Abstract
Chronic kidney diseases (CKD), a common pathway of various glomerular diseases, which carries great morbidity and mortality to people. CKD is characterized by progressive kidney fibrosis and remodeling. CKD is also associated with the depletion of glomerular and tubular cells. Autophagy is a highly conserved process that degrades cellular long-lived proteins and organelles. It plays an important role in both normal and disease states, including immunity, inflammation, and adaptation to stress. Evidence has indicated that impaired autophagic activity is involved in the development of CKD. Here, we review the progress in our understanding of the role of autophagy in the development and progression of CKD. Targeting the autophagic signaling pathways may be a therapeutic strategy for CKD.
Keywords: Autophagy, role, chronic kidney diseases, review
Introduction
Autophagy degrades cellular long-lived proteins and organelles [1]. Autophagy is essential for cellular homeostasis and many biological activities. It is a nonselective degradation system for long-lived cytoplasmic proteins and dysfunctional organelles [2]. It responds to the cellular stress by removing old/damaged proteins/organelles and produces new cellular building blocks [3]. Hence, autophagy plays an important role in keeping the body homeostasis. Dysregulation of autophagy results in the accumulation of autophagosomes, which was observed in a lot of diseases [4]. Autophagy is reported to be involved in the development of many disorders.
Autophagy regulates cellular quality control through the disposal and recycling of cellular components [5]. Under cellular stress, autophagy managed stress by removing the potentially toxic proteins, lipids, and organelles [6]. The cellular stress, such as obesity and diabetes, was likely to cause the damage to kidney. In this sense, we speculated that the stress response of autophagy may protect the kidney indirectly. On the other hand, autophagy plays a role in immunity and inflammation. CKD occurs partly due to the imbalance of cellular mechanisms related to oxidative stress, inflammation and cell death [7]. These risk factors were closely associated with autophagy. Agonist induction of AMPK activity attenuates the albuminuria and podocyte foot process effacement [8]. Hence, autophagy may also play a protective role against the development and progression of CKD. Targeting the autophagy may be a therapeutic way for CKD.
During the past decades, a number of studies have been performed to investigate the role of autophagy in kidney diseases, including chronic kidney diseases (CKD).
The role of autophagy in CKD attracted the nephrologists’ attention. An in-depth understanding of the role of autophagy in CKD will provide a new insight of new molecular therapy strategies or biomarker for CKD patients. Therefore, we performed this review to discuss the important role of autophagy in the pathogenesis of CKD, as well as the signaling pathways of autophagy and the role of the possible interactions between autophagy and other molecules in CKD.
Autophagy
Autophagy is a cellular degradation pathway that involves cytoplasmic content engulfment, delivery and degradation by the lysosome [9]. It is tightly regulated to prevent its unbalanced activation and maintain homeostatic protein or organelle turnover. Autophagy is also a catabolic mechanism required for cellular homeostatic quality control and regeneration as well as a cellular stress response mechanism. It removes damaged organelles, protein aggregates and pathogens by recruiting these substrates into double membrane vesicles called autophagosomes which subsequently fuse with lysosomes [7].
Autophay is a self-digestion process of the cell. It is involved in the initial cell survival phase and regeneration phase. Autophagy is beneficial for the body repair after injury. To date, three major types of autophagy were identified: macroautophagy, microautophagy and chaperone-mediated autophagy (CMA) [10]. Autophagy process includes five parts [11]: (1) the initiation of a double-membrane structure, which is also called phagophore, (2) the expansion of the phagophore, (3) the maturation of this structure into the autophagosome, (4) the fusion of autophagosomes with lysosomes, resulting in autolysosomes, and, finally, (5) the degradation of the ingested biological materials. In a word, autophagy is a process that removes some substances, which is important for keeping the homeostasis of the body. In another word, uncontrolled autophagy may damage the homeostasis.
Autophagy signaling pathways
In the past, a number of studies have been performed to investigate the signaling pathways of autophagy. These investigations indicated that many genes could regulate autophagy expression and autophagy could regulate a number of genes expression. In addition, the possible interaction of autophagy with other proteins may affect the role of autophagy in kidney. We presented the signaling pathways for autophagy (Figure 1).
Figure 1.
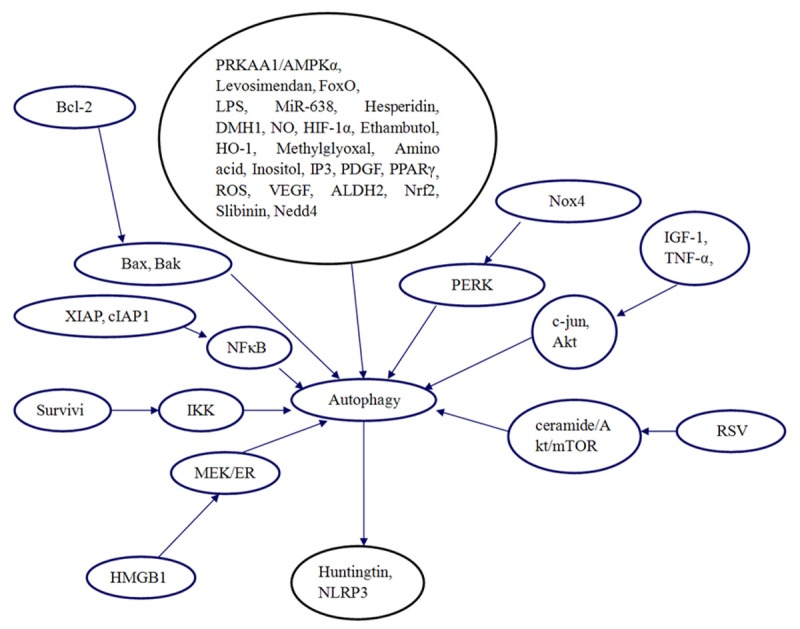
Signaling pathways for autophagy in our review.
