Abstract
Gliomas are the most common brain tumors, leading to significant cancer-related mortality worldwide. Hypoxia-inducible factor 1-alpha (HIF-1α) was shown to be involved in the pathophysiology and management of glioma, and might offer a therapeutic target. However, the results remain inconclusive. The purpose of this study was to systematically investigate the clinical and prognostic significance of HIF-1α expression in patients with glioma. Relevant studies published between 2000 and 2015 were searched in the electronic databases. The odds ratio (OR), risk ratio (RR) and mean difference (MD) with their 95% confidence intervals (CI) were employed to calculate the strength of significance. Finally, a total of 24 articles were retrieved, including 1422 glioma patients. No significant heterogeneity was presented between studies (I2<50%, P>0.01). Overall, our results showed that HIF-1α expression was significantly associated with high WHO grade (III+IV) of glioma (OR=8.59, 95% CI=6.56-11.24, P<0.00001). This significant relationship was also found between HIF-1α expression and microvascular density (MD=26.32, 95% CI=14.48-38.16, P<0.0001), overall survival (OS) (3-year OS: RR=0.48, 95% CI=0.35-0.66, P<0.00001; 2-year OS: RR=0.53, 95% CI=0.38-0.73, P<0.0001; 1-year OS: RR=0.79, 95% CI=0.66-0.95, P=0.01), and the cumulative survival time. However, HIF-1α expression was not associated with age and gender of glioma patients (P>0.05). In conclusions, our results suggested that HIF-1α expression was associated with high grade of glioma and OS, indicating that HIF-1α could predict prognosis and provide clinical insights into the therapeutic strategy for patients with glioma. More studies concerning other populations are also needed in the future research.
Keywords: Glioma, HIF-1α, prognosis, meta-analysis
Introduction
Gliomas, tumors of the neoplastic glial cells, or neuroglia, are the most common primary intracranial and central nervous system tumor [1,2]. It accounts for 81% of all malignant brain tumors in adults, leading to significant mortality and morbidity worldwide [3]. Glioma incidence rate (RA) have remained stable by region and over time during the last decade [4,5], but the RA varies by gender, race, age at diagnosis, histologic type and genetics [6,7]. According to the World Health Organization (WHO) grading system, glioma are divided as astrocytoma, oligodendroglioma, mixed oliogoastrocytoma, and ependymoma based on the degree of malignancy [8]. Although major advances have made, treatment of glioma is still the most challenging problems in oncology and neurosurgery due to resistant to radiation treatment, with the 5-year relative survival less than 5% in patients with the most aggressive glioma histology, glioblastoma [9]. Therefore, there is an urgent need to explore an effective biomarker to predict the grade of gliomas and the overall prognosis so as to enhance patient survival and quality of life.
Hypoxia-inducible factor 1-alpha (HIF-1α), the subunit of HIF-1, is the major transcriptional factor involved in the adaptive response under hypoxic conditions [10]. It controls embryonic and tumorigenic responses to variations in microenvironmental oxygenation [11]. HIF-1α plays a role in hypoxia-mediated apoptosis, tumor angiogenesis and cell proliferation [12]. Its expression was identified in several tumors. Cellular HIF-1α was shown to be an important indicator of prognosis in patients with clear cell renal cell carcinoma, and high HIF-1α expression predicted poor survival [13]. HIF-1 expression was enhanced in meningiomas of higher WHO grade [14]. It was elevated in glioblastoma multiforme as well [15]. Studies have shown that HIF-1α play a role in the pathophysiology and management of glioblastoma [16]. HIF-1α silencing combined with radiation therapy will increase the therapeutic efficacy of glioma treatment via regulation of cell cycle and apoptosis-related signaling pathways [17]. Furthermore, polymorphisms of HIF-1α gene may be used as a molecular marker for gliomas occurrence, grades and clinical outcome in gliomas patients [18]. Thus, it is crucial to understand the role of HIF-1α in grade and prognosis of gliomas, which can provide clinical insights into the efficacious therapeutic strategy.
Although many researches have been conducted on this issue, the value of HIF-1α in clinical data and prognosis of glioma is still unclear. Therefore, we conducted this meta-analysis to systematically review and evaluate all the published articles on the effect of HIF-α expression in glioma patients and obtain a reliable result.
Materials and methods
Publication search
Eligible articles published between January 2000 and 2015 were searched in online electronic databases of CNKI (China National Knowledge Internet), Wanfang, PubMed, Embase and Medline. The following searching terms: “glioma or glioblastoma”, “hypoxia-inducible factor-1 or HIF-1α”, “expression”, “prognosis” and “clinical significance” as well as their combinations were used. References of related articles were searched manually. Only published articles written in English or in Chinese were included.
Inclusion and exclusion criteria
Eligible studies should meet the following criteria: 1) patients were confirmed with the diagnosis of glioma by the department of pathology, and were classified according to current World Health Organization guidelines [19]; 2) HIF-1α expression was evaluated by using immunohistochemistry (IHC) methods; 3) the main results were focused on WHO grade and overall survival; 4) when the same laboratory or authors reported the same issue twice or more, only the most recent full-test was included.
The exclusion criteria were: 1) reviews or conference papers; 2) data cannot be extracted; 3) duplicate articles; and 4) studies were not conducted in humans or studies with incomplete data.
Data extraction
According to the Newcastle Ottawa Quality Assessment Scale (NOS) criteria [20], two experts independently assessed and scored the quality of the methodology of each study. Any disagreement or discrepancies was resolved by consulting with a third investigator to reach a consensus on NOS scores of each study. The NOS score was ranged from 0 to 10 stars. Studies with NOS score over 6 stars were included in the final analysis. The following information was extracted from each included studies: first author, published year, country, sample size, mean age, cutoff point for protein positivity, WHO grade of patients and positive rate.
