Abstract
The oral cavity is the beginning of the aero-digestive tract, which is covered by mucosal epithelium continuously under the threat of invasion of pathogens, it is thus protected by the mucosal immune system. In the early phase of our scientific efforts for the demonstration of mucosal immune system, dental science was one of major driving forces due to their foreseeability to use oral immunity for the control of oral diseases. The mucosal immune system is divided functionally into, but interconnected inductive and effector sites. Intestinal Peyer’s patches (PPs) are an inductive site containing antigen-sampling M cells and immunocompetent cells required to initiate antigen-specific immune responses. At effector sites, PP-originated antigen-specific IgA B cells become plasma cells to produce polymeric IgA and form secretory IgA by binding to poly-Ig receptor expressed on epithelial cells for protective immunity. The development of new-generation mucosal vaccines, including the rice-based oral vaccine MucoRice, on the basis of the coordinated mucosal immune system is a promising strategy for the control of mucosal infectious diseases.
Keywords: innate lymphoid cell, M cell, mast cell, mucosal vaccine, MucoRice, Peyer’s patch
1. Introduction
The mucosa covers the largest surface area of the human body, including the oral cavity, respiratory and digestive tracts, ocular cavity, ear cavity, and genitourinary tract, and it forms the boundary between the interior of the body and its external environment.1) Because of its large surface area and continuous exposure to the external environment, the mucosa is the primary entry route of most pathogens via inhalation, ingestion, or sexual contact.2) Therefore, the host must maintain a dynamic and flexible immunologic barrier at the mucosal surface to protect against invasion by harmful pathogens. At same time, the mucosa has essential roles in the physiologic functions of ingestion and inhalation and therefore is integral to these basic life-supporting systems. These concurrent yet contrasting roles — immunologic barrier and physiologic machinery — are a unique characteristic of the mucosa.
To provide a protective barrier at mucosal surfaces, the innate and acquired immune systems must operate concurrently. Physical barriers — consisting of tight junctions and a dense layer of mucins3) — and biochemical barriers — provided by antimicrobial peptides4) — are crucial elements in the first line of defense, together with components of innate immunity, including Toll-like receptors5–7) and innate immune cells (e.g., natural killer cells, natural killer T cells, mast cells, and eosinophils).8–11) For acquired immunity at the mucosal epithelium, antigen-specific secretory IgA (SIgA) immune responses are key players in preventing pathogen invasion. SIgA directly neutralizes the infectivity of pathogens and their toxins12–14) and contributes to the creation and maintenance of mucosal homeostasis.15)
For the induction of antigen-specific SIgA antibodies, antigens must be presented directly to the mucosal surface — especially its component mucosa-associated lymphoid tissue (MALT), antigen-sampling cells (microfold or membranous cells [M cells]),3,16) and antigen-presenting cells (e.g., dendritic cells).17–19) Therefore, oral and nasal deposition of antigens that target gut-associated lymphoid tissue (GALT) and nasopharynx-associated lymphoid tissue (NALT) leads to the generation of antigen-specific SIgA antibodies in the secretions of the gastrointestinal and upper respiratory tracts. In contrast, although very effective in the induction of systemic immunity (e.g., serum IgG antibody), the systemic presentation of antigens, such as through their injection, apparently does not induce effective antigen-specific SIgA antibody responses.20) In fact, subcutaneous vaccination is often ineffective for the induction of antigen-specific mucosal immune responses.21) These facts indicate that new-generation vaccines must be rationally designed to efficiently elicit antigen-specific mucosal immunity at the entry sites of aero-digestive infectious agents.
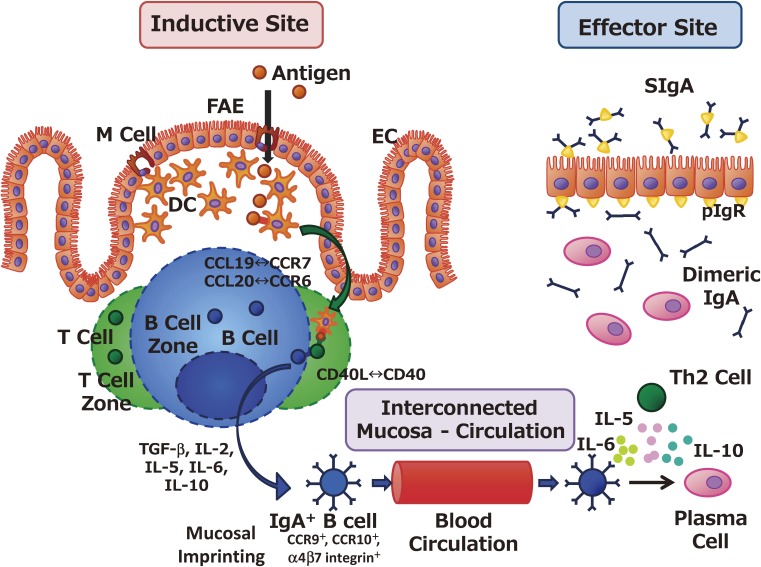
The mucosal immune system consists of coordinated inductive and effector tissues (Fig. 1).2) As inductive sites, MALTs are equipped with all of the immunocompetent cells needed to initiate antigen-specific humoral and cell-mediated immune responses.22–24) For example, at Peyer’s patches (PPs), which are well characterized GALT components, orally administered antigens are taken up by M cells located in the follicle-associated epithelium; M cells promptly deliver antigens to antigen-presenting cells, such as dendritic cells and macrophages, that lie beneath PPs.24) Antigen-presenting cells subsequently present processed antigen to naïve T cells, leading to the differentiation to Th1 cells, Th2 cells, Th17 cells, and cytotoxic T cells; they also induce IgA-committed B cells (IgA+ B cells) with key immunological molecules (e.g., TGF-β [transforming growth factor β], interleukin (IL)-5, IL-6, IL-10, APRIL [a proliferation-inducing ligand] and BAFF [B cell activating factor]), leading to the initiation of antigen-specific immune responses.24) Concurrent with antigen presentation, dendritic cells located in PPs induce gut-imprinting molecules (e.g., CC chemokine receptor [CCR]9, CCR10, α4β7 integrin) on antigen-specific lymphocytes through the retinoic acid cascade for their subsequent migration to the effector tissues (e.g., intestinal lamina propria) (Fig. 1).25,26) The lipid mediator system, which includes sphingosine-1-phosphate and its receptor, sphingosine-1-phosphate receptor type 1, plays an essential role in the egress from PPs of antigen-specific lymphocytes that carry gut-imprinting molecules and in their subsequent immunologic journey to distant effector sites.27–29)
Figure 1.
Coordination between inductive and effector sites for the induction and regulation of antigen-specific mucosal immune responses. Antigens in the lumens of the gastrointestinal tract, nasal cavity, and tear ducts are endocytosed by M cells located on the follicle-associated epithelium (FAE) of the mucosa-associated lymphoid tissues. In the case of gut-associated lymphoid tissue or Peyer’s patches, M cells located in the FAE form the subepithelial dome structure, and antigen-presenting cells such as dendritic cells (DCs) lie immediately beneath the FAE. M-cell–endocytosed antigens are immediately processed by DCs, which transport antigens to underlying T cell zones through molecular interactions such as CCL19–CCR7 and CCL20–CCR6. Antigen-primed T cells support the induction of IgA-committed B cells (IgA+ B cells) owing to the biologic influences of transforming growth factor (TGF)-β, IL-2, IL-5, IL-6, IL-10, and the CD40–CD40 ligand (CD40L) interaction. In addition, IgA+ B cells acquire mucosal-imprinting molecules, such as CCR9, CCR10, and α4β7 integrin, and subsequently migrate to the effector sites. At the effector sites (e.g., the intestinal lamina propria), IgA+ B cells differentiate into plasma cells after stimulation by the IgA-enhancing cytokines IL-5, IL-6, and IL-10, which are secreted by antigen-specific Th2 cells. Dimeric or polymeric IgA secreted from plasma cells is transported to the mucosal surface as secretory IgA (SIgA) through the binding to polymeric Ig receptor expressed on the basal membrane of epithelial cells (ECs).
At the effector sites, PP-originated antigen-specific Th2 cells provide the IgA-enhancing cytokines (including IL-5, IL-6, and IL-10) needed for the final differentiation of IgA+ B cells into plasma cells that produce dimeric or polymeric forms of IgA.2,24,30,31) These IgA antibodies then bind to poly-Ig receptors expressed on the basal membrane of epithelial cells, where they form SIgA and are transported to gut secretions (Fig. 1).32,33) This collaborative and well-orchestrated sequence between the inductive (e.g., PPs) and effector (e.g., intestinal lamina propria) sites provides the immunologic basis for the induction and regulation of antigen-specific immune responses (e.g., SIgA production) at the mucosal surface.
2. Historical insight into the contribution of dental science to mucosal immunology
In recent years, mucosal immunology has become a core entity uniting the biomedical fields of immunology, microbiology, allergology, and pathology. Before the 1970s, few immunologists credited the presence of the immune system at the mucosal surface of the digestive tract. However, a major scientific contribution during that era initiated research into the mucosal immune system, then known as “local immunity.” It was originally shown the presence of IgA antibodies in secretions including saliva by Tomasi T.B. Jr. and his colleagues in the mid-1960s.34,35) They showed that human parotid saliva (and other nonvascular fluids) contained large amounts of IgA relative to IgG and these IgA differed in chemical and immunological properties from serum IgA.34–36) Several investigators, including those in our group, with backgrounds in dentistry and oral biology recognized the important relationship between the oral cavity as the beginning of the digestive tract and the large quantities of IgA antibodies (∼200 mg) generated in the salivary glands and ingested through saliva (∼750–1000 mL) each day.37) In addition, although Streptococcus mutans was first isolated in 1924, most of the research formally proving its role as the causative pathogen in dental caries occurred in the 1960s and 1970s.38,39) These advances yielded the scientific strategy for developing a caries vaccine that induced the production of S. mutans-specific SIgA in the salivary fluids and of gingival fluid IgG produced in the serum.40,41) Our laboratory showed that oral administration of S. mutans induced the production of both antigen-specific IgA in the salivary glands and serum IgG antibodies.42,43) In summary, the efforts of several researchers in the fields of dental science and oral biology together became a driving force behind the wider scientific community’s current acceptance of an immune system at the mucosal surface of the digestive tract and of oral immunization as an effective way to induce antigen-specific SIgA production in mucosal secretions.
