
Author contributions
Brooks D. Cash is the guarantor of the article and contributed to data acquisition, data analysis, drafting of manuscript, and critical revision of manuscript. Michael Epstein was involved in study design, implementation, data acquisition, data analysis, drafting of manuscript, and critical revision of manuscript. Syed Shah contributed to study design, implementation, data acquisition, data analysis, drafting of manuscript, and critical revision of manuscript.
Compliance with ethical standards
Conflict of interest
Michael S. Epstein, MD, AGAF, FACG, is the Chief Medical Advisor for IM HealthScience, LLC. Brooks D. Cash, MD, AGAF, FACG, FASGE, is a consultant for IM HealthScience, LLC. Syed M. Shah, PhD, is the Chief Innovation Officer at IM HealthScience, LLC.
Contributor Information
Brooks D. Cash, Phone: 251-660-5555, Email: bcash@health.southalabama.edu
Michael S. Epstein, Email: michael.epstein@dda.net
Syed M. Shah, Email: sshah@imhealthscience.com
References
- 1.Saito YA, Schoenfeld P, Locke GR., III The epidemiology of irritable bowel syndrome in North America: a systematic review. Am J Gastroenterol. 2002;97:1910–1915. doi: 10.1111/j.1572-0241.2002.05913.x. [DOI] [PubMed] [Google Scholar]
- 2.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712–721. doi: 10.1016/j.cgh.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 3.Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol. 2014;6:71–80. doi: 10.2147/CLEP.S40245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manning AP, Thompson WG, Heaton KW, Morris AF. Towards positive diagnosis of the irritable bowel. Br Med J. 1978;2:653–654. doi: 10.1136/bmj.2.6138.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 6.Cappello G, Spezzaferro M, Grossi L, Manzoli L, Marzio L. Peppermint oil (Mintoil) in the treatment of irritable bowel syndrome: a prospective double blind placebo-controlled randomized trial. Dig Liver Dis. 2007;39:530–536. doi: 10.1016/j.dld.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 7.European Medicines Agency. Guideline on the evaluation of medicinal products for the treatment of irritable bowel syndrome. September 25, 2014. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/09/WC500173457.pdf. Accessed May 4, 2015.
- 8.Drossman DA, Morris CB, Schneck S, Hu YJ, Norton NJ, Norton WF, Weinland SR, Dalton C, Leserman J, Bangdiwala SI. International survey of patients with IBS: symptom features and their severity, health status, treatments, and risk taking to achieve clinical benefit. J Clin Gastroenterol. 2009;43:541–550. doi: 10.1097/MCG.0b013e318189a7f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drossman DA, Chang L, Bellamy N, Gallo-Torres HE, Lembo A, Mearin F, Norton NJ, Whorwell P. Severity in irritable bowel syndrome: a Rome Foundation Working Team report. Am J Gastroenterol. 2011;106:1749–1759. doi: 10.1038/ajg.2011.201. [DOI] [PubMed] [Google Scholar]
- 10.Canavan C, West J, Card T. Review article: the economic impact of the irritable bowel syndrome. Aliment Pharmacol Ther. 2014;40:1023–1034. doi: 10.1111/apt.12938. [DOI] [PubMed] [Google Scholar]
- 11.Thompson WG. Understanding the irritable gut: The functional gastrointestinal disorders. McLean, VA: Degnon and Associates; 2008. pp. 56–59. [Google Scholar]
- 12.Martinez C, Lobo B, Pigrau M, Ramos L, Gonzalez-Castro AM, Alonso C, Guilarte M, Guila M, de Torres I, Azpiroz F, Santos J, Vicario M. Diarrhoea-predominant irritable bowel syndrome: an organic disorder with structural abnormalities in the jejunal epithelial barrier. Gut. 2013;62:1160–1168. doi: 10.1136/gutjnl-2012-302093. [DOI] [PubMed] [Google Scholar]