Autophagy is essential for the cell death and injury. For example, the PRKAA1/ AMPKα1 pathway triggers autophagy during CSF1-induced human monocyte differentiation and is a potential target in CMML [12]. Resveratrol regulates redox signaling and autophagy during cardiovascular diseases [13]. Levosimendan protects hepatocytes against oxidative injuries by autophagic-dependent inhibition of apoptosis and the activation of survival signaling [14]. FoxO transcriptional network regulates autophagy and the ubiquitin-proteasome system during muscle atrophy [15]. Morphine facilitates LPS-induced autophagy, and inhibits autophagolysosomal fusion leading to decreased bacterial clearance and increased bacterial load [16]. MiR-638 promotes melanoma metastasis and protects melanoma cells from apoptosis and autophagy [17]. NADPH oxidase 4 (Nox4) promotes the activation of autophagy and survival in cardiomyocytes in response to nutrient deprivation and ischemia through activation of the PERK (protein kinase RNA-like endoplasmic reticulum kinase) signaling pathway [18]. Hesperidin induces apoptosis and triggers autophagic markers [19]. DMH1 inhibited cellular autophagy responses in a range of cell types [20]. Antiapoptotic Bcl-2 family members affect autophagy through inhibition of Bax and Bak [21]. Nitric oxide and sphingolipids control apoptosis and autophagy with a significant impact on Alzheimer’s disease [22]. Hypoxia inducible factor 1α (HIF-1α) contributes to regulation of autophagy in retinal detachment [23]. Ethambutol induces impaired autophagic flux and apoptosis in the retina [24]. Src/STAT3-dependent heme oxygenase-1 induction mediates chemoresistance of breast cancer cells to doxorubicin by promoting autophagy [25]. Methylglyoxal enhances autophagy flux and suppresses proliferation of human retinal pigment epithelial ARPE-19 cells [26]. Amino acid metabolism inhibits antibody-driven kidney injury by inducing autophagy [27]. Autophagy delays apoptotic death in breast cancer cells following DNA damage [28]. Insulin-like growth factor-1 and TNF-alpha regulate autophagy through c-jun N-terminal kinase and Akt pathways in human atherosclerotic vascular smooth cells [29]. Inositol and IP3 levels regulate autophagy [30]. Autophagy is induced in CD4+ T cells and important for the growth factor-withdrawal cell death [31]. Fasting activates macroautophagy in neurons of Alzheimer’s disease mouse model [32]. Telomere 3’ overhang-specific DNA oligonucleotides induce autophagy in malignant glioma cells [33]. Inhibition of plate-derived growth factor (PDGF) signaling induces autophagy in malignant glioma cells [34]. Presenilin 1 mediates the turnover of telencephalin in hippocampal neurons via an autophagic degradative pathway [35]. Programmed autophagy in the drosophila fat body is induced by ecdysone through regulation of the PI3K pathway [36]. Autophagy suppresses tumor progression by limiting chromosomal instability [37]. Nrf2 induces cisplatin resistance through activation of autophagy in ovarian carcinoma [38]. Inhibition of autophagy enhances the cytotoxic effect of PA-MSHA in breast cancer [39]. Advanced glycation endproducts trigger autophagy in cardiomyocyte via RAGE/PI3K/AKT/mTOR pathway [40]. Celecoxib regulates apoptosis and autophagy via the PI3K/Akt signaling pathway in SGC-7901 gastric cancer cells [41]. Resveratrol triggers protective autophagy through the ceramide/Akt/mTOR pathway in melanoma B16 cells [42]. Survivin-2B promotes autophagy by accumulating IKK alpha in the nucleus of selenite-treated NB4 cells [43]. Remote ischemic preconditioning protects against liver ischemia-reperfusion injury via heme oxygemase-1-induced autophagy [44]. Sphingosylphosphorylcholine protects cardiomyocytes against ischemic apoptosis via lipid raft/PTEN/Akt1/mTOR mediated autophagy [45]. Erα downregulation plays a key role in slibinin-induced autophagy and apoptosis in human breast cancer MCF-7 cells [46]. Nedd4 E3 ubiquitin ligase promotes cell proliferation and autophagy [47]. HMGB1- mediated autophagy modulates sensitivity of colorectal cancer cells to oxaliplatin via MEK/ERK signaling pathway [48]. Neuropeptide Y stimulates autophagy in hypothalamic neurons [49]. Autophagy is upregulated in ovarian endometriosis [50]. Leucine limitation induces autophagy and activation of lysosome-dependent proteolysis in C2C12 myotubes [51]. Bergapten drives autophagy through the upregulation of PTEN expression in breast cancer cells [52]. Tumor necrosis factor (TNF) induced autophagy and mitochondrial morphological abnormalities are mediated by TNFR-I and/or TNFR-II and do not invariably lead to cell death [53]. Autophagy