3. Unique features of the mucosal immune system
3-1) Critical role of antigen-sampling system at mucosal epithelium.
GALT (e.g., PPs) plays important roles as inductive sites for initiating the antigen-specific humoral and cell-mediated immune responses necessary for achieving protective acquired immunity at mucosal surfaces. Mucosal surfaces provide physical and chemical epithelial barriers to prevent the invasion of unwanted antigens;44) thus foreign antigens are thought to lack easy access to the host immune system. However, the host mucosal immune system is equipped with specialized antigen-sampling cells that transport external luminal antigens across the epithelial barrier into the organized lymphoid tissues (e.g., GALT).45) These specialized antigen-sampling cells, known as M cells, typically are located in the follicle-associated epithelium of MALT (Fig. 2).46–49) M cells are characterized by their unique morphology comprising an irregular brush border, few microvilli, and decreased glycocalyx.48) M cells were first identified in the rabbit appendix through the use of transmission electron microscopy in 197349) and were identified in humans in 1974.46) However, the presence of an antigen-entrance site on the organized lymphoid structure of the intestine had already been reported by Dr. Kenzaburo Kumagai in a 1922 Japanese article.50)
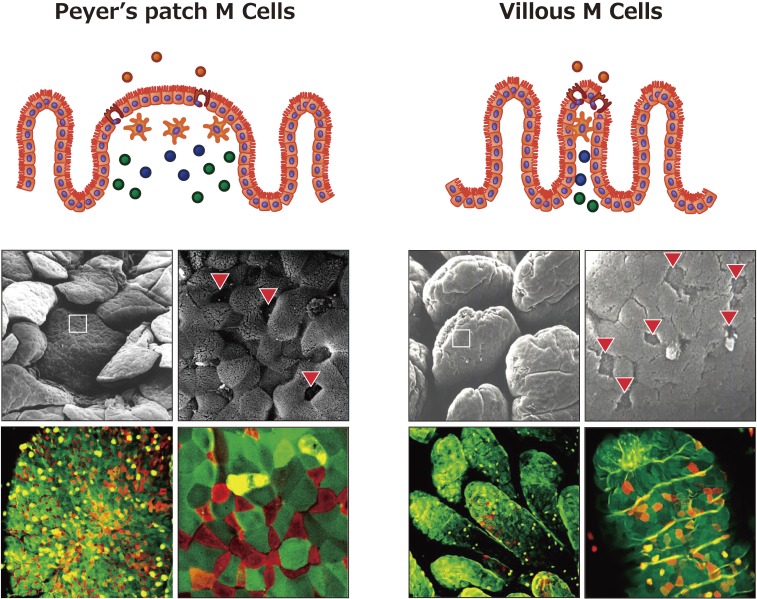
Figure 2.
Characteristics of antigen-sampling M cells located in the follicle-associated epithelium (FAE) of Peyer’s patches (PPs) and villous epithelium. M cells are preferentially located in the FAE of mucosa-associated lymphoid tissues and characterized by their unique morphologies: an irregular brush border, few microvilli, and decreased glycocalyx. NKM 16-2-4 is a monoclonal antibody specifically recognizing an α(1,2) fucose–containing carbohydrate moiety of murine M cells. When NKM 16-2-4 was applied to a tissue section of PPs, it specifically bound the apical surfaces of M cells in the FAE of the PPs (red cells in the bottom left part of the figure). Furthermore, NKM 16-2-4 recognized another M cell (known as villous M cell) located in the villous epithelium (red cells in the bottom right part of the figure), which is an additional “gateway” cell and has the similar morphologic features as M cell. Some contents of the figure have been adopted and modified from our original article reference 65.
B cells are indispensable in the development of M cells, given that the follicle-associated epithelium of PPs of B-cell–deficient mice is devoid of M cell development.51) Among B cells, CCR6HighCD11cInt B cells in PPs have a vital role in the differentiation of M cells through CCL20–CCR6 signaling.52) Our collaborative study showed that, in addition to B cells, the interaction between RANK (receptor activator of nuclear factor κB) and its ligand, the tumor necrosis factor superfamily member RANKL (receptor activator of nuclear factor κB ligand), is a key in the development of M cells.53) RANKL-null mice possess <2% of the M cells in wild-type mice and approximately 10% of the M cells in either B-cell–deficient or CCR6-deficient mice.53) Expression of RANKL is restricted to non-hematopoietic mesenchymal cells underlying the follicle-associated epithelium, whereas RANK is expressed on epithelial cells in the follicle-associated epithelium and other parts of the small intestine.53) This microarchitectural arrangement suggests that RANKL affects the differentiation of M cells through short-range delivery of RANKL to RANK-expressing epithelial precursor cells in follicle-associated epithelium.53)
The use of modern tools, including transcriptome analysis, has led to the identification of the Ets family transcription factor Spi-B as a critical factor in M cell differentiation.54) Our group and others have shown that Spi-B is highly expressed in M cells located in the follicle-associated epithelium of PPs, and Spi-B-deficient mice lack functionally mature M cells in PPs, leading to impaired antigen uptake.54,55) Therefore, the Spi-B-mediated signaling cascade is a critical pathway for the induction of M cells;54,55) however, an alternative cascade may exist as well, given that M cell development is not completely abolished in Spi-B-deficient mice.55)
Under stable physiologic conditions, M cells account for only about 10% of the epithelial cells within follicle-associated epithelium of PPs in the small intestine of mice and 5% of those in humans.48,56) M cells exhibit a unique intrapocket structure at their basal membranes and house antigen-presenting cells (e.g., dendritic cells and macrophages) or lymphocytes (Fig. 1).56) Furthermore, the region beneath the follicle-associated epithelium (including the M-cell pocket region) is enriched with antigen-presenting cells.48,56) This immunologic microstructure thus enables rapid and effective transport of antigens from the lumen into MALTs such as PPs; underlying antigen-presenting cells then process the antigens and present them to lymphocytes, thus initiating an antigen-specific immune response.57)
M cells have been studied and characterized according to their unique morphologic features and have been identified by using the lectin specificity of Ulex europaeus agglutinin 1 (UEA-1), which has strong affinity for an α(1,2) fucose expressed by murine M cells but not neighboring columnar epithelial cells.58–60) The specificity of UEA-1 for M-cell-related glycosylation patterns has therefore led to the use of this agglutinin as an M-cell marker.58–60) However, UEA-1 reacts with not only M cells but also goblet cells and the mucus layer covering the epithelium, suggesting that it is not a marker specific for M cells.61) To this end, our group developed an M-cell-specific monoclonal antibody, NKM 16-2-4, which reacts with a glycosylation site specific to murine M cells (Fig. 2).62) NKM 16-2-4 is a murine M-cell-specific monoclonal IgG2c antibody obtained by immunizing rats with UEA-1-positive cells isolated from murine PPs.62) NKM 16-2-4 also recognizes UEA-1-positive M cells in murine NALT located in the nasal cavity but not UEA-1-positive goblet cells.62) Although NKM 16-2-4 is considered to be an M-cell-specific monoclonal antibody, it also recognizes Paneth cells but not goblet cells or other epithelial cells.62)
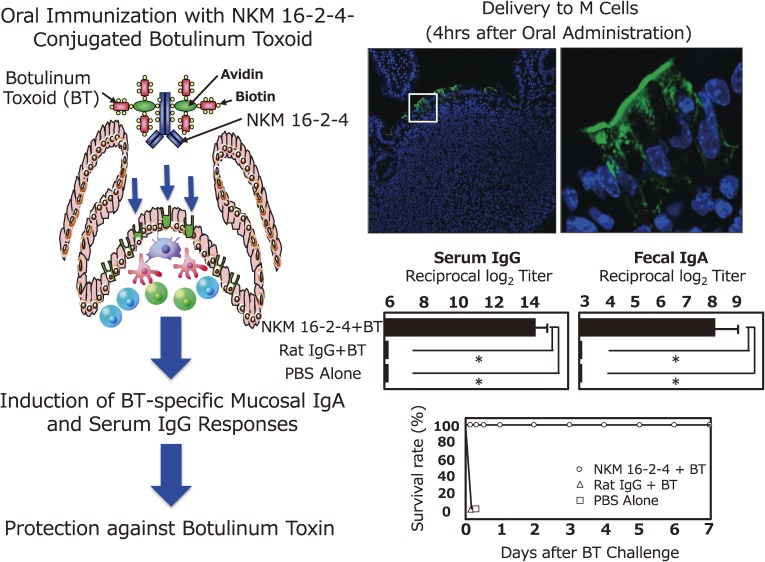
When NKM 16-2-4 was used as a targeting and carrier vehicle for the oral delivery of vaccine antigens, vaccine antigens (e.g., a vaccine complex of NKM 16-2-4-tetanus toxoid or NKM 16-2-4-botulinum toxoid [BT]) were preferentially delivered to M cells located in the follicle-associated epithelium of PPs (Fig. 3).62) Oral administration of NKM 16-2-4-conjugated tetanus toxoid or BT together with the mucosal adjuvant (e.g., cholera toxin [CT]) induced high-levels of antigen-specific serum IgG and mucosal IgA responses.62) In particular, an oral vaccine formulation of NKM 16-2-4-conjugated BT induced full protective immunity against lethal challenge with botulinum toxin.62) These results formally proved the scientific platform that a monoclonal antibody specific for M cells is an effective vehicle for delivering vaccine antigen to mucosal inductive site (e.g., PPs) to induce antigen-specific systemic IgG and mucosal IgA responses.
Figure 3.
Specific delivery of vaccine antigen to M cells located in the follicle associated epithelium (FAE) of Peyer’s patches (PPs) induces antigen-specific mucosal IgA and serum IgG responses. NKM 16-2-4 is an M cell-specific monoclonal antibody (mAb) and used as an antigen delivery vehicle to M cells located in FAE of PPs. When mice were orally immunized with the mAb-conjugated vaccine antigen (e.g., botulinum toxoid [BT]) (NKM 16-2-4-BT), the mAb-conjugated vaccine antigen (NKM 16-2-4-BT) was preferentially delivered to M cells in the FAE of PPs. Furthermore, oral immunization with the chimeric form of vaccine antigen NKM 16-2-4-BT induced BT-specific mucosal IgA and serum IgG antibody responses with full neutralizing activity against botulinum toxin. Some contents of the figure have been adopted and modified from our original article reference 62.
