Abstract
Background
Many patients with cancer experience aggressive care towards the end of life (EOL) despite evidence of an association with poor outcomes such as prolonged pain and overall dissatisfaction with care.
Purpose
To investigate socio-demographic, clinical and community health care service factors associated with aggressive EOL cancer care.
Methods
An analysis of pooled data from two mortality follow-back surveys was performed. Aggressive EOL care was defined as greater than or equal to one of the following indicators occurring during the last 3 months of life: greater than or equal to two emergency department visits, ≥30 days in hospital and death in hospital.
Results
Of the 681 included patients, 50.1 % were men and mean age at death was 75 years. The majority of patients (59.3 %, 95 % confidence interval (CI) 55.6–63.0 %) experienced at least one indicator of aggressive EOL care: 29.7 % experienced greater than or equal to two ED visits, 17.1 % spent ≥30 days in hospital and 37.9 % died in hospital. Patients with prostate or haematological cancer were more likely to experience aggressive EOL care (adjusted odds ratio (AOR) 4.36, 95 % CI 1.39–13.70, and 4.16, 95 % CI 1.38–12.47, respectively, reference group lung cancer). Patients who received greater than five general practitioner (GP) home visits (AOR 0.37, 95 % CI 0.17–0.82, reference group no GP visits) or had contact with district nursing (AOR 0.48, 95 % CI 0.28–0.83, reference group no contact) or contact with community palliative care services (AOR 0.27, 95 % CI 0.15–0.49, reference group no contact) were less likely to experience aggressive EOL care. No association was found between aggressive EOL care and patients’ age, gender, marital, financial or health status.
Conclusions
Community health care services, in particular contact with community palliative care, are associated with a significant reduction in the odds of cancer patients receiving aggressive EOL care. Expansion of such services may help address the current capacity crises faced by many acute health care systems.
Keywords: Neoplasms, Palliative care, Terminal care, Community health services, Emergency service, Hospital, Hospital mortality
Introduction
Towards the end of life (EOL), patients with cancer wish to be comfortable, be afforded dignity and privacy, and have the opportunity to achieve a sense of completion [1–4]. They also wish to avoid overly ‘intensive’ or ‘aggressive’ medical care which can be defined as care that focuses mostly or exclusively on disease-modifying treatments at the expense of good symptom management and/or advance care planning. In 2003, Earle and colleagues identified several markers of potentially overly aggressive EOL cancer care including multiple emergency department (ED) visits towards the EOL, a high number of days spent in hospital or intensive care towards the EOL, death in hospital and an underuse of hospice services [5]. Since then, several studies have further supported these findings with evidence of an association between aggressive EOL care and poor symptom control, reduced patient quality of life and an increased risk of psychiatric illness in bereaved caregivers [6–8]. Furthermore, in a randomised controlled trial of early palliative care for patients with advanced non-small cell lung cancer, Temel and colleagues found that overly aggressive EOL care may even shorten survival [9].
From a societal perspective, it is important to consider the cost-effectiveness of any health care service delivered. In the USA >10 % of the total health care budget and as much as 30 % of the Medicare budget are spent on care for those in the last year of life [10, 11]. In the UK expenditure is similar with an estimated 20 % of the National Health Service budget spent on care for those in the last year of life [12]. For cancer patients, health care spending has been shown to increase substantially in the months prior to death, with the additional costs being mostly attributable to an increased use of acute health care services such as unplanned hospital admissions and ED visits [11, 13, 14].
Yet, despite these potentially negative outcomes for both individuals and society, EOL cancer care is becoming increasingly aggressive over time [15–19]. In a population-based retrospective study of Medicare data, Earle and colleagues reported significant increases over time in the proportion of cancer patients with more than one ED visit (7.2 vs. 9.2 %; P < 0.001), an acute hospitalisation (7.8 vs. 9.1 %; P = 0.008) or admission to intensive care (7.1 vs. 9.4 %; P = 0.009) in the last month of life [15]. A number of studies have investigated this trend with several associated factors identifying gender, ethnicity and age as important determinants [20–24]. However, evidence of an association with clinical characteristics and community health care services is limited [25, 26]. The aim of our study was therefore to investigate socio-demographic, clinical and community health care service factors associated with aggressive EOL care for a cohort of 681 cancer decedents.
Methods
Study design and setting
We analysed pooled data from two mortality follow-back studies: the QUALYCARE study [27] and the International Access, Rights and Empowerment (IARE) study [28]. Mortality follow-back studies involve surveying a cohort of decedents’ significant others to gain information regarding the EOL [29]. Through their design they address a number of challenges commonly encountered when researching EOL care including the accurate identification of people at the EOL and the often high rates of participant withdrawal (typically due to ill health) that are seen with prospectively designed studies [29, 30]. In both the QUALYCARE and IARE studies, bereaved relatives/significant others were surveyed (via a postal questionnaire) regarding the care received by their family member/friend in the last 3 months of life. Both studies were conducted across London, collectively representing a socially and economically diverse urban population. All participants had access to free health care at the point of delivery through the UK’s National Health Service; however, within this broader context, there were important differences between the study samples with regard to the EOL care packages provided; the IARE study sampled patients, all of whom had accessed specialist palliative care services prior to death, whereas the QUALYCARE study included patients who had accessed generalist, specialist or no palliative care services prior to death. Our pooled study sample therefore provided us with information regarding the EOL care experiences of cancer patients across a range of health care packages and levels of palliative care input. Further information regarding each of the original studies is available elsewhere [27, 28].
Study population
Eligibility criteria for each of the original studies are described in Table 3 Appendix. For our analysis, inclusion criteria were as follows: (1) bereaved caregiver aged ≥18 years at time of survey completion, (2) family member/friend died from cancer (ICD-10 codes C00 to C97) and were ≥18 years at time of death, and (3) registration of death occurred 4 to 10 months prior to survey completion.
Bereaved caregivers of patients’ whose underlying cause of death was due to non-malignant disease were excluded.
Study questionnaire
The questionnaire was developed using cognitive interviewing for the QUALYCARE study [31] and then adapted for use by the IARE study. Both questionnaire versions include five questions about the deceased’s health state 3 months prior to death (using the EuroQol Five-Dimensional Questionnaire (EQ5D-3L) [32]) and several questions regarding the number and type of health care services used during the deceased’s last 3 months of life.