is a protective response to Bnip3-mediated apoptotic signaling in the heart [54]. PSMD10/Gankyrin induces autophagy to promote tumor progression through cytoplasmic interaction with ATG7 and nuclear transactivation of ATG7 expression [55]. Autophagy and mTORC1 regulate the stochastic phase of somatic cell reprogramming [56]. Autophagy abrogation was efficacious in boosting cell death and ecto-CRT/ecto-HSP90 in PLX4032-resistant cells upon blockage of MEK hyper-activation by U0126 [57]. PKC delta and tissue transglutaminase are inhibitors of autophagy in pancreatic cancer cells [58]. Second, autophagy also plays a key role in inflammation. For instance, ALDH2 plays a beneficial role in ameliorating chronic alcohol intake-induced hepatic steatosis and inflammation through regulation of autophagy [59]. Autophagy in pulmonary macrophages mediates lung inflammatory injury via NLRP3 inflammasome activation during mechanical ventilation [60]. Autophagy attenuates the catabolic effect during inflammatory conditions in nucleus pulposus cells as sustained by NF-kB and JNK inhibition [61]. Upregulation of autophagy decreases chlorine-induced mitochondrial injury and lung inflammation [62]. Cartilage-specific deletion of mTOR upregulates autophagy and protects mice from osteoarthritis [63]. Finally, autophagy is also closely associated with the metabolic index, which is related to the kidney injury and repair. Autophagy also attenuated ER stress- and c-Jun N-terminal kinase/inhibitory-κB kinase-associated impairment in insulin signaling transduction target of rapamycin-independent manner [64]. Autocrine VEGF maintains endothelial survival through regulation of metabolism and autophagy [65]. A REDD1/TXNIP pro-oxidant complex regulates ATG4B activity to control stress-induced autophagy and sustain exercise capacity [66]. Autophagy mediated the clearance of huntingtin aggregates triggered by the insulin-signaling pathway [67]. Sera from patients with type 2 diabetes and neuropathy induces autophagy and colocalization with mitochondria in SY5Y cells [68]. Reactive oxygen species (ROS) regulate autophagy through redox-sensitive proteases [69]. Modulation of mitochondrial function and autophagy mediates carnosine neuroprotection against ischemic brain damage [70]. The PPARγ agonist troglitazone induces autophagy, apoptosis and necroptosis in bladder cancer cells [71].
In a word, apart from the regulation of cell death, autophagy plays a role against inflammation. Autophagy is also a metabolic regulator. Hence, autophagy might play a role against the development or progression of renal diseases.
Autophagy and CKD
Kidneys are important for healthy living, kidneys regulates the body fluids and blood pressure, waste products excretion and the production of red blood cells [72]. Human kidneys receive nearly 25% of cardiac output and consume 7% of daily energy expenditure [73]. CKD is characterized by a glomerular filtration rate below 60 mLper minute for over 3 months [74]. The incidence of CKD is increasing and a number of CKD patients progress to ESRD. CKD leads to significant morbidity and mortality.
Autophagy is associated with chronic organ dysfunction [75]. CKD patients have altered autophagy function. Oxidative stress and reactive oxygen species (ROS) are important regulators of autophagy. Autophagy may be an adaptive process in CKD. It may also play a protective role against CKD progression. Several facts may account for the role of autophagy in CKD. First, autophagy is very essential for keeping the body homeostasis, autophagy removed the excessive long-lived proteins, which is toxic for the body. A lot of substances aggregate at the stage of CKD. Second, autophagy also plays a role against inflammation. It is well-documented that inflammation is closely associated with the CKD progression [76]. Autophagy may play a protective role against CKD progression through anti-inflammation. Finally, autophagy can regulate a number of metabolic indexes, which are closely associated with the renal injury.
In a word, autophagy may play a protective role against the CKD risk and progression. Here, we presented the potential protective mechanism of autophagy in CKD (Figure 2).
Figure 2.
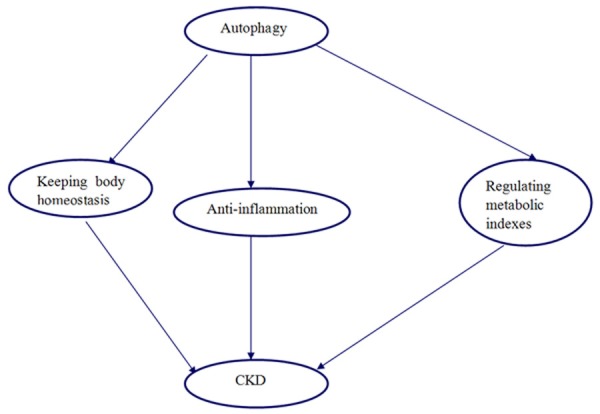
The potential protective role of autophagy against CKD.
Conclusion and future directions
In conclusion, autophagy may confer protection against CKD progression. Autophagy activation in CKD may provide new insight to the therapy of CKD. The precise protective mechanism of autophagy against CKD need to be further clarified, particularly the molecules along the signaling pathways of autophagy.