By using NKM 16-2-4 and UEA-1, populations highly enriched in M cells have been isolated and analyzed to further elucidate the molecular features of this unique cell type; these analyses have included specific DNA profiling of M cells compared with other epithelial cell populations.63,64) Through the efforts of our group and collaborators, glycoprotein 2 (Gp2) has been identified as a molecule that is expressed specifically by murine M cells; Gp2 is expressed by human M cells also.63,64) Furthermore, Gp2 was found to specifically interact with bacterial FimH, a component of type I pili on the outer membrane of various bacteria, including Escherichia coli and Salmonella typhimurium.63) These bacteria therefore use Gp2 expressed on M cells as their port of entry and then are transcytosed through Gp2 into the PPs.63) A Gp2 deficiency in the host, or the deletion of FimH from the bacterium, prevents M-cell-mediated bacterial invasion of the host.63)
M cells are now defined through the combined use of several criteria, including reactivity with UEA-1 and NKM 16-2-4, expression of Gp2, characteristic morphologic features (few surface microvilli and the presence of a pocket structure), and endocytic activity.48,49,58,62,63) Most M cells reside in the follicle-associated epithelium of MALTs, but our work has provided evidence that M-cell-like cells with several of these criteria exist in the villous epithelium even in the absence of follicle-associated epithelium.65) These M-cell-like cells have been named “villous M cells” and can transcytose external antigens from the intestinal lumen to induce antigen-specific immune responses in a PP-independent manner.65) NKM 16-2-4 reacts with villous M cells (Fig. 2, bottom right).62)
3-2) GALT as the inductive site for antigen-specific mucosal immunity.
In addition to containing antigen-sampling M cells, GALTs contain all of the immunocompetent cells required for the generation of antigen-specific immune responses, including dendritic cells, macrophages, T cells, and B cells (Fig. 1).24,66,67) Our early work contributed to the establishment of PPs — components of GALT — as the inductive tissues for the mucosal immune system by showing the presence of antigen-presenting cells (in addition to T and B cells) in PPs.66) After antigens are taken up by M cells, transcytosed antigens are engulfed by antigen-presenting cells such as macrophages and dendritic cells, which process antigens into peptides and transport the resulting peptides on MHC class I or II molecules on the surface of antigen-presenting cells to underlying CD8+ or CD4+ T cells, respectively.47,68,69) The interaction between chemokines produced in the interfollicullar region of the PPs and chemokine receptors, such as between CCL19 and CCR7 and between CCL20 and CCR6, plays an important role in the transport of dendritic cells to T cell zones.70) After arriving at the T cell zones, dendritic cells present antigen peptides to naïve helper T (Th) cells for the induction of antigen-specific T cells. Antigen-primed Th cells support IgA class switching and somatic hypermutation of B cells in the germinal centers and B cell zones.71) Molecular interactions between CD40 on B cells, TGF-β, IL-2, IL-5, IL-6, and IL-10 also are needed to induce the production of IgA+ B cells in GALT and their differentiation into plasma cells in the intestinal lamina propria regions.31,72) Moreover, in PPs, Th17 cells acquire the phenotype of follicular helper T cells; this conversion is dependent on the PP environment but independent of IL-23 and contributes to the development of IgA+ B cell responses.17)
3-3) Role of innate immunity-associated cells in mucosal immunity.
In addition to the acquired mucosal immunity (e.g., SIgA) established by antigen-specific helper T and IgA+ B cells, innate-immunity–associated cells, including mast cells, eosinophils, basophils, macrophages, and innate lymphoid cells, are important in providing mucosal immunity. Among the different cell subsets, innate lymphoid cells have recently been recognized as a novel family of hematopoietic effectors that fulfill several key roles in the innate immune responses to infectious pathogens, the formation of lymphoid tissue, the process of tissue repair, and homeostatic control (with tissue stromal cells).73–76) Innate lymphoid cells can be allocated to three subsets according to their phenotypes, transcription factor requirements, and cytokine production.77,78) Group 1 innate lymphoid cells are characterized by their ability to produce interferon γ. Group 2 innate lymphoid cells produce type 2 cytokines, including IL-5 and IL-13, and depend on GATA-binding protein 3 and retinoic acid receptor α for their development. Group 3 innate lymphoid cells produce IL-17 and IL-22 and are dependent on retinoic acid receptor γt. Our recent studies have shown that intestinal innate lymphoid cells play a critical role in the creation of homeostatic environment with commensal microflora.74,75)
Among the innate lymphoid cell subsets, IL-22-producing group 3 innate lymphoid cells constitutively present in the intestine of healthy mice stimulate IL-22R+ epithelial cells to produce anti-microbial peptides that may help to physically contain intra-tissue habituated Alcaligenes species within the PPs.74,79) IL-22-producing innate lymphoid cell-deficient mice — that is, Rag1−/− mice treated with monoclonal antibody against CD90.2, one of the surface molecules expressed on innate lymphoid cells — show peripheral dissemination of commensal Alcaligenes bacteria, leading to the development of susceptibility to the inflammation associated with Crohn’s disease and hepatitis C virus infection; these inflammatory responses are attenuated by the administration of IL-22.74) In addition to their contribution to the retention of commensal bacteria in GALT (e.g., PPs), IL-22-producing group 3 innate lymphoid cells contribute to epithelial fucosylation in the gastrointestinal tract.75,80) Development and maintenance of the fucosylation of intestinal epithelial cells and homeostasis of the normal flora in the gut require mutual interactions.75,76,81,82) Furthermore, epithelial fucosylation protects the intestinal surface against bacterial invasion.75,76) In particular, group 3 innate lymphoid cells induce the expression of fucosyltransferase 2 on the gut epithelium; this enzyme is a key factor in the regulation of fucosylation of intestinal epithelial cells, the mediators of which include IL-22 and lymphotoxin α.75,76)
Mast cells are involved in innate immunity in addition to their roles in adaptive immune responses, allergy, mucosal secretion, intestinal peristalsis, tissue repair, and peripheral tolerance.83) In the intestine, mast cells are categorized into two major subclasses: mucosal mast cells, which reside in the epithelium, and connective-tissue mast cells, which reside in the connective tissue beneath the epithelium.84) In mice, mucosal mast cells predominantly express the chymase mouse mast cell protease 1, which has an essential role in preventing infestation by the nematode Trichinella spiralis.84,85) A granule serine protease, tryptase α1, is expressed on human pulmonary mast cells and directly attenuates the infection of lung tissue by Klebsiella pneumoniae.86) Thus, mast cells are implicated in mucosal innate immunity.85,86)
Contrary to their participation in beneficial protective immunity, overabundance or abnormal activation of mast cells in the intestine often occurs in patients with inflammatory bowel disease.87,88) The key factors responsible for the activation of mast cells in this mucosal inflammation are still unclear. Our group recently showed that mice lacking the P2X7 receptor (a P2 purinoceptor specific for ATP) on their mast cells were resistant to 2,4,6-trinitrobenzene sulfonic acid–induced intestinal inflammation, whereas the activation of mast cells induced by the interaction between ATP and P2X7 purinoceptor elicited the production of inflammatory cytokines such as IL-6, tumor necrosis factor α, and oncostatin M.76,89) In addition, patients with Crohn’s disease have increased numbers of mast cells and enhanced expression of P2X7 purinoceptor on these cells.89) Taken together, these findings suggest that P2X7-mediated mast-cell activation is an important factor in the development of intestinal inflammation.
4. The mucosal immune system in the development of new-generation vaccines
At present, most of the vaccines available for clinical human use are administered through systemic routes, such as subcutaneous injection. The traditional route of vaccination is effective for inducing protective systemic immune responses but typically elicits only weak, or no, antigen-specific immune responses at mucosal surfaces, which are important sites of pathogen invasion.20) Although the systemic route of vaccination is currently routinely used to induce protective immunity within the body, it may not be suitable for defending against the invasion of harmful mucosal pathogens upon their entry by inhalation, ingestion, or sexual contact. In contrast, mucosal vaccination with appropriate delivery vehicles or co-administered with adjuvant has successfully induced not only systemic immune responses but also protective mucosal immune responses, leading to a double layer of protection against mucosally invasive pathogens.25,26,90–92) In addition to their effectiveness, mucosal vaccines offer additional advantages over injectable vaccines in terms of decreased costs, increased ease of administration, avoidance of needlestick injuries and transmission of blood-borne diseases, and less physical and psychologic discomfort.26)
Despite these obvious advantages, only a few mucosal vaccines have been approved for clinical use in humans: vaccines against poliovirus, rotavirus, Salmonella typhi, and Vibrio cholerae are administered orally, and vaccines against influenza virus are given intranasally (Table 1).26) Most of these currently available mucosal vaccines involve either attenuated or gene-modified live or killed forms of whole microorganisms (Table 1).26) A mucosal vaccine that delivers a component (subunit) or purified form is not yet available for clinical use. Oral administration of a protein antigen for vaccine candidate alone fails to effectively induce antigen-specific immune responses because of intrinsic physiologic mechanisms of the intestinal tract, namely degradation of vaccine antigen by digestive enzymes (e.g., pepsin);91,93) clearance mechanisms (e.g., peristalsis action and mucus secretion); and physiologic and biologic barriers (e.g., gastric acids, mucins, and tight junctions) to the access of vaccine antigen to mucosal inductive tissue (e.g., GALT or PPs).26) Although various obstacles remain, a mucosal vaccination strategy appears attractive and beneficial for the next generation of vaccines. Furthermore, recent scientific advances achieved through the sharing of knowledge and technologies among different fields of science are helping to overcome various hurdles in the development of mucosal vaccines.
Table 1.