Statistic analysis
The clinical significance of HIF-1α expression in glioma patients was estimated by risk ratios (RRs) or odds ratios (ORs) or mean difference (MD) and their 95% confidence intervals (CI). The statistical significance was determined by Z-test with a P-value less than 0.05 considered significant. Extent of heterogeneity between studies was measured by both the Q-test and the I2 test. The fixed-effect model was employed when the effect were homologous (P-value ≥0.01 for the Q-test and I2≤50% for the I2 test), while the random-effect model was used in its opposite. All analyses were calculated using the RevMan5.2 program.
Results
Characteristics of included studies
We firstly identified 85 articles through literature search from Jan 1, 2004 to Dec 31, 2014. Among them, 18 articles were duplicated. After screening the titles and abstracts of the remaining 67 articles, 32 articles were excluded for they did not meet our specified criteria. Finally, a total of 24 articles were screened out, including 1422 glioma patients. All the included articles were considered as high quality articles. The selection process was shown in Figure 1. Of the 24 studies, 2 were written in English [21,22] and 22 in Chinese [23-44]; 23 were conducted in Chinese population and 1 in Japanese population [21]. The sample size ranged from 27 to 120. The cutoff points for HIF-1α expression selected in most studies was that 5% or less positive cell percentage was scored as 0, no staining (-); 6%-25% as 1 points, weak intensity (+); 26%-50% as 2 points, moderate intensity (++); 51% or more as 3 points, strong intensity (+++). The main characteristics of included studies were presented in Table 1.
Figure 1.
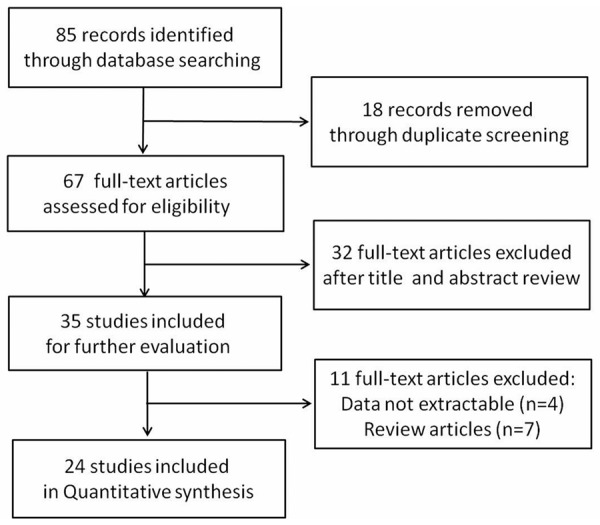
The process of literature selection.
Table 1.
Main characteristics of included studies in this meta-analysis
| First author | Year | Mean age | Sample size (M/F) | Grade | Cutoff | Positive | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Low (I+II) | High (III+IV) | ||||||
| Gao WC | 2004 | 35.4 | 31/32 | 46 | 17 | >1% | 45/63 |
| Fu K | 2005 | 37.63 | 27/18 | 22 | 23 | >1% | 28/45 |
| Wang GK | 2006 | 43.1±12.54 | 25/22 | 13 | 24 | Score =1 | 26/37 |
| Yin J | 2007 | 46.6 | 34/26 | 31 | 29 | >1% | 43/60 |
| Li L | 2008 | 49 | 13/17 | 21 | 9 | >5% | 20/30 |
| Tan YL | 2008 | 4-71 | 31/23 | 26 | 28 | Score =1 | 33/54 |
| Xu X | 2008 | 23-67 | 31/16 | 19 | 28 | Score =2 | 35/47 |
| Zheng KB | 2008 | 40.6 | 34/28 | 28 | 34 | Score =3 | 41/62 |
| Li FC | 2009 | 34 | 56/30 | 36 | 50 | - | 48/80 |
| Liu QD | 2009 | 40.1 | 24/16 | 18 | 22 | Score =2 | 27/40 |
| Liu Y | 2009 | 36.69±13.47 | 61/59 | 60 | 60 | Score =1 | 62/120 |
| Wu WC | 2010 | NA | 33/25 | 26 | 32 | Score =1 | 39/58 |
| Yu G | 2010 | 39 | 30/22 | 32 | 20 | >1% | 34/52 |
| Zhang L | 2010 | 10-67 | 41/39 | 40 | 40 | >1% | 49/80 |
| Zhao SP | 2010 | 41.8±18.3 | 25/20 | 24 | 21 | Score =2 | 32/45 |
| Li J | 2011 | 40.1 | 32/24 | 34 | 22 | >1% | 37/56 |
| Zhang H | 2011 | 37 | 39/23 | 30 | 32 | Score =2 | 40/62 |
| Zhang MY | 2011 | 42.9 | 35/35 | 32 | 38 | NA | 45/70 |
| Jiang JC | 2012 | 16-68 | 36/32 | 34 | 34 | Score =1 | 46/68 |
| Liu XR | 2013 | 38.81 | 45/53 | 50 | 48 | >5% | 70/98 |
| Xing PH-a | 2013 | 24-67 | 35/28 | 27 | 36 | >1% | 42/63 |
| Xing PH-b | 2013 | 27-68 | 32/26 | 25 | 33 | >1% | 41/58 |
| Cao WD | 2014 | 49.4±19.9 | 14/13 | 5 | 22 | Score =2 | 20/27 |
| Liao CC | 2014 | - | 24/17 | 17 | 24 | Score =2 | 27/41 |
-, not applicable; M/F, male/female; cutoff, cutoff points for HIF-1α expression.