Future generation of autophagy-/- mice will advance the understanding of the role of autophagy in the development and progression of CKD and reveal the potential functions of autophagy.
Disclosure of conflict of interest
None.
References
- 1.Murrow L, Debnath J. Autophagy as a stressresponse and quality-control mechanism: implications for cell injury and human disease. Annu Rev Pathol. 2013;8:105–37. doi: 10.1146/annurev-pathol-020712-163918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crotzer VL, Blum JS. Autophagy and adaptive immunity. Immunology. 2010;131:9–17. doi: 10.1111/j.1365-2567.2010.03321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou XJ, Cheng FJ, Zhang H. Emerging view of autophagy in systemic lupus erythematosus. Int Rev Immunol. 2015;34:280–92. doi: 10.3109/08830185.2013.879711. [DOI] [PubMed] [Google Scholar]
- 4.Cortes CJ, La Spada AR. Autophagy in polyglutamine disease: imposing order or contributing to the chaos? Mol Cell Neurosci. 2015;66:53–61. doi: 10.1016/j.mcn.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Komatsu M, Ichimura Y. Selective autophagy regulates various cellular functions. Genes Cells. 2010;15:923–33. doi: 10.1111/j.1365-2443.2010.01433.x. [DOI] [PubMed] [Google Scholar]
- 6.Satriano J, Sharma K. Autophagy and metabolic changes in obesity-related chronic kidney disease. Nephrol Dial Transplant. 2013;28(Suppl 4):iv29–36. doi: 10.1093/ndt/gft229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sureshbabu A, Ryter SW, Choi ME. Oxidative stress and autophagy: crucial modulators of kidney injury. Redox Biol. 2015;4:208–14. doi: 10.1016/j.redox.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eid AA, Ford BM, Block K, Kasinath BS, Gorin Y, Ghosh-Choudhury G, Barnes JL, Abboud HE. AMP-activated protein kinase (AMPK) negatively regulates Nox4-dependent activation of p53 and epithelial cell apoptosis in diabetes. J Biol Chem. 2010;285:37503–12. doi: 10.1074/jbc.M110.136796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gan-Or Z, Dion PA, Rouleau GA. Genetic perspective on the role of the Autophagy-Lysosome Pathway in Parkinson disease. Autophagy. 2015;11:1443–57. doi: 10.1080/15548627.2015.1067364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaushik S, Massey AC, Mizushima N, Cuervo AM. Constitutive activation of chaperone-mediated autophagy in cells with impaired macroautophagy. Mol Biol Cell. 2008;19:2179–92. doi: 10.1091/mbc.E07-11-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abada A, Elazar Z. Getting ready for building: signaling and autophagosome biogenesis. EMBO Rep. 2014;15:839–52. doi: 10.15252/embr.201439076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Obba S, Hizir Z, Boyer L, Selimoglu-Buet D, Pfeifer A, Michel G, Hamouda MA, Gonçalvès D, Cerezo M, Marchetti S, Rocchi S, Droin N, Cluzeau T, Robert G, Luciano F, Robaye B, Foretz M, Viollet B, Legros L, Solary E, Auberger P, Jacquel A. The PRKAA1/AMPKα1 pathway triggers autophagy during CSF1-induced human monocyte differentiation and is a potential target in CMML. Autophagy. 2015;11:1114–29. doi: 10.1080/15548627.2015.1034406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu DG. Regulation of redox signaling and autophagy during cardiovascular diseases-role of resveratrol. Eur Rev Med Pharmacol Sci. 2015;19:1530–6. [PubMed] [Google Scholar]
- 14.Grossini E, Bellofatto K, Farruggio S, Sigaudo L, Marotta P, Raina G, De Giuli V, Mary D, Pollesello P, Minisini R, Pirisi M, Vacca GL. Evosimendan inhibits peroxidation in hepatocytes by modulating apoptosis/autophagy interplay. PLoS One. 2015;10:e0124742. doi: 10.1371/journal.pone.0124742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Milan G, Romanello V, Pescatore F, Armani A, Paik JH, Frasson L, Seydel A, Zhao J, Abraham R, Goldberg AL, Blaauw B, DePinho RA, Sandri M. Regulation of autophagy and the ubiquitinproteasome system by the FoxO transcriptional networkduring muscle atrophy. Nat Commun. 2015;6:6670. doi: 10.1038/ncomms7670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wan J, Ma J, Anand V, Ramakrishnan S, Roy S. Morphine potentiates LPS-induced autophagy initiation but inhibits autophagosomal maturation through distinct TLR4-dependentand independent pathways. Acta Physiol (Oxf) 2015;214:189–99. doi: 10.1111/apha.12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhattacharya A, Schmitz U, Raatz Y, Schönherr M, Kottek T, Schauer M, Franz S, Saalbach A, Anderegg U, Wolkenhauer O, Schadendorf D, Simon JC, Magin T, Vera J, Kunz M. miR-638 promotes melanoma metastasis and protects melanoma cells from apoptosis and autophagy. Oncotarget. 