Mucosal vaccines available for human clinical use
| Target | Route | Type | Protocol | Trade name (Producer) |
|---|---|---|---|---|
| Poliovirus | Oral | Live attenuated poliovirus monovalent (Sabin strain type 1), bivalent (type 1 and 2), or trivalent (type 1, 2 and 3) | 3 doses, with at least 4 weeks between doses (0–5 years old) | Polio Sabin Mono Two (GlaxoSmithKline), Sabin (Biken) and others |
| Rotavirus | Oral | Live reassortant rotavirus containing G1, G2, G3, G4, and P1A | 3 doses, with 4–10 weeks between doses (only infants 6–32 weeks old) | RotaTeq (Merck) |
| Live attenuated human G1P[8] rotavirus | 2 doses at least 4 weeks apart (only infants 6–24 weeks old) | RotaRix (GlaxoSmithKline) | ||
| Salmonella typhi | Oral | Live attenuated S. typhi Ty21a | 4 doses on alternate days (persons older than 6 years) | Vivotif (Crucell) |
| Vibrio cholerae | Oral | Killed whole-cell V. cholerae O1 and recombinant cholera toxin B subunit | 2 doses given 1–6 weeks apart (3 doses for children 2–6 years old) | Dukoral (Crucell) |
| Killed bivalent whole-cell V. cholerae O1 and O139 | 2 doses 2 weeks apart (persons older than 1 year) | Shanchol (Shanta Biotechnics), mORC-Vax (Vabiotech) | ||
| Influenza virus | Nasal | Live attenuated influenza virus reassortants of A/H1N1, A/H3N2, B/Yamagata/16/88, and B/Victoria/2/87 | 1 or 2 doses (persons 2–49 years old) | FluMist Quadrivalent (MedImmune) |
| Live attenuated influenza virus A/H1N1 | 1 or 2 doses (persons older than 3 years) | Nasovac (Serum Institute of India) | ||
4-1) MucoRice, a new-generation rice-based oral vaccine.
In the field of oral vaccine development, transgenic plant-based vaccines are spotlighted because of their practicality, safety, and low cost.25,94–96) Compared with injectable vaccines, oral vaccines require additional adaptations in antigen-delivery techniques, because the antigens must cross mucosal barriers comprising digestive enzymes, mucus, and physiologic mechanisms to be presented to gut-associated MALTs such as GALTs and PPs. Among the various plant candidates (e.g., carrot, potato, rice, soybean, tobacco, and tomato) for transgenic vaccines composed of a bacterial component (such as heat-labile enterotoxin [LT] B subunit, CT B subunit [CTB], or Yersinia pestis) and a viral component (such as hepatitis B virus, rotavirus, or norovirus),91,97–103) rice may be the most suitable antigen-expressing plant because rice seed (especially the protein body) is resistant to digestion by gastric acid.91) Furthermore, protein expressed in rice seeds is stable for a prolonged time in the absence of refrigerated storage.91,104) Rice can thus be considered a viable candidate for the creation of cold-chain–free and needle-free vaccines.
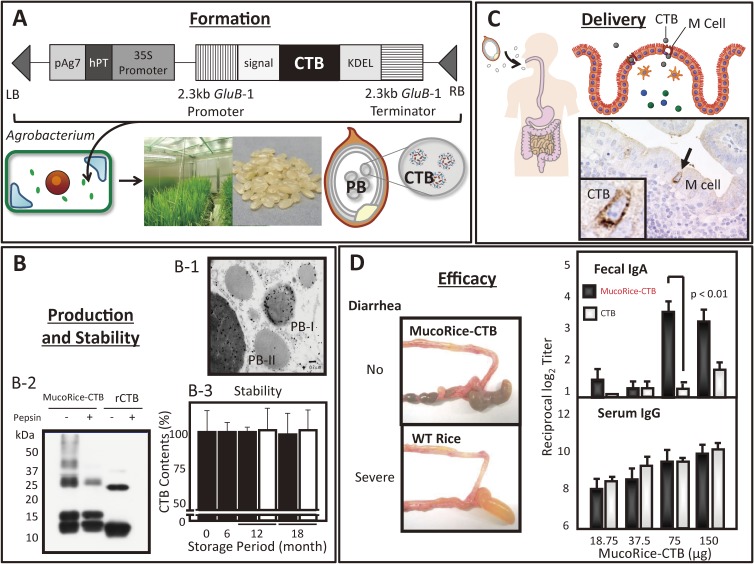
MucoRice-CTB, which consists of CTB expressed in transgenic rice, is one of the most potent plant-based vaccines (Fig. 4).91,104) The gene encoding CTB was transduced into rice seed by means of an Agrobacterium-mediated method (Fig. 4, A), and the amount of resultant recombinant CTB protein reaches approximately 30 µg per transgenic rice seed.91) The recombinant CTB protein accumulates in the rice protein body, a unique protein-storage organelle in rice seed (Fig. 4, B-1).91) The CTB proteins expressed in the protein body are not only stable at room temperature for more than 3 years without loss of immunogenicity but also resistant to digestive enzymes in the gastrointestinal tract (Fig. 4, B-2 and -3),91,104) suggesting that the rice protein body acts as a natural capsule for the oral delivery of vaccine antigens to the gut immune system. Introduction of the MucoRice-CTB (CTB accumulated in protein bodies) in the digestive tract leads to its subsequent uptake by M cells and transport to PPs (Fig. 4, C).91)
Figure 4.
Contributions of fusion science from agriculture, gene engineering, and immunology to rice-based antigen-delivery technology and the potential oral vaccine MucoRice. (A) The gene encoding cholera toxin B subunit (CTB) is transduced into rice seed by means of an Agrobacterium-mediated method. The resultant CTB protein accumulates in the rice protein body (PB), which is a unique protein-storage organelle. (B) When the section of MucoRice-CTB seeds was stained with gold particle-conjugated anti-CTB, electron microscopic analysis showed that MucoRice-CTB preferentially expressed CTB in the protein body (PB)-I and -II of rice seeds (B-1). MucoRice-CTB powder containing 15 µg of CTB and recombinant CTB (rCTB: 15 µg) were treated with or without pepsin (0.5 mg/ml) for 1 hr at 37 ℃ and then subjected to Western blotting analysis. The rCTB protein was easily digested by pepsin (right column with + pepsin), whereas the CTB protein expressed in the protein body of rice (MucoRice-CTB) was resistant to digestive enzymes (left column with + pepsin) (B-2). In addition, MucoRice-CTB was stable at room temperature for years and thus the amount of CT expressed in the rice seeds did not change after 18 months storage period (B-3). (C) Following oral immunization, MucoRice-CTB is delivered effectively to gut mucosal surfaces and is endocytosed by M cells located follicle associated epithelium of Peyer’s patch. (D) Oral administration of MucoRice-CTB in mice effectively elicited CT-neutralizing serum IgG and fecal IgA antibodies and protects mice from the diarrhea induced by orally challenged CT. Some contents of the figure have been adopted and modified from our original article reference 91.
In addition to its advantages of cold-chain-free storage and effective delivery, MucoRice-CTB induces antigen-specific protective immune responses in both the systemic and mucosal compartments (Fig. 4, D). Oral administration of MucoRice-CTB in mice elicits the production of CT-neutralizing serum IgG and fecal IgA antibodies and protects mice from diarrhea induced by orally administered CT (Fig. 4, D).91,104) MucoRice-CTB also induces CT-specific antibody production in cynomolgus macaques.105) Moreover, MucoRice-CTB induces cross-protective immunity against LT after its oral administration.104) CT and LT are multisubunit macromolecules that comprise an active A subunit, which confers the biologic activities of toxicity and adjuvanticity, and a GM1 ganglioside-binding B subunit that lacks toxicity.106,107) The ADP-ribosyltransferase activity of the CTA and LTA subunits activates adenylate cyclase, causing subsequent increases in cAMP,108) triggering the secretion of water and chloride ions from epithelial cells into the small intestinal lumen, and leading to severe diarrhea.109) The cross-protective immunity against LT induced through vaccination with CT is thought to reflect the close similarity between the CTB and LTB subunits.110) Therefore, MucoRice-CTB may be a potential vaccine against the toxin produced by enterotoxic E. coli, which is a major cause of severe diarrhea in children in developing countries.104,111) In terms of safety, because MucoRice-CTB contains lower amounts of the major rice allergens than does wild-type rice, the risk of unexpected allergic reactions against the rice proteins in MucoRice-CTB is minimized.112,113) Moreover, oral immunization with MucoRice-CTB does not induce rice-storage-protein–specific serum IgE responses and other adverse events in cynomolgus macaques.105)
4-2) MucoRice for oral delivery of antibody.
The MucoRice system can be applied to passive immunotherapy because of its advantages of both stability in the absence of refrigeration and effective delivery.114) MucoRice-ARP1 expresses the anti-rotavirus specific variable domain of llama heavy-chain antibody fragment (VHH) in rice seeds and is a candidate for a new passive immunotherapy against rotavirus infection.114) Worldwide, an estimated 1.731 billion episodes of diarrhea and 700,000 deaths due to diarrheal infections occur each year in children younger than 5 years, and rotavirus (associated with 28% of cases) is the most common cause of vaccine-preventable severe diarrhea.115) A pentavalent reassortant vaccine (RotaTeq, Merck) and a monovalent live attenuated G1P[8] rotavirus vaccine (Rotarix, GlaxoSmithKline) are currently in clinical use for oral vaccines against rotavirus infections and effectively protect infants against rotavirus gastroenteritis.26) In developing countries where rotavirus-induced diarrhea is common,115) financial constraints may limit the widespread use of these vaccines against rotavirus. In addition, rehydration is the only effective treatment for rotaviral diarrhea.116) Therefore, MucoRice-ARP1 might provide an alternative strategy for the control of rotavirus infections. Because VHH, which is composed of heavy-chain dimers and is devoid of a light chain, possesses an extensive antigen-binding repertoire,117) and because rotavirus-specific VHH (ARP1) has been efficiently produced in yeast and reduces morbidity from rotaviral diarrhea in mice,118) its application to passive immunotherapy for rotaviral infection offers an attractive alternative strategy for the control of rotavirus infection, in addition to the currently available rotavirus oral vaccines. MucoRice-ARP1 comprises the 12-kDa ARP1 protein expressed in transgenic rice (0.85% of seed weight) and has all the advantages described for MucoRice-CTB, such as stability at room temperature.114) Moreover, oral administration of MucoRice-ARP1 attenuates rotaviral infection in immunocompetent and immunodeficient mice, suggesting that MucoRice-ARP1 can be used for the immunocompromised population in addition to healthy subjects. The combined technologies of the MucoRice expression system and VHH are expected to evolve into passive immunotherapy options for diverse mucosal infectious diseases.114)
5. Conclusion
The mucosal surface harbors a complex but flexible immune network that protects the host against constant exposure to external pathogens. Each of the diverse mucosal surfaces has its own immune inductive tissue — the so-called MALTs, such as GALT or PPs in the gastrointestinal tract. These MALTs possess unique functional characteristics, including antigen sampling by M cells and subsequent processing and presentation by antigen-presenting cells, resulting in the initiation of antigen-specific mucosal immune responses. Th1, Th2, Th17, and follicular helper T cells and cytotoxic T cells, as well as IgA+ B cells, are induced in PPs and then migrate to the distant intestinal lamina propria and epithelium to provide the first line of defense. This mucosal T- and B-cell–mediated network plays an important role in the induction of protective immunity.