Correlation of HIF-1α expression with WHO grade of glioma
Firstly, we divided glioma patients into low (I+II) and high grade (III+IV) according to WHO grade. All the 24 articles contained these data and were available to extract, including 726 high and 696 low grade patients, respectively. No significant heterogeneity was found between studies (P>0.01, I2<50%), and the fixed-effect model was employed. Overall, our results found that HIF-1α was highly expressed in high grade patients compared with that in low grade (86.0% versus 44.0%), indicating HIF-1α expression was significantly associated with high WHO grade of glioma (OR=8.59, 95% CI=6.56-11.24, P<0.00001) as shown in Figure 2.
Figure 2.
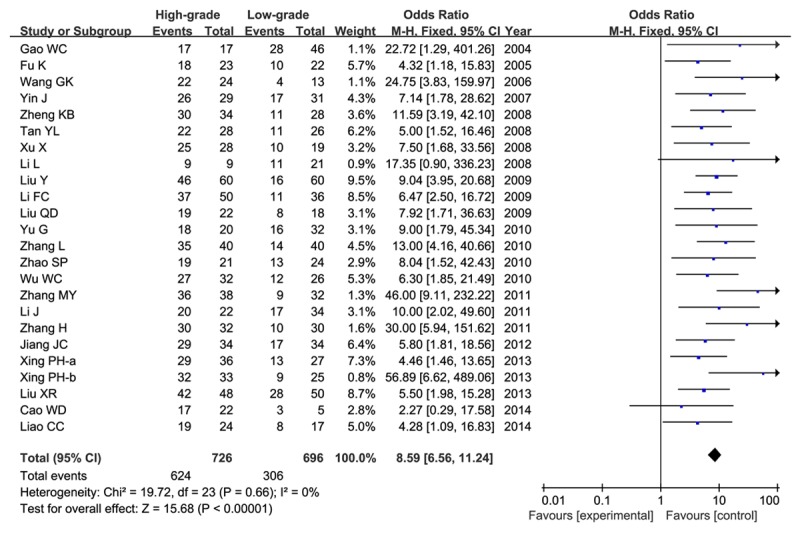
Meta-analysis of HIF-1α expression in high grade (III+IV) and low grade (I+II) gliomas.
Then we analyzed the relationship between HIF-1α expression and glioma at each grade, respectively. Table 2 listed the results of each comparison by grade. Our results showed that the HIF-1α expression in patients with higher grade significantly related with glioma when compared with the lower-grade (P<0.00001).
Table 2.
Meta-analysis of HIF-1α expression in glioma patients by WHO grade
| Comparison (WHO Grade) | N | number | Z-test | Between-study heterogeneity | |||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| OR (95% CI) | P | I2 | Ph | Model | |||
| IV+III vs. II+I | 24 | 1422 | 8.59 (6.56, 11.24) | <0.00001 | 0% | 0.66 | F |
| IV vs. III | 14 | 461 | 2.51 (1.43, 4.42) | 0.001 | 0% | 0.98 | F |
| IV vs. II | 11 | 399 | 9.18 (5.18, 16.28) | <0.00001 | 0% | 0.82 | F |
| IV vs. I | 9 | 272 | 24.23 (12.21, 48.09) | <0.00001 | 0% | 0.45 | F |
| III vs. II | 12 | 458 | 4.59 (2.96, 7.12) | <0.00001 | 0% | 0.74 | F |
| III vs. I | 10 | 304 | 13.34 (7.53, 23.62) | <0.00001 | 0% | 0.83 | F |
| II vs. I | 11 | 365 | 4.19 (2.59, 6.77) | <0.00001 | 10% | 0.35 | F |
N, number of included studies; number, total glioma patients; OR, odds ratio; 95% CI, 95% confidence interval; I2, Ph, between-study heterogeneity; F, the fixed-effect model.
Lastly, we verified the correlation of HIF-1α expression between glioma and normal. Total 11 studies contained 603 glioma patients and 86 controls. This results showed that HIF-1α expression was connected with glioma (OR=27.16, 95% CI=12.03-61.33, P<0.00001) as shown in Figure 3.
Figure 3.
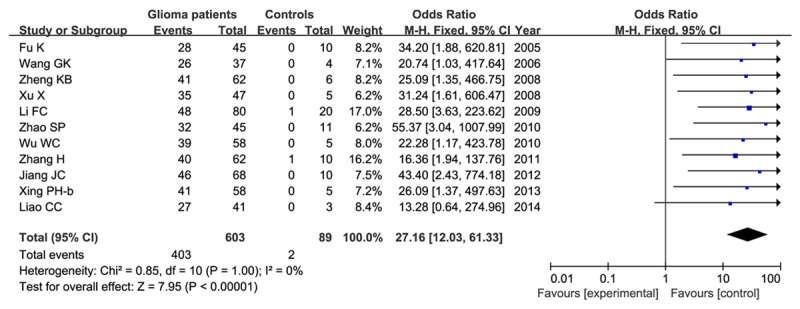
Meta-analysis of HIF-1α expression in patients with gliomas and normal controls.
Combined effect of HIF-1α expression and gender, age, microvascular density (MVD) in patients with glioma
With respect to the gender issue, six studies included 223 male patients and 210 female patients. Even though the frequency of HIF-1α expression was slightly higher in male patients than that in female patients (68.2% versus 63.8%), our results did not find a significant association between HIF-1α expression and gender (OR=1.23, 95% CI=0.82-1.84, P=0.33) in a fixed-effect model as shown in Figure 4.