Outcome measure and explanatory variables
For our primary outcome, we calculated a composite measure, based on markers of potentially aggressive EOL cancer care developed by Earle and colleagues [5, 33], where we scored each patient one point per occurrence of any of the following three indicators: greater than or equal to two ED visits in the last 3 months of life, ≥30 days in hospital in the last 3 months of life and death in hospital. We then dichotomised the composite score into two groups: those who experienced no indicators of aggressive EOL care (composite score = 0) and those who experienced at least one indicator (composite score = 1–3).
We examined three groups of variables potentially associated with our primary outcome: socio-demographics, clinical characteristics and community health care service factors.
Socio-demographics included age at death (categorised into five groups; <60 (reference), 60–69, 70–79, 80–89 and 90+ years), gender (reference female), marital status (married or with partner (reference), widowed, divorced/ separated and single) and living circumstances dichotomised as living with others (reference) or living alone. A subjective measure of patients’ financial hardship was reported from five possible categories and dichotomised for analysis into those described as living comfortably (reference) compared to all other groups (doing alright, just about getting by, finding it quite difficult and finding it very difficult).
Clinical characteristics included patients’ health state 3 months prior to death and underlying cancer diagnosis. Health state was measured using the EQ5D-3L [32] which includes questions on mobility, ability to self-care, activity level, pain/discomfort and anxiety/depression. Underlying type of cancer was categorised into seven groups (lung (reference), breast, prostate, gastrointestinal tract, haematological, unknown primary and other).
Use of community health care services in the last 3 months of life included the number of general practitioner (GP) home visits (categorised as none (reference), one to five and greater than five visits), contact with community palliative care services (yes or no (reference)), and contact with district nursing (yes or no (reference)). Community palliative care services were defined as those that specifically provided palliative and/or EOL care to patients in non-hospital settings and included services such as Hospice at Home and Marie Curie or Macmillan nursing. District nursing was defined as any other nursing care (i.e. not exclusively palliative or EOL care nursing) received by patients in non-hospital settings such as the patients’ home.
Statistical analysis
We used summary statistics to report patient demographic data and describe the aggressiveness of EOL care experienced. Differences between patients who did and did not experience aggressive care were tested using a chi-squared test.
The likelihood of patients experiencing aggressive EOL care was investigated using multivariable logistic regression, where we calculated adjusted odds ratios (AORs) and their corresponding 95 % confidence intervals (CIs). Based on findings of a recently published systematic review [24], the logistic model was constructed with the following variables included a priori: age, gender, financial status, marital status, type of cancer and contact with palliative care services. All additional variables were included if found to be significant (p < 0.10) at univariate analysis. We conducted sensitivity analysis to explore the potential impact to our findings from the two different study samples.
Stata/IC 13 (STATA, College Station, TX, USA) was used for all statistical analysis.
Results
The pooled dataset contains survey responses from 681 bereaved caregivers from across five London health regions (QUALYCARE n = 554, IARE n = 127).
Mean age at death was 75 years; 50.1 % were men. Most lived with others prior to death (69.7 %). The two most common diagnoses were gastrointestinal cancer (24.5 %) and lung cancer (21.3 %) (Table 1).
Table 1.
Socio-demographics, clinical characteristics and community health care service use of study sample
| Entire cohort | Patients not experiencing any aggressive care | Patients experiencing aggressive care | QUALYCARE cohort | QUALYCARE patients experiencing aggressive care | IARE cohort | IARE patients experiencing aggressive care | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | |
| 681 | 100 | 277 | 40.7 | 404 | 59.3 | 554 | 100 | 299 | 54.0 | 127 | 100 | 105 | 82.7 | |
| Age in years | ||||||||||||||
| <60 | 67 | 9.8 | 33 | 11.9 | 34 | 8.4 | 67 | 12.1 | 34 | 11.4 | 0 | 0.0 | 0 | 0.0 |
| 60–69 | 141 | 20.7 | 49 | 17.7 | 92 | 22.8 | 112 | 20.2 | 66 | 22.1 | 29 | 22.8 | 26 | 24.8 |
| 70–79 | 191 | 28.1 | 69 | 24.9 | 122 | 30.2 | 140 | 25.3 | 82 | 27.4 | 51 | 40.1 | 40 | 38.1 |
| 80–89 | 228 | 33.5 | 105 | 37.9 | 123 | 30.5 | 190 | 34.3 | 91 | 30.4 | 38 | 29.9 | 32 | 30.5 |
| 90+ | 54 | 7.9 | 21 | 7.6 | 33 | 8.2 | 45 | 8.1 | 26 | 8.7 | 9 | 7.1 | 7 | 6.7 |
| Gender | ||||||||||||||