- 13.Ford AC, Talley NJ. Mucosal inflammation as a potential etiological factor in irritable bowel syndrome: a systematic review. J Gastroenterol. 2011;46:421–431. doi: 10.1007/s00535-011-0379-9. [DOI] [PubMed] [Google Scholar]
- 14.Martinez C, Vicario M, Ramos L, Lobo B, Mosquera JL, Alonso C, Sanchez A, Guilarte M, Antolin M, de Torres I, Gonzalez-Castro AM, Pigrau M, Saperas E, Azpiroz F, Santos J. The jejunum of diarrhea-predominant irritable bowel syndrome shows molecular alterations in the tight junction signaling pathway that are associated with mucosal pathobiology and clinical manifestations. Am J Gastroenterol. 2012;107:736–746. doi: 10.1038/ajg.2011.472. [DOI] [PubMed] [Google Scholar]
- 15.Kellow JE, Phillips SF. Altered small bowel motility in irritable bowel syndrome is correlated with symptoms. Gastroenterology. 1987;92:1885–1893. doi: 10.1016/0016-5085(87)90620-2. [DOI] [PubMed] [Google Scholar]
- 16.Dunlop SP, Hebden J, Campbell E, Naesdal J, Olbe L, Perkins AC, Spiller RC. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am J Gastroenterol. 2006;101:1288–1294. doi: 10.1111/j.1572-0241.2006.00672.x. [DOI] [PubMed] [Google Scholar]
- 17.Giamarellos-Bourboulis E, Tang J, Pyleris E, Pistiki A, Barbatza C, Brown J, Lee CC, Harkins TT, Kim G, Weitsman S, Barlow GM, Funari VA, Pimentel M. Molecular assessment of differences in the duodenal microbiome in subjects with irritable bowel syndrome. J Clin Pharm TherScand J Gastroenterol. 2015;50:1076–1087. doi: 10.3109/00365521.2015.1027261. [DOI] [PubMed] [Google Scholar]
- 18.Salvioli B, Serra J, Azpiroz F, Lorenzo C, Aguade S, Castell J, Malagelada JR. Origin of gas retention and symptoms in patients with bloating. Gastroenterology. 2005;128:574–579. doi: 10.1053/j.gastro.2004.12.047. [DOI] [PubMed] [Google Scholar]
- 19.Hawthorn M, Ferrante J, Luchowski E, Rutledge A, Wei XY, Triggle DJ. The actions of peppermint oil and menthol on calcium channel dependent processes in intestinal, neuronal and cardiac preparations. Aliment Pharmacol Ther. 1988;2:101–118. doi: 10.1111/j.1365-2036.1988.tb00677.x. [DOI] [PubMed] [Google Scholar]
- 20.Goerg KJ, Spilker T. Effect of peppermint oil and caraway oil on gastrointestinal motility in healthy volunteers: a pharmacodynamic study using simultaneous determination of gastric and gall-bladder emptying and orocaecal transit time. Aliment Pharmacol Ther. 2003;17:445–451. doi: 10.1046/j.1365-2036.2003.01421.x. [DOI] [PubMed] [Google Scholar]
- 21.Harries N, James KC, Pugh WK. Antifoaming and carminative actions of volatile oils. J Clin Pharm Ther. 1977;2:171–177. doi: 10.1111/j.1365-2710.1977.tb00087.x. [DOI] [Google Scholar]
- 22.Galeotti N, Di Cesare ML, Mazzanti G, Bartolini A, Ghelardini C. Menthol: a natural analgesic compound. Neurosci Lett. 2002;322:145–148. doi: 10.1016/S0304-3940(01)02527-7. [DOI] [PubMed] [Google Scholar]
- 23.Hawrelak JA, Cattley T, Myers SP. Essential oils in the treatment of intestinal dysbiosis: a preliminary in vitro study. Altern Med Rev. 2009;14:380–384. [PubMed] [Google Scholar]
- 24.Juergens UR, Stober M, Vetter H. The anti-inflammatory activity of L-menthol compared to mint oil in human monocytes in vitro: a novel perspective for its therapeutic use in inflammatory diseases. Eur J Med Res. 1998;3:539–545. [PubMed] [Google Scholar]