Figure 4.
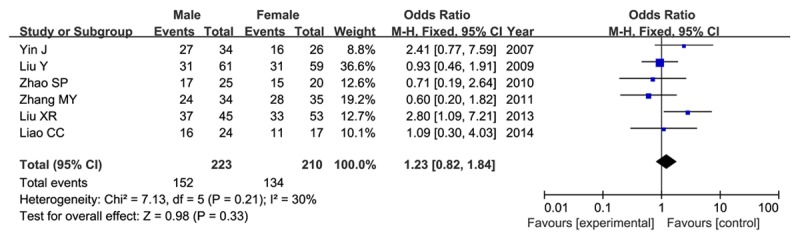
Association between HIF-1α expression and gender in glioma patients in a fixed-effect model.
The same six studies also focused on the age issue. For different age stages were presented, we divided ages into three comparable groups. As shown in Figure 5, our results showed no difference between HIF-1α expression and age (P>0.05).
Figure 5.
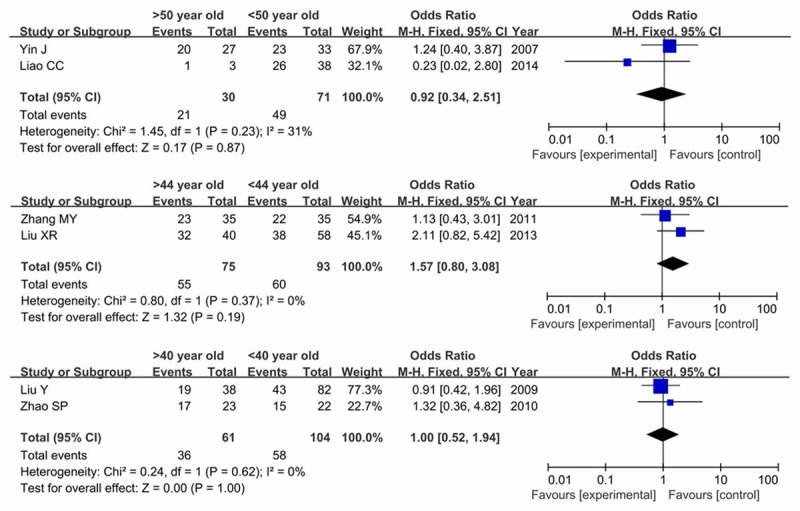
Forest plot of association between HIF-1α expression and age in glioma patients.
Three articles analyzed the relationship between HIF-1α expression and MVD. Our result demonstrated that there was a significant association between HIF-1α expression and MVD (MD=26.32, 95% CI=14.48-38.16, P<0.0001) in a random-effect model as shown in Figure 6.
Figure 6.
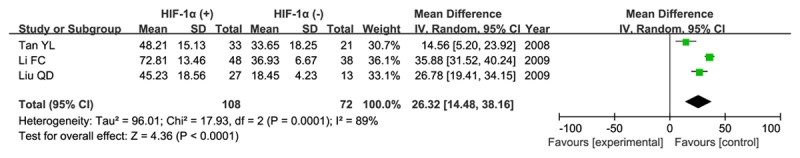
Association between HIF-1α expression and microvascular density (MVD) in patients with glioma.
Correlation of HIF-1α expression with overall survival (OS)
Six articles were obtained, including 310 glioma patients. Two articles concerned the 3-year OS, three in the 2-year OS, two in the 1-year OS, and one in two-month OS. Our result showed that HIF-1α expression was significantly associated with 3-year OS (RR=0.48, 95% CI=0.35-0.66, P<0.00001, Figure 7A) in a fixed-effect model. This significant association was also found with 2-year OS (RR=0.53, 95% CI=0.38-0.73, P<0.0001, Figure 7B), and 1-year OS (RR=0.79, 95% CI=0.66-0.95, P=0.01, Figure 7C).
Figure 7.
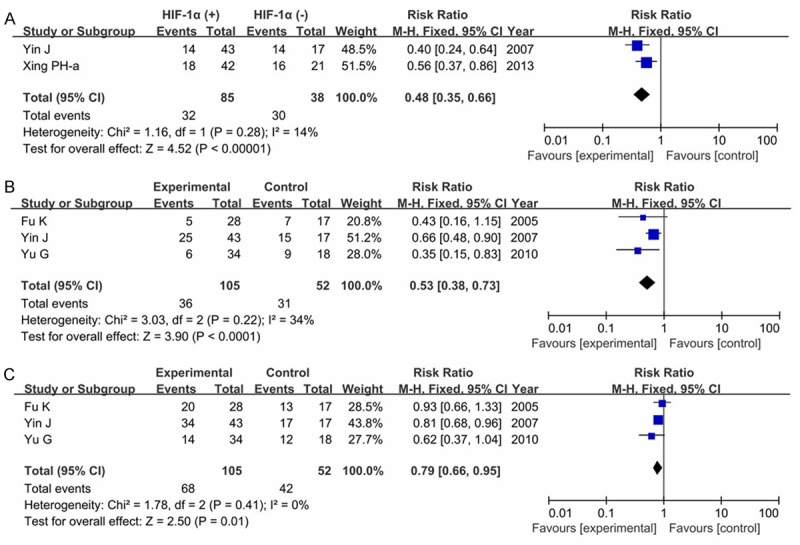
Forest plot of association between HIF-1α expression and overall survive (OS) in glioma patients (A. 3-year OS; B. 2-year OS; C. 1-year OS).