2015;6:2966–80. doi: 10.18632/oncotarget.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sciarretta S, Zhai P, Shao D, Zablocki D, Nagarajan N, Terada LS, Volpe M, Sadoshima J. Activation of NADPH oxidase 4 in the endoplasmic reticulum promotes cardiomyocyteautophagyand survivalduring energy stress through the protein kinase RNA-activated-like endoplasmic reticulum kinase/eukaryotic initiation factor 2α/activating transcription factor 4 pathway. Circ Res. 2013;113:1253–64. doi: 10.1161/CIRCRESAHA.113.301787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saiprasad G, Chitra P, Manikandan R, Sudhandiran G. Hesperidin induces apoptosis andtriggers autophagic markers through inhibition of Aurora-A mediated phosphoinositide-3-kinase/Akt/mammalian target of rapamycin and glycogen synthase kinase-3 beta signalling cascades in experimental colon carcinogenesis. Eur J Cancer. 2014;50:2489–507. doi: 10.1016/j.ejca.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 20.Sheng Y, Sun B, Guo WT, Liu X, Wang YC, Xie X, Xiao XL, Li N, Dong DL. (4-[6-(4-isopropoxyphenyl) pyrazolo [1,5-a] pyrimidin-3-yl] quinoline) is a novel inhibitor of autophagy. Br J Pharmacol. 2014;171:4970–80. doi: 10.1111/bph.12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindqvist LM, Heinlein M, Huang DC, Vaux DL. Prosurvival Bcl-2 family membersaffect autophagy only indirectly, by inhibiting Bax and Bak. Proc Natl Acad Sci U S A. 2014;111:8512–7. doi: 10.1073/pnas.1406425111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cervia D, Perrotta C, Moscheni C, De Palma C, Clementi E. Nitric oxide andsphingolipids control apoptosis and autophagy with a significant impact on Alzheimer’s disease. J Biol Regul Homeost Agents. 2013;272(Suppl):11–22. [PubMed] [Google Scholar]
- 23.Shelby SJ, Angadi PS, Zheng QD, Yao J, Jia L, Zacks DN. Hypoxia induciblefactor1α contributes to regulation of autophagy in retinal detachment. Exp Eye Res. 2015;137:84–93. doi: 10.1016/j.exer.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang SP, Chien JY, Tsai RK. Ethambutol induces impaired autophagicflux and apoptosisin the rat retina. Dis Model Mech. 2015;8:977–87. doi: 10.1242/dmm.019737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan Q, Wang H, Hu Y, Hu M, Li X, Aodengqimuge , Ma Y, Wei C, Song L. Src/STAT3-dependent heme oxygenase-1 induction mediates chemoresistance of breast cancer cells todoxorubicin by promoting autophagy. Cancer Sci. 2015;106:1023–32. doi: 10.1111/cas.12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang YC, Hsieh MC, Wu HJ, Wu WC, Kao YH. Methylglyoxal, a reactive glucose metabolite, enhances autophagy flux and suppresses proliferation of humanretinal pigment epithelial ARPE-19 cells. Toxicol In Vitro. 2015;29:1358–1368. doi: 10.1016/j.tiv.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 27.Chaudhary K, Shinde R, Liu H, Gnana-Prakasam JP, Veeranan-Karmegam R, Huang L, Ravishankar B, Bradley J, Kvirkvelia N, McMenamin M, Xiao W, Kleven D, Mellor AL, Madaio MP, McGaha TL. Amino Acid Metabolism InhibitsAntibody-DrivenKidney Injury by Inducing Autophagy. J Immunol. 2015;194:5713–24. doi: 10.4049/jimmunol.1500277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abedin MJ, Wang D, McDonnell MA, Lehmann U, Kelekar A. Autophagy delays apoptotic death in breast cancer cells following DNA damage. Cell Death Differ. 2007;14:500–10. doi: 10.1038/sj.cdd.4402039. [DOI] [PubMed] [Google Scholar]
- 29.Jia G, Cheng G, Gangahar DM, Agrawal DK. Insulin-like growth factor-1and TNF-alpharegulate autophagy through c-jun N-terminal kinase and Akt pathwaysin human atherosclerotic vascular smooth cells. Immunol Cell Biol. 2006;84:448–54. doi: 10.1111/j.1440-1711.2006.01454.x. [DOI] [PubMed] [Google Scholar]
- 30.Sarkar S, Rubinsztein DC. Inositol and IP3 levels regulate autophagy: biology and therapeutic speculations. Autophagy. 2006;2:132–4. doi: 10.4161/auto.2387. [DOI] [PubMed] [Google Scholar]
- 31.Li C, Capan E, Zhao Y, Zhao J, Stolz D, Watkins SC, Jin S, Lu B. Autophagy is induced in CD4+ T cells and important for the growth factorwithdrawal cell death. J Immunol. 2006;177:5163–8. doi: 10.4049/jimmunol.177.8.5163. [DOI] [PubMed] [Google Scholar]