| Male | 341 | 50.1 | 127 | 45.9 | 214 | 53.0 | 282 | 50.9 | 166 | 44.5 | 59 | 46.5 | 48 | 45.7 |
| Female | 340 | 49.0 | 150 | 54.2 | 190 | 47.0 | 272 | 49.1 | 133 | 55.5 | 68 | 53.5 | 57 | 54.3 |
| Financial status | ||||||||||||||
| Living comfortably | 323 | 48.1 | 142 | 51.6 | 181 | 45.6 | 273 | 49.9 | 138 | 46.9 | 50 | 40.0 | 43 | 41.8 |
| Not living comfortably | 349 | 51.9 | 133 | 48.4 | 216 | 54.4 | 274 | 50.1 | 156 | 53.1 | 75 | 60.0 | 60 | 58.3 |
| Marital status | ||||||||||||||
| Married or with partner | 323 | 50.6 | 147 | 54.7 | 176 | 47.7 | 289 | 53.2 | 147 | 50.5 | 34 | 35.8 | 29 | 37.2 |
| Widowed | 191 | 29.9 | 77 | 28.6 | 114 | 30.9 | 154 | 28.4 | 84 | 28.9 | 37 | 39.0 | 30 | 38.5 |
| Divorced/Separated | 59 | 9.3 | 20 | 7.4 | 39 | 10.6 | 47 | 8.7 | 29 | 10.0 | 12 | 12.6 | 10 | 12.8 |
| Single | 65 | 10.2 | 25 | 9.3 | 40 | 10.8 | 53 | 9.8 | 31 | 10.7 | 12 | 12.6 | 9 | 11.5 |
| Living circumstances | ||||||||||||||
| Living alone | 203 | 30.3 | 77 | 28.3 | 126 | 31.7 | 379 | 69.8 | 198 | 67.6 | 87 | 69.1 | 73 | 70.2 |
| Living with others | 466 | 69.7 | 195 | 71.7 | 271 | 68.3 | 164 | 30.2 | 95 | 32.4 | 39 | 31.0 | 31 | 29.8 |
| Cancer type | ||||||||||||||
| Lung | 145 | 21.3 | 63 | 22.7 | 82 | 20.3 | 119 | 21.5 | 64 | 21.4 | 26 | 20.5 | 18 | 17.1 |
| Breast | 52 | 7.6 | 28 | 10.1 | 24 | 5.9 | 47 | 8.5 | 20 | 6.7 | 5 | 3.9 | 4 | 3.8 |
| Prostate | 46 | 6.8 | 8 | 2.9 | 38 | 9.4 | 37 | 6.7 | 30 | 10.0 | 9 | 7.1 | 8 | 7.6 |
| Gastrointestinal tract | 167 | 24.5 | 78 | 28.2 | 89 | 22.0 | 157 | 28.3 | 81 | 27.1 | 10 | 7.9 | 8 | 7.6 |
| Haematological | 54 | 7.9 | 9 | 3.3 | 45 | 11.1 | 34 | 6.1 | 28 | 9.4 | 20 | 15.8 | 17 | 16.2 |
| Cancer of unknown primary | 61 | 9.0 | 20 | 7.2 | 41 | 10.2 | 47 | 8.5 | 28 | 9.4 | 43 | 33.9 | 13 | 12.4 |
| Othera | 156 | 22.9 | 71 | 25.6 | 85 | 21.0 | 113 | 20.4 | 48 | 16.1 | 43 | 33.9 | 37 | 35.2 |
| Mobility at 3 months before death | ||||||||||||||
| No problem | 159 | 24.6 | 58 | 21.8 | 101 | 26.6 | 132 | 25.0 | 78 | 27.5 | 27 | 23.1 | 23 | 24.0 |
| Some problem | 423 | 65.5 | 176 | 66.2 | 247 | 65.0 | 350 | 66.2 | 184 | 64.8 | 73 | 62.4 | 63 | 65.6 |
| Confined to bed | 64 | 9.9 | 32 | 12.0 | 32 | 8.4 | 47 | 8.9 | 22 | 7.8 | 17 | 14.5 | 10 | 10.4 |
| Self-care at 3 months before death | ||||||||||||||
| No problem | 262 | 41.1 | 93 | 35.5 | 169 | 45.0 | 212 | 40.6 | 128 | 45.7 | 50 | 43.1 | 41 | 42.7 |
| Some problem | 259 | 40.6 | 120 | 45.8 | 139 | 37.0 | 215 | 41.2 | 102 | 36.4 | 44 | 37.9 | 37 | 38.5 |
| Unable to self-care | 117 | 18.3 | 49 | 18.7 | 68 | 18.1 | 95 | 18.2 | 50 | 17.9 | 22 | 19.0 | 18 | 18.8 |
| Activity at 3 months before death | ||||||||||||||
| No problem | 137 | 21.3 | 40 | 15.2 | 97 | 25.6 | 106 | 20.2 | 71 | 25.2 | 31 | 26.5 | 26 | 26.8 |
| Some problem | 286 | 44.5 | 127 | 48.1 | 159 | 42.0 | 241 | 45.8 | 121 | 42.9 | 45 | 38.5 | 38 | 39.2 |
| Unable to walk | 220 | 34.2 | 97 | 36.7 | 123 | 32.5 | 179 | 34.0 | 90 | 31.9 | 41 | 35.0 | 33 | 34.0 |
| Pain/Discomfort at 3 months before death | ||||||||||||||
| No pain | 113 | 17.7 | 46 | 17.4 | 67 | 17.9 | 97 | 18.6 | 54 | 19.3 | 16 | 13.8 | 13 | 13.7 |
| Some pain | 392 | 61.4 | 158 | 59.9 | 234 | 62.4 | 322 | 61.6 | 179 | 63.9 | 70 | 60.3 | 55 | 57.9 |
| Extreme pain | 134 | 21.0 | 60 | 22.7 | 74 | 19.7 | 104 | 19.9 | 47 | 16.8 | 30 | 25.9 | 27 | 28.4 |
| Anxiety/Depression at 3 months before death | ||||||||||||||
| No anxiety/depression | 223 | 35.5 | 91 | 35.1 | 132 | 35.8 | 176 | 34.1 | 97 | 35.0 | 47 | 42.0 | 35 | 38.0 |
| Some anxiety/depression | 316 | 50.3 | 128 | 49.4 | 188 | 51.0 | 267 | 51.7 | 146 | 52.7 | 49 | 43.8 | 42 | 45.7 |
| Extreme anxiety/depression | 89 | 14.2 | 40 | 15.4 | 49 | 13.3 | 73 | 14.2 | 34 | 12.3 | 16 | 14.3 | 15 | 16.3 |
| Number of GP home visits during the last 3 months of life | ||||||||||||||
| None | 181 | 31.4 | 46 | 19.1 | 135 | 40.3 | 138 | 29.2 | 96 | 37.9 | 43 | 41.8 | 39 | 47.6 |
| 1–5 | 338 | 58.7 | 159 | 66.0 | 179 | 53.4 | 287 | 60.7 | 141 | 55.7 | 51 | 49.5 | 38 | 46.3 |
| >5 | 57 | 9.9 | 36 | 14.9 | 21 | 6.3 | 48 | 10.2 | 16 | 6.3 | 9 | 8.7 | 5 | 6.1 |
| Community palliative care | ||||||||||||||
| No | 205 | 31.2 | 36 | 13.2 | 169 | 43.9 | 156 | 29.0 | 121 | 42.2 | 49 | 40.8 | 48 | 49.0 |
| Yes | 453 | 68.8 | 237 | 86.8 | 216 | 56.1 | 382 | 71.0 | 166 | 57.8 | 71 | 59.2 | 50 | 51.0 |
| District nurse | ||||||||||||||
| No | 227 | 34.7 | 56 | 20.8 | 171 | 44.4 | 172 | 32.2 | 121 | 42.2 | 55 | 45.8 | 50 | 51.0 |
| Yes | 427 | 65.3 | 213 | 79.2 | 214 | 55.6 | 362 | 67.8 | 166 | 57.8 | 65 | 54.2 | 48 | 49.0 |
aOther cancers included those of the urinary tract (5.0 %), gynaecological (3.8 %), central nervous system (3.8 %) and skin (1.8 %) with percentage figures referring to entire cohort data
Most patients in our sample (59.3 % (95 % CI 55.6–63.0 %)) experienced at least one indicator of aggressive care during the last 3 months of life: 29.7 % experienced greater than or equal to two ED visits, 17.1 % spent ≥30 days in hospital and 37.9 % died in hospital (Fig. 1). The median composite score of aggressive EOL care was 1 (range 0 to 3).