Although the importance of mucosal immunity is well known, traditional systemic vaccines, which typically are administered subcutaneously, often fail to induce antigen-specific mucosal immune responses and thus cannot prevent pathogenic invasion at epithelial surfaces. Despite our increased understanding of the need to maximize the effective induction of both antigen-specific systemic and mucosal immune responses, few mucosal vaccines are available for human clinical use. However, advances in the fields of biochemical engineering, bioengineering, agricultural science, and plant biology have provided clues for success regarding the creation of new-generation mucosal vaccines (e.g., MucoRice-based oral vaccine). Continued parallel progress in elucidating the fundamental aspects of mucosal immunity and their application to mucosal vaccines relies not only on an increased understanding of the mucosal immune system but also on novel mergers of modern concepts and technologies from related and unrelated fields.
Acknowledgements
We sincerely thank our wonderful past and present colleagues at the University of Alabama at Birmingham, Osaka University, and the University of Tokyo who have contributed their expertise toward the understanding of mucosal immunology and the development of mucosal vaccines. In particular, we thank Drs. Yoshiyuki Goto, Takahiro Nagatake, and Yosuke Kurashima for reading this review article and providing helpful discussion. Our studies were supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (Scientific Research S); the Global Center of Excellence Program of the Center of Education and Research for Advanced Genome-based Medicine; MEXT Translational Research Network Seed C; and the Core Research for Evolutional Science and Technology Program of the Japan Science and Technology Agency.
Abbreviations
- BT
botulinum toxoid
- cCHP
cationic cholesteryl-group bearing pullulan
- CCL
CC chemokine ligand
- CCR
CC chemokine receptor
- CT
cholera toxin
- CTB
CT B subunit
- GALT
gut-associated lymphoid tissue
- Gp2
glycoprotein 2
- IgA+ B cell
IgA-committed B cell
- IL
interleukin
- LT
heat-labile enterotoxin
- MALT
mucosa-associated lymphoid tissue
- M cell
microfold or membranous cell
- NALT
nasopharynx-associated lymphoid tissue
- PP
Peyer’s patch
- RANK
receptor activator of nuclear factor κB
- RANKL
RANK ligand
- SIgA
secretory IgA
- TGF-β
transforming growth factor β
- Th
helper T
- UEA-1
Ulex europaeus agglutinin 1
- VHH
variable domain of llama heavy-chain antibody fragment
Profile
Hiroshi Kiyono was born in 1953. He graduated from Nihon University, School of Dentistry of Matsudo, Japan in 1977 with his D.D.S. degree. After his graduation from the dental school, he majored in mucosal immunology and received his Ph.D. degree in Pathology program in 1983 from the University of Alabama at Birmingham (UAB) Medical Center in USA. He worked as Research and Clinical Assistant Professor at Departments of Oral Biology, Preventive Dentistry and Microbiology, UAB between 1984 and 1986. After worked as Visiting Senior Scientist at Max-Planck Institute for Biology and as Associate Professor at Departments of Oral Biology and Microbiology, UAB in between 1986–1991, he became Professor at Departments of Oral Biology and Microbiology, UAB in 1991. At UAB, he worked as Professor at Departments of Oral Biology and Microbiology (1991–2003), and Co-Director of Immunobiology Vaccine Center (1992–1996) and Adjunct Professor (2004-present) at UAB School of Dentistry. He also worked as Professor and Chairman at Department of Mucosal Immunology, Research Institute for Microbial Diseases, Osaka University in 1994–2003. At present, he has been Professor at Division of Mucosal Immunology, The Institute of Medical Science, The University of Tokyo (IMSUT) since 2001. He was also appointed to Associated Dean (2007–2010) and elected to Dean (2011–2015) at IMSUT, and Director at International Research and Development Center for Mucosal Vaccines of IMSUT (2011-present). He has had a distinguished career in researching the molecular and cellular mechanisms controlling mucosal immune system as well as contributing to the development of Mucosal Immunology. His pioneering works covered a broad range of Mucosal Immunology, such as dentistry, gastroenterology, pneumology, gynecology, and vaccinology. His scientific contributions to the area of Mucosal Immunology include the demonstration of antigen presenting cells in the gut-associated lymphoid tissues, unique functions of intra-epithelial lymphocytes, diversity of mucosal antigen sampling system and distinctive tissue genesis program for the nasopharynx- and tear duct-associated lymphoid tissues. His most recent scientific interests have been focusing on the intra-tissue habitation of commensal bacteria, glycosylation of epithelial cells and unique roles of innate lymphoid cells and mast cells for the elimination and symbiosis at mucosal surface. Further, he has now extending his accomplishment in the basic science aspect of Mucosal Immunology to the translational research including the development of rice-based oral vaccine, MucoRice and nanogel-based nasal vaccine. To reflect his scientific contribution, he has over 450 publications in peer review journals and listed in ISI Highly Cited Researchers’ List (2005). For his accomplishment, he received NIH New Investigator Award (1984) and Research Career Development Award (1988), The 51st Dr. Hideyo Noguchi Memorial Medical Science Award (2007), Takahashi Prize of The Japanese Society for Vaccinology (2007), and Distinguished Scientist Award of The Japanese Society for Food Immunology (2009). He has served as a chairman of board for The Japanese Society for Vaccinology and a board member for The Japanese Society for Immunology. He is currently a chairman of board for The Japan Bifidus Foundation.
References
- 1).Takahashi I., Fujihashi K., Kiyono H. (2010) Mucosal regulatory cells in the gastrointestinal tract and periodontium. Periodontol. 2000 54, 247–256. [DOI] [PubMed] [Google Scholar]
- 2).Kiyono, H., Kunisawa, J., McGhee, J.R. and Mestecky, J. (2008) The mucosal immune system. In Fundamental Immunology (ed. Paul, W.E.). Lippincott Williams & Wilkins, Philadelphia, pp. 983–1030. [Google Scholar]
- 3).Kato, T. and Owen, R.L. (2005) Structure and functional intestinal mucosal epithelium. In Mucosal immunology (eds. Mestecky, J., Strober, W., Russell, M.W., Cheroutre, H., Bambrecht, B.N. and Kelsall, B.L.). Elsevier Academic Press, Amsterdam, pp. 131–153. [Google Scholar]
- 4).Peters B.M., Shirtliff M.E., Jabra-Rizk M.A. (2010) Antimicrobial peptides: primeval molecules or future drugs? PLoS Pathog. 6, e1001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).DePaolo R.W., Kamdar K., Khakpour S., Sugiura Y., Wang W., Jabri B. (2012) A specific role for TLR1 in protective T(H)17 immunity during mucosal infection. J. Exp. Med. 209, 1437–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Shang L., Fukata M., Thirunarayanan N., Martin A.P., Arnaboldi P., Maussang D., Berin C., Unkeless J.C., Mayer L., Abreu M.T., Lira S.A. (2008) Toll-like receptor signaling in small intestinal epithelium promotes B-cell recruitment and IgA production in lamina propria. Gastroenterology 135, 529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Vaishnava S., Behrendt C.L., Ismail A.S., Eckmann L., Hooper L.V. (2008) Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc. Natl. Acad. Sci. U.S.A. 105, 20858–20863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Cella M., Fuchs A., Vermi W., Facchetti F., Otero K., Lennerz J.K., Doherty J.M., Mills J.C., Colonna M. (2009) A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature 457, 722–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Kinjo Y., Wu D., Kim G., Xing G.W., Poles M.A., Ho D.D., Tsuji M., Kawahara K., Wong C.H., Kronenberg M. (2005) Recognition of bacterial glycosphingolipids by natural killer T cells. Nature 434, 520–525. [DOI] [PubMed] [Google Scholar]
- 10).Kurashima Y., Kiyono H. (2014) New era for mucosal mast cells: their roles in inflammation, allergic immune responses and adjuvant development. Exp. Mol. Med. 46, e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Yousefi S., Gold J.A., Andina N., Lee J.J., Kelly A.M., Kozlowski E., Schmid I., Straumann A., Reichenbach J., Gleich G.J., Simon H.U. (2008) Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat. Med. 14, 949–953. [DOI] [PubMed] [Google Scholar]
- 12).Williams R.C., Gibbons R.J. (1972) Inhibition of bacterial adherence by secretory immunoglobulin A: a mechanism of antigen disposal. Science 177, 697–699. [DOI] [PubMed] [Google Scholar]