- 32.Chen X, Kondo K, Motoki K, Homma H, Okazawa H. Fasting activates macroautophagy in neurons of Alzheimer’s disease mouse model but is insufficient to degrade amyloid-beta. Sci Rep. 2015;5:12115. doi: 10.1038/srep12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aoki H, Iwado E, Eller MS, Kondo Y, Fujiwara K, Li GZ, Hess KR, Siwak DR, Sawaya R, Mills GB, Gilchrest BA, Kondo S. Telomere 3’ overhangspecific DNAoligonucleotides induce autophagy in malignant glioma cells. FASEB J. 2007;21:2918–30. doi: 10.1096/fj.06-6941com. [DOI] [PubMed] [Google Scholar]
- 34.Takeuchi H, Kanzawa T, Kondo Y, Kondo S. Inhibition of platelet-derived growth factor signalling induces autophagy in malignant glioma cells. Br J Cancer. 2004;90:1069–75. doi: 10.1038/sj.bjc.6601605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Esselens C, Oorschot V, Baert V, Raemaekers T, Spittaels K, Serneels L, Zheng H, Saftig P, De Strooper B, Klumperman J, Annaert W. Presenilin 1 mediates the turnover of telencephalin in hippocampal neurons via an autophagic degradativepathway. J Cell Biol. 2004;166:1041–54. doi: 10.1083/jcb.200406060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rusten TE, Lindmo K, Juhász G, Sass M, Seglen PO, Brech A, Stenmark H. Programmed autophagy in the Drosophila fat body is inducedby ecdysone throughregulation of the PI3K pathway. Dev Cell. 2004;7:179–92. doi: 10.1016/j.devcel.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 37.Mathew R, Kongara S, Beaudoin B, Karp CM, Bray K, Degenhardt K, Chen G, Jin S, White E. Autophagy suppressestumor progression by limiting chromosomal instability. Genes Dev. 2007;21:1367–81. doi: 10.1101/gad.1545107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bao LJ, Jaramillo MC, Zhang ZB, Zheng YX, Yao M, Zhang DD, Yi XF. Nrf2 induces cisplatin resistance through activation of autophagy in ovarian carcinoma. Int J Clin Exp Pathol. 2014;7:1502–13. [PMC free article] [PubMed] [Google Scholar]
- 39.Xu WH, Liu ZB, Hou YF, Hong Q, Hu DL, Shao ZM. Inhibition of autophagy enhances the cytotoxic effect of PA-MSHA in breast cancer. BMC Cancer. 2014;14:273. doi: 10.1186/1471-2407-14-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hou X, Hu Z, Xu H, Xu J, Zhang S, Zhong Y, He X, Wang N. Advanced glycation endproducts trigger autophagy in cadiomyocyte via RAGE/PI3K/AKT/mTOR pathway. Cardiovasc Diabetol. 2014;13:78. doi: 10.1186/1475-2840-13-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu M, Li CM, Chen ZF, Ji R, Guo QH, Li Q, Zhang HL, Zhou YN. Celecoxib regulates apoptosis and autophagy via the PI3K/Aktsignaling pathway in SGC-7901 gastric cancer cells. Int J Mol Med. 2014;33:1451–8. doi: 10.3892/ijmm.2014.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang M, Yu T, Zhu C, Sun H, Qiu Y, Zhu X, Li J. Resveratrol triggers protective autophagy through the ceramide/Akt/mTOR pathway in melanoma B16 cells. Nutr Cancer. 2014;66:435–40. doi: 10.1080/01635581.2013.878738. [DOI] [PubMed] [Google Scholar]
- 43.Shi K, An J, Shan L, Jiang Q, Li F, Ci Y, Wu P, Duan J, Hui K, Yang Y, Xu C. Survivin-2B promotes autophagy by accumulating IKK alpha in the nucleus of selenite-treated NB4 cells. Cell Death Dis. 2014;5:e1071. doi: 10.1038/cddis.2014.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Shen J, Xiong X, Xu Y, Zhang H, Huang C, Tian Y, Jiao C, Wang X, Li X. Remote ischemic preconditioning protects against liver ischemia-reperfusion injury via heme oxygenase-1-induced autophagy. PLoS One. 2014;9:e98834. doi: 10.1371/journal.pone.0098834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yue HW, Liu J, Liu PP, Li WJ, Chang F, Miao JY, Zhao J. Sphingosyl phosphoryl choline protects cardiomyocytes against ischemic apoptosis via lipidraft/PTEN/Akt1/mTOR mediated autophagy. Biochim Biophys Acta. 2015;1851:1186–1193. doi: 10.1016/j.bbalip.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 46.Zheng N, Zhang P, Huang H, Liu W, Hayashi T, Zang L, Zhang Y, Liu L, Xia M, Tashiro SI, Onodera S, Ikejima T. ERα down-regulation plays a key role in silibinin-induced autophagy and apoptosis in human breast cancer MCF-7 cell. J Pharmacol Sci. 2015;128:97–107. doi: 10.1016/j.jphs.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 47.Li Y, Zhang L, Zhou J, Luo S, Huang R, Zhao C, Diao A. Nedd4 E3 ubiquitin ligase promotes cell proliferation and autophagy. Cell Prolif. 