Fig. 1.
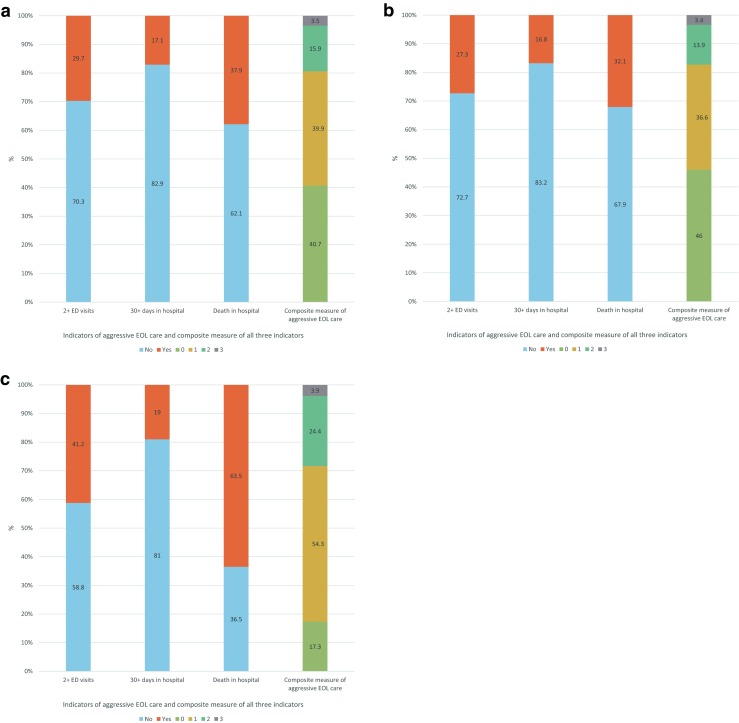
a Graph showing number of patients experiencing greater than or equal to two emergency department (ED) visits in the last 3 months of life, ≥30 days spent in hospital in the last 3 months of life and death in hospital, and composite measure of aggressive end of life (EOL) care calculated from these indicators for entire study cohort. b Graph showing number of patients experiencing greater than or equal to two ED visits in the last 3 months of life, ≥30 days spent in hospital in the last 3 months of life and death in hospital, and composite measure of aggressive EOL care calculated from these indicators for the QUALYCARE cohort of sample. c Graph showing number of patients experiencing greater than or equal to two ED visits in the last 3 months of life, ≥30 days spent in hospital in the last 3 months of life and death in hospital, and composite measure of aggressive EOL care calculated from these indicators for the IARE cohort of sample
Relative to those with lung cancer, patients with prostate or haematological cancer were significantly more likely to experience aggressive care during their last 3 months of life (AOR 4.36, 95 % CI 1.39–13.70, and AOR 4.16, 95 % CI 1.38–12.47, respectively). No association was found between aggressive EOL care and cancer patients’ health status 3 months prior to death (Table 2).
Table 2.
Findings from multivariable analysis for factors associated with cancer patients experiencing aggressive care during the last 3 months of life
| Variable | OR | 95 % CI | AOR | 95 % CI |
|---|---|---|---|---|
| Age in years | ||||
| <60 | 1.00 | – | – | – |
| 60–69 | 1.82 | 1.00–3.32 | 1.82 | 0.82–4.03 |
| 70–79 | 1.72 | 0.97–3.03 | 1.70 | 0.78–3.71 |
| 80–89 | 1.14 | 0.66–1.96 | 0.98 | 0.45–2.13 |
| >90 | 1.53 | 0.73–3.18 | 1.44 | 0.49–4.26 |
| Gender | ||||
| Female | 1.00 | – | – | – |
| Male | 1.33 | 0.98–1.81 | 1.35 | 0.84–2.18 |
| Financial status | ||||
| Living comfortably | 1.00 | – | – | – |
| Not living comfortably | 1.27 | 0.94–1.74 | 1.34 | 0.88–2.05 |
| Marital status | ||||
| Married or with partner | 1.00 | – | – | – |
| Widowed | 1.24 | 0.86–1.78 | 1.50 | 0.85–2.65 |
| Divorced/Separated | 1.63 | 0.91–2.92 | 1.04 | 0.48–2.24 |
| Single | 1.34 | 0.77–2.31 | 1.65 | 0.70–3.88 |
| Cancer type | ||||
| Lung | 1.00 | – | – | – |
| Breast | 0.66 | 0.35–1.25 | 0.98 | 0.38–2.52 |
| Prostate | 3.65 | 1.55–8.58 | 4.36 | 1.39–13.70 |
| Gastrointestinal tract | 0.88 | 0.56–1.37 | 1.35 | 0.74–2.44 |
| Haematological | 3.84 | 1.70–8.68 | 4.16 | 1.38–12.47 |
| Cancer of unknown primary | 1.58 | 0.84–2.96 | 1.34 | 0.62–2.92 |
| Other | 0.92 | 0.58–1.45 | 0.91 | 0.48–1.74 |
| Self-care at 3 months before death | ||||
| No problem | 1.00 | – | – | – |
| Some problem | 0.64 | 0.45–0.91 | 0.93 | 0.53–1.62 |
| Unable to self-care | 0.76 | 0.49–1.19 | 1.43 | 0.63–3.22 |
| Activity at 3 months before death | ||||
| No problem | 1.00 | – | – | – |
| Some problem | 0.52 | 0.33–0.80 | 0.74 | 0.40–1.39 |
| Unable to walk | 0.52 | 0.33–0.83 | 0.69 | 0.31–1.49 |
| Number of GP home visits during the last 3 months of life | ||||
| None | 1.00 | – | – | – |
| 1–5 | 0.38 | 0.26–0.58 | 0.59 | 0.35–1.00 |
| >5 | 0.20 | 0.10–0.39 | 0.37 | 0.17–0.82 |
| Community palliative care | ||||
| No | 1.00 | – | – | – |
| Yes | 0.19 | 0.13–0.30 | 0.27 | 0.15–0.49 |
| District nurse | ||||
| No | 1.00 | – | – | – |
| Yes | 0.33 | 0.23–0.48 | 0.48 | 0.28–0.83 |
Numbers in bold represent statistically significant findings, p < 0.05
OR odds ratio, 95 % CI 95 % confidence interval, AOR adjusted odds ratio
Patients who had contact with community health care services (GP home visits, district nursing, and community palliative care) were significantly less likely to experience aggressive care during their last 3 months of life (Table 2). For GP home visits, an incremental pattern was found whereby patients with greater than five visits had a greater reduction in odds than those who had one to five visits (AOR 0.37, 95 % CI 0.17–0.82, and AOR 0.59, 95 % CI 0.35–1.00, respectively (reference group no GP home visit)). Compared to patients who had no contact with district nursing, those with contact were less likely to experience aggressive EOL care (AOR 0.48, 95 % CI 0.28–0.83); however, the greatest reduction in odds was found for cancer patients who had contact with community palliative care services (AOR 0.27, 95 % CI 0.15–0.49 (reference group no contact)).
We found no association between aggressive EOL care and cancer patients’ age, gender, marital status or financial status (Table 2). No changes to the effect outcomes were found at sensitivity analysis.
Discussion
We used pooled data from two mortality follow-back surveys to examine the aggressiveness of EOL care received by 681 deceased cancer patients. We found statistically and clinically significant variations based on patients’ underlying cancer type and their contact with community health care services.