- 25.Walstab J, Wohlfarth C, Hovius R, Schmitteckert S, Roth R, Lasitschka F, Wink M, Bonisch H, Niesler B. Natural compounds boldine and menthol are antagonists of human 5-HT3 receptors: implications for treating gastrointestinal disorders. Neurogastroenterol Motil. 2014;26:810–820. doi: 10.1111/nmo.12334. [DOI] [PubMed] [Google Scholar]
- 26.Enck P, Junne F, Klosterhalfen S, Zipfel S, Martens U. Therapy options in irritable bowel syndrome. Eur J Gastroenterol Hepatol. 2010;22:1402–1411. doi: 10.1097/MEG.0b013e3283405a17. [DOI] [PubMed] [Google Scholar]
- 27.Ford AC, Moayyedi P, Lacy BE, Lembo AJ, Saito YA, Schiller LR, Soffer EE, Spiegel BM, Quigley EM. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol. 2014;109:S2–S26. doi: 10.1038/ajg.2014.187. [DOI] [PubMed] [Google Scholar]
- 28.National Collaborating Center for Nursing and Supportive Care (UK). Irritable bowel syndrome in adults: diagnosis and management of irritable bowel syndrome in primary care. 2008. http://www.ncbi.nlm.nih.gov/books/NBK51953/. Accessed May 4, 2015. [PubMed]
- 29.Lech Y, Olesen KM, Hey H, Rask-Pedersen E, Ostergaard O, Vilien M. Treatment of irritable bowel syndrome with peppermint oil. A double-blind study with a placebo. Ugeskr Laeger. 1988;150:2388–2389. [PubMed] [Google Scholar]
- 30.Merat S, Khalili S, Mostajabi P, Ghorbani A, Ansari R, Malekzadeh R. The effect of enteric-coated, delayed-release peppermint oil on irritable bowel syndrome. Dig Dis Sci. 2010;55:1385–1390. doi: 10.1007/s10620-009-0854-9. [DOI] [PubMed] [Google Scholar]
- 31.Liu JH, Chen GH, Yeh HZ, Huang CK, Poon SK. Enteric-coated peppermint-oil capsules in the treatment of irritable bowel syndrome: a prospective, randomized trial. J Gastroenterol. 1997;32:765–768. doi: 10.1007/BF02936952. [DOI] [PubMed] [Google Scholar]
- 32.Rees WD, Evans BK, Rhodes J. Treating irritable bowel syndrome with peppermint oil. Br Med J. 1979;2:835–836. doi: 10.1136/bmj.2.6194.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gelal A, Jacob P, III, Yu L, Benowitz NL. Disposition kinetics and effects of menthol. Clin Pharmacol Ther. 1999;66:128–135. doi: 10.1053/cp.1999.v66.100455001. [DOI] [PubMed] [Google Scholar]
- 34.Lembo T, Naliboff B, Munakata J, Fullerton S, Saba L, Tung S, Schmulson M, Mayer EA. Symptoms and visceral perception in patients with pain-predominant irritable bowel syndrome. Am J Gastroenterol. 1999;94:1320–1326. doi: 10.1111/j.1572-0241.1999.01009.x. [DOI] [PubMed] [Google Scholar]
- 35.Khanna R, MacDonald JK, Levesque BG. Peppermint oil for the treatment of irritable bowel syndrome: a systematic review and meta-analysis. J Clin Gastroenterol. 2014;48:505–512. doi: 10.1097/MCG.0b013e3182a88357. [DOI] [PubMed] [Google Scholar]
- 36.Somerville KW, Richmond CR, Bell GD. Delayed release peppermint oil capsules (Colpermin) for the spastic colon syndrome: a pharmacokinetic study. Br J Clin Pharmacol. 1984;18:638–640. doi: 10.1111/j.1365-2125.1984.tb02519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.European Medicines Agency. Guideline on quality of oral modified release products. March 20, 2014. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/07/WC500170465.pdf. Accessed May 4, 2015.
- 38.U.S.Department of Health and Human Services Food and Drug Administration. Guidance for industry irritable bowel syndrome– Clinical evaluation of drugs for treatment. 2012. http://www.fda.gov/downloads/Drugs/Guidances/UCM205269.pdf. Accessed March 29, 2015.