Three articles concerned the survival times of patients with glioma. The data from the study conducted by Cao et al could not be extracted, and the result revealed a significant effect of HIF-1α expression on the cumulative survival time (P<0.05). The meta-analysis of the other two studies also showed HIF-1α expression played a role on survival time.
Sensitivity analysis and publication bias
We deleted each study at a time to reveal single study on the overall effect, and the result indicated that the pooled OR, RR and MD were not significantly influenced by omitting any single study at a given time. The funnel plot also demonstrated no publication bias in this meta-analysis as shown in Figure 8.
Figure 8.
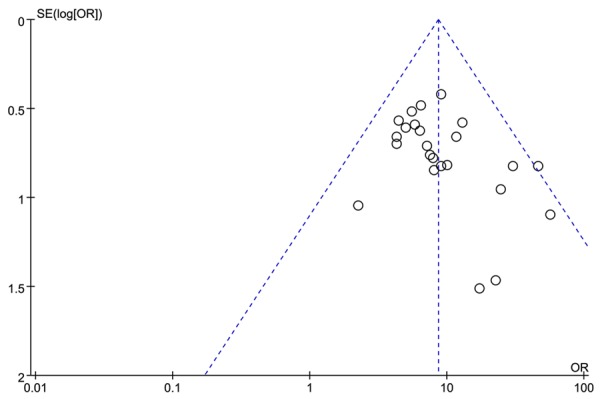
Funnel plot for publication bias in selection of studies.
Discussion
Glioma is a neurological disease with a poor prognosis and a clinical course characterized by progressive functional and cognitive impairment [45]. Heterogeneity between individual patients increasingly limits therapeutic progress for glioma [46]. It is meaningful to investigate biomarkers in each grade of glioma to enhance patient survival and quality of life. In this meta-analysis, we discussed the significance of HIF-1α expression in patients with glioma. Our results found that HIF-1α expression was significantly associated with high WHO grade of glioma, MVD, 3 (2 or 1) year overall survival and cumulative survival time (P<0.01). This significant association was not found in age and gender. Our results indicated that HIF-1α expression might predict the prognosis of patients with glioma and provide an insight into the treatment strategy. Furthermore, this is the first meta-analysis to systematically analyze the role of HIF-1α expression in glioma.
HIF-1α, an O2-sensitive subunit, is rapidly split by ubiquitination and proteasomal degradation under a normoxic condition, whereas is stabilized and translocates from the cytoplasm to the nucleus under a hypoxic condition [47], which contributes to the regulation of multiple adaptive responses, such as cell proliferation, metabolism, and angiogenesis. It also regulates lymphangiogenic cytokine expression in chronic sterile inflammation [48]. HIF-1α expression is shown to be associated with disease progression. Patients in primary hepatocellular carcinoma who exhibited high HIF-1α expression had significantly poorer overall survival than those with low expression [49]. HIF-1α expression might be a predicative factor of poor prognosis for gastric cancer particularly in Asia [50]. In patients with invasive breast cancer, it correlated with tumor clinicopathological parameters [51].
HIF-1α affected glioma tumor growth, and might play a role in clinical applications for patients with malignant glioma [52]. Studies have exhibited a significant relationship between HIF-1α overexpression and poor prognosis in glioma patients [53]. In majority of glioblastoma, HIF-1α was up-regulated, and the activated regulation of HIF-1α made glioblastoma stem cells more sensitive to hypoxia-mediated maintenance, which provided HIF-1α as an attractive target for glioblastoma therapy [54]. Silencing HIF-1α expression appeared to inhibit the proliferation, invasion, and migration of glioblastoma U87 cells [55]. HIF-1α was shown to be a useful prognostic factor in astrocytic tumor [56].
HIF-1α might play a role in the survival and self-renewal potential of cancer stem cells. It may affect the biology of glioma through regulating or interacting with other proteins. HIF-1 regulated anterior gradient protein 2 expression, which involved in control of glioblastoma growth and vascularity [57]. Positive expression for HIF-1α was correlated with VEGF and PDGF-C expression in glioma [58]. Up-regulation of miR-183 in malignant gliomas induced HIF-1α expression and might play a role in glioma biology [59]. Nuclear factor E2-related factor 2 (Nrf2) and HIF-1α were shown to be overexpressed in glioblastoma tissues, and there was significant correlation between HIF-1α level and Nrf2 status overall survive and progression-free survival [60]. HIF-1α and HIF-2α competitively binded to NICD and regulated the activation of Notch signaling, which provided improved therapeutic opportunities for malignant gliomas [61]. Knock down of HIF-1α in human and murine glioma cells might impair the migration in vitro and their invasion in vivo [62].
Several limitations were presented in this meta-analysis. Firstly, most of the included studies were conducted in Chinese population, other populations should be considered. Secondly, the cutoff points for HIF-1a expression were not in unison, which might influence the reliability of results. Thirdly, the included studies for some comparisons were small, such as age, 1-year overall survival. Lastly, other causes, such as genetic polymorphisms of HIF-1α or factors which may interact with HIF-1α expression should be focused on.
In conclusions, our results suggested that HIF-1α expression was significantly associated with high WHO grade of glioma, MVD, 3 (2 or 1) year overall survival and cumulative survival time, indicating that HIF-1α might be a valuable biomarker for glioma grade and therapeutic treatment. However, further studies concerning other non-Asian populations should be included in the future research.
Disclosure of conflict of interest
None.