Supporting previously published studies, we found that patients with haematological cancers were more likely to experience aggressive EOL care compared to those with lung cancer [16, 34]. Features related to both the disease process and the discipline of haemato-oncology are likely to contribute to this effect, for example, chemotherapy remains the main and often only form of therapy available, clinical trial involvement is particularly high and haemato-oncology clinical services have historically remained distinct from those of solid tumours with less collaboration between disciplines, including with palliative care [35]. Our finding that patients with prostate cancer also have an increased risk of experiencing aggressive EOL care is interesting, and additional research exploring patterns of acute care towards the EOL by cancer sub-groups is warranted. In our sample, although the proportion of prostate cancer patients who spent ≥30 days in hospital during the last 3 months of life was similar to patients with other cancer types, we found that prostate cancer patients were more likely to have greater than or equal to two ED visits in the last 3 months of life and/or die in hospital (Table 4 Appendix). Metastatic bone disease is commonly seen in patients with advanced prostate cancer, and complications from this pattern of disease spread, in particular pathological fractures, typically result in ED visits. This may explain some of the higher rates of ED use that we found in our sub-group of prostate cancer patients; however, further research exploring this finding is required. Our finding of lower odds of aggressive EOL care associated with GP home visits supports Almaawiy and colleagues [25]. In their study of 9467 cancer decedents in Canada, increased family physician visits were associated with reduced odds for both hospital death and an ED visit in the last 2 weeks of life [25]. However, Almaawiy et al. found that patients with greater than four visits per week had increased odds of hospitalisations and hospital death, the opposite of our study which found that care was less aggressive for patients who had greater than five GP home visits than for those who had one to five or no GP visits.
Our data support growing observational and experimental evidence that community palliative care is associated with lower odds of aggressive EOL care [9, 24, 36, 37]. Expansion of palliative care services may therefore be one approach towards helping address the current capacity crises faced by many acute health care systems. Of note, the effect size found in our study (AOR 0.27, 95 % CI 0.15–0.49) was greater than those previously reported which may be related to our study time period (the last 3 months of life) as this is longer than those reported by several similar studies [20, 37]. This is particularly relevant given the small but emerging body of evidence indicating a greater reduction in risk of patients receiving aggressive EOL care with earlier palliative care involvement [9, 37, 38]. Further investigation of the effect according to timing of palliative care interventions is necessary.
Several previously published studies have reported that men and patients of lower financial status have an increased risk of experiencing aggressive EOL care [15, 16, 20–22, 25, 26, 34]. In our study, although we found a similar pattern, our results for these factors did not reach statistical significance. This may be related to our study sample size which when compared to similar studies reporting significant findings is much smaller, with the later mostly analysing population-based routinely collected data. We also used a subjective measure of financial hardship which in health research in less common than objective socio-economic status measures [39]. Subjective assessments of financial status are valid alternatives to objective measures and are particularly valuable when objective measures, which require responses to multiple questions and are prone to having high levels of missing data, are felt to be inappropriate [40, 41]. The lack of association found between patient age and aggressive EOL care is inconsistent with the wider scientific literature where a decrease in aggressiveness with increasing patient age has generally been reported [15, 17, 20, 42, 43]. This requires further investigation but may reflect a specific change in UK policy towards cancer treatment for older people, with equality legislation in 2012 [44].
We also found no association between aggressive EOL care and cancer patients’ health status (mobility, ability to self-care, activity level, pain/discomfort and anxiety/depression) 3 months prior to death, suggesting that socio-demographic and/or environmental factors may be of greater importance when determining the type of care that patients are likely to receive towards the EOL. In a systematic review of place of death by Gomes and Higginson in 2006, environmental factors were also found to be more influential than factors relating to the underlying illness [45]. These findings have important policy implications when considering how future acute health care services are delivered, especially given the ageing population and anticipated rise in cancer cases [46].