2015;48:338–47. doi: 10.1111/cpr.12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu W, Zhang Z, Zhang Y, Chen X, Guo S, Lei Y, Xu Y, Ji C, Bi Z, Wang K. HMGB1-mediated autophagy modulates sensitivity of colorectal cancer cells to oxaliplatin via MEK/ERK signaling pathway. Cancer Biol Ther. 2015;16:511–7. doi: 10.1080/15384047.2015.1017691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aveleira CA, Botelho M, Carmo-Silva S, Pascoal JF, Ferreira-Marques M, Nóbrega C, Cortes L, Valero J, Sousa-Ferreira L, Álvaro AR, Santana M, Kügler S, Pereira de Almeida L, Cavadas C. Neuropeptide Y stimulates autophagy in hypothalamic neurons. Proc Natl Acad Sci U S A. 2015;112:E1642–51. doi: 10.1073/pnas.1416609112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Allavena G, Carrarelli P, Del Bello B, Luisi S, Petraglia F, Maellaro E. Autophagy is upregulated in ovarian endometriosis: a possible interplay with p53 and heme oxygenase-1. Fertil Steril. 2015;103:1244–51. e1. doi: 10.1016/j.fertnstert.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 51.Mordier S, Deval C, Béchet D, Tassa A, Ferrara M. Leucine limitation induces autophagy and activation of lysosome-dependent proteolysis in C2C12 myotubesthrough a mammalian target of rapamycin-independent signaling pathway. J Biol Chem. 2000;275:29900–6. doi: 10.1074/jbc.M003633200. [DOI] [PubMed] [Google Scholar]
- 52.De Amicis F, Aquila S, Morelli C, Guido C, Santoro M, Perrotta I, Mauro L, Giordano F, Nigro A, Andò S, Panno ML. Bergapten drives autophagy through the up-regulationof PTEN expression in breast cancer cells. Mol Cancer. 2015;14:130. doi: 10.1186/s12943-015-0403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tarhini AA, Lin Y, Yeku O, LaFramboise WA, Ashraf M, Sander C, Lee S, Kirkwood JM. A four-marker signature of TNF-RII, TGF-α, TIMP-1 and CRP is prognostic of worse survival in high-risk surgically resected melanoma. J Transl Med. 2014;12:19. doi: 10.1186/1479-5876-12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hamacher-Brady A, Brady NR, Gottlieb RA, Gustafsson AB. Autophagy as a protective response to Bnip3-mediated apoptotic signaling in the heart. Autophagy. 2006;2:307–9. doi: 10.4161/auto.2947. [DOI] [PubMed] [Google Scholar]
- 55.Luo T, Fu J, Xu A, Su B, Ren Y, Li N, Zhu J, Zhao X, Dai R, Cao J, Wang B, Qin W, Jiang J, Li J, Wu M, Feng G, Chen Y, Wang H. PSMD10/Gankyrin Induces Autophagy toPromoteTumor Progression through CytoplasmicInteraction with ATG7and Nuclear Transactivation of ATG7 Expression. Autophagy. 2015 [Epub ahead of print] [Google Scholar]
- 56.Wu Y, Li Y, Zhang H, Huang Y, Zhao P, Tang Y, Qiu X, Ying Y, Li W, Ni S, Zhang M, Liu L, Xu Y, Zhuang Q, Luo Z, Benda C, Song H, Liu B, Lai L, Liu X, Tse HF, Bao X, Chan WY, A Esteban M, Qin B, Pei D. Autophagy and mTORC1 regulate the stochastic phase of somatic cell reprogramming. Nat Cell Biol. 2015;17:715–25. doi: 10.1038/ncb3172. [DOI] [PubMed] [Google Scholar]
- 57.Martin S, Dudek-Perić AM, Maes H, Garg AD, Gabrysiak M, Demirsoy S, Swinnen JV, Agostinis P. Concurrent MEK and autophagy inhibition is required to restore cell death associated danger-signalling in Vemurafenib-resistant melanoma cells. Biochem Pharmacol. 2015;93:290–304. doi: 10.1016/j.bcp.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 58.Ozpolat B, Akar U, Mehta K, Lopez-Berestein G. PKC delta and tissue transglutaminase are novel inhibitors of autophagy in pancreatic cancer cells. Autophagy. 2007;3:480–3. doi: 10.4161/auto.4349. [DOI] [PubMed] [Google Scholar]
- 59.Guo R, Xu X, Babcock SA, Zhang Y, Ren J. Aldehyde dedydrogenase-2plays a beneficialrole in ameliorating chronic alcohol-induced hepatic steatosis and inflammation through regulation of autophagy. J Hepatol. 2015;62:647–56. doi: 10.1016/j.jhep.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 60.Zhang Y, Liu G, Dull RO, Schwartz DE, Hu G. Autophagy in pulmonary macrophages mediates lung inflammatory injury via NLRP3 inflammasome activation during mechanical ventilation. Am J Physiol Lung Cell Mol Physiol. 2014;307:L173–85. doi: 10.1152/ajplung.00083.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu K, Chen W, Wang X, Peng Y, Liang A, Huang D, Li C, Ye W. Autophagy attenuates the catabolic effect during inflammatory conditions in nucleus pulposus cells, as sustained by NF-κB and JNK inhibition. Int J Mol Med. 2015;36:661–8. doi: 10.3892/ijmm.2015.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jurkuvenaite A, Benavides GA, Komarova S, Doran SF, Johnson M, Aggarwal S, Zhang J, Darley-Usmar VM, Matalon S. Upregulation of autophagy decreaseschlorine-induced mitochondrial injury and lung inflammation. Free Radic Biol Med. 2015;85:83–94. doi: 10.1016/j.freeradbiomed.2015.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Y, Vasheghani F, Li YH, Blati M, Simeone K, Fahmi H, Lussier B, Roughley P, Lagares D, Pelletier JP, Martel-Pelletier J, Kapoor M. Cartilage-specific deletion ofmTORupregulates autophagy and protects mice from osteoarthritis. Ann Rheum Dis. 2015;74:1432–40. doi: 10.1136/annrheumdis-2013-204599. [DOI] [PubMed] [Google Scholar]