References
- 1.Marumoto T, Saya H. Molecular biology of glioma, in Glioma. Springer; 2012. pp. 2–11. [DOI] [PubMed] [Google Scholar]
- 2.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jovčevska I, Kočevar N, Komel R. Glioma and glioblastoma‑how much do we (not) know? (Review) Mol Clin Oncol. 2013;1:935–941. doi: 10.3892/mco.2013.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sehmer EA, Hall GJ, Greenberg DC, O’Hara C, Wallingford SC, Wright KA, Green AC. Incidence of glioma in a northwestern region of England, 2006-2010. Neuro Oncol. 2014;16:971–4. doi: 10.1093/neuonc/not301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Houben M, Aben K, Teepen J, Schouten-Van Meeteren AY, Tijssen CC, Van Duijn CM, Coebergh JW. Stable incidence of childhood and adult glioma in The Netherlands, 1989-2003. Acta Oncologica. 2006;45:272–279. doi: 10.1080/02841860500543190. [DOI] [PubMed] [Google Scholar]
- 6.Butowski NA, Berger M. Malignant Gliomas: Part I: Epidemiology, Risk Factors, Prognostic Factors, and Imaging Findings. Contemporary Neurosurgery. 2012;34:1–5. [Google Scholar]
- 7.Efird JT. Epidemiology of Glioma. INTECH Open Access Publisher; 2011. [Google Scholar]
- 8.Nakazato Y. The 4th Edition of WHO Classification of Tumours of the Central Nervous System published in 2007. No Shinkei Geka. 2008;36:473–91. [PubMed] [Google Scholar]
- 9.Ostrom QT, Bauchet L, Davis FG, Deltour I, Fisher JL, Langer CE, Pekmezci M, Schwartzbaum JA, Turner MC, Walsh KM, Wrensch MR, Barnholtz-Sloan JS. The epidemiology of glioma in adults: a “state of the science” review. Neuro Oncol. 2014;16:896–913. doi: 10.1093/neuonc/nou087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Semenza GL. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J Clin Invest. 2013;123:3664–71. doi: 10.1172/JCI67230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryan HE, Lo J, Johnson RS. HIF-1 alpha is required for solid tumor formation and embryonic vascularization. EMBO J. 1998;17:3005–15. doi: 10.1093/emboj/17.11.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P, Koch CJ, Ratcliffe P, Moons L, Jain RK, Collen D, Keshert E. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394:485–90. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- 13.Minardi D, Lucarini G, Santoni M, Mazzucchelli R, Burattini L, Conti A, Principi E, Bianconi M, Scartozzi M, Milanese G, Di Primio R, Montironi R, Cascinu S, Muzzonigro G. Survival in Patients with Clear Cell Renal Cell Carcinoma Is Predicted by HIF-1α Expression. Anticancer Res. 2015;35:433–438. [PubMed] [Google Scholar]
- 14.Reszec J, Hermanowicz A, Rutkowski R, Bernaczyk P, Mariak Z, Chyczewski L. Evaluation of mast cells and hypoxia inducible factor-1 expression in meningiomas of various grades in correlation with peritumoral brain edema. J Neurooncol. 2013;115:119–125. doi: 10.1007/s11060-013-1208-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaynar MY, Sanus GZ, Hnimoglu H, Kacira T, Kemerdere R, Atukeren P, Gumustas K, Canbaz B, Tanriverdi T. Expression of hypoxia inducible factor-1alpha in tumors of patients with glioblastoma multiforme and transitional meningioma. J Clin Neurosci. 2008;15:1036–42. doi: 10.1016/j.jocn.2007.07.080. [DOI] [PubMed] [Google Scholar]
- 16.Womeldorff M, Gillespie D, Jensen RL. Hypoxia-inducible factor-1 and associated upstream and downstream proteins in the pathophysiology and management of glioblastoma. Neurosurg Focus. 2014;37:E8. doi: 10.3171/2014.9.focus14496. [DOI] [PubMed] [Google Scholar]
- 17.Luo Z, Bai M, Xiao X, Zhang W, Liu X, Yang X, Li S, Huan Y, Wu Z, Zhang X, Cao W. Silencing of HIF-1alpha enhances the radiation sensitivity of human glioma growth in vitro and in vivo. Neuropharmacology. 2015;89:168–74. doi: 10.1016/j.neuropharm.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Yi L, Hou X, Zhou J, Xu L, Ouyang Q, Liang H, Zheng Z, Chen H, Xu M. HIF-1alpha genetic variants and protein expression confer the susceptibility and prognosis of gliomas. Neuromolecular Med. 2014;16:578–86. doi: 10.1007/s12017-014-8310-1. [DOI] [PubMed] [Google Scholar]
- 19.Kleihues P, Burger P, Scheithauer B. Histological Typing of Tumors of the Central Nervous System: World Health Organization International Histologic Classification of Tumors. Berlin: Springer-Verlag; 1993. [Google Scholar]
- 20.Wells G, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses [Internet], 2014. 2015 [Google Scholar]