Limitations
Mortality follow-back surveys have recognised limitations primarily relating to the validity of bereaved caregivers’ responses as proxies for the decedents. For objective measures, caregiver responses have been shown to have moderate to good agreement with patients’; however, for subjective experiences, such as pain or anxiety, less overall agreement has been reported and it is therefore possible that the responses received in our study may not be truly representative of the patients’ experiences at that time [47, 48].
As is the case with all secondary analysis, our choice of variables was limited by the data collected for the purposes of the primary studies. For our dependent variable, this meant that two of the three EOL care indicators that we used to calculate our composite outcome measure have not themselves been validated (greater than or equal to two ED visits in the last 3 months of life and ≥30 days in hospital in the last 3 months). However, both indicators were considered to be clinically relevant and were based on well-established validated markers [5, 33]. The third indicator, death in hospital, is commonly used as a marker of potentially aggressive EOL care arising from consistent evidence that the majority of cancer patients would prefer to die at home [49]. With regard to the independent variables investigated, we explored socio-demographic factors, clinical characteristics and patient receipt of GP home visits, district nursing and community palliative care. Further information regarding the local availability of health and social care services was not available. As our study included patients from across London, it is therefore possible that regional variations in care provision, for example, bed availability and/or community hospice services, may have influenced the parameters that we used to define our primary outcome of aggressive EOL care. Finally, because of the different sampling approaches used by each of the primary studies, the prevalence of aggressive EOL care in our pooled sample may not be representative of, and therefore generalisable to, the wider cancer population. However, these different sampling approaches were not expected to impact the factors associated with aggressive EOL care which was the primary focus of our study, and benefits to pooling the datasets included being able to explore the EOL care experiences of patients receiving a range of different health care packages including various levels of palliative care input. Furthermore, the QUALYCARE study sampled participants according to place of death, with deaths in hospital undersampled. It is therefore likely that our estimation of aggressive EOL care, which included death in hospital as one of its indicators, is actually lower than that of the true population.
Conclusions
Our results reveal an association between aggressive care in the last 3 months of life and a diagnosis of prostate or haematological cancer. We also found that community health care services, in particular contact with community palliative care, are associated with a significant reduction in the odds of cancer patients receiving aggressive care towards the EOL. Expansion of such services may help address the current capacity crises faced by many acute health care systems. Contrary to earlier studies, we found that older age did not appear to influence the risk of cancer patients receiving aggressive EOL care.
Acknowledgments
This study was conducted as part of the project BuildCARE; supported by Cicely Saunders International and The Atlantic Philanthropies, led by King’s College London, Cicely Saunders Institute, Department of Palliative Care, Policy and Rehabilitation, UK. CI: Higginson. Grant leads: Higginson, McCrone, Normand, Lawlor, Meier and Morrison. Project Co-ordinator/principal investigator (PI): Daveson. Study arm PIs: Pantilat, Selman, Normand, Ryan, McQuillan, Morrison and Daveson. We thank all collaborators and advisors including service users. BuildCARE members: Emma Bennett, Francesca Cooper, Barbara A Daveson, Susanne de Wolf-Linder, Mendwas Dzingina, Clare Ellis-Smith, Catherine J Evans, Taja Ferguson, Lesley A Henson, Irene J Higginson, Bridget Johnston, Paramjote Kaler, Pauline Kane, Peter Lawlor, Paul McCrone, Regina McQuillan, Diane Meier, Sean Morrison, Fliss E Murtagh, Charles Normand, Caty Pannell, Steve Pantilat, Ana Reison, Karen Ryan, Lucy Selman, Melinda Smith, Katy Tobin, Rowena Vohora and Gao Wei.
This study involved data from two studies: the QUALYCARE study and the IARE study. We thank all participants, the researchers (Natalia Calanzani, Paul McCrone, Sue Hall, Caty Pannell, Melinda Smith and Susanne de Wolf-Linder) and the funders of these original studies (Cicely Saunders International and The Atlantic Philanthropies).
Conflict of interest
The authors indicate no potential conflicts of interest.
Funding
This study was supported by Cicely Saunders International, The Atlantic Philanthropies, Marie Curie Cancer Care and the Collaboration for Leadership in Applied Health Research and Care (CLAHRC) South London, National Institute for Health Research (NIHR). The CLAHRC is a partnership between King’s Health Partners, St George’s, University of London, and St George’s Healthcare NHS Trust.
Author contributions
Conception and design: Lesley A Henson, Wei Gao and Irene J Higginson.
Collection and assembly of data: all authors.
Data analysis: Lesley A Henson, supported by Wei Gao, Barbara Gomes and Irene J Higginson.
Data interpretation: all authors.
Manuscript writing: Lesley A Henson and Wei Gao supported by Irene J Higginson, with critical revisions by all other authors.
Final approval of manuscript: all authors.
Appendix
Table 3
Table 3.
Eligibility criteria for each of the primary studies included in secondary analysis
| Eligibility criteria | Additional comments | |
|---|---|---|
| QUALYCARE [27] | Inclusion criteria | The four Primary Care Trusts were specifically chosen as they provided contrasting cancer home death rates and contrasting deprivation levels within London. The study sample was then stratified by Primary Care Trust and place of death so that in each area the sample included all deaths that occurred at home, all hospice deaths, all nursing home deaths, and a random sample of NHS acute hospital deaths. |
| Study participants: Bereaved relatives of people aged ≥18 years who died from cancer over a 1-year period and who lived in one of four London Primary Care Trusts: Bromley, Islington, Sutton and Merton or Westminster |
1.Deceased last resident in one of the four following Primary Care Trusts as recorded in the death registration: Bromley, Islington, Sutton and Merton or Westminster. 2.Date of death registration within 4 to 10 months before sampling. 3.Deceased aged ≥18 at time of death. 4.Cancer (ICD10 codes C00–D48) recorded as ‘underlying cause of death’ or in the lowest completed cause of death line in death certificate. |
|
| Exclusion criteria | ||
| 1.Place of death other than an NHS acute hospital, the deceased’s own home, hospice or nursing home. 2.Place of death unknown. 3.Deaths registered by a coroner. | ||
| IARE [28] Study participants: Bereaved carers of older patients who had accessed specialist palliative care prior to death. |
Inclusion criteria | |
| 1.Deceased aged ≥65 years at time of death and had accessed specialist palliative care prior to death. 2.Main informal carer aged ≥18 years and known to the palliative care team as the primary informal carer that was most involved in providing information and unpaid care for the patient. 3.Date of death registration within 4 to 10 months before sampling. | ||
| Exclusion criteria | ||
| 1.Adults who did not provide informal (unpaid) care of an eligible patient. 2.Carers aged <18 years. |
Table 4
Table 4.