- 64.Wang H, Sun RQ, Zeng XY, Zhou X, Li S, Jo E, Molero JC, Ye JM. Restoration of autophagy alleviates hepatic ER stress and impaired insulin signalling transduction in high fructose-fed male mice. Endocrinology. 2015;156:169–81. doi: 10.1210/en.2014-1454. [DOI] [PubMed] [Google Scholar]
- 65.Domigan CK, Warren CM, Antanesian V, Happel K, Ziyad S, Lee S, Krall A, Duan L, Torres-Collado AX, Castellani LW, Elashoff D, Christofk HR, van der Bliek AM, Potente M, Iruela-Arispe ML. Autocrine VEGF maintains endothelial survivalthroughregulation of metabolism and autophagy. J Cell Sci. 2015;128:2236–48. doi: 10.1242/jcs.163774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Qiao S, Dennis M, Song X, Vadysirisack DD, Salunke D, Nash Z, Yang Z, Liesa M, Yoshioka J, Matsuzawa S, Shirihai OS, Lee RT, Reed JC, Ellisen LW. A REDD1/TXNIP pro-oxidant complex regulates ATG4B activity to control stressinduced autophagy and sustain exercise capacity. Nat Commun. 2015;6:7014. doi: 10.1038/ncomms8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamamoto A, Cremona ML, Rothman JE. Autophagy-mediated clearance of huntingtin aggregates triggered by the insulin-signaling pathway. J Cell Biol. 2006;172:719–31. doi: 10.1083/jcb.200510065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Towns R, Kabeya Y, Yoshimori T, Guo C, Shangguan Y, Hong S, Kaplan M, Klionsky DJ, Wiley JW. Sera from patients with type 2 diabetes and neuropathy induce autophagy and colocalization with mitochondria inSY5Y cells. Autophagy. 2005;1:163–70. doi: 10.4161/auto.1.3.2068. [DOI] [PubMed] [Google Scholar]
- 69.Liu Z, Lenardo MJ. Reactive oxygen species regulate autophagy through redox-sensitive proteases. Dev Cell. 2007;12:484–5. doi: 10.1016/j.devcel.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 70.Baek SH, Noh AR, Kim KA, Akram M, Shin YJ, Kim ES, Yu SW, Majid A, Bae ON. Modulation of mitochondrial function and autophagy mediates carnosine neuroprotection against ischemic brain damage. Stroke. 2014;45:2438–43. doi: 10.1161/STROKEAHA.114.005183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yan S, Yang X, Chen T, Xi Z, Jiang X. The PPARγ agonist Troglitazone induces autophagy, apoptosis and necroptosis in bladder cancer cells. Cancer Gene Ther. 2014;21:188–93. doi: 10.1038/cgt.2014.16. [DOI] [PubMed] [Google Scholar]
- 72.Guyton AC. Blood pressure control--special role of the kidneys and body fluids. Science. 1991;252:1813–6. doi: 10.1126/science.2063193. [DOI] [PubMed] [Google Scholar]
- 73.Macdonald JH, Fearn L, Jibani M, Marcora SM. Exertional fatigue in patients with CKD. Am J Kidney Dis. 2012;60:930–9. doi: 10.1053/j.ajkd.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 74.Brück K, Jager KJ, Dounousi E, Kainz A, Nitsch D, Ärnlöv J, Rothenbacher D, Browne G, Capuano V, Ferraro PM, Ferrieres J, Gambaro G, Guessous I, Hallan S, Kastarinen M, Navis G, Gonzalez AO, Palmieri L, Romundstad S, Spoto B, Stengel B, Tomson C, Tripepi G, Völzke H, Więcek A, Gansevoort R, Schöttker B, Wanner C, Vinhas J, Zoccali C, Van Biesen W, Stel VS European CKD Burden Consortium. Methodology used in studies reporting chronic kidney disease prevalence: a systematic literature review. Nephrol Dial Transplant. 2015;30(Suppl 4):iv6–iv16. doi: 10.1093/ndt/gfv131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hua Y, Zhang Y, Ceylan-Isik AF, Wold LE, Nunn JM, Ren J. Chronic Akt activation accentuates aging-induced cardiac hypertrophy and myocardial contractile dysfunction: role of autophagy. Basic Res Cardiol. 2011;106:1173–91. doi: 10.1007/s00395-011-0222-8. [DOI] [PubMed] [Google Scholar]
- 76.Impellizzeri D, Esposito E, Attley J, Cuzzocrea S. Targeting inflammation: new therapeutic approaches in chronic kidney disease (CKD) Pharmacol Res. 2014;81:91–102. doi: 10.1016/j.phrs.2014.02.007. [DOI] [PubMed] [Google Scholar]