- 21.Cao WD, Kawai N, Miyake K, Zhang X, Fei Z, Tamiya T. Relationship of 14-3-3zeta (ζ), HIF-1α, and VEGF expression in human brain gliomas. Brain Tumor Pathol. 2014;31:1–10. doi: 10.1007/s10014-013-0135-3. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Li YM, Tian RF, Liu WP, Fei Z, Long QF, Wang XA, Zhang X. The expression and significance of HIF-1α and GLUT-3 in glioma. Brain Res. 2009;1304:149–154. doi: 10.1016/j.brainres.2009.09.083. [DOI] [PubMed] [Google Scholar]
- 23.Liao CC, Fan YH, Xiao B, Wu L, Zhu XG. The correlation between the expressions of hypoxiainducible factor-1α and centromere protein W in glioma tissues and cells. Tumor. 2014;34:1045–1051. [Google Scholar]
- 24.Xing P, Lv Z, Zhang X, Yuan J. Expression and significance of HIF-1α, MGMT, CD133 in human glioblastoma. Chinese Journal of Clinicians Electronic Edition. 2013;7:6258–6264. [Google Scholar]
- 25.Gao W, Guo K, Yu R, Pan X. Study of relativity between expressions of hypoxia-inducible factor-1 alpha and PCNA in human gliomas and prognosis of patients with gliomas. Chinese Journal of Clinical Neurosurgery. 2004;9:354–356. [Google Scholar]
- 26.Fu K, Yuan X. The expression of HIF-1αand its relationship to apoptosis and prognostic significance in human glioma. Wuhan University. 2005 [Google Scholar]
- 27.Wang G, Yang X. Study on the expression of HIF-1α and VEGF in human gliomas. Tianjin Medical University. 2006 [Google Scholar]
- 28.Yin J, Zhao S, Fan S, Zhu X. Hypoxia inducible factor-1α expression in gliomas and its effect on prognosis. Hunan Medical Journal. 2007;18:25–27. [Google Scholar]
- 29.Zheng K, Jiao B, Geng S. Expression of HIF-1α and VEGF in human glioma and their significance in the pathogenesis thereof. Journal of Brain and Nervous Diseases. 2008;16:55–58. [Google Scholar]
- 30.Tan Y, Fang C, Sun H, Liu H. Expression and clinical significance of hypoxia inducible factor-1α and vascular endothlal growth factor in human gliomas. Shandong Medical Journal. 2008;48:4–6. [Google Scholar]
- 31.Li L, Guo Z. The expressions of HIF-1α and nNOS in human cerebral glioma. Chinese Medical Sciences University. 2008 [Google Scholar]
- 32.XXu X, Fan Z. The expression of hypoxia-inducible factor-1α and glucose transport-3 in human gliomas. Hebei Medical University. 2008 [Google Scholar]
- 33.Liu Q, Sun G, Liu A, Zheng L, Xiong F. Relationship between expressions of HIF-1a and MMP-9 and angiogenesis and peritumorous edema of glioma. Chinese Journal of Cancer Prevention and Treatment. 2009;16:1654–1656. [Google Scholar]
- 34.Li F, Yang X. The significance of HIF-1a, MMP-9, and VEGF expressions in gliomas. Acta Academiae Medicinae Xuzhou. 2009;29:680–681. [Google Scholar]
- 35.Zhao S, Niu G, Tao S. Expression of ING4 and HIF-1a in human brain astrocytoma. Zhengzhou University. 2010 [Google Scholar]
- 36.Wu W, Zhang Z. Expression and clinical significance of hypoxia inducible factor-1a in gliomas. Journal of Huaihai Medicine. 2010;28:105–106. [Google Scholar]
- 37.Yu G, Ge X, Song Y, Fu R, Chen C. Study on relationship between the expression of HIF-1a and postoperative radiotherapy of glioma. Journal of Qiqihar Medical College. 2010;31:3199–3201. [Google Scholar]
- 38.Zhang L, Liu Y, Yang F. Expression and clinical significance of HIF-1a in gliomas. Chinese Journal of Neurosurgical Disease Research. 2010;9:325–327. [Google Scholar]
- 39.Li J, Yu G. The expression and their clinical significance of HIF-1a and Ki-67 in human brain gliomas. Chinese Medicine Guides. 2011;8:21–23. [Google Scholar]
- 40.Zhang M, Fan H, Han X, Wang D. Expression of HIF-1a, MMP-2 and VEGF in gliomas and their relativity analysis. Cancer Research on Prevention and Treatment. 2011;38:460–461. [Google Scholar]
- 41.Zhang H, Chu S, Ma Y, et al. The expression and significance of SLC22A18 and hypoxia-inducible facotr-1a in brain gliomas. Chinese Journal of Clinical Physicians. 2011;5:4148–4151. [Google Scholar]
- 42.Jiang J, Lv Y, Ji Y, LI X. Significance and expressions of PTEN, Survivin and HIF-1a in gliomas. Chinese Journal of Cancer Prevention and Treatment. 2012;19:1532–1534. [Google Scholar]
- 43.Xing P, Zhang X, Li J, Yuan J. Study on the relationship between the expression of HIF-1, MGMT and psot-operation chemotherapy in various grade glioma. Chinese Journal of Difficult and Complicated Cases. 2013;12:852–854. [Google Scholar]
- 44.Liu X, Gu Y. The correlation and clinical significance of neuroglobin and HIF-1a expressed in gliomas. Zhongnan University. 2013 [Google Scholar]
- 45.Diamond EL, Corner GW, De Rosa A, Breitbart W, Applebaum AJ. Prognostic awareness and communication of prognostic information in malignant glioma: a systematic review. J Neurooncol. 2014;119:227–34. doi: 10.1007/s11060-014-1487-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reardon DA, Wen PY. Glioma in 2014: Unravelling tumour heterogeneity-implications for therapy. Nat Rev Clin Oncol. 2015;12:69–70. doi: 10.1038/nrclinonc.2014.223. [DOI] [PubMed] [Google Scholar]