- 13).Taylor H.P., Dimmock N.J. (1985) Mechanism of neutralization of influenza virus by secretory IgA is different from that of monomeric IgA or IgG. J. Exp. Med. 161, 198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Alvarez N., Otero O., Camacho F., Borrero R., Tirado Y., Puig A., Aquilar A., Rivas C., Cervantes A., Falero-Díaz G., Cádiz A., Sarmiento M.E., Morazmi M.N., Hernández-Pando R., Acosta A. (2013) Passive administration of purified secretory IgA from human colostrum induces protection against Mycobacterium tuberculosis in a murine model of progressive pulmonary infection. BMC Immunol. 14 (Suppl 1), S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Johansen F.E., Pekna M., Norderhaug I.N., Haneberg B., Hietala M.A., Krajci P., Betsholtz C., Brandtzaeg P. (1999) Absence of epithelial immunoglobulin A transport, with increased mucosal leakiness, in polymeric immunoglobulin receptor/secretory component-deficient mice. J. Exp. Med. 190, 915–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Owen R.L. (1977) Sequential uptake of horseradish peroxidase by lymphoid follicle epithelium of Peyer’s patches in the normal unobstructed mouse intestine: an ultrastructural study. Gastroenterology 72, 440–451. [PubMed] [Google Scholar]
- 17).Hirota K., Turner J.E., Villa M., Duarte J.H., Demengeot J., Steinmetz O.M., Stockinger B. (2013) Plasticity of Th17 cells in Peyer’s patches is responsible for the induction of T cell-dependent IgA responses. Nat. Immunol. 14, 372–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Tezuka H., Abe Y., Asano J., Sato T., Liu J., Iwata M., Ohteki T. (2011) Prominent role for plasmacytoid dendritic cells in mucosal T cell-independent IgA induction. Immunity 34, 247–257. [DOI] [PubMed] [Google Scholar]
- 19).Fukuyama Y., Tokuhara D., Sekine S., Aso K., Kataoka K., Kavydova J., Yamamoto M., Gilbert R.S., Tokuhara Y., Fujihashi K., Kunisawa J., Yuki Y., Kiyono H., McGhee J.R., Fujihashi K. (2013) Potential roles of CCR5(+) CCR6(+) dendritic cells induced by nasal ovalbumin plus Flt3 ligand expressing adenovirus for mucosal IgA responses. PLoS One 8, e60453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Babiuk S., Asper D.J., Rogan D., Mutwiri G.K., Potter A.A. (2008) Subcutaneous and intranasal immunization with type III secreted proteins can prevent colonization and shedding of Escherichia coli O157:H7 in mice. Microb. Pathog. 45, 7–11. [DOI] [PubMed] [Google Scholar]
- 21).Ohmura M., Yamamoto M., Tomiyama-Miyaji C., Yuki Y., Takeda Y., Kiyono H. (2005) Nontoxic Shiga toxin derivatives from Escherichia coli possess adjuvant activity for the augmentation of antigen-specific immune responses via dendritic cell activation. Infect. Immun. 73, 4088–4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).London S.D., Rubin D.H., Cebra J.J. (1987) Gut mucosal immunization with reovirus serotype 1/L stimulates virus-specific cytotoxic T cell precursors as well as IgA memory cells in Peyer’s patches. J. Exp. Med. 165, 830–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Zuercher A.W., Coffin S.E., Thurnheer M.C., Fundova P., Cebra J.J. (2002) Nasal-associated lymphoid tissue is a mucosal inductive site for virus-specific humoral and cellular immune responses. J. Immunol. 168, 1796–1803. [DOI] [PubMed] [Google Scholar]
- 24).Kunisawa J., Kurashima Y., Kiyono H. (2012) Gut-associated lymphoid tissues for the development of oral vaccines. Adv. Drug. Deliv. Rev. 64, 523–530. [DOI] [PubMed] [Google Scholar]
- 25).Yuki Y., Kiyono H. (2009) Mucosal vaccines: novel advances in technology and delivery. Expert Rev. Vaccines 8, 1083–1097. [DOI] [PubMed] [Google Scholar]
- 26).Azegami T., Yuki Y., Kiyono H. (2014) Challenges in mucosal vaccines for the control of infectious diseases. Int. Immunol. 26, 517–528. [DOI] [PubMed] [Google Scholar]
- 27).Kunisawa J., Kurashima Y., Higuchi M., Gohda M., Ishikawa I., Ogahara I., Kim N., Shimizu M., Kiyono H. (2007) Sphingosine 1-phosphate dependence in the regulation of lymphocyte trafficking to the gut epithelium. J. Exp. Med. 204, 2335–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Gohda M., Kunisawa J., Miura F., Kagiyama Y., Kurashima Y., Higuchi M., Ishikawa I., Ogahara I., Kiyono H. (2008) Sphingosine 1-phosphate regulates the egress of IgA plasmablasts from Peyer’s patches for intestinal IgA responses. J. Immunol. 180, 5335–5343. [DOI] [PubMed] [Google Scholar]
- 29).Kunisawa J., Kiyono H. (2012) Immunological function of sphingosine 1-phosphate in the intestine. Nutrients 4, 154–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Mestecky J., McGhee J.R. (1987) Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv. Immunol. 40, 153–245. [DOI] [PubMed] [Google Scholar]
- 31).Cerutti A. (2008) The regulation of IgA class switching. Nat. Rev. Immunol. 8, 421–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Mostov K.E., Kraehenbuhl J.P., Blobel G. (1980) Receptor-mediated transcellular transport of immunoglobulin: synthesis of secretory component as multiple and larger transmembrane forms. Proc. Natl. Acad. Sci. U.S.A. 77, 7257–7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Mostov K.E., Friedlander M., Blobel G. (1984) The receptor for transepithelial transport of IgA and IgM contains multiple immunoglobulin-like domains. Nature 308, 37–43. [DOI] [PubMed] [Google Scholar]
- 34).Tomasi T.B., Jr., Zigelbaum S. (1963) The selective occurrence of gamma-1a globulins in certain body fluids. J. Clin. Invest. 42, 1552–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Chodirker W.B., Tomasi T.B., Jr. (1963) Gamma-globulins: quantitative relationships in human serum and nonvascular fluids. Science 142, 1080–1081. [DOI] [PubMed] [Google Scholar]
- 36).Tomasi T.B., Jr., Tan E.M., Solomon A., Prendergast R.A. (1965) Characteristics of an immune system common to certain external secretions. J. Exp. Med. 121, 101–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Fujihashi, K. and Kiyono, H. (2012) The Nasopharyngeal-oral mucosal immune system. In Principles of Mucosal Immunology (eds. Smith, P.D., MacDonald, T.T. and Blumberg, R.S.). Garland Science, Danvers, pp. 293–307. [Google Scholar]
- 38).Hirasawa M., Kiyono H., Babb J.L., Shiota T., Michalek S.M., McGhee J.R. (1980) Virulence of Streptococcus mutans: in vivo reversion of a low-virulence mutant results in partial displacement and pathogenesis. Infect. Immun. 27, 1003–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Hamada, S. (1986) Overview of the biology of Streptococcus mutans In Molecular Microbiology and Immunology of Streptococcus mutans (eds. Hamada, S., Michalek, S.M., Kiyono, H., Menaker, S.L. and McGhee, J.R.). Elsevier, Amsterdam, pp. 7–20. [Google Scholar]
- 40).Kiyono, H., Czerkinsky, C., Koga, T., Kimura, S., Kurita, T. and McGhee, J.R. (1986) Induction and T cell regulation of IgA responses to Streptococcus mutans antigens. In The Borderland between Caries and Periodontal Diseases III (eds. Cimasoni, G. and Lehner, T.). Editions Medecine et Hygiene, Geneva, pp. 249–267. [Google Scholar]
- 41).Hamada, S. and Kodama, Y. (1996) Passive immunity for protection against mucosal infections and vaccination for dental caries. In Mucosal Vaccines (eds. Kiyono, H., Ogra, P.L. and McGhee, J.R.). Academic Press, San Diego, pp. 187–197. [Google Scholar]
- 42).Kiyono H., Michalek S.M., Mosteller L.M., Torii M., Hamada S., McGhee J.R. (1982) Enhancement of murine immune responses to orally administered haptenated Streptococcus mutans. Scand. J. Immunol. 16, 455–463. [DOI] [PubMed] [Google Scholar]
- 43).Saito M., Otake S., Ohmura M., Hirasawa M., Takada K., Mega J., Takahashi I., Kiyono H., McGhee J.R., Takeda Y., Yamamoto M. (2001) Protective immunity to Streptococcus mutans induced by nasal vaccination with surface protein antigen and mutant cholera toxin adjuvant. J. Infect. Dis. 183, 823–826. [DOI] [PubMed] [Google Scholar]
- 44).Peterson L.W., Artis D. (2014) Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 14, 141–153. [DOI] [PubMed] [Google Scholar]
- 45).Kunisawa J., Kiyono H. (2013) Immune regulation and monitoring at the epithelial surface of the intestine. Drug Discov. Today 18, 87–92. [DOI] [PubMed] [Google Scholar]
- 46).Owen R.L., Jones A.L. (1974) Epithelial cell specialization within human Peyer’s patches: an ultrastructural study of intestinal lymphoid follicles. Gastroenterology 66, 189–203. [PubMed] [Google Scholar]
- 47).Wolf J.L., Rubin D.H., Finberg R., Kauffman R.S., Sharpe A.H., Trier J.S., Fields B.N. (1981) Intestinal M cells: a pathway for entry of reovirus into the host. Science 212, 471–472. [DOI] [PubMed] [Google Scholar]
- 48).Kraehenbuhl J.P., Neutra M.R. (2000) Epithelial M cells: differentiation and function. Annu. Rev. Cell Dev. Biol. 16, 301–332. [DOI] [PubMed] [Google Scholar]
- 49).Bockman D.E., Cooper M.D. (1973) Pinocytosis by epithelium associated with lymphoid follicles in the bursa of Fabricius, appendix, and Peyer’s patches. An electron microscopic study. Am. J. Anat. 136, 455–477. [DOI] [PubMed] [Google Scholar]
- 50).Kumagai K. (1922) Ueber den Resorptionsvorgang der korpuskulären Elemente aus dem Darmtraktus. Mitteilungen der Medizinischen Gesellschaft zu Osaka 21, 497–522. [Google Scholar]
- 51).Golovkina T.V., Shlomchik M., Hannum L., Chervonsky A. (1999) Organogenic role of B lymphocytes in mucosal immunity. Science 286, 1965–1968. [DOI] [PubMed] [Google Scholar]