Number of patients experiencing greater than or equal to two emergency department (ED) visits in the last 3 months of life, ≥30 days spent in hospital in the last 3 months of life and death in hospital for each cancer type
| Entire cohort | Patients with greater than or equal to two ED visits in the last 3 months of life | Patients who spent ≥30 days in hospital in the last 3 months of life | Patients who died in hospital | ||||
|---|---|---|---|---|---|---|---|
| N | N | % | N | % | N | % | |
| 681 | 194 | 29.8 | 108 | 15.9 | 258 | 38.0 | |
| Cancer type | |||||||
| Lung | 145 | 37 | 26.6 | 15 | 10.3 | 53 | 36.6 |
| Breast | 52 | 10 | 20.0 | 4 | 7.7 | 13 | 25.0 |
| Prostate | 46 | 22 | 52.4 | 11 | 23.9 | 27 | 58.7 |
| Gastrointestinal tract | 167 | 42 | 25.3 | 27 | 16.2 | 52 | 31.1 |
| Haematological | 54 | 18 | 38.3 | 16 | 29.6 | 32 | 59.3 |
| Cancer of unknown primary | 61 | 24 | 39.3 | 11 | 18.0 | 26 | 42.6 |
| Othera | 156 | 41 | 27.9 | 24 | 15.4 | 55 | 35.5 |
aOther cancers included those of the urinary tract (5.0 %), gynaecological (3.8 %), central nervous system (3.8 %) and skin (1.8 %) with percentage figures referring to entire cohort data
References
- 1.Smith R. A good death. An important aim for health services and for us all. BMJ. 2000;320(7228):129–30. doi: 10.1136/bmj.320.7228.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinhauser KE, et al. Factors considered important at the end of life by patients, family, physicians, and other care providers. JAMA. 2000;284(19):2476–82. doi: 10.1001/jama.284.19.2476. [DOI] [PubMed] [Google Scholar]
- 3.Heyland DK, et al. What matters most in end-of-life care: perceptions of seriously ill patients and their family members. CMAJ. 2006;174(5):627–33. doi: 10.1503/cmaj.050626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voogt E, et al. Attitudes of patients with incurable cancer toward medical treatment in the last phase of life. J Clin Oncol. 2005;23(9):2012–9. doi: 10.1200/JCO.2005.07.104. [DOI] [PubMed] [Google Scholar]
- 5.Earle CC, et al. Identifying potential indicators of the quality of end-of-life cancer care from administrative data. J Clin Oncol. 2003;21(6):1133–8. doi: 10.1200/JCO.2003.03.059. [DOI] [PubMed] [Google Scholar]
- 6.Wright AA, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665–73. doi: 10.1001/jama.300.14.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wright AA, et al. Place of death: correlations with quality of life of patients with cancer and predictors of bereaved caregivers’ mental health. J Clin Oncol. 2010;28(29):4457–64. doi: 10.1200/JCO.2009.26.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teno JM, et al. Family perspectives on end-of-life care at the last place of care. JAMA. 2004;291(1):88–93. doi: 10.1001/jama.291.1.88. [DOI] [PubMed] [Google Scholar]
- 9.Temel JS, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733–42. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 10.Emanuel EJ. Cost savings at the end of life—what do the data show? JAMA. 1996;275(24):1907–1914. doi: 10.1001/jama.1996.03530480049040. [DOI] [PubMed] [Google Scholar]
- 11.Riley GF, Lubitz JD. Long-term trends in Medicare payments in the last year of life. Health Serv Res. 2010;45(2):565–576. doi: 10.1111/j.1475-6773.2010.01082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnato AE. End-of-life spending: can we rationalise costs? Crit Q. 2007;49(3):84–92. doi: 10.1111/j.1467-8705.2007.00788.x. [DOI] [Google Scholar]
- 13.Georghiou T, Davies S, Davies A, Bardsley M (2012) Understanding patterns of health and social care at the end of life. Nuffield Trust, London
- 14.Huang J, et al. Time spent in hospital in the last six months of life in patients who died of cancer in Ontario. J Clin Oncol. 2002;20(6):1584–92. doi: 10.1200/JCO.20.6.1584. [DOI] [PubMed] [Google Scholar]
- 15.Earle CC, et al. Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol. 2004;22(2):315–321. doi: 10.1200/JCO.2004.08.136. [DOI] [PubMed] [Google Scholar]
- 16.Ho TH, et al. Trends in the aggressiveness of end-of-life cancer care in the Universal Health Care system of Ontario, Canada. J Clin Oncol. 2011;29(12):1587–1591. doi: 10.1200/JCO.2010.31.9897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Earle CC, et al. Aggressiveness of cancer care near the end of life: is it a quality-of-care issue? J Clin Oncol. 2008;26(23):3860–6. doi: 10.1200/JCO.2007.15.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooke CR, et al. Aggressiveness of intensive care use among patients with lung cancer in the surveillance, epidemiology, and end results-Medicare registry. Chest. 2014;146(4):916–923. doi: 10.1378/chest.14-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aldridge MD, et al. Has hospice use changed? 2000–2010 utilization patterns. Med Care. 2015;53(1):95–101. doi: 10.1097/MLR.0000000000000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barbera L, Paszat L, Chartier C. Indicators of poor quality end-of-life cancer care in Ontario. J Palliat Care. 2006;22(1):12–7. [PubMed] [Google Scholar]
- 21.Miesfeldt S, et al. Association of age, gender, and race with intensity of end-of-life care for Medicare beneficiaries with cancer. J Palliat Med. 2012;15(5):548–54. doi: 10.1089/jpm.2011.0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maddison AR, et al. Inequalities in end-of-life care for colorectal cancer patients in Nova Scotia, Canada. J Palliat Care. 2012;28(2):90–96. [PMC free article] [PubMed] [Google Scholar]