- 47.Kaur B, Khwaja FW, Severson EA, Matheny SL, Brat DJ, Van Meir EG. Hypoxia and the hypoxiainducible-factor pathway in glioma growth and angiogenesis. Neuro Oncol. 2005;7:134–53. doi: 10.1215/S1152851704001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zampell JC, Daluvoy S, Tomer A, Yan A, Mehrara BJ. HIF-1a regulates lymphangiogenic cytokine expression in chronic sterile inflammation. Journal of the American College of Surgeons. 2011;213:S97. [Google Scholar]
- 49.Xiang ZL, Zeng ZC, Fan J, Tang ZY, He J, Zeng HY, Chang JY. The expression of HIF-1alpha in primary hepatocellular carcinoma and its correlation with radiotherapy response and clinical outcome. Mol Biol Rep. 2012;39:2021–9. doi: 10.1007/s11033-011-0949-1. [DOI] [PubMed] [Google Scholar]
- 50.Zhu Cl, Huang Q, Liu CH, Lin XS, Xie F. Prognostic value of HIF-1α expression in patients with gastric cancer. Mol Biol Rep. 2013;40:6055–6062. doi: 10.1007/s11033-013-2715-z. [DOI] [PubMed] [Google Scholar]
- 51.Papatheodorou H, et al. in Virchows Archiv. New York, NY 10013, USA: Springer 233 Spring ST; 2011. Hypoxia inducing factor 1a (HIF-1a) expression in invasive breast cancer: correlation with tumor clinicopathological parameters. [Google Scholar]
- 52.Jensen RL, Ragel BT, Whang K, Gillespie D. Inhibition of hypoxia inducible factor-1alpha (HIF-1alpha) decreases vascular endothelial growth factor (VEGF) secretion and tumor growth in malignant gliomas. J Neurooncol. 2006;78:233–47. doi: 10.1007/s11060-005-9103-z. [DOI] [PubMed] [Google Scholar]
- 53.Oliver L, Olivier C, Marhuenda FB, Campone M, Vallette FM. Hypoxia and the malignant glioma microenvironment: regulation and implications for therapy. Curr Mol Pharmacol. 2009;2:263–84. doi: 10.2174/1874467210902030263. [DOI] [PubMed] [Google Scholar]
- 54.Qiang L, Wu T, Zhang HW, Lu N, Hu R, Wang YJ, Zhao L, Chen FH, Wang XT, You QD, Guo QL. HIF-1α is critical for hypoxia-mediated maintenance of glioblastoma stem cells by activating Notch signaling pathway. Cell Death Differ. 2012;19:284–294. doi: 10.1038/cdd.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shen S, Kwan A, Chen Y, Wang Z. Effect of silencing HIF-1α on proliferation, invasion and migration of glioblastoma U87 cells. Neurol Sci. 2013;34:365–371. doi: 10.1007/s10072-012-1010-4. [DOI] [PubMed] [Google Scholar]
- 56.Mashiko R, Takano S, Ishikawa E, Yamamoto T, Nakai K, Matsumura A. Hypoxia-inducible factor 1α expression is a prognostic biomarker in patients with astrocytic tumors associated with necrosis on MR image. J Neurooncol. 2011;102:43–50. doi: 10.1007/s11060-010-0292-8. [DOI] [PubMed] [Google Scholar]
- 57.Hong XY, Wang J, Li Z. AGR2 expression is regulated by HIF-1 and contributes to growth and angiogenesis of glioblastoma. Cell Biochem Biophys. 2013;67:1487–1495. doi: 10.1007/s12013-013-9650-4. [DOI] [PubMed] [Google Scholar]
- 58.Clara CA, Marie SK, de Almeida JR, Wakamatsu A, Oba-Shinjo SM, Uno M, Neville M, Rosemberg S. Angiogenesis and expression of PDGFC, VEGF, CD105 and HIF-1alpha in human glioblastoma. Neuropathology. 2014;34:343–52. doi: 10.1111/neup.12111. [DOI] [PubMed] [Google Scholar]
- 59.Tanaka H, Sasayama T, Tanaka K, Nakamizo S, Nishihara M, Mizukawa K, Kohta M, Koyama J, Miyake S, Taniguchi M, Hosoda K, Kohmura E. MicroRNA-183 upregulates HIF-1alpha by targeting isocitrate dehydrogenase 2 (IDH2) in glioma cells. J Neurooncol. 2013;111:273–83. doi: 10.1007/s11060-012-1027-9. [DOI] [PubMed] [Google Scholar]
- 60.Ji X, Wang H, Zhu J, Tang Y, Zhou Y, Zhu L, Gao C, Li W, You W, Yu B, Xia Q. Correlation of Nrf2 and HIF-1α in glioblastoma and their relationships to clinicopathologic features and survival. Neurol Res. 2013;35:1044–1050. doi: 10.1179/1743132813Y.0000000251. [DOI] [PubMed] [Google Scholar]
- 61.Hu YY, Fu LA, Li SZ, Chen Y, Li JC, Han J, Liang L, Li L, Ji CC, Zheng MH, Han H. Hif-1α and Hif-2α differentially regulate Notch signaling through competitive interaction with the intracellular domain of Notch receptors in glioma stem cells. Cancer Lett. 2014;349:67–76. doi: 10.1016/j.canlet.2014.03.035. [DOI] [PubMed] [Google Scholar]
- 62.Méndez O, Zavadil J, Esencay M, Lukyanov Y, Santovasi D, Wang SC, Newcomb EW, Zagzag D. Research Knock down of HIF-1α in glioma cells reduces migration in vitro and invasion in vivo and impairs their ability to form tumor spheres. Mol Cancer. 2010;9:133. doi: 10.1186/1476-4598-9-133. [DOI] [PMC free article] [PubMed] [Google Scholar]