- 52).Ebisawa M., Hase K., Takahashi D., Kitamura H., Knoop K.A., Williams I.R., Ohno H. (2011) CCR6hiCD11c(int) B cells promote M-cell differentiation in Peyer’s patch. Int. Immunol. 23, 261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53).Knoop K.A., Kumar N., Butler B.R., Sakthivel S.K., Taylor R.T., Nochi T., Akiba H., Yagita H., Kiyono H., Williams I.R. (2009) RANKL is necessary and sufficient to initiate development of antigen-sampling M cells in the intestinal epithelium. J. Immunol. 183, 5738–5747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54).Kanaya T., Hase K., Takahashi D., Fukuda S., Hoshino K., Sasaki I., Hemmi H., Knoop K.A., Kumar N., Sato M., Katsuno T., Yokosuka O., Toyooka K., Nakai K., Sakamoto A., Kitahara Y., Jinnohara T., McSorley S.J., Kaisho T., Williams I.R., Ohno H. (2012) The Ets transcription factor Spi-B is essential for the differentiation of intestinal microfold cells. Nat. Immunol. 13, 729–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55).Sato S., Kaneto S., Shibata N., Takahashi Y., Okura H., Yuki Y., Kunisawa J., Kiyono H. (2013) Transcription factor Spi-B-dependent and -independent pathways for the development of Peyer’s patch M cells. Mucosal Immunol. 6, 838–846. [DOI] [PubMed] [Google Scholar]
- 56).Nicoletti C. (2000) Unsolved mysteries of intestinal M cells. Gut 47, 735–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57).Siebers A., Finlay B.B. (1996) M cells and the pathogenesis of mucosal and systemic infections. Trends Microbiol. 4, 22–29. [DOI] [PubMed] [Google Scholar]
- 58).Clark M.A., Jepson M.A., Simmons N.L., Booth T.A., Hirst B.H. (1993) Differential expression of lectin-binding sites defines mouse intestinal M-cells. J. Histochem. Cytochem. 41, 1679–1687. [DOI] [PubMed] [Google Scholar]
- 59).Clark M.A., Jepson M.A., Simmons N.L., Hirst B.H. (1994) Differential surface characteristics of M cells from mouse intestinal Peyer’s and caecal patches. Histochem. J. 26, 271–280. [PubMed] [Google Scholar]
- 60).Giannasca P.J., Giannasca K.T., Falk P., Gordon J.I., Neutra M.R. (1994) Regional differences in glycoconjugates of intestinal M cells in mice: potential targets for mucosal vaccines. Am. J. Physiol. 267, G1108–G1121. [DOI] [PubMed] [Google Scholar]
- 61).Kandori H., Hirayama K., Takeda M., Doi K. (1996) Histochemical, lectin-histochemical and morphometrical characteristics of intestinal goblet cells of germfree and conventional mice. Exp. Anim. 45, 155–160. [DOI] [PubMed] [Google Scholar]
- 62).Nochi T., Yuki Y., Matsumura A., Mejima M., Terahara K., Kim D.Y., Fukuyama S., Iwatsuki-Horimoto K., Kawaoka Y., Kohda T., Kozaki S., Igarashi O., Kiyono H. (2007) A novel M cell-specific carbohydrate-targeted mucosal vaccine effectively induces antigen-specific immune responses. J. Exp. Med. 204, 2789–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63).Hase K., Kawano K., Nochi T., Pontes G.S., Fukuda S., Ebisawa M., Kadokura K., Tobe T., Fujimura Y., Kawano S., Yabashi A., Waguri S., Nakato G., Kimura S., Murakami T., Iimura M., Hamura K., Fukuoka S., Lowe A.W., Itoh K., Kiyono H., Ohno H. (2009) Uptake through glycoprotein 2 of FimH(+) bacteria by M cells initiates mucosal immune response. Nature 462, 226–230. [DOI] [PubMed] [Google Scholar]
- 64).Terahara K., Yoshida M., Igarashi O., Nochi T., Pontes G.S., Hase K., Ohno H., Kurokawa S., Mejima M., Takeyama N., Yuki Y., Lowe A.W., Kiyono H. (2008) Comprehensive gene expression profiling of Peyer’s patch M cells, villous M-like cells, and intestinal epithelial cells. J. Immunol. 180, 7840–7846. [DOI] [PubMed] [Google Scholar]
- 65).Jang M.H., Kweon M.N., Iwatani K., Yamamoto M., Terahara K., Sasakawa C., Suzuki T., Nochi T., Yokota Y., Rennert P.D., Hiroi T., Tamagawa H., Iijima H., Kunisawa J., Yuki Y., Kiyono H. (2004) Intestinal villous M cells: an antigen entry site in the mucosal epithelium. Proc. Natl. Acad. Sci. U.S.A. 101, 6110–6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66).Kiyono H., McGhee J.R., Wannemuehler M.J., Frangakis M.V., Spalding D.M., Michalek S.M., Koopman W.J. (1982) In vivo immune response to a T-cell-dependent antigen by cultures of disassociated murine Peyer’s patch. Proc. Natl. Acad. Sci. U.S.A. 79, 596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67).Frangakis M.V., Koopman W.J., Kiyono H., Michalek S.M., McGhee J.R. (1982) An enzymatic method for preparation of dissociated murine Peyer’s patch cells enriched for macrophages. J. Immunol. Methods 48, 33–44. [DOI] [PubMed] [Google Scholar]
- 68).Kelsall B.L., Strober W. (1996) Distinct populations of dendritic cells are present in the subepithelial dome and T cell regions of the murine Peyer’s patch. J. Exp. Med. 183, 237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69).MacDonald T.T., Carter P.B. (1982) Isolation and functional characteristics of adherent phagocytic cells from mouse Peyer’s patches. Immunology 45, 769–774. [PMC free article] [PubMed] [Google Scholar]
- 70).Sato A., Iwasaki A. (2005) Peyer’s patch dendritic cells as regulators of mucosal adaptive immunity. Cell Mol. Life Sci. 62, 1333–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71).Mora J.R., Iwata M., Eksteen B., Song S.Y., Junt T., Senman B., Otipody K.L., Yokota A., Takeuchi H., Ricciardi-Castagnoli P., Rajewsky K., Adams D.H., von Andrian U.H. (2006) Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science 314, 1157–1160. [DOI] [PubMed] [Google Scholar]
- 72).Fagarasan S., Kawamoto S., Kanagawa O., Suzuki K. (2010) Adaptive immune regulation in the gut: T cell-dependent and T cell-independent IgA synthesis. Annu. Rev. Immunol. 28, 243–273. [DOI] [PubMed] [Google Scholar]
- 73).Spits H., Di Santo J.P. (2011) The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat. Immunol. 12, 21–27. [DOI] [PubMed] [Google Scholar]
- 74).Sonnenberg G.F., Monticelli L.A., Alenghat T., Fung T.C., Hutnick N.A., Kunisawa J., Shibata N., Grunberg S., Sinha R., Zahm A.M., Tardif M.R., Sathaliyawala T., Kubota M., Farber D.L., Collman R.G., Shaked A., Fouser L.A., Weiner D.B., Tessier P.A., Friedman J.R., Kiyono H., Bushman F.D., Chang K.M., Artis D. (2012) Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science 336, 1321–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75).Goto Y., Obata T., Kunisawa J., Sato S., Ivanov I.I., Lamichhane A., Takeyama N., Kamioka M., Sakamoto M., Matsuki T., Setoyama H., Imaoka A., Uematsu S., Akira S., Domino S.E., Kulig P., Becher B., Renauld J.C., Sasakawa C., Umesaki Y., Benno Y., Kiyono H. (2014) Innate lymphoid cells regulate intestinal epithelial cell glycosylation. Science 345, 1254009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76).Kurashima Y., Goto Y., Kiyono H. (2013) Mucosal innate immune cells regulate both gut homeostasis and intestinal inflammation. Eur. J. Immunol. 43, 3108–3115. [DOI] [PubMed] [Google Scholar]
- 77).Walker J.A., Barlow J.L., McKenzie A.N. (2013) Innate lymphoid cells — how did we miss them? Nat. Rev. Immunol. 13, 75–87. [DOI] [PubMed] [Google Scholar]
- 78).Spits H., Artis D., Colonna M., Diefenbach A., Di Santo J.P., Eberl G., Koyasu S., Locksley R.M., McKenzie A.N., Mebius R.E., Powrie F., Vivier E. (2013) Innate lymphoid cells — a proposal for uniform nomenclature. Nat. Rev. Immunol. 13, 145–149. [DOI] [PubMed] [Google Scholar]
- 79).Obata T., Goto Y., Kunisawa J., Sato S., Sakamoto M., Setoyama H., Matsuki T., Nonaka K., Shibata N., Gohda M., Kagiyama Y., Nochi T., Yuki Y., Fukuyama Y., Mukai A., Shinzaki S., Fujihashi K., Sasakawa C., Iijima H., Goto M., Umesaki Y., Benno Y., Kiyono H. (2010) Indigenous opportunistic bacteria inhabit mammalian gut-associated lymphoid tissues and share a mucosal antibody-mediated symbiosis. Proc. Natl. Acad. Sci. U.S.A. 107, 7419–7424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80).Pickard J.M., Maurice C.F., Kinnebrew M.A., Abt M.C., Schenten D., Golovkina T.V., Bogatyrev S.R., Ismagilov R.F., Pamer E.G., Turnbaugh P.J., Chervonsky A.V. (2014) Rapid fucosylation of intestinal epithelium sustains host-commensal symbiosis in sickness. Nature 514, 638–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81).Rausch P., Rehman A., Kunzel S., Hasler R., Ott S.J., Schreiber S., Rosenstiel P., Franke A., Baines J.F. (2011) Colonic mucosa-associated microbiota is influenced by an interaction of Crohn disease and FUT2 (Secretor) genotype. Proc. Natl. Acad. Sci. U.S.A. 108, 19030–19035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82).Bry L., Falk P.G., Midtvedt T., Gordon J.I. (1996) A model of host-microbial interactions in an open mammalian ecosystem. Science 273, 1380–1383. [DOI] [PubMed] [Google Scholar]
- 83).Bischoff S.C. (2007) Role of mast cells in allergic and non-allergic immune responses: comparison of human and murine data. Nat. Rev. Immunol. 7, 93–104. [DOI] [PubMed] [Google Scholar]
- 84).Gurish M.F., Austen K.F. (2012) Developmental origin and functional specialization of mast cell subsets. Immunity 37, 25–33. [DOI] [PubMed] [Google Scholar]