- 23.Smith AK, Earle CC, McCarthy EP. Racial and ethnic differences in end-of-life care in fee-for-service Medicare beneficiaries with advanced cancer. J Am Geriatr Soc. 2009;57(1):153–8. doi: 10.1111/j.1532-5415.2008.02081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henson LA, et al. Emergency department attendance by patients with cancer in their last month of life: a systematic review and meta-analysis. J Clin Oncol. 2015;33(4):370–376. doi: 10.1200/JCO.2014.57.3568. [DOI] [PubMed] [Google Scholar]
- 25.Almaawiy U, et al. Are family physician visits and continuity of care associated with acute care use at end-of-life? A population-based cohort study of homecare cancer patients. Palliat Med. 2014;28(2):176–183. doi: 10.1177/0269216313493125. [DOI] [PubMed] [Google Scholar]
- 26.Seow H, et al. Using more end-of-life homecare services is associated with using fewer acute care services: a population-based cohort study. Med Care. 2010;48(2):118–124. doi: 10.1097/MLR.0b013e3181c162ef. [DOI] [PubMed] [Google Scholar]
- 27.Gomes B, et al. Variations in the quality and costs of end-of-life care, preferences and palliative outcomes for cancer patients by place of death: the QUALYCARE study. BMC Cancer. 2010;10:400. doi: 10.1186/1471-2407-10-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.UK Clinical Research Network (2014) The IARE study. UK Clinical Research Network: portfolio database. UK Clinical Research Network, London
- 29.Lawson B, Van Aarsen K, Burge F (2013) Challenges and strategies in the administration of a population based mortality follow-back survey design. BMC Palliat Care 12:28. doi:10.1186/1472-684X-12-28 [DOI] [PMC free article] [PubMed]
- 30.Teno JM. Measuring end-of-life care outcomes retrospectively. J Palliat Med. 2005;8(Suppl 1):S42–9. doi: 10.1089/jpm.2005.8.s-42. [DOI] [PubMed] [Google Scholar]
- 31.Gomes B, et al. Cognitive interviewing of bereaved relatives to improve the measurement of health outcomes and care utilisation at the end of life in a mortality followback survey. Support Care Cancer. 2013;21(10):2835–44. doi: 10.1007/s00520-013-1848-x. [DOI] [PubMed] [Google Scholar]
- 32.EuroQol (2015) EQ5D. http://www.euroqol.org/home.html. Accessed 5 March 2015
- 33.Earle CC, et al. Evaluating claims-based indicators of the intensity of end-of-life cancer care. Int J Qual Health Care. 2005;17(6):505–9. doi: 10.1093/intqhc/mzi061. [DOI] [PubMed] [Google Scholar]
- 34.Tang ST, et al. Determinants of aggressive end-of-life care for Taiwanese cancer decedents, 2001 to 2006. J Clin Oncol. 2009;27(27):4613–8. doi: 10.1200/JCO.2008.20.5096. [DOI] [PubMed] [Google Scholar]
- 35.National Institute for Clinical Excellence (2003) Improving outcomes in haematological cancers. National Institute for Clinical Excellence, London
- 36.Bergman J, et al. Hospice use and high-intensity care in men dying of prostate cancer. Arch Intern Med. 2011;171(3):204–210. doi: 10.1001/archinternmed.2010.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gonsalves WI, et al. Effect of palliative care services on the aggressiveness of end-of-life care in the Veteran’s Affairs cancer population. J Palliat Med. 2011;14(11):1231–1235. doi: 10.1089/jpm.2011.0131. [DOI] [PubMed] [Google Scholar]
- 38.Saito AM, et al. Hospice care and survival among elderly patients with lung cancer. J Palliat Med. 2011;14(8):929–939. doi: 10.1089/jpm.2010.0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Howe LD, et al. Subjective measures of socio-economic position and the wealth index: a comparative analysis. Health Policy Plan. 2011;26(3):223–32. doi: 10.1093/heapol/czq043. [DOI] [PubMed] [Google Scholar]
- 40.Nobles J, Weintraub MR, Adler NE. Subjective socioeconomic status and health: relationships reconsidered. Soc Sci Med. 2013;82:58–66. doi: 10.1016/j.socscimed.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh-Manoux A, Marmot MG, Adler NE. Does subjective social status predict health and change in health status better than objective status? Psychosom Med. 2005;67(6):855–861. doi: 10.1097/01.psy.0000188434.52941.a0. [DOI] [PubMed] [Google Scholar]
- 42.Van den Block L, et al. Hospitalisations at the end of life: using a sentinel surveillance network to study hospital use and associated patient, disease and healthcare factors. BMC Health Serv Res. 2007;7(1):69. doi: 10.1186/1472-6963-7-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Braga S, et al. The aggressiveness of cancer care in the last three months of life: a retrospective single centre analysis. Psychooncology. 2007;16(9):863–8. doi: 10.1002/pon.1140. [DOI] [PubMed] [Google Scholar]
- 44.Department of Health (2012) Cancer services coming of age: learning from the improving cancer treatment assessment and support for older people project. Department of Health, UK
- 45.Gomes B, Higginson IJ. Factors influencing death at home in terminally ill patients with cancer: systematic review. BMJ. 2006;332(7540):515–21. doi: 10.1136/bmj.38740.614954.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moller H, et al. The future burden of cancer in England: incidence and numbers of new patients in 2020. Br J Cancer. 2007;96(9):1484–8. doi: 10.1038/sj.bjc.6603746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McPherson CJ, Addington-Hall JM. Judging the quality of care at the end of life: can proxies provide reliable information? Soc Sci Med. 2003;56(1):95–109. doi: 10.1016/S0277-9536(02)00011-4. [DOI] [PubMed] [Google Scholar]
- 48.Stajduhar KI, et al. Bereaved family members’ assessments of the quality of end-of-life care: what is important? J Palliat Care. 2011;27(4):261–9. [PubMed] [Google Scholar]
- 49.Gomes B, et al. Heterogeneity and changes in preferences for dying at home: a systematic review. BMC Palliat Care. 2013;12:7. doi: 10.1186/1472-684X-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