- 85).Knight P.A., Wright S.H., Lawrence C.E., Paterson Y.Y., Miller H.R. (2000) Delayed expulsion of the nematode Trichinella spiralis in mice lacking the mucosal mast cell-specific granule chymase, mouse mast cell protease-1. J. Exp. Med. 192, 1849–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86).Huang C., De Sanctis G.T., O’Brien P.J., Mizgerd J.P., Friend D.S., Drazen J.M., Brass L.F., Stevens R.L. (2001) Evaluation of the substrate specificity of human mast cell tryptase beta I and demonstration of its importance in bacterial infections of the lung. J. Biol. Chem. 276, 26276–26284. [DOI] [PubMed] [Google Scholar]
- 87).Nolte H., Spjeldnaes N., Kruse A., Windelborg B. (1990) Histamine release from gut mast cells from patients with inflammatory bowel diseases. Gut 31, 791–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88).Hamilton M.J., Sinnamon M.J., Lyng G.D., Glickman J.N., Wang X., Xing W., Krilis S.A., Blumberg R.S., Adachi R., Lee D.M., Stevens R.L. (2011) Essential role for mast cell tryptase in acute experimental colitis. Proc. Natl. Acad. Sci. U.S.A. 108, 290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89).Kurashima Y., Amiya T., Nochi T., Fujisawa K., Haraguchi T., Iba H., Tsutsui H., Sato S., Nakajima S., Iijima H., Kubo M., Kunisawa J., Kiyono H. (2012) Extracellular ATP mediates mast cell-dependent intestinal inflammation through P2X7 purinoceptors. Nat. Commun. 3, 1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90).Lamichhane A., Azegami T., Kiyono H. (2014) The mucosal immune system for vaccine development. Vaccine 32, 6711–6723. [DOI] [PubMed] [Google Scholar]
- 91).Nochi T., Takagi H., Yuki Y., Yang L., Masumura T., Mejima M., Nakanishi U., Matsumura A., Uozumi A., Hiroi T., Morita S., Tanaka K., Takaiwa F., Kiyono H. (2007) Rice-based mucosal vaccine as a global strategy for cold-chain- and needle-free vaccination. Proc. Natl. Acad. Sci. U.S.A. 104, 10986–10991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92).Moldoveanu Z., Clements M.L., Prince S.J., Murphy B.R., Mestecky J. (1995) Human immune responses to influenza virus vaccines administered by systemic or mucosal routes. Vaccine 13, 1006–1012. [DOI] [PubMed] [Google Scholar]
- 93).Brenneman K.E., Willingham C., Kilbourne J.A., Curtiss R., 3rd, Roland K.L. (2014) A low gastric pH mouse model to evaluate live attenuated bacterial vaccines. PLoS One 9, e87411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94).Azegami T., Itoh H., Kiyono H., Yuki Y. (2015) Novel Transgenic Rice-Based Vaccines. Arch. Immunol. Ther. Exp. (Warsz) 63, 87–99. [DOI] [PubMed] [Google Scholar]
- 95).Fujkuyama Y., Tokuhara D., Kataoka K., Gilbert R.S., McGhee J.R., Yuki Y., Kiyono H., Fujihashi K. (2012) Novel vaccine development strategies for inducing mucosal immunity. Expert. Rev. Vaccines 11, 367–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96).Takahashi I., Nochi T., Yuki Y., Kiyono H. (2009) New horizon of mucosal immunity and vaccines. Curr. Opin. Immunol. 21, 352–358. [DOI] [PubMed] [Google Scholar]
- 97).Thanavala Y., Mahoney M., Pal S., Scott A., Richter L., Natarajan N., Goodwin P., Arntzen C.J., Mason H.S. (2005) Immunogenicity in humans of an edible vaccine for hepatitis B. Proc. Natl. Acad. Sci. U.S.A. 102, 3378–3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98).Li J.T., Fei L., Mou Z.R., Wei J., Tang Y., He H.Y., Wang L., Wu Y.Z. (2006) Immunogenicity of a plant-derived edible rotavirus subunit vaccine transformed over fifty generations. Virology 356, 171–178. [DOI] [PubMed] [Google Scholar]
- 99).Zhang X., Buehner N.A., Hutson A.M., Estes M.K., Mason H.S. (2006) Tomato is a highly effective vehicle for expression and oral immunization with Norwalk virus capsid protein. Plant Biotechnol. J. 4, 419–432. [DOI] [PubMed] [Google Scholar]
- 100).Santi L., Giritch A., Roy C.J., Marillonnet S., Klimyuk V., Gleba Y., Webb R., Arntzen C.J., Mason H.S. (2006) Protection conferred by recombinant Yersinia pestis antigens produced by a rapid and highly scalable plant expression system. Proc. Natl. Acad. Sci. U.S.A. 103, 861–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101).Moravec T., Schmidt M.A., Herman E.M., Woodford-Thomas T. (2007) Production of Escherichia coli heat labile toxin (LT) B subunit in soybean seed and analysis of its immunogenicity as an oral vaccine. Vaccine 25, 1647–1657. [DOI] [PubMed] [Google Scholar]
- 102).Jiang X.L., He Z.M., Peng Z.Q., Qi Y., Chen Q., Yu S.Y. (2007) Cholera toxin B protein in transgenic tomato fruit induces systemic immune response in mice. Transgenic Res. 16, 169–175. [DOI] [PubMed] [Google Scholar]
- 103).Rosales-Mendoza S., Soria-Guerra R.E., Lopez-Revilla R., Moreno-Fierros L., Alpuche-Solis A.G. (2008) Ingestion of transgenic carrots expressing the Escherichia coli heat-labile enterotoxin B subunit protects mice against cholera toxin challenge. Plant Cell Rep. 27, 79–84. [DOI] [PubMed] [Google Scholar]
- 104).Tokuhara D., Yuki Y., Nochi T., Kodama T., Mejima M., Kurokawa S., Takahashi Y., Nanno M., Nakanishi U., Takaiwa F., Honda T., Kiyono H. (2010) Secretory IgA-mediated protection against V. cholerae and heat-labile enterotoxin-producing enterotoxigenic Escherichia coli by rice-based vaccine. Proc. Natl. Acad. Sci. U.S.A. 107, 8794–8799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105).Nochi T., Yuki Y., Katakai Y., Shibata H., Tokuhara D., Mejima M., Kurokawa S., Takahashi Y., Nakanishi U., Ono F., Mimuro H., Sasakawa C., Takaiwa F., Terao K., Kiyono H. (2009) A rice-based oral cholera vaccine induces macaque-specific systemic neutralizing antibodies but does not influence pre-existing intestinal immunity. J. Immunol. 183, 6538–6544. [DOI] [PubMed] [Google Scholar]
- 106).Gill D.M. (1976) The arrangement of subunits in cholera toxin. Biochemistry 15, 1242–1248. [DOI] [PubMed] [Google Scholar]
- 107).Gill D.M., Clements J.D., Robertson D.C., Finkelstein R.A. (1981) Subunit number and arrangement in Escherichia coli heat-labile enterotoxin. Infect. Immun. 33, 677–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108).Dallas W.S., Falkow S. (1980) Amino acid sequence homology between cholera toxin and Escherichia coli heat-labile toxin. Nature 288, 499–501. [DOI] [PubMed] [Google Scholar]
- 109).Field M., Rao M.C., Chang E.B. (1989) Intestinal electrolyte transport and diarrheal disease (1). N. Engl. J. Med. 321, 800–806. [DOI] [PubMed] [Google Scholar]
- 110).Peltola H., Siitonen A., Kyronseppa H., Simula I., Mattila L., Oksanen P., Kataja M.J., Cadoz M. (1991) Prevention of travellers’ diarrhoea by oral B-subunit/whole-cell cholera vaccine. Lancet 338, 1285–1289. [DOI] [PubMed] [Google Scholar]
- 111).Abba K., Sinfield R., Hart C.A., Garner P. (2009) Pathogens associated with persistent diarrhoea in children in low and middle income countries: systematic review. BMC Infect. Dis. 9, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112).Kurokawa S., Nakamura R., Mejima M., Kozuka-Hata H., Kuroda M., Takeyama N., Oyama M., Satoh S., Kiyono H., Masumura T., Teshima R., Yuki Y. (2013) MucoRice-cholera toxin B-subunit, a rice-based oral cholera vaccine, down-regulates the expression of alpha-amylase/trypsin inhibitor-like protein family as major rice allergens. J. Proteome. Res. 12, 3372–3382. [DOI] [PubMed] [Google Scholar]
- 113).Kurokawa S., Kuroda M., Mejima M., Nakamura R., Takahashi Y., Sagara H., Takeyama N., Satoh S., Kiyono H., Teshima R., Masumura T., Yuki Y. (2014) RNAi-mediated suppression of endogenous storage proteins leads to a change in localization of overexpressed cholera toxin B-subunit and the allergen protein RAG2 in rice seeds. Plant Cell Rep. 33, 75–87. [DOI] [PubMed] [Google Scholar]
- 114).Tokuhara D., Alvarez B., Mejima M., Hiroiwa T., Takahashi Y., Kurokawa S., Kuroda M., Oyama M., Kozuka-Hata H., Nochi T., Sagara H., Aladin F., Marcotte H., Frenken L.G., Iturriza-Gómara M., Kiyono H., Hammarström L., Yuki Y. (2013) Rice-based oral antibody fragment prophylaxis and therapy against rotavirus infection. J. Clin. Invest. 123, 3829–3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115).Walker C.L., Rudan I., Liu L., Nair H., Theodoratou E., Bhutta Z.A., O’Brien K.L., Campbell H., Black R.E. (2013) Global burden of childhood pneumonia and diarrhoea. Lancet 381, 1405–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116).Sack D.A., Chowdhury A.M., Eusof A., Ali M.A., Merson M.H., Islam S., Black R.E., Brown K.H. (1978) Oral hydration rotavirus diarrhoea: a double blind comparison of sucrose with glucose electrolyte solution. Lancet 2, 280–283. [DOI] [PubMed] [Google Scholar]
- 117).Hamers-Casterman C., Atarhouch T., Muyldermans S., Robinson G., Hamers C., Songa E.B., Bendahman N., Hamers R. (1993) Naturally occurring antibodies devoid of light chains. Nature 363, 446–448. [DOI] [PubMed] [Google Scholar]
- 118).van der Vaart J.M., Pant N., Wolvers D., Bezemer S., Hermans P.W., Bellamy K., Sarker S.A., van der Logt C.P., Svensson L., Verrips C.T., Hammarstrom L., van Klinken B.J. (2006) Reduction in morbidity of rotavirus induced diarrhoea in mice by yeast produced monovalent llama-derived antibody fragments. Vaccine 24, 4130–4137. [DOI] [PubMed] [Google Scholar]